Abstract
Background and Objectives
The concept of person-centered care has been utilized/adapted to various interventions to enhance health-related outcomes and ensure the quality of care delivered to persons living with dementia. A few systematic reviews have been conducted on the use of person-centered interventions in the context of dementia care, but to date, none have analyzed intervention effect by intervention type and target outcome. This study aimed to review person-centered interventions used in the context of dementia care and examine their effectiveness.
Research Design and Methods
A systematic review and meta-analysis were conducted. We searched through 5 databases for randomized controlled trials that utilized person-centered interventions in persons living with dementia from 1998 to 2019. Study quality was assessed using the National Institute for Health and Clinical Excellence checklist. The outcomes of interest for the meta-analysis were behavioral and psychological symptoms in dementia (BPSD) and cognitive function assessed immediately after the baseline measurement.
Results
In total, 36 studies were systematically reviewed. Intervention types were reminiscence, music, and cognitive therapies, and multisensory stimulation. Thirty studies were included in the meta-analysis. Results showed a moderate effect size for overall intervention, a small one for music therapy, and a moderate one for reminiscence therapy on BPSD and cognitive function.
Discussion and Implications
Generally speaking, person-centered interventions showed immediate intervention effects on reducing BPSD and improving cognitive function, although the effect size and significance of each outcome differed by intervention type. Thus, health care providers should consider person-centered interventions as a vital element in dementia care.
Keywords: Behavioral and psychological symptoms in dementia, Cognitive function, Reminiscence therapy
Cognitive impairment is a hallmark symptom of dementia; more than 90% of persons living with dementia experience behavioral and psychological symptoms in dementia (BPSD; Ballard & Corbett, 2010). Additional studies showed that common experiences of people living with dementia are BPSD (Zhao et al., 2016). In particular, a study highlighted that all of these common occurrences are major sources of caregiver burden and reasons to consider institutionalization for this population group (Brodaty et al., 2014; Eska et al., 2013; Voutilainen et al., 2018). Relatedly, person-centered care is recommended as a first line of choice to manage BPSD (Kales et al., 2019) and to provide care for persons living with cognitive impairments.
Person-centered care refers to care in which the “individual’s values and preference are elicited and, once expressed, guide all aspects of their health care, supporting their realistic health and life goals” (The American Geriatrics Society Expert Panel on Person-Centered Care, 2016, p. 16). This concept of care has been emphasized as an essential element to help maintain care quality; in the United States, to encourage person-centered care, the Center for Medicare & Medicaid Services launched a regulation that enforces the documentation of resident’s preferences in long-term care contexts (U.S. Department of Health and Human Services, 2015). In dementia care, the term “person-centered care” was first used in 1998, by Tom Kitwood (1988), and it is now considered a fundamental part of dementia care quality (Fazio et al., 2018).
Since the introduction of this term in the dementia care field of research, many studies using person-centered care have been published. Specifically, two review papers have synthesized the evidence on the effects of person-centered care in persons living with dementia (Chenoweth et al., 2019; Li & Porock, 2014); nonetheless, they did not provide the specific effect size of the person-centered interventions. Partially filling in this research gap, a recent meta-analysis that analyzed 16 studies reported the overall effect size of person-centered care interventions for four specific outcomes (i.e., agitation, neuropsychiatric symptoms, depression, and quality of life); results showed that person-centered interventions were significantly effective on reducing agitation, neuropsychiatric symptoms, and depression, and on improving quality of life (Kim & Park, 2017). However, Kim and Park (2017) did not examine which intervention was effective for each studied outcome.
This lack of examination provides for an important research gap, because various studies have applied different kinds of interventions (e.g., music, reminiscence, cognitive, and multisensory therapies) using the concept of person-centered care, and there are huge differences between each kind; for instance, in music therapy, choosing which music to play during therapy is done based on individuals’ preferences, whereas in cognitive therapy, this type of customization is done based on individuals’ cognitive abilities. Namely, the intervention effect might differ by intervention type and target outcome. Thus, it appears important to understand not only the overall effect of person-centered interventions, but also the effect of each intervention type in persons living with dementia.
Thus, it seems important to evaluate whether person-centered interventions are effective for improving cognitive function and reducing BPSD in persons living with dementia. Accordingly, this study aimed to: (a) systematically review studies using person-centered care and (b) conduct a meta-analysis to examine the overall effectiveness and the effectiveness of each type of person-centered intervention on BPSD and cognitive function of persons living with dementia.
Method
Protocol and Registration
This systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Moher et al., 2009; Supplementary Table 1) and the protocol for this systematic review was registered at International Prospective Register of Systematic Reviews (ID: CRD42020169376).
Search Strategies
We searched the following electronic databases: PubMed, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, Embase, and the Cochrane Database of Systematic Reviews. As mentioned, the term “person-centered care” was introduced in dementia research in 1998; thus, we included articles published in English from January 1998 to September 2019. The search strategies included three key terms: person-centered care, dementia, and randomized controlled trial (RCT). Detailed descriptions are presented herein:
For person-centered care, the combinations of (“person” or “patient” or “resident” “client” or “relationship”) AND (“centered” or “directed” or “oriented” or “focused”) AND (“care” or “intervention”) were used. We also used potential key terms that referred to specific types of interventions using person-centered care: “dementia care mapping” OR “touch therapy” OR “music therapy” OR “aroma therapy” OR “light therapy” OR “validation therapy” OR “Montessori-based activities” OR “reminiscence” OR “emotion-oriented care” OR “sensory stimulation” OR “snoezelen” OR “ability-focused approach.”
For dementia, the terms “dementia” OR “Alzheimer Disease” were used.
For RCT, the terms “randomized controlled trial” OR “controlled clinical trial” OR “randomized” OR “randomly” OR “trial” were used.
Selection of Studies
To prevent the deletion of relevant articles, two reviewers used an online program (http://rayyan.qcri.org) to independently assess the titles and abstracts of the articles that were identified by the search strategy. The eligible studies were included in the full-text review only after consensus was reached; if there was a disagreement between the two reviewers regarding eligibility, a third reviewer intervened.
The analyzed studies were included based on the following criteria:
Participants: Studies with a study population comprising participants who were diagnosed with any type of dementia.
Intervention: Studies that applied the concept of person-centered care, which was defined as “promoting patient involvement and individualization of care” based on a previous study (Robinson et al., 2008). Moreover, we ensured that studies utilized this concept when there were phrases such as: “Meet individualized needs,” “maintain the personhood,” “understanding of personal experience,” “enabling them to make choices about their care,” and “respecting the person’s beliefs, values, and preferences” (Robinson et al., 2008).
Comparison: Studies in which participants were assigned to either an experimental intervention group or a control group with traditional care.
Outcomes: Studies that measured the effects of interventions in cognitive function and BPSD (e.g., depression, agitation, neuropsychiatric symptoms).
Study design: To ensure study homogeneity, we only included RCTs.
Among the eligible studies, we applied an additional criterion for eligibility to the meta-analysis procedure: The quantitative results of the studies had to include adequate statistical values (e.g., mean, standard deviation, and median with range) for computing an effect size.
