Abstract
Introduction: Depression represents a public health issue because it significantly increases the risk of disabilities and premature mortality, decreases the quality of life, and increases the costs of care. The incomplete remissions favor the aggravation of neurobiological dysfunctions and pathogenesis of severe somatic comorbidities. The etiopathogenic mechanisms of depression are complex and involve multisystemic risk factors (genetic, neuroanatomic, neurobiochemical, neuroendocrine and psychosocial). Cognitive behavioral therapy (CBT) is used in all the stages of depression, as independent therapeutic method or as support of pharmacotherapy. Patients, Materials and Methods: This study has evaluated the therapeutic response of depression in M (medication) group with 136 patients under pharmacotherapy, compared with P (psychotherapy and medication) group with 137 patients treated simultaneously with medication and CBT, and the factors that can improve therapeutic management. Results: Patients with depression had predominantly a reactive onset, recurrent evolution of at least four episodes, and frequent somatic comorbidities. After treatment, a significant improvement of depressive symptomatology was recorded especially in M group (72.06%), compared to P group (88.32%), p<0.01, as well as a significant difference in regaining functional skills (69.12% – M group, 93.43% – P group; p<0.001). The therapeutic response was significantly correlated with age (p<0.01), social-economical involvement and education level. Conclusions: CBT demonstrated efficiency in the treatment of major depressive disorder in association with pharmacotherapy. The therapeutic approach should rely on the pathogenic biological models that would highlight the prediction indicators for the therapeutic response and for the evolution of depression, as well as considering the psychological profile of each patient.
Keywords: depression, cognitive behavioral therapy, psychosocial function, somatic comorbidities
⧉ Introduction
The depressive disorder remains one of the most common psychiatric disorders at global level, current estimations amounting to over 320 million people currently suffering from this disorder. The global estimates show that 4.4% of the total population is affected by depression, with a higher incidence in women (5.1%) compared with men (3.6%) [1,2]. The approach with cognitive behavioral therapy (CBT) of depression must consider the individual neurobiological background of each patient and the influence of pharmacological therapies over cognitive circuits and emotional functions. Although most psychotherapists consider the depressive disorder a mental health disorder, it should be considered that current translational research underline a multisystemic pathogenesis for this disorder [3,4].
If the clinical diagnosis of depression is based on the diagnosis criteria from Diagnostic and Statistical Manual of Mental Disorders 5 (DSM-5) and International Statistical Classification of Diseases and Related Health Problems 10 (ICD-10), the efficiency of therapy is determined by the multifactorial neurobiological underlayer influencing the integrity of the cognitive and emotional circuits. The depression activates the neuroendocrine system, the hypothalamic–pituitary–adrenal (HPA) axis, intensifies the activity of proinflammatory factors and the endothelial dysfunction at cerebral, cardiac, and renal level. The incomplete remissions favor the continuation of neurobiological dysfunctions determining the pathogenesis of severe somatic comorbidities (diabetes, cardiometabolic syndrome, cardiovascular diseases, stroke, neurodegenerative diseases, and significant cognitive impairment) [5,6].
In this context, the use of singular and uncustomed therapeutic strategies can have a limited efficiency, while the unfavorable evolution of somatic comorbidities may trigger secondary stress factors lowering the patient’s resilience capacity. Hence, the chronic forms of depression and the severity of symptomatology with multiple psychic and somatic consequences become a real public health issue. The depressive disorder represents a major cause for disabilities, significantly increases the risk of premature mortality, decreases the quality of life, and generates a substantial burden on the health care system. The patients with depression show diminished social and economic functioning capacity, independent functioning, social, professional, and family productiveness, as well as personal development activities, especially educational. The most dramatic consequence of depression remains the suicidal act, given that approximately 700 000 persons die every year on this account, and suicide is the second main death cause for teenagers and youngsters aged between 15 and 29 [7].
Depression has a major impact on public health, even more important than some somatic diseases, such as coronary diseases, rheumatoid arthritis, and hyperglycemia [8], and additionally the considerable economical and financial impact [9]. The association of depression with somatic diseases determines a negative prognosis and increases the risk of invalidity and mortality. The onset and evolution of the depressive disorder can also be influenced by socio-demographic factors. The gender of the patient, the age and the marital status were proven to be significantly associated with depression, with a twice higher risk of development of depression in women compared to men, in separated or divorced, as well as in elderly [1, 10,11]. The etiopathogenic mechanisms of depression are complex and have not been totally explained, since it involves a complex of multisystemic risk factors from genetic, neuroanatomical, neurobiochemical, neuroendocrine and psychosocial specters. This approach allows the elaboration of pathogenic biological models that underline indicators for the prediction of therapeutic efficiency and of the evolution of the depressive disorder.
The treatment of depression includes also non-pharmacological interventions represented by psychotherapeutic techniques, used in all stages of the disorder, both as an independent therapeutic method and as support or means to consolidate the pharmacological therapy. The usefulness and efficacy of these methods determined their inclusion as therapeutic recommendations in the international guidelines for depression treatment [12,13,14,15,16], and their integration in the psychiatric management of depression. Psychotherapy is considered a useful instrument especially in addressing psychological and psychosocial stress factors that are responsible both for the onset and for the negative evolution of depressive symptomatology. This type of non-pharmacological therapy had higher beneficial effects also regarding long-term efficacy, by maintaining the remission, in comparison with the pharmacological therapy after termination of the active treatment [17,18]. In the context of associating pharmacotherapy with psychotherapy, important beneficial effects were recorded both in obtaining and maintaining remissions, but also in reacquiring social skills and self-esteem affected by depression [19].
CBT is targeted at the cognitive patterns of the persons with depression and their behavioral reflection, its combined action being oriented on the irrational beliefs and distorted attitude towards self and environment, leading to depressive symptomatology. This therapy aims at instigating and reversing the negative factors to eliminate prejudice and maladaptive behavior [20]. CBT proved an efficient treatment method in the case of patients with major depressive disorder, similar to other types of such interventions (interpersonal or behavioral therapy), but there are no conclusive results yet regarding comparative efficacy with psychopharmacological therapies [21,22]. The non-conclusive results seem to be in direct relation with the functional or lesional modification of cognitive circuits during the evolution of depression, correlated with the drug side effects or with associated vascular and metabolic pathology. Evaluation of these mechanisms may allow for customization of each case and a CBT approach adapted to the patient and his cognitive resilience.
Aim
This study aimed to highlight the role of some clinical, neurobiological, and pharmacological indicators that allow for CBT personalization, especially in association with psychopharmacological treatment. The use of these indicators in the current practice will help in the eternal dilemma of the clinical therapist regarding the psychopharmacological, psychotherapeutic or combined treatment options.
⧉ Patients, Materials and Methods
This study targeted the evaluation of the therapeutic response in patients with depression that were administered only pharmacological treatment, compared with patients that received medication therapy associated with CBT interventions, as well as identifying the factors that may contribute to improving the strategies for therapeutic management. The study included patients diagnosed with major depressive disorder according to ICD-10 criteria, with other psychiatric comorbidities, admitted in the Psychiatry Clinic I, Clinical Neuropsychiatry Hospital, Craiova, Romania, during 2017–2019, split in two groups. The criteria for inclusion in the study were: age over 18, absence of other psychiatric diagnostics and Mini-Mental State Examination (MMSE) score ≥18. M (medication) group included 136 patients that received during hospitalization pharmacological treatment according to treatment protocols and guides in force. P (psychotherapy and medication) group enrolled 137 patients that benefited during hospitalization both from pharmacological treatment and CBT, performed in the medical institution by the psychotherapist–psychologist certified in using this technique.
