Abstract
OBJECTIVES
This study sought to investigate outcomes of heart failure (HF) in veterans living with human immunodeficiency virus (HIV).
BACKGROUND
Data on outcomes of HF among people living with human immunodeficiency virus (PLHIV) are limited.
METHODS
We performed a retrospective cohort study of Veterans Health Affairs data to investigate outcomes of HF in PLHIV. We identified 5,747 HIV+ veterans with diagnosis of HF from 2000 to 2018 and 33,497 HIV− frequency-matched controls were included. Clinical outcomes included all-cause mortality, HF hospital admission, and all-cause hospital admission.
RESULTS
Compared with HIV− veterans with HF, HIV+ veterans with HF were more likely to be black (56% vs. 14%), be smokers (52% vs. 29%), use alcohol (32% vs. 13%) or drugs (37% vs. 8%), and have a higher comorbidity burden (Elixhauser comorbidity index 5.1 vs. 2.6). The mean ejection fraction (EF) (45 ± 16%) was comparable between HIV+ and HIV− veterans. HIV+ veterans with HF had a higher age-, sex-, and race-adjusted 1-year all-cause mortality (30.7% vs. 20.3%), HF hospital admission (21.2% vs. 18.0%), and all-cause admission (50.2% vs. 38.5%) rates. Among veterans with HIV and HF, those with low CD4 count (<200 cells/ml) and high HIV viral load (>75 copies/μl) had worse outcomes. The associations remained statistically significant after adjusting for extensive list of covariates. The incidence of all-cause mortality and HF admissions was higher among HIV+ veterans with ejection fraction <45%
CONCLUSIONS
HIV+ veterans with HF had higher risk of hospitalization and mortality compared with their HIV− counterparts, with worse outcomes reported for individuals with lower CD4 count, higher viral load, and lower ejection fraction.
Keywords: correlates, epidemiology, heart failure, HIV, prognosis
Human immunodeficiency virus (HIV) infection is an important disease domestically and globally, affecting 1.1 million people in the United States and 36 million in the world (1). The success of antiretroviral therapy (ART) has converted HIV infection into a chronic illness (2). With this, cardiovascular (CV) disease, including HIV–associated cardiac dysfunction and heart failure (HF), have become important causes of morbidity and mortality in people living with HIV (PLHIV) (3–5). An epidemiological study based on Veterans Health Affairs (VHA) data showed that HIV infection is associated with increased risk of HF among US veterans independent of CV risk factors and incident myocardial infarction (3). Recently, we undertook a meta-analysis of extant observational studies of HIV–associated cardiac dysfunction. We found that PLHIV have a substantial burden of cardiac dysfunction and HF (6), with combined prevalence of 12.3% (95% confidence interval [CI]: 6.4% to 19.7%) for left ventricular systolic dysfunction, 11.7% (95% CI: 8.5% to 15.3%) for advanced diastolic dysfunction, and 6.5% (95% CI: 4.4% to 9.6%) for prevalent HF (6).
HIV–associated cardiac dysfunction in the pre-ART era was mainly associated with uncontrolled viremia and opportunistic infections, and had a grave prognosis, with an average life expectancy of few months reported in some studies, for individuals presenting with dilated cardiomyopathy (7–10). Limited observations suggest that with the advent of ART, HIV–associated cardiac dysfunction is presenting with a less fulminant course, whereas diastolic dysfunction is becoming more common (7,11). However, even in the post-ART era, small-scale observational studies have shown that PLHIV have worse HF outcomes compared with individuals without HIV (12,13). Several factors including differences in comorbidity burden, CV risk factor control, HIV–related factors, and type of ART used may potentially contribute to the observed disparities (12,14,15). For instance, a recent observational study involving 2,038 patients (including 374 HIV+ individuals) admitted to a tertiary medical center with decompensated HF found that HIV+ patients with low CD4 count or detectable viral load had higher 30-day readmission and CV mortality compared with patients without HIV. Understanding the prognosis, predictors and potential mediators of HF outcomes in PLHIV has relevance to designing secondary HF prevention strategies in this population. However, data on patient characteristic, prognosis, and predictors of outcomes of HF in PLHIV are limited (16,17). To help fill this gap, we undertook a large-scale analysis of characteristics and clinical outcomes of patients with HIV and HF, using national linkage data from the VHA.
METHODS
DATA SOURCE AND STUDY DESIGN.
