Abstract
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a common and disabling disorder primarily characterized by persistent fatigue and exercise intolerance, with associated sleep disturbances, autonomic dysfunction, and cognitive problems. The causes of ME/CFS are not well understood but may coincide with immune and inflammatory responses following viral infections. During the current SARS-CoV2 coronavirus pandemic, ME/CFS has been increasingly reported to overlap with persistent “long COVID” symptoms, also called the post-acute sequelae of COVID-19 (PASC). Given the prominence of activity and sleep problems in ME/CFS, circadian rhythm disruption has been examined as a contributing factor in ME/CFS. While these studies of circadian rhythms have been pursued for decades, evidence linking circadian rhythms to ME/CFS remains inconclusive. A major limitation of older chronobiology studies of ME/CFS was the unavailability of modern molecular methods to study circadian rhythms and incomplete understanding of circadian rhythms outside the brain in peripheral organ systems. Major methodological and conceptual advancements in chronobiology have since been made. Over the same time, biomarker research in ME/CFS has progressed. Together, these new developments may justify renewed interest in circadian rhythm research in ME/CFS. Presently, we review ME/CFS from the perspective of circadian rhythms, covering both older and newer studies that make use of modern molecular methods. We focus on transforming growth factor beta (TGFB), a cytokine that has been previously associated with ME/CFS and has an important role in circadian rhythms, especially in peripheral cells. We propose that disrupted TGFB signaling in ME/CFS may play a role in disrupting physiological rhythms in sleep, activity, and cognition, leading to the insomnia, energy disturbances, cognition problems, depression, and autonomic dysfunction associated with ME/CFS. Since SARS-like coronavirus infections cause persistent changes in TGFB and previous coronavirus outbreaks have caused ME/CFS-like syndromes, chronobiological considerations may have immediate implications for understanding ME/CFS in the context of the COVID-19 pandemic and possibly suggest new avenues for therapeutic interventions.
Keywords: Myalgic encephalomyelitis, Chronic fatigue syndrome, Sleep, Activity, Temperature, Circadian rhythm, Cytokine, Transforming growth factor beta, COVID-19
Highlights
-
•
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is characterized by disrupted sleep and activity implicating circadian clocks.
-
•
The incidence of ME/CFS is expected to increase as a result of the post-acute sequelae of COVID-19.
-
•
Biomarker studies in ME/CFS patients implicate Transforming Growth Factor B (TGFB).
-
•
TGFB has roles in synchronizing circadian rhythms in peripheral cells.
-
•
Identification of biomarkers and new methodologies may facilitate progress in the chronobiological basis of ME/CFS.
1. Introduction
1.1. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a disabling disorder characterized by persistent fatigue, activity intolerance, post-exertional malaise, pain, sleep disturbances, and cognitive impairment often described by patients as “brain fog” (Afari and Buchwald, 2003; Clayton, 2015; Beyond Myalgic Encephalom, 2015). The disorder affects women more than men and may affect minority populations disproportionately. Average age of onset of ME/CFS is 33 y but can affect children as young as 10 y and older people into the eighth decade of life (Afari and Buchwald, 2003). While some individuals with ME/CFS recover, many have enduring symptoms and may not return to their previous state of health, with up to 25% remaining unable to perform basic occupational functions. There is no diagnostic test for ME/CFS and therefore, diagnosis is made entirely by clinical evaluation. The most recent Institute of Medicine (IOM) Diagnostic criteria for ME/CFS require the presence of disabling fatigue, post-exertional malaise, and unrefreshing sleep as well as cognitive impairment or orthostatic intolerance. The duration of symptoms must last at least six months and other explanatory disorders must be excluded (Clayton, 2015). Many ME/CFS patients also suffer from psychiatric disorders, most commonly depression and anxiety which may either pre-date or follow the diagnosis of ME/CFS and overlap in symptom presentation to further increase disability (Afari and Buchwald, 2003). Prior to the current SARS-CoV2 coronavirus pandemic, ME/CFS was thought to affect as many as 2.5 million people in the USA (Beyond Myalgic Encephalom, 2015; Komaroff and Bateman, 2020). With reports that the post-acute sequelae of COVID-19 (PASC) may affect 40–60% of recovering patients and the concern that many of these will progress to ME/CFS, this number is expected to increase dramatically (Komaroff and Bateman, 2020). ME/CFS usually starts suddenly and is often preceded by a febrile illness, causing many to believe that post-inflammatory and/or autoimmune mechanisms are involved, possibly affecting mitochondrial function, causing oxidative stress, and/or disrupting immune functions. Infectious triggers have been variously implicated. Prospective studies in adolescents demonstrate an association of ME/CFS with infection by Epstein Barr virus and/or infectious mononucleosis (Hickie et al., 2006; Jason et al., 2021; Katz et al., 2009). Other agents such as human herpes virus 6, B19 parvovirus and enteroviruses, have shown association with ME/CFS through retrospective serological association studies, but none have been definitively proven as having causal roles, suggesting a lack of specificity in terms of etiology (Rasa et al., 2018). Accordingly, the pathophysiology of ME/CFS remains poorly understood, and most cases are sporadic, lacking a specific, identifiable trigger and leading to uncertainty about the contributing factors, causing substantial heterogeneity in the clinical presentation. During the current SARS-CoV2 coronavirus pandemic, ME/CFS has been increasingly reported to overlap with persistent “long COVID” symptoms, termed the post-acute sequelae of COVID-19 (PASC). It is possible that because of the COVID-19 pandemic and subsequent rise of PASC-associated ME/CFS cases, that a large and more homogeneous group of patients will be identifiable in coming years (Komaroff and Bateman, 2020). Renewed work on biological mechanisms of PASC-related ME/CFS is therefore urgently needed.
1.2. Circadian rhythms
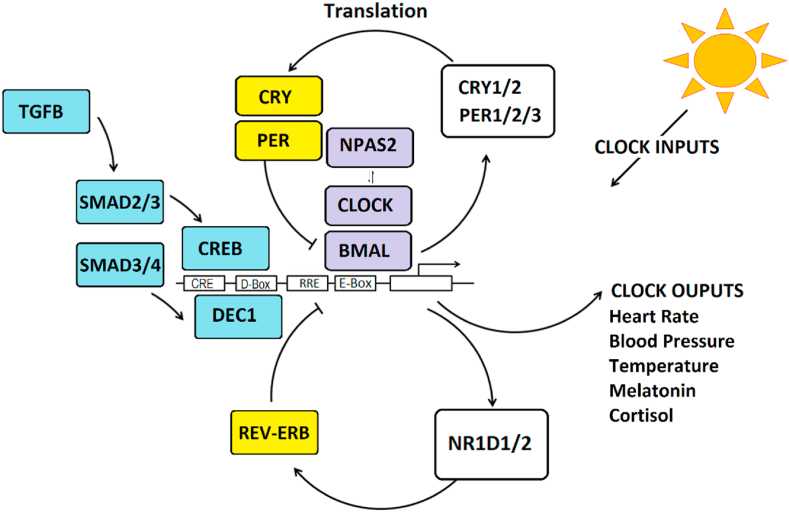
Human physiology has evolved to anticipate changes in the daily light/dark cycle and associated changes in behavior such as sleep, and activity (termed “circadian rhythms”). Nearly all physiological processes in humans have circadian rhythms including body temperature, heart rate, blood pressure, respiration and immune function (Scheiermann et al., 2018; Mohawk et al., 2012). As such, most cells in the body contain autonomous circadian clocks that maintain rhythms through a circuit of interconnected “clock genes” (Mohawk et al., 2012; Partch et al., 2014). Clock genes are organized in transcriptional-translational feedback loops that oscillate over a period of ∼24 h (Fig. 1). The positive limb of the circuit is driven by BMAL1 protein dimers including either CLOCK or NPAS2 to activate transcription. BMAL1-containing dimers activate the negative loop consisting of PER1/2/3, CRY1/2 and NR1D1/2 genes that yield transcriptional repressor proteins. Other proteins, such as DEC1 modify circadian rhythms through binding at D-box DNA elements and light-responsive transcription factors like CREB that bind at E-Box elements. Circadian rhythms are coordinated by the suprachiasmatic nucleus (SCN) in the hypothalamus which acts as a master clock (Mohawk et al., 2012). Under healthy circumstances, central rhythms in the SCN are coordinated with peripheral rhythms elsewhere in the body. However, under some conditions (e.g. jet lag, shift work), physiological rhythms become desynchronized leading to health problems such as insomnia, depression, and metabolic disorders (Scheer et al., 2009; McCarthy and Welsh, 2012). To maintain synchronization with the environment, the SCN receives light input from the retina. Light synchronizes rhythms in the SCN central pacemaker which then coordinates with peripheral clocks using melatonin. In the absence of photoreceptors, temporal cues such as food, exercise, oxygen levels and pH also affect circadian rhythms in peripheral tissues (Kon et al., 2008; Youngstedt et al., 2019; Pendergast and Yamazaki, 2018). In humans, circadian phase of the SCN can be estimated in the laboratory by measuring the onset of melatonin under dim light conditions (DLMO). Measures of clock gene expression oscillations can also be used to measure circadian rhythms in peripheral cells such as buccal epithelial cells (Moon et al., 2016) and hair follicles (Akashi et al., 2010).
