Abstract
Ginsenoside Re is a protopanaxatriol-type saponin extracted from the berry, leaf, stem, flower bud, and root of Panax ginseng. In recent years, ginsenoside Re (Re) has been attracting attention as a dietary phytochemical. In this review, studies on Re were compiled by searching a combination of keywords, namely “pharmacology,” “pharmacokinetics,” and “toxicology,” in the Google Scholar, NCBI, PubMed, and Web of Science databases. The aim of this review was to provide an exhaustive overview of the pharmacological activities, pharmacokinetics, and toxicity of Re, focusing on clinical evidence that has shown effectiveness in specific diseases, such as diabetes mellitus, nervous system diseases, inflammation, cardiovascular disease, and cancer. Re is also known to eliminate virus, enhance the immune response, improve osteoporosis, improve skin barrier function, enhance intracellular anti-oxidant actions, regulate cholesterol metabolism, alleviate allergic responses, increase sperm motility, reduce erectile dysfunction, promote cyclic growth of hair follicles, and reduce gastrointestinal motility dysfunction. Furthermore, this review provides data on pharmacokinetic parameters and toxicological factors to examine the safety profile of Re. Such data will provide a theoretical basis and reference for Re-related studies and future applications.
Keywords: ginsenoside Re, pharmacological activities, pharmacokinetics, toxicology, bioactive component
Introduction
Ginseng is a perennial herb belonging to the family Araliaceae and genus Panax (P.). The plant has been used as a tonic in Chinese traditional medicine for more than 2000 years. It is also extensively used as a medicinal supplement across Asia and America (Jiang et al., 2020; Xu et al., 2020). P. ginseng Meyer (Asian ginseng), P. quinquefolium L. (American ginseng), and Eleutherococcus senticosus (Siberian ginseng) are the most common types of ginseng (Kiefer and Pantuso, 2003). All of these species are in the Araliaceae plant family. Extensive preclinical and clinical evidence in scientific literature support the significant beneficial effects of P. ginseng and P. quinquefolius L. in significant central nervous system, metabolic, infectious, and neoplastic diseases (Mancuso and Santangelo, 2017). Active components of most P. ginseng species include ginsenoside, polysaccharide, peptide, polyacetylenic alcohol and fatty acids (Dong et al., 2017). Of the active components, ginsenoside (i.e., ginseng saponin or triterpene saponin) is an important component responsible for many biochemical and pharmacological properties of the herb (Gillis, 1997). Currently, more than 30 natural ginsenosides have been extracted and their chemical structures have been identified. The main active ginsenosides are categorized into two groups based on the types of aglycone. The 20(S)-protopanaxadiol group includes ginsenosides Rb1, Rb2, Rb3, Rc, Rd, Rh2, compound K, and Rg3, and the 20(S)-protopanaxatriol group (PPT) comprises ginsenosides Re, Rf, Rg1, Rg2, and Rh1 (Ma et al., 2005). Of these, Re (C48H82O18, PubChem CID: 441921) is a major component (0.15%) of P. ginseng. We chose Re in the present study because of its high concentration in a number of commercially available P. ginseng extracts (Harkey et al., 2001). This water-soluble compound (Xie et al., 2005b) accounts for 23% of total saponins and is abundant in the leaves, stems, flower buds, berries, and roots of the plant (Joo et al., 2010; Bae et al., 2012; Kim et al., 2009). Previous research has shown that Re is more abundant in leaves, berries, and flower buds than in roots, and that it is the major saponin in P. ginseng fruits (Attele et al., 2002; Xie et al., 2004; Su et al., 2014). The percentage weight of Re extracts from American P. ginseng were 4.79, 3.5, and 0.4% in leaves, berries, and roots, respectively (Xie et al., 2005a; Han et al., 2012). This work showed that P. ginseng leaves and berries had the highest Re concentration, and that Re is the major ginsenoside in P. ginseng leaves. These findings also revealed that the Re content is different in various parts of the P. ginseng plant. In recent years, Re has been attracting attention as a dietary phytochemical, likely attributed to advantages such as ease of availability, low cost, high efficacy, straightforward isolation and purification techniques, and low side effects and toxicity risks (Quan et al., 2012). Re is a white crystalline powder that is readily soluble in methanol and ethanol. Its chemical properties include; melting point: 201–203°C; boiling point: 1011.8 ± 65.0°C; density: 1.38 ± 0.1 g/cm3; and acidity coefficient: 12.85 ± 0.70 (https://www.chemicalbook.com/ProductChemicalPropertiesCB5210824.htm). Previous research revealed in vivo and in vitro mechanisms that mediated diverse pharmacological activities of Re. Re has anti-diabetic (Table 1), neuroregulatory (Table 2), anti-inflammatory (Table 3), pro-cardiac (Table 4), anti-cancer (Table 5), anti-viral, anti-fungal and anti-oxidant effects. It is also known to improve skin barrier function, regulate cholesterol metabolism, alleviate allergic responses, enhance the immune response, improve osteoporosis, increase sperm motility, reduce erectile dysfunction, promote cyclic growth of hair follicles, and reduce gastrointestinal motility dysfunction (Table 6). In this review, the pharmacological actions and associated molecular mechanisms, pharmacokinetic characteristics, and toxicology of Re were summarized after researching major online databases. This review also describes the limitations of Re.
TABLE 1.
Summary of anti-diabetes effects of Re.
| Inducer | Experimental Model | Outcome and Proposed Mechanism | Reference(s) |
|---|---|---|---|
| C57BL/6J ob/ob mice | FBG↑, IPGTT↑ | Attele et al. (2002) | |
| C57BL/6J ob/ob mice | BG↑, FBG↑ | Xie et al. (2005a) | |
| HFD | Wistar rats | IR↑, GLUT4↑ | Han et al. (2012) |
| HFD, GPL | C57BL/6J mice, HepG2 cells | p-LKB1↑, p-AMPK↑, SHP↓, SREBP1c↓, FAS↓, SCD1↓ | Quan et al. (2012) |
| HFD, DII | Wistar rats, 3T3-L1 adipocytes | Glucose uptake↑, p-IRS-1↑, p-PI3K↑, Akt/PKCγ/λ↑, p-JNK↓, NF-κB↓ | Zhang et al. (2008) |
| HSHF; HSHF+AM; HSHF+STZ | Wistar rats | BG↓, TC↓, TG↓, Lp-a↓, VEGF↓, IL-6↓, p-p38↓,insulin levles↑, HDL-C↑ | Shi et al. (2016) |
| STZ | SD rats | BG↓, MDA↓, TC↓, TG↓, GSH↑ | Cho et al. (2006) |
| STZ | SD rats | FBG↓, TNF-α↓, MDA↓, GSH↑ | Liu et al. (2012) |
| HFD | C57BL/6 mice | TG↓, TC↓, LDL-C↓, GOT↓, GPT↓, MDA↓, p-JNK↓, p-IRS↓, p-tau↓, BG↑, HDL-C↑, Ach↑, GSH↑, SOD↑ | Kim et al. (2017) |
| HFD | C57BL/6 mice | FG↓, TG↓, TC↓, LDL-C↓, AChE↓, MDA↓ | Park et al. (2015) |
| DII | 3T3-L1 cells | Glucose uptake↑, GLUT4↑, IRS-1↑, PI3K↑ | Lee et al. (2011) |
| DII | 3T3-L1 cells | TNF-α↓,TG↑, Glucose uptake↑, PPARγ-2↑, ap2↑, IRS-1↑, GLUT4↑, Adiponectin↑ | Gao et al. (2013) |
| High glucose | RF/6A cells | LDH↓, MDA↓, p-Akt↓,ROS↑, CAT↑, GSH-Px↑, HIF-1α↑, Caspase-3↑, VEGF↑, Caspase-9↑ | Xie et al. (2020) |
TABLE 2.
Summary of nervous system disease effects of Re.
| Inducer | Experimental Model | Outcome and Proposed Mechanism | Reference(s) |
|---|---|---|---|
| Surgery | SD rats, Schwann cell | PCNA↑, GAP-43↑, S100↑, p-ERK1/2↓, p-JNK1/2↓ | Wang et al. (2015) |
| MCAO model | SD rats | SOD↑, GSH-Px↑, Average microviscosity↓, MDA↓ | Zhou et al. (2006) |
| MCAO model | SD rats | H+-ATPase activity↑, MDA↓ | Chen et al. (2008) |
| TMT | IL-6(−/+) C57BL/6 mice | c-FOS-IR↑, IL-6↑, p-Akt↑, IFN-γ↓, TNF-α↓, IL-1β↓, MDA↓, ROS↓ | Tu et al. (2017) |
| PCP | C57BL/6mice, GPx-1 knockout mice | GPx-1↑, PHOX activity↑ | Tran et al. (2017) |
| RIS | SD rats | BDNF↑, Behavioral deficits↓, TH↓ | Lee I et al. (2012) |
| CRS | C57BL/6J mice | BDNF↑, Nrf2↑, HO-1↑, SYP↑, PSD95↑, NLRP3↓, ASC↓, Caspase-1↓ | Wang et al. (2021) |
| MPTP | C57BL mice | Bcl-2↑, iNOS↑, caspase-3↑, TH-positive neurons↑, Bax↓ | Xu et al. (2005) |
| MA | PKCδ(+/−) C57BL/6 mice | SOD↑, catalase↑, GPx↑, DA↑, dopaminergic degeneration↓, PKCδ↓ | Shin et al. (2014) |
| MA | DYN KO mice | κ-opioid receptor↓, P-mediated NK1 receptor↓ | Dang et al. (2018) |
| CCl4 | Primary dopaminergic cell | Neurites of TH cells↑, Neuritic lengths↓ | Zhang et al. (2016) |
| MA | SH-SY5Y cell | Cell viability↑, GPx↑, GSH↑, TH activity↑, PKCδ↓ | Nam et al. (2015) |
| Dopaminergic neuronal cell, Hsp60 KD cell, PINK1 null dopaminergic cell lines | Hsp90↑, LRPPRC↑, Hsp60↑ | Kim et al. (2012) | |
| Rotenone | SH-SY5Y cells | SOD↑, GSH/GSSG↑, aconitase↑, Nrf2↑, ROS↓, Caspase-3↓, Bax/Bcl2↓, Cytochrome c↓ | Gonzalez-Burgos et al. (2017) |
| 6-OHDA | SH-SY5Y cells | Cell viability↑, GPX4↑, p-Akt↑, p-ERK↑, LDH↓, ROS↓, lipid peroxidation↓ | Lee et al. (2020) |
| Scopolamine | CR mice, Wistar rats | Escape latency↓ | Wang et al. (2010) |
| Tg2576 mice | Aβ-40↓, Aβ-42↓ | Zhou et al. (2020) | |
| CHO 2B7 cells, Aβ-lesioned mice | Aβ-40↓, Aβ-42↓ | Chen et al. (2006) | |
| Aβ-25-35 peptide | Kunming mice | phenylalanine↓, tryptophan↑, hexadecasphinganine↑, phytosphingosine↑, LPCs↑ | Li et al. (2018) |
| Surgery and microdialysis | SD rats | DA↑, Ach↑, mPFC | Shi et al. (2013) |
| N2a/APP695 cells | PPARγ↑, Aβ1-40↓, Aβ1-42↓, β-amyloid, BACE1↓ | Cao et al. (2016) | |
| Aβ+serum free | PC12 cells | LDH↓, cell toxicity↓ | Ji et al. (2006) |
| Aβ | SH-SY5Y cells | GSH↑, SOD↑, GPx↑, ROS↓, Bcl2/Bax↓, Nrf2↓, Caspase-3/9↓, Cytochrome c↓, p-ASK-1↓, p-JNK↓, HO-1↓ | Liu et al. (2019) |
| Neuro-2a cells | MAP-2↑, p75↑, p21↑, TrkA↑, ChAT/VAChT↑ | Kim M et al. (2014) |
TABLE 3.
Summary of anti-inflammation effects of Re.
| Inducer | Experimental Model | Outcome and Proposed Mechanism | Reference(s) |
|---|---|---|---|
| C48/80, LPS | HMC-1 cell, A549 cell | Histamine secretion↓, IL-1α↓, IL-8↓, IL-10↓, RANTES↓ | Bae et al. (2012) |
| TPA | BALB/c mice, Raw 264.7 cells | NO↓, MDA↓, ear edema↓, inflammatory cell infiltration↓, IL-1β↓, TNF-α↓ | Paul et al. (2012) |
| LPS | SD rats, BALB/c mice, RAW264.7 cells | WBCs↑, neutrophil counts↑, TNF-α↓, IL-1β↓, IL-6↓, COX-2↓, iNOS↓, NO production↓, PGE2↓ | Su et al. (2015) |
| LPS, TNBS | ICR mice | ZO-1↑, claudin-1↑, occludin↑, IL-1β↓, TNF-α↓, COX-2↓, iNOS↓, IL-6↓, colon shortening↓ | Lee J et al. (2012) |
| LPS | C57BL/6 mice | ERs↑, PI3K/Akt↑, INF-γ↓, MCP-1↓, LDH↓, CK↓, AST↓, TNF-α↓, IL-1β↓, IL-6↓, p-p65↓, MAPKs↓ | Chen et al. (2016) |
| LPS | ICR mice, A549, MH-S cells | Neutrophil↓, macrophage infiltration↓, NF-κB↓, MAPKs↓, c-Fos↓ | Lee et al. (2018) |
| LPS | N9 microglia cells | NO↓, TNF-α↓, NF-κB↓, p-ERK↓, p-JNK↓, p-jun↓, p-IκB-α↓ | Wu et al. (2007)) |
| LPS | BV2 microglial cells | Cell viability↑, iNOS↓, COX-2↓, p-P38↓ | Lee K et al. (2012) |
| LPS | RAW264.7 cells and primary rat hepatocytes | TNF-α↓, IL-6↓, PGE2↓, NO secreation↓, MAPKs↓, NF-κB↓ | Quan et al. (2019) |
| TNF-α | EAhy926, HEK 293 cells | Cell viability↑, LDH↓, IL-6↓, p-IKK/IKK↓, p-IκB↓, p-NF-κB↓ | Li Z et al. (2016) |
TABLE 4.
Summary of cardiovascular disease effects of Re.
| Inducer | Experimental Model | Outcome and Proposed Mechanism | Reference(s) |
|---|---|---|---|
| I/R | SD rats | Haemodynamic change↑, [Ca2 +]i↓ | Kim et al. (2011) |
| Cardiomyocytes, Guinea pig ventricular myocytes | I(Ks) ↑, I(Ca,L) ↓ | Bai et al. (2003), Bai et al. (2004) | |
| LADCA ligation | Wistar rats, SD rat | Infarct size↓, MPO↓, PMN infiltration↓, ICAM-1↓ | Jing et al. (2010), Li et al. (2013) |
| I/R | SD rats | Hemodynamic parameter↑, QRS complex↓, QT interval↓, R-R interval↓, TNF-α↓ | Lim et al. (2013) |
| Isoproterenol | Wistar rats | TGF-β↓, p-Smad3↓, collagen I↓ | Wang et al. (2019) |
| MI | SD rats | Heart rate↑, LVEF↑, LVPWd↑, LVPWs↑, IVSTd↑, IVSTs↑, SOD↑, FAK↑, PI3K↑, Akt↑, AMPKα↑, LVDd↓, LVDs↓, EDV↓, ESV↓, CK-MB↓, cTnT↓, MDA↓, Ang II↓, ANP↓, BNP↓, TGF-β1↓, Smad↓ | Yu et al. (2020) |
| tBHP, MI/R | H9c2 cells, SD rats | miR-30c-5p↑, Apoptosis↓, LDH↓, p53↓ | Wang et al. (2020) |
| GD | H9c2 cells | Cell viability↑, SOD↑, ATP depletion↑, LC3B-2↑, MDA↓ | Zhang et al. (2020) |
| H/R | HL-1 cells | Cell viability↑, ATP Levels↑, LC3B-2↑, p-AMPK↑ | Sun et al. (2020) |
| Cat and human cardiomyocytes | [Ca2+]i transient amplitude↑, Sarcoplasmic reticulum Ca2+ content↓ | Wang et al. (2008b) | |
| Guinea pig ventricular myocytes | IKs↑, eNOS↑, PI3K↑, Akt↑ | Furukawa et al. (2006) | |
| VSMCs | KCa↑, eNOS↑, PI3K↑, Akt↑ | Nakaya et al. (2007) | |
| HUVEC | [Ca2+]i↑, NO↑, eNOS↑ | Leung et al. (2007) | |
| HCAEC | Outward currents↑, SKCa currents↑ | Sukrittanon et al. (2014) | |
| Balloon | SD rats | vessel lumen↑, NO↑, cGMP↑, eNOS↑, PCNA positive cells↓ | Gao et al. (2018) |
| PDGF-BB | VSMCs | cGMP↑, NO↑, p-eNOS/eNOS↑, p21↑, PCNA↓, cyclin D1↓, CDK4↓ | Gao et al. (2019) |
| H2O2 | HUVECs | NO↑, eNOS↑, SOD↑, GSH-Px↑, LDH↓, MDA↓ | Huang et al. (2016) |
| Ox-LDL | HUVECs | ERα↑, PI3K↑, PKB↑, LOX-1↓, NADPH oxidase↓, NF-κB↓, p-p38↓ | Yang et al. (2018) |
| bFGF | HUVECs, Wistar rats | Cell proliferation↑, hemoglobin content in ECMs↑, migration, tube formation↑, neo-collagen regenerate↑ | Huang et al. (2005) |
| bFGF, Matrigel | HUVECs, C57/BL6 mice | Cell proliferation and migration↑, tube formation↑, neo-vessels density↓ | Yu et al. (2007) |
TABLE 5.