The characteristics of patients were evaluated from sociodemographic, clinical, and psychological perspectives, to contribute to a better quality of the results of the therapeutic intervention on the evolution and prognosis of the disorder. The severity of the depressive symptomatology was assessed using Hamilton Depression Rating Scale (HAM-D) and the social adaptation level with Global Assessment of Functioning (GAF). These tests were done on admission (initial evaluation) and after 12 weeks of treatment (final evaluation). The cognitive status was evaluated with the MMSE. The MMSE assessment was done only initially, being a criterion for inclusion in the study. The groups in the study included only patients without cognitive deficit or with mild cognitive deficit, with MMSE score ≥18, because CBT efficiency is dependent on the patient’s cognitive status [23].
This study was performed in accordance with the provisions of the Helsinki Declaration and was approved by the Ethics Commission of the University for Medicine and Pharmacy of Craiova. All patients were voluntarily enrolled in the study based on informed consent, and the data collection and storage respected the principles of anonymity and security.
The statistical analysis used Microsoft Excel software (Microsoft Corp., Redmond, WA, USA), together with XLSTAT suite for MS Excel (Addinsoft SARL, Paris, France). The information was stored in Microsoft Excel files, then statistically processed, to analyze the relations between clinical and paraclinical data from the patients. The secondary processing of data, represented by the descriptive analysis of the groups of patients according to various parameters, calculation of fundamental statistical parameters, such as average and standard deviation, was done with Excel software. For performing complex statistical tests [χ2 (chi-squared), Kendall’s tau] the XLSTAT was used.
⧉ Results
The analysis of socio-demographic indicators showed that the average age was 52.83±3.48 years for both groups, 52.23±3.13 years for women and 54.52±3.85 years for men. The enrolled patients were mainly women (73.99%), with an urban residence (91.94%), married or involved in a couple (84.98%), with no professional employment (90.11%), and graduates from general education (73.16%) (Table 1).
Table 1.
Socio-demographic characteristics of the patients in the two groups
|
Study group |
Socio-demographic characteristics |
|||||||||||
|
Mean age |
Women |
Men |
||||||||||
|
M group [years] |
52.41±3.19 |
54.66±3.56 |
||||||||||
|
P group [years] |
52.04±3.07 |
54.41±4.1 |
||||||||||
|
Total [years] |
52.23±3.13 |
54.52±3.8 |
||||||||||
|
Age group [years] |
40–44 |
45–49 |
50–54 |
55–59 |
60–64 |
|||||||
|
M group, n (%) |
3 (2.21%) |
18 (13.24%) |
69 (50.74%) |
43 (31.62%) |
3 (2.21%) |
|||||||
|
P group, n (%) |
5 (3.65%) |
18 (3.14%) |
71 (51.82%) |
41 (29.93%) |
2 (1.46%) |
|||||||
|
Total, n (%) |
8 (2.93%) |
36 (13.19%) |
140 (51.28%) |
84 (30.77%) |
5 (1.83%) |
|||||||
|
Gender |
Women |
Men |
||||||||||
|
M group, n (%) |
104 (76.47%) |
32 (23.53%) |
||||||||||
|
P group, n (%) |
98 (71.53%) |
39 (28.47%) |
||||||||||
|
Total, n (%) |
202 (73.99%) |
71 (26.01%) |
||||||||||
|
Place of residence |
Rural |
Urban |
||||||||||
|
M group, n (%) |
9 (6.62%) |
127 (93.38%) |
||||||||||
|
P group, n (%) |
13 (9.49%) |
124 (90.51%) |
||||||||||
|
Total, n (%) |
22 (8.06%) |
251 (91.94%) |
||||||||||
|
Marital status |
Married |
Unmarried |
Widower |
Divorced |
||||||||
|
M group, n (%) |
114 (83.82%) |
4 (2.94%) |
8 (5.88%) |
10 (7.35%) |
||||||||
|
P group, n (%) |
118 (86.13%) |
4 (2.92%) |
11 (8.03%) |
4 (2.92%) |
||||||||
|
Total, n (%) |
232 (84.98%) |
8 (2.93%) |
19 (6.96%) |
14 (5.13%) |
||||||||
|
Employment |
Employed |
Retired |
Unemployed |
No job |
||||||||
|
M group, n (%) |
10 (7.35%) |
115 (84.56%) |
4 (2.94%) |
7 (5.15%) |
||||||||
|
P group, n (%) |
17 (12.41%) |
102 (74.45%) |
11 (8.03%) |
7 (5.11%) |
||||||||
|
Total, n (%) |
27 (9.89%) |
217 (79.49%) |
15 (5.49%) |
14 (5.13%) |
||||||||
|
Education |
No education |
Primary school |
Secondary school |
Vocational school |
High school |
Post high school |
Faculty |
|||||
|
M group, n (%) |
2 (1.47%) |
1 (0.74%) |
32 (23.53%) |
37 (27.21%) |
59 (43.38%) |
1 (0.74%) |
4 (2.94%) |
|||||
|
P group, n (%) |
6 (4.38%) |
2 (1.46%) |
30 (21.90%) |
30 (21.90%) |
53 (38.69%) |
2 (1.46%) |
14 (10.22%) |
|||||
|
Total, n (%) |
8 (2.93%) |
3 (1.10%) |
62 (22.71%) |
67 (24.54%) |
112 (41.03%) |
3 (1.10%) |
18 (6.59%) |
|||||
M: Medication group; n: No. of cases; P: Psychotherapy and medication group
Analysis of the clinical evolution indicators showed that the onset of depression was mostly reactive (71.79%), patients having over four previous admissions (42.65% in M group, respectively 42.34% in P group), previous psychological trauma had a significantly lower frequency (28.68% in M group, respectively 25.55% in P group) and the presence of other psychiatric diagnostics in the medical history of the patients in the study was similar for both groups (22.79% for M group, respectively 18.98% in P group) (Table 2).
Table 2.
Comparison of clinical evolution prediction indicators in the two groups of patients
|
Study group |
Indicator |
|||||
|
|
Form of onset |
|||||
|
Insidious |
Reactive |
|||||
|
M group, n (%) |
34 (25.00%) |
102 (75.00%) |
||||
|
P group, n (%) |
43 (31.39%) |
94 (68.61%) |
||||
|
Total, n (%) |
77 (28.21%) |
196 (71.79%) |
||||
|
|
No. of previous admissions |
|||||
|
4 |
5 |
6 |
7+ |
|||
|
M group, n (%) |
58 (42.65%) |
24 (17.65%) |
21 (15.44%) |
33 (24.26%) |
||
|
P group, n (%) |
58 (42.34%) |
29 (21.17%) |
20 (14.60%) |
30 (21.90%) |
||
|
Total, n (%) |
116 (42.49%) |
53 (19.41%) |
41 (15.02%) |
63 (23.08%) |
||
|
Previous psychological trauma |
||||||
|
Absent |
Present |
|||||
|
M group, n (%) |
97 (71.32%) |
39 (28.68%) |
||||
|
P group, n (%) |
102 (74.45%) |
35 (25.55%) |
||||
|
Total, n (%) |
199 (72.89%) |
74 (27.11%) |
||||
|
Psychiatric history |
||||||
|
Absent |
Present |
|||||
|
M group, n (%) |
105 (77.21%) |
31 (22.79%) |
||||
|
P group, n (%) |
111 (81.02%) |
26 (18.98%) |
||||
|
Total, n (%) |
216 (79.12%) |
57 (20.88%) |
||||
|
|
Somatic comorbidities |
|||||
|
Absent |
Present |
|
||||
|
M group, n (%) |
35 (25.74%) |
101 (74.26%) |
|
|||
|
P group, n (%) |
40 (29.20%) |
97 (70.80%) |
|
|||
|
Total, n (%) |
75 (27.47%) |
198 (72.53%) |
|
|||
|
|
Suicidal behavior and ideation |
|
||||
|
Absent |
Present |
|
||||
|
M group, n (%) |
125 (91.91%) |
11 (8.09%) |
|
|||
|
P group, n (%) |
124 (90.51%) |
13 (9.49%) |
|
|||
|
Total, n (%) |
249 (91.21%) |
24 (8.79%) |
|
|||
|
|
Smoking |
|
||||
|
No |
Yes |
|
||||
|
M group, n (%) |
104 (76.47%) |
32 (23.53%) |
|
|||
|
P group, n (%) |
114 (83.21%) |
23 (16.79%) |
|
|||
|
Total, n (%) |
218 (79.85%) |
55 (20.15%) |
|
|||
|
|
Alcohol consumption |
|
||||
|
Abstinence / occasional |
Moderate / abusive |
|
||||
|
M group, n (%) |
68 (50.00%) |
68 (50.00%) |
|
|||
|
P group, n (%) |
74 (54.01%) |
63 (45.99%) |
|
|||
|
Total, n (%) |
142 (52.01%) |
131 (47.99%) |
|
|||
M: Medication group; n: No. of cases; P: Psychotherapy and medication group
A major factor with significant influence on the evolution of the depressive disorder is the presence of somatic comorbidities, these being present in approximately two thirds of the patients (74.26% M group, respectively 70.83% P group). The suicidal behavior and ideation had a reduced percentage in both study groups (8.09% M group, respectively 9.49% P group). In M group, 23.53% of the patients are smokers and 50% have a moderate or abusive alcohol consumption, while in P group, 16.79% of the cases smoke and 45.99% have a moderate or abusive alcohol consumption.