Fig. 1.
The molecular clock is comprised of clock genes, organized in transcriptional/translational feedback loops. Transcriptional activator proteins (purple), BMAL1 and CLOCK/NPAS2 bind to DNA elements in promoters (E-Box) to stimulate transcription of CRY1/2, PER1/2/3, and NR1D1/2 genes that encode transcriptional repressors (yellow) to regulate activity through protein and DNA interactions. Other regulators include DEC1 that binds to D-box elements. These interactions yield oscillatory changes in gene expression and translation over ∼24 h cycles causing cellular rhythms. Light and environmental entrainment signals can phase shift the clock through intracellular signals that stimulate the cAMP-response element binding protein (CREB) that binds to CRE DNA elements. Cytokine pathways (blue) directed by Transforming Growth Factor Beta (TGFB) and SMAD proteins can shift rhythms in response to changes in pH (through DEC1) and peripheral synchronization cues (through CREB). The circadian clock affects the expression of clock-controlled genes that are rhythmically expressed and control numerous physiological processes that require precise timing across the daily 24 h cycle. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2. Circadian disruption in ME/CFS
Given the presentation of clinical symptoms in ME/CFS, researchers have focused on the hypothalamus, and particularly the suprachiasmatic nucleus (SCN), the master circadian pacemaker in mammals. Energy level, activity, alertness, and mood all follow daily circadian rhythms in healthy people, while in circadian rhythm disorders, such as shift work syndrome and jet lag, these processes can be adversely affected causing adverse changes in physiology (Scheer et al., 2009). Given the prominence of sleep and activity disturbances in ME/CFS, one hypothesis is that circadian rhythm disruptions desynchronize the mechanisms that coordinate physiological activity across organ systems, disrupting metabolism and energy balance, leading to suboptimal physiological function, and causing symptoms of fatigue, cognitive disturbance, and dysautonomia. Circadian disruption in ME/CFS has been studied and reviewed previously (Bonsall and Harrington, 2013). In these studies, ME/CFS has been associated with genetic variation in clock genes (Smith et al., 2011), as well as rhythm alterations in autonomic functions such as body temperature (Wyller et al., 2007), heart rate and blood pressure (van de Luit et al., 1998; Bou-Holaigah et al., 1995) and cortisol levels (Demitrack et al., 1991).
2.1. Genetic association studies
Twin studies reveal that ME/CFS has a heritable component accounting for 55% of the phenotypic variance (Buchwald et al., 2001). A genome-wide association study of >116,000 single nucleotide variants (SNPs) in 40 ME/CFS patients and 40 controls reported nominally significant association of ME/CFS with variants in 65 SNPs, including markers in the clock gene NPAS2 (Smith et al., 2011). The study was not sufficiently powered to identify genome-wide significant studies after correcting for multiple comparisons. However, in a gene-expression study using peripheral blood cells from the same cohort, NPAS2 was among only two genes (with GRIK2 encoding a glutamate receptor) that was found to be significantly different both in the genetic association and gene expression analyses. Compared to controls, samples from ME/CFS patients showed 10-fold higher expression of NPAS2. The UK Biobank study of activity levels in a population sample of 85,000 people, the same NPAS2 SNP was nominally associated (p = 0.008) with activity level. NPAS2 is an accessory component of the core transcriptional circadian oscillator and is commonly expressed with a 24 h rhythm (Fig. 1). However, because the data were examined at only a single time point, in this instance they did not evaluate rhythms and cannot distinguish a potential phase difference from an amplitude difference between ME/CFS cases and controls.
2.2. Sleep and activity
Lower daytime activity and less regularity across days in activity cycles was reported in 8 patients and 10 control predominantly female adults over 5–7 days using actigraphy (Tryon et al., 2004). In a later study of activity over 5 days in 15 ME/CFS patients and 15 control adults, the ME/CFS group reported earlier sleep times and less quality sleep (Rahman et al., 2011). There were no differences in overall activity or rhythms. However, in this study patients were matched at baseline on self-reported activity which may have limited the ability to identify group differences. Using sleep diaries and actigraphy, ME/CFS patients showed considerable difficulty with sleep with longer sleep latency, more waking after sleep onset, more time in bed and later wake times in a study of 10 middle aged women with ME/CFS and 10 age and sex-matched controls (Cambras et al., 2018). Compared to controls, the ME/CFS group also showed irregular activity patterns with decreased total activity, lower activity rhythm amplitude and less stable rhythms. Collectively, these small studies reveal generally lower activity, more irregular activity patterns, and less efficient sleep with more subjective sleep complaints. However, multiple factors can affect sleep/activity patterns, many of which do not relate to the circadian clock. Therefore, masking effects from the light/dark cycle as well as environmental and social factors complicate the interpretation of these studies with respect to circadian rhythms. To date, no studies of ME/CFS have been conducted under rigorous laboratory conditions (e.g., constant routine, forced desynchrony) that could more clearly quantify the contribution of the circadian clock.
2.3. Motivation, mood and cognition
Fatigue is the defining feature of ME/CFS and a multi-faceted construct that overlaps with motivation, energy, affect and cognition. Circadian regulation has been described for a variety of motivated behaviors including eating, sex, and exercise (Antle and Silver, 2016). Notably, exercise has bidirectional effects on circadian rhythms. Exercise not only shows daily patterns of occurrence but also provides feedback to the circadian clock, functioning as an entrainment signal. In humans, effects of exercise on melatonin metabolites (urinary 6-sulphatoxymelatonin) show a phase response curve similar to light (Youngstedt et al., 2019). Therefore, fatigue and inactivity in the context of ME/CFS may be both a cause and effect of circadian disruption. Depression is present in about 30% of cases with ME/CFS. Positive affect follows a circadian rhythm in humans assessed in ecological samples and forced desynchrony laboratory studies (Murray et al., 2009), and circadian disruption has been linked with depression and depression-like behaviors in human and animal studies (Li et al., 2013; Landgraf et al., 2016). “Brain fog” associated with ME/CFS may indicate cognitive impairments, particularly in attention, reaction time and memory (Cockshell and Mathias, 2010). Attention shows diurnal variation in humans and is regulated both by circadian and sleep-homeostatic mechanisms (Valdez, 2019; Burke et al., 2015). Therefore, cognitive dysfunction in ME/CFS may also reflect a circadian phase abnormality. Interestingly, despite the strong theoretical basis for studying motivational, mood and attentional rhythms in ME/CFS, relatively little work has been done in this area. ME/CFS patients reported diurnal variation in mood, positive affect and subjective energy with similar temporal patterns in healthy individuals, but with reduced levels in ME/CFS vs healthy subjects regardless of time (Wood et al., 1992). A study of 30 patients with ME/CFS compared cognitive performance against matched patients with major depression and healthy controls (Lawrie et al., 2000). Across the majority of neuropsychological, physical effort and motor performance measures, ME/CFS and depressed patients performed worse than controls, regardless of time, indicating a general decrease in performance in ME/CFS, but no pronounced effect of timing.
2.4. Temperature
Williams et al. measured 24 h core temperature measured by rectal thermometer in 20 ME/CFS (10 male and 10 female) patients aged 23–49 and 17 age and sex-matched controls (Williams et al., 1996). In the same subjects, plasma dim-light melatonin onset (DLMO) was measured in the laboratory. While no differences were identified in temperature rhythms or the timing of DLMO, the relationship between DLMO and temperature rhythm was significantly different in the two groups. While controls showed significant positive correlation between temperature acrophase and DLMO, this relationship was absent in the ME/CFS group. In a subsequent study of 10 ambulatory ME/CFS patients and 10 controls, core body temperature rhythms were monitored by telemetry over 48 h (Hamilos et al., 2001). Temperature rhythms in the ME/CFS group were again similar to controls, with the only observed differences being a trend towards more variability and more frequent ultradian rhythms among ME/CFS patients. The most recent study measured activity, sleep, light, and distal skin temperature measurements across days and seasons (Cambras et al., 2018). This study reported less light exposure in the ME/CFS group and irregular coupling of activity and temperature rhythms whereby ME/CFS patients lack the midday temperature increase observed in controls and show a distinct evening temperature drop. Therefore, as with activity and sleep cycles there is some indication of greater variability in rhythms and perhaps loss of synchronization and coupling across temperature, activity and/or melatonin rhythms. Notably, these data raise the possibility that inactivity may lead to rhythm disruptions through poor entrainment to environmental light. In this context, insufficient light exposure may be a primary mechanism underlying altered rhythms or a secondary factor that exacerbates existing endogenous rhythm deficits arising within cells.
