Abstract
Non-small cell lung cancer (NSCLC), a common type of cancer worldwide, is normally associated with a poor prognosis. It is difficult to treat successfully as it often metastasizes into brain or bone. Methods to facilitate the induction of effective programmed cell death (PCD) in NSCLC cells to reverse drug resistance, or to inhibit the invasion and migration of NSCLC cells, are currently under investigation. The present study summarized the regulatory functions of PCD, including apoptosis, autophagy and ferroptosis, in the context of NSCLC metastasis. It further summarized how regulatory agents, including long non-coding RNAs, circular RNAs and microRNAs, regulate PCD during the metastasis of NSCLC and characterized new potential diagnostic biomarkers of NSCLC metastasis.
Keywords: apoptosis, autophagy, ferroptosis, metastasis, non-small cell lung cancer
1. Features of NSCLC
Non-small cell lung cancer (NSCLC) is the primary histological type of lung cancer worldwide; it accounts for ~85% of all lung cancer cases and is a leading cause of cancer-related mortality. NSCLC is histologically divided into adenocarcinoma, squamous cell carcinoma and large cell carcinoma (1). Epidemiology has revealed that lung cancer accounts for ~18% of all cancer-related mortalities and remains the major cause of cancer-related mortality worldwide. The 5-year survival rate ranges from 50 to 70% for stage I and from 1 to 5% for stage IV; however, only ~20% of patients are diagnosed with stage I disease and the majority of patients with NSCLC are diagnosed at an advanced stage (stage IV) with distant metastasis and poor prognosis (2). Thus, it is important to investigate the regulatory mechanisms of lung cancer metastasis, and discover new predictive methods and therapeutic targets. Findings from these investigations may aid in the identification of new treatments for patients with NSCLC and improve their prognosis.
Tumor metastasis occurs as a complex cascade involving invasion, dissemination and distant colonization. Specifically, primary tumor cells invade the surrounding tissues, enter the circulatory system, survive during blood circulation, enter the parenchyma of distant tissues and finally colonize distant sites for subsequent proliferation (3). Postsurgical recurrence is the major cause of mortality in the majority of patients with NSCLC (3). Brain and bone metastases can be easily observed in patients with advanced NSCLC; for example, bone metastasis occurs in 30-40% of patients with NSCLC (4). The present study summarized the regulatory processes underlying metastasis of NSCLC, and analyzed the diagnostic and prognostic factors related to tumor metastasis of NSCLC to further clarify the molecular mechanisms underlying metastasis in NSCLC.
2. The role of PCD in the metastasis of NSCLC
Programmed cell death (PCD) refers to orderly and autonomous cell death that is controlled by a series of endogenous genes. PCD includes apoptosis, autophagy, pyroptosis, ferroptosis and necroptosis (5). PCD serves a key role in the treatment of various metastatic tumors. The present study summarized the regulatory molecular mechanisms involved in PCD and their corresponding regulatory factors, including non-coding (nc)RNAs, proteins and compounds, during the process of NSCLC bone metastasis. Simultaneously, it drew attention to future research trends and identified several unsolved problems relevant to NSCLC metastasis.
Apoptosis in NSCLC metastasis
Aberrant apoptosis often leads to the progression and metastasis of various types of human cancer. Cell apoptosis inhibits metastasis at multiple stages ranging from invasion from the primary site to distant colonization (3). During the progression and metastasis of NSCLC, apoptosis is regulated by various factors, such as proteins, ncRNAs and other compounds. Previously, research examining the metastasis of NSCLC has primarily focused on the regulatory mechanisms of novel proteins, microRNAs (miRNAs/miRs), long ncRNAs (lncRNAs) and circular RNAs (circRNAs) (5,6).
Novel proteins regulate apoptosis during the metastasis of NSCLC
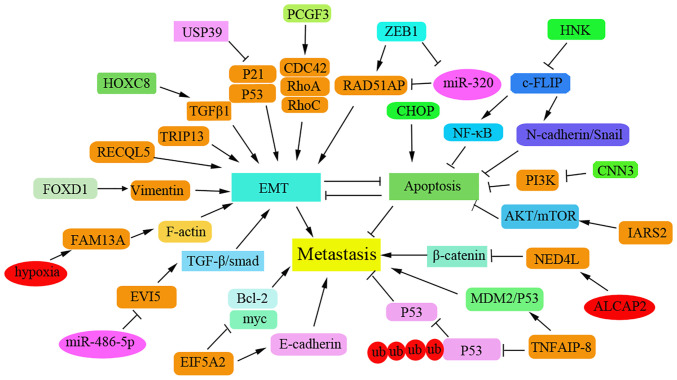
Recently, a series of novel and important proteins have been reported to be associated with apoptosis, metastasis and prognosis of NSCLC by modulating epithelial-mesenchymal transition (EMT) progression or chemotherapeutic drug resistance, and by regulating various cancer-related signaling pathways in patients with NSCLC. The proteins identified in the regulation of NSCLC metastasis are summarized in Fig. 1. Interference with forkhead box D1 (FOXD1) has been shown to inhibit proliferation and metastasis in vivo by increasing the apoptotic rate of NSCLC cells (6). In addition, the oncogene ecotropic viral integration site 5 (EVI5) has been reported to be upregulated in NSCLC tissues. Knockdown of EVI5 may suppress cell invasion via the TGF-β/Smad signaling pathway in NSCLC cells (7). The natural compound honokiol may also inhibit the migration of NSCLC cells by targeting cellular FLICE-inhibitory protein via the NF-κB pathway and the N-cadherin/Snail pathway (8). Knockdown of eukaryotic translation initiation factor 5A2 can significantly inhibit cell proliferation and metastasis by inducing the apoptosis of NSCLC cells (9).
Figure 1.
Newly identified proteins that regulate the metastasis of NSCLC via promoting apoptosis. NSCLC, non-small cell lung cancer; TRIP13, thyroid hormone receptor interacting protein 13; HOX8, homeobox 8; USP39, ubiquitin-specific protease 39; PCGF3, polycomb group ring finger 3; ZEB1, zinc finger E-box binding homeobox 1; CNN3, Calponin 3; EVI5, ecotropic viral integration site 5; EIF5A2, eukaryotic translation initiation factor 5A2; CHAF1B, chromatin assembly factor 1 subunit B; FOXD1, forkhead box D1.
Several regulators inhibit NSCLC metastasis by inducing cell cycle arrest and apoptosis. Upregulation of thyroid hormone receptor interacting protein 13 (TRIP13) has been reported to be associated with tumor metastasis via EMT. TRIP13 knockdown can promote cell apoptosis by inducing cell cycle arrest at the S phase, and can inhibit the invasion and migration of NSCLC cells (10). Calponin 3 overexpression can also suppress the migration of NSCLC cells by inhibiting the PI3K/AKT pathway and promoting G1-phase arrest (11). Furthermore, inhibition of isoleucyl-tRNA synthetase 2, mitochondrial (IARS2) has been shown to induce cell cycle arrest at the G0/G1 stage and mitochondrial apoptosis; thus, IARS2 may function as an oncogene to promote NSCLC tumorigenesis by activating the AKT/mTOR pathway (12). The deubiquitinating enzyme ubiquitin-specific protease 39 (USP39) has been reported to be overexpressed in NSCLC tissues. Notably, interference with the expression of USP39 has been demonstrated to induce cell cycle arrest at G2/M and to induce apoptosis by activating the p53 and p21 pathways, thus resulting in inhibition of metastasis (13). Tumor necrosis factor-α-induced protein 8 may promote drug resistance in NSCLC via the MDM2/p53 pathway (14). Chromatin assembly factor 1 subunit B (CHAF1B) has been shown to be highly expressed in NSCLC tissues, and high CHAF1B levels can lead to poor clinical outcomes and lymph node metastasis (15). By contrast, CHAF1B knockdown may contribute to cell cycle arrest and the induction of apoptosis by activating the p53-dependent apoptotic pathway.
Some newly identified regulators have been reported to induce apoptosis and thus inhibit metastasis of NSCLC cells and this has been demonstrated to be associated with the clinical prognosis of patients with NSCLC. For example, homeobox (HOCX) 8 has been shown to be upregulated in NSCLC specimens and to function as a transcriptional activator that induces the expression of TGF-β1. High levels of HOXC8 have been reported to be associated with tumor-node metastasis and poor relapse-free survival in patients with NSCLC (16). Polycomb group ring finger 3 has been demonstrated to be overexpressed in tumors of patients with NSCLC and to be positively associated with lymph node metastasis; it can promote migration via the PI3K/AKT pathway (17). Conversely, miR-320a expression levels have been shown to be reduced in NSCLC tissues and cells. Zinc finger E-box binding homeobox 1 can inhibit the expression of miR-320a that contributes to the high expression level of RAD51AP1 to promote tumor growth and metastasis in NSCLC (18). Vitamin D may suppress metastasis of NSCLC cells by promoting apoptosis via the PI3K/AKT/mTOR signaling pathway (19). In addition, C/EBP homologous protein has been reported to suppress metastasis and to sensitize NSCLC cells to cisplatin (DPP) by blocking the Bcl-2/JNK pathway (20). RecQ-like helicase 5 (RECQL5), a member of the RecQ helicase family, may promote the migration of NSCLC cells and has been shown to be strongly associated with the poor prognosis of lung adenocarcinoma; knockdown of RECQL5 can increase the level of apoptosis in DPP-resistant A549 cells (21). Elevated levels of FOXD1 have been revealed to be even higher in NSLC tissues compared with normal controls and to be associated with poor prognosis in patients with NSCLC (6).
lncRNAs regulate apoptosis and metastasis of NSCLC
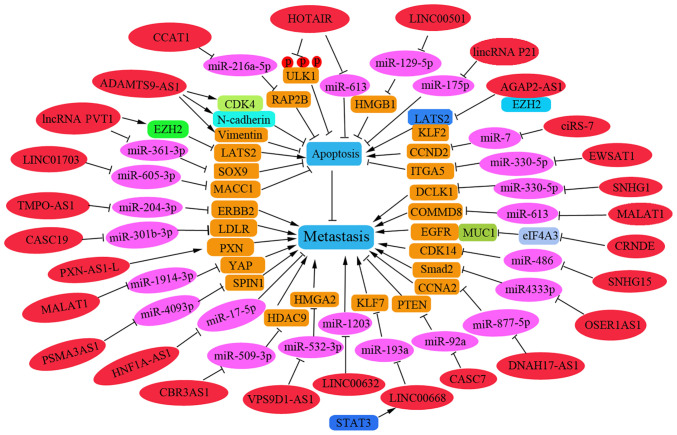
A number of lncRNAs have been reported to be involved in NSCLC metastasis by regulating apoptosis-related pathways (22-25). As presented in Fig. 2, these regulatory lncRNAs promote or inhibit the apoptosis process during the metastasis of NSCLC cells. In addition, several lncRNAs and associated miRNAs have been reported to be associated with the prognosis of NSCLC, and thus could be used as effective therapeutic and diagnostic targets in patients with NSCLC.
