Abstract
Background and Objectives
This secondary analysis examined (a) the association between illness perceptions (perceived understanding and cause of mild cognitive impairment [MCI]) and self-management behaviors for cognitive health, and (b) whether sociodemographic and clinical factors moderate such relationships among persons with MCI.
Research Design and Methods
We conducted a cross-sectional study of 85 participants using baseline data from the Return of Amyloid Imaging Scan Results Study. The coherence and causality subscales of the Revised Illness Perceptions Questionnaires were used. Self-management behaviors (dietary changes, physical activity, mental activities, dietary supplements) were assessed using the Risk Evaluation and Education for ALzheimer’s disease health behavior measure. Sociodemographic and clinical information was extracted from patients’ medical records. We performed hierarchical linear regression and binary logistic regression.
Results
We found no main effects for illness perceptions and self-management of cognitive health. Interaction effects were detected, including (a) coherence and age on the total number of self-management behaviors (b = 0.01, p = .04) and on physical activity (p = .04, odds ratio [OR] = 1.02, 95% confidence interval [CI] = 1.00–1.03), (b) causality and age on dietary supplements (p = .03, OR = 1.31, 95% CI = 1.02–1.67), and (c) causality and education on mental activities (p = .02, OR = 0.44, 95% CI = 0.22–0.88).
Discussion and Implications
Findings suggest that age and education moderate the relationship between illness perceptions and self-management behaviors. Health care professionals should consider subjective perceptions about MCI in light of sociodemographic and clinical factors when discussing cognitive health self-management.
Keywords: Age, Dementia, Education, Illness perceptions, Self-management
Mild cognitive impairment (MCI), a syndrome in which individuals may experience slight cognitive changes with minimal impairment of everyday activities, may represent the first clinical expression of dementia (Petersen et al., 1999; Petersen, 2004). Although MCI is widely recognized as a risk state for dementia, persons with MCI may also remain cognitively stable (Clem et al., 2017) or return to normal cognition (Koepsell & Monsell, 2012). While age, genetics (e.g., apolipoprotein [APOE-ε4] gene), and family history are strong risk factors for dementia development (Alzheimer’s Association, 2021; Elias-Sonnenschein et al., 2011), efforts to better understand pathological cognitive aging have elucidated modifiable risk factors and identified lifestyle modifications for promoting cognitive health in those at risk for Alzheimer’s disease (AD) and related dementias (Livingston et al., 2020). Regarding MCI in particular, researchers and public health professionals agree that, even as disease-modifying pharmacological treatments become available, lifestyle modifications will be required to stabilize or reverse the course of MCI (Karssemeijer et al., 2017; Petersen et al., 2018).
Research in other populations with chronic conditions suggests that disease management, especially the performance of self-management behaviors like healthy diets and exercise, may be influenced by the way that an individual views the nature and cause of a disease or its symptoms (Hagger et al., 2017). However, the relationship between one’s views about MCI and the performance of cognitive health self-management behaviors has not been investigated. The purpose of this secondary analysis, therefore, was to examine the relationship between perceptions of cognitive changes and self-management behaviors among persons with MCI.
Illness Perceptions in MCI
Given the subtle nature of an MCI diagnosis and its symptoms, an important research question is, “what circumstances would encourage lifestyle changes to promote cognitive health?” Receiving a diagnosis of, or experiencing symptoms related to, a particular ailment often leads individuals to form thoughts and feelings about their health condition, which may affect subsequent self-management behaviors to mitigate the impact of disease (Leventhal et al., 1984, 2003). One way to conceptualize such thoughts, referred to as illness perceptions, is Leventhal et al.’s (1984, 2003) Common Sense Model (CSM) of Self-Regulation. This empirically validated framework explains how individuals formulate beliefs about their health condition. The literature suggests that two dimensions of illness perception, coherence and causality, may be particularly salient in persons with MCI. The dimension of coherence refers to one’s perceived understanding of the disease on a continuum of understandable versus confusing (Moss-Morris et al., 2002). Qualitative research has shown that there is variability in the levels of illness coherence among persons with cognitive diagnoses like MCI (Lingler et al., 2006; Matchwick et al., 2014; Portacolone et al., 2018). Reports of relatively low levels of illness coherence in MCI suggest that individuals who receive an MCI diagnosis live with considerable uncertainty about what their cognitive changes mean for the future (Lingler et al., 2016). The second dimension of interest, causality, refers to beliefs about what causes a disease or set of symptoms (Leventhal et al., 1984, 2003). Studies have shown that older adults attribute decline in memory and thinking to an array of controllable (e.g., lifestyle) and uncontrollable (e.g., heredity) factors, with many perceiving such changes as an unavoidable consequence of aging (Anderson et al., 2011; Rodakowski et al., 2014). The extent to which such perceptions of MCI affect the adoption of self-management behaviors for the condition has not been documented.
Self-Management in MCI
Although self-management is a promising strategy to enhance treatment regimens and quality of life (National Institute of Nursing Research, 2016), the unfamiliar concept and limited approved effective treatments for MCI may impede the uptake of cognitively healthy lifestyle changes. Indeed, research on self-management cognitive health behaviors among persons at risk for AD dementia has shown mixed results. Individuals at risk of AD by virtue of genetic risk (i.e., APOE-ε4 carriers) have reported altering their self-management behaviors, including engaging in mental activities and dietary changes upon learning their test results (Christensen et al., 2015). Among those who are at risk by virtue of an MCI diagnosis, cognitive stimulation (Lin & Heidrich, 2012; Morgan et al., 2012) and physical activity (Lin & Heidrich, 2012) were the most frequently or readily performed self-management behaviors.
Although illness perceptions have been posited to influence self-management behaviors across an array of chronic health conditions (Leventhal et al., 2016), studies of the MCI population have explored either illness perceptions (Lin et al., 2012; Lingler et al., 2006, 2016) or health behaviors (Christensen et al., 2015; Morgan et al., 2012), but not the association between the two.
Demographic and clinical characteristics (e.g., age, education, and comorbidity) are also receiving attention from researchers and clinicians as factors that may influence self-management behaviors for cognitive health. While older age and fewer years of education are the greatest risk factors for AD dementia (Livingston et al., 2020), little is known about the role of such factors in the relationship between illness perceptions and self-management behaviors in MCI. Thus, in this study, we also examined underlying demographic (i.e., age, sex, education) and clinical (i.e., comorbid health conditions, time since first MCI diagnosis) factors along with illness perceptions to better understand strategies for promoting cognitive health among persons with MCI.
