Abstract
Epithelial–mesenchymal transition (EMT) is an essential biological process involved in the initiation and progression of cancer by which epithelial tumor cells lose their differentiated characteristics, such as cell–cell adhesion and apical–basal polarity and acquire a more invasive and/or metastatic mesenchymal phenotype. The present study investigated the expression of immunomarkers with a role in EMT of non-melanoma skin cancers (NMSCs), such as E-cadherin, fibronectin and Slug, for a number of 50 NMSCs, represented by 30 cases of basal cell carcinomas (BCCs) and 20 cases of squamous cell carcinomas (SCCs). For BCC, the statistical analysis of the investigated immunomarkers indicated significantly differences in relation to the depth of invasion, and for E-cadherin and fibronectin with the degree of risk. In the case of SCC, the statistical analysis indicated significant differences of E-cadherin and Slug with the degree of tumor differentiation, and for fibronectin and Slug with the depth of invasion. The analysis of the distribution for the percentage values of the investigated immunomarkers in the case of BCC indicated a significant negative linear relation between E-cadherin/fibronectin and E-cadherin/Slug, and in SCC a significant negative linear relation between E-cadherin/fibronectin, E-cadherin/Slug and a positive linear one in the case of fibronectin/Slug. The study indicates through the statistically significant relation between E-cadherin/fibronectin and E-cadherin/Slug, the EMT intervention in carcinogenesis of NMSC.
Keywords: non-melanoma skin cancer, E-cadherin, fibronectin, Slug
⧉ Introduction
Epithelial–mesenchymal transition (EMT) is an essential biological process involved in the initiation and progression of cancer [1]. Neoplastic EMT is a process by which epithelial tumor cells lose their differentiated characteristics, such as cell–cell adhesion and apical–basal polarity and acquire a more invasive and/or metastatic mesenchymal phenotype [2,3,4].
Loss of keratins expression, accompanied by de novo expression of vimentin, is a particularity of EMT in carcinomas [5]. The cadherin switch is primarily the result of transcriptional regulation of cadherin expression by several factors, such as Snail, Slug, Twist, Zeb1, and Zeb2 [6,7]. These factors act by repressing E-cadherin transcription by direct binding to its promoter [8]. While initially considered only a phenotypic conversion from epithelial to mesenchymal cells, recent studies indicate the critical role that this process plays in metabolic reprogramming, immune evasion, and resistance to therapy [1]. Currently, EMT has a rather flexible, plasticity status, known as the “partial EMT program”, rather than the status of carrier of the complete transformation phenotype during the tumor progression [9]. However, the roles of EMT-associated proteins in basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) have not been fully elucidated [10].
Aim
Due to numerous studies indicating the involvement of EMT in the progression of cancer with various locations, we aimed to evaluate the different aspects of this process in the most common malignant skin tumors, respectively in non-melanoma skin cancers (NMSCs) whose incidence has an alarming growth rate, but with quite limited therapeutic targets. The present study investigated the expression of some immunomarkers with a role in EMT of NMSC, such as E-cadherin, fibronectin and Slug.
⧉ Materials and Methods
We investigated a number of 50 cases of NMSC from the Clinics of Dermatology and Plastic Surgery, Emergency County Hospital, Craiova, Romania. The surgical excision specimens were fixed in 10% neutral buffered formalin, processed by paraffin embedding technique and Hematoxylin–Eosin (HE) staining. The histopathological classification of the lesions allowed the identification of 30 cases of BCC and 20 cases of SCC. The histopathological study of both tumor varieties was completed by identification of two categories: low-risk grade BCC (LRG–BCC) and high-risk grade BCC (HRG–BCC), and forms of SCC with high grade of differentiation (HGD–SCC) and low grade of differentiation (LGD–SCC) [11].
From the paraffin blocks, we performed serial sections that were immunohistochemically processed using a detection system based on amplification polymer (EnVision™ FLEX System, code K8002, Dako, Agilent). Visualization of the reactions was done using the 3,3’-Diaminobenzidine (DAB) tetrahydrochloride chromogen included in the same kit, and for the validation of the reactions, we used positive and negative external controls (by omitting the primary antibody) (Table 1).
Table 1.