Data Extraction
We extracted outcome measures that included baseline and post intervention results. If a study had multiple measurement time points, to ensure that we would identify only the effect size of the intervention that was of interest to our review, we compared the results that were measured immediately after the baseline measurement.
Additionally, if the study had more than three intervention arms, we paired them as “person-centered care versus usual care interventions,” based on what the respective study described as the person-centered intervention. To examine the effectiveness of person-centered interventions in persons living with dementia through the meta-analysis, after reaching consensus among the three reviewers, we divided the studies into four categories: (a) music therapy (i.e., doing movements accompanied by music or listening to a preferred music); (b) reminiscence therapy (i.e., the recalling and sharing of personal memories or experiences); (c) cognitive therapy (i.e., general stimulation using concentration, thinking, or learning); and (d) multisensory stimulation (i.e., touch, and additional sensory stimulations to people’s vision, audition, and smell).
Quality Assessment
The quality of the studies was assessed by two independent authors who used the National Institute for Health and Clinical Excellence (NICE) checklist (National Institute for Health and Care Excellence, 2012). If there was a disagreement between the two authors, further discussions were conducted with the intervention of a third author until consensus was reached.
The NICE methodology checklist comprised 14 items regarding four biases (i.e., selection, performance, attrition, and detection biases) that could occur in RCTs. Checklist items were answered based on a four-item scale: “yes,” “no,” “unclear,” and “not applicable.” An item was rated as “yes” when the study minimized the risk of bias for that item, as “no” when the study did not meet the condition proposed by the item and had a potentially high risk of bias, as “unclear” when the item was not reported or if the report was unclear, and as “not applicable” when the item was not applicable for the study.
We allocated points to individual studies according to how items were rated: Two points were allocated if the item was rated as “yes,” one point if “unclear,” and no points if “no.” Then, we calculated the average risk of bias for each study by summing up the points and dividing this sum by the number of checklist items. We excluded the “not applicable” items in the point rating and calculation. Finally, we classified the overall risk of bias for a study as high (<1), moderate (1 to <1.5), and low (≥1.5).
Data Analysis
All data analyses were performed using Comprehensive Meta-Analysis software, version 3 and Review Manager (RevMan) software, version 5. To decrease the effects of between-study heterogeneity (DerSimonian & Laird, 1986), we evaluated the pooled treatment effects using random-effects models. Because the included studies used different measures to assess the outcomes of interest, the standardized mean difference (SMD) was calculated with a 95% confidence interval (CI).
We evaluated the effect size in accordance with Cohen’s criteria: An SMD of ≥0.20 and <0.50 was considered small; ≥0.50 and <0.8 was moderate; and ≥0.8 was large (Cohen, 1992, 2013). The direction of the effect size was defined according to the methodology utilized in the measurement of each outcome; to be included in the meta-analysis, an outcome needed to appear in more than two studies. To confirm between-intervention heterogeneity according to the outcomes of interest, we conducted additional subgroup analyses using the I2 value.
Results
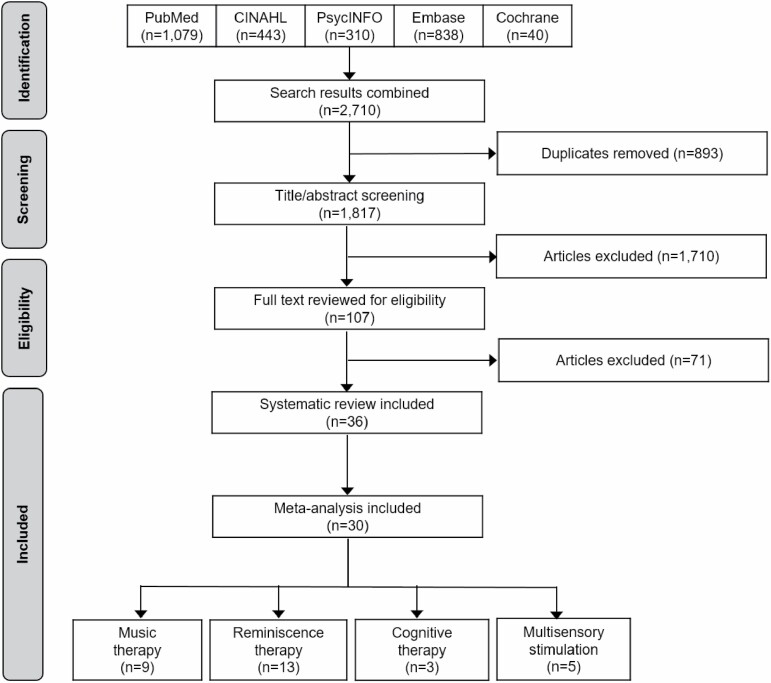
Figure 1 illustrates study selection procedures. The initial search in five databases generated 2,710 articles. After removing duplicates, 1,817 articles remained; after reviewing the titles and abstracts, 107 articles remained.
Figure 1.
Flow diagram of study selection.
Then, we excluded 71 articles through full-text review; the reasons for exclusion are described herein: 34 articles did not use a control group with usual care; 19 articles did not provide a definition for person-centered care that concurred with our definition; seven articles did not use a RCT design; six articles did not include outcomes of interest; three articles did not focus on persons living with dementia as the study population (e.g., they focused on the caregivers, families, and staff); and two articles were published abstracts.
Consequently, 36 articles were included in the systematic review. Among them, six articles were not included in the meta-analysis because they did not provide usable data for computing effect size. Therefore, 30 articles including 2,551 participants underwent meta-analysis.
Characteristics of the Included Studies
Table 1 describes the characteristics of the included studies; the included studies were published from 1998 to 2019: one in 1998, nine from 1999 to 2010, and 26 studies from 2011 to 2019. Participants’ mean age ranged from 69.1 to 94.9 years in the studies.
Table 1.