Guided by Leventhal et al.’s (1984, 2003) CSM of Self-Regulation, we examined illness perceptions, specifically perceived understanding and cause of MCI, and their associations with self-management behaviors. We also aimed to determine how interactions between demographics (i.e., age and education) and illness perceptions (i.e., perceived understanding and cause of MCI) are associated with self-management behaviors in MCI. As an exploratory aim, other demographic and clinical information, including sex, comorbid conditions, and time since the MCI diagnosis, were tested for interactions with illness perceptions and how such interactions are associated with self-management behaviors. We hypothesized that participants who believed they have a better understanding of their diagnosis and those who perceived MCI is attributable to controllable factors (e.g., lifestyle) would be more likely to perform self-management behaviors for cognitive health. We further predicted that such relationships would be moderated by participants’ age and years of education.
Methods
Participants and Setting
We conducted a cross-sectional study using baseline data from the Return of Amyloid Imaging Scan Results (RAISR) Study (Lingler et al., 2020), a clinical trial to examine how persons with MCI and their care partners undertake decisions to pursue and react to the results of AD biomarker testing. Ninety individuals with MCI were recruited from the University of Pittsburgh Alzheimer’s Disease Research Center (ADRC). Individuals were included in the parent study if they (a) were 50 years of age or older, (b) had an ADRC consensus diagnosis of MCI via a multidisciplinary consensus meeting based on the National Alzheimer’s Coordinating Center and The National Institute on Aging and Alzheimer’s Association (NIA-AA) guidelines for MCI classification (Albert et al., 2011; Lopez et al., 1999; Snitz et al., 2018), (c) were community-dwelling, (d) had a care partner (e.g., family members), and (e) provided written informed consent to participate. The RAISR Study excluded individuals with active, untreated psychiatric disorders. We used the same enrollment criteria for this analysis, but care partner participants were excluded. This study was approved by the University of Pittsburgh Institutional Review Board.
Measures
Illness perceptions: perceived understanding and cause of MCI
Participants’ perceived understanding of their cognitive changes was measured using the coherence subscale of the Revised Illness Perception Questionnaire (IPQ-R; Moss-Morris et al., 2002), which consisted of five items with a 5-point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). The responses from these items were summed to yield an overall rating with the first four items being reversed so that higher scores indicate a better-perceived understanding of their MCI. The general term “my illness” was replaced with “my memory or thinking difficulties” as recommended by Moss-Morris et al. (2002).
We used the causality subscale of the IPQ-R (Moss-Morris et al., 2002) to measure the perceived cause of cognitive changes. Participants listed in rank order the three most important perceived causes of their memory or thinking difficulties and the top-ranked cause was used in this study. While the RAISR Study used the original open-ended question, Rodakowski et al.’s (2014) categories, potentially controllable factors (1; e.g., lifestyle, eating habits, stress/worry) or uncontrollable factors (0; e.g., heredity, personality, normal aging), were adapted in this study.
Self-management behaviors in MCI
Self-management behaviors were assessed using the Risk Evaluation and Education for ALzheimer’s disease (REVEAL) health behavior measure (Chao et al., 2008). While the REVEAL consists of eight items with dichotomous response options (i.e., yes or no) related to AD prevention strategies, we used five of these items in the study: dietary changes, physical activity, vitamins, herbal supplements, and mental activities. Vitamins and herbal supplements were combined and are reported as “dietary supplements” based on the most recent definition reported by the Food and Drug Administration (2018). The final four self-management behavior items deployed were the following: (a) dietary changes, (b) physical activity, (c) mental activities (e.g., puzzles), and (d) dietary supplements. The count of self-management behaviors was also used in this analysis.
Sociodemographic and clinical information
We extracted sociodemographic and clinical information of participants from ARDC records. Sociodemographic data comprised age (in years), education (in years), sex (male or female), race (Caucasian, African American, or Native Hawaiian/Pacific Islander), and marital status (currently married/living as married, widowed, divorced, or separated). Clinical information included the number of comorbid conditions (except for MCI), time since the first MCI diagnosis (in months), and MCI type (amnestic or nonamnestic). The total scores of the Mini-Mental State Examination (Folstein et al., 1975) were retrieved from the last annual visit records to characterize the global cognitive status of each participant. We used the 17-item Hamilton Depression Rating Scale (Hamilton, 1960) to assess participants’ depressive symptoms with each item being rated on a 3- or 5-point Likert scale.
Procedure
In the RAISR Study, trained research assistants conducted face-to-face interviews in a private location (i.e., participants’ home or a private room at the ADRC). For the causality item, the principal investigator of this study and a trained research assistant coded and entered data separately to categorize original open-ended responses into two broad domains (i.e., potentially controllable or uncontrollable factors; Rodakowski et al., 2014). The interrater reliability was assessed with Cohen’s kappa coefficient (κ) using the IBM SPSS Statistics Version 27.0. κ for the causality subscale was 0.91 in this study, indicating an excellent agreement between two raters (Fleiss et al., 1969). Disagreements regarding the categorization of the items (e.g., one rater coded as controllable and the other coded as uncontrollable factors) were resolved through further discussion between the raters for the final decision.
Data Analysis
Data were first screened for anomalies (e.g., outliers, missing data, and violations of assumptions of linear regression) using descriptive and exploratory analyses. The amount and pattern of missing data were evaluated, and five (5.56%) participants were identified as missing data for the causality item. Of these five, three did not answer the item, one believed that he/she does not have cognitive issues, and one indicated both potentially controllable (high cholesterol and diet) and uncontrollable (previous health history) factors. Little’s missing completely at random (MCAR) test was used to assess whether data were MCAR or not. Covariates to be considered in models comprised age, sex, education, time since the MCI diagnosis, and the number of comorbid conditions based on data screening and literature reviews (Chao et al., 2008; Smee et al., 2012).
We used hierarchical linear regression to examine the association between the predictors of interest (i.e., perceived understanding and cause of MCI) and the total number of self-management behaviors while controlling for covariates. This model was expanded to include the two-way interaction of the targeted predictors and each covariate. In the first block, we entered covariates, and primary predictors were added in the second block. In the final block, interaction terms between each predictor and covariate were included to assess the possible moderating effect of the covariate on the relationship between predictors and the total number of self-management behaviors.
To explore how each self-management behavior is associated with potential predictors, we performed hierarchical binary logistic regression. Similar to how we performed the hierarchical linear regression analysis, we entered predictors while controlling for identified covariates. Covariates were included in the first block; subsequently, we added coherence and causality variables to the second block. In the final block, interaction terms between a particular predictor and each covariate were added to the model. Mean centering was applied to the continuous type variables of age, education, time since the MCI diagnosis, and the number of comorbid conditions to minimize multicollinearity between these covariates and their interactions with the targeted predictors. The level of statistical significance for two-tailed hypothesis testing was set at .05, and all analyses were performed using IBM SPSS Statistics for Windows, Version 27.0.