The cognitive status of the patients was evaluated on admission by using the MMSE scale, the resulting scores splitting the patients from the cognitive function perspective in two categories: without cognitive deficit (MMSE score 24–30), respectively mild cognitive deficit (MMSE score 18–23). Mild cognitive impairment was present in most of the patients from both study groups (58.82% in M group, 64.96% in P group, respectively 61.90% in total group), but following statistical analysis showed no significant difference between the two groups (χ2 test, p=0.2962439, p>0.05). The other patients did not have cognitive impairment.
The seriousness of depressive symptoms was evaluated using the HAM-D scale upon admission and further on after a 12-weeks interval. The HAM-D scores were clustered in three categories HAM-D score <14 (mild depression), HAM-D score 14–17 (moderate depression), respectively HAM-D score >17 (severe depression). A prevalence of moderate severity symptoms was apparent in both groups (50.74% in M group, respectively 48.91% in P group), but the patients distribution depending on this variable did not show statistically significant differences (χ2 test, p=0.581994273, p>0.05). At the second moment of evaluation, the severity of depressive symptoms decreased significantly in P group, where mild depression was present in 88.32% of cases, compared with 72.06% in M group. This result confirms the efficiency of the therapeutic approach associating pharmacotherapy with psychotherapy, correlated with the highly significant statistical difference recorded among the values of HAM-D scores in the two analyzed groups (χ2 test, p=0.003336489, p<0.01). Following treatment, the patients with moderate or severe depression showed residual post-therapeutic symptoms quantified as mild depression.
The level of social adjustment was measured in the initial and final evaluations, by using GAF questionnaire, that was quantified, depending on the scores, as deficient (GAF<50), respectively positive (GAF>50). Both groups recorded GAF scores demonstrating the impact of depression on the capacity for functioning and social networking of the affected individuals (98.53% M group, respectively 97.08% P group) (χ2 test, p=0.41418423, p>0.05). At the final evaluation, the observed situation was similar as in the evaluation of the symptomatology severity, the statistical analysis shows a highly significant statistical difference between the two groups (χ2 test, p=2.55897E-07, p<0.001). The results show that the social skills of the patients in P group improved from the perspective of the results obtained on GAF scale in most patients (93.43%), probably because of the CBT training, compared with only 69.12% in M group which received only medication treatment (Table 3).
Table 3.
Comparative evaluation of the levels of depression and social adjustment in the two groups of patients
|
Study group |
HAM-D scores initial evaluation |
|||
|
HAM-D<14 |
HAM-D 14–17 |
HAM-D>17 |
||
|
M group, n (%) |
15 (11.03%) |
69 (50.74%) |
52 (38.24%) |
|
|
P group, n (%) |
11 (8.03%) |
67 (48.91%) |
59 (43.07%) |
|
|
Total, n (%) |
26 (9.52%) |
136 (49.82%) |
111 (40.66%) |
|
|
Study group |
HAM-D scores final evaluation |
|||
|
HAM-D<14 |
HAM-D 14–17 |
HAM-D>17 |
||
|
M group, n (%) |
98 (72.06%) |
30 (22.06%) |
8 (5.88%) |
|
|
P group, n (%) |
121 (88.32%) |
13 (9.49%) |
3 (2.19%) |
|
|
Total, n (%) |
219 (80.22%) |
43 (15.75%) |
11 (4.03%) |
|
|
Study group |
GAF scores initial evaluation |
|||
|
GAF<50 |
GAF>50 |
|||
|
M group, n (%) |
134 (98.53%) |
2 (1.47%) |
||
|
P group, n (%) |
133 (97.08%) |
4 (2.92%) |
||
|
Total, n (%) |
267 (97.80%) |
6 (2.20%) |
||
|
Study group |
GAF scores final evaluation |
|||
|
GAF<50 |
GAF>50 |
|||
|
M group, n (%) |
42 (30.88%) |
94 (69.12%) |
||
|
P group, n (%) |
9 (6.57%) |
128 (93.43%) |
||
|
Total, n (%) |
51 (18.68%) |
222 (81.32%) |
||
GAF: Global Assessment of Functioning; HAM-D: Hamilton Depression Rating Scale; M: Medication group; n: No. of cases; P: Psychotherapy and medication group
In both groups, the initial evaluation showed a prevalence of social dysfunctionality (GAF<50), statistically significantly correlated with the high severity of depression (HAM-D>17) both in M group and in P group (Kendall’s tau test, p<0.001). The same level of significant association (Kendall’s tau test, p<0.001) was observed in the final evaluation, especially in the patients with combined therapy, the improvement of symptomatology being correlated with the significant improvement in the global functionality level (Table 4).
Table 4.
Correlations between the severity of depression and the level of social adjustment
|
M group |
GAF scores initial evaluation |
||
|
HAM-D scores initial evaluation |
GAF<50 |
GAF>50 |
Total |
|
HAM-D<14, n (%) |
13 (86.67%) |
2 (13.33%) |
15 (100.00%) |
|
HAM-D 14–17, n (%) |
69 (100.00%) |
0 (0.00%) |
69 (100.00%) |
|
HAM-D>17, n (%) |
52 (100.00%) |
0 (0.00%) |
52 (100.00%) |
|
Total |
134 (98.53%) |
2 (1.47%) |
136 (100.00%) |
|
M group |
GAF scores final evaluation |
||
|
HAM-D scores final evaluation |
GAF<50 |
GAF>50 |
Total |
|
HAM-D<14, n (%) |
4 (4.08%) |
94 (95.92%) |
98 (100.00%) |
|
HAM-D 14–17, n (%) |
30 (100.00%) |
0 (0.00%) |
30 (100.00%) |
|
HAM-D>17, n (%) |
8 (100.00%) |
0 (0.00%) |
8 (100.00%) |
|
Total |
42 (30.88%) |
94 (69.12%) |
136 (100.00%) |
|
P group |
GAF scores initial evaluation |
||
|
HAM-D scores initial evaluation |
GAF<50 |
GAF>50 |
Total |
|
HAM-D<14, n (%) |
7 (63.64%) |
4 (36.36%) |
11 (100.00%) |
|
HAM-D 14–17, n (%) |
67 (100.00%) |
0 (0.00%) |
67 (100.00%) |
|
HAM-D>17, n (%) |
59 (100.00%) |
0 (0.00%) |
59 (100.00%) |
|
Total |
133 (97.08%) |
4 (2.92%) |
137 (100.00%) |
|
P group |
GAF scores final evaluation |
||
|
HAM-D scores final evaluation |
GAF<50 |
GAF>50 |
Total |
|
HAM-D<14, n (%) |
0 (0.00%) |
121 (100.00%) |
121 (100.00%) |
|
HAM-D 14–17, n (%) |
6 (46.15%) |
7 (53.85%) |
13 (100.00%) |
|
HAM-D>17, n (%) |
3 (100.00%) |
0 (0.00%) |
3 (100.00%) |
|
Total |
9 (6.57%) |
128 (93.43%) |
137 (100.00%) |
GAF: Global Assessment of Functioning; HAM-D: Hamilton Depression Rating Scale; M: Medication group; n: No. of cases; P: Psychotherapy and medication group
In the patients receiving pharmacological treatment associated with CBT the levels of correlation between the socio-demographic data and the severity of depression were analyzed as well, resulting a highly significant correlation between the age distribution and the results of the combined therapeutic process including psychotherapy (χ2 test, p=0.001168343, p<0.01), as well as with their level of involvement in social and professional activities (χ2 test, p=0.009275363, p<0.01). Improvement of HAM-D scores consecutive with the complex therapeutic approach showed a highly significant statistical correlation with the educational level of the patients (χ2 test, p=4.10163E-06, p<0.001).