2.5. Hypothalamic-pituitary axis
In a report on hypothalamic-pituitary axis (HPA) activity in 30 ME/CFS patients and 72 controls, reduced levels of evening cortisol and elevated ACTH levels in ME/CFS vs controls were reported. Measuring 24-h urine cortisol, the ME/CFS group also had reduced evening cortisol compared to controls (Demitrack et al., 1991). The study did not directly measure diurnal variation in either hormone. In a later study of cortisol and ACTH at two time points, 12 h apart in 30 ME/CFS patients and 15 controls, mean hormone levels did not vary by group at either time, but the diurnal change in cortisol was higher in controls compared to ME/CFS (MacHale et al., 1998). In a smaller study of 15 patients without co-morbid psychiatric disorders and 10 controls (Di Giorgio et al., 2005), Di Giorgio et al. found that upon repeated sampling over 24 h, ME/CFS patient had reduced ACTH in the morning vs controls and earlier acrophase. They found no difference in mean levels of cortisol, prolactin or growth hormone. Taken together, these findings indicate ME/CFS-associated differences in both cortisol and ACTH hormone, but most studies did not have the sample size or temporal resolution to fully assess 24 h hormone rhythms.
2.6. Heart rate and blood pressure
In a study of 17 CFS patients and 12 controls, circadian rhythms in heart rate, systolic and diastolic blood pressures showed significantly higher amplitudes in the patients vs controls leading to night time hypotension in the CFS group (van de Luit et al., 1998). These data indicate that circadian oscillations in heart rate and blood pressure may be exaggerated in ME/CFS causing more erratic diurnal fluctuations compared to healthy subjects.
2.7. Chronobiological interventions for ME/CFS
Given the realization that circadian disruption may play a role in ME/CFS, therapeutic chronobiological interventions have been attempted. Melatonin and light therapy were given individually in series for 12 weeks each (Williams et al., 2002). While unsuccessful in terms of overall symptom reduction, there was improvement in sleep after light therapy, despite the fact that the light intensity used (2500 lux) was lower than typically used clinically (10,000 lux). Interestingly, preferential response to melatonin over 3 months was observed in a subset of ME/CFS patients with phase delayed rhythms (van Heukelom et al., 2006). Exercise, especially graded exercise therapy has been attempted in ME/CFS and has been shown in some studies to have a beneficial impact on fatigue, sleep and mood (White et al., 2011; Larun et al., 2016). However, graded exercise may worsen symptoms of post-exertional fatigue in some patients and its role in treating ME/CSF is controversial (Wilshire et al., 2018; Larun et al., 2019). While the chronobiological implications of exercise remain unexplored, it is interesting to consider the possibility that the lack of activity characteristically observed in ME/CFS may contribute to rhythm desynchronization in peripheral tissues and that exercise may mitigate some of these negative effects (Youngstedt et al., 2019). Time restricted eating has been shown to be helpful across a range of health conditions and may show similar effects on peripheral rhythms (Longo and Panda, 2016). Future studies in these domains around exercise and lifestyle modification are needed.
3. Cytokine involvement in ME/CFS and circadian rhythms
3.1. Cytokine biomarkers studies of ME/CFS
Studies of ME/CFS have identified serum cytokines contributing to the disease phenotype and/or that correlate with severity. Both pro- and anti-inflammatory pathways have been implicated but very few markers show consistent experimental support (Blundell et al., 2015; Montoya et al., 2017). In a meta-analysis including data from 38 studies, 77 different cytokines have been examined in at least one study. The most commonly studied were the pro-inflammatory markers IL-1β, IL-6 and TNF. The majority of pro-inflammatory marker studies failed to identify any significant differences between controls and ME/CFS among these cytokines. However, a large study of 298 adult ME/CFS cases and 348 controls found differences in IL-1β, IL-6 and TNF and a number of other cytokines associated duration of illness, typically with more pronounced changes in early stages (<3 y) compared to chronic cases (Hornig et al., 2015). In other studies IL-1β, IL-6 and TNF were associated with severity of sleep disturbance and fatigue among ME/CFS cases (Milrad et al., 2017). In meta-analyses, the cytokine with the best support of involvement in ME/CFS was the peptide, transforming growth factor beta (TGFB) (Blundell et al., 2015). Support for increased TGFB in ME/CFS was reported in five out of eight studies and with no studies showing lower levels in ME/CFS (Blundell et al., 2015). In a subsequent large, independent study of 584 adults, the result was replicated with TGFB significantly increased in ME/CFS cases compared to controls (Montoya et al., 2017). Across most studies of ME/CFS cases, TGFB was not associated with severity of fatigue (Montoya et al., 2017; Jonsjo et al., 2020) or exercise (Clark et al., 2017), but was associated with symptoms of pain and cognitive processing deficits (Jonsjo et al., 2020). In mice, injection of TGFB into the lateral ventricles of the brain modeled several features of ME/CFS, showing reduced activity in the forced swim test, increased pain sensitivity, elevated peripheral markers of muscle fatigue and decreased striatal dopamine (Lee et al., 2021). However, the role of TGFB may differ in adults and children with ME/CFS. In adolescents with ME/CFS, TGFB was not statistically different from the control group, but was significantly correlated with stress biomarkers and fatigue scores (Wyller et al., 2017).
Resistin is only other marker that distinguished ME/CFS cases from controls in the largest human study (Montoya et al., 2017). Resistin is a hormone made by adipocytes with roles in energy metabolism and inflammation (Jamaluddin et al., 2012). However, 17 additional peptides, while not significantly different from controls in ME/CFS, were significantly associated with ME/CFS illness severity including several chemokines (CCL11, CXCL1, CXCL10), interleukin (IL) cytokines (IL-4, IL-5, IL-7, IL-12p70, IL-13, IL-17F, interferon-α), hormones (leptin), and growth factors (G-CSF, GM-CSF, LIF, NGF, TGFA, SCF) (Montoya et al., 2017).
3.2. Organization of the TGFB system
Given the totality of experimental support for association with ME/CFS, the potential role of TGFB in the chronobiology of ME/CFS will be considered in detail. TGFB is encoded from three different genes (TGFB1/2/3). The resulting peptides are highly similar with 71–80% identity in humans, but are structurally and functionally distinct with different cellular distribution and distinct biological roles in immune function, cell growth and development (Huang et al., 2014). TGFB is translated as latent pro-peptides that undergo enzymatic cleavage, and act upon target cells simultaneously through three receptors (TGFBR1, TGFBR2, TGFBR3). TGFB forms a complex with TGFBR3, which then binds TGFBR2. Upon receptor binding, dimerization of TGFBR1/2 occurs. Formation of the TGFB1/2/3 complex stimulates kinase activity in TGFBR1 that phosphorylates intracellular targets, including SMAD proteins (primarily SMAD1-4). Activated SMAD proteins are transported into the nucleus to form transcription factor complexes that regulate gene expression.
3.3. TGFB and the circadian clock
In the SCN and paraventricular nucleus, TGFB protein is rhythmically expressed in young mice but these rhythms are lost in older animals that show higher, constant levels of expression, suggesting a developmentally limited role in maintaining circadian rhythms in the central nervous system (CNS) (Beynon et al., 2009). In peripheral cells and blood, TGFB is upregulated by alkaline pH, activity and feeding (Kon et al., 2008; Han et al., 2020), and in human subjects, serum levels of TGFB1 were shown to have diurnal changes (Kong et al., 2006). TGFB peptides have been shown to act directly on the circadian clock in peripheral cells causing effects on clock gene expression. For instance, in response to alkaline pH, fibroblasts signal using TGFB and SMAD2/3 to upregulate DEC1 and phase shift circadian rhythms (Fig. 1), an effect can be reproduced by pulsatile treatment of cells for 1 h with recombinant TGFB1 peptide (Kon et al., 2008). In another study, fibroblasts and neurons were treated for longer periods of time (4–24 h) using TGFB2, a peptide associated with overexpression and cognitive disturbance in humans with dementia (Gast et al., 2012). In contrast to short exposures to TGFB1, longer, longer exposures (>4 h) to TGFB2 strongly inhibited clock gene expression (PER1, PER3, CRY1) in peripheral cells and neurons (Gast et al., 2012). These findings indicate that different TGFB peptides (TGFB 1 vs. TGFB 2) may play distinct roles in rhythms, perhaps depending on the duration of exposure and intensity of the TGFB signal.