Figure 2.
circRNAs regulate cell apoptosis and metastasis of NSCLC. The regulatory circRNAs on NSCLC metastasis are shown on a green background. The targeted miRNAs are shown on a pink background and their corresponding target genes are shown on a brown background; blue background indicates metastatic phenotype. circRNAs, circular RNAs; NSCLC, non-small cell lung cancer; miRNA, microRNA; FSCN1, fascin homolog 1, actin-bundling protein 1; AKT3, AKT serine/threonine kinase 3; NEAT, nuclear paraspeckle assembly transcript; EZH2, enhancer of zeste homolog 2(); KIF2A, kinesin family member 2A; TIPRL, TOR signaling pathway regulator-like.
Certain lncRNAs have been reported to act as suppressor genes in NSCLC metastasis by promoting apoptosis. LINC00632 was shown to be downregulated in NSCLC tissues, and low levels of LINC00632 can promote lymph node metastasis and distant metastasis. Furthermore, LINC00632 was revealed to inhibit tumor growth in nude mice with NSCLC by downregulating miR-1203(22). The lncRNA cancer susceptibility candidate 7 may inhibit the growth and invasion of NSCLC cells and promote apoptosis in vitro through phosphatase and tensin homolog upregulation by sponging miR-92a (23). LINC00520 can inhibit cell proliferation, metastasis and EMT, and increase the extent of apoptosis in NSCLC cells. Mechanistically, LINC00520 has been shown to serve as a competing endogenous (ce)RNA involved in the regulation of miR-577 that affects the target gene CCNE2(24). Upregulated lncRNA-SNHG7 (SNHG7) has been reported to be positively correlated with Fas apoptosis inhibitory molecule 2 (FAIM2) in lung cancer cells and to function as a molecular sponge to sequester endogenous miRNA (25). Knockdown of SNHG7 in vivo has been reported to significantly inhibit tumor growth and decrease tumor volume, which is accompanied by enhanced miR-193b expression and reduced FAIM2 levels (25). Nuclear paraspeckle assembly transcript (NEAT) 1 may act as a ceRNA against let-7a, and IGF-2 is a direct target of let-7a; thus, knockdown of NEAT1 can inhibit cell viability and reduce migration by increasing apoptosis of NSCLC cells by regulating the NEAT1/let-7a/IGF2 pathway (26).
LncRNAs have recently been identified as oncogenes that promote the invasion and migration of NSCLC cells. For example, the ncRNA plasmacytoma variant translocation 1 (PVT1) was shown to be highly expressed in 105 patients with NSCLC and to promote tumor-to-node metastasis. Knockdown of PVT1 was revealed to inhibit metastasis and proliferation by increasing LATS2 transcription via the Mdm2-p53 signaling pathway (27). In addition, PVT1 may act as a miRNA sponge and could promote cell metastasis of NSCLC by regulating miR-361-3p/SOX9 and activating the Wnt/β-catenin signaling pathway (28). AGAP2-AS1 has been reported to function as an oncogene to inhibit the transcription of the tumor-suppressors LATS2 and KLF2; thus, interference with AGAP2-AS1 may suppress migration and induce apoptosis in NSCLC cells (29). The lncRNA HOTAIR has been reported to be overexpressed in NSCLC specimens and can negatively regulate the levels of miR-613. Conversely, knockdown of HOTAIR expression may promote cell apoptosis and suppress the migration of NSCLC by down-regulating miR-613(30). Furthermore, interference with lncRNA ADAMTS9-AS1 has been shown to suppress EMT in patients with NSCLC, and cause cell cycle arrest at G0/G1 and the induction of cell apoptosis, which is associated with downregulation of CDK4, N-cadherin and vimentin, and upregulation of Bad and E-cadherin (31). The lncRNA PXN-AS1-L has been shown to be upregulated in NSCLC tissues compared with levels in noncancerous lung tissues and to be further upregulated in NSCLC bone metastasis tissues, thus suggesting that higher levels of PXN-AS1-L may be positively related to advanced TNM stage and poor prognosis. This lncRNA can also promote NSCLC progression and metastasis by upregulating PXN during NSCLC progression (32).
The lncRNA HNF1A-AS1 may promote cell invasion by targeting miR-17-5p in patients with NSCLC (33). By contrast, lincRNAp21 can effectively inhibit migration and promote the apoptosis of NSCLC cells by targeting miR-175p (34). The lncRNA CBR3AS1 can promote metastasis of NSCLC by sponging miR-509-3p and competitively upregulating histone deacetylase 9 expression (35). Overexpression of SNHG15 has been reported to promote NSCLC tumorigenesis through regulating the CDK14 protein and by sponging miR-486(36). An elevated expression level of OSER1AS1 has been associated with lymph node metastasis in patients with NSCLC. OSER1AS1 may promote the malignant properties of NSCLC by sponging miR-4333p and upregulating Smad2 expression (37).
CircRNAs regulate NSCLC metastasis
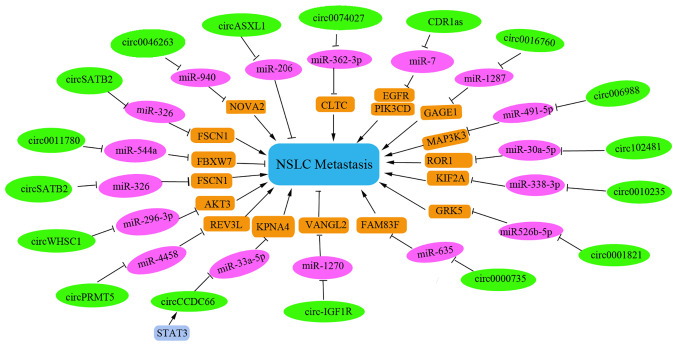
CircRNA is a new member of the ncRNA family that functions as a miRNA sponge to influence gene transcription. These RNAs are highly tissue-specific in mammals. An increasing number of circRNAs have been identified that regulate apoptosis in NSCLC metastasis. The specific and regulatory circRNAs involved in NSCLC metastasis that function by inducing or suppressing apoptosis in NSCLC cells have been summarized in the present study.
As presented in Fig. 3, hsa_circ_0046263 can function as an oncogene in NSCLC by sponging miR-940 and increasing the expression levels of NOVA2(38). circ-IGF1R has been reported to suppress migration of NSCLC cells via the circ-IGF1R/miR-1270/VANGL2 pathway (39). STAT3-induced upregulation of circCCDC66 has been reported to facilitate NSCLC progression by targeting the miR-33a-5p/KPNA4 axis (40). Furthermore, knockdown of circWHSC1 may suppress the migration of NSCLC cells and increase apoptosis in NSCLC cells by sponging miR-296-3p and increasing the expression levels of AKT3(41). Circ_0000735 has also been reported to enhance metastasis and glycolysis, and inhibit apoptosis in NSCLC cells, by regulating the miR-635/family via the sequence similarity 83 member F axis (42). Knockdown of CircGLIS3 has been observed to be highly expressed in NSCLC tissues and cell lines, and high circGLIS3 levels have been revealed to be correlated with malignant characteristics and poor prognosis of NSCLC (43). In addition, circGLIS3 can sponge multiple anti-cancer miRNAs, including miR-526b, miR-198, miR-498 and miR-664a. Notably, PTBP1 has been revealed as a novel target of miR-644a (43). Therefore, a circGLIS3/miR-644a/PTBP1 positive feedback loop can promote the malignant progression of NSCLC (43). The levels of circ_0006988 have been demonstrated to be increased in NSCLC tissues and cells. Notably, knockdown of circ_0006988 can inhibit metastasis and angiogenesis, and induce apoptosis in NSCLC cells by modulating the miR-491-5p/MAP3K3 axis (44). In addition, circ_0010235 has been shown to be highly upregulated in patients with NSCLC. Overexpression of circ_0010235 may promote NSCLC cell metastasis in vitro and in vivo by sponging miR-338-3p to upregulate the expression of kinesin family member 2A (45).
Figure 3.
lncRNAs regulate cell apoptosis and metastasis of NSCLC. The regulatory lncRNAs are shown on a red background. The targeted miRNAs are shown on a pink background and their corresponding target genes are shown on a brown background; blue background indicates phenotype; green background indicates circRNA. lncRNAs, long non-coding RNAs; NSCLC, non-small cell lung cancer; miRNA, microRNA; PVT1, plasmacytoma variant translocation1; SOX9, sex determining region Y-related high mobility group box9; MACC1, metastasis associated with colon cancer 1; DCLK1, doublecortin-like kinase 1; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; COMMD8, copper metabolism MURR1 domain-containing 8; CRNDE, colorectal neoplasia differentially expressed; PSMA3AS1, PSMA3 antisense RNA 1; HMGA2, spindlin 1 high mobility group AT-hook 2; CASC7, cancer susceptibility candidate 7; CBR3AS1, CBR3 antisense RNA 1; Smad2, mothers against decapentaplegic homolog 2.
Certain circRNAs serve as tumor suppressor gene. For instance, low hsa_circ_11780 expression levels have been reported to be associated with distant metastasis and poor overall survival. Overexpression of hsa_circ_11780 can significantly suppress the metastasis of NSCLC cells in vitro and in vivo by decreasing FBXW7 level to suppress the function of miR-544a (46).
Several circRNAs have been reported to be involved in chemotherapeutic drug resistance and the metastasis of NSCLC cells. Knockdown of circ_0001821 may inhibit metastasis and paclitaxel resistance in NSCLC cells via the miR-526b-5p/GRK5 pathway (47). Furthermore, circASXL1 silencing can inhibit DDP resistance and tumorigenesis by sponging miR-206 in NSCLC tissues and cells (48). Interference with circ-PRMT5 has also been reported to induce apoptosis, reverse DDP resistance and reduce metastasis in DDP-resistant NSCLC. Notably, circ-PRMT5 can sponge miR-4458 to increase REV3L expression levels and to promote DDP resistance in patients with NSCLC (49). Tumor-derived exosomal circRNA_102481 has been reported to correlate with TNM stage, tumor differentiation status, brain metastasis, progression-free survival and overall survival duration. This RNA can promote cell proliferation, inhibit cell apoptosis and induce EGFR-TKI resistance by sponging miR-30a-5p to increase ROR1 levels in NSCLC (50).
Furthermore, the expression of several circRNAs has been shown to be associated with clinicopathological parameters and prognostic value in patients with NSCLC. For example, CDR1as has been considered an independent prognostic factor for patients with NSCLC and knockdown of CDR1as can upregulate miR-7, a process that leads to the induction of cell apoptosis and G1/S arrest. Furthermore, high expression levels of CDR1as have been shown to be correlated with advanced TNM stage, increased lymph node metastasis and shorter overall survival time (51). Notably, circSATB2 has also been reported to be highly expressed in NSCLC cells and tissues, and to be associated with lung cancer metastasis. This circRNA can promote the progression and invasion of NSCLC cells via upregulation of fascin homolog 1 and actin-bundling protein 1, and downregulation of miR-326; this can provide a potential biomarker for the diagnosis of NSCLC (52). Furthermore, upregulation of circ_0016760 can be indicative of increased lymph node metastasis and poor prognosis in NSCLC, and can also promote cell progression and metastasis and inhibit apoptosis through the miR-1287/GAGE1 axis (53).
miRNAs regulate metastasis of NSCLC
miRNAs are the most abundant endogenous small ncRNAs and are abnormally expressed in various types of human cancer. The miRNAs affecting NSCLC metastasis are summarized in Fig. 4. Several miRNAs function as oncogenes that promote the invasion and migration of NSCLC cells. Specifically, miR-4286 is considered an oncogenic miRNA in NSCLC that is significantly enhanced in NSCLC tissues, and positively related to lymph node metastasis and poor prognosis in patients with NSCLC. Inhibition of miR-4826 has been reported to cause cell cycle arrest at the G1 stage by regulating Runt-related transcription factor 3 in NSCLC cells (54). miR-619-5p transported in tumor-derived exosomes has been shown to promote angiogenesis and metastasis of NSCLC by inhibiting RCAN1.4, which is a target of miR-619-5p (55).