Results
Sample Characteristics
Table 1 reports participants’ sociodemographic and clinical characteristics. Little’s MCAR test (χ 2 = 9.110, df = 9, p = .427) demonstrated that the missingness of causality data was completely at random, supporting our decision to include only participants who completed the causality item with a valid response. Our sample was on average 72.1 (SD = 8.5, range = 52–87) years of age and exhibited high levels of educational attainment, with an average of 16.5 (SD = 2.6) years of education. Over half of the participants were male (58.8%; n = 50), and most were Caucasian (92%, n = 78), married or living as married (79%, n = 67), and diagnosed with amnestic MCI (86%, n = 73). Our sample exhibited moderate levels of perceived understanding of MCI with the coherence mean score of 15.84 (SD = 4.96), and the majority (80%; n = 68) attributed their cognitive changes to uncontrollable factors (e.g., aging, heredity). Most participants (87.1%, n = 74) reported that they engage in one or more self-management behaviors for cognitive health. Mental activities were the most commonly reported behavior (60%; n = 51), and dietary changes were the least frequently performed behaviors (28.2%; n = 24) for cognitive health.
Table 1.
Sociodemographic and Clinical Characteristics (N = 85)
| Characteristic | Mean ± SD (Min–Max)/Median | n (%) |
|---|---|---|
| Age (years) | 72.07 ± 8.47 (52–87)/73.0 | |
| Education (years) | 16.45 ± 2.55 (12–21)/18.0 | |
| Sex | ||
| Male | 50 (58.8) | |
| Female | 35 (41.2) | |
| Race | ||
| Caucasian | 78 (91.8) | |
| African American | 6 (7.1) | |
| Native Hawaiian/Pacific Islander | 1 (1.2) | |
| Marital status | ||
| Currently married | 67 (78.8) | |
| Widowed | 10 (11.8) | |
| Divorced | 7 (8.2) | |
| Separated | 1 (1.2) | |
| Number of comorbid conditions (excluding MCI) | 1.88 ± 1.28 (0–6)/2 | |
| Time since MCI diagnosis (months) | 30.60 ± 37.89 (1–195)/13.2 | |
| MCI type | ||
| Amnestic MCI | 73 (85.9) | |
| Nonamnestic MCI | 12 (14.1) | |
| Dimension of illness perception | ||
| Coherence | 15.84 ± 4.96 (5–25)/16 | |
| Causality | ||
| Potentially controllable factors | 17 (20.0) | |
| Uncontrollable factors | 68 (80.0) | |
| MMSE total score | 27.09 ± 1.94 (22–30)/27.0 | |
| 17-item HDRS total score | 3.76 ± 4.16 (0–22)/2 | |
| Self-management behaviorsa | 1.59 ± 1.25 (0–4)/1 | 74 (87.1) |
| Dietary changes | 24 (28.2) | |
| Physical activity | 30 (35.3) | |
| Mental activities (e.g., crossword puzzles) | 51 (60.0) | |
| Dietary supplements | 30 (35.3) |
Note: SD = standard deviation; Min = minimum; Max = maximum; MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination; HDRS = Hamilton Depression Rating Scale.
aSelf-management behaviors counted as yes if any of the behaviors were performed (one or more) and counted as no if the behaviors were never performed.
Self-Management in MCI: Links to Perceived Understanding and Cause of Cognitive Changes
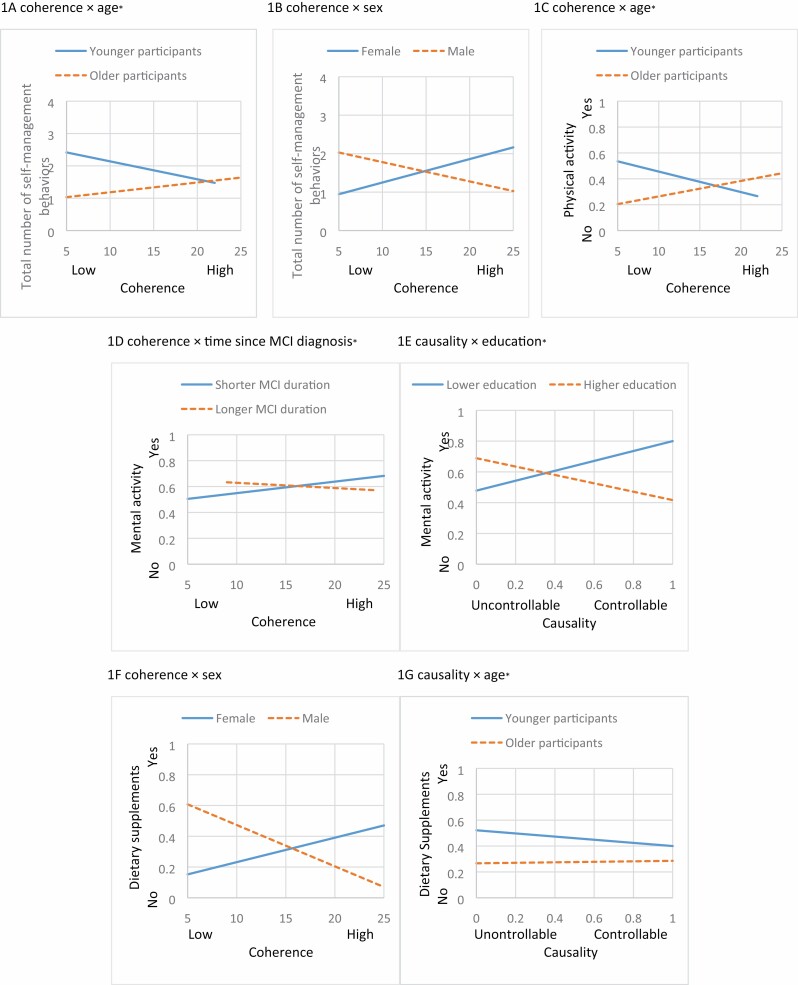
Table 2 summarizes the results of the hierarchical linear regression of the total number of self-management behaviors performed among participants. The independent variables, coherence and causality, were not significant predictors in Block 2. However, we found a significant interaction between coherence and age (b = 0.01, p = .04) on the total number of self-management behaviors (Table 2, Figure 1A), indicating that while the relationship between coherence and the total number of self-management behaviors was not significant, this relationship became positive as participants’ age increased. We also found a significant interaction between coherence and sex (b = −0.13, p = .03; Table 2, Figure 1B), suggesting that perceived understanding of MCI was negatively associated with the total number of self-management behaviors among male participants, whereas there was no association among female participants. Indeed, adding interaction terms between covariates and illness perceptions to the model explained an additional 9% of the variation in self-management behaviors for cognitive health, and this change in R2 was significant (F [17, 67] = 1.486, p = .04).