Circadian rhythms can be observed in single cells and are therefore often considered to be cell autonomous. The SCN was previously thought to be unique in its ability to coordinate rhythms across cellular networks (Welsh et al., 2010). However, recent work shows that peripheral cells may also coordinate rhythms through networks mediated by paracrine signals and that TGFB is a key regulator of this intercellular synchronization in non-neuronal cells incapable of forming synapses (Finger et al., 2021). In U2OS cells expressing the Bmal:luc reporter, rhythm synchronization could be blocked by antibodies or small molecule inhibitors of TGFB signaling. In conditioned media showing synchronizing activity, TGFB2 was identified as an active component, and recombinant TGFB was found to strongly upregulate PER2 expression through a pathway involving the TGFBR1, SMAD3/4 and stimulation of cyclic-AMP responsive element (CRE) DNA binding sites (Fig. 1). Taken together, these data clearly indicate that TGFB peptides are capable of directing circadian rhythms. While both brain and peripheral clocks may be subject to regulation by TGFB signals, in vivo evidence in mice suggests that the brain effects are not prominent in adults, and that TGF may play a larger role in the synchronization of peripheral rhythms. Given the findings of increased TGFB in ME/CFS patient serum as well as rhythm findings characterized by uncoupling across systems and variability, alterations in TGFB signaling may be a strong candidate for future chronobiological studies of ME/CFS (Williams et al., 1996).
3.4. Other ME/CFS-associated cytokines and circadian rhythms
Resistin does not have any know direct influence on the circadian clock. However, in laboratory studies of humans undergoing circadian disruption, resistin levels have been shown to be increased and associated with elevated blood pressure and decreased vagal modulation of heart rate (Morris et al., 2016). Similar findings were observed in community studies of 439 shift workers with +18% increased resistin levels compared to 255 workers on a daytime work schedule (Burgueno et al., 2010). Among the cytokines linked with ME/CFS illness severity in high quality studies (Montoya et al., 2017), none have well substantiated roles as mediators of the cellular clock. However, as the immune system is tightly linked to the circadian clock (Scheiermann et al., 2018), many have potential points of overlap with circadian rhythms or may be markers of circadian disruption. For example, levels of IL-6 and IL-7 show circadian rhythms in blood and CSF and/or vary during sleep in humans (Benedict et al., 2007; Agorastos et al., 2014). The cytokine CXCL1 is increased in arrhythmic mutant mice (Narasimamurthy et al., 2012) and both G-CSF and CCL11 (Eotaxin-1) are increased by peripheral-central rhythm desynchronization in animal models (Oyama et al., 2014). These finding may indicate that illness severity markers in ME/CFS overlap at least in part with the effects of circadian disruption. Despite the similar names, TGFA is biologically distinct from TGFB. Conflicting evidence exists with respect to the role TGFA has in the circadian clock (Li et al., 2002; Lindley et al., 2008).
4. ME/CFS and coronavirus related illnesses
4.1. ME/CFS and SARS Coronvirus
Among survivors of the 2003 severe acute respiratory syndrome (SARS) pandemic, 27–40% of confirmed cases in Hong Kong met criteria for ME/CFS more than 3 years after recovery (Lam et al., 2009). Psychiatric disorders were common with 54% endorsing post-traumatic stress disorder (PTSD), 39% reporting depression, 36% reporting somatoform pain conditions and 34% reporting panic disorder. Survivors with a psychiatric disorder were more likely to endorse ME/CFS symptoms, but symptoms were common even among survivors without a psychiatric condition. A similar study from Canada described a strikingly high proportion of cases (22/273 total cases, 8% of total) who remained disabled by core symptoms of ME/CFS 13–36 months after infection (Moldofsky and Patcai, 2011). The authors did not make the criteria-based diagnoses of ME/CFS in this study, but symptoms of fatigue, myalgia, weakness, depression, and sleep abnormalities were commonly reported.
4.2. ME/CFS and COVID-19
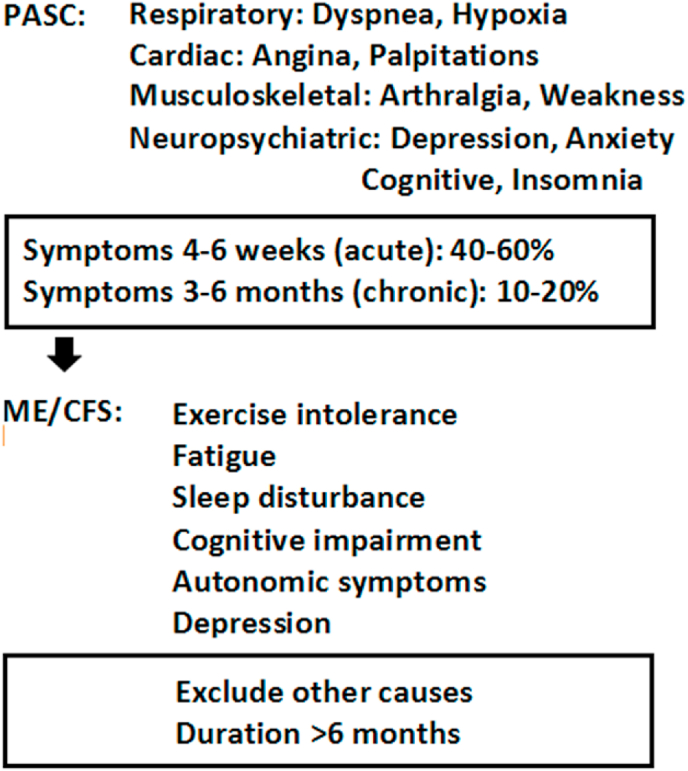
The current COVID-19 pandemic has affected hundreds of millions of people worldwide. While the majority of affected patients ultimately recover from the acute respiratory and febrile illness, many continue to suffer from disabling symptoms long after the virus has been cleared. Recent studies indicate that 40–60% of people with COVID-19 continue to have significant symptoms months after the initial illness (Fig. 2) (Ayoubkhani et al., 2021; Nalbandian et al., 2021). This syndrome of “long COVID-19” has been officially termed the post-acute sequalae of COVID-19 (PASC) (Nalbandian et al., 2021; Alwan, 2021). Among PASC patients, fibrotic and ischemic damage to the heart, lungs, brain and kidneys suffered during the acute illness may explain some of the lingering effects such as dyspnea, angina, palpitations, and kidney failure (Nalbandian et al., 2021). However, there exists a population of PASC patients who suffered only mild cases of COVID-19, who were never hospitalized, and never developed detectable organ damage. Disabling symptoms among these PASC patients include fatigue, exercise intolerance, sleep disruption, cognitive disturbances, autonomic instability and depression, symptoms that overlap with ME/CFS (Fig. 2) (Komaroff and Bateman, 2020). With the massive scale of the COVID-19 pandemic, it is expected that the incidence of PASC-associated ME/CFS is now growing rapidly (Komaroff and Bateman, 2020). To date, no studies have looked specifically at circadian rhythm disruption in ME/CFS following COVID-19 and/or PASC.
Fig. 2.
Progression from COVID-19 to PASC and ME/CFS. Acute COVID-19 illness is commonly resolved fully within 2–4 weeks of infection. For a variety of reasons, a significant number of recovering individuals continue to suffer symptoms 1–6 months after infection. This heterogeneous collection of patients are commonly diagnosed with “long COVID” or the post-acute sequalae of COVID-19 (PASC). A diagnosis of ME/CFS may be made for those who fail to recover by 6 months, have no objective medical explanation for their continuing symptoms and endorse fatigue, cognitive disturbances (“brain fog”), non-restorative sleep and psychiatric symptoms. Given the early stage of recovery for many with COVID-19 related illnesses and corresponding lack of data, it is not yet clear what fraction of people will progress from PASC to ME/CFS.