Summary anti-cancer effects of Re.
| Inducer | Experimental Model | Outcome and Proposed Mechanism | Reference(s) |
|---|---|---|---|
| CDDP | LLC-PK1 cells, Wistar rats | Cell viability↑, DPPH radical-scavenging activity↑, Caspase-3↑, Renal cortex tissue tubular damage↓ | Lee W et al. (2012), |
| Kim J et al. (2014) | |||
| CDDP | ICR mice | CAT↑, GSH↑, Bcl2/Bax↑, CRE↓, BUN↓, MDA↓, 4-HNE↓, CYP2E1↓, COX-2↓, iNOS | Wang et al. (2018c) |
| CTX | BALB/c mice | Erythropoietin↑, thrombopoietin↑, TPO↑, RBCs↑, hemoglobin↑, platelets S phase↑, Bcl-2↑, WBCs↓, thymus index↓, BMNC↓, spleen index↓, Bax↓, Caspase-3↓ | Han et al. (2019) |
| SW480 cells | Apoptosis↑, Cell proliferation↓ | Xie et al. (2011) | |
| 293T, MCF-7, A375, HepG2 cells | LDH release↑, Cell viability↓, ROS↓, Caspase-3↓ | Yao et al. (2018) |
TABLE 6.
Summary of other disease effects of Re.
| Effect | Experimental Model | Outcome and Proposed Mechanism | Reference(s) |
|---|---|---|---|
| Anti-viral | CVB3, and HRV3 infection HeLa and Vero cells | Cytotoxicity↓ | Song et al. (2014) |
| Anti-viral and immune response | RV-induced ICR mice | Splenocyte proliferative↑, IL-4↑, IL-10↑, IL-12↑, IFN-γ↑, CD4+ cells↓, CD8+ cells↓ | Su et al. (2014) |
| H3N2-induced ICR mice | Th1↑, Th2↑ | Song et al. (2010) | |
| Anti-viral | Avian influenza H9N2 infected HUVEC cells | miR-15b↑, Cell viability↑, IP-10↓, DNA damage↓ | Chan et al. (2011) |
| Immune response | CD4+ T cells | Cell viability↑, IFN-γ↓, IL-13↓, IRGM↓ | Son et al. (2010) |
| OVA-induced ICR mice | Th1↑, Th2↑ | Sun et al. (2006) | |
| Osteoblast differentiation | RANKL-induced Zebrafish | ERK↓, TRAP↓, cathepsin K↓ | Feng and McDonald, (2011) |
| MC3T3-E1 cells and Zebrafish model | ALP↑, Runx2↑, Colla1↑, Alp↑, Ocn↑ | Park et al. (2016) | |
| Against UVB radiation | UVB-induced HaCaT keratinocytes | GSH↑, SOD↑, ROS↓, MMP-2↓, MMP-9↓ | Kim et al. (2016) |
| Improve skin barrier function | HaCaT keratinocytes | Filaggrin↑, Cornified envelope formation↑, Caspase-14↑ | Shin et al. (2018) |
| Anti-oxidant | HaCaT keratinocytes | GSH↑, SOD↑, ROS, MMP-2↓, MMP-9↓ | Oh et al. (2016) |
| H2O2-induced E.coli | Fpg↑, ROS↓ | Lim et al. (2016) | |
| H2O2 or ATA-induced chick cardiomyocytes | Cell viability↑, DCF fluorescence↓ | Lee B et al. (2012) | |
| Regulating Cholesterol Metabolism | High cholesterol-induced Wistar rats | CYP8B1↑ | Kawase et al. (2014) |
| Alleviating allergic response | Histamine-induced ICR mice | IL-4↓, TNF-α↓, NF-κB↓, c-jun↓ | Jang et al. (2012) |
| Increasing sperm motility | Fertile volunteer, Asthenozoospermic infertile patients | iNOS↑, NO↑ | Zhang et al. (2006) |
| Restoring erectile dysfunction | Ethanol-induced SD rats | Nitrite↑, cGMP↑, ICP↑ | Pyo et al. (20I6) |
| Promoting cyclic growth of hair follicles | Immunodeficient mice, C57BL/6 mice, HeLa cells | Hair shaft growth↑, P-Smad 2/3↑, p-FAK↑, p-ERK↑, p-JNK↑, TGF-β↓, SAMD↓ | Li et al. (20I6) |
| Reducing gastrointestinal motility dysfunction | CP SD rats, DP SD rats | p-MLC20↑, MLCK↓, NO↑, adrenaline↑ | Xiong et al. (2014) |
| Cajal interstitial cells | Amplitude↓, frequency↓, cGMP↑ | Hong et al. (2015) | |
| C48/80-induced Wistar rats | Hexosamine↑, adherent mucus↑, TBARS↓, XO↓, MPO↓, Bax↓, Bcl2↑ | Lee et al. (2014) |
Pharmacokinetics of Re
Pharmacokinetic studies are necessary for observing and predicting the actions and interactions of drugs and for determining their efficacy and toxicity. The pharmacokinetics of Re have been studied in both animals and humans (Table 7), with major parameters, such as maximum concentration (Tmax), T1/2, and bioavailability examined. However, there is still little known about its metabolic and pharmacokinetic profiles.
TABLE 7.
The main pharmacokinetic parameters of Re.
| Route Adminstration | Dose | Model | Parameters | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (0-t) (ng/ml·h) | AUC (0-∞) (ng/ml·h) | T½ (h) | Tmax (h) | Cmax (ng/ml) | MRT (h) | Vd (L/kg) | CL (L/h/kg) | RC | f (%) | F (%) | ||||
| i.v. | 1 mg/kg | ICR mice (♀) | 638.8 ± 197.0 | 639.3 ± 196.8 | 0.2 ± 0.03 | — | — | 0.2 ± 0.07 | 0.3 ± 0.2 | 1.7 ± 0.7 | — | — | — | Joo et al. (2010) |
| 1 mg/kg | ICR mice (♂) | 1437.6 ± 271.2 | 1442.0 ± 271.0 | 0.5 ± 0.08 | — | — | 0.5 ± 0.08 | 0.2 ± 0.07 | 0.7 ± 0.11 | — | — | — | ||
| p.o. | 10 mg/kg | ICR mice | — | 17.7 ± 4.5 | — | 0.4 ± 0.2 | 29 ± 25.4 | 0.76 ± 0.20 | — | — | — | — | 0.28 | |
| 50 mg/kg | — | 61.5 ± 37.0 | — | 0.7 ± 0.7 | 35 ± 4.3 | 2.0 ± 1.2 | — | — | — | — | 0.19 | |||
| p.o. | 200 mg | Healthy volunteers | 2.476 ± 2.281 | 2.699 ± 2.284 | 1.82 ± 0.75 | 1.19 ± 0.44 | 0.939 ± 0.549 | — | — | 124.054 ± 84.725 | — | — | — | Liu et al. (2011) |
| i.v. | 152.91 mg/kg | Rabbits | — | — | 0.83 | — | — | — | 0.246 | — | 0.61 | 17 | — | Chen et al. (1980) |
| i.p. | 1.165 | — | 0.72 | 18 | 35 | |||||||||
| s.c. | 12.5 mg/kg | SD rats | 2.771 | 2.963 | 2.399 | 1 | 0.56 | — | — | — | — | — | — | Shi et al. (2013) |
| 25 mg/kg | 6.328 | 8.073 | 2.531 | 1 | 2.19 | — | — | — | — | — | — | |||
| 50 mg/kg | 12.630 | 14.295 | 2.157 | 1 | 3.72 | — | — | — | — | — | — | |||
| p.o. | 200 mg/kg | SD rats | 9,896.68 ± 1,234.48 | 11,830.85 ± 2,366.47 | 8.343 ± 6.148 | 0.9 ± 0.22 | 1,703.85 ± 104.15 | 14.924 ± 5.205 | 250.73 ± 159.7 | 0.32 ± 0.044 | — | — | — | Chen et al. (2017) |
| p.o. | 800 mg/kg XSTDT | SD rats | 6 × 105 ± 1 × 105 | 6 × 105 ± 1 × 105 | 6 ± 3 | 6 ± 1 | 6 × 104 ± 2 × 104 | 8.6 ± 2.2 | 12.9 ± 3.5 | 1.45 ± 0.58 | — | — | — | Dai et al. (2016) |
| p.o. | 600 mg/kg QXSBP | SD rats | 823.15 ± 97.94 | 958.34 ± 157.26 | 1.71 ± 0.39 | 0.56 ± 0.10 | 412.35 ± 89.16 | — | — | — | — | — | — | Chen et al. (2021) |
| 60 mg/kg QXSBP | 1,764.19 ± 265.38 | 1,906.79 ± 239.45 | 1.32 ± 0.38 | 0.50 ± 0.16 | 867.69 ± 103.29 | |||||||||
| i.v. | 5 ml/kg GGSQ | SD rats | 2.16 × 106 ± 0.59 × 106 | 2.24 × 106 ± 0.76 × 106 | 2.25 ± 0.84 | — | — | 1.4 ± 0.65 | 39.08 ± 5.21 | — | — | — | — | Ji et al. (2017) |
| i.v. | 7.2 ml/kg SFI | SD rats | 639.70 ± 134.61 | 653.77 ± 121.07 | 0.14 ± 0.03 | — | 3176.44 ± 515.91 | 0.18 ± 0.03 | 0.29 ± 0.04 | 1.48 ± 0.28 | — | — | — | Shen et al. (2021) |
Absorption and Distribution
The time for saponins to reach Tmax in rat plasma was less than 2 h, indicating that saponins are rapidly absorbed and readily distributed in tissues (Li et al., 2006; Gui et al., 2007). In humans, Liu et al. (2011) reported that the Tmax of Re was 1.19 ± 0.44 h after oral ingestion. Another study showed that the Tmax of Re was 0.75 h after oral administration of total P. notoginsenoside powder in rats, suggesting rapid absorption of Re in the gastrointestinal tract. The absolute bioavailability of Re was 7.06% (Li et al., 2006). Joo et al. (2010) revealed that the Tmax of Re was 0.4 ± 0.2 h in ICR mice. The same study also showed that the oral bioavailability was 0.19–0.28%, suggesting that the absorption rate of Re was lower after oral administration. Shi et al. (2013) demonstrated that Re (12.5, 25 and 50 mg/kg, s.c. injection) was rapidly distributed to the cerebrospinal fluid and exhibited linear pharmacokinetics in rats, and that the Tmax of Re was 1 h for all doses. However, for the lowest dose of 12.5 mg/kg, Re was not detectable in dialysates after 4 h. Extensive gastrointestinal metabolism, poor membrane permeability, and low solubility of deglycosylated products may limit the absorption of ginsenosides in the intestines. Therefore, the dose of test compounds must be high to detect ginsenoside content in plasma (Qi et al., 2011).
Metabolism and Biotransformation
According to preclinical trials, several types of saponins, including ginsenosides Rg2, Rh1, F1, Rg1, and protopanaxatriol, may be metabolites of Re in human plasma and urine samples (Liu et al., 2011). After administration of Re (200 mg/kg, p.o. for 24 h), the major excreted ginsenoside metabolites in rat urine included Rg1 and Re. In feces, the main metabolite was Rg1, but other deglycosylated metabolites, including F1 and protopanaxatriol, were also detected (Kim et al., 2013). Yang et al. (2009) identified 11 and nine metabolites together with Re in rat urine collected after intravenous (50 mg/kg, i.v.) and oral (100 mg/kg, p.o.) administration of Re, respectively. The metabolites included Rg1, Rg2, Rh1, and F1. Oral and intravenous doses of Re showed distinct metabolism patterns in the rat, but there were also certain characteristics in common. Deglycosylation was found to be the major metabolic pathway of Re in rats, indicating that a large part of Re was metabolized and transformed in the gastrointestinal tract to ginsenosides with more biological effects (Christensen, 2009). The Re may be metabolized into ginsenosides Rh1 and F1 by human intestinal microflora, and subsequently absorbed into the blood (Bae et al., 2005). After oral administration of 100 mg/kg Re to rats, Chen et al. (2009) detected six metabolites of Re in feces, including ginsenosides Rg2, Rh1, Rh1, F1, Rh1, and PPT. In general, Re may be hydrolyzed by gastric fluids to ginsenoside Rg2 that is then converted in the intestine into ginsenoside Rh1 by the elimination of rhamnose through intestinal bacteria. Intact Re also reaches the large intestine where it can be metabolized by bacteria into ginsenoside F1 and 20(S)-PPT via ginsenoside Rg1. Like intestinal bacteria, several food microorganisms produce specific forms of ginsenosides. (Chi and Ji, 2005) tested the biotransformation of Re by cell extracts from various food-grade edible microorganisms, and found Re was transformed into Rh1 via Rg2 by Bifidobacterium sp. Int57 and SJ32, Re was transformed into Rh1 via Rg1 by Aspergillus niger KCTC 6906, and Re was transformed into Rg2 by A. usamii var. shirousamii KCTC 6956.
Elimination
Joo et al. (2010) found that Re was rapidly cleared from the bodies of male or female mice within 0.2 ± 0.03 and 0.5 ± 0.08 h, respectively, after intravenous administration. Chen et al. (1980) estimated that the half-life of Ren in rabbits, after intravenous administration, was about 0.83 h, and the elimination half-life of Re after i.p. injection could be measured from urine (1.165 h) but not plasma samples. In healthy volunteers, the half-life of Re after oral ingestion of Re tablets (200 mg/tablets, p.o.) was reported to be 1.82 ± 0.75 h (Liu et al., 2011). A randomized, double-blind, placebo-controlled trial reported that researchers were unable to detect Re in plasma of obese adults, even though the subjects were prescribed large daily oral doses of P. ginseng and Re for 30 days and ingested the last dose 30 min before collection of blood samples to assess Re concentrations. The absence of Re may be explained by the quick elimination of ginsenoside (Reeds et al., 2011). Pharmacokinetic studies of Re in rats and human volunteers were consistent with this statement. After intragastric (i.g.) administration of Banxia Xiexin Decoction in rats, plasma concentrations of Re at most time points were lower than the lower limit of quantification (Wang et al., 2008a). Pharmacokinetic studies of Re in rats and volunteers following i.v. administration of Shen Mai indicated that Re was quickly eliminated in the body, and that pharmacokinetic characteristics fitted the two-compartment model (Liu et al., 2005; Xia et al., 2008). Altogether, evidence from pharmacokinetic and metabolic studies of Re demonstrated that 1) the absorption of Re was fast in the gastrointestinal tract; 2) Re may be metabolized mainly into Rh1 and F1 by intestinal microflora before absorption into blood; and 3) Re was quickly cleared from the body (Peng et al., 2012).
Search Method
We included articles that were published from January 2000 to March 2021. Because more than 344 articles were found, we opted to focus on those specifically pertaining to new reports of the pharmacology, pharmacokinetics, and toxicology of Re. We searched four electronic databases, Google Scholar, NCBI, PubMed, and Web of Science, and compiled data according to the grade of evidence that was found. Systematic searches were performed in four electronic databases and the reference lists of most papers in the past 20 years were checked for further relevant publications. All articles containing original data on pharmacological activity, pharmacokinetics, and toxicology of Re were included. In addition, we only included studies written in English. Approximately 140 articles were used in the review process, across a variety of in vitro and in vivo studies, case reports, and randomized controlled trials.