Table 2.
Hierarchical Linear Regression Model of Predictors of the Total Number of Self-Management Behaviors for Cognitive Health (N = 85)
| Unstandardized regression coefficients | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | b | SE (b) | p | b | SE (b) | p | b | SE (b) | p |
| Model 1 | |||||||||
| (Constant) | 3.57 | 1.37 | .01 | 3.51 | 1.43 | .02 | 3.75 | 5.45 | .49 |
| Age (years) | −0.03 | 0.02 | .06 | −0.03 | 0.02 | .08 | −0.15 | 0.05 | .01 |
| Malea | −0.06 | 0.28 | .83 | −0.06 | 0.29 | .83 | 2.12 | 1.00 | .04 |
| Education (years) | 0.03 | 0.06 | .64 | 0.03 | 0.06 | .66 | 0.45 | 0.22 | .05 |
| Time since MCI diagnosis (months) | <0.01 | 0.00 | .90 | <0.01 | <0.01 | .88 | 0.02 | 0.01 | .14 |
| Comorbid conditionsb | −0.02 | 0.11 | .82 | −0.03 | 0.12 | .80 | −0.53 | 0.48 | .27 |
| Model 2 | |||||||||
| Coherence | <0.01 | 0.03 | .92 | 0.08 | 0.05 | .08 | |||
| Causality | 0.05 | 0.38 | .89 | −0.12 | 0.66 | .85 | |||
| Model 3c | |||||||||
| Coherence × Age | 0.01 | 0.00 | .04 | ||||||
| Coherence × Male | −0.13 | 0.06 | .03 | ||||||
| Coherence × Education | −0.02 | 0.01 | .06 | ||||||
| Coherence × Time since MCI diagnosis | <−0.01 | <0.01 | .12 | ||||||
| Coherence × comorbid conditions | 0.02 | 0.03 | .41 | ||||||
| Causality × Age | 0.09 | 0.06 | .14 | ||||||
| Causality × Male | 0.47 | 0.84 | .58 | ||||||
| Causality × Education | −0.16 | 0.14 | .26 | ||||||
| Causality × Time since MCI diagnosis | −0.01 | 0.01 | .60 | ||||||
| Causality × Comorbid conditions | 0.42 | 0.24 | .08 | ||||||
| R 2 | 0.05 | 0.05 | 0.27 | ||||||
| Adjusted R2 | <0.01 | <0.01 | 0.09 |
Note: SE = standard error; MCI = mild cognitive impairment.
aFemale was treated as the reference category for sex.
bThe number of comorbid conditions was calculated excluding MCI.
cDue to multicollinearity, age, years of education, time since MCI diagnosis, and the number of comorbid conditions were mean centered for main and interaction effects.
Figure 1.
Interaction effects between primary predictors (coherence and causality) and covariates (sociodemographic and clinical factors) on self-management behaviors for cognitive health (aage, time since MCI diagnosis, and education variables were dichotomized for interaction plots based on data distribution). MCI = mild cognitive impairment.
The results of the hierarchical multivariable binary logistic regression for each self-management behavior (i.e., dietary changes, physical activity, mental activities, dietary supplements) are reported in Tables 3 and 4. Although we did not observe the main effects for coherence and causality in all models, several interaction effects were identified (Figure 1C–G).
Table 3.
Hierarchical Binary Logistic Regression Models of Predictors of Dietary Changes and Physical Activity (N = 85)
| Dietary changes | Physical activity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | b | OR [95% CI] | b | OR [95% CI] | b | OR [95% CI] | b | OR [95% CI] | b | OR [95% CI] | b | OR [95% CI] |
| Model 1 | ||||||||||||
| (Constant) | 2.16 | 8.64 | 1.47 | 4.36 | 3.97 | 52.97 | 0.06 | 1.06 | −0.12 | 0.88 | 6.86 | 951.00 |
| Age (years) | −0.08 | 0.93* [0.87–0.99] | −0.07 | 0.94 [0.87–1.00] | −0.14 | 0.87 [0.70–1.08] | −0.04 | 0.97 [0.91–1.02] | −0.04 | 0.96 [0.91–1.02] | −0.28 | 0.75* [0.59–0.97] |
| Malea | −0.41 | 0.66 [0.24–1.84] | −0.47 | 0.62 [0.22–1.76] | 3.00 | 20.06 [0.26–1527.26] | −0.41 | 0.66 [0.26–1.69] | −0.40 | 0.67 [0.26–1.73] | 2.00 | 7.41 [0.11–515.19] |
| Education (years) | 0.12 | 1.13 [0.91–1.40] | 0.11 | 1.11 [0.90–1.38] | 0.21 | 1.23 [0.50–3.01] | 0.10 | 1.11 [0.91–1.34] | 0.10 | 1.10 [0.91–1.34] | 0.66 | 1.93 [0.71–5.20] |
| Time since MCI diagnosis (months) | 0.01 | 1.01 [0.99–1.02] | 0.01 | 1.01 [0.99–1.02] | 0.01 | 1.01 [0.95–1.07] | <0.01 | 1.00 [0.99–1.02] | <0.01 | 1.00 [0.99–1.02] | 0.01 | 1.01 [0.95–1.07] |
| Comorbid conditionsb | 0.18 | 1.20 [0.82–1.75] | 0.11 | 1.11 [0.75–1.66] | −0.47 | 0.63 [0.08–5.10] | 0.18 | 1.20 [0.83–1.72] | 0.17 | 1.18 [0.81–1.73] | 0.14 | 1.15 [0.15–8.64] |
| Model 2 | ||||||||||||
| Coherence | 0.01 | 1.01 [0.91–1.13] | 0.13 | 1.14 [0.93–1.38] | 0.02 | 1.02 [0.93–1.13] | 0.08 | 1.09 [0.89–1.33] | ||||
| Causality | 0.79 | 2.21 [0.63–7.73] | 1.87 | 6.49 [0.38–110.21] | 0.04 | 1.04 [0.31–3.55] | −0.43 | 0.65 [0.02–26.95] | ||||
| Model 3c | ||||||||||||
| Coherence × Age | <0.01 | 1.00 [0.99–1.02] | 0.02 | 1.02* [1.00–1.03] | ||||||||
| Coherence × Male | −0.21 | 0.81 [0.63–1.04] | −0.12 | 0.89 [0.70–1.13] | ||||||||
| Coherence × Education | −0.01 | 0.99 [0.94–1.05] | −0.04 | 0.97 [0.91–1.02] | ||||||||
| Coherence × Time since MCI diagnosis | <−0.01 | 1.00 [0.99–1.00] | <−0.01 | 1.00 [0.10–1.00] | ||||||||
| Coherence × Comorbid conditions | 0.03 | 1.03 [0.91–1.17] | −0.01 | 0.99 [0.88–1.11] | ||||||||
| Causality × Age | −0.02 | 0.98 [0.72–1.32] | −0.15 | 0.86 [0.55–1.35] | ||||||||
| Causality × Male | −1.56 | 0.21 [<0.01–16.79] | −3.28 | 0.04 [<0.01–11.31] | ||||||||
| Causality × Education | 0.35 | 1.41 [0.56–3.60] | 0.10 | 1.12 [0.51–2.41] | ||||||||
| Causality × Time since MCI diagnosis | 0.06 | 1.06 [0.98–1.15] | −0.07 | 0.94 [0.84–1.05] | ||||||||
| Causality × Comorbid conditions | 0.32 | 1.38 [0.43–4.38] | 1.25 | 3.49 [0.78–15.54] | ||||||||
| Model GOF test (Hosmer– Lemeshow, p) | .51 | .22 | .45 | .68 | .98 | .71 | ||||||
| Omnibus test (χ 2 (df), p) | 7.51 (5), .19 | 9.12 (7), .24 | 18.08 (17), .38 | 3.56 (5), .61 | 3.79 (7), .80 | 18.43 (17), .36 | ||||||
| Model predictivity (Nagelkerke R2) | 0.12 | 0.15 | 0.28 | 0.06 | 0.06 | 0.27 |
Note: b = unstandardized regression coefficients; CI = confidence interval; GOF = goodness of fit; OR = odds ratio; MCI = mild cognitive impairment.