The global functioning deficit leads to the depreciation of social and professional status of the person affected by depression, a situation identified also in the patients in the study receiving CBT (χ2 test, p=0.0278, p<0.05). Another significant indicator correlated with the severity of depression expressed by the global dysfunctionality level was represented by the educational level of the depressive patients, a predominantly medium level (vocational and high school studies) which was significantly associated with the scores obtained on GAF scale in P group (χ2 test, p=0.038432336, p<0.05).
⧉ Discussions
The major depression disorder has negative effects on the individual in all domains of life, but especially on the personal abilities for functioning and social networking, thus determining a high burden of the disorder that is experienced both by the afflicted person and by the members of the family or community that person is part of. The socio-demographic characteristics play an important role in the onset and evolution of depression. The results of the study show the prevalence of women (73.99%), compared with men (26.01%), this 3/1 ratio for women aged over 30 being mentioned in the specialized literature [24]. The evaluation of the marital status pointed out that 84.98% of the patients are married or involved in a couple relationship, while some data in the specialized literature indicate high rates of the depressive disorder in single, divorced or widowed persons, this element being considered as a risk factor for the onset and unfavorable evolution of the disorder [11, 25]. The socio-professional status and educational level of patients, two indicators that are interdependent, show an extremely reduced involvement in socio-professional activities (9.89%), while 73.26% of them have at least a medium education level. These results are explained by the severe disability caused by depression over individual lucrative capacities, which determines a major suffering for the individual and his family [26,27,28].
The depression onset comes most frequently with a reactive form (71.79%), with predisposing factors as psychotraumatic events, from recent history and from childhood or adolescence, respectively acute or chronic exposure to stress factors being most often incriminated for the major depressive disorder, both in the diathesis–stress model [29,30,31,32], and in the theories supporting the depressogenic role of emotional trauma [33,34,35]. From this point of view, the results of this study confirm the hypothesis that there are no protective factors for the onset of the disorder, even if socio-demographic differences are present. The data related to the patient’s medical history underlines a reduced frequency of presence of a psychotraumatic event (27.11%) or of psychiatric history other than depression (20.88%). This fact leads to the conclusion that prolonged exposure to stress factors represents the main source for the onset of depression, in this respect being evident the socio-economic issues creating the favorable ground for exacerbating the depressive symptomatology [26,27,28, 36,37].
The somatic comorbidities, present in two thirds of the patients, represent simultaneous both vulnerability factors for the onset of depression and consequences of psychiatric disorders. It is highly accepted the negative role that the major depressive disorder has both on the general health status of the patient [38,39], and on the quality of the evolution and prognosis of the associated somatic diseases [5, 39]. In the relation between depressive disorder and somatic comorbidities, risky behaviors are directly involved, smoking and alcohol consumption playing a major role in exacerbating the symptomatology and the negative evolution of depression [40,41,42]. On the other hand, the alcohol consumption aggravates the suicidal behavior, this risk being amplified in the presence of psychiatric disorders, such as psychosis, mood disorders, anxiety disorders or stress susceptibility, given the reciprocal influence between them [43]. Since suicide is directly and strongly associated with the depressive disorder, as one and the most severe of its consequences [44,45,46], the evaluation of the suicidal behavior is necessary by identifying suicidal attempts and suicidal ideation in the patients’ medical history.
Considering all these factors, the therapeutic approach requires customization, considering that the association of pharmacological treatment with CBT is with high statistical significance more efficient than the exclusively medication therapy. The advantages of the multidisciplinary therapeutic approach were important for the patients with mild or severe depression, for whom after 12 weeks of combined interdisciplinary treatment, the final evaluation showed elements of symptomatic remission or just mild depressive symptomatology, an argument that is supported by other studies as well [47,48,49].
At the same time, the combined therapy, compared with the effects of the medication therapy, demonstrated a highly significant statistical improvement of the global functioning (psychological, social, and occupational) of the patients. In such case, we can support that the results were not only due to the recognized efficacy of this technique as interventional assembly in the management of the depressive disorder [19], but more to the complex levels it acts upon, thus involving the biopsychosocial triangle specific to the depressive disorder [50,51,52,53].
Secondly, to be efficient, the psychotherapeutic interventions, especially the cognitive–behavioral ones, must benefit from a well-preserved cognitive support, that is able to deal both with the challenges required by these techniques [20], and to the potential cognitive deficits generated as side effects of the antidepressant or psychotropic medication used in the treatment of depression [12,13,14,15,16]. The MMSE score facilitate the psychotherapeutic intervention, by signaling the dysfunction of the cognitive circuits that can be influenced by somatic comorbidities, alcohol addiction or pharmacological strategies. The presence of the cognitive deficit cautions the clinical psychotherapist of the need to customize the psychotherapeutic techniques and to evaluate the neurobiological resilience, factors which may influence the efficiency of CBT.
The biological background of the depressive disorder is determined first of all by the activation of the HPA axis, emphasized by the presence of social and professional stress, acute or chronic. The main detrimental consequence of this hyperactivity is represented by the increase of endogenous cortisol, damaging the gamma-aminobutyric acid (GABA)/glutamate balance in the hippocampus, leading to the atrophy of this cerebral area. The hippocampal atrophy objectified by neuroimaging evaluation is correlated with the cognitive deficit and increased risk of important somatic comorbidities, such as hyperglycemia, cardiometabolic syndrome, obesity, stroke [54]. The recurrence of depression and incomplete remissions amplify the intensity of the atrophy. Ventriculomegaly can be an indirect indicator for the diminished capacities for activating the stem cells repairing hippocampal circuits, due to the lesioning of the subventricular granular area [55].
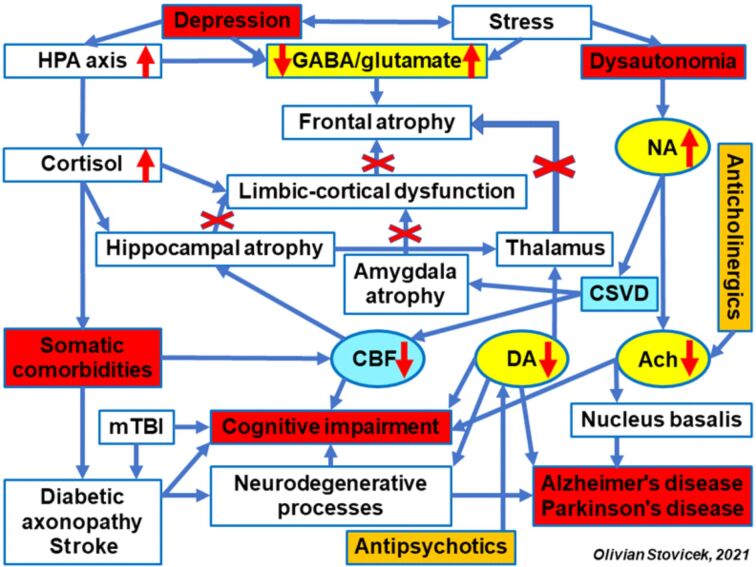
Another important consequence of hippocampal atrophy is the missing connectivity of the cognitive circuits in this area with limbic structures, especially amygdala, but also with thalamus and nucleus accumbens. The thalamus is an area integrating the cognitive circuits with the emotional ones. The dysfunctionality of this area disturbs the thalamic–cortical circuit, triggering the hypofrontality syndrome that may associate adynamia, anhedonia and apathy. This clinical presentation represents a characteristic of the depression associating the dysfunction of the frontal lobe, with alteration of the working memory and attention and learning deficits (Figure 1).