Characteristics of Included Studies
| Study; country | Dementia type | Intervention | Sample size (mean age) | % of female | Duration (frequency)c | Main instructor | Outcome (instrument) | PCC definitione | |
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | ||||||||
| Amieva et al. (2016); France | AD | Personal CT | 156 (78.90) | 153 (78.70) | 60.22 | 90 (1) | Psychologist | NPS (NPI) | 2 |
| Group CT | 168 (78.50) | ||||||||
| RT | 169 (78.80) | ||||||||
| Aslakson (2010); United States | Dementia | MT | 21 (85.00) | 19 (86.68) | 90.00 | 35 (3) | Music therapist | Agitation (WAI) | 1 |
| Bailey et al. (2017); United States | Dementia | RT | 26 (84.35) | 25 (83.92) | 90.20 | 30 (2) | Psychologist | Depression (CSDD) | 1, 2 |
| Bakshi (2004); Canada | Dementia | MSS | 20 (78.00) | 20 (78.00) | 40.00 | 35 (3) | Occupational therapist | Agitation (CMAI) | 2 |
| Chu et al. (2014); Taiwan | Dementia | MT | 49 (82.00a) | 51 (82.00a) | 53.00 | 30 (2) | Music therapist | Cognition (MMSE), depression (CSDD) | 1 |
| Clare et al. (2010); England | AD, mixed | Personal CT | 22 (76.32) | 22 (78.18) | 58.82 | 60 (1) | Occupational therapist | Depression (HADS) | 2 |
| Duru Aşiret & Kapucu (2016); Turkey | AD | RT | 31 (81.83) | 31 (82.26) | 67.74 | 35 (1) | Nurse | Cognition (MMSE), depression (GDS) | 1 |
| Hong & Choi (2011); Korea | AD, VD, PDD | MT | 15 (78.30a) | 15 (78.30a) | 93.33 | 60 (1) | Music therapist | Cognition (MMSE) | 1 |
| Hsieh et al. (2010); Taiwan | Dementia | RT | 29 (77.90) | 32 (77.25) | 40.98 | 45 (1) | Nurse | Depression (CSDD) | 1 |
| Hsu et al. (2015); England | AD, VD, DFT, DLB, mixed, UNS | MT | 9 (84.56) | 8 (82.50) | 94.12 | 30 (1) | Music therapist | NPS (NPI) | 1 |
| Hutson et al. (2014); England | AD, VD, DLB, mixed, UNS | MSS | 21 (86.80a) | 18 (86.80a) | 86.11 | 50 (2) | Staff | Depression (CSDD), NPS (NPI) | 1 |
| Ito et al. (2007); Japan | VD | RT | 18 (82.90) | 17 (82.10) | 61.76 | 60 (1) | Specialistd | Cognition (MMSE) | 1, 2 |
| Kallio et al. (2018); Finland | AD, VD, PDD, DLB, UNS | CT | 76 (82.60) | 71 (83.60) | 72.00 | 45 (2) | Psychologist | Cognition (MMSE) | 2 |
| Lai et al. (2004); Hong Kong | Dementia | RT | 36 (86.20) | 30 (86.80) | 67.44 | 30 (1) | RA | Cognition (MMSE) | 1, 2 |
| Lawton et al. (1998); England | Dementia | MSS | 88 (NA) | 94 (NA) | NA | NA | Nurse | Depression (MOSES) | 1, 2 |
| Lök et al. (2019); Turkey | AD, unspecified | RT | 30 (NA) | 30 (NA) | 56.67 | 60 (1) | Nurse | Cognition (MMSE), depression (CSDD) | 1, 2 |
| Lopes et al. (2016); Portugal | Dementia | RT | 20 (83.85) | 20 (83.62) | 77.50 | 35 (1) | Nurse | Cognition (MoCA), depression (CSDD) | 1 |
| Lyu et al. (2018); China | AD | MT | 97 (68.90) | 95 (69.90) | 59.03 | 35 (14) | Music therapist | Cognition (MMSE), NPS (NPI) | 1 |
| Maseda et al. (2014); Spain | Dementia | MSS | 10 (87.20) | 10 (86.70) | 95.00 | 30 (2) | Psychologist or occupational therapist |
Agitation (CMAI), cognition (MMSE), depression CSDD), NPS (NPI) | 1 |
| Nakamae et al. (2014); Japan | AD, VD | RT | 17 (84.76) | 19 (87.16) | 100.00 | 40 (1) | Occupational therapist | Cognition (MMSE), depression (CSDD) | 1, 2 |
| Pérez-Ros et al. (2019); Spain | Dementia | MT | 47 (80.06) | 72 (80.80) | 50.85 | 60 (5) | Staff | Cognition (MMSE), depression (CSDD) | 1 |
| Raglio et al. (2015); Italy | Dementia | MT | 40 (81.00) | 40 (82.40) | 78.33 | 30 (2) | Music therapist | Depression (CSDD), NPS (NPI) | 2 |
| Ridder et al. (2013); Denmark and Norway | Dementia | MT | 21 (82.17) | 21 (80.20) | 69.00 | 33.8 (2) | Music therapist | Agitation (CMAI) | 1 |
| Sánchez et al. (2016); Spain | Dementia | MSS | 11 (86.40) | 10 (82.30) | 78.10 | 30 (2) | Occupational therapist | Agitation (CMAI), cognition (MMSE), depression (CSDD), NPS (NPI) | 1, 2 |
| Shiltz et al. (2018); United States | Dementia | MT | 47 (80.00) | 45 (76.00) | 52.17 | 30 (3) | Staff | Agitation (CMAI), cognition (MMSE) | 1 |
| Staal et al. (2007); United States | Dementia | MSS | 12 (80.33) | 12 (72.00) | 66.67 | 30 (3) | RA | Agitation (PAS) | 1, 2 |
| Tadaka & Kanagawa (2007); Japan | Dementia | RT | 30 (84.20) | 30 (82.40) | 75.00 | 90 (1) | Specialistd | Cognition (MMSE), depression (MOSES) | 1, 2 |
| Tanaka et al. (2017); Japan | AD, VD, DLB, mixed, UNS | Personal CT | 20 (86.00) | 20 (86.50) | 90.00 | 60 (2) | Staff | Cognition (MMSE), depression (GDS) | 2 |
| Group CT | 20 (84.90) | 20 (2) | |||||||
| Van Bogaert et al. (2013); Belgium | AD | RT | 41 (83.00) | 41 (85.00) | 82.93 | 45 (2) | Nurse | Cognition (MMSE), depression (CSDD), NPS (NPI) | 1, 2 |
| Van Bogaert et al. (2016); Belgium | Dementia | RT | 29 (84.00b) | 31 (84.00b) | 80.00 | 45 (2) | Nurse | Cognition (MMSE), depression (CSDD), NPS (NPI) | 1, 2 |
| van der Ploeg et al. (2013); Australia | Dementia | MSS | 15 (78.10a) | 29 (78.10a) | 68.18 | 30 (2) | Psychologist | Agitation (CMAI) | 1 |
| Wang (2007); Taiwan | Dementia | RT | 51 (79.76) | 51 (78.92) | 50.98 | 60 (1) | Nurse | Cognition (MMSE), depression (CSDD) | 2 |
| Wang et al. (2018); China | AD | MT | 30 (70.40) | 30 (69.10) | 63.33 | 40 (21) | Music therapist | Cognition (MMSE), NPS (NPI) | 1, 2 |
| Weise et al. (2019); Germany | Dementia | MT | 10 (85.05a) | 10 (85.05a) | 80.00 | 30 (3.5) | Staff | Agitation (CMAI) | 2 |
| Wu & Koo (2016); Taiwan | AD, DFT | RT | 53 (73.50) | 50 (73.60) | 68.93 | 10 (1) | Psychologist | Cognition (MMSE) | 1 |
| Yamagami et al. (2012); Japan | AD, VD, DFT mixed, UNS | CT | 28 (85.50) | 26 (84.90) | 90.74 | 60 (2) | Staff | Depression (MOSES) | 1, 2 |
Notes: AD = Alzheimer’s disease; CMAI = Cohen-Mansfield Agitation Inventory; CSDD = Cornell Scale for Depression in Dementia; CT = cognitive therapy; DFT = dementia of frontal lobe type; DLB = dementia with Lewy bodies; GDS = Geriatric Depression Scale; HADS = Hospital Anxiety and Depression Scale; MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment; MOSES = Multidimensional Observation Scale for Elderly Subjects; MSS = multisensory stimulation; MT = music therapy; NA = not applicable; NPI = Neuropsychiatric Inventory; NPS = neuropsychiatric symptoms; PAS = Pittsburgh Agitation Scale; PCC = person-centered care; PDD = Parkinson disease dementia; RA = research assistant; RT = reminiscence therapy; VD = vascular dementia; UNS = unspecified; WAI = Wisconsin Agitation Inventory.
aAge of all participants. bMedian age. cDuration refers to the number of minutes per session and frequency refers to the number of sessions per week. dSpecialist refers to various types of therapists. ePCC definition (1 = promoting patient involvement, 2 = individualization of care).