Antibodies used: clone, dilution, antigen retrieval and external positive control
|
Antibody |
Clone |
Manufacturer |
Dilution |
Antigen retrieval |
External control |
|
E-cadherin |
36B5 |
Leica Biosystems |
1:50 |
Microwaving in citrate buffer, pH 6 |
Mammary gland |
|
Fibronectin |
A024502-2 |
Dako, Agilent |
1:200 |
Pepsin |
Kidney |
|
Slug |
ab27568 |
Dako, Agilent |
1:150 |
Microwaving in citrate buffer, pH 6 |
Placenta |
The examination of the semiquantitative expression of the investigated immunomarkers was performed by an adapted system, by two specialists, who assessed the intensity of the immunostaining and the rate of positive cells [12], using the Panthera L research binocular microscope with 5 Megapixel digital camera (Motic manufacturer) and the software integrated in the microscope. The intensity of the score was assessed as following: 1 (low), 2 (moderate), 3 (high). The percentage of positive cells was noted as following: 1 (6–25%), 2 (26–50%), 3 (51–75%) and 4 (>75%), with a cutoff value of over 5% for the positivity of the reactions, in case of a reaction with a number of positive cells below this value or without a reaction the aspect being considered negative. The multiplying of these two scores of intensity and percentage, allowed the calculation of the final staining scores (FSS), and then the mean value for each group, these being considered low for values between 1–6 and high for values between 8–12. Low, high, and negative scores were used for statistical comparison.
For statistical analysis, we used the mean values and the comparison tests [chi-squared (χ2) and Pearson] within Statistical Package for the Social Sciences (SPSS) 10 automatic software.
This study follows the general ethical guidelines of scientific research and has been approved by the Local Ethics Committee.
⧉ Results
This study included a number of 50 NMSC cases of which 30 cases of BCC (20 cases of LRG–BCC and 10 cases of HRG–BCC), and 20 cases of SCC (10 HGD–SCC and 10 LGD–SCC). For each lesion category, we selected cases appropriate to the various primary tumor (pT) subcategories: for pT1 a number of 26 cases (15 BCC cases and 11 SCC cases), for pT2 12 cases (eight BCC cases and four SCC cases), for pT3 10 cases (six BCC cases and four SCC cases) and for pT4 two cases (one BCC case and one SCC case). The results of the immunohistochemical study for E-cadherin, fibronectin and Slug are shown in Table 2.
Table 2.
Distribution of BCC and SCC according to the number of positive cases and the average values of FSS for E-cadherin, fibronectin and Slug
|
NMSC |
BCC |
SCC |
||||||||
|
LRG |
HRG |
χ2 test |
HGD |
LGD |
χ2 test |
|||||
|
No. of cases |
FSS |
No. of cases |
FSS |
No. of cases |
FSS |
No. of cases |
FSS |
|||
|
E-cadherin | ||||||||||
|
pT1 |
10 |
8 |
5 |
6 |
p<0.001 |
6 |
12 |
3 |
4 |
p=0.117 |
|
pT2 |
6 |
6 |
2 |
6 |
2 |
6 |
1 |
1 |
||
|
pT3 |
4 |
6 |
– |
– |
2 |
6 |
1 |
1 |
||
|
pT4 |
– |
– |
– |
– |
– |
– |
– |
– |
||
|
χ2 test |
p=0.003 |
p=0.004 |
||||||||
|
Fibronectin | ||||||||||
|
pT1 |
– |
– |
– |
– |
p=0.001 |
– |
– |
– |
– |
p<0.001 |
|
pT2 |
– |
– |
– |
– |
1 |
3 |
2 |
4 |
||
|
pT3 |
– |
– |
1 |
1 |
2 |
3 |
2 |
9 |
||
|
pT4 |
– |
– |
1 |
1 |
– |
– |
1 |
9 |
||
|
χ2 test |
p=0.038 |
p=0.311 |
||||||||
|