aFemale was treated as the reference category for sex.
bThe number of comorbid conditions was calculated with the exception of MCI.
cFor interaction terms, age, years of education, and the number of comorbid conditions were mean centered due to multicollinearity.
*p < .05.
Table 4.
Hierarchical Binary Logistic Regression Model of Predictors of Mental Activities and Dietary Supplements (N = 85)
| Mental activities | Dietary supplements | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | b | OR [95% CI] | b | OR [95% CI] | b | OR [95% CI] | b | OR [95% CI] | b | OR [95% CI] | b | OR [95% CI] |
| Model 1 | ||||||||||||
| (Constant) | 0.39 | 1.48 | 0.58 | 1.78 | −2.52 | 0.08 | 4.89 | 133.61 | 5.35 | 209.86 | 1.15 | 3.17 |
| Age (years) | 0.01 | 1.01 [0.96–1.07] | 0.01 | 1.01 [0.95–1.07] | −0.17 | 0.84 [0.66–1.07] | −0.06 | 0.94* [0.89–1.00] | −0.06 | 0.94† [0.89–1.00] | −0.28 | 0.75* [0.58–0.98] |
| Malea | 0.43 | 1.54 [0.62–3.83] | 0.47 | 1.59 [0.63–4.02] | 3.78 | 43.66 [0.42–4568.95] | 0.01 | 1.01 [0.39–2.63] | −0.01 | 0.99 [0.37–2.62] | 4.91 | 136.02† [0.90–20,609.80] |
| Education (years) | −0.03 | 0.97 [0.81–1.16] | −0.03 | 0.97 [0.81–1.17] | 0.83 | 2.29†[0.88–5.93] | −0.05 | 0.95 [0.79–1.15] | −0.05 | 0.96 [0.79–1.16] | 0.90 | 2.45† [0.89–6.78] |
| Time since MCI diagnosis (months) | −0.01 | 0.99 [0.98–1.01] | −0.01 | 0.99 [0.98–1.01] | 0.07 | 1.07† [1.00–1.15] | <0.01 | 1.00 [0.99–1.01] | < −0.01 | 1.00 [0.99–1.01] | 0.06 | 1.06 [0.99–1.13] |
| Comorbid conditionsb | −0.19 | 0.83 [0.58–1.19] | −0.16 | 0.85 [0.59–1.24] | −1.58 | 0.21 [0.02–1.77] | −0.26 | 0.77 [0.53–1.14] | −0.23 | 0.79 [0.53–1.18] | −0.34 | 0.71 [0.11–4.72] |
| Model 2 | ||||||||||||
| Coherence | 0.01 | 1.01 [0.92–1.11] | 0.14 | 1.15 [0.93–1.41] | −0.03 | 0.97† [0.88–1.07] | 0.16 | 1.17 [0.92–1.49] | ||||
| Causality | −0.33 | 0.72 [0.22–2.39] | −1.69 | 0.18 [0.01–3.58] | −0.23 | 0.79 [0.22–2.82] | −1.55 | 0.21 [0.01–4.40] | ||||
| Model 3c | ||||||||||||
| Coherence × Age | 0.01 | 1.01 [0.99–1.03] | 0.01 | 1.01 [0.99–1.03] | ||||||||
| Coherence × Male | −0.20 | 0.82 [0.62–1.07] | −0.30 | 0.74* [0.56–0.99] | ||||||||
| Coherence × Education | −0.05 | 0.96 [0.90–1.01] | −0.06 | 0.94† [0.89–1.00] | ||||||||
| Coherence × Time since MCI diagnosis | <−0.01 | 0.99* [0.99–1.00] | <−0.01 | 1.00† [0.99–1.00] | ||||||||
| Coherence × Comorbid conditions | 0.06 | 1.06 [0.95–1.20] | <−0.01 | 1.00 [0.89–1.11] | ||||||||
| Causality × Age | 0.28 | 1.32 [0.98–1.79] | 0.27 | 1.31* [1.02–1.67] | ||||||||
| Causality × Male | 2.85 | 17.29 [0.27–1095.56] | 3.31 | 27.50 [0.78–966.93] | ||||||||
| Causality × Education | −0.81 | 0.44* [0.22–0.88] | −0.15 | 0.86 [0.48–1.54] | ||||||||
| Causality × Time since MCI diagnosis | −0.03 | 0.97 [0.92–1.03] | −0.02 | 0.98 [0.93–1.03] | ||||||||
| Causality × Comorbid conditions | 0.86 | 2.35 [0.84–6.63] | 0.44 | 1.55 [0.58–4.14] | ||||||||
| Model GOF test (Hosmer–Lemeshow, p) | .93 | .50 | .91 | .74 | .90 | .25 | ||||||
| Omnibus test (χ 2 (df), p) | 3.09 (5), .69 | 3.45 (7), .84 | 26.39 (17), .69 | 7.21 (5), .21 | 7.80 (7), .35 | 25.71 (17), .08 | ||||||
| Model predictivity (Nagelkerke R2) | 0.05 | 0.05 | 0.36 | 0.11 | 0.12 | 0.36 |
Note: b = unstandardized regression coefficients; CI = confidence interval; GOF = goodness of fit; OR = odds ratio; MCI = mild cognitive impairment.
aFemale was treated as the reference category for sex.
bThe number of comorbid conditions was calculated excluding MCI.
cFor interaction terms, age, years of education, and the number of comorbid conditions were mean centered due to multicollinearity.