Figure 1.
Neurobiological and pharmacological aspects involved in cognitive impairment from the depressive disorder. Ach: Acetylcholine; CBF: Cerebral blood flow; CSVD: Cerebral small vessel disease; DA: Dopamine; NA: Noradrenaline; HPA: Hypothalamic–pituitary–adrenal; GABA: Gamma-aminobutyric acid; mTBI: Mild traumatic brain injury
The thalamic lesion can also be the consequence of involvement of vascular factors, endothelial dysfunction, and cerebral small vessel disease (CSVD) syndrome associating major cognitive dysfunctions. This context brings up the topic of a veritable thalamic vascular syndrome [56]. Evaluation of the thalamic lesion and thalamo–frontal dysconnectivity can become an indirect indicator of a neurodegenerative process. The onset of Alzheimer’s disease can be predicted in persons over 60 years old with mild cognitive impairment (MCI) syndrome [57]. The presence of stress amplifies the endogenous cortisol secretion and damages the GABA/glutamate balance in the frontal cortex, glutamate hyperactivity determining frontal atrophy. Neuroimaging evaluation and going from the relatively normal cognitive status to progressing cognitive deficit, suggested by the onset of disturbance in the verbal fluency, can be indicators suggesting the evolution towards a frontotemporal dementia [58]. Cognitive dysfunction can be accentuated by CSVD, chronic cerebral ischemia, with or without stroke, diabetic axonopathy or mild traumatic brain injury (mTBI) [59].
The association of stress with the depressive disorder leads to the functional disturbance of the autonomous nervous system, with the increase of noradrenaline (NA) release, provoking spasms in the cerebral arterioles and significant decrease in the cerebral blood flow (CBF). An acute ischemic syndrome can appear at coronary level, which can be involved in sudden death of patients. However, the arterial spasm and the chronic cerebral ischemia induces a decreased release of acetylcholine (Ach) in the Meynert nucleus and in the cerebral vessels, determining a CBF decrease by affecting the self-regulatory mechanisms [60]. The anticholinergic effects of antidepressants, especially tricyclic, may favor the lesional mechanism in the frontal cortex and hippocampus [61]. The cholinergic blockade with atropine that was objectified in the animal model, by histopathological examination. This morphological injury and frontal cortex disconnectivity can be amplified by cerebral ischemia (Figures 2,3,4). The animal model also underlined an increase in frontal and hippocampal lesions in large doses of exogenous cortical steroids, such as Dexamethasone [62].
Figure 2.
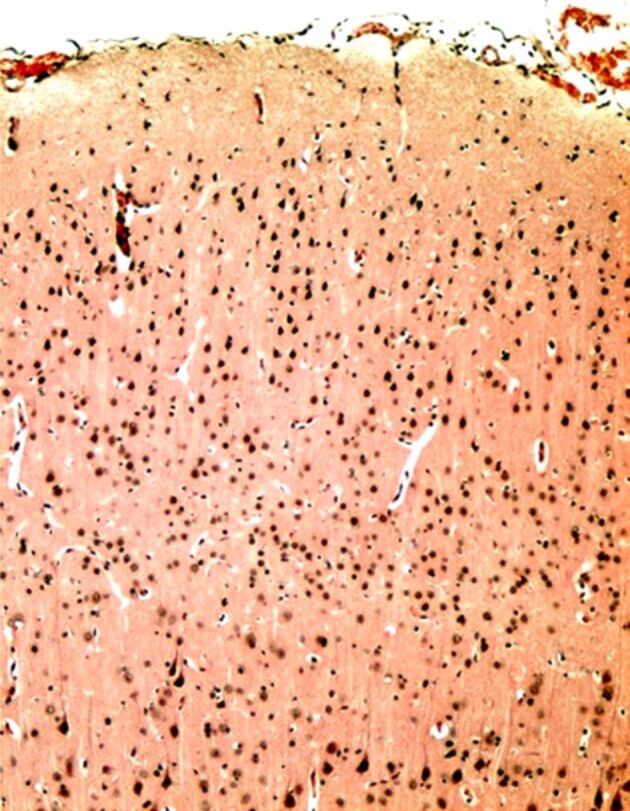
Microscopic image of normal cerebral cortex, without lesions of neuronal cytoarchitecture. Hematoxylin–Eosin (HE) staining ×100
Figure 3.
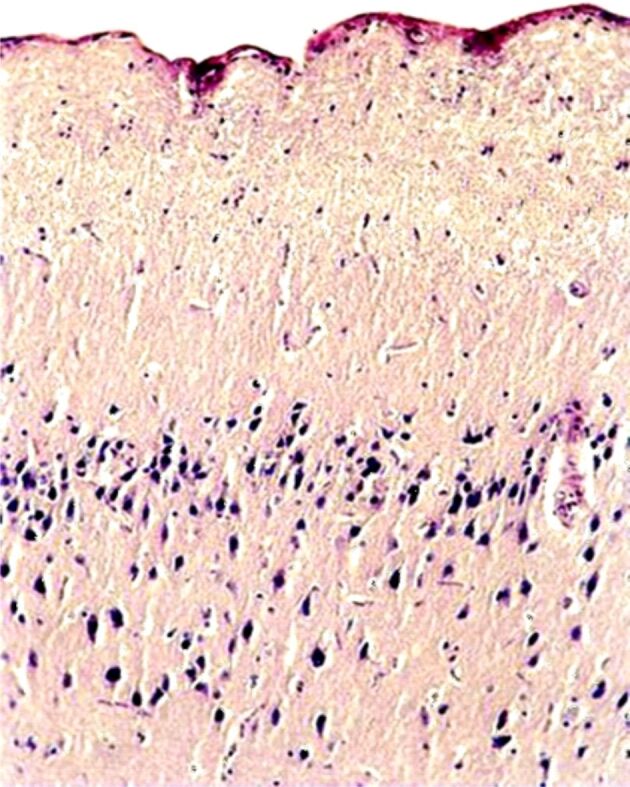
Cholinergic blockade: microscopic image of the cerebral cortex showing a reduction in the number of neurons, especially in the superficial layers (molecular, external granular and external pyramidal layers), with neuropil fragmentation in these areas (fragmentation of neuronal prolongation) due to neuronal cell death. In the deep layers, the condensation of neurons with tachychromatic nucleus is observed. HE staining ×100
Figure 4.