The mean sample size (including intervention and control groups) was of approximately 88 persons living with dementia, ranging from 17 to 646. The percentage of female participants in each study ranged from 40% to 100%. Most studies comprised two arms (n = 27), but could include up to four. Most studies were conducted in Japan (n = 5), followed by the United Kingdom (n = 4), Taiwan (n = 4), and the United States (n = 4).
The types of interventions varied widely; reminiscence therapy (n = 14) was the most frequent, but music therapy (n = 11; e.g., music listening, singing, or song writing), multisensory therapy (n = 7; e.g., Montessori-based activities), and cognitive therapy (n = 4) were also included. The persons who conducted the interventions varied by intervention type: nurses (n = 8), music therapists (n = 8), nursing home staff (n = 6), psychologists (n = 6), occupational therapists (n = 5), and so on. The duration (i.e., minutes per session) of each intervention ranged from 10 to 90 min, and the frequency (i.e., number of sessions per week) ranged from 1 to 21.
Among the selected studies, the effectiveness of the interventions was assessed by the following outcomes: agitation (n = 9), cognition (n = 21), depression (n = 20), and neuropsychiatric symptoms (n = 10). Particularly, cognitive therapies evaluated depression (n = 3) or cognition (n = 2); music therapies evaluated cognition (n = 6), neuropsychiatric symptoms (n = 4), agitation (n = 4), or depression (n = 3); multisensory stimulation mainly evaluated agitation (n = 5) or depression (n = 3); reminiscence therapies mostly measured cognition (n = 11) or depression (n = 10).
Moreover, the studies used different tools to measure these outcomes. For agitation, most studies used the Cohen-Mansfield Agitation Inventory (n = 7). For cognition, almost all studies used the Mini-Mental State Examination (MMSE; n = 20). For depression, studies used the Cornell Scale for Depression in Dementia (n = 14), the Multidimensional Observation Scale for Elderly Subjects (n = 3), the Geriatric Depression Scale (n = 2), and the Hospital Anxiety and Depression Scale (n = 1). For neuropsychiatric symptoms, all studies used the Neuropsychiatric Inventory (n = 10). The included studies applied person-centered care interventions by promoting participants’ involvement (n = 15), individualizing care (n = 8), or applying both concepts (n = 13).
Quality Assessment
We evaluated all included studies by the NICE methodological checklist (Supplementary Table 2). Results showed that 26 studies (72.2%) had a low risk of bias, 10 (27.8%) had a moderate risk, and no study had a high risk of bias. Thus, most included studies had high quality.
Effects of Intervention
Table 2 shows the overall effect size of the person-centered interventions on BPSD and cognitive function by intervention type. The overall effects of the interventions on BPSD (SMD = −0.52, 95% CI: −0.67 to −0.36) and cognitive function (SMD = 0.52, 95% CI: 0.27–0.76) were moderate. Specifically, the subgroup analyses confirmed the immediate effect of the interventions on reducing BPSD and improving cognitive functions. Additionally, between-subgroup comparisons showed heterogeneous effects on BPSD (I2 = 66%) and cognitive function (I2 = 73%).
Table 2.
Meta-analytic Result of Person-Centered Intervention Effect
| Variable | k | d | 95% CI | Z | I 2 |
|---|---|---|---|---|---|
| Overall effect on BPSD | 31 | −0.52 | −0.67, −0.36 | 6.60** | 66 |
| Overall effect on depression | 19 | −0.51 | −0.71, −0.32 | 5.24** | 63 |
| Overall effect on NPS | 7 | −0.51 | −0.84, −0.18 | 3.04** | 77 |
| Overall effect on agitation | 5 | −0.59 | −1.13, −0.04 | 2.11* | 68 |
| Overall effect on cognitive function | 15 | 0.52 | 0.27, 0.76 | 4.16** | 73 |
| MT effect on BPSD | 9 | −0.33 | −0.48, −0.18 | 4.33** | 0 |
| MT effect on depression | 3 | −0.33 | −0.56, −0.09 | 2.71** | 0 |
| MT effect on NPS | 4 | −0.35 | −0.68, −0.03 | 2.13* | 47 |
| MT effect on agitation | 2 | −0.26 | −0.80, 0.27 | 0.96 | 0 |
| MT effect on cognitive function | 5 | 0.35 | 0.01, 0.69 | 2.04* | 68 |
| RT effect on BPSD | 13 | −0.74 | −1.02, −0.47 | 5.28** | 77 |
| RT effect on depression | 10 | −0.77 | −1.03, −0.51 | 5.74** | 59 |
| RT effect on NPS | 3 | −0.69 | −1.44, 0.05 | 1.82 | 90 |
| RT effect on cognitive function | 10 | 0.59 | 0.27, 0.91 | 3.60** | 72 |
| CT effect on depression | 4 | −0.21 | −0.53, 0.11 | 1.30 | 0 |
| MSS effect on BPSD | 5 | −0.48 | −0.95, −0.01 | 2.01* | 75 |
| MSS effect on depression | 2 | −0.10 | −0.37, 0.16 | 0.76 | 0 |
| MSS effect on agitation | 3 | −0.81 | −1.71, 0.09 | 1.76 | 83 |
Notes: k = number of comparisons; d = standardized mean difference calculated under random-effect model; I2 = heterogeneity statistic; Z = test statistic used to derive the p value; BPSD = behavioral and psychological symptoms of dementia; CI = confidence interval; CT = cognitive therapy; MSS = multisensory stimulation; MT = music therapy; NPS = neuropsychiatric symptoms; RT = reminiscence therapy.
*p < .05. **p < .01.
For music therapy, the immediate effects on BPSD (SMD = −0.33, 95% CI: = −0.48 to −0.18) and cognitive function (SMD = 0.35, 95% CI: 0.01–0.69) were small. Nonetheless, results confirmed that, regardless of the effect size, music therapy reduced BPSD and improved cognitive function.
For reminiscence therapy, the immediate effects on BPSD (SMD = −0.74, 95% CI: −1.02 to −0.47) and cognitive function (SMD = 0.59, 95% CI: 0.27–0.91) were both moderate. Thus, results confirmed that reminiscence therapy reduced BPSD and improved cognitive function.