Pharmacological Effects of Re on Diabetes Mellitus (DM)
Anti-DM Effects In Vivo
Attele et al. (2002) found that Re (20 mg/kg, i.p. for 12 days) had marked anti-hyperglycemic activities, with no effect on the body weight of C57BL/6J ob/ob mice. This finding suggests that Re has potential as an anti-diabetic agent. Re (10 mg/kg, i.p. for 12 days) significantly reduced fasting blood glucose levels and promoted glucose tolerance (GT) and systemic insulin sensitivity (IS) in ob/ob mice without affecting body weight (Xie et al., 2005a). These findings suggest Re may provide a therapeutic role in ameliorating GT and insulin resistance (IR) in patients with type 2 diabetes mellitus (T2DM). Administration of Re (0.2 mg/ml for 90 min) rapidly normalized IR and muscle glucose transport induced by high-fat diet (HFD) in the epitrochlearis and soleus muscles of rats (Han et al., 2012). Re may have specifically acted to ameliorate IR in muscles of rats because it failed to modify HFD-induced muscle glucose transport resistance following stimulation by contraction or hypoxia. Muscle contraction and hypoxia exert an insulin-like-stimulating effect on glucose transport. However, Re did not affect basal or insulin-stimulated muscle glucose transport in chow-fed rats. According to these animal studies, P. ginseng or ginsenoside appeared to improve oral GT and accelerate insulin-stimulated glucose disposal (Xie et al., 2004). The Re-induced improvement in IS may or may not be associated with weight loss. Therefore, it remains unclear whether the amelioration was due to weight loss or insulin-sensitizing traits. These studies demonstrated the association between the anti-hyperglycemic activity of Re and improved IS, whereas body weight was unaffected. The improvement may be attributed to the insulin-sensitizing properties of Re. Quan et al. (2012) studied the potential anti-glycemic role of Re in HFD-induced diabetes in mice. Administration of Re (20 mg/kg, i.g. for 3 weeks) markedly lowered BG and triglyceride levels and prevented hepatic steatosis in C57BL/6J mice on a HFD. The hypoglycemic effect was associated with suppression of hepatic gluconeogenesis, possibly associated with AMP-activated protein kinase (AMPK) activation. In rats on a HFD, Re (40 mg/kg, i.p. for 2 weeks, twice a day) improved IR by inhibiting c-Jun N-terminal kinase (JNK) and nuclear factor (NF)-kB activation (Zhang et al., 2008). Several studies have concluded that the anti-hyperglycemic effect of Re was primarily responsible for improved microvasculopathy or reduced cognitive impairment in HFD-induced diabetic mouse models. In such models, Re (20 mg/kg, i.g. for 8 weeks) exerted a protective and anti-angiopathy effect in DM, such as the initial stages of high-sucrose-HFD (HSHF)-induced diabetes, HSHF+alloxan monohydrate-induced Type 1 diabetes mellitus (T1DM), and HSHF+streptozotocin (STZ)-induced T2DM. Administration of Re reduced BG levels, regulated increasing insulin levels, improved lipid metabolism, and reduced endothelial cell dysfunction. The underlying mechanism was possibly associated with p38 mitogen-activated protein kinase (MAPK) activation, and extracellular signal-regulated kinase (ERK) 1/2 and JNK signaling (Shi et al., 2016). In addition, Re (20 mg/kg, i.g. for 2 weeks) had an anti-diabetic microvasculopathy effect, including protective actions against oxidative stress in the kidneys and eyes, and increased BG and lipid levels in rats with STZ-induced diabetes (Cho et al., 2006). In rats with STZ-induced T1DM, Re (40 mg/kg, i.g. for 8 weeks) improved diabetes-related cognitive decline while decreasing fasting BG levels, although it did not affect BG, which was associated with oxidative stress and inflammation (Liu et al., 2012). In mice, Re improved HFD-induced IR through amelioration of hyperglycemia by protecting the brain cholinergic and antioxidant systems (Kim et al., 2017). Specifically, Re (5, 10 and 20 mg/kg/d, i.g. for 4 weeks) improved diabetes-associated cognitive impairment, and was possibly associated with improvement of the anti-oxidant and cholinergic systems in brain tissue. In HFD-induced hyperglycemic C57BL/6 mice, Re played a positive role through amelioration of insulin tolerance and BG levels. Re possibly improved learning and memory disorders related to HFD-induced diabetes. As the major ginsenoside in the P. ginseng berry ethyl acetate fraction (blended with drinking water 20 and 50 mg/kg, p.o. for 4 weeks), Re ameliorated cognitive decline in a dose-dependent manner because of its cholinergic activity, and it decreased oxidative stress in mice with HFD-induced T2DM and behavioral deficiency (Park et al., 2015).
Anti-DM Effects In Vitro
In 3T3-L1 adipocytes, Re (10 μM for 24 h) improved IR by inhibiting the inflammatory signaling cascade and activating the insulin signaling pathway (Zhang et al., 2008). Further results demonstrated that Re (1–10 μΜ for 0.5 h) increased glucose uptake in mature 3T3-L1 cells by significantly enhancing glucose transporter 4 (GLUT4) mRNA expression through the phosphoinositide 3-kinase (PI3K)-dependent pathway involving insulin receptor substrate-1 (IRS-1) in the glucose transport system cascade (Lee et al., 2011). Gao et al. (2013) demonstrated that Re (30, 60 μM for 5 days) reduced IR in adipocytes by directly enhancing the expression of peroxisome proliferator-activated receptor-γ (PPARγ)-2 and the corresponding AP2 genes, increasing adiponectin and IRS-1 expression, inhibiting inflammatory cytokine tumor nuclear factor-α (TNF-α) expression and production, and promoting GLUT4 translocation. The regulation of these factors facilitated adipocyte glucose uptake and disposal, although it failed to enhance GLUT4 expression. Another study found that Re (20 μM for 3 h) suppressed glucose generation in HepG2 cells, possibly by triggering the expression of the orphan nuclear receptor small heterodimer partner gene via AMPK activation (Quan et al., 2012). These results indicate that Re improved IR through reduction of lipotoxicity in the muscles and liver by enhancing adipocyte lipid storage capacity and promoting GLUT4 translocation to plasma membranes. Thus, Re compound regulation of insulin-stimulated glucose ingestion led to improved IR. Furthermore, Re (3 μM for 24 h) was proposed to exert anti-angiogenetic effects in diabetic retinopathy through the PI3K/Akt-mediated hypoxia-inducible factor-1-alpha (HIF-1α)/vascular endothelial growth factor (VEGF) signaling pathway in high-glucose-induced retinal endothelial RF/6A cells (Xie et al., 2020).
Overall, in vivo and in vitro data suggest four possible mechanisms underlying Re-induced improvement of diabetes and diabetes-related complications: 1) regulation of insulin resistance and insulin secretion, 2) modulation of glucose or lipid metabolism, 3) modulation of inflammatory cytokines, and 4) activation of oxidative stress.
Pharmacological Effects of Re on Nervous Diseases
Anti-Peripheral Nerve Injuries Effects In Vivo and Vitro
In rats with sciatic nerve crush injury, Re (2.0 mg/kg, i.p. for 4 weeks) promoted functional recovery, nerve regeneration, and proliferation of injured sciatic nerves. The Re compound promoted Schwann cell proliferation, differentiation, and migration during the course of peripheral neural repair after crush injury. This effect was possibly mediated by the regulation of ERK1/2 and JNK1/2 signaling pathways (Wang et al., 2015).
Anti-Cerebral Ischemia Effects In Vivo
One study reported the anti-oxidant effects of Re (5, 10 and 20 mg/kg, i.g. for 1 week) in rats with cerebral ischemia-reperfusion (I/R) injury. The Re compound considerably increased membrane fluidity of brain mitochondria, activated anti-oxidative enzymes, and decreased lipid peroxidation products, including malondialdehyde (Zhou et al., 2006). Neuroprotective effects of Re (5, 10 and 20 mg/kg, i.g. for 1 week) against cerebral I/R injury in rats were associated with a reduction in malondialdehyde levels and mitochondrial swelling, leading to an increase in H+-ATPase activity (Chen et al., 2008).
Anti-Neurotoxicity Effects In Vivo
Tu et al. (2017) reported that Re (20 mg/kg, i.p. for 3 days) attenuated convulsive behaviors, oxidative damage, pro-apoptotic potential and neuronal degeneration through the interleukin-6 (IL-6)-dependent PI3K/Akt signaling pathway in mice with trimethyltin-induced neurotoxicity. Treatment with Re (20 mg/kg, i.p. for 1 day) markedly decreased phencyclidine-induced neurotoxic alterations, including behavioral changes and mitochondrial dysfunction. These Re-mediated alterations were due to interactive modulation between glutathione peroxidase-1 (GPx-1) and NADPH oxidase in mice (Tran et al., 2017).
Anti-Depression and Anti-Cognitive Dysfunction Effects
Administration of Re (50 mg/kg, i.p. for 10 days) before immobilization stress markedly improved body weight, serum corticosterone levels, behavioral alterations, and cognitive deficits in rats. These effects were mediated through modulation of the central noradrenergic system and hypothalamic corticotrophin-releasing factor in the brain (Lee B et al., 2012). Another study showed Re (20, 40 mg/kg, i.p. for 3 weeks) inhibited memory deficits induced by chronic restraint stress (Wang et al., 2021). The protective effects were related to anti-inflammatory and anti-oxidant activities of the Re compound, as well as positive regulation of brain-derived neurotrophic factor and plasticity-associated proteins in the hippocampus.
Anti-Parkinson’s Disease (PD) Effects In Vivo
Administration of Re can effectively prevent onset of Alzheimer’s disease (AD) by improving the activity of dopamine (DA) neurons. One study found that Re (6.5, 13 and 26 mg/kg, i.g. for 13 days) prevented apoptosis of substantia nigra dopaminergic neurons induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL mice (Xu et al., 2005). The effect was mediated by reversing the abnormal expression of apoptosis regulatory proteins and inhibiting caspase-3 activation. Administration of Re (10, 20 mg/kg, i.p. for 2 weeks, twice a day) rescued methamphetamine-induced dopaminergic neurotoxicity. The effect was associated with potentiating oxidative burdens, compensative induction of GPx activity, mitochondrial dysfunction, pro-inflammatory changes, apoptotic cellular degeneration, and dopaminergic degeneration through inactivation of the protein kinase Cẟ (PKCδ) gene (Shin et al., 2014). Another study reported that Re (20 mg/kg, i.p. for 5 days, twice a day) protected methamphetamine-treated prodynorphin knockout mice against dopaminergic neurotoxicity through anti-oxidant, anti-inflammatory, and anti-apoptotic actions. The effects were facilitated by dynorphin-induced upregulation of the κ-opioid receptor, followed by substance P-mediated downregulation of the NK1 receptor (Dang et al., 2018).
Anti-PD Effects In Vitro
Administration of Re (10 µM) and ginsenoside Rd (5 µM for 48 h) provided considerable neuroprotective effects on primary dopaminergic midbrain neurons treated with CCl4. The neuroprotective effects were in part due to the lowering of oxidative stress and alleviation of inflammatory responses (Zhang et al., 2016). In addition, Re treatment (50, 100 μM for 24 h) of SH-SY5Y cells rescued methamphetamine-induced mitochondrial burden (compensative induction of cytosolic and mitochondrial GPx activity, mitochondrial oxidative stress, mitochondrial dysfunction, and mitochondrial translocation of cleaved PKCδ, and pro-apoptosis through genetic inhibition of PKCδ) (Nam et al., 2015). Kim et al. (2012) investigated the actions of Re on mitochondrial dysfunction in a PD model. They found that Re (3 µM) targeted mitochondrial dysfunction and rescued the defective PINK1-Hsp90/LRPPRC-Hsp60-complex IV signaling axis of PINK1-null neurons by restoring nitric oxide (NO) levels. Co-treatment using Rd and Re (0.5, 1 μM for 24 h) protected SH-SY5Y cells against rotenone-induced toxicity by regulating molecular mechanisms that enhanced cell viability, including prevention of morphological changes, lowered oxidative stress, improved mitochondrial integrity and function, and inhibited apoptosis owing to oxidative stress (Gonzalez-Burgos et al., 2017). The anti-oxidant mechanism of Re in PD remains unclear. In SH-SY5Y cells treated with 6-hydroxydopamine to induce oxidative stress, the Re compound (25 µM for 9 h) mediated its anti-oxidant effect by upregulating a key antioxidant gene GPX4 via PI3K/Akt and ERK cascades (Lee et al., 2020).
Anti-AD Effects In Vivo
Kai-Xin-San, a Chinese herbal formula, has been clinically administered at 3 g/kg (i.g. for 4 weeks) to treat animals with AD and neurosis. P. ginseng, a component of Kai-Xin-San, is known to enhance learning ability and memory. In addition, positive effects of Re and Rb1, the most abundant saponins, on learning ability and memory were reported (Wang et al., 2010). Amyloid β (Aβ) peptide plays an important role in AD. Zhou et al. reported that Re may interfere with AD progression by affecting the Aβ peptide (Zhou et al., 2020). Oral administration of Re (25 mg/kg, i.g. for 18 h) considerably reduced Aβ1-40 and Aβ1-42 levels in brains of Tg2576 mice (Chen et al., 2006). Furthermore, Li et al. (2018) demonstrated that Re (4 mg/kg, i.g. for 40 days) improved cognitive impairment, reduced Aβ accumulation, and restored biomarker levels, including amino acids, lecithin, and sphingolipids in the plasma of AD mice. Because of its effect on Aβ peptides, Re is increasingly considered a potential alternative drug for AD treatment. In addition, Re exhibits anti-dementia activity. The Re compound improved extracellular levels of DA and acetylcholine (Ach), particularly in the hippocampus. Also, Re (12.5, 25 and 50 mg/kg, s.c.) increased extracellular levels of DA and Ach in the medial prefrontal cortex (Shi et al., 2013).
Anti-AD Effects In Vitro
Treatment with Re has been reported to improve AD by affecting Aβ peptide levels in several cell models. Liang et al. reported that Re markedly reduced the generation of Aβ proteins in N2a/APP695 cells. The effect of Re (50–100 μM for 24 h) on Aβ generation was mediated by PPARγ activation in combination with Aβ-site precursor protein-cleaving enzyme 1 inhibition (Cao et al., 2016). Treatment with Re (0.1–100 μM for 2 h) considerably reduced cell toxicity and increased the release of lactate dehydrogenase, thereby attenuating PC12 cell damage induced by Aβ peptides (Ji et al., 2006). In addition, Re (25 µM for 48 h) exhibited neuroprotective activity against neurotoxicity arising from Aβ25-35 in SH-SY5Y cells by reducing oxidative damage and neuronal cell apoptosis. The neuroprotective activity was associated with the activation of nuclear factor erythroid-2 associated factor 2/heme oxygenase-1 anti-oxidant response pathways and inhibition of reactive oxygen species (ROS)-dependent apoptosis signal-regulated kinase 1/JNK/Bax apoptosis pathways (Liu et al., 2019). Furthermore, Kim et al. demonstrated that Re (5 μg/ml for 48 h) effectively upregulated the expression of choline acetyltransferase and vesicular acetylcholine transporter, and Ach production in Neuro-2a cells, thus countering symptoms during AD progression (Kim J et al., 2014).
In vivo and in vitro data suggest six possible mechanisms of Re-mediated improvement of complications associated with nervous system diseases: 1) regulation of central cholinergic pathways, 2) modulation of the apoptotic signaling pathway, 3) modulation of inflammatory responses, 4) modulation of mitochondrial burden, 5) regulation of anti-oxidant signaling pathways, and 6) reduction of Aβ peptide accumulation and loss of midbrain DA neurons.