Figure 4.
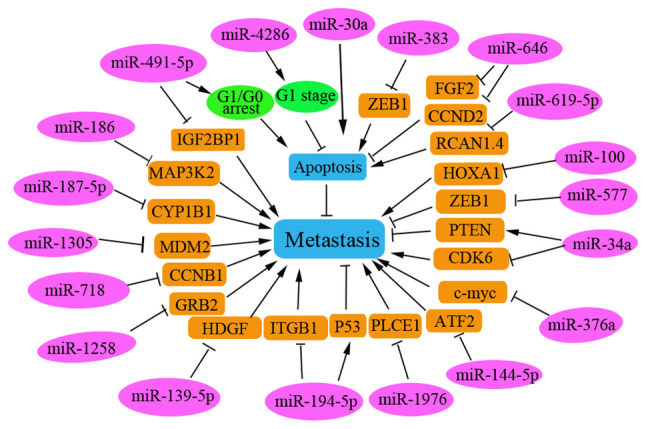
Regulatory miRNAs in human NSCLC. Several miRNAs serve a role as oncogenes to inhibit cell apoptosis and promote the metastasis of NSCLC, whereas others function as tumor suppressors to induce cell apoptosis and inhibit NSCLC metastasis. The regulatory miRNAs are shown on a pink background; corresponding target genes are shown on a brown background; green background indicates circRNA. miRNA, microRNA; NSCLC, non-small cell lung cancer; ATF2, transcription factor 2; ZEB1, zinc finger E-box binding homeobox 1; Runx3, runt-related transcription factor 3; MDM2, mouse/murine double minute 2; TEXs, tumor-derived exosomes; FGF2, fibroblast growth factor 2; CCND2, cyclin D2; CCNB1, cyclin B1.
Furthermore, certain miRNAs have been reported to function as tumor suppressors to promote apoptosis in NSCLC cells. Tumor-suppressive miRNAs are significantly downregulated in NSCLC tissues and cell lines. Downregulation of a number of tumor-suppressive miRNAs has been reported to be correlated with the poor prognosis of patients with NSCLC, including miR-187-5p (56), miR-101(57), miR-718(58), miR-374b (59) and miR-30a (60). These miRNAs can exert anti-tumor effects by inhibiting the invasion and migration of NSCLC cells by targeting various key proteins to regulate their corresponding signaling pathways. For example, miR-186 has been shown to inhibit the metastasis of NSCLC cells by targeting MAP3K2(61). miR-491-5p can suppress NSCLC cell proliferation by arresting cells in the G0/G1 phase and promoting cell apoptosis. In addition, miR-491-5p can negatively regulate insulin-like growth factor 2 mRNA binding protein 1 expression in malignant NSCLC (62) and miR-187-5p can negatively regulate the target gene CYP1B1 to inhibit NSCLC cell metastasis (56).
miR-718 has been demonstrated to serve a tumor suppressive role by suppressing NSCLC progression in vivo by directly targeting cyclin B1 mRNA (58). Overexpression of miR-374b may inhibit metastasis of NSCLC by directly downregulating integrin β1 and upregulating p53(59). Furthermore, overexpression of miR-139-5p in vitro can induce apoptosis through a process that is partly mediated by inhibiting hepatoma-derived growth factor (HDGF) expression in NSCLC cells (63). In addition, miR-139-5p may also induce apoptosis in NSCLC cells by targeting and regulating YAF1 via the AKT/p38 MAPK signaling pathway (64). The overexpression of miR-34a has been shown to inhibit NSCLC growth and metastasis by increasing PTEN and decreasing CDK6 expression levels, as reported in tin miners in Gejiu County and farmers in Xuanwei (65). miR-30a may sensitize NSCLC cells by promoting DDP/pemetrexed-induced apoptosis and by enhancing autophagy in NSCLC cells (60). miR-1258 has been reported to inhibit GRB2 expression and inactivate the Ras/ERK pathway in NSCLC, thus inhibiting NSCLC progression (66). Phospholipase C ε1 has been demonstrated to promote metastasis of NSCLC cells, and to be directly and negatively regulated by miR-1976 in NSCLC cells (67). These identified miRNAs function as tumor suppressors and could be used as therapeutic targets for patients with NSCLC.
Autophagy regulation in metastasis of NSCLC
Autophagy is critical in cancer development and metastasis, and serves a complex role in tumorigenesis in various types of cancer (68). Transfection with the autophagy-related gene ATG16L1-300T (vs. 300A) has been shown to promote the brain metastasis of NSCLC cells, suggesting that autophagy may be closely associated with the metastasis of NSCLC (68). Autophagy is involved in cancer cell metastasis by regulating EMT. The autophagy protein Beclin1 is a key regulator of tumorigenesis and metastasis in NSCLC cells, and the expression levels of Beclin1 have been shown to be significantly reduced in NSCLC tissues (69,70). Low Beclin1 expression levels have also been observed in patients with NSCLC with more advanced stages of cancer, increased lymph node metastasis and more poorly differentiated tumors (70). Reduced Beclin1 expression has also been associated with shorter survival of patients with NSCLC, thus suggesting that autophagy, which is an independent prognostic indicator for the survival of patients with NSCLC, may be suppressed in these patients (69). Additionally, an increased level of Nrf2 has been suggested as an independent prognostic factor in patients with NSCLC (71). Thus, the key autophagy-related proteins may be strongly associated with the tumor stage and node metastasis of NSCLC, and this could help to identify new independent prognostic biomarkers for patients with NSCLC.
Previous research has suggested that autophagy induction inhibits EMT or metastasis in NSCLC cells (72). Certain agents are involved in regulating autophagy during the metastasis of NSCLC. For example, the expression of transcription factor 21 (TCF21) has been reported to be strongly associated with tumor stage, metastasis and invasion of NSCLC, and to be significantly regulated by its methylation level in patients with NSCLC. Promoter methylation of TCF21 may suppress autophagy and increase cell apoptosis in lung cancer metastasis (72). Furthermore, knockdown of EHMT2 can decrease the proliferation of NSCLC cells by inducing autophagy (73).
The combination of apigenin and gefitinib has been shown to inhibit the activation of multiple oncogenes, including c-Myc, HIF-1α and phosphorylated EGFR, and this combination can also inhibit the AMPK signaling pathway in H1975 cells (74). These data revealed that apigenin combined with gefitinib may promote apoptosis and inhibit autophagy of EGFR L858R-T790M-mutated H1975 lung cancer cells and this could be used as an alternative therapy for acquired resistance in patients with NSCLC (74). The drug 10-hydroxycamptothecin (HCPT) may promote autophagy in H1299 cells by suppressing MAP4K3 levels and downregulating the mTOR pathway. HCPT in combination with Astragalus polysaccharide (APS) has been reported to reduce the migration and invasion of NSCLC cells compared with that observed in response to individual treatments; this may be partly due to the induction of HCPT-induced autophagy (75). Wnt inhibitory factor 1-mediated autophagy may inhibit Wnt/βcatenin signaling by downregulating dishevelled2 and suppressing activation of the PI3K/AKT/mTOR pathway that contributes to the inhibition of proliferation and promotion of apoptosis in NSCLC cells (76). In addition, treatment with miR-16 mimics can suppress TGF-β1-induced EMT by inducing autophagy in NSCLC cells (76). Cell division cycle-associated 4 can inhibit EMT and metastasis in NSCLC by interacting with coactivator-associated arginine methyltransferase 1 to activate autophagy (77).
Consistently, previous studies have reported that the induction of autophagy enhances the sensitivity of NSCLC cells to chemotherapeutic drugs. For example, knockdown of NIPBL has been shown to enhance the chemosensitivity of NSCLC cells via the DNA damage response and autophagy pathways (78). Combined therapy with rapamycin and suberoylanilide hydroxamic acid may also increase radiosensitivity in these cells and inhibiting the levels of autophagy can markedly weaken the radiotherapy sensitivity caused by this combined treatment, thus suggesting that the inhibitory effect of combination therapy may be partially due to the induction of autophagy in NSCLC (79). Cediranib has also been shown to exhibit effective anti-tumor activities in NSCLC cells by inducing G1 phase cell cycle arrest and autophagy via the MAPK/Erk1/2 and Akt/mTOR pathways in A549 cells (80).
Notably, there is an opposing view that inducing autophagy increases chemoresistance or radioresistance in NSCLC cells. For example, troponin C1, slow skeletal and cardiac type can promote gemcitabine chemoresistance in NSCLC by inducing autophagy and this is negatively regulated by FOXO3(81). Inhibition of autophagy by chloroquine has been shown to prevent the development of paclitaxel resistance and to alleviate the metastatic potential of NSCLC cells (82). Furthermore, downregulation of CLDN1 can induce apoptosis in A549/CDDP cells, whereas overexpression of CLDN1 can increase drug resistance and metastasis of NSCLC by inducing autophagy through upregulating the phosphorylation level of ULK1(83). Lung cancer-associated transcript 1 upregulation has also been shown to increase DPP resistance by inducing autophagy, promoting metastasis and inhibiting the apoptosis of NSCLC cells by regulating the miR-514a-3p/ULK1 axis in human NSCLC (84).
Several studies have reported that autophagy induction promotes metastasis of NSCLC cells. miR-21 has been shown to be highly expressed in NSCLC tissues, and to be positively associated with lymphatic metastasis and clinical staging. This miRNA can promote the migration and invasion of A549 cells by regulating autophagy activity via the AMPK/ULK1 signaling pathway (85). In addition, UBE2C has been demonstrated to selectively inhibit cell autophagy in NSCLC, which is essentially associated with clonogenicity and invasion of NSCLC, thus suggesting that UBE2C-mediated autophagy may contribute to NSCLC progression (86). Interference with PINK1 expression has been shown to inhibit cell autophagy, whereas PINK1 overexpression can promote cell migration by promoting autophagy and is associated with poor prognosis in patients with lung cancer (87). Hsa_circ_0010235 can sponge miR-433-3p to upregulate TOR signaling pathway regulator-like expression, thus promoting proliferation and autophagy but inhibiting apoptosis in NSCLC cells. Knockdown of hsa_circ_0010235 or gain of miR-433-3p can block tumor growth in vivo (88). Damage-regulated autophagy modulator 2 (DRAM2) expression may contribute to autophagy inhibition and has been shown to be highly expressed in NSCLC tissues. Higher expression levels of DRAM2 in NSCLC are associated with TNM stage and lymph node metastasis. As an oncogene, interference with DRAM2 can induce upregulation of p53 and p21 expression in NSCLC cells (89). Quercetin, a chemical extracted from a traditional Chinese drug, has been shown to promote HDGF secretion and to induce autophagy. High serum levels of HDGF may contribute to bone metastasis and poor prognosis in patients with NSCLC (90) (Fig. 5).