For cognitive therapy, the only outcome subgroup that had enough cases to be included in the meta-analysis was that of depression; however, there was no significant effect of cognitive therapy on depression (SMD = −0.21, 95% CI: −0.53 to 0.11), and there was no heterogeneity among cases (I2 = 0%).
For multisensory stimulation, subgroup analyses were used to explore the effect on two outcomes (i.e., depression and agitation); nevertheless, there were no significant effects of multisensory stimulation on depression (SMD = −0.10, 95% CI: −0.37 to 0.16) or agitation (SMD = −0.81, 95% CI: −1.71 to 0.09). However, there was a significant effect of the overall cases toward favoring the use of multisensory stimulation therapies (SMD = −0.48, 95% CI: −0.95 to −0.01), and there was heterogeneity among cases (I2 = 75%). The forest plots showing the effects of each intervention have been provided as Supplementary Figures 1–5.
Discussion
Our systematic review summarized the applied interventions and characteristics of various studies that utilized the concept of person-centered care. Considerable high-quality studies have applied person-centered interventions to persons living with dementia. These interventions have utilized multidisciplinary experts, according to the nature of each intervention and measurement tools suitable for dementia, and consequentially contributed to improving BPSD and cognitive function of persons living with dementia. Our meta-analysis highlighted that the effect size of the interventions on BPSD and cognition differed by intervention type. To the best of our knowledge, this was the first study to examine the effectiveness of person-centered interventions on persons living with dementia by outcome and intervention type; until now, only three reviews—including one meta-analysis—have focused on the outcomes of interventions aimed at persons living with dementia (Chenoweth et al., 2019; Kim & Park, 2017; Li & Porock, 2014). Although these reviews have explored the effect of our analyzed interventions in our population of interest, they did not differentially consider intervention type. Thus, our unique contribution to dementia research was to identify the effect size of each intervention type on the outcomes of interest.
Since the concept of person-centered care was first introduced to the dementia context in 1998, it has been applied to numerous studies. Although studies have applied person-centered care in different ways in the last two decades, humanity is its essence (Li & Porock, 2014; Powers, 2005). Specially in the dementia care context, respecting patients and treating them as human beings that have the right to dignity and autonomy is very important because persons living with dementia are often treated as not having self-determination owing to the cognitive impairments inherent to their condition. Relatedly, a study showed they can express emotions even during the later stages of dementia and that their psychological needs should be respected (Lee et al., 2013).
To ensure that all studies applied person-centered care on their interventions, we used a definition set forth by a prior study that was devoted to analyzing person-centered care and its definitions (Robinson et al., 2008). As a result, we were able to include a sizable amount of studies in our analysis (n = 36). Specifically, our results showed that the number of studies on the topic has increased in recent years worldwide, and that person-centered care was applied to various intervention types (e.g., music, cognitive, and reminiscence therapies, and multisensory stimulation). The increase in the number and scope of application of person-centered care may reflect a philosophical shift on dementia care to consider an individual’s values and preferences, which is both meaningful and inspiring. Regarding quality, over 72% of the studies were classified as low risk of bias, and no studies were classified as high risk of bias with respect to their methodologies. Thus, the results from the included studies were deemed valid and reliable.
Our meta-analysis revealed that person-centered interventions showed the immediate effects on reducing BPSD and improving cognitive function, but effect size differed by intervention type. Specifically, reminiscence therapy showed a moderate effect size, while music therapy and multisensory stimulation had a small effect size. Despite effect size differences, we speculated that music and reminiscence therapies each had their own merits; music therapy distracts people from their unpleasant emotions by musical stimulation and allows for the expression of people’s current emotions through musical methods (Chu et al., 2014), and reminiscence therapy uses empathy and interactions with others through the re-experience of past memories to help people with their problems (Aşiret & Kapucu, 2016; Hsieh et al., 2010; Lopes et al., 2016; Scales et al., 2018). Specifically, expressing one’s thoughts or feelings through the remaining memory (i.e., reminiscence therapy) could be a meaningful activity to persons living with dementia; it might promote their self-understanding through communication with others and further improves ego-integrity by reconstructing their memories (Haight & Burnside, 1993). However, we were not able to compare whether the effect size of reminiscence therapy was bigger than that of other interventions regarding BPSD owing to study heterogeneity; thus, further and more elaborate studies are warranted to reveal whether this type of intervention is indeed superior in reducing BPSD.
Moreover, our results emphasized that music and reminiscence therapies were effective in improving MMSE scores, namely, the cognitive function of people living with dementia. This is in line with the finding that music stimulates a number of brain regions and can help with cognitive rehabilitation (Li et al., 2015; Lyu et al., 2018); additionally, remembering and discussing past events (i.e., core components of reminiscence therapy) could be beneficial for the rehabilitation of cognitive function of persons living with dementia (Lök et al., 2019).
Our results also showed that music and reminiscence therapies were effective in decreasing depression, whereas cognitive therapy and multisensory stimulation did not show statistically significant effects on depression. This between-intervention difference may owe to what each intervention type focused on; for example, music and reminiscence therapies have their main procedures intricately related to individuals’/patients’ values and preferences (e.g., listing their preferred songs or creating opportunities to communicate about one’s good memories), and such characteristics have been shown to help people relax and improve their moods (Aşiret & Kapucu, 2016; Chu et al., 2014). These cited studies confirm our results that show significant effects for these two types of therapy. Conversely, cognitive therapy might not focus on the mood or feelings of persons living with dementia because it is usually designed to enhance cognitive function (Wilson, 2002), and the multisensory stimulation studies in our review had a relatively smaller scale compared to the others, which might have influenced the nonsignificant results. Furthermore, music therapy was the only intervention type that was effective to reduce neuropsychiatric symptoms, indicating that listening to a preferred music can promote relaxation (Lin et al., 2011).
Study Limitations and Strengths
Our study had some limitations that we would like to highlight. First, it may have incurred reporting bias because we only included studies published in the English language and from the main search engines that show up exclusively scholarly journals and dissertations.
Second, although we endeavored to include all studies that utilized the concept of person-centered care, we acknowledge that there may be studies that applied the concept and that were not included in our review; this is because we decided to include studies solely based on their descriptions of the interventions or of the intervention components.
Third, the reviewed studies utilized a wide array of measurement tools, and there were instances in which the same outcome was measured by different tools. Although we tried to overcome this limitation by calculating SMD, interpretations regarding our results on the effect size of each outcome should be made with caution.
Despite these limitations, our study also has strengths to be mentioned. First, we only included RCTs; this was done because RCTs are less likely to be influenced by confounding factors and bias, compared to observational or other types of experimental studies, thus conferring reliability to our study results. Moreover, to date, this was the first meta-analysis to examine the effect of person-centered interventions on persons living with dementia by intervention type and targeted outcome, to the best of our knowledge. Thus, this may be a valuable resource for researchers endeavoring to know which person-centered intervention may be more effective for a specific health outcome.