Slug | ||||||||||
|
pT1 |
2 |
2 |
4 |
7 |
p=0.041 |
1 |
1 |
2 |
7 |
p=0.024 |
|
pT2 |
5 |
9 |
2 |
10 |
2 |
5 |
2 |
10.5 |
||
|
pT3 |
4 |
11 |
1 |
12 |
2 |
6 |
2 |
12 |
||
|
pT4 |
– |
– |
1 |
12 |
– |
– |
1 |
12 |
||
|
χ2 test |
p=0.350 |
p=0.034 |
||||||||
BCC: Basal cell carcinoma; FSS: Final staining score; HGD: High grade of differentiation; HRG: High-risk grade; LGD: Low grade of differentiation; LRG: Low-risk grade; NMSC: Non-melanoma skin cancer; pT: Primary tumor; SCC: Squamous cell carcinoma
E-cadherin immunoexpression
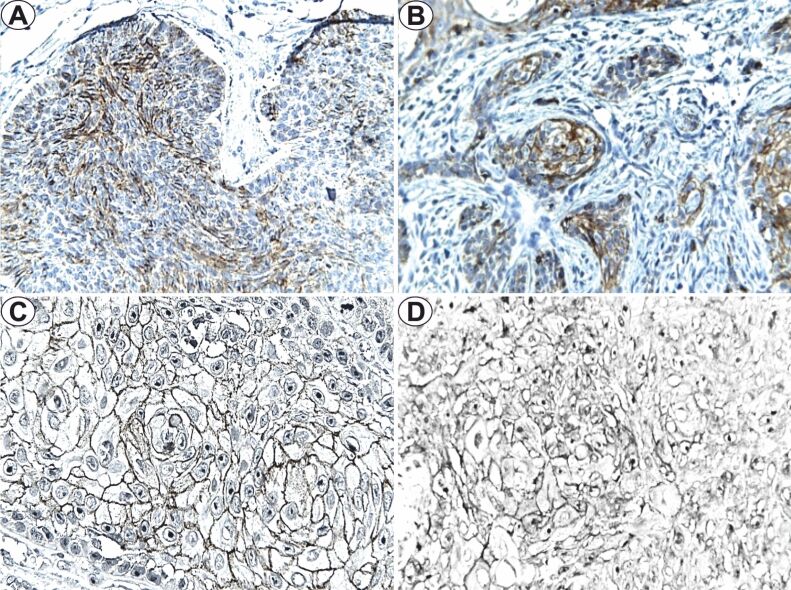
The study of E-cadherin immunoexpression indicated positivity in 27 (90%) of the BCC cases, with membranous topography, in the tumoral parenchyma, respectively 20 cases of LRG–BCC and seven cases of HRG–BCC. For LRG–BCC, the mean FSS value was between 6–8 depending on the pT category, and for the mean FSS value it was 6 regardless pT. The immunostaining intensity for LRG–BCC was moderate, with a positive cell average of 80% in pT1 tumors, 66.6% in pT2 tumors, and 70% in pT3 tumors (Figure 1A). In HRG–BCC, the immunostaining intensity was also moderate with a different mean value of positive cells, respectively 75% for pT1 and 65% for pT2 (Figure 1B). The statistical analysis indicated significant lower differences of the E-cadherin immunoexpression in HRG–BCC (p=0.003, χ2 test) with deep invasion (p<0.001, χ2 test).
Figure 1.
E-cadherin immunoexpression (×400): (A) LRG–BCC, pT1; (B) HRG–BCC, pT1; (C) HGD–SCC, pT1; (D) LGD–SCC, pT1. BCC: Basal cell carcinoma; HGD: High grade of differentiation; HRG: High-risk grade; LGD: Low grade of differentiation; LRG: Low-risk grade; pT: Primary tumor; SCC: Squamous cell carcinoma
For SCC, the immunoexpression of E-cadherin was positive in 15 (75%) cases, in the tumoral parenchyma, with membranous topography (pT1 and pT2 tumors) or membranous and cytoplasmic localization (pT3 tumors). In the HGD–SCC, the mean FSS value was 12 for pT1 tumors and 6 for pT2 and pT3 stages, with mostly moderate/high intensity of the immunoreactions and labeled cells of 80%, 72.5% and 65.5% in pT1–pT3 SCC (Figure 1C). In the LGD-SCC cases, immunoexpression was identified in the pT1–pT3 tumor categories, with FSS values between 1–4. For LGD–SCC, the immunostaining distribution was variable, with 15–50% positive cells, moderate intensity in pT1 tumors and low in pT2 and pT3 tumors and mean FSS values of 4.1 and 1 (Figure 1D). The statistical analysis indicated significantly lower differences of the E-cadherin immunoexpression in LGD–SCC (p=0.004, χ2 test) and a nonsignificant difference in the case of pT category (p=0.117, χ2 test).
Fibronectin immunoexpression
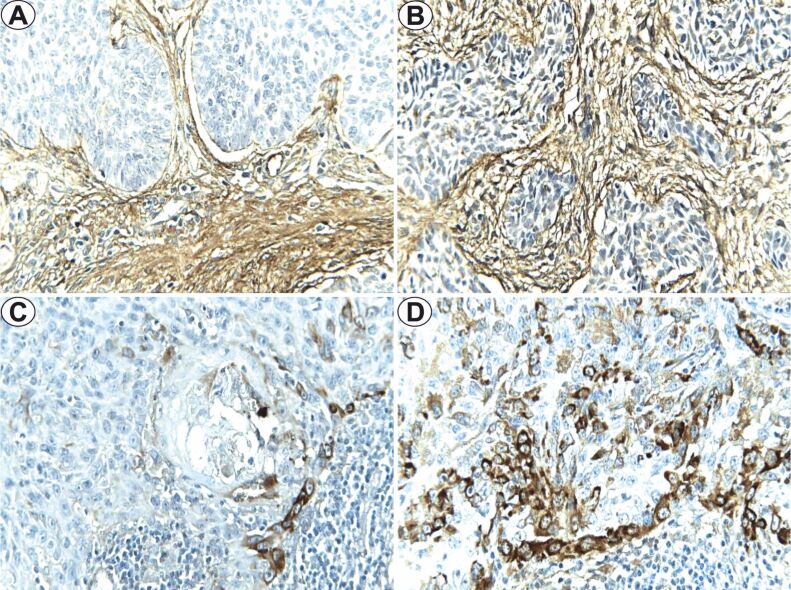
The fibronectin immunoexpression study revealed positivity only in eight (6.7%) of the BCC cases, in the tumoral parenchyma, for HRG tumors with deep invasion. In addition, we observed positivity of this immunomarker in the tumoral stroma, with moderate intensity. FSS values for HRG–BCC were in both cases 1, with limited distribution (10% and 15%, respectively) and low intensity (Figure 2, A and B). The statistical analysis indicated significantly higher differences in fibronectin in HRG–BCC (p=0.038, χ2 test) and with deep invasion (p=0.001, χ2 test).