Pharmacological Effects of Re on Inflammation
Anti-Inflammatory Effects In Vivo
Treatment with Re considerably inhibited neutrophil infiltration in a model of skin inflammation arising from 12-O-tetradecanoylphorbol-13-acetate. It also improved paw and ear oedema, increased malondialdehyde levels in paw fluid during c-carrageenan-induced edema, and suppressed interleukin-1β (IL-1β) and TNF-α expression in lipopolysaccharide (LPS)-stimulated murine Raw 264.7 macrophages (Paul et al., 2012). Moreover, Re (1 mg/kg, i.v. for 15 min) suppressed the LPS-induced increase in body temperature, white blood cell count, and pro-inflammatory mediators (Su et al., 2015). In LPS-induced systemic inflammation, Re (10, 20 mg/kg, i.g. for 4 h) suppressed serum levels of IL-1β and TNF-α in mice. Similarly, in 2,4,6-trinitrobenzene sulfonic acid-induced colitic mice, Re (10, 20 mg/kg, i.g. for 3 days) suppressed the expression of IL-1β, TNF-α, cyclooxygenase-2, and inducible nitric oxide synthase, and the activation of transcription factor NF-κB. However, it enhanced the expression of anti-inflammatory cytokine IL-10, indicating that Re can suppress Th1 rather than Th2 cell activation (Lee I et al., 2012). Administration of Re (15 mg/kg, i.g. for 1 week) also prevented NF-κB activation and LPS-induced myocardial inflammation in mice. The action of Re in cardiac dysfunction involves both MAPK inhibition and preserved activation of estrogen receptors and the PI3K/Akt signaling pathway (Chen et al., 2016). Treatment with Re (6–50 mg/kg, p.o. for 2 h) produced strong and significant inhibitory actions against LPS-induced lung inflammation in mice, and decreased inflammatory cell infiltration into lung tissue. The effect was mediated by inhibiting the activation of MAPK and transcription factors NF-κB and c-Fos (Lee et al., 2018).
Anti-Inflammatory Effects In Vitro
An in vitro investigation of the anti-inflammatory effects of Re (5, 10 μΜ for 30 min) in macrophages showed that it suppressed the expression of pro-inflammatory cytokines (TNF-α and IL-1β) and activation of transcription factor NF-κB by preventing the binding between LPS and toll-like receptor 4 (TLR4). However, Re did not suppress pro-inflammatory cytokines in peptidoglycan- or TNF-α-stimulated peritoneal macrophages (Lee J et al., 2012), highlighting its action in reducing inflammation by suppressing the LPS and TLR4 interaction in macrophages. Su et al. (2015) demonstrated that Re (50 μg/ml for 1 h) competed with LPS binding to the TLR4, and blocked the LPS-triggered signaling pathway in LPS-stimulated RAW264.7 cells. Extracellular Re was shown to compete with LPS binding to the TLR4, consistent with its role in the activation of extracellular TLR4 (Su et al., 2012). In addition, Wu et al. reported an anti-inflammatory role of Re (10–100 μΜ for 48 h) in LPS-induced activated N9 microglial cells. Re mediated its effects by inhibiting the generation of NO and TNF-α through downregulation of NF-κB activation (Wu et al., 2007). Treatment with Re (2 μg/ml for 24 h) reduced neuroinflammation by reducing the levels of inducible nitric oxide synthase and cyclooxygenase-2, and activating p38 MAPK in LPS-treated BV2 microglial cells (Lee K et al., 2012). Moreover, Quan H et al. (2019) reported that Re (10–40 μΜ for 24 h) inhibited LPS-induced TNF-α and IL-6 production in RAW264.7 cells, and reduced IL-6, NO, prostaglandin E2, and TNF-α secretion in primary rat hepatocytes via MAPK and NF-κB signaling pathways. Re is an effective component of Shen Fu, and was reported to exert anti-inflammatory effects by suppressing the NF-κB signaling pathway in TNF-α-stimulated EAhy926 cells (Li P et al., 2016). Incubation with Re (1.7 μg/ml for 24 h) decreased histamine secretion in human mast cells, and reduced IL-1α, IL-8, and IL-10 levels, and regulated T-cell-expressed and secreted protein secretion in A549 cells (Bae et al., 2012).
Altogether, in vivo and in vitro study data indicate that the possible mechanism of anti-inflammatory activities of Re involves NF-κB inactivation and reduced inflammatory cytokine release.
Pharmacological Effects of Re on Cardiovascular Diseases (CVDs)
Anti-Myocardial Injury Effects In Vivo
Kim et al. (2011) showed that Re improved ischemia/reperfusion (I/R) dysfunction by reversing the hemodynamic change (aortic flow, coronary flow, perfusion pressure, and cardiac output) and inhibiting the level of intracellular Ca2+ ([Ca2+]i). This study indicated that the anti-ischemic effect of Re was mediated by inhibiting an increase of [Ca2+]i. Additionally, Re prevented heart mitochondrial Ca2+ accumulation in I/R injury. In isolated single cardiomyocytes, Re suppressed the L-type Ca2+ current and strengthened the slowly activating delayed rectifier K+ current (IKs). This may be the underlying mechanism that prevented mitochondrial Ca2+ overload (Bai et al., 2003; Bai et al., 2004).
A rat model showed that Re (20 mg/kg, i.g. for 15 days) provided an effective treatment for myocardial infraction arising from left anterior descending coronary artery ligation. Treatment with Re improved the parameters of myocardial injury by downregulating the expression of intercellular adhesion molecule-1 and inhibiting polymorphonuclear leukocyte infiltration (Jing et al., 2010; Li et al., 2013). In this research, Re was reported to exhibit a protective role in ischemia-induced myocardial injury by regulating calcium transport, preserving mitochondrial structure and function, enhancing anti-oxidant capacity, and recovering myocardial blood flow.
In addition, Re lowered myocardial injury and suppressed cardiac hypertrophy in experimental models with cardiac dysfunction. Lim et al. (2013) proposed that Re (100 μM, injected into the aortic line for 3 min) exerted beneficial effects on cardiac function in rats with I/R injury, considerably improved hemodynamic functions and left ventricular developed pressure, ameliorated electrocardiographic abnormalities, and decreased the production of TNF-α. Treatment with Re (5, 20 mg/kg, i.g. for 4 weeks) also reduced isoproterenol-induced myocardial fibrosis, increased heart weight and hydroxyproline content, and reduced heart failure. The molecular mechanisms underlying the protective role of Re were possibly related to regulation of the transforming growth factor-beta 1 (TGF-β1)/Smad3 pathway (Wang et al., 2019). In a rat model of myocardial injury, Re (135 mg/kg, i.g. for 4 weeks) preserved cardiac function and structure, reduced myocardial injury and stress, and decreased left ventricular fibrosis by regulating the AMPK/TGF-β1/Smad2/3 and FAK/PI3K/Akt signaling pathways (Yu et al., 2020). These findings suggest a possible therapeutic role for Re in suppressing ventricular remodeling and promoting postinfarction healing. Overall, Re restored blood supply quickly and also delayed detrimental ventricular remodeling during chronic myocardial infraction rehabilitation.
Anti-Myocardial Injury Effects In Vitro
Wang et al. found that Re (200 μg/ml for 24 h) increased H9c2 cell viability after tertbutyl hydroperoxide treatment and reduced lactate dehydrogenase release and cell apoptosis (Wang et al., 2020). Treatment with Re (100 μM for 3 h) inhibited glucose deprivation-induced autophagy of H9c2 cardiac muscle cells, an effect which may be associated with the inhibition of autophagy, increase in cellular ATP content and viability, and alleviation of oxidative stress (Zhang et al., 2020). In addition, in the hypoxia/reoxygenation injury model, Re (100 µM for 21 h) increased HL-1 cell viability and ATP levels. The possible mechanism was that Re acted on the binding interface between HIF-1α and von Hippel-Lindau protein to prevent the binding of these proteins, thereby suppressing HIF-1α ubiquitination (Sun et al., 2020).
Adjusting Electrophysiological Activities
Administration of Re (≥10 nM) effectively suppressed the electromechanical alternans of cardiomyocytes in cats and humans by increasing sarcoplasmic reticulum Ca2+-release channels, and thereby improving arrhythmia (Wang et al., 2008b). Furukawa et al. (2006) showed that Re (3 μM) increased IKs, [Ca2+]i, activation of eNOS, and NO production through a c-Src/PI3K/Akt-dependent mechanism related to the non-genomic pathway of sex steroid receptors. Similarly, in vascular smooth muscle cells (VSMCs), Re non-genomically and dose dependently activated KCa currents and eNOS (EC50 = 4.1 ± 0.3 μM) through the c-Src/PI3-kinase/Akt pathway of the estrogen receptor (Nakaya et al., 2007). A study on human umbilical vein endothelial cells (HUVECs) revealed that Re augmented [Ca2+]i and NO production in a dose-dependent manner (EC50 of 316 and 615 nM, respectively) (Leung et al., 2007). In human coronary artery endothelial cells, Re (1 μM) induced vasorelaxation by increasing small-conductance Ca2+-activated K+ (SKCa) channel activity, stimulating NO production, and promoting vasodilation (Sukrittanon et al., 2014).
Anti-Atherosclerosis Effects
Abnormal structure and function of VSMCs may result in the development and progression of arteriosclerosis. Enhanced proliferation and migration of VSMCs represent critical events during the course of atherosclerotic lesion development (Bennett et al., 2016). Gao et al. (2018) demonstrated that Re (25 or 50 mg/kg, i.g. for 2 weeks) inhibited VSMC proliferation by suppressing phenotypic modulation and inhibiting vascular neointimal hyperplasia in balloon-injured rats through the eNOS/NO/cyclic guanosine monophosphate (cGMP) pathway. Re improved platelet-derived growth factor-BB-induced VSMC proliferation through G0/G1 cell cycle arrest, which was associated with eNOS/NO/cGMP pathway activation (Gao et al., 2019).
In contrast, endothelial cells provide an interface between circulating blood in the lumen and other vessel walls. Endothelial cells exhibit great sensitivity and vulnerability to toxic substances circulating in blood vessels. Endothelial dysfunction is an important contributor to the pathobiology of atherosclerosis (Gimbrone and García-Cardeña, 2016). Huang et al. (2016) found that Re (4, 16, and 64 μmol/L for 24 h) attenuated oxidative damage in H2O2-induced HUVECs and increased the production of NO and eNOS, superoxide dismutase (SOD), and GPx activities. The protective effects were associated with an oxidative stress response, protein synthesis and mitochondrial function. In addition, Yang et al. demonstrated that Re (120 μg/ml for 12 h) improved oxidized low-density lipoprotein-induced endothelial cell apoptosis. The effect was possibly elicited through regulation of oxidative stress, inhibition of inflammatory mediators, and recovery of balanced pro- and anti-apoptotic protein expression via p38/MAPK/NF-κB and PI3K/Akt/NF-κB pathways. These pathways may be regulated by the lectin-like oxidized low-density lipoprotein receptor-1, NADPH oxidase, and estrogen receptor α (Yang et al., 2018). Therefore, Re is a potential anti-oxidant that may be used to protect HUVECs from damage by oxidative stress through the anti-oxidant defense system. The Re compound also inhibited VSMC proliferation, attenuated endothelial dysfunction, and possibly promoted NO production, thereby reducing atherosclerosis.
Promoting Angiogenesis
Re is a pro-angiogenic compound with high stability that upregulates in vitro proliferation, migration, chemo-invasion, and tube formation of HUVECs. It also affects ex vivo aortic sprouting and in vivo neovascularization. In vitro results revealed that Re (10–30 μg/ml for 48 h) dose dependently enhanced the proliferation, migration, and tube formation of HUVECs (Huang et al., 2005). Additionally, extracellular matrix incorporating Re (70 μg for 1 week and 1 month) induced angiogenesis and enhanced tissue regeneration by increasing neocapillary density and tissue hemoglobin in a rat model (Yu et al., 2007). These findings indicate that Re can serve as an angiogenic agent to accelerate tissue regeneration.
In summary, in vivo and in vitro reports suggest five possible mechanisms by which Re may improve the cardiovascular system: 1) attenuation of myocardial ischemia, 2) inhibition of [Ca2+]i and activation of IKs, 3) increased NO production, 4) reduced cardiomyocyte apoptosis autophagy, and 5) the regulation of oxidative stress.
Pharmacological Effects of Re on Cancer
Reduction In Side Effects of Chemotherapy
A combination of Re and cisplatin increased the survival rate of LLC-PK1 cells by 21.4%. However, the renoprotective effects of Re were weaker than that of Maillard reaction products in Re-leucine/serine and glucose-leucine mixtures. Moreover, Maillard reaction products reduced cisplatin-induced oxidative kidney damage by increasing 1,1-diphenyl-2 picrylhydrazyl radical-scavenging activity and decreasing the expression of cleaved caspase-3 protein in rats (Lee W et al., 2012; Kim M et al., 2014). (Wang et al., 2008c) found that Re (25 mg/kg, i.g. for 10 days) considerably suppressed acute kidney injury induced by cisplatin in mice, by inhibiting the oxidative stress damage, inflammatory response, and apoptosis. Re (5, 10 mg/kg, i.p. for 1 week) also improved cyclophosphamide-induced myelosuppression, alleviated clinical symptoms of myelosuppression, and promoted recovery of bone marrow hematopoietic functions. The possible mechanisms involved the regulation of hematopoiesis-related cytokine levels, promotion of cellular entry to the normal cell cycle, and improvement of bone marrow nucleated cell apoptosis-related protein expression (Han et al., 2019).
Anti-Cancer Effects In Vitro
One mg/mL of American P. ginseng berry extract (containing 15.1 mg/g of Re for 72 h) exhibited strong anti-proliferative effects and triggered morphological alterations in SW480 human colorectal cancer cells (Xie et al., 2011). Re-carbon dots (0.5 mg/ml for 4 h) inhibited cancer cell proliferation (A375, HepG2, and MCF-7 cells) through the ROS-mediated pathway. However, the inhibitory effect on A375 cells was higher than that on other cells. Re induced apoptosis via the ROS- and caspase-mediated pathways (Yao et al., 2018). These findings demonstrate that Re can be used as a potential anti-cancer adjuvant for preventing and treating various cancers.
Altogether, in vivo and in vitro data show three possible mechanisms underlying the anti-cancer activities of Re: 1) inhibition of cell proliferation, 2) induction of cell apoptosis and 3) modulation of oxidative damage.
Pharmacological Effects of Re on Other Diseases
Anti-Viral and Enhancement of Immune Response
Song et al. (2014) demonstrated that Re (100 μg/ml for 48 h) had potential therapeutic efficacy in CVB3 and HRV3 infections in HeLa and Vero cells, respectively. Su et al. (2014) showed that co-administration of Re (5.0 mg/kg. s.c. for 3 weeks) with the rabies virus vaccine remarkably increased the serum antibody response in mice. Other studies have shown that co-administration of Re (50 μg, s.c. for 3 weeks) with inactivated influenza virus A/Fujian/411/2002 (H3N2) markedly amplified serum-specific antibody responses (IgG, IgG1, IgG2a, and IgG2b), hemagglutination inhibition titers, lymphocyte proliferation responses, and IL-5 and IFN-γ production (Song et al., 2010). Chan et al. (2011) reported that Re (50 μΜ for 16 h) protected HUVECs from H9N2/G1 influenza virus-induced apoptosis. CD4+ T cells are important immune cells in the human immune system. Son et al. found that Re (10, 20 and 40 μg/ml for 24 h) enhanced the viability of activated CD4+ T cells by downregulating IFN-γ production, which interfered with autophagy by reducing immunity-associated GTPase family proteins (Son et al., 2010). Re also enhanced the expression of Th1-type-related and Th2-type-related cytokines (Su et al., 2014). Administration of Re (10, 25 and 50 μg, s.c. for 2 weeks) had considerable adjuvant effects on specific antibody and cellular responses in ovalbumin-immunized mice, affecting the immune system favoring Th1- or Th2-type responses, as shown by enhanced titers of IgG1 and IgG2b isotypes (Sun et al., 2006). These results indicated Re-mediated activation of Th1 and Th2 immune responses in mouse models. Therefore, these studies indicate that Re can enhance the host immune system as a vaccine adjuvant.