Conclusions
Our results underlined that various types of person-centered interventions have been applied to reduce BPSD or improve cognitive function of persons living with dementia in the past two decades (i.e., from 1998 to 2019). They also showed that person-centered interventions can be effective to decrease BPSD in the studied population. We recommend that health care providers utilize person-centered care as an essential part of the treatment when trying to reduce the BPSD and rehabilitate the cognitive function of persons living with dementia. Specifically, given that reminiscence therapy was effective on both BPSD and cognitive function and that its effect size was superior to that of other interventions (i.e., cognitive and music therapy and multisensory stimulation), we suggest this type of therapy as the first choice of nonpharmacological intervention that should be applied to manage BPSD and maintain the cognitive function of our population of interest.
Supplementary Material
Contributor Information
Kyung Hee Lee, Yonsei University College of Nursing, Seoul, South Korea; Mo-Im Kim Nursing Research Institute, Seoul, South Korea.
Ji Yeon Lee, Yonsei University College of Nursing, Seoul, South Korea.
Bora Kim, Yonsei University College of Nursing, Seoul, South Korea.
Funding
This work was supported by the Ministry of Education through the Basic Science Research Program of the National Research Foundation of Korea (grant number NRF-2020R1A6A1A03041989).
Conflict of Interest
None declared.
References
- Amieva, H., Robert, P. H., Grandoulier, A. S., Meillon, C., De Rotrou, J., Andrieu, S., Berr, C., Desgranges, B., Dubois, B., Girtanner, C., Joël, M. E., Lavallart, B., Nourhashemi, F., Pasquier, F., Rainfray, M., Touchon, J., Chêne, G., & Dartigues, J. F. (2016). Group and individual cognitive therapies in Alzheimer’s disease: The ETNA3 randomized trial. International Psychogeriatrics, 28(5), 707–717. doi: 10.1017/S1041610215001830 [DOI] [PubMed] [Google Scholar]
- Aşiret, G. D., & Kapucu, S. (2016). The effect of reminiscence therapy on cognition, depression, and activities of daily living for patients with Alzheimer disease. Journal of Geriatric Psychiatry and Neurology, 29(1), 31–37. doi: 10.1177/0891988715598233 [DOI] [PubMed] [Google Scholar]
- Aslakson, M. (2010). The effects of a music therapy intervention on agitation in people with dementia (Publication No. 3442003) [Doctoral dissertation, The University of Wisconsin-Milwaukee]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Bailey, E. M., Stevens, A. B., LaRocca, M. A., & Scogin, F. (2017). A randomized controlled trial of a therapeutic intervention for nursing home residents with dementia and depressive symptoms. Journal of Applied Gerontology, 36(7), 895–908. doi: 10.1177/0733464815627956 [DOI] [PubMed] [Google Scholar]
- Bakshi, R. (2004). Assessing the effectiveness of sensory stimulation on individuals who have moderate to severe dementia (Publication No. 3124938) [Doctoral dissertation, New York University]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Ballard, C., & Corbett, A. (2010). Management of neuropsychiatric symptoms in people with dementia. CNS Drugs, 24(9), 729–739. doi: 10.2165/11319240-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Brodaty, H., Connors, M. H., Xu, J., Woodward, M., & Ames, D.; PRIME Study Group . (2014). Predictors of institutionalization in dementia: A three year longitudinal study. Journal of Alzheimer’s Disease, 40(1), 221–226. doi: 10.3233/JAD-131850 [DOI] [PubMed] [Google Scholar]
- Chenoweth, L., Stein-Parbury, J., Lapkin, S., Wang, A., Liu, Z., & Williams, A. (2019). Effects of person-centered care at the organisational-level for people with dementia. A systematic review. PLoS ONE, 14(2), e0212686. doi: 10.1371/journal.pone.0212686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, H., Yang, C. Y., Lin, Y., Ou, K. L., Lee, T. Y., O’Brien, A. P., & Chou, K. R. (2014). The impact of group music therapy on depression and cognition in elderly persons with dementia: A randomized controlled study. Biological Research for Nursing, 16(2), 209–217. doi: 10.1177/1099800413485410 [DOI] [PubMed] [Google Scholar]
- Clare, L., Linden, D. E., Woods, R. T., Whitaker, R., Evans, S. J., Parkinson, C. H., van Paasschen, J., Nelis, S. M., Hoare, Z., Yuen, K. S., & Rugg, M. D. (2010). Goal-oriented cognitive rehabilitation for people with early-stage Alzheimer disease: A single-blind randomized controlled trial of clinical efficacy. The American Journal of Geriatric Psychiatry, 18(10), 928–939. doi: 10.1097/JGP.0b013e3181d5792a [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1992). A power primer. Psychological Bulletin, 112(1), 155–159. doi: 10.1037//0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Cohen, J. (2013). Statistical power analysis for the behavioral sciences. Academic Press. [Google Scholar]
- DerSimonian, R., & Laird, N. (1986). Meta-analysis in clinical trials. Controlled Clinical Trials, 7(3), 177–188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Duru Aşiret, G., & Kapucu, S. (2016). The effect of reminiscence therapy on cognition, depression, and activities of daily living for patients with Alzheimer disease. Journal of Geriatric Psychiatry and Neurology, 29(1), 31–37. doi: 10.1177/0891988715598233 [DOI] [PubMed] [Google Scholar]
- Eska, K., Graessel, E., Donath, C., Schwarzkopf, L., Lauterberg, J., & Holle, R. (2013). Predictors of institutionalization of dementia patients in mild and moderate stages: A 4-year prospective analysis. Dementia and Geriatric Cognitive Disorders Extra, 3(1), 426–445. doi: 10.1159/000355079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio, S., Pace, D., Flinner, J., & Kallmyer, B. (2018). The fundamentals of person-centered care for individuals with dementia. Gerontologist, 58(suppl. 1), S10–S19. doi: 10.1093/geront/gnx122 [DOI] [PubMed] [Google Scholar]
- Haight, B. K., & Burnside, I. (1993). Reminiscence and life review: Explaining the differences. Archives of Psychiatric Nursing, 7(2), 91–98. doi: 10.1016/s0883-9417(09)90007-3 [DOI] [PubMed] [Google Scholar]
- Hong, I. S., & Choi, M. J. (2011). Songwriting oriented activities improve the cognitive functions of the aged with dementia. The Arts in Psychotherapy, 38(4), 221–228. doi: 10.1016/j.aip.2011.07.002 [DOI] [Google Scholar]
- Hsieh, C.-J., Chang, C., Su, S.-F., Hsiao, Y.-L., Shih, Y.-W., Han, W.-H., & Lin, C.-C. (2010). Reminiscence group therapy on depression and apathy in nursing home residents with mild-to-moderate dementia. Journal of Experimental & Clinical Medicine, 2(2), 72–78. doi: 10.1016/S1878-3317(10)60012-5 [DOI] [Google Scholar]