Figure 2.
Fibronectin immunoexpression (×400): (A) LRG–BCC, pT3; (B) HRG–BCC, pT4; (C) HGD–SCC, pT3; (D) LGD–SCC, pT3. BCC: Basal cell carcinoma; HGD: High grade of differentiation; HRG: High-risk grade; LGD: Low grade of differentiation; LRG: Low-risk grade; pT: Primary tumor; SCC: Squamous cell carcinoma
In SCC, fibronectin positivity was identified in eight (40%) cases, with cytoplasmic topography, in the tumoral parenchyma, especially at the interface level of epithelium–stroma. HGD–SCCs were positive in three cases with mean FSS values of 3, high intensity immunoexpression, with 15% of positive cells, constant in the peripheral area of the tumoral islands (Figure 2C). For LGD–SCC, we found positivity in five cases, with an average number of labelled cells of 37.5±24, with moderate or high intensity (Figure 2D). The statistical analysis indicated significant higher differences of the fibronectin immunoexpression in SCC with deep invasion (p<0.001, χ2 test) and non-significant with the degree of differentiation (p=0.311, χ2 test).
Slug immunoexpression
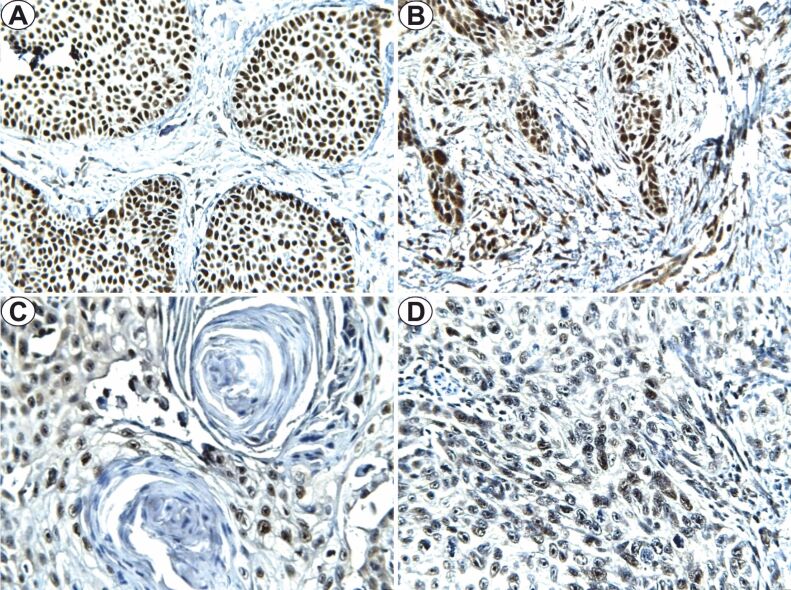
The study of Slug immunoexpression revealed positivity in 19 (63.3%) of the analyzed BCC cases, with nuclear topography, in the tumoral parenchyma. In the LRG–BCC cases, tumors from the pT1 category presented a lower intensity, with an average number of 35% positive cells, with a mean FSS value of 2, and for the pT2 and pT3 tumors, we observed high intensity of the immunostaining, with an average of 73% and respectively 82.5% positive cells, FSS mean values being 9 and 11 (Figure 3A). For HRG–BCC, the intensity of the immunostaining was moderate, with an average of 77.5% positive cells for pT1 tumors and FSS mean value of 7, high intensity with an average of 77.5% positive cells and mean FSS value of 10 for the pT2 tumors, 82.5% for pT3 and 85% for pT4, both with a mean FSS value of 12 (Figure 3B). The statistical analysis indicated significant high differences of Slug immunoexpression in tumors with deep invasion (p=0.041, χ2 test), the statistical aspects being nonsignificant for BCC risk groups (p=0.350, χ2 test).