Anti-Osteoporotic Effects
An optimal balance of osteoblasts and osteoclasts is crucial for bone remodeling. Impaired bone homeostasis potentially causes bone disease, such as bone fracture and osteoporosis (Feng and McDonald, 2011). It was demonstrated that Re had dual effects promoting osteoblast differentiation and inhibiting osteoclast differentiation. This research showed that Re (2.5, 5 and 10 μM for 48 h) dose dependently inhibited osteoclast differentiation and decreased nuclear factor of activated T cell cytoplasmic 1 and tartrate-resistant acid phosphatase mRNA levels, which are osteoclast differentiation markers. These effects were elicited by blocking the ERK signaling pathway in bone marrow-derived macrophages stimulated with the receptor activator of NF-κB ligand. Osteoclast generation in zebrafish scales was inhibited by Re (10 μM for 5 weeks), shown by reduced expression of osteoclast marker genes tartrate-resistant acid phosphatase and cathepsin K (Park et al., 2016). Kim et al. (2016) found that Re affected the differentiation and mineralization of osteoblasts both in vitro and in vivo models. Treatment with Re (50 µM for 5 weeks) promoted the expression of osteoblastic markers, including alkaline phosphatase activity, and mRNA levels of alkaline phosphatase, type 1 collagen, and osteocalcin in mouse osteoblast precursor MC3T3-E1 cells. Moreover, Re amplified the mineralization of osteoblasts in mouse MC3T3-E1 cells and zebrafish scales.
Improving Skin Barrier Function
Treatment with Re (5, 12, and 30 μM for 0.5 h) provided potential anti-photo-ageing activity in HaCaT keratinocytes under UVB radiation. This activity was possibly elicited through downregulation of UVB-induced intracellular ROS formation, production and secretion of pro-matrix metalloproteinase-2 and -9, and upregulation of total GPx levels and SOD activity (Shin et al., 2018). In addition, Oh et al. (2016) found that Re (5, 12 and 30 μM for 1 h) improved skin barrier functions, shown by enhanced cornified cell envelope formation, filaggrin levels and caspase-14 activity in HaCaT keratinocytes. Furthermore, Re (5, 12 and 30 μM for 24 h) demonstrated anti-oxidative activity through the upregulation of anti-oxidant components including total GPx and SOD under normal conditions. Re also prevented oxidative stress in HaCaT keratinocytes (Lim et al., 2016).
Anti-Oxidative Effects
Re (0.05, 0.1 and 0.5 mg/ml for 2 h) protected chick cardiomyocytes from exogenous H2O2- and endogenous antimycin A-induced oxidative stress. The underlying mechanism for this protective effect involved scavenging of H2O2 and hydroxyl radicals. However, in an electron spin resonance spectroscopy study, Re did not reduce the 1,1-diphenyl-2 picrylhydrazyl-induced electron spin resonance signals for xanthine oxidase or H2O2 (Xie et al., 2006). Therefore, direct scavenging of free radicals was impossible through a single anti-oxidation pathway in vivo. The anti-oxidative effects of Re were achieved through activation or enhancement of the intracellular anti-oxidant system.
Regulating Cholesterol Metabolism
Kawase et al. (2014) reported that Re (0.1–1 μM for 24 h) exerted a positive effect on cholesterol metabolism, increasing the expression level of sterol 12a-hydroxylase mRNA in rat primary hepatocytes, thereby facilitating cholic acid generation within bile acids.
Alleviating Allergic Response
Jang et al. (2012) reported that Re (25 mg/kg, p.o. for 6 h) potently alleviated scratching behavior in mice with histamine-induced itch, by inhibiting the activation of transcription factors (NF-κB and c-jun), as well as the expression of IL-4 and TNF-α.
Increasing Sperm Motility
Zhang et al. (2006) demonstrated that Re (100 μM for 2 h) improved sperm motility from fertile and asthenozoospermic infertile human subjects by enhancing NOS activity to promote endogenous NO generation.
Restoring Erectile Dysfunction
The Re-enriched fraction (containing 109.0 mg/g of Re, 54.5 mg/kg, i.g. for 5 weeks) of P. ginseng berries effectively restored ethanol-induced erectile dysfunction in male rats through the NO-cGMP pathway (Pyo et al., 2016).
Promoting Cyclic Growth of Hair Follicles
(Li Z et al., 2016) reported that topical treatment (5 mg/day, topical application on the back for 9 weeks) with Re markedly triggered hair shaft growth through selective suppression of hair growth phase transition-associated signaling pathways and TGF-β signaling cascades in nude mice.
Reducing Gastrointestinal Motility Dysfunction
Re-mediated bidirectional regulation is dependent on the jejunal contractile status and requires the co-existence of the enteric nervous system, Ca2+, and Cajal interstitial cells. The stimulatory role of Re (10 μM) on jejunal contractility of rat isolated jejunal segments was associated with cholinergic stimulation, whereas its inhibitory role was associated with adrenergic activation and the NO-relaxing mechanism (Xiong et al., 2014). In addition, Re (40 μΜ) inhibited pacemaker potentials through ATP-sensitive K+ channels and the cGMP/NO-dependent pathway in cultured Cajal interstitial cells obtained from the small intestine of mice (Hong et al., 2015). Re (20, 100 mg/kg, i.g. for 30 min) ameliorated acute gastric mucosal lesions induced by compound 48/80, possibly by triggering mucus secretion and decreasing neutrophil infiltration, inflammation, and oxidative stress in gastric mucosa (Lee et al., 2014).
Toxicology of Re
An acute toxicity study in mice treated with P. ginseng extract found LD50 values of 10–30 g/kg (Brekhman and Dardymov, 1969). Chronic treatment of mice and rats with P. ginseng extract (5 g/kg, p.o. for 2 years) produced almost no toxic effects, and the appearance, behavior, weight, and various physiological/histological indexes were within reasonable ranges (National Toxicology Program, 2011). Likewise, (Lu et al., 2012) found that the LD50 of Re was 5.0 g/kg in mice. In addition, in a chronic toxicity study, male and female SD rats treated with 375 mg/kg/day (orally) Re for 26 weeks, well below the typically non-toxic range (5–15 g/kg) of chemical substances (Hayes and Loomis, 1996), did not exhibit death, adverse reactions, and organ abnormalities (Lu et al., 2012).
Reproductive and Developmental Toxicology
In vitro rat embryo cultures found that 50 μg/ml Re induced severe developmental delay and significantly reduced the morphological scores of all organ systems, but was not teratogenic to specific organ systems (Chan et al., 2004). However, in vitro embryotoxicity may not reflect the human situation, and limited information about the blood concentration of Re in humans was available from the medical literature. Further investigations are necessary to evaluate the pharmacokinetics and placental transfer of ginsenosides in humans.
Carcinogenicity
No chronic carcinogenicity studies of Re in experimental animals have been found in the literature.
Adverse Effects
Several studies reported that some patients had vaginal bleeding and breast pain owing to the estrogen-like effects of P. ginseng (Palmer et al., 1978; Greenspan, 1983; Kabalak et al., 2004). The Re compound has an estrogen-like effect (Bae et al., 2005), and may have similar side effects, but these have not been reported in the literature.
Conclusions
Previous studies have shown that Re is abundant in the leaves, berries, flower buds, and roots of P. ginseng plants (Gao et al., 2018), in which the Re compound accounts for more than 30% of the total ginsenoside content (Wang et al., 2008a). Its pharmaco-economical merits support its use in natural supplements or drug formulations. Although Re is a relatively abundant ginsenoside with well-known pharmacological effects, to date, little is known about its pharmacokinetic profiles. Several studies have shown that because of its low bioavailability after oral absorption, its therapeutic effect is poor. Therefore, in-depth pharmacokinetic studies of Re should be performed to examine the presence of active metabolites. The identification of these metabolites may provide pivotal information regarding the bioactive forms of the ginsenoside Re and its pharmacological mechanisms. The potential therapeutic effect of Re may be improved by modifying the mode of administration or chemical structure. Structural changes in ginsenoside after heat processing may be strongly related to improvement in biological activity. After heat processing, Re demonstrated improved therapeutic efficacy, including anti-oxidant and anti-cancer activities (Lee B et al., 2012). Therefore, this area could be a new focus for future research.
Studies have shown that the Re compound has therapeutic efficacy on DM, neurological disorders, inflammatory responses, CVD and cancer. Moreover, multiple studies had shown a role for Re in treating hyperglycemia and hyperlipidemia in models of diabetes. Literature searches indicated that Re-induced improvement in the above-mentioned conditions were associated with anti-oxidant and anti-inflammatory properties, part of which were elicited through suppression of the p38-MAPK-mediated signaling pathway or activation of the PI3K/Akt and NF-κB signaling pathways. The anti-oxidant effect of Re was achieved by activating or enhancing the intracellular anti-oxidant system.
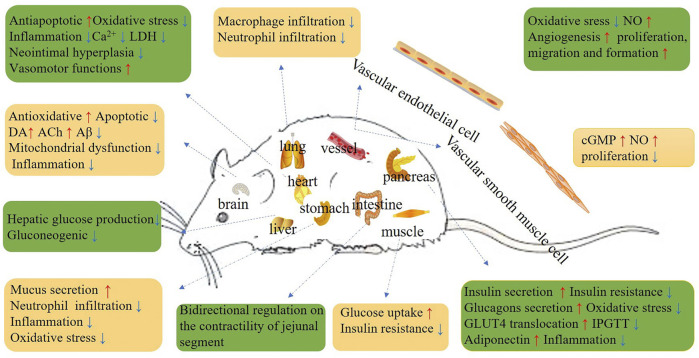
In conclusion, the beneficial properties of Re for DM, nervous system diseases, inflammatory responses, CVD, cancers, viral infections, oxidative stress, cholesterol metabolism, allergic and immune responses (Figure 1) indicate its potential as a novel treatment agent, but these properties need to be verified by future clinical experiments.
FIGURE 1.
Schematic diagram depicting the beneficial effects of Re.
Author Contributions
X-YG completed the document collection and manuscript writing with the help of G-CL, J-XZ, L-HW, CX, Z-AY, AW, and Y-FS. The revision of the manuscript was collaboratively finished by G-CP and finally approved by H-DY.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82060674), Science and Technology Planning Project of the Jilin Provincial Education Department (JJKH20210587KJ), Higher Education Discipline Innovation Project (111 Project, D18012), and Jilin Scientific and Technological development program (20190304055YY). We thank Liwen Bianji (Edanz) (www.liwenbianji.cn), for editing the English text of a draft of this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- 4-HNE
4-hydroxynonenal
- 6-HODA
6-hydroxydopamine
- Aβ
amyloid β
- ACh
acetylcholine
- AChE
acetylcholinesterase
- AD
alzheimer disease
- ALP
alkalin phosphatase
- AM
alloxan monohydrate
- AMPK
AMP-activated protein kinase
- ASK1
apoptosis signal-regulated kinase-1
- ATA
antimycin A
- AUC
area under curve
- BDNF
brain-derived neurotrophic factor
- BACE1
Aβ-site precursor protein-cleaving enzyme 1
- bFGF
basic fibroblast growth factor
- BG
blood glucose
- BMNC
bone marrow nucleated cell
- BNP
brain natriuretic peptide
- BUN
blood urea nitrogen
- BW
body weight
- [Ca2+]i
intracellular Ca2+ homeostasis
- cGMP
cyclic guanosine monophosphate
- c-FOS-IR
c-Fos-immunoreactivity
- CAT
catalase
- ChAT
choline acetyltransferase
- colla1
type 1 collagen
- CRE
creatinine
- CRS
chronic restraint stress
- CTX
cyclophosphamide
- CVDs
cardiovascular diseases
- CYP8B1
sterol 12a-hydroxylase
- CP
constipation-prominent
- Cmax
peak concentration
- CL
clearance
- SFI
Shenfu Injection
- DA
dopamine
- DBP
diastolic blood pressure
- DII
dexamethasone + 3-isobutyl-1-methylxanthine + insulin
- DM
diabetes mellitus
- DPPH
1,1-diphenyl-2 picrylhydrazyl
- DP
diarrhea-prominent
- EDV
end-diastolic volume
- ERs
oestrogen receptors
- ERK
extracellular signal-regulated kinase
- ESV
end-systolic volume
- f
fraction excreted unchanged in the urine
- FAS
fatty acid synthase
- FBG
fasting blood glucose
- GD
glucose deprivation
- GOT
glutamic oxaloacetic transaminase
- GPL
Glucose + sodium pyruvate + sodium lactate
- GPT
glutamic pyruvic transaminase
- GSH
glutathione
- GT
glucose tolerance
- GLUT4
glucose transporter 4
- GPx
glutathione peroxidase
- GGSQ
Gegen-Sanqi
- HDL-C
high density lipoprotein cholesterol
- HFD
high-fat diet
- HGB
hemoglobin
- HIF-1α
hypoxia-inducible factor-1-alpha
- HO-1
heme oxygenase-1
- HSHF
high-sucrose-HFD
- HUVEC
human umbilical vein endothelial cell
- ICa,L
L-type Ca2+ current
- iNOS
nitric oxide synthase
- JNK
c-Jun N-terminal kinase
- ICAM-1
intercellular adhesion molecule-1
- IFN-γ
interferon-γ
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- Lp-α
lipoprotein α
- IPGTT
intraperitoneal glucose tolerance test
- I/R
ischamia/reperfusion
- IR
insulin resistance
- IRS-1
insulin receptor substrate
- LRPPRC
leucine-rich pentatricopeptide repeat-containing
- IS
insulin sensitivity
- IVS
interventricular septum
- IVSTd
IVS end-diastolic thickness
- ICP
intracavernous pressure
- LADCA
left anterior descending coronary artery
- LC3B-2
microtubule-associated protein 1A/1B-light chain 3
- LDH
lactate dehydrogenase
- LDL-C
low density lipoprotein cholesterol
- LKB1
liver kinase B1
- LPC
lysophosphatidylcholine
- LPS
lipopolysaccharide
- LV
left ventricular
- LVD
LV dimension
- LVDd
LV end-diastolic dimension
- ILVDs
LV endsystolic dimension
- LVPWTs
LV posteriorend-systolic thickness
- LVPWTd
LV posteriorend-diastolic thickness
- LVEDP
Left ventricular end diastolic pressure
- MA
methamphetamine
- MAPK
mitogen-activated protein kinase
- MCAO
middle cerebral artery occlusion
- MDA
malondialdehyde
- MI
myocardial infraction
- MMP
metalloproteinase
- MPO
myeloperoxidase
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MRPs