Figure 5.
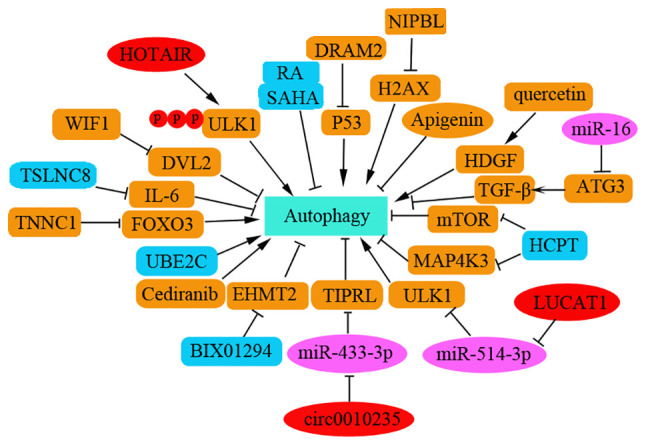
Autophagy regulation in metastasis of NSCLC. The key targets involved in autophagy in metastasis of NSCLC are shown here. The role of autophagy is complex in tumorigenesis and metastasis of NSCLC. NSCLC, non-small cell lung cancer; Dvl2, dishevelled2; CDCA4, cell division cycle associated 4; CARM1, coactivator associated arginine methyltransferase 1; DRAM2, damage-regulated autophagy modulator 2; WIF1, Wnt inhibitory factor1; SAHA, suberoylanilide hydroxamic acid; NIPBL, nipped-B-like protein; TIPRL, TOR signaling pathway regulator-like; FOXO3, forkhead box 03.
Ferroptosis and the regulators of NSCLC metastasis
Ferroptosis is a newly reported iron-dependent PCD process that is characterized by the accumulation of an iron-dependent lethal lipid reactive oxygen species (ROS) response. To date, the induction of cell ferroptosis has provided a new insight into the design of anti-tumor drugs and is considered a promising strategy to inhibit tumor metastasis or overcome chemotherapeutic drug resistance (60,91). Diphenyldifluoroketone (EF24) has been shown to exhibit cytotoxicity against NSCLC via facilitating ROS accumulation (91). In patients with osteosarcoma, it has been determined that EF24 can induce ferroptosis by suppressing GPX4 via the upregulation of HMOX1, thus leading to increased MDA, ROS and intracellular ferric ion levels. EF24 could therefore be considered a potential anti-cancer agent for the clinical therapy of patients with NSCLC and osteosarcoma (60). The light chain of the cystine/glutamic acid reverse transporter System Xc (-) (xCT) has been shown to be overexpressed in human cancer and to be associated with tumor metastasis. Erastin/sorafenib can induce ferroptosis in DPP-resistant NSCLC cells by inhibiting the Nrf2/xCT pathway (92); thus, ferroptosis inducers, such as erastin and sorafenib, may be used as novel therapies for patients with NSCLC, particularly those with advanced NSCLC and DDP failure. Metal-organic networks (MONs) encapsulated with a p53 plasmid (MON-p53) can evoke ferroptosis to prolong the survival time of tumor-bearing mice (93). In a study examining lung carcinoma, dihydroisotanshinone I, extracted from Danshen (Salvia miltiorrhiza Bunge), inhibited metastasis of lung carcinoma in vivo by triggering apoptosis and ferroptosis (94). The iron-sulfur cluster biosynthetic enzyme NFS1 has been reported to be highly expressed in lung adenocarcinoma. Inhibition of NFS1 and of cysteine transport can induce ferroptosis in vitro to suppress tumor growth in lung adenocarcinomas (95). GPX4 is a negative regulator of ferroptosis and metastatic cells are resistant to ferroptosis. Notably, high exposure to 27-hydroxycholesterol, an abundant circulating cholesterol metabolite, can increase tumorigenic and metastatic capacity that requires sustained expression of GPX4(96). Fibroblast activation protein α (FAP) has been reported to be overexpressed in >90% of types of human cancer (97). A newly discovered FAP gene-engineered tumor cell-derived exosome-like nanovesicle (eNVs-FAP) vaccine can induce tumor cell ferroptosis to overcome immunosuppression of the tumor microenvironment (97). Thus, ferroptosis exhibits promising potential to trigger an anti-cancer immune response (98).
Recently, studies examining the regulation of ferroptosis during cancer progression and metastasis have focused primarily on ncRNAs. Regulatory ncRNAs that regulate NSCLC progression and metastasis, including lncRNAs and circRNAs, have been gradually and widely reported (99-106). lncRNA MIR503HG serves as a sponge of miR-1273c and has been reported to significantly increase NSCLC progression by regulating SRY-box 4 expression and inhibiting ferroptosis (99). Furthermore, overexpression of metallothionein 1D pseudogene can sensitize A549 and H1299 cells to erastin-induced ferroptosis by downregulating Nrf2 in NSCLC cells (100). LINC00336 can inhibit ferroptosis by binding to ELAV-like RNA-binding protein 1 in human lung cancer (101). Additionally, H19 may promote cancer progression and suppress ferroptosis by regulating the miR-106b-5p/ACSL4 axis (102). PVT1 can also regulate ferroptosis via miR-214-mediated TFR1 and TP53 expression (104); notably, GPX4 has also been demonstrated to be a target of miR-214-3p. Notably, ketamine has been reported to inhibit the proliferation of liver cancer cells in vitro and in vivo by inducing ferroptosis via the lncRNA PVT1/miR-214-3p/GPX4 pathway (104). lncRNA ZFAS1 can also induce ferroptosis by functioning as a ceRNA through the miR-150-5p/SLC38A1 axis (105). Additionally, LINC00618(106), lncRNA GABPB1-AS1, lncRNA ZFAS1, lncRNA P53RRA and lncRNA MEG8 may all be involved in regulating ferroptosis during cancer progression (105). These RNAs function in addition to the reported circRNAs, which include circ_008035(107), circRNA cIARS (108), circ_0067934(109), circ_0007142(110), circIL4R (111), circTTBK2(112) and circKIF4A (113), and the lncRNAs such as lncRNA OIP5-AS1(114). However, the relationship between these newly identified ncRNAs and ferroptosis in NSCLC metastasis has not been clearly clarified and requires further investigation (Fig. 6; Table I).
Figure 6.
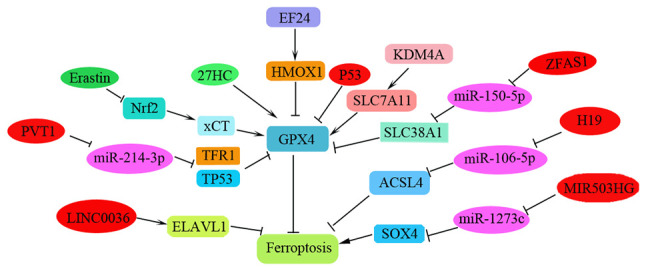
Ferroptosis in tumorigenesis and metastasis of NSCLC. The key target genes and its corresponding miRNAs and lncRNAs are shown. NSCLC, non-small cell lung cancer; miRNA, microRNA; lncRNAs, long non-coding RNAs; EF24, diphenyldifluoroketone; SOX4, regulating SRY-box 4; ELAVL1, ELAV-like RNA-binding protein 1; xCT, System Xc (-); 27HC, 27-hydroxycholesterol.
Table I.
Regulators of ferroptosis in NSCLC metastasis.
| Protein or non-coding RNA | Target gene | Function in human cancer metastasis |
|---|---|---|
| Proteins | ||
| EF24 | HMOX1 | EF24 exerted cytotoxicity against NSCLC via ROS accumulation (91). EF24 induced ferroptosis by upregulating HMOX1 to inhibit the GPX4 expression in patients with osteosarcoma (60). |
| xCT | / | xCT was highly expressed in a variety of tumors and associated with tumor metastasis. Erastin/sorafenib induced cisplatin-resistant NSCLC cell ferroptosis through inhibition of the Nrf2/xCT pathway (92). |
| NFS1 | / | NFS1 was highly expressed in lung adenocarcinoma. Inhibition of NFS1 induced ferroptosis in vitro to suppress progression of lung adenocarcinomas adenocarcinoma (95). |
| GPX4 | / | Metastatic cells exhibited resistance to ferroptosis. High exposure to 27-hydroxycholesterol inhibited ferroptosis to increase metastatic capacity of tumor cells by inducing sustained expression of GPX4(96) |
| lncRNAs | ||
| lncOIP5-AS1 | miR-128/SLC1A11 | OIP5-AS1 was reported to induce metastatic phenotypes of NSCLC via miR-140-5p (118). It also promoted prostate cancer progression and inhibited ferroptosis via regulating miR-128-3p/SLC7A11. |
| MIR503HG | miR-1273c/SOX4; SLC7A11 | Interference with the expression of MIR503HG inhibited NSCLC tumorigenesis and promoted cell apoptosis by regulating miR-489-3p and miR-625-5p (119). XAV939 induced downregulation of lncRNA MIR503HG, inhibited NSCLC progression via sponging miR-1273c and regulating SOX4 expression, and decreased expression of SLC7A11 via the ferroptosis pathway in NSCLC cells (99). |
| MT1DP | miR-365a-3p/Nrf2 | MT1DP sensitized erastin-induced ferroptosis via regulating miR-365a-3p/Nrf2 axis in NSCLC cells (100). |
| H19 | miR-106b-5p/ACSL4 | Knockdown of H19 promoted tumor growth and suppressed ferroptosis by regulating the miR-106b-5p/ACSL4 pathway (102). |
| PVT1 | miR-214/p53; GPX4 | PVT1 regulated ferroptosis through miR-214-mediated TFR1 and TP53 expression (103). Ketamine inhibited the viability of liver cancer cells and induced ferroptosis via lncPVT1/miR-214-3p/GPX4 pathway (104). |
| ZFAS1 | miR-150-5p/SLC38A1 | Silencing of lncRNA ZFAS1 attenuated ferroptosis and pulmonary fibrosis progression by lncRNA ZFAS1 acting as a competing endogenous RNA and sponging miR-150-5p to downregulate SLC38A1 expression (105). |
| LINC00618 | SLC7A11 | LINC00618 induced ferroptosis by decreasing the expression of LSH and increasing the level of SLC7A11(106). |
| LINC00336 | miR-6852/CBS | miR-6852 inhibited lung cancer cell growth by promoting ferroptosis and LINC00336 promoted tumorigenesis through LINC00336/miR-6852/CBS pathway (101). |
| circRNAs | ||
| circ_008035 | miR-599/EIF4A1 axis | Circ_0008035 promoted gastric cancer progression and suppressed ferroptosis by upregulating EIF4A1 through sponging miR-599(107). |
| circRNA c IARS | ALKBH5 | cIARS may be an important circRNA, positively regulating sorafenib-induced ferroptosis through suppressing the ALKBH5-mediated autophagy inhibition (108). |
| circ_0067934 | miR-545-3p/SLC7A11 | Circ_0067934 knockdown enhanced the levels of ferroptosis and induced thyroid cancer cell apoptosis by sponging and inhibiting miR-545-3p in thyroid cancer cells. Thus, circ_0067934 attenuated ferroptosis of thyroid cancer cells via the miR-545-3p/SLC7A11 pathway (109). |
| circ_0007142 | miR-874-3p/GDPD5 | Circ_0007142 was overexpressed in CRC. Inhibiting circ_0007142 promoted apoptosis and ferroptosis in CRC cells via the miR-874-3p/GDPD5 axis (110). |
| non-coding RNA | Target gene | Function in human cancer metastasis |
| circIL4R | miR-541-3p/GPX4 | CircIL4R was abnormally overexpressed in HCC tissues and cells. CircIL4R functioned as an oncogene and inhibited ferroptosis in HCC via miR-541-3p/GPX4 pathway (111). |
| circTTBK2 | miR-761/ITGB8 | circTTBK2 knockdown, or overexpression of miR-761, suppressed invasion and promoted ferroptosis via targeting ITGB8 by sponging miR-761 in glioma (112). |
| circKIF4A | miR-1231/GPX4 | circKIF4A facilitated the malignant progress of papillary thyroid tumor by sponging miR-1231 and upregulating GPX4 expression (113). |
| circDTL | miR-1287-5p/GPX4 | CircDTL functioned as an oncogene and promoted NSCLC progression via the miR-1287-5p/GPX4 pathway (120). |
| circEPSTI1 | miR-375/409-3P/515-5p-SLC7A11 | CircEPSTI1 regulated ferroptosis in cervical cancer by sponging miR-375, miR-409-3p and miR-515-5p to upregulate SLC7A11 expression (121). |
| miRNAs | ||
| miR-375 | SLC7A11 | miR-375 triggered ferroptosis via targeting SLC7A11 and attenuated the stemness of gastric cancer cells (122). |
| miR-4443 | FSP1/METLL3 | miR-4443 promoted cisplatin resistance in NSCLC cells by regulating FSP1 in an m6A manner via METLL3, a direct target gene of miR-4443(123). |
| miR-324-3p | GPX | miR-324-3p enhanced ferroptosis in cisplatin-resistant A549 cells via miR-324-3p/GPX4 in NSCLC cells (124). |
NSCLC, non-small cell lung cancer; ROS, reactive oxygen species; lncRNA, long non-coding RNA; miRNA/miR, microRNA; circRNA, circular RNAs; CRC, colorectal cancer; HCC, hepatocellular carcinoma.