- Hsu, M. H., Flowerdew, R., Parker, M., Fachner, J., & Odell-Miller, H. (2015). Individual music therapy for managing neuropsychiatric symptoms for people with dementia and their carers: A cluster randomised controlled feasibility study. BMC Geriatrics, 15, 84. doi: 10.1186/s12877-015-0082-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson, C., Orrell, M., Dugmore, O., & Spector, A. (2014). Sonas: A pilot study investigating the effectiveness of an intervention for people with moderate to severe dementia. American Journal of Alzheimer’s Disease and Other Dementias, 29(8), 696–703. doi: 10.1177/1533317514534756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, T., Meguro, K., Akanuma, K., Ishii, H., & Mori, E. (2007). A randomized controlled trial of the group reminiscence approach in patients with vascular dementia. Dementia and Geriatric Cognitive Disorders, 24(1), 48–54. doi: 10.1159/000103631 [DOI] [PubMed] [Google Scholar]
- Kales, H. C., Lyketsos, C. G., Miller, E. M., & Ballard, C. (2019). Management of behavioral and psychological symptoms in people with Alzheimer’s disease: An international Delphi consensus. International Psychogeriatrics, 31(1), 83–90. doi: 10.1017/S1041610218000534 [DOI] [PubMed] [Google Scholar]
- Kallio, E. L., Öhman, H., Hietanen, M., Soini, H., Strandberg, T. E., Kautiainen, H., & Pitkälä, K. H. (2018). Effects of cognitive training on cognition and quality of life of older persons with dementia. Journal of the American Geriatrics Society, 66(4), 664–670. doi: 10.1111/jgs.15196 [DOI] [PubMed] [Google Scholar]
- Kim, S. K., & Park, M. (2017). Effectiveness of person-centered care on people with dementia: A systematic review and meta-analysis. Clinical Interventions in Aging, 12, 381–397. doi: 10.2147/CIA.S117637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitwood, T. (1988). The technical, the personal, and the framing of dementia. Social Behaviour, 3(2), 161–179. [Google Scholar]
- Lai, C. K., Chi, I., & Kayser-Jones, J. (2004). A randomized controlled trial of a specific reminiscence approach to promote the well-being of nursing home residents with dementia. International Psychogeriatrics, 16(1), 33–49. doi: 10.1017/s1041610204000055 [DOI] [PubMed] [Google Scholar]
- Lawton, M. P., Van Haitsma, K., Klapper, J., Kleban, M. H., Katz, I. R., & Corn, J. (1998). A stimulation-retreat special care unit for elders with dementing illness. International Psychogeriatrics, 10(4), 379–395. doi: 10.1017/s104161029800547x [DOI] [PubMed] [Google Scholar]
- Lee, K. H., Algase, D. L., & McConnell, E. S. (2013). Daytime observed emotional expressions of people with dementia. Nursing Research, 62(4), 218–225. doi: 10.1097/NNR.0b013e31829999d7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., & Porock, D. (2014). Resident outcomes of person-centered care in long-term care: A narrative review of interventional research. International Journal of Nursing Studies, 51(10), 1395–1415. doi: 10.1016/j.ijnurstu.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Li, H. C., Wang, H. H., Chou, F. H., & Chen, K. M. (2015). The effect of music therapy on cognitive functioning among older adults: A systematic review and meta-analysis. Journal of the American Medical Directors Association, 16(1), 71–77. doi: 10.1016/j.jamda.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Lin, Y., Chu, H., Yang, C. Y., Chen, C. H., Chen, S. G., Chang, H. J., Hsieh, C. J., & Chou, K. R. (2011). Effectiveness of group music intervention against agitated behavior in elderly persons with dementia. International Journal of Geriatric Psychiatry, 26(7), 670–678. doi: 10.1002/gps.2580 [DOI] [PubMed] [Google Scholar]
- Lök, N., Bademli, K., & Selçuk-Tosun, A. (2019). The effect of reminiscence therapy on cognitive functions, depression, and quality of life in Alzheimer patients: Randomized controlled trial. International Journal of Geriatric Psychiatry, 34(1), 47–53. doi: 10.1002/gps.4980 [DOI] [PubMed] [Google Scholar]
- Lopes, T. S., Afonso, R. M., & Ribeiro, Ó. M. (2016). A quasi-experimental study of a reminiscence program focused on autobiographical memory in institutionalized older adults with cognitive impairment. Archives of Gerontology and Geriatrics, 66, 183–192. doi: 10.1016/j.archger.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Lyu, J., Zhang, J., Mu, H., Li, W., Champ, M., Xiong, Q., Gao, T., Xie, L., Jin, W., Yang, W., Cui, M., Gao, M., & Li, M. (2018). The effects of music therapy on cognition, psychiatric symptoms, and activities of daily living in patients with Alzheimer’s disease. Journal of Alzheimer’s disease, 64(4), 1347–1358. doi: 10.3233/JAD-180183 [DOI] [PubMed] [Google Scholar]
- Maseda, A., Sánchez, A., Marante, M. P., González-Abraldes, I., Buján, A., & Millán-Calenti, J. C. (2014). Effects of multisensory stimulation on a sample of institutionalized elderly people with dementia diagnosis: A controlled longitudinal trial. American Journal of Alzheimer’s Disease and Other Dementias, 29(5), 463–473. doi: 10.1177/1533317514522540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G.; PRISMA Group . (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamae, T., Yotsumoto, K., Tatsumi, E., & Hashimoto, T. (2014). Effects of productive activities with reminiscence in occupational therapy for people with dementia: A pilot randomized controlled study. Hong Kong Journal of Occupational Therapy, 14(1), 13–19. doi: 10.1016/j.hkjot.2014.01.003 [DOI] [Google Scholar]
- National Institute for Health and Care Excellence. (2012). The guidelines manual: Process and methods.https://www.nice.org.uk/process/pmg6/resources/the-guidelines-manual-appendices-bi-2549703709/chapter/appendix-c-methodology-checklist-randomised-controlled-trials
- Pérez-Ros, P., Cubero-Plazas, L., Mejías-Serrano, T., Cunha, C., & Martínez-Arnau, F. M. (2019). Preferred music listening intervention in nursing home residents with cognitive impairment: A randomized intervention study. Journal of Alzheimer’s Disease, 70(2), 433–442. doi: 10.3233/JAD-190361 [DOI] [PubMed] [Google Scholar]
- Powers, B. A. (2005). Everyday ethics in assisted living facilities: A framework for assessing resident-focused issues. Journal of Gerontological Nursing, 31(1), 31–37. doi: 10.3928/0098-9134-20050101-10 [DOI] [PubMed] [Google Scholar]
- Raglio, A., Bellandi, D., Baiardi, P., Gianotti, M., Ubezio, M. C., Zanacchi, E., Granieri, E., Imbriani, M., & Stramba-Badiale, M. (2015). Effect of active music therapy and individualized listening to music on dementia: A multicenter randomized controlled trial. Journal of the American Geriatrics Society, 63(8), 1534–1539. doi: 10.1111/jgs.13558 [DOI] [PubMed] [Google Scholar]