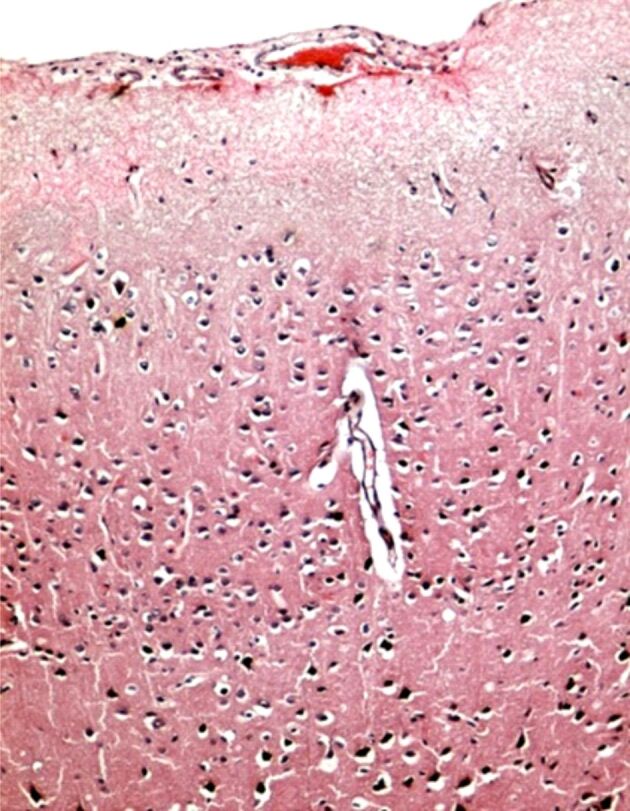
Ischemia: microscopic image of the cerebral cortex showing a reduction in the number of neurons in the superficial (molecular) layer, with marked perineuronal and perivascular edema. HE staining ×100
The lowered Ach level inhibits the release of dopamine (DA) in the basal ganglia. The use of antipsychotics blocking D2 DA receptors amplifies the DA deficit, and the occurrence of extrapyramidal symptoms (EPS) can be an indicator anticipating Parkinson’s disease [63].
CBF decrease and the hyperglutamatergic activity leads to disconnection of the nucleus accumbens from the cognitive and emotional circuits with low of reward capacity and risk of treatment-resistant addiction and anhedonia comorbidities [64]. The nucleus accumbens, a mainly dopaminergic structure, becomes dysfunctional also from administration of medication with antidopaminergic effect.
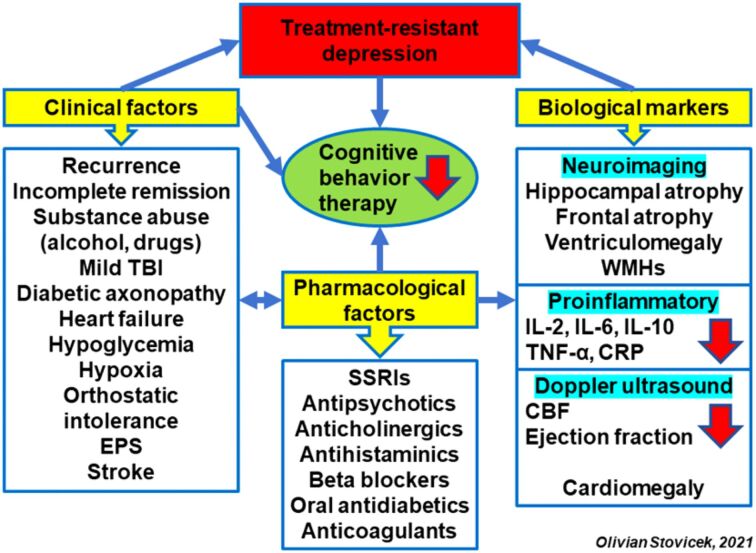
In the clinical approach, the use of theoretical biological and psychopharmacological models allows to highlight some risk factors. From the clinical point of view, the main risk factors are multiple relapses, incomplete remissions, alcohol or illicit substances consumption, mTBI, diabetic axonopathy, diabetic neuropathy pain, heart failure, hypoglycemia, hypoxia, orthostatic intolerance syndrome, EPS, decrease in verbal flow fluency, stroke. Secondly, the pharmacological factors can be correlated with DA decrease, following the blockage of D2 receptors with antipsychotics or selective serotonin reuptake inhibitors (SSRIs) antidepressants. In this case, the clinical marker if the onset of EPS, as side effects. Administration of anticholinergics, especially in the case of gastrointestinal or neurological comorbidities (medication parkinsonism) decrease the Ach level, the main mediator involved in maintaining the cognitive function (Figure 5).
Figure 5.
Clinical, neuroimaging and pharmacological factors with negative influence on cognitive behavioral psychotherapy. CBF: Cerebral blood flow; CRP: C-reactive protein; EPS: Extrapyramidal symptoms; IL: Interleukin; SSRIs: Selective serotonin reuptake inhibitors; TBI: Traumatic brain injury; TNF-α: Tumor necrosis factor-alpha; WMHs: White matter hyperintensities
Antihistaminic medication can determine hypodopaminergia indirectly, which can be correlated with cognitive deficit in the working memory and attention, but also with increased prolactin levels. Beta-blockers, frequently used in cardiovascular comorbidities can block the NA release in locus coeruleus, favoring the functional disturbance of the autonomous nervous system with the orthostatic hypotension. Due to the CBF decrease, the beta-blocking medication can be involved in deterioration of the cognitive circuits. Hypoglycemia consequent to administration of insulin or oral antidiabetic medication for the hyperglycemic patients can lead to alteration of hippocampus and exacerbate the hippocampal atrophy induced by the increase endogenous cortisol. This structural modification is specific for the depressive disorder with cognitive impairment.
Anticoagulants drugs administered in the treatment of cardiac comorbidities (atrial fibrillation, thromboembolic accidents) can generate microhemorrhages in the cerebral areas strategic for cognitive circuits. Important caution should be considered in the patients with depressive disorder and infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), who may have an increased risk of cerebral microbleeds under administration of anticoagulant therapies.
The main markers supporting this pathogenic model are the neuroimaging markers (hippocampal atrophy, frontal atrophy, ventriculomegaly, white matter hyperintensities) and the proinflammatory ones [increase level of interleukin (IL)-2, IL-6, IL-10, tumor necrosis factor-alpha (TNF-α), C-reactive protein]. The evaluation of cerebral circulation with transcranial Doppler (TCD) ultrasound may bring information on CBF decrease following the diminished blood speed in the circle of Willis, especially in the anterior pole [65]. The Doppler echocardiography evaluation can highlight ventriculomegaly and ejection fraction decrease, which can be correlated with lower DA level following SSRI or antipsychotic treatment blocking D2 receptors.
Initiation of CBT, disregarding the screening of the aforementioned risk factors, can determine therapeutic resistance, both in pharmacological therapy and in psychotherapy. The occurrence of diminished verbal fluency during CBT is an important clinical and psychological indicator for the clinical psychologist. When approaching the depressive disorder with CBT, the case must be customized depending on the risk factors and the scores obtained with specific evaluation scales (MMSE, GAF, HAM-D), to initiate a therapeutic strategy adjusted to the functional or lesional status of the cognitive circuits.
⧉ Conclusions
The major depressive disorder is caused by multisystemic pathogenic mechanisms, but also by the presence of incomplete remission and frequent relapses, becoming a major issue for the clinical psychologist, as well as for the psychiatrist or clinical pharmacologist. The cognitive deterioration, through lack of antidepressant therapeutic response, is a market for the negative prognosis of depression. Identifying the risk factors for cognitive deficit becomes compulsory at the start of associated psychotherapy, since it no longer allows the integration of precognitive information in the resilience mechanisms. Customization of pharmacological treatment and Continuous Performance Test (CPT) according to risk factor and depression pathogenic models can significantly improve the therapeutic response, with decrease in incomplete remissions and risk for developing somatic comorbidities. The results of the current research demonstrate the efficiency of CBT used in the treatment of major depressive disorder as auxiliary to pharmacotherapy, but the psychological profile of each patient needs evaluation. The interdisciplinary cooperation is paramount in monitoring and maintaining the therapeutic effects obtained.