Crosstalk among apoptosis, autophagy and ferroptosis
Different types of PCD, such as apoptosis and autophagy, apoptosis and necroptosis, and necroptosis and ferroptosis, exist in close crosstalk (115). ROS-induced lipid peroxidation has been shown to serve an important role in the crosstalk among apoptosis, autophagy and ferroptosis (115). Lipid peroxidation products are harmful as they destroy DNA, proteins and enzymes in various cell types to inactivate PCD. For example, the products of lipid peroxidation can induce cell apoptosis through the NF-κB, MAPK and protein kinase C-related signaling pathways. Lipid peroxidation can also interact with upstream regulators of autophagy-related signaling pathways, such as AMP-activated protein kinase and Akt-mTOR signaling to interfere with autophagy (115). In addition, GPX4 activity can significantly affect lipid peroxidation and regulate ferroptosis induction.
The association between lncRNA, circRNA and miRNAs via miRNA recognition elements and negatively regulated the expression of miRNAs. The ceRNA regulatory network can be mRNA-miRNA-lncRNA, mRNA-miRNA-circRNA or mRNA-miRNA-lncRNA-circRNA. Each miRNA can have multiple target genes and multiple miRNAs can co-regulate the same gene. During the regulation of NSCLC metastasis, lncRNAs, circRNAs and miRNAs may interact with each other as ceRNAs, and can be involved in the progression and metastasis of NSCLC (105,15).
3. Conclusion
The rapid progress in RNA sequencing and analysis has revealed that a number of ncRNAs regulate tumor proliferation, invasion and migration of NSCLC cells. The present study summarized the regulatory role of PCD, including apoptosis, autophagy-related cell death and ferroptosis, in NSCLC metastasis. It also discussed the regulatory ncRNAs that promote PCD or inhibit the induction of PCD by acting as oncogenes or tumor suppressor genes.
In addition, necroptosis and pyroptosis have been newly identified as PCD mechanisms associated with various inflammatory or autoimmune diseases. The functions of necroptosis or pyroptosis in the context of cancer metastasis are complex; they possess both pro- and anti-tumor effects according to different pathological tissues, cell types or cancer stages during metastasis (116). Additionally, receptor-interacting kinase 3, the key regulator of necroptosis, is normally silenced in types of cancer that are unable to induce necroptosis. There are relatively few studies examining necroptosis and pyroptosis in NSCLC metastasis and the present study did not discuss the recent achievements in pyroptosis and necroptosis in the context of NSCLC metastasis (117). Furthermore, several aspects remain that must be addressed further: i) Novel and effective biomarkers for identifying the progression and prognosis of patients with NSCLC must be screened; ii) drug resistance remains a major obstacle in the treatment of patients with NSCLC; therefore, the effective induction of PCD, including apoptosis, ferroptosis and autophagy, to overcome drug resistance in NSCLC cells must be investigated in the future.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by the Special Scientific Research Project of Venous Thromboembolism Prevention and Treatment (Heng Rui) of Sichuan Medical Association (grant no. 2019HR23).
Availability of data and materials
Not applicable.
Authors' contributions
XH was responsible for article selection and literature review. XH, LX, YL, WX and HZ wrote the manuscript. WX constructed figures. Data authentication is not applicable. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Indini A, Rijavec E, Grossi F. Circulating biomarkers of response and toxicity of immunotherapy in advanced non-small cell lung cancer (NSCLC): A comprehensive review. Cancers (Basel) 2021;13(1794) doi: 10.3390/cancers13081794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan MV, Huo YR, Cao C, Ridley L. Survival outcomes for surgical resection versus CT-guided percutaneous ablation for stage I non-small cell lung cancer (NSCLC): A systematic review and meta-analysis. Eur Radiol. 2021;31:5421–5433. doi: 10.1007/s00330-020-07634-7. [DOI] [PubMed] [Google Scholar]
- 3.Chen YS, Lin WH, Zhang AL, Lin Y. Application of CT perfusion imaging in NSCLC and its correlation with angiogenesis and lymph node metastasis. Eur Rev Med Pharmacol Sci. 2021;25:2511–2516. doi: 10.26355/eurrev_202103_25414. [DOI] [PubMed] [Google Scholar]
- 4.Lombardi M, D'Ascanio M, Scarpino S, Scozzi D, Giordano M, Costarelli L, Raj ER, Mancini R, Cardillo G, Cardaci V, et al. Full-length TrkB variant in NSCLC is associated with brain metastasis. Biomed Res Int. 2020;2020(4193541) doi: 10.1155/2020/4193541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su Z, Yang Z, Xu Y, Chen Y, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015;14(48) doi: 10.1186/s12943-015-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li D, Fan S, Yu F, Zhu X, Song Y, Ye M, Fan L, Lv Z. FOXD1 promotes cell growth and metastasis by activation of vimentin in NSCLC. Cell Physiol Biochem. 2018;51:2716–2731. doi: 10.1159/000495962. [DOI] [PubMed] [Google Scholar]
- 7.Cai T, Zhou J, Zeng Y, Du W, Zhang Y, Liu T, Fu Y, Huang JA, Qian Q, Zhu J, et al. EVI5 is an oncogene that regulates the proliferation and metastasis of NSCLC cells. J Exp Clin Cancer Res. 2020;39(84) doi: 10.1186/s13046-020-01585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv XQ, Qiao XR, Su L, Chen SZ. Honokiol inhibits EMT-mediated motility and migration of human non-small cell lung cancer cells in vitro by targeting c-FLIP. Acta Pharmacol Sin. 2016;37:1574–1586. doi: 10.1038/aps.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Zhang B, Wu S, Song Y, Li J. Knockdown of EIF5A2 inhibits the malignant potential of non-small cell lung cancer cells. Oncol Lett. 2018;15:4541–4549. doi: 10.3892/ol.2018.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu R, Zhou Q, Ju L, Chen L, Wang F, Shao J. Upregulation of TRIP13 promotes the malignant progression of lung cancer via the EMT pathway. Oncol Rep. 2021;46(172) doi: 10.3892/or.2021.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang C, Zhu S, Feng W, Chen X. Calponin 3 suppresses proliferation, migration and invasion of non-small cell lung cancer cells. Oncol Lett. 2021;22(634) doi: 10.3892/ol.2021.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di X, Jin X, Ma H, Wang R, Cong S, Tian C, Liu J, Zhao M, Li R, Wang K. The oncogene IARS2 promotes non-small cell lung cancer tumorigenesis by activating the AKT/MTOR Pathway. Front Oncol. 2019;9(393) doi: 10.3389/fonc.2019.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan J, Zhang G, Li X, Ma Q, Cheng W, Wang W, Zhang B, Hu T, Song G. Knocking down USP39 inhibits the growth and metastasis of non-small-cell lung cancer cells through activating the p53 pathway. Int J Mol Sci. 2020;21(8949) doi: 10.3390/ijms21238949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing Y, Liu Y, Liu T, Meng Q, Lu H, Liu W, Hu J, Li C, Cao M, Yan S, et al. TNFAIP8 promotes the proliferation and cisplatin chemoresistance of non-small cell lung cancer through MDM2/p53 pathway. Cell Commun Signal. 2018;16(43) doi: 10.1186/s12964-018-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan Y, Liu T, Li S, Huang M, Li X, Zhao H, Li J. CHAF1B promotes proliferation and reduces apoptosis in 95D lung cancer cells and predicts a poor prognosis in nonsmall cell lung cancer. Oncol Rep. 2019;41:2518–2528. doi: 10.3892/or.2019.6994. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Zhang M, Xu S, Zhang J, Zou J, Yang C, Zhang Y, Gong C, Kai Y, Li Y. HOXC8 promotes proliferation and migration through transcriptional up-regulation of TGFbeta1 in non-small cell lung cancer. Oncogenesis. 2018;7(1) doi: 10.1038/s41389-017-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Y, Cheng Y, Jiang X, Zhang Y, Wang H, Ren H, Xu Y, Jiang J, Wang Q, Su H, et al. PCGF3 promotes the proliferation and migration of non-small cell lung cancer cells via the PI3K/AKT signaling pathway. Exp Cell Res. 2021;400(112496) doi: 10.1016/j.yexcr.2021.112496. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Dong H, Qiao L, Wu Y, Wu B, Jin X. ZEB1 induces non-small cell lung cancer development by targeting microRNA-320a to increase the expression of RAD51AP1. Exp Cell Res. 2021;405(112687) doi: 10.1016/j.yexcr.2021.112687. [DOI] [PubMed] [Google Scholar]
- 19.Songyang Y, Song T, Shi Z, Li W, Yang S, Li D. Effect of vitamin D on malignant behavior of non-small cell lung cancer cells. Gene. 2021;768(145309) doi: 10.1016/j.gene.2020.145309. [DOI] [PubMed] [Google Scholar]
- 20.Wang LL, Hu RC, Dai AG, Tan SX, Xu M, Kong CC, Chen YR, Fu DY. CHOP overexpression sensitizes human non-small cell lung cancer cells to cisplatin treatment by Bcl-2/JNK pathway. Am J Transl Res. 2021;13:6279–6287. [PMC free article] [PubMed] [Google Scholar]
- 21.Xia HW, Zhang ZQ, Yuan J, Niu QL. Human RECQL5 promotes metastasis and resistance to cisplatin in non-small cell lung cancer. Life Sci. 2021;265(118768) doi: 10.1016/j.lfs.2020.118768. [DOI] [PubMed] [Google Scholar]
- 22.Luo T, Yan L, Liu H. LINC00632 inhibits the malignant development of non-small cell lung cancer by downregulating miR-1203. J BUON. 2020;25:1517–1524. [PubMed] [Google Scholar]