maillard reaction products
- MLCK
myosin light chain kinase
- MRT
mean residence time
- NF-kB
nuclear factor-kB
- NLRP3
NOD‐like receptor protein 3
- NK1
neurokinin1
- NO
nitric oxide
- Nrf2
nuclear factor erythroid-2 associated factor 2
- ox-LDL
oxidized low density lipoprotein
- OVA
ovalbumin
- QXSBP
QixueShuang bu Prescription
- p75
nerve growth factor receptor
- PCNA
proliferating cell nuclear antigen
- PCP
phencyclidine
- PD
parkinson disease
- PEPCK
phosphoenolpyruvate carboxykinase
- PG
peptidoglycan
- PGE2
prostaglandin E2
- PHOX
NADPH oxidase
- PI3K
phosphoinositide3-kinase
- PMN
polymorphonuclear leukocyte
- PKCδ
protein kinase Cδ
- PPARγ
peroxisome proliferator-activated receptor-γ
- PSD95
postsynaptic density 95
- RC
renal clearance
- RANKL
receptor activator of NF-κB ligand
- RANTES
T-cell-expressed and secreted
- RBCs
red blood cells
- RIS
repeated immobilization stress
- RV
rabies virus vaccine
- SCD1
stearoyl-CoA desaturase-1
- SHP
small heterodimer partner
- SNC
sciatic nerve crush injury
- SOD
superoxide dismutase
- SREBP
sterol regulatory element-binding protein
- STZ
streptozotocin
- SYP
synaptophysin
- T1DM
type 1 diabetes
- T2DM
type 2 diabetes
- tBHP
tertbutyl hydroperoxide
- TC
total cholesterol
- TG
triglyceride
- TH
tyrosine hydroxylase
- TLR4
toll-like receptor 4
- TMT
trimethyltin
- TNBS
2,4,6-trinitrobenzene sulfonic acid
- TNF-α
tumour nuclear factor-α
- TPA
12-O-tetradecanoylphorbol-13-acetate
- TPO
thrombopoietin
- TRAP
tartrate-resistant acid phosphatase
- TGF-β
transforming growth factor-β
- TRARS
thiobarbituric acid reactive substances
- T1/2
elimination half-life
- Tmax
time for maximum concentration
- VAchT
vesicular acetylcholine transporter
- VEGF
vascular endothelial growth factor
- VHL
von Hippel-Lindau
- VSTs
IVS end-systolic thickness
- Vd
volume of distribution
- WBC
white blood cell
- XO
xanthine oxidase
- XSTDT
Xue saitong dispersible table
References
- Attele A. S., Zhou Y. P., Xie J. T., Wu J. A., Zhang L., Dey L., et al. (2002). Antidiabetic Effects of Panax Ginseng berry Extract and the Identification of an Effective Component. Diabetes 51 (6), 1851–1858. 10.2337/diabetes.51.6.1851 [DOI] [PubMed] [Google Scholar]
- Bae E. A., Shin J. E., Kim D. H. (2005). Metabolism of Ginsenoside Re by Human Intestinal Microflora and its Estrogenic Effect. Biol. Pharm. Bull. 28 (10), 1903–1908. 10.1248/bpb.28.1903 [DOI] [PubMed] [Google Scholar]
- Bae H. M., Cho O. S., Kim S. J., Im B. O., Cho S. H., Lee S., et al. (2012). Inhibitory Effects of Ginsenoside Re Isolated from Ginseng berry on Histamine and Cytokine Release in Human Mast Cells and Human Alveolar Epithelial Cells. J. Ginseng Res. 36 (4), 369–374. 10.5142/jgr.2012.36.4.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai C. X., Sunami A., Namiki T., Sawanobori T., Furukawa T. (2003). Electrophysiological Effects of Ginseng and Ginsenoside Re in guinea Pig Ventricular Myocytes. Eur. J. Pharmacol. 476 (1-2), 35–44. 10.1016/s0014-2999(03)02174-5 [DOI] [PubMed] [Google Scholar]
- Bai C. X., Takahashi K., Masumiya H., Sawanobori T., Furukawa T. (2004). Nitric Oxide-dependent Modulation of the Delayed Rectifier K+ Current and the L-type Ca2+ Current by Ginsenoside Re, an Ingredient of Panax Ginseng, in guinea-pig Cardiomyocytes. Br. J. Pharmacol. 142 (3), 567–575. 10.1038/sj.bjp.0705814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Sinha S., Owens G. K. (2016). Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 118 (4), 692–702. 10.1161/CIRCRESAHA.115.306361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekhman I. I., Dardymov I. V. (1969). New Substances of Plant Origin Which Increase Nonspecific Resistance. Annu. Rev. Pharmacol. 9, 419–430. 10.1146/annurev.pa.09.040169.002223 [DOI] [PubMed] [Google Scholar]
- Cao G., Su P., Zhang S., Guo L., Zhang H., LiangQin Y. C., et al. (2016). Ginsenoside Re Reduces Aβ Production by Activating PPARγ to Inhibit BACE1 in N2a/APP695 Cells. Eur. J. Pharmacol. 793, 101–108. 10.1016/j.ejphar.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Chan L. Y., Chiu P. Y., Lau T. K. (2004). Embryotoxicity Study of Ginsenoside Rc and Re in In Vitro Rat Whole Embryo Culture. Reprod. Toxicol. 19 (1), 131–134. 10.1016/j.reprotox.2004.06.001 [DOI] [PubMed] [Google Scholar]
- Chan L. Y., Kwok H. H., Chan R. W., Peiris M. J., Mak N. K., Wong R. N., et al. (2011). Dual Functions of Ginsenosides in Protecting Human Endothelial Cells against Influenza H9N2-Induced Inflammation and Apoptosis. J. Ethnopharmacol. 137 (3), 1542–1546. 10.1016/j.jep.2011.08.022 [DOI] [PubMed] [Google Scholar]
- Chen F., Eckman E. A., Eckman C. B. (2006). Reductions in Levels of the Alzheimer's Amyloid Beta Peptide after Oral Administration of Ginsenosides. FASEB. J. 20 (8), 1269–1271. 10.1096/fj.05-5530fje [DOI] [PubMed] [Google Scholar]
- Chen G., Yang M., Guo D. (2009). [Metabolic Study of Ginsenoside Re in Rats]. Zhongguo Zhong Yao Za Zhi 34 (12), 1540–1543. [PubMed] [Google Scholar]
- Chen L., Liu L., Wang Q., JiangTian Y. H., Tian H. (2021). Comparative Pharmacokinetics Study of Six Effective Components between Two Dosage Forms of Qixue-Shuangbu Prescription in Rats by UPLC-MS/MS. Biomed. Chromatogr. 35 (10), e5179. 10.1002/bmc.5179 [DOI] [PubMed] [Google Scholar]
- Chen L. M., Zhou X. M., Cao Y. L., Hu W. X. (2008). Neuroprotection of Ginsenoside Re in Cerebral Ischemia-Reperfusion Injury in Rats. J. Asian Nat. Prod. Res. 10 (5-6), 439–445. 10.1080/10286020801892292 [DOI] [PubMed] [Google Scholar]
- Chen R. C., Wang J., Yang L., Sun G. B., Sun X. B. (2016). Protective Effects of Ginsenoside Re on Lipopolysaccharide-Induced Cardiac Dysfunction in Mice. Food Funct. 7 (5), 2278–2287. 10.1039/c5fo01357g [DOI] [PubMed] [Google Scholar]
- Chen S. E., Sawchuk R. J., Staba E. J. (1980). American Ginseng. III. Pharmacokinetics of Ginsenosides in the Rabbit. Eur. J. Drug Metab. Pharmacokinet. 5 (3), 161–168. 10.1007/bf03189460 [DOI] [PubMed] [Google Scholar]
- Chen Y. B., Wang Y. F., Hou W., Wang Y. P., Xiao S. Y., Fu Y. Y., et al. (2017). Effect of B-Complex Vitamins on the Antifatigue Activity and Bioavailability of Ginsenoside Re after Oral Administration. J. Ginseng. Res. 41 (2), 209–214. 10.1016/j.jgr.2016.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H., Ji G. E. (2005). Transformation of Ginsenosides Rb1 and Re from Panax Ginseng by Food Microorganisms. Biotechnol. Lett. 27 (11), 765–771. 10.1007/s10529-005-5632-y [DOI] [PubMed] [Google Scholar]
- Cho W. C., Chung W. S., Lee S. K., Leung A. W., Cheng C. H., Yue K. K. (2006). Ginsenoside Re of Panax Ginseng Possesses Significant Antioxidant and Antihyperlipidemic Efficacies in Streptozotocin-Induced Diabetic Rats. Eur. J. Pharmacol. 550 (1-3), 173–179. 10.1016/j.ejphar.2006.08.056 [DOI] [PubMed] [Google Scholar]
- Christensen L. P. (2009). Ginsenosides Chemistry, Biosynthesis, Analysis, and Potential Health Effects. Adv. Food Nutr. Res. 55, 1–99. 10.1016/s1043-4526(08)00401-4 [DOI] [PubMed] [Google Scholar]
- Dai G., Jiang Z., Zhu L., Zhang Q., Zong Y., Liu S., et al. (2016). Simultaneous Determination of Notoginsenoside R1 and Ginsenoside Re in Rat Plasma by Ultra High Performance Liquid Chromatography with Tandem Mass Spectrometry and its Application to a Pharmacokinetic Study. J. Sep. Sci. 39 (17), 3368–3374. 10.1002/jssc.201600522 [DOI] [PubMed] [Google Scholar]
- Dang D. K., Shin E. J., Kim D. J., Tran H. Q., Jeong J. H., Jang C. G., et al. (2018). Ginsenoside Re Protects Methamphetamine-Induced Dopaminergic Neurotoxicity in Mice via Upregulation of Dynorphin-Mediated κ-opioid Receptor and Downregulation of Substance P-Mediated Neurokinin 1 Receptor. J. Neuroinflammation 15 (1), 52. 10.1186/s12974-018-1087-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W.-W., Zhao J., Zhong F.-L., Zhu W.-J., Jiang J., Wu S., et al. (2017). Biotransformation of Panax Ginseng Extract by Rat Intestinal Microflora: Identification and Quantification of Metabolites Using Liquid Chromatography-Tandem Mass Spectrometry. J. Ginseng Res. 41 (4), 540–547. 10.1016/j.jgr.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., McDonald J. M. (2011). Disorders of Bone Remodeling. Annu. Rev. Pathol. 6, 121–145. 10.1146/annurev-pathol-011110-130203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Bai C. X., Kaihara A., Ozaki E., Kawano T., Nakaya Y., et al. (2006). Ginsenoside Re, a Main Phytosterol of Panax Ginseng, Activates Cardiac Potassium Channels via a Nongenomic Pathway of Sex Hormones. Mol. Pharmacol. 70 (6), 1916–1924. 10.1124/mol.106.028134 [DOI] [PubMed] [Google Scholar]
- Gao Y., Gao C. Y., Zhu P., Xu S. F., Luo Y. M., DengYang J. D. L., et al. (2018). Ginsenoside Re Inhibits Vascular Neointimal Hyperplasia in Balloon-Injured Carotid Arteries through Activating the eNOS/NO/cGMP Pathway in Rats. Biomed. Pharmacother. 106, 1091–1097. 10.1016/j.biopha.2018.07.044 [DOI] [PubMed] [Google Scholar]
- Gao Y., Yang M. F., Su Y. P., Jiang H. M., You X. J., Yang Y. J., et al. (2013). Ginsenoside Re Reduces Insulin Resistance through Activation of PPAR-γ Pathway and Inhibition of TNF-α Production. J. Ethnopharmacol. 147 (2), 509–516. 10.1016/j.jep.2013.03.057 [DOI] [PubMed] [Google Scholar]
- Gao Y., Zhu P., Xu S. F., Li Y. Q., Deng J., Yang D. L. (2019). Ginsenoside Re Inhibits PDGF-BB-Induced VSMC Proliferation via the eNOS/NO/cGMP Pathway. Biomed. Pharmacother. 115, 108934. 10.1016/j.biopha.2019.108934 [DOI] [PubMed] [Google Scholar]
- Gillis C. N. (1997). Panax Ginseng Pharmacology: a Nitric Oxide Link? Biochem. Pharmacol. 54 (1), 1–8. 10.1016/s0006-2952(97)00193-7 [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr., García-Cardeña G. (2016). Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 118 (4), 620–636. 10.1161/CIRCRESAHA.115.306301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Burgos E., Fernández-Moriano C., Lozano R., Iglesias I., Gómez-Serranillos M. P. (2017). Ginsenosides Rd and Re Co-treatments Improve Rotenone-Induced Oxidative Stress and Mitochondrial Impairment in SH-Sy5y Neuroblastoma Cells. Food Chem. Toxicol. 109 (Pt 1), 38–47. 10.1016/j.fct.2017.08.013 [DOI] [PubMed] [Google Scholar]
- Greenspan E. M. (1983). Ginseng and Vaginal Bleeding. Jama 249 (15), 2018. 10.1001/jama.1983.03330390026012 [DOI] [PubMed] [Google Scholar]
- Gui F. J., Yang X. W., Li L. Y., Tian J. M. (2007). Simultaneous Enantiomer Determination of 20 (R)- and 20 (S)-ginsenoside-Rg2 in Rat Plasma after Intravenous Administration Using HPLC Method. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 850 (1-2), 1–6. 10.1016/j.jchromb.2006.11.008 [DOI] [PubMed] [Google Scholar]
- Han D. H., Kim S. H., Higashida K., Jung S. R., Polonsky K. S., Klein S., et al. (2012). Ginsenoside Re Rapidly Reverses Insulin Resistance in Muscles of High-Fat Diet Fed Rats. Metabolism 61 (11), 1615–1621. 10.1016/j.metabol.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Xia J., Zhang L., Cai E., Zhao Y., Fei X., et al. (2019). Studies of the Effects and Mechanisms of Ginsenoside Re and Rk3 on Myelosuppression Induced by Cyclophosphamide. J. Ginseng Res. 43 (4), 618–624. 10.1016/j.jgr.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkey M. R., Henderson G. L., Gershwin M. E., Stern J. S., Hackman R. M. (2001). Variability in Commercial Ginseng Products: an Analysis of 25 Preparations. Am. J. Clin. Nutr. 73 (6), 1101–1106. 10.1093/ajcn/73.6.1101 [DOI] [PubMed] [Google Scholar]
- Hayes A. W., Loomis T. A. (1996). Loomis's Essentials of Toxicology. Elsevier. [Google Scholar]
- Hong N. R., Park H. S., Ahn T. S., Kim H. J., Ha K. T., Kim B. J. (2015). Ginsenoside Re Inhibits Pacemaker Potentials via Adenosine Triphosphate-Sensitive Potassium Channels and the Cyclic Guanosine Monophosphate/nitric Oxide-dependent Pathway in Cultured Interstitial Cells of Cajal from Mouse Small Intestine. J. Ginseng Res. 39 (4), 314–321. 10.1016/j.jgr.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G. D., Zhong X. F., Deng Z. Y., Zeng R. (2016). Proteomic Analysis of Ginsenoside Re Attenuates Hydrogen Peroxide-Induced Oxidative Stress in Human Umbilical Vein Endothelial Cells. Food Funct. 7 (5), 2451–2461. 10.1039/c6fo00123h [DOI] [PubMed] [Google Scholar]
- Huang Y. C., Chen C. T., Chen S. C., Lai P. H., Liang H. C., Chang Y., et al. (2005). A Natural Compound (Ginsenoside Re) Isolated from Panax Ginseng as a Novel Angiogenic Agent for Tissue Regeneration. Pharm. Res. 22 (4), 636–646. 10.1007/s11095-005-2500-3 [DOI] [PubMed] [Google Scholar]
- Jang S. E., Jung I. H., Joh E. H., Han M. J., Kim D. H. (2012). Antibiotics Attenuate Anti-scratching Behavioral Effect of Ginsenoside Re in Mice. J. Ethnopharmacol. 142 (1), 105–112. 10.1016/j.jep.2012.04.022 [DOI] [PubMed] [Google Scholar]
- Ji B., Zhao X., Yu P., Meng L., Zhao Y., Yu Z. (2017). Simultaneous Determination and Pharmacokinetics of Fourteen Bioactive Compounds in Rat Plasma by LC-ESI-MS/MS Following Intravenous Injection of Gegen-Sanqi Compatibility Solution. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 1068-1069-1069, 164–172. 10.1016/j.jchromb.2017.10.023 [DOI] [PubMed] [Google Scholar]
- Ji Z. N., Dong T. T., Ye W. C., Choi R. C., Lo C. K., Tsim K. W. (2006). Ginsenoside Re Attenuate Beta-Amyloid and Serum-free Induced Neurotoxicity in PC12 Cells. J. Ethnopharmacol. 107 (1), 48–52. 10.1016/j.jep.2006.02.004 [DOI] [PubMed] [Google Scholar]
- Jiang J., Sun X., Akther M., Lian M. L., Quan L. H., Koppula S., et al. (2020). Ginsenoside Metabolite 20(S)-protopanaxatriol from Panax Ginseng Attenuates Inflammation-Mediated NLRP3 Inflammasome Activation. J. Ethnopharmacol. 251, 112564. 10.1016/j.jep.2020.112564 [DOI] [PubMed] [Google Scholar]