4.3. TGFB and the post-acute sequalae of COVID-19
To promote viral proliferation, SARS-CoV2 reduces the ability of infected cells to undergo apoptosis by altering TGFB signaling through a variety of direct and indirect mechanisms (Mirzaei and Faghihloo, 2018; Hamidi and KadamboorVeethil, 2021). SARS-CoV nucleocapsid protein alters TGFB signaling pathways by interacting directly with SMAD3-4 proteins (Zhao et al., 2008). In host tissue, SARS-COV2 upregulates expression of TGFB in peripheral blood mononuclear (Xiong et al., 2020), alveolar (Xu et al., 2020), and plasma cells (Ferreira-Gomes et al., 2021). Accordingly, TGFB has a well-established role as a driver of pathological tissue damage and has been proposed as a likely contributor to the long-term pathophysiological changes associated with PASC, particularly related to solid organ fibrosis (Wynn, 2008; Oronsky et al., 2021). In light of the information reviewed previously, TGFB is a plausible link between PASC and subsequent development of ME/CFS.
4.4. Implications of the post-inflammatory circadian model: progression from PASC to ME/CFS
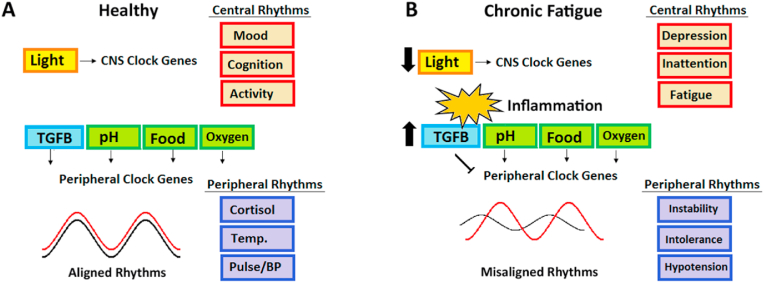
Beyond the putative role for TGFB in PASC, individuals with pre-existing circadian disruption may be at particular risk for developing ME/CFS (Fig. 3). Based on the circadian rhythm hypothesis of ME/CFS, we speculate that other factors in addition to TGFB may interact physiologically to increase the risk of developing ME/CFS in recovering COVID-19 patients with PASC. Among these are light exposure and hypoxia which are considered below.
Fig. 3.
Proposed model by which PASC-associated increases in TGFB may disrupt circadian rhythms, uncouple central and peripheral circadian rhythms and cause progression to ME/CFS. A) Healthy rhythms in the central nervous system (CNS) and peripheral organs are coordinated by multiple mechanisms that coincide with daily activity and routines to reinforce rhythms through multiple overlapping mechanisms. With optimal synchronization rhythmic processes in brain function (red) and physiology (purple) are timed and mutually reinforced. B) Post-inflammatory cytokine changes may increase levels of TGFB to disrupt the alignment of circadian rhythms, especially in peripheral organ systems. Other factors such as hypoxia and poor light exposure may further contribute to misalignment, and/or cause a negative feedback loop and further weakening central rhythms. Misalignment of CNS and peripheral physiology may lead to symptoms of ME/CFS including fatigue, inattention, depression, autonomic instability, cold intolerance and orthostatic hypotension. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4.5. Interaction with light and circadian rhythms in PASC
In an online patient survey of 640 people self-reporting PASC symptoms, Vitamin D deficiency was commonly reported in 13.4% of respondents (Assaf et al., 2020). However, this prevalence of Vitamin D deficiency falls within the range of estimates for general populations in industrialized nations (Bakaloudi and Chourdakis, 2022). A meta-analysis of SARS-CoV-2 infections confirmed that Vitamin D deficiency was associated with severe disease (Oscanoa et al., 2021), but prospective studies in 149 PASC patients followed a median of 79 days found no correlation of Vitamin D deficiency with fatigue (Townsend et al., 2021). Therefore, the link between Vitamin D and PASC is not supported by high quality evidence and is overall weak. An indirect link to Vitamin D could be mediated by insufficient light exposure. Light is the most potent entrainment signal for the circadian clock and low 25-OH Vitamin D is a proxy marker for inadequate light exposure (Brouwer-Brolsma et al., 2016). In a study of 25,534 individuals, 25-OH Vitamin D status alone did not predict sleep levels, but low 25-OH Vitamin D in addition to low light exposure was linked with excessive sleep duration (Choi et al., 2020). This difference in sleep may indicate the presence circadian disruption owing to inadequate light exposure that may be correlated in some cases with Vitamin D deficiency. The topic needs further study in PASC and in patients progressing to ME/CFS.
Recent research has shown that shift-workers are at increased risk for developing COVID-19, even after correcting for sleep duration (Maidstone et al., 2021). While this cohort of people has not yet been studied in terms of PASC and subsequent ME/CFS, the circadian disruption associated with shift work may conceivably make this group particularly vulnerable given their over-representation in the recovering population.
Hospitalization, especially the need for critical care has been associated with higher levels of developing PASC (Nalbandian et al., 2021). These patients are not only more likely to suffer organ damage from the severity of their illness, but may be affected by poor light conditions in intensive care units (ICU) incompatible with maintenance of a healthy circadian rhythm (Danielson et al., 2018). Ability to maintain physiological circadian rhythms has been associated with better outcomes in ICU (Davidson et al., 2021), and could in principle, protect patients from circadian disruption and possibly altering the risk of PASC and ME/CFS.
4.6. Interaction of hypoxemia and circadian rhythms in PASC
Oxygen consumption and metabolic release of carbon dioxide (CO2) follow circadian rhythms that are tightly coupled to activity (Adamovich et al., 2019). Interestingly, these rhythms are at least partly dissociable with oxygen rhythms following locomotor activity and CO2 rhythms following feeding schedules (Adamovich et al., 2019). Arrhythmic mutant mice lacking Per1/2 or Bmal1 show reduced amplitude of both oxygen and CO2 rhythms, while cellular rhythms respond to changes in ambient CO2 causing phase shifts similar to those observed with temperature pulses (Adamovich et al., 2019). Therefore, hypoxic episodes resulting in hypercapnia and/or changes in pH may cause phase shifts and rhythm misalignment across tissues (Kon et al., 2008; Adamovich et al., 2019; Manella et al., 2020). Recovering PASC patients commonly report dyspnea (Nalbandian et al., 2021). Circadian clocks in these patients with compromised lung function and post-inflammatory cytokines changes may therefore have multiple points of vulnerability. Future research in PASC and conversion to ME/CFS should investigate the role of hypoxia and hypercapnia as potential factors.
4.7. Agenda for future research
Key challenges in ME/CFS research revolve around phenotypic heterogeneity and uncertain etiology. While the COVID-19 pandemic has been a catastrophe for its victims and society, there exists an advantage to studying long-term PASC patients as a relatively homogenous cohort of ME/CFS cases with a known cause, widespread diagnostic testing and a well described and similarly timed illness course. Much of the work reviewed remains preliminary and our ability to draw firm conclusions regarding circadian rhythms in ME/CFS remains incomplete. Nonetheless, in light of the data reviewed, several testable hypotheses can be readily formulated. These include but are not limited to: 1) Cytokine factors in ME/CFS serum (primarily TGFB) have distinct effects on cellular circadian clocks 2) ME/CFS cases with greater circadian rhythm disruption have corresponding elevations in TGFB or other serum factors 3) Persistently elevated TGFB may be a biomarker predicting conversion from PASC to ME/CFS and 3) Circadian rhythm disturbances in ME/CFS may be amenable to interventions targeting TGFB and/or therapeutics that entrain circadian rhythms such as bright light therapy, melatonin, or carefully timed eating, and exercise schedules (“chronotherapuetics”). Future work in this area will provide important insight and is urgently needed.
5. Conclusions
The current COVID-19 pandemic has affected millions worldwide and created a large cohort of people with PASC, people who may be at high risk for converting to ME/CFS. This situation has created an urgent need to better understand the mechanisms underlying ME/CFS. Based on decades of data, circadian rhythm disruption is commonly observed in ME/CFS. The majority of this evidence suggests that while a total loss of rhythm is unlikely, circadian misalignment and/or more variability is common. Moreover, peripheral rhythms (e.g., temperature, heart rate, blood pressure) appear more strongly affected than central rhythms (motor control, cognition, affect). Sleep and activity are often affected in ME/CFS, but the circadian contribution to these processes is indirect and is hard to estimate based on available evidence. Previous studied may not have fully appreciated to cell-autonomous nature of peripheral clocks and ability to dissociate peripheral rhythms from central rhythms through the actions of cytokines, exercise, feeding, acidic/alkaline pH and hypoxia. In this context, the preliminary identification of TGFB as a cytokine biomarker has implications for the circadian rhythm hypothesis of ME/CFS. TGFB is not only elevated in ME/CFS but plays an important role in the synchronization of peripheral rhythms. With modern chronobiological methods, new avenues of ME/CFS research in TGFB and chronobiology is now approachable. By helping to identify risk factors for conversion to ME/CFS from PASC, insights from new work in this area may have immediate and direct relevance to the COVID-19 pandemic. If validated, circadian rhythms may be the target for interventions to prevent or reverse progression to ME/CFS and improve quality of life for the millions who survived COVID-19.