† p < .10, *p < .05.
An interaction between coherence and age (p = .04, odds ratio [OR] = 1.02, 95% confidence interval [CI] = 1.00–1.03) on physical activity (Table 3, Figure 1C) indicates that older participants with a better-perceived understanding of MCI had an increased likelihood of engaging in physical activity for cognitive health. As indicated in Table 4 and Figure 1D, we also found an interaction between coherence and time since the MCI diagnosis (p = .03, OR = 0.99, 95% CI = 0.99–1.00) on mental activities. This finding suggests that among participants with a longer duration of MCI diagnosis, the effect of the perceived understanding of cognitive changes on the likelihood of engaging in mental activities was less pronounced than those with a shorter duration. While the perceived cause of MCI was not associated with mental activities in Block 2, such association was moderated by participants’ levels of education (p = .02, OR = 0.44, 95% CI = 0.22–0.88; Table 4, Figure 1E). This suggests that the sensitivity of mental activities to the perceived cause of cognitive changes was negative among participants with more years of education. Taking dietary supplements was associated with either participants’ levels of perceived understanding or cause of MCI after adding interaction terms of sex and age (Table 4, Figure 1F and G). While no significant association was found between coherence and taking dietary supplements in the female participants, male participants with a higher perceived understanding of MCI were less likely than those with a lower perceived understanding to take dietary supplements (p = .04, OR = 0.74, 95% CI = 0.56–0.99). An interaction between causality and age (p = .03, OR = 1.31, 95% CI = 1.02–1.67) indicates that as participants’ age increased, the likelihood of taking dietary supplements was greater for those who believed that MCI is attributable to controllable factors.
Discussion and Implications
This study extends the literature on self-management for cognitive health in MCI by examining perceptions of MCI (i.e., perceived understanding of and cause of cognitive changes) and their associations with self-management behaviors (i.e., dietary changes, physical activity, mental activities, and taking dietary supplements), and whether such associations were moderated by sociodemographic or clinical factors. Our analyses did not support the first two hypotheses testing the main effects of illness perceptions of MCI on either the total number of or each of the self-management behaviors. However, participants’ illness perceptions were associated with self-management behaviors among subgroups defined by sociodemographic or clinical factors. Despite the fact that persons with MCI are at increased risk for AD dementia (Petersen et al., 2018), the transition to AD from MCI still carries many unknowns, which may lead to difficulty in engaging in self-management behaviors for cognitive health among persons with MCI.
A meta-analysis (Hagger et al., 2017) indicated that when a condition has medically explained etiology with a clear prognosis and treatment, affected individuals have a better sense of understanding of and causality of their condition, which may result in the adoption of self-management behaviors. The findings from our study are consistent with prior investigations of MCI samples that adopted or used the IPQ-R (Lin et al., 2012; Lingler et al., 2016). However, the levels of perceived understanding observed in our MCI participants were lower than has been observed in other populations with chronic diseases such as hypertension (Stallings, 2016) and type 2 diabetes (Kim et al., 2021). This suggests that persons with MCI may not easily understand their diagnosis due to ambiguity related to the course or treatment of MCI compared with other medically explained health conditions. Similar to previous research on the perceived cause of cognitive changes in the MCI population (Lin &Heidrich, 2012; Rodakowski et al., 2014), the majority of our participants believed that uncontrollable factors such as aging and heredity caused their cognitive changes.
The findings of interaction effects for coherence and age, causality and age, and causality and education supported our hypotheses that age and education would moderate the effect of perceived understanding or perceived cause of MCI on reported self-management behaviors. The moderating effects of age, in particular, should be considered in future efforts to promote self-management for cognitive health as different interventions or messaging may be warranted for the old-old versus other older adults. Interestingly, this relationship was also moderated by sex, which was an exploratory aim of this study. One possible explanation of a negative association between coherence and self-management behaviors in the male group is that male participants with a better-perceived understanding of MCI may tend to engage in other behaviors such as seeking information or testing their cognitive function more frequently rather than the lifestyle type behaviors listed on our checklist.
Regarding education, we found a negative association between perceived causality and participation in mental training activities among participants with more years of education. A previous study of healthy behavior changes following the diagnosis of hypertension (Hernandez et al., 2018) also found that education was a strong predictor of the complex behavior changes to manage the disease. Although our data did not fully explain the education difference, similar to our speculation about gender, participants with higher education attainment may be engaged in other behaviors (e.g., evaluations of their cognitive impairment) for their cognitive health (Lingler et al., 2016) that were not assessed by our interview tool. However, the current finding must be interpreted with caution because our participants recruited from the ADRC were, on average, highly educated. Time since the diagnosis also moderated the relationship between perceived understanding of MCI and mental activities, which was the focus of another exploratory analysis. This relationship was negative among participants with a longer time since the diagnosis; however, there is a need for additional studies on how the time since the diagnosis affects such a relationship. Quite possibly, individuals are more motivated to pursue cognitive health behaviors in the period immediately following diagnosis, making it an ideal time for an intervention.
The interaction between causality and age on taking dietary supplements raises the possibility that younger participants may be more likely to heed recommendations to take dietary supplements for cognitive health when they perceive the cause of MCI to be controllable. Nevertheless, the majority of our participants attributed their memory issues to uncontrollable factors such as aging, and this pattern of findings is congruent with previous quantitative and qualitative investigations of persons with cognitive impairment (Clare et al., 2016; Lin et al., 2012). Such beliefs among persons with MCI may limit taking dietary supplements. Our male participants who believed that they have a better-perceived understanding of their cognitive changes were also less likely to take dietary supplements. One may question the clinical relevance of a disinclination for taking supplements within certain subgroups; however, a recent population-based longitudinal study (Zhao et al., 2020) found that higher vitamin D intake was significantly associated with a reduced risk of dementia among older adults without cognitive impairment, suggesting that such an approach may one day have a role in the comprehensive management of AD risk in at-risk groups of older adults.
Interventions aiming at changing illness perceptions in a positive way may be critical in enhancing self-management across an array of chronic conditions (Hagger et al., 2017). Nevertheless, based on the current findings, there is little to suggest that participants who believed they have a better understanding or those who attributed their cognitive changes to controllable factors necessarily performed more self-management behaviors for cognitive health. Instead, it would be productive to systematically identify how individuals’ sociodemographic and clinical characteristics affect the direction or strength of the relationship between perceptions of MCI and self-management behaviors. The findings from our study suggest that clinicians and health care experts should assess perceptions of MCI with related factors (e.g., sociodemographics) when discussing patients’ self-management behaviors for cognitive health. Indeed, health care providers should provide patients with up-to-date information on MCI and recommend them to keep physically and cognitively active, although more robust evidence of specific activities preventing AD dementia is needed (Livingston et al., 2020).