- Jing L., Wang M., Zheng Z., Peng J., Wei Y., Zheng Z., et al. (2010). Ginsenoside Re Downregulates ICAM-1 Expression, Inhibits Polymorphonuclear Infiltration, and Ameliorates Ischemia-Reperfusion Injury. Med. Chem. Res. 19 (8), 962–969. 10.1007/s.00044-009-9242-410.1007/s00044-009-9242-4 [DOI] [Google Scholar]
- Joo K. M., Lee J. H., Jeon H. Y., Park C. W., Hong D. K., Jeong H. J., et al. (2010). Pharmacokinetic Study of Ginsenoside Re with Pure Ginsenoside Re and Ginseng berry Extracts in Mouse Using Ultra Performance Liquid Chromatography/mass Spectrometric Method. J. Pharm. Biomed. Anal. 51 (1), 278–283. 10.1016/j.jpba.2009.08.013 [DOI] [PubMed] [Google Scholar]
- Kabalak A. A., Soyal O. B., Urfalioglu A., Saracoglu F., Gogus N. (2004). Menometrorrhagia and Tachyarrhythmia after Using Oral and Topical Ginseng. J. Womens Health (Larchmt) 13 (7), 830–833. 10.1089/jwh.2004.13.830 [DOI] [PubMed] [Google Scholar]
- Kawase A., Yamada A., Gamou Y., Tahara C., Takeshita F., Murata K., et al. (2014). Effects of Ginsenosides on the Expression of Cytochrome P450s and Transporters Involved in Cholesterol Metabolism. J. Nat. Med. 68 (2), 395–401. 10.1007/s11418-013-0791-y [DOI] [PubMed] [Google Scholar]
- Kiefer D., Pantuso T. (2003). Panax Ginseng. Am. Fam. Physician 68 (8), 1539–1542. [PubMed] [Google Scholar]
- Kim H. B., Lim K. H., Kang C.-W., Kim B. S., Roh Y. S., Kwon J., et al. (2011). Influence of Ginsenoside-Re against Myocardial Infarction in Isolated Heart. Mol. Cel. Toxicol. 7 (1), 15–24. 10.1007/s13273-011-0003-3 [DOI] [Google Scholar]
- Kim H. M., Kim D. H., Han H. J., Park C. M., Ganipisetti S. R., Valan Arasu M., et al. (2016). Ginsenoside Re Promotes Osteoblast Differentiation in Mouse Osteoblast Precursor MC3T3-E1 Cells and a Zebrafish Model. Molecules 22 (1), 42. 10.3390/molecules22010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Han I. H., Yamabe N., Kim Y. J., Lee W., Eom D. W., et al. (2014). Renoprotective Effects of Maillard Reaction Products Generated during Heat Treatment of Ginsenoside Re with Leucine. Food Chem. 143, 114–121. 10.1016/j.foodchem.2013.07.075 [DOI] [PubMed] [Google Scholar]
- Kim J. M., Park C. H., Park S. K., Seung T. W., Kang J. Y., Ha J. S., et al. (2017). Ginsenoside Re Ameliorates Brain Insulin Resistance and Cognitive Dysfunction in High Fat Diet-Induced C57BL/6 Mice. J. Agric. Food Chem. 65 (13), 2719–2729. 10.1021/acs.jafc.7b00297 [DOI] [PubMed] [Google Scholar]
- Kim K. H., Song K., Yoon S. H., Shehzad O., Kim Y. S., Son J. H. (2012). Rescue of PINK1 Protein Null-specific Mitochondrial Complex IV Deficits by Ginsenoside Re Activation of Nitric Oxide Signaling. J. Biol. Chem. 287 (53), 44109–44120. 10.1074/jbc.M112.408146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. S., Yu J. M., Kim H. J., Kim H. B., Kim S. T., Jang S. K., et al. (2014). Ginsenoside Re and Rd Enhance the Expression of Cholinergic Markers and Neuronal Differentiation in Neuro-2a Cells. Biol. Pharm. Bull. 37 (5), 826–833. 10.1248/bpb.b14-00011 [DOI] [PubMed] [Google Scholar]
- Kim U., Park M. H., Kim D. H., Yoo H. H. (2013). Metabolite Profiling of Ginsenoside Re in Rat Urine and Faeces after Oral Administration. Food Chem. 136 (3-4), 1364–1369. 10.1016/j.foodchem.2012.09.050 [DOI] [PubMed] [Google Scholar]
- Kim Y. K., Yoo D. S., Xu H., Park N. I., Kim H. H., Choi J. E., et al. (2009). Ginsenoside Content of Berries and Roots of Three Typical Korean Ginseng (Panax Ginseng) Cultivars. Nat. Prod. Commun. 4 (7), 903–906. 10.1177/1934578X0900400704 [DOI] [PubMed] [Google Scholar]
- Lee B., Shim I., Lee H., Hahm D. H. (2012). Effect of Ginsenoside Re on Depression- and Anxiety-like Behaviors and Cognition Memory Deficit Induced by Repeated Immobilization in Rats. J. Microbiol. Biotechnol. 22 (5), 708–720. 10.4014/jmb.1112.12046 [DOI] [PubMed] [Google Scholar]
- Lee G. H., Lee W. J., Hur J., Kim E., Lee H. G., Seo H. G. (2020). Ginsenoside Re Mitigates 6-Hydroxydopamine-Induced Oxidative Stress through Upregulation of GPX4. Molecules 25 (1), 188. 10.3390/molecules25010188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I. A., Hyam S. R., Jang S. E., Han M. J., Kim D. H. (2012). Ginsenoside Re Ameliorates Inflammation by Inhibiting the Binding of Lipopolysaccharide to TLR4 on Macrophages. J. Agric. Food Chem. 60 (38), 9595–9602. 10.1021/jf301372g [DOI] [PubMed] [Google Scholar]
- Lee J. H., Lee W., Lee S., Jung Y., Park S. H., Choi P., et al. (2012). Important Role of Maillard Reaction in the Protective Effect of Heat-Processed Ginsenoside Re-serine Mixture against Cisplatin-Induced Nephrotoxicity in LLC-PK1 Cells. Bioorg. Med. Chem. Lett. 22 (17), 5475–5479. 10.1016/j.bmcl.2012.07.018 [DOI] [PubMed] [Google Scholar]
- Lee J. H., Min D. S., Lee C. W., Song K. H., Kim Y. S., Kim H. P. (2018). Ginsenosides from Korean Red Ginseng Ameliorate Lung Inflammatory Responses: Inhibition of the MAPKs/NF-κB/c-Fos Pathways. J. Ginseng Res. 42 (4), 476–484. 10.1016/j.jgr.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. W., Jung S. Y., Choi S. M., Yang E. J. (2012). Effects of Ginsenoside Re on LPS-Induced Inflammatory Mediators in BV2 Microglial Cells. BMC Complement. Altern. Med. 12, 196. 10.1186/1472-6882-12-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee O. H., Lee H. H., Kim J. H., Lee B. Y. (2011). Effect of Ginsenosides Rg3 and Re on Glucose Transport in Mature 3T3-L1 Adipocytes. Phytother. Res. 25 (5), 768–773. 10.1002/ptr.3322 [DOI] [PubMed] [Google Scholar]
- Lee S., Kim M. G., Ko S. K., Kim H. K., Leem K. H., Kim Y. J. (2014). Protective Effect of Ginsenoside Re on Acute Gastric Mucosal Lesion Induced by Compound 48/80. J. Ginseng Res. 38 (2), 89–96. 10.1016/j.jgr.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Park S. H., Lee S., Chung B. C., Song M. O., Song K. I., et al. (2012). Increase in Antioxidant Effect of Ginsenoside Re-alanine Mixture by Maillard Reaction. Food Chem. 135 (4), 2430–2435. 10.1016/j.foodchem.2012.06.108 [DOI] [PubMed] [Google Scholar]
- Leung K. W., Leung F. P., Huang Y., Mak N. K., Wong R. N. (2007). Non-genomic Effects of Ginsenoside-Re in Endothelial Cells via Glucocorticoid Receptor. FEBS Lett. 581 (13), 2423–2428. 10.1016/j.febslet.2007.04.055 [DOI] [PubMed] [Google Scholar]
- Li J., Liu Y., Li W., Wang Z., Guo P., Li L., et al. (2018). Metabolic Profiling of the Effects of Ginsenoside Re in an Alzheimer's Disease Mouse Model. Behav. Brain Res. 337, 160–172. 10.1016/j.bbr.2017.09.027 [DOI] [PubMed] [Google Scholar]
- Li L., Sheng Y. X., Zhang J. L., Wang S. S., Guo D. A. (2006). High-performance Liquid Chromatographic Assay for the Active Saponins from Panax Notoginseng in Rat Tissues. Biomed. Chromatogr. 20 (4), 327–335. 10.1002/bmc.567 [DOI] [PubMed] [Google Scholar]
- Li P., Lv B., Jiang X., Wang T., Ma X., Chang N., et al. (2016). Identification of NF-Κb Inhibitors Following Shenfu Injection and Bioactivity-Integrated UPLC/Q-TOF-MS and Screening for Related Anti-inflammatory Targets In Vitro and In Silico. J. Ethnopharmacol. 194, 658–667. 10.1016/j.jep.2016.10.052 [DOI] [PubMed] [Google Scholar]
- Li Z., Ryu S. W., Lee J., Choi K., Kim S., Choi C. (2016). Protopanaxatirol Type Ginsenoside Re Promotes Cyclic Growth of Hair Follicles via Inhibiting Transforming Growth Factor β Signaling Cascades. Biochem. Biophys. Res. Commun. 470 (4), 924–929. 10.1016/j.bbrc.2016.01.148 [DOI] [PubMed] [Google Scholar]
- Lim H. W., Kim K., Lim C. J. (2016). Contribution of Ginsenoside Re to Cellular Redox Homeostasis via Upregulating Glutathione and Superoxide Dismutase in HaCaT Keratinocytes under normal Conditions. Pharmazie 71 (7), 413–419. 10.1691/ph.2016.6518 [DOI] [PubMed] [Google Scholar]
- Lim K. H., Lim D. J., Kim J. H. (2013). Ginsenoside-Re Ameliorates Ischemia and Reperfusion Injury in the Heart: a Hemodynamics Approach. J. Ginseng Res. 37 (3), 283–292. 10.5142/jgr.2013.37.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Huang J., Hu X., Li K., Sun C. (2011). Simultaneous Determination of Ginsenoside (G-Re, G-Rg1, G-Rg2, G-F1, G-Rh1) and Protopanaxatriol in Human Plasma and Urine by LC-MS/MS and its Application in a Pharmacokinetics Study of G-Re in Volunteers. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 879 (22), 2011–2017. 10.1016/j.jchromb.2011.05.018 [DOI] [PubMed] [Google Scholar]
- Liu M., Bai X., Yu S., Zhao W., Qiao J., Liu Y., et al. (2019). Ginsenoside Re Inhibits ROS/ASK-1 Dependent Mitochondrial Apoptosis Pathway and Activation of Nrf2-Antioxidant Response in Beta-Amyloid-Challenged SH-Sy5y Cells. Molecules 24 (15), 2687. 10.3390/molecules24152687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. M., Yang L., Zeng X., Deng Y. H., Feng Y., Liang W. X. (2005). [Pharmacokinetics of Ginsenosides Rg1 and Re in Shenmai Injection]. Yao Xue Xue Bao 40 (4), 365–368. [PubMed] [Google Scholar]
- Liu Y. W., Zhu X., Li W., Lu Q., Wang J. Y., Wei Y. Q., et al. (2012). Ginsenoside Re Attenuates Diabetes-Associated Cognitive Deficits in Rats. Pharmacol. Biochem. Behav. 101 (1), 93–98. 10.1016/j.pbb.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Lu D., Liu J., Zhao W., Li P. (2012). Chronic Toxicity of Ginsenoside Re on Sprague-Dawley Rats. J. Ethnopharmacol. 144 (3), 656–663. 10.1016/j.jep.2012.10.007 [DOI] [PubMed] [Google Scholar]
- Ma X., Xu Q., Liang X. (2005). [Analysis of Ginsenosides in Ginseng by Liquid Chromatographyatmospheric Pressure Chemical Ionization Mass Spectrometry]. Se Pu 23 (4), 389–393. [PubMed] [Google Scholar]
- Mancuso C., Santangelo R. (2017). Panax Ginseng and Panax Quinquefolius: From Pharmacology to Toxicology. Food Chem. Toxicol. 107 (Pt A), 362–372. 10.1016/j.fct.2017.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya Y., Mawatari K., Takahashi A., Harada N., Hata A., Yasui S. (2007). The Phytoestrogen Ginsensoside Re Activates Potassium Channels of Vascular Smooth Muscle Cells through PI3K/Akt and Nitric Oxide Pathways. J. Med. Invest. 54 (3-4), 381–384. 10.2152/jmi.54.381 [DOI] [PubMed] [Google Scholar]
- Nam Y., Wie M. B., Shin E. J., Nguyen T. T., Nah S. Y., Ko S. K., et al. (2015). Ginsenoside Re Protects Methamphetamine-Induced Mitochondrial Burdens and Proapoptosis via Genetic Inhibition of Protein Kinase C δ in Human Neuroblastoma Dopaminergic SH-Sy5y Cell Lines. J. Appl. Toxicol. 35 (8), 927–944. 10.1002/jat.3093 [DOI] [PubMed] [Google Scholar]
- National Toxicology Program (2011). Toxicology and Carcinogenesis Studies of Ginseng (CAS No. 50647-08-0) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser. 567, 1–149. [PubMed] [Google Scholar]
- Oh Y., Lim H. W., Kim K., Lim C. J. (2016). Ginsenoside Re Improves Skin Barrier Function in HaCaT Keratinocytes under normal Growth Conditions. Biosci. Biotechnol. Biochem. 80 (11), 2165–2167. 10.1080/09168451.2016.1206808 [DOI] [PubMed] [Google Scholar]
- Palmer B. V., Montgomery A. C., Monteiro J. C. (1978). Gin Seng and Mastalgia. Br. Med. J. 1 (6122), 1284. 10.1136/bmj.1.6122.1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. H., Park S. K., Seung T. W., Jin D. E., Guo T., Heo H. J. (2015). Effect of Ginseng (Panax Ginseng) berry EtOAc Fraction on Cognitive Impairment in C57BL/6 Mice under High-Fat Diet Inducement. Evid. Based Complement. Alternat Med. 2015, 316527. 10.1155/2015/316527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. M., Kim H. M., Kim D. H., Han H. J., Noh H., Jang J. H., et al. (2016). Ginsenoside Re Inhibits Osteoclast Differentiation in Mouse Bone Marrow-Derived Macrophages and Zebrafish Scale Model. Mol. Cell 39 (12), 855–861. 10.14348/molcells.2016.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S., Shin H. S., Kang S. C. (2012). Inhibition of Inflammations and Macrophage Activation by Ginsenoside-Re Isolated from Korean Ginseng (Panax Ginseng C.A. Meyer). Food Chem. Toxicol. 50 (5), 1354–1361. 10.1016/j.fct.2012.02.035 [DOI] [PubMed] [Google Scholar]
- Peng D., Wang H., Qu C., Xie L., Wicks S. M., Xie J. (2012). Ginsenoside Re: Its Chemistry, Metabolism and Pharmacokinetics. Chin. Med. 7, 2. 10.1186/1749-8546-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo M. K., Park K.-H., Oh M. H., Lee H., Park Y. S., Kim N. Y., et al. (2016). Ginsenoside Re Enriched Fraction (GS-F3k1) from Ginseng Berries Ameliorates Ethanol-Induced Erectile DysfunctionviaNitric Oxide-cGMP Pathway. Nat. Prod. Sci. 22 (1), 46–52. 10.20307/nps.2016.22.1.46 [DOI] [Google Scholar]
- Qi L. W., Wang C. Z., Du G. J., Zhang Z. Y., Calway T., Yuan C. S. (2011). Metabolism of Ginseng and its Interactions with Drugs. Curr. Drug Metab. 12 (9), 818–822. 10.2174/138920011797470128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan H., Jin X., Cui E., Zhang Q. (2019). Lipopolysaccharide-induced Inflammation Is Inhibited by Ginsenoside Re through NF-Κb Signaling in RAW264.7 Cells and Primary Rat Hepatocytes. Lat. Am. J. Pharm. 38 (10), 1969–1978. 10.1016/j.ejphar.2006.11.023 [DOI] [Google Scholar]
- Quan H. Y., Yuan H. D., Jung M. S., Ko S. K., Park Y. G., Chung S. H. (2012). Ginsenoside Re Lowers Blood Glucose and Lipid Levels via Activation of AMP-Activated Protein Kinase in HepG2 Cells and High-Fat Diet Fed Mice. Int. J. Mol. Med. 29 (1), 73–80. 10.3892/ijmm.2011.805 [DOI] [PubMed] [Google Scholar]
- Reeds D. N., Patterson B. W., Okunade A., Holloszy J. O., Polonsky K. S., Klein S. (2011). Ginseng and Ginsenoside Re Do Not Improve β-cell Function or Insulin Sensitivity in Overweight and Obese Subjects with Impaired Glucose Tolerance or Diabetes. Diabetes Care 34 (5), 1071–1076. 10.2337/dc10-2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B. Q., Qu C., Mi L., Wang H. Y., Yang H. (2021). Simultaneous Quantification of Twenty-Eight Components of Shenfu Injection in Rat Plasma by UHPLC-QQQ MS and its Application to a Pharmacokinetic Study. J. Pharm. Biomed. Anal. 203, 114211. 10.1016/j.jpba.2021.114211 [DOI] [PubMed] [Google Scholar]