Conflict of interest
The authors declare that they have no conflict of interests.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. . Lancet. 2018;2019;392393(10159)(10190):1789–1858. e44–e44. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Depression and other common mental disorders global health estimates.Document No. WHO/MSD/MER/2017.2, License: CC BY-NC-SA 3.0 IGO. Geneva, Switzerland: WHO; 2017. https://apps.who.int/iris/handle/10665/254610 [Google Scholar]
- 3.Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138–162. doi: 10.1016/j.neuroscience.2015.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasler G. Pathophysiology of depression: do we have any solid evidence of interest to clinicians. World Psychiatry. 2010;9(3):155–161. doi: 10.1002/j.2051-5545.2010.tb00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steffen A, Nübel J, Jacobi F, Bätzing J, Holstiege J. Mental and somatic comorbidity of depression: a comprehensive cross-sectional analysis of 202 diagnosis groups using German nationwide ambulatory claims data. BMC Psychiatry. 2020;20(1):142–142. doi: 10.1186/s12888-020-02546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otte C. Incomplete remission in depression: role of psychiatric and somatic comorbidity. Dialogues Clin Neurosci. 2008;10(4):453–460. doi: 10.31887/DCNS.2008.10.4/cotte. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization (WHO) Suicide, key facts. Geneva, Switzerland: WHO; 2021. http://www.who.int/news-room/fact-sheets/detail/suicide [Google Scholar]
- 8.Egede LE. Depression: greater effect on overall health than angina, arthritis, asthma or diabetes. Evid Based Ment Health. 2008;11(2):57–57. doi: 10.1136/ebmh.11.2.57. [DOI] [PubMed] [Google Scholar]
- 9.Kessler RC. The costs of depression. Psychiatr Clin North Am. 2012;35(1):1–14. doi: 10.1016/j.psc.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W, Dragomirecka E, Kohn R, Keller M, Kessler RC, Kawakami N, Kiliç C, Offord D, Ustun TB, Wittchen HU. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. . Int J Methods Psychiatr Res. 2003;1212(1)(3):3–21. 165–165. doi: 10.1002/mpr.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van de Velde S, Bracke P, Levecque K. Gender differences in depression in 23 European countries. Cross-national variation in the gender gap in depression. Soc Sci Med. 2010;71(2):305–313. doi: 10.1016/j.socscimed.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 12.National Collaborating Centre for Mental Health (UK) Depression: the treatment and management of depression in adults (updated edition). Leicester, UK: British Psychological Society; 2010. [PubMed] [Google Scholar]
- 13.Baghai TC, Blier P, Baldwin DS, Bauer M, Goodwin GM, Fountoulakis KN, Kasper S, Leonard BE, Malt UF, Stein D, Versiani M, Möller HJ, Section of Pharmacopsychiatry, World Psychiatric Association General and comparative efficacy and effectiveness of antidepressants in the acute treatment of depressive disorders: a report by the WPA section of pharmacopsychiatry. Eur Arch Psychiatry Clin Neurosci. 2011;261(Suppl 3):207–245. doi: 10.1007/s00406-011-0259-6. [DOI] [PubMed] [Google Scholar]
- 14.Soleimani L, Lapidus KAB, Iosifescu DV. Diagnosis and treatment of major depressive disorder. Neurol Clin. 2011;29(1):177–193, ix. doi: 10.1016/j.ncl.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Bauer M, Pfennig A, Severus E, Whybrow PC, Angst J, Möller HJ, World Federation of Societies of Biological Psychiatry. Task Force on Unipolar Depressive Disorders World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14(5):334–385. doi: 10.3109/15622975.2013.804195. [DOI] [PubMed] [Google Scholar]
- 16.Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, Dickens C, Ferrier IN, Geddes J, Gilbody S, Haddad PM, Katona C, Lewis G, Malizia A, McAllister-Williams RH, Ramchandani P, Scott J, Taylor D, Uher R, Members of the Consensus Meeting Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology Guidelines. J Psychopharmacol. 2015;29(5):459–525. doi: 10.1177/0269881115581093. [DOI] [PubMed] [Google Scholar]
- 17.Vittengl JR, Clark LA, Dunn TW, Jarrett RB. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive–behavioral therapy’s effects. J Consult Clin Psychol. 2007;75(3):475–488. doi: 10.1037/0022-006X.75.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobson KS, Hollon SD, Dimidjian S, Schmaling KB, Kohlenberg RJ, Gallop RJ, Rizvi SL, Gollan JK, Dunner DL, Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J Consult Clin Psychol. 2008;76(3):468–477. doi: 10.1037/0022-006X.76.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markowitz JC. Psychotherapy of the postdysthymic patient. J Psychother Pract Res. 1993;2(2):157–163. [PMC free article] [PubMed] [Google Scholar]
- 20.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. The Guilford Clinical Psychology and Psychotherapy Series. New York, USA: The Guilford Press; 1979. https://www.worldcat.org/title/cognitive-therapy-of-depression/oclc/1030551738?referer=di&ht=edition [Google Scholar]
- 21.Parker G, Roy K, Eyers K. Cognitive behavior therapy for depression? Choose horses for courses. Am J Psychiatry. 2003;160(5):825–834. doi: 10.1176/appi.ajp.160.5.825. [DOI] [PubMed] [Google Scholar]
- 22.Basch MF. Understanding psychotherapy: the science behind the art. New York, USA: Basic Books Inc; 1988. https://www.worldcat.org/title/understanding-psychotherapy-the-science-behind-the-art/oclc/17953071 [Google Scholar]
- 23.Regan B, Varanelli L. Adjustment, depression, and anxiety in mild cognitive impairment and early dementia: a systematic review of psychological intervention studies. Int Psychogeriatr. 2013;25(12):1963–1984. doi: 10.1017/S104161021300152X. [DOI] [PubMed] [Google Scholar]
- 24.Gutiérrez-Lobos K, Scherer M, Anderer P, Katschnig H. The influence of age on the female/male ratio of treated incidence rates in depression. BMC Psychiatry. 2002;2:3–3. doi: 10.1186/1471-244X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulloch AG, Williams JV, Lavorato DH, Patten SB. The relationship between major depression and marital disruption is bidirectional. Depress Anxiety. 2009;26(12):1172–1177. doi: 10.1002/da.20618. [DOI] [PubMed] [Google Scholar]
- 26.Barrett AE, Turner RJ. Family structure and mental health: the mediating effects of socioeconomic status, family process, and social stress. J Health Soc Behav. 2005;46(2):156–169. doi: 10.1177/002214650504600203. [DOI] [PubMed] [Google Scholar]
- 27.Banks KH, Kohn-Wood LP. The influence of racial identity profiles on the relationship between racial discrimination and depressive symptoms. J Black Psychol. 2007;33(3):331–354. https://journals.sagepub.com/doi/10.1177/0095798407302540 [Google Scholar]
- 28.Gee GC, Spencer M, Chen J, Yip T, Takeuchi DT. The association between self-reported racial discrimination and 12-month DSM-IV mental disorders among Asian Americans nationwide. Soc Sci Med. 2007;64(10):1984–1996. doi: 10.1016/j.socscimed.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 30.Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- 31.Mazure CM. Life stressors as risk factors in depression. Clin Psychol Sci Pract. 1998;5(3):291–313. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1468-2850.1998.tb00151.x [Google Scholar]
- 32.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156(6):837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 33.Shih JH, Eberhart NK, Hammen CL, Brennan PA. Differential exposure and reactivity to interpersonal stress predict sex differences in adolescent depression. J Clin Child Adolesc Psychol. 2006;35(1):103–115. doi: 10.1207/s15374424jccp3501_9. [DOI] [PubMed] [Google Scholar]