We must acknowledge the limitations of this study. First, secondary analysis prevented us from examining other important factors that may be related to our key variables, including other dimensions of illness perception. Second, the cross-sectional design precludes investigation of any temporal relationship between illness perceptions and self-management behaviors in MCI. Third, our findings cannot readily be generalized to the broader MCI population as the majority of our participants were Caucasian with relatively high levels of education. Finally, our data relied heavily on self-report.
Conclusions
This study adds essential insights into how perceptions of MCI, sociodemographics, and clinical factors are associated with self-management behaviors for cognitive health. Persons with MCI may engage in a wide range of self-management behaviors based on their own perceptions of cognitive changes together with their age, education, sex, or time since the diagnosis. Educating health care professionals who are counseling persons with MCI to consider an individual’s perceptions of MCI and other factors may prove critical to promoting self-management behaviors.
Acknowledgments
This study is adapted from a portion of Hyejin Kim’s dissertation. The authors would thank the participants of the RAISR Study, RAISR research staff (Lisa Tamres, Melissa Knox, and Uchenna Mbawuike), Director of International Affairs at the University of Pittsburgh School of Nursing (Dr. Brian Greene), data manager at the Alzheimer’s Disease Research Center (Rocco Mercurio), and the undergraduate research assistant (Jeong Eun Kim) for their contributions to this work.
Contributor Information
Hyejin Kim, Department of Biobehavioral Nursing and Health Informatics, University of Washington School of Nursing, Seattle, USA.
Susan M Sereika, Department of Health and Community Systems, University of Pittsburgh School of Nursing, Pittsburgh, USA.
Steven M Albert, Department of Behavioral and Community Health Sciences, University of Pittsburgh Graduate School of Public Health, Pittsburgh, USA.
Catherine M Bender, Department of Health and Community Systems, University of Pittsburgh School of Nursing, Pittsburgh, USA.
Jennifer H Lingler, Department of Health and Community Systems, University of Pittsburgh School of Nursing, Pittsburgh, USA; Alzheimer’s Disease Research Center, University of Pittsburgh School of Medicine, Pittsburgh, USA.
Funding
This work was supported by Judith A. Erlen Nursing PhD Student Research Award from the University of Pittsburgh School of Nursing (PI: Kim). The parent study was supported by the National Institutes of Health/National Institute on Aging (P50-AG005133 to O. L. Lopez) and the National Institutes of Health/National Institute on Aging (R01-AG046906 to J. H. Lingler).
Conflict of Interest
None declared.
References
- Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., Gamst, A., Holtzman, D. H., Jagust, W. J., Petersen, R. C., Snyder, P. J., Carrillo, M. C., Thies, B., & Phelps, C. H. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 270–279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association . (2021). 2021 Alzheimer’s disease facts and figures.https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf
- Anderson, L. N., McCaul, K. D., & Langley, L. K. (2011). Common-sense beliefs about the prevention of Alzheimer’s disease. Aging & Mental Health, 15(7), 922–931. doi: 10.1080/13607863.2011.569478 [DOI] [PubMed] [Google Scholar]
- Chao, S., Roberts, J. S., Marteau, T. M., Silliman, R., Cupples, L. A., & Green, R. C. (2008). Health behavior changes after genetic risk assessment for Alzheimer disease: The REVEAL Study. Alzheimer Disease and Associated Disorders, 22(1), 94–97. doi: 10.1097/WAD.0b013e31815a9dcc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, K. D., Roberts, J. S., Zikmund-Fisher, B. J., Kardia, S. L., McBride, C. M., Linnenbringer, E., & Green, R. C.; REVEAL Study Group . (2015). Associations between self-referral and health behavior responses to genetic risk information. Genome Medicine, 7(1), 10. doi: 10.1186/s13073-014-0124-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare, L., Quinn, C., Jones, I. R., & Woods, R. T. (2016). “I don’t think of it as an illness”: Illness representations in mild to moderate dementia. Journal of Alzheimer’s Disease, 51(1), 139–150. doi: 10.3233/JAD-150794 [DOI] [PubMed] [Google Scholar]
- Clem, M. A., Holliday, R. P., Pandya, S., Hynan, L. S., Lacritz, L. H., & Woon, F. L. (2017). Predictors that a diagnosis of mild cognitive impairment will remain stable 3 years later. Cognitive and Behavioral Neurology, 30(1), 8–15. doi: 10.1097/WNN.0000000000000119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias-Sonnenschein, L. S., Viechtbauer, W., Ramakers, I. H., Verhey, F. R., & Visser, P. J. (2011). Predictive value of APOE-ε4 allele for progression from MCI to AD-type dementia: A meta-analysis. Journal of Neurology, Neurosurgery, and Psychiatry, 82(10), 1149–1156. doi: 10.1136/jnnp.2010.231555 [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration . (2018). Dietary supplement labeling guide: Chapter I. general dietary supplement labeling.https://www.fda.gov/food/dietary-supplements-guidance-documents-regulatory-information/dietary-supplement-labeling-guide-chapter-i-general-dietary-supplement-labeling#1-14
- Fleiss, J. L., Cohen, J., & Everitt, B. S. (1969). Large sample standard errors of kappa and weighted kappa. Psychological Bulletin, 72(5), 323. doi: 10.1037/H0028106 [DOI] [Google Scholar]
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Hagger, M. S., Koch, S., Chatzisarantis, N. L. D., & Orbell, S. (2017). The common sense model of self-regulation: Meta-analysis and test of a process model. Psychological Bulletin, 143(11), 1117–1154. doi: 10.1037/bul0000118 [DOI] [PubMed] [Google Scholar]
- Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, E. M., Margolis, R., & Hummer, R. A. (2018). Educational and gender differences in health behavior changes after a gateway diagnosis. Journal of Aging and Health, 30(3), 342–364. doi: 10.1177/0898264316678756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karssemeijer, E. E., Aaronson, J. J., Bossers, W. W., Smits, T. T., & Kessels, R. R. (2017). Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Research Reviews, 40, 75–83. doi: 10.1016/j.arr.2017.09.003 [DOI] [PubMed] [Google Scholar]