Funding
MJM is an employee of the Federal Government of the USA and receives research support from the Department of Veterans Affairs ORD/BLR&D (BX003431).
Role of the funding source
The opinions expressed in the paper solely reflect those of the author. The sponsors had no direct role in the formulation or preparation of the work or the decision to publish.
Declaration of competing interest
The author has no conflicts of interest to report.
Acknowledgements
Thanks to Liam Murphy who made helpful contributions to the organization of this material.
References
- Adamovich Y., Ladeuix B., Sobel J., Manella G., Neufeld-Cohen A., Assadi M.H., Golik M., Kuperman Y., Tarasiuk A., Koeners M.P., et al. Oxygen and carbon dioxide rhythms are circadian clock controlled and differentially directed by behavioral signals. Cell Metabol. 2019;29(5):1092–1103 e1093. doi: 10.1016/j.cmet.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Afari N., Buchwald D. Chronic fatigue syndrome: a review. Am. J. Psychiatr. 2003;160(2):221–236. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- Agorastos A., Hauger R.L., Barkauskas D.A., Moeller-Bertram T., Clopton P.L., Haji U., Lohr J.B., Geracioti T.D., Jr., Patel P.M., Chrousos G.P., et al. Circadian rhythmicity, variability and correlation of interleukin-6 levels in plasma and cerebrospinal fluid of healthy men. Psychoneuroendocrinology. 2014;44:71–82. doi: 10.1016/j.psyneuen.2014.02.020. [DOI] [PubMed] [Google Scholar]
- Akashi M., Soma H., Yamamoto T., Tsugitomi A., Yamashita S., Nishida E., Yasuda A., Liao J.K., Node K. Noninvasive method for assessing the human circadian clock using hair follicle cells. Proc. Natl. Acad. Sci. U. S. A. 2010;107(35):15643–15648. doi: 10.1073/pnas.1003878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwan N.A. The road to addressing Long Covid. Science. 2021;373(6554):491–493. doi: 10.1126/science.abg7113. [DOI] [PubMed] [Google Scholar]
- Antle M.C., Silver R. Circadian insights into motivated behavior. Curr. Top. Behav. Neurosci. 2016;27:137–169. doi: 10.1007/7854_2015_384. [DOI] [PubMed] [Google Scholar]
- Assaf G., Davis H., McCorkell L., Wei H., O'Neil B., Akrami A., Low R., Mercier J., Adetutu A. 2020. An analysis of the prolonged COVID-19 symptoms survey by patient-led research team; pp. 1–52. [Google Scholar]
- Ayoubkhani D., Khunti K., Nafilyan V., Maddox T., Humberstone B., Diamond I., Banerjee A. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaloudi D.R., Chourdakis M. A critical update on the role of mild and serious vitamin D deficiency prevalence and the COVID-19 epidemic in Europe. Nutrition. 2022;93:111441. doi: 10.1016/j.nut.2021.111441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C., Dimitrov S., Marshall L., Born J. Sleep enhances serum interleukin-7 concentrations in humans. Brain Behav. Immun. 2007;21(8):1058–1062. doi: 10.1016/j.bbi.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Beynon A.L., Thome J., Coogan A.N. Age and time of day influences on the expression of transforming growth factor-beta and phosphorylated SMAD3 in the mouse suprachiasmatic and paraventricular nuclei. Neuroimmunomodulation. 2009;16(6):392–399. doi: 10.1159/000228914. [DOI] [PubMed] [Google Scholar]
- Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. Mil. Med. 2015;180(7):721–723. doi: 10.7205/MILMED-D-15-00085. [DOI] [PubMed] [Google Scholar]
- Blundell S., Ray K.K., Buckland M., White P.D. Chronic fatigue syndrome and circulating cytokines: a systematic review. Brain Behav. Immun. 2015;50:186–195. doi: 10.1016/j.bbi.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Bonsall D.R., Harrington M.E. Circadian rhythm disruption in chronic fatigue syndrome. Adv. Neuroimmune Biol. 2013;4(4):265–274. [Google Scholar]
- Bou-Holaigah I., Rowe P.C., Kan J., Calkins H. The relationship between neurally mediated hypotension and the chronic fatigue syndrome. JAMA. 1995;274(12):961–967. [PubMed] [Google Scholar]
- Brouwer-Brolsma E.M., Vaes A.M.M., van der Zwaluw N.L., van Wijngaarden J.P., Swart K.M.A., Ham A.C., van Dijk S.C., Enneman A.W., Sohl E., van Schoor N.M., et al. Relative importance of summer sun exposure, vitamin D intake, and genes to vitamin D status in Dutch older adults: the B-PROOF study. J. Steroid Biochem. Mol. Biol. 2016;164:168–176. doi: 10.1016/j.jsbmb.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Buchwald D., Herrell R., Ashton S., Belcourt M., Schmaling K., Sullivan P., Neale M., Goldberg J. A twin study of chronic fatigue. Psychosom. Med. 2001;63(6):936–943. doi: 10.1097/00006842-200111000-00012. [DOI] [PubMed] [Google Scholar]
- Burgueno A., Gemma C., Gianotti T.F., Sookoian S., Pirola C.J. Increased levels of resistin in rotating shift workers: a potential mediator of cardiovascular risk associated with circadian misalignment. Atherosclerosis. 2010;210(2):625–629. doi: 10.1016/j.atherosclerosis.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Burke T.M., Scheer F., Ronda J.M., Czeisler C.A., Wright K.P., Jr. Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions. J. Sleep Res. 2015;24(4):364–371. doi: 10.1111/jsr.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambras T., Castro-Marrero J., Zaragoza M.C., Diez-Noguera A., Alegre J. Circadian rhythm abnormalities and autonomic dysfunction in patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.H., Lee B., Lee J.Y., Kim C.H., Park B., Kim D.Y., Kim H.J., Park D.Y. Relationship between sleep duration, sun exposure, and serum 25-hydroxyvitamin D status: a cross-sectional study. Sci. Rep. 2020;10(1):4168. doi: 10.1038/s41598-020-61061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L.V., Buckland M., Murphy G., Taylor N., Vleck V., Mein C., Wozniak E., Smuk M., White P.D. Cytokine responses to exercise and activity in patients with chronic fatigue syndrome: case-control study. Clin. Exp. Immunol. 2017;190(3):360–371. doi: 10.1111/cei.13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E.W. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: an IOM report on redefining an illness. JAMA. 2015;313(11):1101–1102. doi: 10.1001/jama.2015.1346. [DOI] [PubMed] [Google Scholar]
- Cockshell S.J., Mathias J.L. Cognitive functioning in chronic fatigue syndrome: a meta-analysis. Psychol. Med. 2010;40(8):1253–1267. doi: 10.1017/S0033291709992054. [DOI] [PubMed] [Google Scholar]
- Danielson S.J., Rappaport C.A., Loher M.K., Gehlbach B.K. Looking for light in the din: an examination of the circadian-disrupting properties of a medical intensive care unit. Intensive Crit. Care Nurs. 2018;46:57–63. doi: 10.1016/j.iccn.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S., Villarroel M., Harford M., Finnegan E., Jorge J., Young D., Watkinson P., Tarassenko L. Day-to-day progression of vital-sign circadian rhythms in the intensive care unit. Crit. Care. 2021;25(1):156. doi: 10.1186/s13054-021-03574-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demitrack M.A., Dale J.K., Straus S.E., Laue L., Listwak S.J., Kruesi M.J., Chrousos G.P., Gold P.W. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J. Clin. Endocrinol. Metab. 1991;73(6):1224–1234. doi: 10.1210/jcem-73-6-1224. [DOI] [PubMed] [Google Scholar]
- Ferreira-Gomes M., Kruglov A., Durek P., Heinrich F., Tizian C., Heinz G.A., Pascual-Reguant A., Du W., Mothes R., Fan C., et al. SARS-CoV-2 in severe COVID-19 induces a TGF-beta-dominated chronic immune response that does not target itself. Nat. Commun. 2021;12(1):1961. doi: 10.1038/s41467-021-22210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger A.M., Jaschke S., Del Olmo M., Hurwitz R., Granada A.E., Herzel H., Kramer A. Intercellular coupling between peripheral circadian oscillators by TGF-beta signaling. Sci. Adv. 2021;7(30) doi: 10.1126/sciadv.abg5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gast H., Gordic S., Petrzilka S., Lopez M., Muller A., Gietl A., Hock C., Birchler T., Fontana A. Transforming growth factor-beta inhibits the expression of clock genes. Ann. N. Y. Acad. Sci. 2012;1261:79–87. doi: 10.1111/j.1749-6632.2012.06640.x. [DOI] [PubMed] [Google Scholar]