- Ridder, H. M., Stige, B., Qvale, L. G., & Gold, C. (2013). Individual music therapy for agitation in dementia: An exploratory randomized controlled trial. Aging & Mental Health, 17(6), 667–678. doi: 10.1080/13607863.2013.790926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, J. H., Callister, L. C., Berry, J. A., & Dearing, K. A. (2008). Patient-centered care and adherence: Definitions and applications to improve outcomes. Journal of the American Academy of Nurse Practitioners, 20(12), 600–607. doi: 10.1111/j.1745-7599.2008.00360.x [DOI] [PubMed] [Google Scholar]
- Sánchez, A., Marante-Moar, M. P., Sarabia, C., de Labra, C., Lorenzo, T., Maseda, A., & Millán-Calenti, J. C. (2016). Multisensory stimulation as an intervention strategy for elderly patients with severe dementia: A pilot randomized controlled trial. American Journal of Alzheimer’s Disease and Other Dementias, 31(4), 341–350. doi: 10.1177/1533317515618801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales, K., Zimmerman, S., & Miller, S. J. (2018). Evidence-based nonpharmacological practices to address behavioral and psychological symptoms of dementia. The Gerontologist, 58(suppl. 1), S88–S102. doi: 10.1093/geront/gnx167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiltz, D. L., Lineweaver, T. T., Brimmer, T., Cairns, A. C., Halcomb, D. S., Juett, J., Beer, L., Hay, D. P., & Plewes, J. (2018). “Music first”: An alternative or adjunct to psychotropic medications for the behavioral and psychological symptoms of dementia. GeroPsych, 31(1), 17–30. doi: 10.1024/1662-9647/a000180 [DOI] [Google Scholar]
- Staal, J. A., Sacks, A., Matheis, R., Collier, L., Calia, T., Hanif, H., & Kofman, E. S. (2007). The effects of Snoezelen (multi-sensory behavior therapy) and psychiatric care on agitation, apathy, and activities of daily living in dementia patients on a short term geriatric psychiatric inpatient unit. International Journal of Psychiatry in Medicine, 37(4), 357–370. doi: 10.2190/PM.37.4.a [DOI] [PubMed] [Google Scholar]
- Tadaka, E., & Kanagawa, K. (2007). Effects of reminiscence group in elderly people with Alzheimer disease and vascular dementia in a community setting. Geriatrics & Gerontology International, 7(2), 167–173. doi: 10.1111/j.1447-0594.2007.00381.x [DOI] [Google Scholar]
- Tanaka, S., Honda, S., Nakano, H., Sato, Y., Araya, K., & Yamaguchi, H. (2017). Comparison between group and personal rehabilitation for dementia in a geriatric health service facility: Single-blinded randomized controlled study. Psychogeriatrics, 17(3), 177–185. doi: 10.1111/psyg.12212 [DOI] [PubMed] [Google Scholar]
- The American Geriatrics Society Expert Panel on Person-Centered Care. (2016). Person-centered care: A definition and essential elements. Journal of the American Geriatrics Society, 64(1), 15–18. doi: 10.1111/jgs.13866 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2015). Medicare and medicaid programs: Reform of requirements for long-term care facilities.http://www.regulations.gov/document?D=CMS-2015-0083-0001 [DOI] [PubMed]
- Van Bogaert, P., Tolson, D., Eerlingen, R., Carvers, D., Wouters, K., Paque, K., Timmermans, O., Dilles, T., & Engelborghs, S. (2016). SolCos model-based individual reminiscence for older adults with mild to moderate dementia in nursing homes: A randomized controlled intervention study. Journal of Psychiatric and Mental Health Nursing, 23(9-10), 568–575. doi: 10.1111/jpm.12336 [DOI] [PubMed] [Google Scholar]
- Van Bogaert, P., Van Grinsven, R., Tolson, D., Wouters, K., Engelborghs, S., & Van der Mussele, S. (2013). Effects of SolCos model-based individual reminiscence on older adults with mild to moderate dementia due to Alzheimer disease: A pilot study. Journal of the American Medical Directors Association, 14(7), 528.e9–528.13. doi: 10.1016/j.jamda.2013.01.020 [DOI] [PubMed] [Google Scholar]
- van der Ploeg, E. S., Eppingstall, B., Camp, C. J., Runci, S. J., Taffe, J., & O’Connor, D. W. (2013). A randomized crossover trial to study the effect of personalized, one-to-one interaction using Montessori-based activities on agitation, affect, and engagement in nursing home residents with dementia. International Psychogeriatrics, 25(4), 565–575. doi: 10.1017/S1041610212002128 [DOI] [PubMed] [Google Scholar]
- Voutilainen, A., Ruokostenpohja, N., & Välimäki, T. (2018). Associations across caregiver and care recipient symptoms: Self-organizing map and meta-analysis. The Gerontologist, 58(2), e138–e149. doi: 10.1093/geront/gnw251 [DOI] [PubMed] [Google Scholar]
- Wang, J. J. (2007). Group reminiscence therapy for cognitive and affective function of demented elderly in Taiwan. International Journal of Geriatric Psychiatry, 22(12), 1235–1240. doi: 10.1002/gps.1821 [DOI] [PubMed] [Google Scholar]
- Wang, Z., Li, Z., Xie, J., Wang, T., Yu, C., & An, N. (2018). Music therapy improves cognitive function and behavior in patients with moderate Alzheimer’s disease. International Journal of Clinical and Experimental Medicine, 11(5), 4808–4814. doi: 10.1002/central/CN-01616811 [DOI] [Google Scholar]
- Weise, L., Töpfer, N. F., Deux, J., & Wilz, G. (2019). Feasibility and effects of individualized recorded music for people with dementia: A pilot RCT study. Nordic Journal of Music Therapy, 29(1), 39–56. doi: 10.1080/08098131.2019.1661507 [DOI] [Google Scholar]
- Wilson, B. A. (2002). Towards a comprehensive model of cognitive rehabilitation. Neuropsychological Rehabilitation, 12(2), 97–110. doi: 10.1080/09602010244000020 [DOI] [Google Scholar]
- Wu, L. F., & Koo, M. (2016). Randomized controlled trial of a six-week spiritual reminiscence intervention on hope, life satisfaction, and spiritual well-being in elderly with mild and moderate dementia. International Journal of Geriatric Psychiatry, 31(2), 120–127. doi: 10.1002/gps.4300 [DOI] [PubMed] [Google Scholar]
- Yamagami, T., Takayama, Y., Maki, Y., & Yamaguchi, H. (2012). A randomized controlled trial of brain-activating rehabilitation for elderly participants with dementia in residential care homes. Dementia and Geriatric Cognitive Disorders Extra, 2(1), 372–380. doi: 10.1159/000342614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Q. F., Tan, L., Wang, H. F., Jiang, T., Tan, M. S., Tan, L., Xu, W., Li, J. Q., Wang, J., Lai, T. J., & Yu, J. T. (2016). The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. Journal of Affective Disorders, 190, 264–271. doi: 10.1016/j.jad.2015.09.069 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.