- 23.Chen L, Li X, Lu C, Zhao Y, Zhu J, Yang L. The long noncoding RNA CASC7 inhibits growth and invasion of nonsmall cell lung cancer cells through phosphatase and tensin homolog upregulation via sequestration of miR92a. Int J Oncol. 2020;57:466–477. doi: 10.3892/ijo.2020.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang JF, Xi ZN, Su HJ, Bao Z, Qiao YH. SP1-induced overexpression of LINC00520 facilitates non-small cell lung cancer progression through miR-577/CCNE2 pathway and predicts poor prognosis. Hum Cell. 2021;34:952–964. doi: 10.1007/s13577-021-00518-y. [DOI] [PubMed] [Google Scholar]
- 25.She K, Yan H, Huang J, Zhou H, He J. miR-193b availability is antagonized by LncRNA-SNHG7 for FAIM2-induced tumour progression in non-small cell lung cancer. Cell Prolif. 2018;51(e12406) doi: 10.1111/cpr.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi L, Liu F, Zhang F, Zhang S, Lv L, Bi Y, Yu Y. lncRNA NEAT1 competes against let-7a to contribute to non-small cell lung cancer proliferation and metastasis. Biomed Pharmacother. 2018;103:1507–1515. doi: 10.1016/j.biopha.2018.04.053. [DOI] [PubMed] [Google Scholar]
- 27.Wan L, Sun M, Liu GJ, Wei CC, Zhang EB, Kong R, Xu TP, Huang MD, Wang ZX. Long noncoding RNA PVT1 promotes non-small cell lung cancer cell proliferation through epigenetically regulating LATS2 expression. Mol Cancer Ther. 2016;15:1082–1094. doi: 10.1158/1535-7163.MCT-15-0707. [DOI] [PubMed] [Google Scholar]
- 28.Qi G, Li L. Long non-coding RNA PVT1 contributes to cell growth and metastasis in non-small-cell lung cancer by regulating miR-361-3p/SOX9 axis and activating Wnt/beta-catenin signaling pathway. Biomed Pharmacother. 2020;126(110100) doi: 10.1016/j.biopha.2020.110100. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Sun M, Zang C, Ma P, He J, Zhang M, Huang Z, Ding Y, Shu Y. Upregulated long non-coding RNA AGAP2-AS1 represses LATS2 and KLF2 expression through interacting with EZH2 and LSD1 in non-small-cell lung cancer cells. Cell Death Dis. 2016;7(e2225) doi: 10.1038/cddis.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang C, Yang Y, Yang Y, Guo L, Huang J, Liu X, Wu C, Zou J. Long noncoding RNA (lncRNA) HOTAIR affects tumorigenesis and metastasis of non-small cell lung cancer by upregulating miR-613. Oncol Res. 2018;26:725–734. doi: 10.3727/096504017X15119467381615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Yue G, Zhang T, Wu J, Tian X. LncRNA ADAMTS9-AS1 knockdown restricts cell proliferation and EMT in non-small cell lung cancer. Histol Histopathol. 2021;36:1063–1072. doi: 10.14670/HH-18-347. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Peng Z, Cao J, Wang J, Hao Y, Song K, Wang Y, Hu W, Zhang X. Long noncoding RNA PXN-AS1-L promotes non-small cell lung cancer progression via regulating PXN. Cancer Cell Int. 2019;19(20) doi: 10.1186/s12935-019-0734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang G, An X, Zhao H, Zhang Q, Zhao H. Long non-coding RNA HNF1A-AS1 promotes cell proliferation and invasion via regulating miR-17-5p in non-small cell lung cancer. Biomed Pharmacother. 2018;98:594–599. doi: 10.1016/j.biopha.2017.12.080. [DOI] [PubMed] [Google Scholar]
- 34.Ao X, Jiang M, Zhou J, Liang H, Xia H, Chen G. lincRNAp21 inhibits the progression of nonsmall cell lung cancer via targeting miR175p. Oncol Rep. 2019;41:789–800. doi: 10.3892/or.2018.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan Y, Yang J, Liu X, Chu L. Long noncoding RNA CBR3 antisense RNA 1 promotes the aggressive phenotypes of nonsmallcell lung cancer by sponging microRNA5093p and competitively upregulating HDAC9 expression. Oncol Rep. 2020;44:1403–1414. doi: 10.3892/or.2020.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin B, Jin H, Wu HB, Xu JJ, Li B. Long non-coding RNA SNHG15 promotes CDK14 expression via miR-486 to accelerate non-small cell lung cancer cells progression and metastasis. J Cell Physiol. 2018;233:7164–7172. doi: 10.1002/jcp.26543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Huang S, Guan Y, Zhang Q. Long noncoding RNA OSER1AS1 promotes the malignant properties of nonsmall cell lung cancer by sponging microRNA4333p and thereby increasing Smad2 expression. Oncol Rep. 2020;44:599–610. doi: 10.3892/or.2020.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li G, Zhao C, Zhang H, Yu J, Sun Y, Zhang Y. Hsa_circ_0046263 drives the carcinogenesis and metastasis of non-small cell lung cancer through the promotion of NOVA2 by absorbing Mir-940 as a molecular sponge. Cancer Manag Res. 2020;12:12779–12790. doi: 10.2147/CMAR.S272603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Z, Xiang W, Chen W, Sun Y, Qin F, Wei J, Yuan L, Zheng L, Li S. Circ-IGF1R inhibits cell invasion and migration in non-small cell lung cancer. Thorac Cancer. 2020;11:875–887. doi: 10.1111/1759-7714.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Zhao W, Zhang S. STAT3-induced upregulation of circCCDC66 facilitates the progression of non-small cell lung cancer by targeting miR-33a-5p/KPNA4 axis. Biomed Pharmacother. 2020;126(110019) doi: 10.1016/j.biopha.2020.110019. [DOI] [PubMed] [Google Scholar]
- 41.Shi F, Yang Q, Shen D, Chen J. CircRNA WHSC1 promotes non-small cell lung cancer progression via sponging microRNA-296-3p and up-regulating expression of AKT serine/threonine kinase 3. J Clin Lab Anal. 2021;35(e23865) doi: 10.1002/jcla.23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tai G, Zhang M, Liu F. Circ_0000735 enhances the proliferation, metastasis and glycolysis of non-small cell lung cancer by regulating the miR-635/FAM83F axis. Exp Lung Res. 2021;47:136–148. doi: 10.1080/01902148.2021.1881188. [DOI] [PubMed] [Google Scholar]
- 43.Wu Z, Jiang H, Fu H, Zhang Y. A circGLIS3/miR-644a/PTBP1 positive feedback loop promotes the malignant biological progressions of non-small cell lung cancer. Am J Cancer Res. 2021;11:108–122. [PMC free article] [PubMed] [Google Scholar]
- 44.Yang C, Shi J, Wang J, Hao D, An J, Jiang J. Circ_0006988 promotes the proliferation, metastasis and angiogenesis of non-small cell lung cancer cells by modulating miR-491-5p/MAP3K3 axis. Cell Cycle. 2021;20:1334–1346. doi: 10.1080/15384101.2021.1941612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Y, Ma C, Lv A, Kou C. Circular RNA circ_0010235 sponges miR-338-3p to play oncogenic role in proliferation, migration and invasion of non-small-cell lung cancer cells through modulating KIF2A. Ann Med. 2021;53:693–706. doi: 10.1080/07853890.2021.1925736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Yang C, Cao C, Li Q, Jin X, Shi H. Hsa_circ_RNA_0011780 Represses the Proliferation and Metastasis of Non-Small Cell Lung Cancer by Decreasing FBXW7 via Targeting miR-544a. Onco Targets Ther. 2020;13:745–755. doi: 10.2147/OTT.S236162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Liu Y, Li C, Liu H, Wang J. Circ_0001821 knockdown suppresses growth, metastasis, and TAX resistance of non-small-cell lung cancer cells by regulating the miR-526b-5p/GRK5 axis. Pharmacol Res Perspect. 2021;9(e00812) doi: 10.1002/prp2.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu L, Li J, Peng B, Cai P, Zhao B, Chen Y, Zhu H. CircASXL1 knockdown restrains hypoxia-induced DDP resistance and NSCLC progression by sponging miR-206. Cancer Manag Res. 2021;13:5077–5089. doi: 10.2147/CMAR.S276964. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Pang J, Ye L, Zhao D, Zhao D, Chen Q. Circular RNA PRMT5 confers cisplatin-resistance via miR-4458/REV3L axis in non-small-cell lung cancer. Cell Biol Int. 2020;44:2416–2426. doi: 10.1002/cbin.11449. [DOI] [PubMed] [Google Scholar]
- 50.Yang B, Teng F, Chang L, Wang J, Liu DL, Cui YS, Li GH. Tumor-derived exosomal circRNA_102481 contributes to EGFR-TKIs resistance via the miR-30a-5p/ROR1 axis in non-small cell lung cancer. Aging (Albany NY) 2021;13:13264–13286. doi: 10.18632/aging.203011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Yang D, Wei Y. Overexpressed CDR1as functions as an oncogene to promote the tumor progression via miR-7 in non-small-cell lung cancer. Onco Targets Ther. 2018;11:3979–3987. doi: 10.2147/OTT.S158316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang N, Nan A, Chen L, Li X, Jia Y, Qiu M, Dai X, Zhou H, Zhu J, Zhang H, Jiang Y. Circular RNA circSATB2 promotes progression of non-small cell lung cancer cells. Mol Cancer. 2020;19(101) doi: 10.1186/s12943-020-01221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y, Hu J, Li L, Cai S, Zhang H, Zhu X, Guan G, Dong X. Upregulated circular RNA circ_0016760 indicates unfavorable prognosis in NSCLC and promotes cell progression through miR-1287/GAGE1 axis. Biochem Biophys Res Commun. 2018;503:2089–2094. doi: 10.1016/j.bbrc.2018.07.164. [DOI] [PubMed] [Google Scholar]
- 54.An X, Ge J, Guo H, Mi H, Zhou J, Liu Y, Weiyue , Wu Z. Overexpression of miR-4286 is an unfavorable prognostic marker in individuals with non-small cell lung cancer. J Cell Biochem. 2019;120:17573–17583. doi: 10.1002/jcb.29024. [DOI] [PubMed] [Google Scholar]
- 55.Kim DH, Park S, Kim H, Choi YJ, Kim SY, Sung KJ, Sung YH, Choi CM, Yun M, Yi YS, et al. Tumor-derived exosomal miR-619-5p promotes tumor angiogenesis and metastasis through the inhibition of RCAN1.4. Cancer Lett. 2020;475:2–13. doi: 10.1016/j.canlet.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 56.Mao M, Wu Z, Chen J. MicroRNA-187-5p suppresses cancer cell progression in non-small cell lung cancer (NSCLC) through down-regulation of CYP1B1. Biochem Biophys Res Commun. 2016;478:649–655. doi: 10.1016/j.bbrc.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Han L, Chen W, Xia Y, Song Y, Zhao Z, Cheng H, Jiang T. MiR-101 inhibits the proliferation and metastasis of lung cancer by targeting zinc finger E-box binding homeobox 1. Am J Transl Res. 2018;10:1172–1183. [PMC free article] [PubMed] [Google Scholar]