Figure 3.
Slug immunoexpression (×400): (A) LRG–BCC, pT2; (B) HRG–BCC, pT2; (C) HGD–SCC, pT3; (D) LGD–SCC, pT3. BCC: Basal cell carcinoma; HGD: High grade of differentiation; HRG: High-risk grade; LGD: Low grade of differentiation; LRG: Low-risk grade; pT: Primary tumor; SCC: Squamous cell carcinoma
In SCC, the Slug immunoexpression revealed positivity in 12 (60%) cases, with nuclear topography, in the tumoral parenchyma. For HGD–SCC, FSS mean values were between 1–6, and for LGD–SCC varied between 7–12. The intensity of the immunostaining in HGD–SCC was low in the pT1 tumors with 20% marked cells, in the pT2, pT3 moderate intensity with an average of 47.5±3.5 and 82.5±3.53 marked cells (Figure 3C). In the case of LGD–SCC from the pT3/pT4 category, high intensity was observed, with approximately 85% of the tumoral cells being marked. In the pT1/T2 category, the intensity of the immunoreaction was moderate/high, with an average number of marked cells of 60% and 78% (Figure 3D). The statistical analysis indicated significant higher differences of Slug immunoexpression in LGD–SCC (p=0.034, χ2 test) with deep invasion (p=0.024, χ2 test).
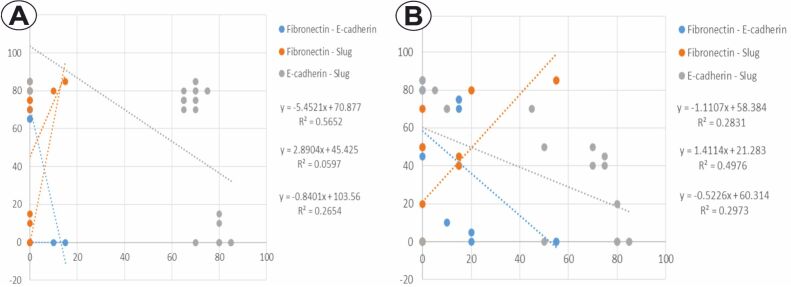
The analysis of the distribution for the percentage values of the investigated immunomarkers in BCC cases indicated a significant negative linear relation between E-cadherin/fibronectin (p<0.001, Pearson’s test), and E-cadherin/Slug (p=0.004, Pearson’s test), as well as a nonsignificant positive linear relation in the case of fibronectin/Slug (p=0.193, Pearson’s test) (Figure 4A). In SCC, the analysis of the distribution for the percentage values of the same immunomarkers indicated a significant negative linear relation between E-cadherin/fibronectin (p=0.016, Pearson’s test) and E-cadherin/Slug (p=0.013, Pearson’s test), as well as a positive linear relation between fibronectin/Slug (p=0.001, Pearson’s test) (Figure 4B).
Figure 4.
The distribution of percentage values of E-cadherin, fibronectin and Slug: (A) BCC; (B) SCC. BCC: Basal cell carcinoma; SCC: Squamous cell carcinoma
⧉ Discussions
The EMT is based on complex molecular and cellular mechanisms [3]. EMT involves concomitant downregulation of epithelial cell surface markers (cytokeratins, cadherins, etc.), an increased expression of the mesenchymal markers (vimentin, fibronectin), and upregulation and/or nuclear translocation of the specific transcription factors (Snail, Slug, Zeb1/2, and Twist1/2, etc.) [13].
E-cadherin is a cell surface adhesion molecule that mediates cell adhesion in the normal epidermis [14] and is essential in maintaining cellular integrity and epithelial tissue architecture and in mechanical regeneration of the skin through stretching and can serve as a potential therapeutic target for promoting skin regeneration [15,16]. In carcinomas, loss of E-cadherin immunoexpression has been described in the late stages of carcinogenesis, with decreased expression being a particularity of EMT [17]. By losing E-cadherin-mediated cell–cell adhesion and acquiring mesenchymal properties, carcinomatous cells acquire mobility and invasiveness, thus being able to penetrate the surrounding stroma [15].
Studies that have analyzed the immunoexpression of E-cadherin in BCC have provided quite a variety of results. While some authors have shown that E-cadherin immunoexpression in BCC cells was frequently reduced and its decrease or loss was associated with a more aggressive tumor biology [18,19,20], the results of other studies did not confirm such an assumption [21]. In our study, the statistical analysis of E-cadherin immunoexpression indicated significantly lower differences in high-grade and deeply invasive tumors.