- Di Giorgio A., Hudson M., Jerjes W., Cleare A.J. 24-hour pituitary and adrenal hormone profiles in chronic fatigue syndrome. Psychosom. Med. 2005;67(3):433–440. doi: 10.1097/01.psy.0000161206.55324.8a. [DOI] [PubMed] [Google Scholar]
- Hamidi S.H., Kadamboor Veethil S. Role of pirfenidone in TGF-beta pathways and other inflammatory pathways in acute respiratory syndrome coronavirus 2 (SARS-Cov-2) infection: a theoretical perspective. Pharmacol. Rep. 2021;73:712–727. doi: 10.1007/s43440-021-00255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilos D.L., Nutter D., Gershtenson J., Ikle D., Hamilos S.S., Redmond D.P., Di Clementi J.D., Schmaling K.B., Jones J.F. Circadian rhythm of core body temperature in subjects with chronic fatigue syndrome. Clin. Physiol. 2001;21(2):184–195. doi: 10.1046/j.1365-2281.2001.00321.x. [DOI] [PubMed] [Google Scholar]
- Han A.J., Alexander L.C., Jr., Huebner J.L., Reed A.B., Kraus V.B. Increase in free and total plasma TGF-beta1 following physical activity. Cartilage. 2020 doi: 10.1177/1947603520916523. 1947603520916523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heukelom R.O., Prins J.B., Smits M.G., Bleijenberg G. Influence of melatonin on fatigue severity in patients with chronic fatigue syndrome and late melatonin secretion. Eur. J. Neurol. 2006;13(1):55–60. doi: 10.1111/j.1468-1331.2006.01132.x. [DOI] [PubMed] [Google Scholar]
- Hickie I., Davenport T., Wakefield D., Vollmer-Conna U., Cameron B., Vernon S.D., Reeves W.C., Lloyd A. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333(7568):575. doi: 10.1136/bmj.38933.585764.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig M., Montoya J.G., Klimas N.G., Levine S., Felsenstein D., Bateman L., Peterson D.L., Gottschalk C.G., Schultz A.F., Che X., et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci. Adv. 2015;1(1) doi: 10.1126/sciadv.1400121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T., Schor S.L., Hinck A.P. Biological activity differences between TGF-beta1 and TGF-beta3 correlate with differences in the rigidity and arrangement of their component monomers. Biochemistry. 2014;53(36):5737–5749. doi: 10.1021/bi500647d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamaluddin M.S., Weakley S.M., Yao Q., Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br. J. Pharmacol. 2012;165(3):622–632. doi: 10.1111/j.1476-5381.2011.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jason L.A., Cotler J., Islam M.F., Sunnquist M., Katz B.Z. Risks for developing myalgic encephalomyelitis/chronic fatigue syndrome in college students following infectious mononucleosis: a prospective cohort study. Clin. Infect. Dis. 2021;73(11):e3740–e3746. doi: 10.1093/cid/ciaa1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsjo M.A., Olsson G.L., Wicksell R.K., Alving K., Holmstrom L., Andreasson A. The role of low-grade inflammation in ME/CFS (Myalgic Encephalomyelitis/Chronic Fatigue Syndrome) - associations with symptoms. Psychoneuroendocrinology. 2020;113:104578. doi: 10.1016/j.psyneuen.2019.104578. [DOI] [PubMed] [Google Scholar]
- Katz B.Z., Shiraishi Y., Mears C.J., Binns H.J., Taylor R. Chronic fatigue syndrome after infectious mononucleosis in adolescents. Pediatrics. 2009;124(1):189–193. doi: 10.1542/peds.2008-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaroff A.L., Bateman L. Will COVID-19 lead to myalgic encephalomyelitis/chronic fatigue syndrome? Front. Med. 2020;7:606824. doi: 10.3389/fmed.2020.606824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon N., Hirota T., Kawamoto T., Kato Y., Tsubota T., Fukada Y. Activation of TGF-beta/activin signalling resets the circadian clock through rapid induction of Dec1 transcripts. Nat. Cell Biol. 2008;10(12):1463–1469. doi: 10.1038/ncb1806. [DOI] [PubMed] [Google Scholar]
- Kong S.Y., Stabler T.V., Criscione L.G., Elliott A.L., Jordan J.M., Kraus V.B. Diurnal variation of serum and urine biomarkers in patients with radiographic knee osteoarthritis. Arthritis Rheum. 2006;54(8):2496–2504. doi: 10.1002/art.21977. [DOI] [PubMed] [Google Scholar]
- Lam M.H., Wing Y.K., Yu M.W., Leung C.M., Ma R.C., Kong A.P., So W.Y., Fong S.Y., Lam S.P. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch. Intern. Med. 2009;169(22):2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- Landgraf D., Long J.E., Proulx C.D., Barandas R., Malinow R., Welsh D.K. Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice. Biol. Psychiatr. 2016;80(11):827–835. doi: 10.1016/j.biopsych.2016.03.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larun L., Brurberg K.G., Odgaard-Jensen J., Price J.R. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst. Rev. 2016;2:CD003200. doi: 10.1002/14651858.CD003200.pub4. [DOI] [PubMed] [Google Scholar]
- Larun L., Brurberg K.G., Odgaard-Jensen J., Price J.R. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst. Rev. 2019;10:CD003200. doi: 10.1002/14651858.CD003200.pub8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie S.M., MacHale S.M., Cavanagh J.T., O'Carroll R.E., Goodwin G.M. The difference in patterns of motor and cognitive function in chronic fatigue syndrome and severe depressive illness. Psychol. Med. 2000;30(2):433–442. doi: 10.1017/s0033291799001816. [DOI] [PubMed] [Google Scholar]
- Lee W.K., Kim Y., Jang H., Sim J.H., Choi H.J., Shin Y., Choi J.J. Exogenous transforming growth factor-beta in brain-induced symptoms of central fatigue and suppressed dopamine production in mice. Int. J. Mol. Sci. 2021;22(5) doi: 10.3390/ijms22052580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Sankrithi N., Davis F.C. Transforming growth factor-alpha is expressed in astrocytes of the suprachiasmatic nucleus in hamster: role of glial cells in circadian clocks. Neuroreport. 2002;13(16):2143–2147. doi: 10.1097/00001756-200211150-00031. [DOI] [PubMed] [Google Scholar]
- Li J.Z., Bunney B.G., Meng F., Hagenauer M.H., Walsh D.M., Vawter M.P., Evans S.J., Choudary P.V., Cartagena P., Barchas J.D., et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc. Natl. Acad. Sci. U. S. A. 2013;110(24):9950–9955. doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley J., Deurveilher S., Rusak B., Semba K. Transforming growth factor-alpha and glial fibrillary acidic protein in the hamster circadian system: daily profile and cellular localization. Brain Res. 2008;1197:94–105. doi: 10.1016/j.brainres.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Longo V.D., Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metabol. 2016;23(6):1048–1059. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Luit L., van der Meulen J., Cleophas T.J., Zwinderman A.H. Amplified amplitudes of circadian rhythms and nighttime hypotension in patients with chronic fatigue syndrome: improvement by inopamil but not by melatonin. Angiology. 1998;49(11):903–908. doi: 10.1177/000331979804901105. [DOI] [PubMed] [Google Scholar]
- MacHale S.M., Cavanagh J.T., Bennie J., Carroll S., Goodwin G.M., Lawrie S.M. Diurnal variation of adrenocortical activity in chronic fatigue syndrome. Neuropsychobiology. 1998;38(4):213–217. doi: 10.1159/000026543. [DOI] [PubMed] [Google Scholar]
- Maidstone R., Anderson S.G., Ray D.W., Rutter M.K., Durrington H.J., Blaikley J.F. Shift work is associated with positive COVID-19 status in hospitalised patients. Thorax. 2021;76(6):601–606. doi: 10.1136/thoraxjnl-2020-216651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manella G., Aviram R., Bolshette N., Muvkadi S., Golik M., Smith D.F., Asher G. Hypoxia induces a time- and tissue-specific response that elicits intertissue circadian clock misalignment. Proc. Natl. Acad. Sci. U. S. A. 2020;117(1):779–786. doi: 10.1073/pnas.1914112117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M.J., Welsh D.K. Cellular circadian clocks in mood disorders. J. Biol. Rhythm. 2012;27(5):339–352. doi: 10.1177/0748730412456367. [DOI] [PubMed] [Google Scholar]