- Kim, H., Sereika, S. M., Lingler, J. H., Albert, S. M., & Bender, C. M. (2021). Illness perceptions, self-efficacy, and self-reported medication adherence in persons aged 50 and older with type 2 diabetes. Journal of Cardiovascular Nursing, 36(4), 312–328. doi: 10.1097/JCN.0000000000000675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell, T. D., & Monsell, S. E. (2012). Reversion from mild cognitive impairment to normal or near-normal cognition: Risk factors and prognosis. Neurology, 79(15), 1591–1598. doi: 10.1212/WNL.0b013e31826e26b7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal, H., Brissette, I., & Leventhal, E. A. (2003). The Common-Sense Model of Self-Regulation of health and illness. In Cameron L. D. & Leventhal H. (Eds.), The self-regulation of health and illness behavior (pp. 42–65). Routledge. [Google Scholar]
- Leventhal, H., Nerenz, D. R., & Steele, D. J. (1984). Illness representation and coping with health threats. In Baum A., Taylor S. E., & Singer J. E. (Eds.), Handbook of psychology and health (pp. 219–252). Routledge. [Google Scholar]
- Leventhal, H., Phillips, L. A., & Burns, E. (2016). The Common-Sense Model of Self-Regulation (CSM): A dynamic framework for understanding illness self-management. Journal of Behavioral Medicine, 39(6), 935–946. doi: 10.1007/s10865-016-9782-2 [DOI] [PubMed] [Google Scholar]
- Lin, F., Gleason, C. E., & Heidrich, S. M. (2012). Illness representations in older adults with mild cognitive impairment. Research in Gerontological Nursing, 5(3), 195–206. doi: 10.3928/19404921-20120605-04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, F., & Heidrich, S. M. (2012). Role of older adult’s illness schemata in coping with mild cognitive impairment. Journal of Psychosomatic Research, 72(5), 357–363. doi: 10.1016/j.jpsychores.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Lingler, J. H., Nightingale, M. C., Erlen, J. A., Kane, A. L., Reynolds, C. F.3rd, Schulz, R., & DeKosky, S. T. (2006). Making sense of mild cognitive impairment: A qualitative exploration of the patient’s experience. The Gerontologist, 46(6), 791–800. doi: 10.1093/geront/46.6.791 [DOI] [PubMed] [Google Scholar]
- Lingler, J. H., Sereika, S. M., Butters, M. A., Cohen, A. D., Klunk, W. E., Knox, M. L., McDade, E., Nadkarni, N. K., Roberts, J. S., Tamres, L. K., & Lopez, O. L. (2020). A randomized controlled trial of amyloid positron emission tomography results disclosure in mild cognitive impairment. Alzheimer’s & Dementia, 16(9), 1330–1337. doi: 10.1002/alz.12129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingler, J. H., Terhorst, L., Schulz, R., Gentry, A., & Lopez, O. (2016). Dyadic analysis of illness perceptions among persons with mild cognitive impairment and their family members. The Gerontologist, 56(5), 886–895. doi: 10.1093/geront/gnv029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., Brayne, C., Burns, A., Cohen-Mansfield, J., Cooper, C., Costafreda, S. G., Dias, A., Fox, N., Gitlin, L. N., Howard, R., Kales, H. C., Kivimäki, M., Larson, E. B., Ogunniyi, A., … Mukadam, N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England), 396(10248), 413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez, O. L., Litvan, I., Catt, K. E., Stowe, R., Klunk, W., Kaufer, D. I., Becker, J. T., & DeKosky, S. T. (1999). Accuracy of four clinical diagnostic criteria for the diagnosis of neurodegenerative dementias. Neurology, 53(6), 1292–1299. doi: 10.1212/wnl.53.6.1292 [DOI] [PubMed] [Google Scholar]
- Matchwick, C., Domone, R., Leroi, I., & Simpson, J. (2014). Perceptions of cause and control in people with Alzheimer’s disease. The Gerontologist, 54(2), 268–276. doi: 10.1093/geront/gnt014 [DOI] [PubMed] [Google Scholar]
- Morgan, G. H., Garand, L. I., & Lingler, J. H. (2012). Self-initiated health behaviors following a diagnosis of mild cognitive impairment. Research in Gerontological Nursing, 5(2), 94–100. doi: 10.3928/19404921-20110831-02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss-Morris, R., Weinman, J., Petrie, K., Horne, R., Cameron, L., & Buick, D. (2002). The revised illness perception questionnaire (IPQ-R). Psychology and Health, 17(1), 1–16. doi: 10.1080/08870440290001494 [DOI] [Google Scholar]
- National Institute of Nursing Research . (2016). The NINR strategic plan: Advancing science, improving lives. https://www.ninr.nih.gov/sites/files/docs/NINR_StratPlan2016_reduced.pdf
- Petersen, R. C. (2004). Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine, 256(3), 183–194. doi: 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- Petersen, R. C., Lopez, O., Armstrong, M. J., Getchius, T. S. D., Ganguli, M., Gloss, D., Gronseth, G. S., Marson, D., Pringsheim, T., Day, G. S., Sager, M., Stevens, J., & Rae-Grant, A. (2018). Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology, 90(3), 126–135. doi: 10.1212/WNL.0000000000004826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Tangalos, E. G., & Kokmen, E. (1999). Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology, 56(3), 303–308. doi: 10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- Portacolone, E., Johnson, J. K., Covinsky, K. E., Halpern, J., & Rubinstein, R. L. (2018). The effects and meanings of receiving a diagnosis of mild cognitive impairment or Alzheimer’s disease when one lives alone. Journal of Alzheimer’s Disease, 61(4), 1517–1529. doi: 10.3233/JAD-170723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodakowski, J., Schulz, R., Gentry, A., Garand, L., & Lingler, J. H. (2014). Attribution of mild cognitive impairment etiology in patients and their care partners. International Journal of Geriatric Psychiatry, 29(5), 464–469. doi: 10.1002/gps.4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee, D. J., Anson, J. M., Waddington, G. S., & Berry, H. L. (2012). Association between physical functionality and falls risk in community-living older adults. Current Gerontology and Geriatrics Research, 2012, 864516. doi: 10.1155/2012/864516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz, B. E., Wang, T., Cloonan, Y. K., Jacobsen, E., Chang, C. H., Hughes, T. F., Kamboh, M. I., & Ganguli, M. (2018). Risk of progression from subjective cognitive decline to mild cognitive impairment: The role of study setting. Alzheimer’s & Dementia, 14(6), 734–742. doi: 10.1016/j.jalz.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalling, D. (2016). Illness perceptions and health behaviors of Black women. Journal of Cardiovascular Nursing, 31(6), 492–499. doi: 10.1097/JCN.0000000000000276 [DOI] [PubMed] [Google Scholar]
- Zhao, C., Tsapanou, A., Manly, J., Schupf, N., Brickman, A. M., & Gu, Y. (2020). Vitamin D intake is associated with dementia risk in the Washington Heights–Inwood Columbia Aging Project (WHICAP). Alzheimer’s & Dementia, 16(10), 1393–1401. doi: 10.1002/alz.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]