Among malignant skin tumors, reduced E-cadherin immunoexpression has been better documented in SCC [22]. Jang demonstrated a reduced immunoexpression of membrane E-cadherin in SCC, compared to its precursor lesions and normal skin, indicating EMT as an important process in tumor progression [23]. The membrane immunoexpression of E-cadherin seems to be related to the degree of tumor differentiation through high expression in low-grade SCC and decreased or absent expression in high-grade SCC [24,25]. Wu et al. reported the E-cadherin immunoexpression in 93–100% of the normal adjacent squamous epithelia or distant from the tumor and in most well-differentiated SCC (75–100%), unlike poorly-differentiated SCC which expressed E-cadherin in less than 40% of the cases [14]. In the present study, statistical analysis indicated significantly lower differences in E-cadherin immunoexpression in high-grade tumors and nonsignificant in relation with the pT category. In some studies, LGD–SCC seem to have a high cytoplasmic E-cadherin immunoexpression [24], and the translocation from the membrane to the intracytoplasmic region is considered by several authors as a functional loss of E-cadherin, which decreases cellular integrity and thus it promotes malignant transformation and metastasis [26,27].
Fibronectins bind various components of the extracellular matrix (ECM), such as collagen and proteoglycans, and also serve as binding sites for components of the ECM with adjacent cells [28,29]. The expression and organization of fibronectin 1 (FN1) is altered in cancer and appears to be significantly correlated with an immunosuppressive environment and associated with primary resistance to immunotherapy in melanoma patients [30] which opens a new perspective in the study of FN1 in immuno-oncology, with potential of high clinical impact [31]. A study reported high fibronectin immunoexpression in the BCC stroma, the maximum expression being identified in the immediate vicinity of the tumoral islands, which may explain the characteristic biological behavior of BCC, including the low metastatic potential and the local destructive nature of the tumors [32]. In SCC, as well as other cancers with various locations, degradation of the FN1 expression has been associated with tumor progression [33]. For the analyzed cases, fibronectin immunoreactions had a negative FSS value in LRG–BCC, no matter the tumor stage and a low FSS mean value in HRG–BCC, diagnosed in advanced stages (pT3, pT4 categories), compared to SCC immunoreactions in which FSS value was variable, being present in HGD–SCC, as well as in LGD–SCC, starting with pT2 tumors, an aspect with statistical significance.
Slug transcription factor also plays an important role in the ultraviolet radiation (UVR)-induced cutaneous carcinogenesis, especially in EMT which takes place during tumor progression. An experimental study indicated the fact that Slug and Snail have similar immunoexpression patterns in both UVR exposure, similar to chemical carcinogenesis, under certain conditions Slug and Snail may be adjusted differently, but the in vitro results may not be similar to the in vivo ones [34].
One study reported high immunoexpression of Slug in SCC induced by UVRs, with epithelial and mesenchymal morphology (spindle cells), but Slug messenger ribonucleic acid (mRNA) expression is considerably higher in spindle cells than in epithelial ones, suggesting that high Slug expression precedes high Snail expression during SCC progression [5]. Another study reported, for preinvasive lesions and SCC, significant negative correlations between Snail and E-cadherin expression and between Slug and E-cadherin expression, the immunostaining intensity for Snail and Slug being associated with decreased E-cadherin immunostaining, which may promote EMT [35]. In our study, statistical analysis indicated significant higher differences in Slug immunoexpression in deep invasion BCC and nonsignificant in cases of risk grades. In SCC, the differences were significantly higher in LGD–SCC with deep invasion.
⧉ Conclusions
The study indicates through the negative linear correlation between E-cadherin/fibronectin and E-cadherin/Slug the EMT intervention in NMSC carcinogenesis. Using the relation between grading groups, risk groups and depth of invasion, the immunomarkers used in this study may be useful in identifying BCC and SCC with aggressive biological behavior.
Conflict of interest
The authors declare that they have no conflict of interests.