- Shi J., Xue W., Zhao W. J., Li K. X. (2013). Pharmacokinetics and Dopamine/acetylcholine Releasing Effects of Ginsenoside Re in hippocampus and mPFC of Freely Moving Rats. Acta Pharmacol. Sin. 34 (2), 214–220. 10.1038/aps.2012.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Wan X., Shao N., Ye R., Zhang N., Zhang Y. (2016). Protective and Anti-angiopathy E-ffects of ginsenoside Re against D-iabetes M-ellitus via the A-ctivation of P-38 MAPK, ERK1/2 and JNK S-ignaling. Mol. Med. Rep. 14 (5), 4849–4856. 10.3892/mmr.2016.5821 [DOI] [PubMed] [Google Scholar]
- Shin D., Moon H. W., Oh Y., Kim K., Kim D. D., Lim C. J. (2018). Defensive Properties of Ginsenoside Re against UV-B-Induced Oxidative Stress through Up-Regulating Glutathione and Superoxide Dismutase in HaCaT Keratinocytes. Iran. J. Pharm. Res. 17 (1), 249–260. [PMC free article] [PubMed] [Google Scholar]
- Shin E. J., Shin S. W., Nguyen T. T., Park D. H., Wie M. B., Jang C. G., et al. (2014). Ginsenoside Re Rescues Methamphetamine-Induced Oxidative Damage, Mitochondrial Dysfunction, Microglial Activation, and Dopaminergic Degeneration by Inhibiting the Protein Kinase Cδ Gene. Mol. Neurobiol. 49 (3), 1400–1421. 10.1007/s12035-013-8617-1 [DOI] [PubMed] [Google Scholar]
- Son Y. M., Kwak C. W., Lee Y. J., Yang D. C., Park B. C., Lee W. K., et al. (2010). Ginsenoside Re Enhances Survival of Human CD4+ T Cells through Regulation of Autophagy. Int. Immunopharmacol. 10 (5), 626–631. 10.1016/j.intimp.2010.03.002 [DOI] [PubMed] [Google Scholar]
- Song J. H., Choi H. J., Song H. H., Hong E. H., Lee B. R., Oh S. R., et al. (2014). Antiviral Activity of Ginsenosides against Coxsackievirus B3, Enterovirus 71, and Human Rhinovirus 3. J. Ginseng Res. 38 (3), 173–179. 10.1016/j.jgr.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Chen J., Sakwiwatkul K., Li R., Hu S. (2010). Enhancement of Immune Responses to Influenza Vaccine (H3N2) by Ginsenoside Re. Int. Immunopharmacol. 10 (3), 351–356. 10.1016/j.intimp.2009.12.009 [DOI] [PubMed] [Google Scholar]
- Su F., Xue Y., Wang Y., Zhang L., Chen W., Hu S. (2015). Protective Effect of Ginsenosides Rg1 and Re on Lipopolysaccharide-Induced Sepsis by Competitive Binding to Toll-like Receptor 4. Antimicrob. Agents Chemother. 59 (9), 5654–5663. 10.1128/AAC.01381-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F., Yuan L., Zhang L., Hu S. (2012). Ginsenosides Rg1 and Re Act as Adjuvant via TLR4 Signaling Pathway. Vaccine 30 (27), 4106–4112. 10.1016/j.vaccine.2012.03.052 [DOI] [PubMed] [Google Scholar]
- Su X., Pei Z., Hu S. (2014). Ginsenoside Re as an Adjuvant to Enhance the Immune Response to the Inactivated Rabies Virus Vaccine in Mice. Int. Immunopharmacol. 20 (2), 283–289. 10.1016/j.intimp.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Sukrittanon S., Watanapa W. B., Ruamyod K. (2014). Ginsenoside Re Enhances Small-Conductance Ca(2+)-Activated K(+) Current in Human Coronary Artery Endothelial Cells. Life Sci. 115 (1-2), 15–21. 10.1016/j.lfs.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Sun H., Ling S., Zhao D., Li J., Li Y., Qu H., et al. (2020). Ginsenoside Re Treatment Attenuates Myocardial Hypoxia/Reoxygenation Injury by Inhibiting HIF-1α Ubiquitination. Front. Pharmacol. 11, 532041. 10.3389/fphar.2020.532041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H. X., Chen Y., Ye Y. (2006). Ginsenoside Re and Notoginsenoside R1: Immunologic Adjuvants with Low Haemolytic Effect. Chem. Biodivers. 3 (7), 718–726. 10.1002/cbdv.200690074 [DOI] [PubMed] [Google Scholar]
- Tran T. V., Shin E. J., Dang D. K., Ko S. K., Jeong J. H., Nah S. Y., et al. (2017). Ginsenoside Re Protects against Phencyclidine-Induced Behavioral Changes and Mitochondrial Dysfunction via Interactive Modulation of Glutathione Peroxidase-1 and NADPH Oxidase in the Dorsolateral Cortex of Mice. Food Chem. Toxicol. 110, 300–315. 10.1016/j.fct.2017.10.019 [DOI] [PubMed] [Google Scholar]
- Tu T. T., Sharma N., Shin E. J., Tran H. Q., Lee Y. J., Jeong J. H., et al. (2017). Ginsenoside Re Protects Trimethyltin-Induced Neurotoxicity via Activation of IL-6-Mediated Phosphoinositol 3-Kinase/Akt Signaling in Mice. Neurochem. Res. 42 (11), 3125–3139. 10.1007/s11064-017-2349-y [DOI] [PubMed] [Google Scholar]
- Wang H., Lv J., Jiang N., Huang H., Wang Q., Liu X. (2021). Ginsenoside Re Protects against Chronic Restraint Stress‐induced Cognitive Deficits through Regulation of NLRP3 and Nrf2 Pathways in Mice. Phytotherapy Res. 35, 2523–2535. 10.1002/ptr.6947 [DOI] [PubMed] [Google Scholar]
- Wang L., Chen X., Wang Y., Zhao L., Zhao X., Wang Y. (2020). MiR-30c-5p Mediates the Effects of Panax Notoginseng Saponins in Myocardial Ischemia Reperfusion Injury by Inhibiting Oxidative Stress-Induced Cell Damage. Biomed. Pharmacother. 125, 109963. 10.1016/j.biopha.2020.109963 [DOI] [PubMed] [Google Scholar]
- Wang L., Yuan D., Zhang D., Zhang W., Liu C., Cheng H., et al. (2015). Ginsenoside Re Promotes Nerve Regeneration by Facilitating the Proliferation, Differentiation and Migration of Schwann Cells via the ERK- and JNK-dependent Pathway in Rat Model of Sciatic Nerve Crush Injury. Cell. Mol. Neurobiol. 35 (6), 827–840. 10.1007/s10571-015-0177-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. W., Yu X. F., Xu H. L., Zhao X. Z., Sui D. Y. (20192019). Ginsenoside Re Improves Isoproterenol-Induced Myocardial Fibrosis and Heart Failure in Rats. Evid. Based Complement. Alternat. Med. 2019, 3714508. 10.1155/2019/3714508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Liao Q. P., Quan L. H., Liu C. Y., Chang Q., Liu X. M., et al. (2010). The Effect of Acorus Gramineus on the Bioavailabilities and Brain Concentrations of Ginsenosides Rg1, Re and Rb1 after Oral Administration of Kai-Xin-San Preparations in Rats. J. Ethnopharmacol. 131 (2), 313–320. 10.1016/j.jep.2010.06.034 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang Y., Xiao J., Xu R., Wang Q., Wang X. (2018b). Simultaneous Determination of Baicalin, Baicalein, Wogonoside, Wogonin, Scutellarin, Berberine, Coptisine, Ginsenoside Rb1 and Ginsenoside Re of Banxia Xiexin Decoction in Rat Plasma by LC-MS/MS and its Application to a Pharmacokinetic Study. Biomed. Chromatogr. 32 (2), 1. 10.1002/bmc.4083 [DOI] [PubMed] [Google Scholar]
- Wang Y. G., Zima A. V., Ji X., Pabbidi R., Blatter L. A., Lipsius S. L. (2008a). Ginsenoside Re Suppresses Electromechanical Alternans in Cat and Human Cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 295 (2), H851–H859. 10.1152/ajpheart.01242.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Li Y. F., Han X. Y., Sun Y. S., Zhang L. X., Liu W., et al. (2018c). Kidney protection Effect of Ginsenoside Re and its Underlying Mechanisms on Cisplatin-Induced Kidney Injury. Cell. Physiol. Biochem. 48 (5), 2219–2229. 10.1159/000492562 [DOI] [PubMed] [Google Scholar]
- Wu C. F., Bi X. L., Yang J. Y., Zhan J. Y., Dong Y. X., Wang J. H., et al. (2007). Differential Effects of Ginsenosides on NO and TNF-Alpha Production by LPS-Activated N9 Microglia. Int. Immunopharmacol. 7 (3), 313–320. 10.1016/j.intimp.2006.04.021 [DOI] [PubMed] [Google Scholar]
- Xia C., Wang G., Sun J., Hao H., Xiong Y., Gu S., et al. (2008). Simultaneous Determination of Ginsenoside Rg1, Re, Rd, Rb1 and Ophiopogonin D in Rat Plasma by Liquid Chromatography/electrospray Ionization Mass Spectrometric Method and its Application to Pharmacokinetic Study of 'SHENMAI' Injection. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 862 (1-2), 72–78. 10.1016/j.jchromb.2007.11.020 [DOI] [PubMed] [Google Scholar]
- Xiang L., Jiang P., Wang S., Hu Y., Liu X., Yue R., et al. (2013). Metabolomic Strategy for Studying the Intervention and the Synergistic Effects of the Shexiang Baoxin Pill for Treating Myocardial Infarction in Rats. Evid. Based. Complement. Alternat. Med. 2013, 823121. 10.1155/2013/823121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J.-T., Du G.-J., McEntee E., Aung H. H., He H., Mehendale S. R., et al. (2011). Effects of Triterpenoid Glycosides from Fresh Ginseng Berry on SW480 Human Colorectal Cancer Cell Line. Cancer Res. Treat. 43 (1), 49–55. 10.4143/crt.2011.43.1.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J. T., Mehendale S. R., Li X., Quigg R., Wang X., Wang C. Z., et al. (2005a). Anti-diabetic Effect of Ginsenoside Re in Ob/ob Mice. Biochim. Biophys. Acta 1740 (3), 319–325. 10.1016/j.bbadis.2004.10.010 [DOI] [PubMed] [Google Scholar]
- Xie J. T., Mehendale S. R., Wang A., Han A. H., Wu J. A., Osinski J., et al. (2004). American Ginseng Leaf: Ginsenoside Analysis and Hypoglycemic Activity. Pharmacol. Res. 49 (2), 113–117. 10.1016/j.phrs.2003.07.015 [DOI] [PubMed] [Google Scholar]
- Xie J. T., Shao Z. H., Vanden Hoek T. L., Chang W. T., Li J., Mehendale S., et al. (2006). Antioxidant Effects of Ginsenoside Re in Cardiomyocytes. Eur. J. Pharmacol. 532 (3), 201–207. 10.1016/j.ejphar.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Xie J. T., Wang C. Z., Wang A. B., Wu J., Basila D., Yuan C. S. (2005b). Antihyperglycemic Effects of Total Ginsenosides from Leaves and Stem of Panax Ginseng. Acta Pharmacol. Sin. 26 (9), 1104–1110. 10.1111/j.1745-7254.2005.00156.x [DOI] [PubMed] [Google Scholar]
- Xie W., Zhou P., Qu M., Dai Z., Zhang X., Zhang C., et al. (2020). Ginsenoside Re Attenuates High Glucose-Induced RF/6A Injury via Regulating PI3K/AKT Inhibited HIF-1α/VEGF Signaling Pathway. Front. Pharmacol. 11, 695. 10.3389/fphar.2020.00695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Chen D., Lv B., Liu F., Yao Q., Tang Z., et al. (2014). Effects of Ginsenoside Re on Rat Jejunal Contractility. J. Nat. Med. 68 (3), 530–538. 10.1007/s11418-014-0831-2 [DOI] [PubMed] [Google Scholar]
- Xu B. B., Liu C. Q., Gao X., Zhang W. Q., Wang S. W., Cao Y. L. (2005). Possible Mechanisms of the protection of Ginsenoside Re against MPTP-Induced Apoptosis in Substantia Nigra Neurons of Parkinson's Disease Mouse Model. J. Asian Nat. Prod. Res. 7 (3), 215–224. 10.1080/10286020410001690172 [DOI] [PubMed] [Google Scholar]
- Xu X., Jin L., Jiang T., Lu Y., Aosai F., Piao H. N., et al. (2020). Ginsenoside Rh2 Attenuates Microglial Activation against Toxoplasmic Encephalitis via TLR4/NF-Κb Signaling Pathway. J. Ginseng Res. 44 (5), 704–716. 10.1016/j.jgr.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Luo Y., Lu S., Hu R., Du Y., Liao P., et al. (2018). Salvianolic Acid B and Ginsenoside Re Synergistically Protect against Ox-LDL-Induced Endothelial Apoptosis through the Antioxidative and Antiinflammatory Mechanisms. Front. Pharmacol. 9, 662. 10.3389/fphar.2018.00662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Xu S., Liu C., Su Z. (2009). In Vivo metabolism Study of Ginsenoside Re in Rat Using High-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry. Anal. Bioanal. Chem. 395 (5), 1441–1451. 10.1007/s00216-009-3121-1 [DOI] [PubMed] [Google Scholar]
- Yao H., Li J., Song Y., Zhao H., Wei Z., Li X., et al. (2018). Synthesis of Ginsenoside Re-based Carbon Dots Applied for Bioimaging and Effective Inhibition of Cancer Cells. Int. J. Nanomedicine 13, 6249–6264. 10.2147/IJN.S176176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L. C., Chen S. C., Chang W. C., Huang Y. C., Lin K. M., Lai P. H., et al. (2007). Stability of Angiogenic Agents, Ginsenoside Rg1 and Re, Isolated from Panax Ginseng: In Vitro and In Vivo Studies. Int. J. Pharm. 328 (2), 168–176. 10.1016/j.ijpharm.2006.08.009 [DOI] [PubMed] [Google Scholar]
- Yu Y., Sun J., Liu J., Wang P., Wang C. (2020). Ginsenoside Re Preserves Cardiac Function and Ameliorates Left Ventricular Remodeling in a Rat Model of Myocardial Infarction. J. Cardiovasc. Pharmacol. 75 (1), 91–97. 10.1097/FJC.0000000000000752 [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhou Q. M., Li X. D., Xie Y., Duan X., Min F. L., et al. (2006). Ginsenoside R(e) increases fertile and asthenozoospermic infertile human sperm motility by induction of nitric oxide synthase. Arch. Pharm. Res. 29 (2), 145–151. 10.1007/bf02974276 [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang Y., Ma C., Yan Y., Yang Y., Wang X., et al. (2016). Ginsenoside Rd and Ginsenoside Re Offer Neuroprotection in a Novel Model of Parkinson's Disease. Am. J. Neurodegener. Dis. 5 (1), 52–61. [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Li X., Lv W., Yang Y., Gao H., Yang J., et al. (2008). Ginsenoside Re Reduces Insulin Resistance through Inhibition of C-Jun NH2-terminal Kinase and Nuclear Factor-kappaB. Mol. Endocrinol. 22 (1), 186–195. 10.1210/me.2007-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. L., Liu M. L., Huang Y. S., Liang W. Y., Zhang M. M., Fan Y. D., et al. (2020). Ginsenoside Re Enhances the Survival of H9c2 Cardiac Muscle Cells through Regulation of Autophagy. J. Asian Nat. Prod. Res. 22 (8), 774–787. 10.1080/10286020.2019.1632834 [DOI] [PubMed] [Google Scholar]
- Zhou X. M., Cao Y. L., Dou D. Q. (2006). Protective Effect of Ginsenoside-Re against Cerebral Ischemia/reperfusion Damage in Rats. Biol. Pharm. Bull. 29 (12), 2502–2505. 10.1248/bpb.29.2502 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Chen S., Qiao J., Cui Y., Yuan C., He L., et al. (2020). Study of the Noncovalent Interactions of Ginsenosides and Amyloid-β-Peptide by CSI-MS and Molecular Docking. J. Mass. Spectrom. 55 (1), e4463. 10.1002/jms.4463 [DOI] [PubMed] [Google Scholar]