- Milrad S.F., Hall D.L., Jutagir D.R., Lattie E.G., Ironson G.H., Wohlgemuth W., Nunez M.V., Garcia L., Czaja S.J., Perdomo D.M., et al. Poor sleep quality is associated with greater circulating pro-inflammatory cytokines and severity and frequency of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) symptoms in women. J. Neuroimmunol. 2017;303:43–50. doi: 10.1016/j.jneuroim.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei H., Faghihloo E. Viruses as key modulators of the TGF-beta pathway; a double-edged sword involved in cancer. Rev. Med. Virol. 2018;28(2) doi: 10.1002/rmv.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk J.A., Green C.B., Takahashi J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldofsky H., Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011;11:37. doi: 10.1186/1471-2377-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya J.G., Holmes T.H., Anderson J.N., Maecker H.T., Rosenberg-Hasson Y., Valencia I.J., Chu L., Younger J.W., Tato C.M., Davis M.M. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc. Natl. Acad. Sci. U. S. A. 2017;114(34):E7150–E7158. doi: 10.1073/pnas.1710519114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J.H., Cho C.H., Son G.H., Geum D., Chung S., Kim H., Kang S.G., Park Y.M., Yoon H.K., Kim L., et al. Advanced circadian phase in mania and delayed circadian phase in mixed mania and depression returned to normal after treatment of bipolar disorder. EBioMedicine. 2016;11:285–295. doi: 10.1016/j.ebiom.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C.J., Purvis T.E., Hu K., Scheer F.A. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. U. S. A. 2016;113(10):E1402–E1411. doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G., Nicholas C.L., Kleiman J., Dwyer R., Carrington M.J., Allen N.B., Trinder J. Nature's clocks and human mood: the circadian system modulates reward motivation. Emotion. 2009;9(5):705–716. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimamurthy R., Hatori M., Nayak S.K., Liu F., Panda S., Verma I.M. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. U. S. A. 2012;109(31):12662–12667. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oronsky B., Larson C., Hammond T.C., Oronsky A., Kesari S., Lybeck M., Reid T.R. A review of persistent post-COVID syndrome (PPCS) Clin. Rev. Allergy Immunol. 2021 doi: 10.1007/s12016-021-08848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscanoa T.J., Amado J., Vidal X., Laird E., Ghashut R.A., Romero-Ortuno R. The relationship between the severity and mortality of SARS-CoV-2 infection and 25-hydroxyvitamin D concentration - a metaanalysis. Adv. Respir. Med. 2021;89(2):145–157. doi: 10.5603/ARM.a2021.0037. [DOI] [PubMed] [Google Scholar]
- Oyama Y., Iwasaka H., Koga H., Shingu C., Matsumoto S., Noguchi T. Uncoupling of peripheral and master clock gene rhythms by reversed feeding leads to an exacerbated inflammatory response after polymicrobial sepsis in mice. Shock. 2014;41(3):214–221. doi: 10.1097/SHK.0000000000000094. [DOI] [PubMed] [Google Scholar]
- Partch C.L., Green C.B., Takahashi J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24(2):90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast J.S., Yamazaki S. The mysterious food-entrainable oscillator: insights from mutant and engineered mouse models. J. Biol. Rhythm. 2018;33(5):458–474. doi: 10.1177/0748730418789043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman K., Burton A., Galbraith S., Lloyd A., Vollmer-Conna U. Sleep-wake behavior in chronic fatigue syndrome. Sleep. 2011;34(5):671–678. doi: 10.1093/sleep/34.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasa S., Nora-Krukle Z., Henning N., Eliassen E., Shikova E., Harrer T., Scheibenbogen C., Murovska M., Prusty B.K. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) J. Transl. Med. 2018;16(1):268. doi: 10.1186/s12967-018-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer F.A., Hilton M.F., Mantzoros C.S., Shea S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U. S. A. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C., Gibbs J., Ince L., Loudon A. Clocking in to immunity. Nat. Rev. Immunol. 2018;18(7):423–437. doi: 10.1038/s41577-018-0008-4. [DOI] [PubMed] [Google Scholar]
- Smith A.K., Fang H., Whistler T., Unger E.R., Rajeevan M.S. Convergent genomic studies identify association of GRIK2 and NPAS2 with chronic fatigue syndrome. Neuropsychobiology. 2011;64(4):183–194. doi: 10.1159/000326692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L., Dyer A.H., McCluskey P., O'Brien K., Dowds J., Laird E., Bannan C., Bourke N.M., Ni Cheallaigh C., Byrne D.G., et al. Investigating the relationship between vitamin D and persistent symptoms following SARS-CoV-2 infection. Nutrients. 2021;13(7) doi: 10.3390/nu13072430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryon W.W., Jason L., Frankenberry E., Torres-Harding S. Chronic fatigue syndrome impairs circadian rhythm of activity level. Physiol. Behav. 2004;82(5):849–853. doi: 10.1016/j.physbeh.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Valdez P. Circadian rhythms in attention. Yale J. Biol. Med. 2019;92(1):81–92. [PMC free article] [PubMed] [Google Scholar]
- Welsh D.K., Takahashi J.S., Kay S.A. Suprachiasmatic nucleus: cell autonomy and network properties. Annu. Rev. Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P.D., Goldsmith K.A., Johnson A.L., Potts L., Walwyn R., DeCesare J.C., Baber H.L., Burgess M., Clark L.V., Cox D.L., et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet. 2011;377(9768):823–836. doi: 10.1016/S0140-6736(11)60096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G., Pirmohamed J., Minors D., Waterhouse J., Buchan I., Arendt J., Edwards R.H. Dissociation of body-temperature and melatonin secretion circadian rhythms in patients with chronic fatigue syndrome. Clin. Physiol. 1996;16(4):327–337. doi: 10.1111/j.1475-097x.1996.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Williams G., Waterhouse J., Mugarza J., Minors D., Hayden K. Therapy of circadian rhythm disorders in chronic fatigue syndrome: no symptomatic improvement with melatonin or phototherapy. Eur. J. Clin. Invest. 2002;32(11):831–837. doi: 10.1046/j.1365-2362.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- Wilshire C.E., Kindlon T., Courtney R., Matthees A., Tuller D., Geraghty K., Levin B. Rethinking the treatment of chronic fatigue syndrome-a reanalysis and evaluation of findings from a recent major trial of graded exercise and CBT. BMC Psychol. 2018;6(1):6. doi: 10.1186/s40359-018-0218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood C., Magnello M.E., Sharpe M.C. Fluctuations in perceived energy and mood among patients with chronic fatigue syndrome. J. R. Soc. Med. 1992;85(4):195–198. doi: 10.1177/014107689208500405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyller V.B., Godang K., Morkrid L., Saul J.P., Thaulow E., Walloe L. Abnormal thermoregulatory responses in adolescents with chronic fatigue syndrome: relation to clinical symptoms. Pediatrics. 2007;120(1):e129–137. doi: 10.1542/peds.2006-2759. [DOI] [PubMed] [Google Scholar]
- Wyller V.B., Nguyen C.B., Ludviksen J.A., Mollnes T.E. Transforming growth factor beta (TGF-beta) in adolescent chronic fatigue syndrome. J. Transl. Med. 2017;15(1):245. doi: 10.1186/s12967-017-1350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microb. Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Xu X., Jiang L., Dua K., Hansbro P.M., Liu G. SARS-CoV-2 induces transcriptional signatures in human lung epithelial cells that promote lung fibrosis. Respir. Res. 2020;21(1):182. doi: 10.1186/s12931-020-01445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstedt S.D., Elliott J.A., Kripke D.F. Human circadian phase-response curves for exercise. J. Physiol. 2019;597(8):2253–2268. doi: 10.1113/JP276943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Nicholls J.M., Chen Y.G. Severe acute respiratory syndrome-associated coronavirus nucleocapsid protein interacts with Smad3 and modulates transforming growth factor-beta signaling. J. Biol. Chem. 2008;283(6):3272–3280. doi: 10.1074/jbc.M708033200. [DOI] [PMC free article] [PubMed] [Google Scholar]