References
- 1.Cho ES, Kang HE, Kim NH, Yook JI. Therapeutic implications of cancer epithelial-mesenchymal transition (EMT) Arch Pharm Res. 2019;42(1):14–24. doi: 10.1007/s12272-018-01108-7. [DOI] [PubMed] [Google Scholar]
- 2.De Craene B, Denecker G, Vermassen P, Taminau J, Mauch C, Derore A, Jonkers J, Fuchs E, Berx G. Epidermal Snail expression drives skin cancer initiation and progression through enhanced cytoprotection, epidermal stem/progenitor cell expansion and enhanced metastatic potential. Cell Death Differ. 2014;21(2):310–320. doi: 10.1038/cdd.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial–mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Savagner P. Epithelial–mesenchymal transitions: from cell plasticity to concept elasticity. Curr Top Dev Biol. 2015;112:273–300. doi: 10.1016/bs.ctdb.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Newkirk KM, Parent AE, Fossey SL, Choi C, Chandler HL, Rajala-Schultz PJ, Kusewitt DF. Snai2 expression enhances ultraviolet radiation-induced skin carcinogenesis. Am J Pathol. 2007;171(5):1629–1639. doi: 10.2353/ajpath.2007.070221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tam WL, Weinberg RA. The epigenetics of epithelial–mesenchymal plasticity in cancer. Nat Med. 2013;19(11):1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121(Pt 6):727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Weinberg RA. Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Nieto MA, Huang RYJ, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166(1):21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 10.Kim YU, Kim KJ, Heo EP. The epithelial–mesenchymal transition and E-cadherin and vimentin expression in basal cell carcinoma and squamous cell carcinoma. Korean J Dermatol. 2015;53(2):96–105. https://koreamed.org/SearchBasic.php?RID=2246223 [Google Scholar]
- 11.Elder DE, Massi D, Scolyer RA, Willemze R, editors. World Health Organization (WHO) Classification of skin tumours. 4. Vol. 11. Lyon, France: International Agency for Research on Cancer (IARC) Press; 2018. pp. 7–14.https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Skin-Tumours-2018 [Google Scholar]
- 12.Wang N, Dong CR, Jiang R, Tang C, Yang L, Jiang QF, Chen GG, Liu ZM. Overexpression of HIF-1α, metallothionein and SLUG is associated with high TNM stage and lymph node metastasis in papillary thyroid carcinoma. Int J Clin Exp Pathol. 2013;7(1):322–330. [PMC free article] [PubMed] [Google Scholar]
- 13.Pearlman RL, Montes de Oca MK, Pal HC, Afaq F. Potential therapeutic targets of epithelial–mesenchymal transition in melanoma. Cancer Lett. 2017;391:125–140. doi: 10.1016/j.canlet.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H, Lotan R, Menter D, Lippman SM, Xu XC. Expression of E-cadherin is associated with squamous differentiation in squamous cell carcinomas. Anticancer Res. 2000;20(3A):1385–1390. [PubMed] [Google Scholar]
- 15.Călinescu A, Scheau C, Zurac S, Nedelcu RI, Brînzea A, Turcu G, Coman A, Antohe M, Balaban M, Hulea I, Andrei R, Ion DA, Bădărău IA. Analysis of E-cadherin expression in a group of primary cutaneous squamous cell carcinomas. Rom J Clin Res. 2019;2(2):80–85. https://rjcronline.com/index.php/rjcr/article/view/33 [Google Scholar]
- 16.Huang X, Liang X, Zhou Y, Li H, Du H, Suo Y, Liu W, Jin R, Chai B, Duan R, Li H, Li Q. CDH1 is identified as a therapeutic target for skin regeneration after mechanical loading. Int J Biol Sci. 2021;17(1):353–367. doi: 10.7150/ijbs.51309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze’ev A. Autoregulation of E-cadherin expression by cadherin–cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J Cell Biol. 2003;163(4):847–857. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papanikolaou S, Bravou V, Gyftopoulos K, Nakas D, Repanti M, Papadaki H. ILK expression in human basal cell carcinoma correlates with epithelial–mesenchymal transition markers and tumour invasion. Histopathology. 2010;56(6):799–809. doi: 10.1111/j.1365-2559.2010.03556.x. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto T, Soeno Y, Maeda G, Taya Y, Aoba T, Nasu M, Kawashiri S, Imai K. Progression of oral squamous cell carcinoma accompanied with reduced E-cadherin expression but not cadherin switch. PLoS One. 2012;7(10):e47899–e47899. doi: 10.1371/journal.pone.0047899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanjaka-Rogošić L, Puizina-Ivić N, Mirić L, Rogošić V, Kuzmić-Prusac I, Babić MS, Vuković D, Mardešić S. Matrix metalloproteinases and E-cadherin immunoreactivity in different basal cell carcinoma histological types. Acta Histochem. 2014;116(5):688–693. doi: 10.1016/j.acthis.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Bozdogan O, Yulug IG, Vargel I, Cavusoglu T, Karabulut AA, Karahan G, Sayar N. Differential expression patterns of metastasis suppressor proteins in basal cell carcinoma. Int J Dermatol. 2015;54(8):905–915. doi: 10.1111/ijd.12581. [DOI] [PubMed] [Google Scholar]