- 58.Wang S, Sun H, Zhan X, Wang Q. MicroRNA718 serves a tumorsuppressive role in nonsmall cell lung cancer by directly targeting CCNB1. Int J Mol Med. 2020;45:33–44. doi: 10.3892/ijmm.2019.4396. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Zhao M, Tong C, Hao Z, Zhao R, Wang L. MicroRNA-374b mediates the initiation of non-small cell lung cancer by regulating ITGB1 and p53 expressions. Thorac Cancer. 2020;11:1670–1678. doi: 10.1111/1759-7714.13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin X, Lai X, Feng W, Yu X, Gu Q, Zheng X. MiR-30a sensitized lung cancer against neoadjuvant chemotherapy by depressing autophagy. Jpn J Clin Oncol. 2021;51:675–684. doi: 10.1093/jjco/hyaa272. [DOI] [PubMed] [Google Scholar]
- 61.Huang T, She K, Peng G, Wang W, Huang J, Li J, Wang Z, He J. MicroRNA-186 suppresses cell proliferation and metastasis through targeting MAP3K2 in non-small cell lung cancer. Int J Oncol. 2016;49:1437–1444. doi: 10.3892/ijo.2016.3637. [DOI] [PubMed] [Google Scholar]
- 62.Gong F, Ren P, Zhang Y, Jiang J, Zhang H. MicroRNAs-491-5p suppresses cell proliferation and invasion by inhibiting IGF2BP1 in non-small cell lung cancer. Am J Transl Res. 2016;8:485–495. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Zhang Z, Li W, Jiang D, Liu C, Lai Z. MicroRNA-139-5p inhibits cell viability, migration and invasion and suppresses tumor growth by targeting HDGF in non-small cell lung cancer. Oncol Lett. 2020;19:1806–1814. doi: 10.3892/ol.2020.11296. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Yan Y, Jin X, Sun H, Pang S, Kong X, Bu J, Xu S. MiR-139-5p targetedly regulates YAF2 and mediates the AKT/P38 MAPK signaling pathway to alleviate the metastasis of non-small cell lung cancer cells and their resistance against cisplatin. Cancer Manag Res. 2021;13:3639–3650. doi: 10.2147/CMAR.S254671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y, Hou C, Zhao LX, Cai QC, Zhang Y, Li DL, Tang Y, Liu HY, Liu YY, Zhang YY, et al. The association of microRNA-34a with high incidence and metastasis of lung cancer in Gejiu and Xuanwei Yunnan. Front Oncol. 2021;11(619346) doi: 10.3389/fonc.2021.619346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang W, Wei K, Pan C, Li H, Cao J, Han X, Tang Y, Zhu S, Yuan W, He Y, et al. MicroRNA-1258 suppresses tumour progression via GRB2/Ras/Erk pathway in non-small-cell lung cancer. Cell Prolif. 2018;51(e12502) doi: 10.1111/cpr.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen G, Hu J, Huang Z, Yang L, Chen M. MicroRNA-1976 functions as a tumor suppressor and serves as a prognostic indicator in non-small cell lung cancer by directly targeting PLCE1. Biochem Biophys Res Commun. 2016;473:1144–1151. doi: 10.1016/j.bbrc.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 68.Li QX, Zhou X, Huang TT, Tang Y, Liu B, Peng P, Sun L, Wang YH, Yuan XL. The Thr300Ala variant of ATG16L1 is associated with decreased risk of brain metastasis in patients with non-small cell lung cancer. Autophagy. 2017;13:1053–1063. doi: 10.1080/15548627.2017.1308997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang L, Liang X, Liu M, Wang W, Ma J, Guo Q, Han L, Yang C, Nan K. Reduced expression of liver kinase B1 and Beclin1 is associated with the poor survival of patients with non-small cell lung cancer. Oncol Rep. 2014;32:1931–1938. doi: 10.3892/or.2014.3432. [DOI] [PubMed] [Google Scholar]
- 70.Du H, Chen L, Luo F, Chen X, Li Y, Cheng Q. Beclin-1 expression is associated with prognosis in a Bcl-2-dependent manner in non-small cell lung cancer. Oncol Lett. 2020;20(9) doi: 10.3892/ol.2020.11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu S, Cheng C, Wang J, Wang J, Qu Z, Ren H, Li Y, Ning Q, Chen M, Hu T. Loss of Beclin1 expression and Nrf2 overexpression are associated with poor survival of patients with non-small cell lung cancer. Anticancer Agents Med Chem. 2018;18:1680–1687. doi: 10.2174/1871520618666180830110700. [DOI] [PubMed] [Google Scholar]
- 72.Chen B, Zeng C, Ye Y, Wu D, Mu Z, Liu J, Xie Y, Wu H. Promoter methylation of TCF21 may repress autophagy in the progression of lung cancer. J Cell Commun Signal. 2018;12:423–432. doi: 10.1007/s12079-017-0418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim H, Choi SY, Lim J, Lindroth AM, Park YJ. EHMT2 inhibition induces cell death in human non-small cell lung cancer by altering the cholesterol biosynthesis pathway. Int J Mol Sci. 2020;21(1002) doi: 10.3390/ijms21031002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Z, Tian D, Liao X, Zhang Y, Xiao J, Chen W, Liu Q, Chen Y, Li D, Zhu L, Cai S. Apigenin combined with gefitinib blocks autophagy flux and induces apoptotic cell death through inhibition of HIF-1a, c-Myc, p-EGFR, and glucose metabolism in EGFR L858R+T790M-mutated H1975 cells. Front Pharmacol. 2019;10(260) doi: 10.3389/fphar.2019.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y, Hong T, Tong L, Liu W, Yang X, Luo J, Wang F, Li J, Yan L. Astragalus polysaccharide combined with 10-hydroxycamptothecin inhibits metastasis in non-small cell lung carcinoma cell lines via the MAP4K3/mTOR signaling pathway. Int J Mol Med. 2018;42:3093–3104. doi: 10.3892/ijmm.2018.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang H, Zhang Y, Wu Q, Wang YB, Wang W. miR-16 mimics inhibit TGF-beta1-induced epithelial-to-mesenchymal transition via activation of autophagy in non-small cell lung carcinoma cells. Oncol Rep. 2018;39:247–254. doi: 10.3892/or.2017.6088. [DOI] [PubMed] [Google Scholar]
- 77.Xu C, Cao H, Sui Y, Zhang H, Shi C, Wu J, Ma R, Feng J. CDCA4 suppresses epithelial-mesenchymal transtion (EMT) and metastasis in Non-small cell lung cancer through modulating autophagy. Cancer Cell Int. 2021;21(48) doi: 10.1186/s12935-021-01754-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng L, Zhou H, Guo L, Xu X, Zhang S, Xu W, Mao W. Inhibition of NIPBL enhances the chemosensitivity of non-small-cell lung cancer cells via the DNA damage response and autophagy pathway. Onco Targets Ther. 2018;11:1941–1948. doi: 10.2147/OTT.S158655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y, Liu F, Fang C, Xu L, Chen L, Xu Z, Chen J, Peng W, Fu B, Li Y. Combination of rapamycin and SAHA enhanced radiosensitization by inducing autophagy and acetylation in NSCLC. Aging (Albany NY) 2021;13:18223–18237. doi: 10.18632/aging.203226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo M, Liu Z, Si J, Zhang J, Zhao J, Guo Z, Xie Y, Zhang H, Gan L. Cediranib induces apoptosis, G1 phase cell cycle arrest, and autophagy in non-small-cell lung cancer cell A549 in vitro. Biomed Res Int. 2021;2021(5582648) doi: 10.1155/2021/5582648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye X, Xie G, Liu Z, Tang J, Cui M, Wang C, Guo C, Tang J. TNNC1 reduced gemcitabine sensitivity of nonsmall-cell lung cancer by increasing autophagy. Med Sci Monit. 2020;26(e922703) doi: 10.12659/MSM.922703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Datta S, Choudhury D, Das A, Mukherjee DD, Dasgupta M, Bandopadhyay S, Chakrabarti G. Autophagy inhibition with chloroquine reverts paclitaxel resistance and attenuates metastatic potential in human nonsmall lung adenocarcinoma A549 cells via ROS mediated modulation of beta-catenin pathway. Apoptosis. 2019;24:414–433. doi: 10.1007/s10495-019-01526-y. [DOI] [PubMed] [Google Scholar]
- 83.Zhao Z, Li J, Jiang Y, Xu W, Li X, Jing W. CLDN1 increases drug resistance of non-small cell lung cancer by activating autophagy via up-regulation of ULK1 phosphorylation. Med Sci Monit. 2017;23:2906–2916. doi: 10.12659/msm.904177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shen Q, Xu Z, Xu S. Long noncoding RNA LUCAT1 contributes to cisplatin resistance by regulating the miR514a3p/ULK1 axis in human nonsmall cell lung cancer. Int J Oncol. 2020;57:967–979. doi: 10.3892/ijo.2020.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li S, Zeng X, Ma R, Wang L. MicroRNA-21 promotes the proliferation, migration and invasion of non-small cell lung cancer A549 cells by regulating autophagy activity via AMPK/ULK1 signaling pathway. Exp Ther Med. 2018;16:2038–2045. doi: 10.3892/etm.2018.6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo J, Wu Y, Du J, Yang L, Chen W, Gong K, Dai J, Miao S, Jin D, Xi S. Deregulation of UBE2C-mediated autophagy repression aggravates NSCLC progression. Oncogenesis. 2018;7(49) doi: 10.1038/s41389-018-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu X, Liu QX, Zhang J, Zhou D, Yang GX, Li MY, Qiu Y, Chen Q, Zheng H, Dai JG. PINK1 Overexpression promotes cell migration and proliferation via regulation of autophagy and predicts a poor prognosis in lung cancer cases. Cancer Manag Res. 2020;12:7703–7714. doi: 10.2147/CMAR.S262466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang F, Cheng R, Li P, Lu C, Zhang G. Hsa_circ_0010235 functions as an oncogenic drive in non-small cell lung cancer by modulating miR-433-3p/TIPRL axis. Cancer Cell Int. 2021;21(73) doi: 10.1186/s12935-021-01764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wudu M, Ren H, Hui L, Jiang J, Zhang S, Xu Y, Wang Q, Su H, Jiang X, Dao R, Qiu X. DRAM2 acts as an oncogene in non-small cell lung cancer and suppresses the expression of p53. J Exp Clin Cancer Res. 2019;38(72) doi: 10.1186/s13046-019-1068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang G, Liu Z, Chen Y, Zhang Y. High serum HDGF levels are predictive of bone metastasis and unfavorable prognosis in non-small cell lung cancer. Tohoku J Exp Med. 2017;242:101–108. doi: 10.1620/tjem.242.101. [DOI] [PubMed] [Google Scholar]