- 34.Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective. Child Dev. 1999;70(3):660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- 35.Maciejewski PK, Prigerson HG, Mazure CM. Sex differences in event-related risk for major depression. Psychol Med. 2001;31(4):593–604. doi: 10.1017/s0033291701003877. [DOI] [PubMed] [Google Scholar]
- 36.Muntaner C, Eaton WW, Miech R, O’Campo P. Socioeconomic position and major mental disorders. Epidemiol Rev. 2004;26:53–62. doi: 10.1093/epirev/mxh001. [DOI] [PubMed] [Google Scholar]
- 37.Malik NM, Boris NW, Heller SS, Harden BJ, Squires J, Chazan-Cohen R, Beeber LS, Kaczynski KJ. Risk for maternal depression and child aggression in Early Head Start families: a test of ecological models. Infant Ment Health J. 2007;28(2):171–191. doi: 10.1002/imhj.20128. [DOI] [PubMed] [Google Scholar]
- 38.Kang HJ, Kim SY, Bae KY, Kim SW, Shin IS, Yoon JS, Kim JM. Comorbidity of depression with physical disorders: research and clinical implications. Chonnam Med J. 2015;51(1):8–18. doi: 10.4068/cmj.2015.51.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.IsHak WW, Steiner AJ, Klimowicz A, Kauzor K, Dang J, Vanle B, Elzahaby C, Reid M, Sumner L, Danovitch I. Major depression comorbid with medical conditions: analysis of quality of life, functioning, and depressive symptom severity. Psychopharmacol Bull. 2018;48(1):8–25. doi: 10.64719/pb.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breslau N, Peterson EL, Schultz LR, Chilcoat HD, Andreski P. Major depression and stages of smoking. A longitudinal investigation. Arch Gen Psychiatry. 1998;55(2):161–166. doi: 10.1001/archpsyc.55.2.161. [DOI] [PubMed] [Google Scholar]
- 41.Fluharty M, Taylor AE, Grabski M, Munafò MR. The association of cigarette smoking with depression and anxiety: a systematic review. Nicotine Tob Res. 2017;19(1):3–13. doi: 10.1093/ntr/ntw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tseng I, Ganz A, Mitton Andrew G, Tsuang J. Comorbidity of alcohol use disorder and depression: a case report and review of the literature. Addict Disord Their Treat. 2017;16(3):121–128. https://journals.lww.com/addictiondisorders/Abstract/2017/09000/Comorbidity_of_Alcohol_Use_Disorder_and.4.aspx [Google Scholar]
- 43.Pompili M, Serafini G, Innamorati M, Dominici G, Ferracuti S, Kotzalidis GD, Serra G, Girardi P, Janiri L, Tatarelli R, Sher L, Lester D. Suicidal behavior and alcohol abuse. Int J Environ Res Public Health. 2010;7(4):1392–1431. doi: 10.3390/ijerph7041392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi Y. Depression and suicide. Jpn Med Assoc J. 2001;44(8):359–363. https://www.suicideinfo.ca/resource/siecno-20080313/ https://www.med.or.jp/english/pdf/2001_08/359_363.pdf [Google Scholar]
- 45.Oquendo MA, Galfalvy H, Russo S, Ellis SP, Grunebaum MF, Burke A, Mann JJ. Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. Am J Psychiatry. 2004;161(8):1433–1441. doi: 10.1176/appi.ajp.161.8.1433. [DOI] [PubMed] [Google Scholar]
- 46.Cavanagh JTO, Carson AJ, Sharpe M, Lawrie SM. Psychological autopsy studies of suicide: a systematic review. Psychol Med. 2003;3333(3)(5):395–405. 947–947. doi: 10.1017/s0033291702006943. [DOI] [PubMed] [Google Scholar]
- 47.Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, Gallop R, McGlinchey JB, Markley DK, Gollan JK, Atkins DC, Dunner DL, Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006;74(4):658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- 48.Pampallona S, Bollini P, Tibaldi G, Kupelnick B, Munizza C. Combined pharmacotherapy and psychological treatment for depression: a systematic review. Arch Gen Psychiatry. 2004;61(7):714–719. doi: 10.1001/archpsyc.61.7.714. [DOI] [PubMed] [Google Scholar]
- 49.Fennell MJV. In: New Oxford textbook of psychiatry. 2. Gelder M, Andreasen N, Lopez-Ibor J, Geddes J, editors. New York USA: Oxford University Press; 2012. Cognitive behaviour therapy for depressive disorders; pp. 1304–1312.https://oxfordmedicine.com/view/10.1093/med/9780199696758.001.0001/med-9780199696758-chapter-0020042 [Google Scholar]
- 50.Boone T, Reilly AJ, Sashkin M. Social learning theory Albert Bandura Englewood Cliffs, NJ. Prentice-Hall 1977 247 pp paperbound. Group Organ Stud. 1977;2(3):384–385. https://journals.sagepub.com/doi/10.1177/105960117700200317 [Google Scholar]
- 51.Lewinsohn PM, Hoberman HM. In: International handbook of behavior modification and therapy. Bellack AS, Hersen M, Kazdin AE, editors. Boston MA USA: Springer; 1982. Depression; pp. 397–431.https://link.springer.com/book/10.1007/978-1-4615-7275-6 [Google Scholar]
- 52.Nezu AM. Efficacy of a social problem-solving therapy approach for unipolar depression. J Consult Clin Psychol. 1986;54(2):196–202. doi: 10.1037//0022-006x.54.2.196. [DOI] [PubMed] [Google Scholar]
- 53.Martell CR, Addis ME, Jacobson NS. Depression in context: strategies for guided action. New York, USA: W.W. Norton & Co; 2001. https://psycnet.apa.org/record/2001-06573-000 [Google Scholar]
- 54.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93(9):3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental–degenerative divide. Neurosci Biobehav Rev. 2009;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003;34(9):2264–2278. doi: 10.1161/01.STR.0000087786.38997.9E. [DOI] [PubMed] [Google Scholar]
- 57.Marinescu I, Enătescu VR, Ghelase ŞM, Marinescu D. Neurobiological arguments for a pathogenic multifactorial disconnective model of cognitive disorders from Alzheimer’s disease in elderly people. Rom J Morphol Embryol. 2017;58(4):1165–1173. [PubMed] [Google Scholar]
- 58.Libon DJ, McMillan C, Gunawardena D, Powers C, Massimo L, Khan A, Morgan B, Farag C, Richmond L, Weinstein J, Moore P, Coslett HB, Chatterjee A, Aguirre G, Grossman M. Neurocognitive contributions to verbal fluency deficits in frontotemporal lobar degeneration. Neurology. 2009;73(7):535–542. doi: 10.1212/WNL.0b013e3181b2a4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stovicek PO, Friedmann C, Marinescu D, Văduva IA, Bondari S, Trifu SC, Marinescu I. Mild TBI in the elderly – risk factor for rapid cognitive impairment in Alzheimer’s disease. Rom J Morphol Embryol. 2020;61(1):61–72. doi: 10.47162/RJME.61.1.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamner JW, Tan CO, Tzeng YC, Taylor JA. Cholinergic control of the cerebral vasculature in humans. J Physiol. 2012;590(24):6343–6352. doi: 10.1113/jphysiol.2012.245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mogoanta L, Marinescu D, Udristoiu T, Udristoiu I, Pirici D. P5d004 Neuroprotective effect of Cerebrolysin and Erythropoietin versus Haloperidol in an Alzheimer’s disease - animal model. Eur Neuropsychopharmacol. 2010;20(Suppl 3):S565–S566. [Google Scholar]
- 62.Marinescu IP, Predescu A, Udriştoiu T, Marinescu D. Comparative study of neuroprotective effect of tricyclics vs. Trazodone on animal model of depressive disorder. Rom J Morphol Embryol. 2012;53(2):397–400. [PubMed] [Google Scholar]
- 63.Mutică M, Marinescu I, Militaru F, Pîrlog MC, Udriştoiu I. Clinical and biological outcomes of prolonged treatment with Haloperidol in schizophrenia. Rom J Morphol Embryol. 2016;57(2):477–481. [PubMed] [Google Scholar]
- 64.Nestler EJ. Role of the brain’s reward circuitry in depression: transcriptional mechanisms. Int Rev Neurobiol. 2015;124:151–170. doi: 10.1016/bs.irn.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roher AE, Garami Z, Tyas SL, Maarouf CL, Kokjohn TA, Belohlavek M, Vedders LJ, Connor D, Sabbagh MN, Beach TG, Emmerling MR. Transcranial Doppler ultrasound blood flow velocity and pulsatility index as systemic indicators for Alzheimer’s disease. Alzheimers Dement. 2011;7(4):445–455. doi: 10.1016/j.jalz.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]