- 22.Lyakhovitsky A, Barzilai A, Fogel M, Trau H, Huszar M. Expression of E-cadherin and beta-catenin in cutaneous squamous cell carcinoma and its precursors. Am J Dermatopathol. 2004;26(5):372–378. doi: 10.1097/00000372-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Jang TJ. Epithelial to mesenchymal transition in cutaneous squamous cell carcinoma is correlated with COX-2 expression but not with the presence of stromal macrophages or CD10-expressing cells. Virchows Arch. 2012;460(5):481–487. doi: 10.1007/s00428-012-1227-x. [DOI] [PubMed] [Google Scholar]
- 24.Hesse K, Satzger I, Schacht V, Köther B, Hillen U, Klode J, Schaper K, Gutzmer R. Characterisation of prognosis and invasion of cutaneous squamous cell carcinoma by podoplanin and E-cadherin expression. Dermatology. 2016;232(5):558–565. doi: 10.1159/000450920. [DOI] [PubMed] [Google Scholar]
- 25.Lan YJ, Chen H, Chen JQ, Lei QH, Zheng M, Shao ZR. Immunolocalization of vimentin, keratin 17, Ki-67, involucrin, β-catenin and E-cadherin in cutaneous squamous cell carcinoma. Pathol Oncol Res. 2014;20(2):263–266. doi: 10.1007/s12253-013-9690-5. [DOI] [PubMed] [Google Scholar]
- 26.Toll A, Masferrer E, Hernández-Ruiz ME, Ferrandiz-Pulido C, Yébenes M, Jaka A, Tuneu A, Jucglà A, Gimeno J, Baró T, Casado B, Gandarillas A, Costa I, Mojal S, Peña R, de Herreros , García-Patos V, Pujol RM, Hernández-Muñoz I. Epithelial to mesenchymal transition markers are associated with an increased metastatic risk in primary cutaneous squamous cell carcinomas but are attenuated in lymph node metastases. J Dermatol Sci. 2013;72(2):93–102. doi: 10.1016/j.jdermsci.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Vinicius de, Scapulatempo C, Perpetuo NM, Mohamed F, de Carvalho TS, de Oliveira ATT, Segalla JGM, Carvalho AL. Prognostic and risk factors in patients with locally advanced cutaneous squamous cell carcinoma of the trunk and extremities. J Skin Cancer. 2011;2011:420796–420796. doi: 10.1155/2011/420796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(Pt 24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieffer Y, Hocine HR, Gentric G, Pelon F, Bernard C, Bourachot B, Lameiras S, Albergante L, Bonneau C, Guyard A, Tarte K, Zinovyev A, Baulande S, Zalcman G, Vincent-Salomon A, Mechta-Grigoriou F. Single-cell analysis reveals fibroblast clusters linked to immunotherapy resistance in cancer. Cancer Discov. 2020;10(9):1330–1351. doi: 10.1158/2159-8290.CD-19-1384. [DOI] [PubMed] [Google Scholar]
- 30.Spada S, Tocci A, Di Modugno F, Nisticò P. Fibronectin as a multiregulatory molecule crucial in tumor matrisome: from structural and functional features to clinical practice in oncology. J Exp Clin Cancer Res. 2021;40(1):102–102. doi: 10.1186/s13046-021-01908-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwarzbauer JE, DeSimone DW. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb Perspect Biol. 2011;3(7):a005041–a005041. doi: 10.1101/cshperspect.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peltonen J, Jaakkola S, Lask G, Virtanen I, Uitto J. Fibronectin gene expression by epithelial tumor cells in basal cell carcinoma: an immunocytochemical and in situ hybridization study. J Invest Dermatol. 1988;91(4):289–293. doi: 10.1111/1523-1747.ep12475415. [DOI] [PubMed] [Google Scholar]
- 33.Yen CY, Huang CY, Hou MF, Yang YH, Chang CH, Huang HW, Chen CH, Chang HW. Evaluating the performance of fibronectin 1 (FN1), integrin α4β1 (ITGA4), syndecan-2 (SDC2), and glycoprotein CD44 as the potential biomarkers of oral squamous cell carcinoma (OSCC) Biomarkers. 2013;18(1):63–72. doi: 10.3109/1354750X.2012.737025. [DOI] [PubMed] [Google Scholar]
- 34.von Maltzan K, Li Y, Rundhaug JE, Hudson LG, Fischer SM, Kusewitt DF. Role of the Slug transcription factor in chemically-induced skin cancer. J Clin Med. 2016;5(2):21–21. doi: 10.3390/jcm5020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H, Takahara M, Xie L, Takeuchi S, Tu Y, Nakahara T, Uchi H, Moroi Y, Furue M. Levels of the EMT-related protein Snail/Slug are not correlated with p53/p63 in cutaneous squamous cell carcinoma. J Cutan Pathol. 2013;40(7):651–656. doi: 10.1111/cup.12142. [DOI] [PubMed] [Google Scholar]