- 91.Chang M, Shang M, Yuan F, Guo W, Wang C. EF24 exerts cytotoxicity against NSCLC via inducing ROS accumulation. Cancer Cell Int. 2021;21(531) doi: 10.1186/s12935-021-02240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin H, Chen X, Zhang C, Yang T, Deng Z, Song Y, Huang L, Li F, Li Q, Lin S, Jin D. EF24 induces ferroptosis in osteosarcoma cells through HMOX1. Biomed Pharmacother. 2021;136(111202) doi: 10.1016/j.biopha.2020.111202. [DOI] [PubMed] [Google Scholar]
- 93.Li Y, Yan H, Xu X, Liu H, Wu C, Zhao L. Erastin/sorafenib induces cisplatin-resistant non-small cell lung cancer cell ferroptosis through inhibition of the Nrf2/xCT pathway. Oncol Lett. 2020;19:323–333. doi: 10.3892/ol.2019.11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu CY, Yang YH, Lin YS, Chang GH, Tsai MS, Hsu CM, Yeh RA, Shu LH, Cheng YC, Liu HT. Dihydroisotanshinone I induced ferroptosis and apoptosis of lung cancer cells. Biomed Pharmacother. 2021;139(111585) doi: 10.1016/j.biopha.2021.111585. [DOI] [PubMed] [Google Scholar]
- 95.Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S, Sabatini DM, Birsoy K, Possemato R. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature. 2017;551:639–643. doi: 10.1038/nature24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu W, Chakraborty B, Safi R, Kazmin D, Chang CY, McDonnell DP. Dysregulated cholesterol homeostasis results in resistance to ferroptosis increasing tumorigenicity and metastasis in cancer. Nat Commun. 2021;12(5103) doi: 10.1038/s41467-021-25354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu S, Ma J, Su C, Chen Y, Shu Y, Qi Z, Zhang B, Shi G, Zhang Y, Zhang Y, et al. Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis. Acta Biomater. 2021;135:567–581. doi: 10.1016/j.actbio.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 98.Song R, Li T, Ye J, Sun F, Hou B, Saeed M, Gao J, Wang Y, Zhu Q, Xu Z, Yu H. Acidity-activatable dynamic nanoparticles boosting ferroptotic cell death for immunotherapy of cancer. Adv Mater. 2021;33(e2101155) doi: 10.1002/adma.202101155. [DOI] [PubMed] [Google Scholar]
- 99.Yu H, Han Z, Xu Z, An C, Xu L, Xin H. RNA sequencing uncovers the key long non-coding RNAs and potential molecular mechanism contributing to XAV939-mediated inhibition of non-small cell lung cancer. Oncol Lett. 2019;17:4994–5004. doi: 10.3892/ol.2019.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gai C, Liu C, Wu X, Yu M, Zheng J, Zhang W, Lv S, Li W. MT1DP loaded by folate-modified liposomes sensitizes erastin-induced ferroptosis via regulating miR-365a-3p/NRF2 axis in non-small cell lung cancer cells. Cell Death Dis. 2020;11(751) doi: 10.1038/s41419-020-02939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang M, Mao C, Ouyang L, Liu Y, Lai W, Liu N, Shi Y, Chen L, Xiao D, Yu F, et al. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ. 2019;26:2329–2343. doi: 10.1038/s41418-019-0304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen B, Wang H, Lv C, Mao C, Cui Y. Long non-coding RNA H19 protects against intracerebral hemorrhage injuries via regulating microRNA-106b-5p/acyl-CoA synthetase long chain family member 4 axis. Bioengineered. 2021;12:4004–4015. doi: 10.1080/21655979.2021.1951070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lu J, Xu F, Lu H. LncRNA PVT1 regulates ferroptosis through miR-214-mediated TFR1 and p53. Life Sci. 2020;260(118305) doi: 10.1016/j.lfs.2020.118305. [DOI] [PubMed] [Google Scholar]
- 104.He GN, Bao NR, Wang S, Xi M, Zhang TH, Chen FS. Ketamine induces ferroptosis of liver cancer cells by targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des Devel Ther. 2021;15:3965–3978. doi: 10.2147/DDDT.S332847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang Y, Tai W, Lu N, Li T, Liu Y, Wu W, Li Z, Pu L, Zhao X, Zhang T, Dong Z. lncRNA ZFAS1 promotes lung fibroblast-to-myofibroblast transition and ferroptosis via functioning as a ceRNA through miR-150-5p/SLC38A1 axis. Aging (Albany NY) 2020;12:9085–9102. doi: 10.18632/aging.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Z, Chen X, Liu N, Shi Y, Liu Y, Ouyang L, Tam S, Xiao D, Liu S, Wen F, Tao Y. A nuclear long non-coding RNA LINC00618 accelerates ferroptosis in a manner dependent upon apoptosis. Mol Ther. 2021;29:263–274. doi: 10.1016/j.ymthe.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li C, Tian Y, Liang Y, Li Q. Circ_0008035 contributes to cell proliferation and inhibits apoptosis and ferroptosis in gastric cancer via miR-599/EIF4A1 axis. Cancer Cell Int. 2020;20(84) doi: 10.1186/s12935-020-01168-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 108.Liu Z, Wang Q, Wang X, Xu Z, Wei X, Li J. Circular RNA cIARS regulates ferroptosis in HCC cells through interacting with RNA binding protein ALKBH5. Cell Death Discov. 2020;6(72) doi: 10.1038/s41420-020-00306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang HH, Ma JN, Zhan XR. Circular RNA Circ_0067934 attenuates ferroptosis of thyroid cancer cells by miR-545-3p/SLC7A11 signaling. Front Endocrinol (Lausanne) 2021;12(670031) doi: 10.3389/fendo.2021.670031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y, Chen H, Wei X. Circ_0007142 downregulates miR-874-3p-mediated GDPD5 on colorectal cancer cells. Eur J Clin Invest. 2021;51(e13541) doi: 10.1111/eci.13541. [DOI] [PubMed] [Google Scholar]
- 111.Xu Q, Zhou L, Yang G, Meng F, Wan Y, Wang L, Zhang L. CircIL4R facilitates the tumorigenesis and inhibits ferroptosis in hepatocellular carcinoma by regulating the miR-541-3p/GPX4 axis. Cell Biol Int. 2020;44:2344–2356. doi: 10.1002/cbin.11444. [DOI] [PubMed] [Google Scholar]
- 112.Zhang HY, Zhang BW, Zhang ZB, Deng QJ. Circular RNA TTBK2 regulates cell proliferation, invasion and ferroptosis via miR-761/ITGB8 axis in glioma. Eur Rev Med Pharmacol Sci. 2020;24:2585–2600. doi: 10.26355/eurrev_202003_20528. [DOI] [PubMed] [Google Scholar]
- 113.Chen W, Fu J, Chen Y, Li Y, Ning L, Huang D, Yan S, Zhang Q. Circular RNA circKIF4A facilitates the malignant progression and suppresses ferroptosis by sponging miR-1231 and upregulating GPX4 in papillary thyroid cancer. Aging (Albany NY) 2021;13:16500–16512. doi: 10.18632/aging.203172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Y, Guo S, Wang S, Li X, Hou D, Li H, Wang L, Xu Y, Ma B, Wang H, Jiang X. LncRNA OIP5-AS1 inhibits ferroptosis in prostate cancer with long-term cadmium exposure through miR-128-3p/SLC7A11 signaling. Ecotoxicol Environ Saf. 2021;220(112376) doi: 10.1016/j.ecoenv.2021.112376. [DOI] [PubMed] [Google Scholar]
- 115.Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, Jiang F, Peng ZY. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. 2019;2019(5080843) doi: 10.1155/2019/5080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y, Chen Q, Zhu Y, Wang T, Ye L, Han L, Yao Z, Yang Z. Non-coding RNAs in necroptosis, pyroptosis and ferroptosis in cancer metastasis. Cell Death Discov. 2021;7(210) doi: 10.1038/s41420-021-00596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tang R, Xu J, Zhang B, Liu J, Liang C, Hua J, Meng Q, Yu X, Shi S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13(110) doi: 10.1186/s13045-020-00946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mei J, Liu G, Wang W, Xiao P, Yang D, Bai H, Li R. OIP5-AS1 modulates epigenetic regulator HDAC7 to enhance non-small cell lung cancer metastasis via miR-140-5p. Oncol Lett. 2020;20(7) doi: 10.3892/ol.2020.11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dao R, Wudu M, Hui L, Jiang J, Xu Y, Ren H, Qiu X. Knockdown of lncRNA MIR503HG suppresses proliferation and promotes apoptosis of non-small cell lung cancer cells by regulating miR-489-3p and miR-625-5p. Pathol Res Pract. 2020;216(152823) doi: 10.1016/j.prp.2020.152823. [DOI] [PubMed] [Google Scholar]
- 120.Shanshan W, Hongying M, Jingjing F, Yiming Y, Yu R, Rui Y. CircDTL Functions as an Oncogene and Regulates Both Apoptosis and Ferroptosis in Non-small Cell Lung Cancer Cells. Front Genet. 2021;12(743505) doi: 10.3389/fgene.2021.743505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu P, Li C, Ye DM, Yu K, Li Y, Tang H, Xu G, Yi S, Zhang Z. Circular RNA circEPSTI1 accelerates cervical cancer progression via miR-375/409-3P/515-5p-SLC7A11 axis. Aging (Albany NY) 2021;13:4663–4673. doi: 10.18632/aging.202518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ni H, Qin H, Sun C, Liu Y, Ruan G, Guo Q, Xi T, Xing Y, Zheng L. MiR-375 reduces the stemness of gastric cancer cells through triggering ferroptosis. Stem Cell Res Ther. 2021;12(325) doi: 10.1186/s13287-021-02394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Song Z, Jia G, Ma P, Cang S. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci. 2021;276(119399) doi: 10.1016/j.lfs.2021.119399. [DOI] [PubMed] [Google Scholar]
- 124.Deng SH, Wu DM, Li L, Liu T, Zhang T, Li J, Yu Y, He M, Zhao YY, Han R, Xu Y. miR-324-3p reverses cisplatin resistance by inducing GPX4-mediated ferroptosis in lung adenocarcinoma cell line A549. Biochem Biophys Res Commun. 2021;549:54–60. doi: 10.1016/j.bbrc.2021.02.077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.