This is a retrospective cohort study of HIV–infected veterans with HF (cases) and a frequency-matched subset of HIV–veterans with HF (controls). Patients with HF who received care through the VHA from 1999 through 2018 were identified using the Veterans Administration (VA) Informatics and Computing Infrastructure. HF was defined by the presence of at least 1 inpatient or 2 outpatient International Classification of Diseases (ICD)-9 or ICD-10 codes for HF (Supplemental Table 1). Accordingly, 1.4 million veterans with HF were identified in the VHA database for the specified period. Among these veterans, those with HIV infection were identified using relevant ICD-9 (042, V08) and ICD-10 (B20, Z21) codes for asymptomatic or symptomatic HIV infection and AIDS, an approach that has already been previously validated (3,18). Controls were selected from the HIV− veterans with HF, matched to the HIV+ HF cases by VA medical center, index date of HF, and inpatient versus outpatient status of the index HF. To maximize the study power, 6 controls were selected for every case. HIV+ status was recorded before the index HF date for 85% of the cases. For an additional 5% of cases, such documentation was present within 2 years of the index HF date. The study has received appropriate approvals from the Providence VA Medical Center institutional review board.
COVARIATES.
Data on relevant covariates obtained from the VHA electronic medical record system include information on demographic variables (e.g., age, sex, race), biophysical variables (e.g., blood pressure, weight, height), behavioral factors (smoking, alcohol abuse, illicit drug use), and laboratory variables (e.g., brain natriuretic peptide, hemoglobin, serum creatinine). Smoking was dichotomized as current or never/former smoker. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. For laboratory variables that were measured repeatedly, we took the value that was recorded closest to the date of the index HF. Echocardiographic parameters (i.e., ejection fraction and pulmonary artery systolic pressure) were obtained with an automated information extraction application using natural language processing algorithms validated for the VHA (19). For echocardiographic variables, we took the value that was recorded closest to the first HF diagnosis, between 1 year before and 1 year after the index date. Treatment with HF medications (i.e., angiotensin-converting enzyme inhibitors/angiotensin receptor blocker, beta-blocker, and mineralocorticoid receptor antagonist) was assessed within 1-year time frame (6 months before and 6 months after) index date of HF. HIV–specific variables including information on antiretroviral therapy, viral load, and CD4 T-cell count were obtained for the HIV+ veterans. We took the value that was recorded closest to the first HF diagnosis, between 1 year before and 1 year after the index date. CD4 count was dichotomized into <200 versus ≥200 cells/μl. Viral load was dichotomized as undetectable (or <75 copies/ml) versus detectable (≥75 copies/ml). Antiretroviral therapy was defined as being on 1 or more antiretroviral medication within 2 years (1 year before and 1 year after) of congestive HF index date. Presence of comorbidity was defined by the presence of at least 1 inpatient or outpatient ICD-9 or ICD-10 code for the respective diagnoses recorded before the index date of HF. The burden of comorbidity was assessed using Elixhauser comorbidity index (20). Specific comorbid conditions of interest included hypertension, diabetes, renal failure, myocardial infarction, anemia, drug abuse, alcohol abuse, and depression.
OUTCOMES.
Because HF has high short-term admission and mortality rates, we studied 1-year clinical outcomes. The primary outcomes of interest were: 1) 1-year all-cause mortality; 2) 1-year all-cause admission rate; and 3) 1-year HF admission rate after index HF date. Mortality data were obtained from the Beneficiary Identification Records Locator Subsystem National Death Index, VA vital status file, the Social Security Administration death master file, and the VHA Medical Statistical Analysis Systems inpatient datasets.
STATISTICAL ANALYSES.
The primary comparison was between HIV+ and HIV− veterans. Because inpatient and outpatient HF could represent different aspect and spectrum of the disease, we performed analyses overall as well as stratified by inpatient and outpatient status. Continuous variables are presented as mean and standard deviation or median and interquartile range, as appropriate. Categorical variables are presented as number and proportion. We calculated standard differences (STD) to enable comparison between HIV+ and HIV− veterans with HF; an STD <0.2 is considered small difference, and an STD >0.5 is considered moderate difference. The p values were calculated using Student’s t-test and chi-square tests as appropriate. We compared the incidence of the clinical outcomes by HIV status overall, and within the following categories: 1) by inpatient versus outpatient status of the index HF; 2) by ejection fraction (<45% vs. ≥45%); and 3) for the HIV+ veterans by categories of CD4 count (<200 vs. ≥200 cells/μl) and viral load (<75 copies/μl vs. ≥75 copies/ml). Event-free survivor curves for the outcomes were depicted using Kaplan-Meier survivor plots, adjusted for age, sex, and race. Cox regression models were used to estimate relative risks, adjusting for clustering effect of the VHA medical centers. Hazard ratios (HRs) and 95% CIs were calculated. We performed a progressive adjustment for covariates that may explain the association. We constructed 6 models by dividing the covariate list into 5 groups: 1) basic model: age, sex, and race; 2) selected comorbidity: diabetes, hypertension, and renal failure; 3) biophysical and biochemical factors: systolic blood pressure, BMI, and serum glucose and creatinine concentration; 4) common behavioral CV risk factors: history of smoking and alcohol abuse; 5) additional psychosocial risk factors: drug abuse, depression, and homelessness; and 6) complete list of 29 Elixhauser comorbidities. The regression models were constructed by progressively adding the groups of covariates 1 to 6 to the basic model. Missing values for covariates were imputed using multiple imputation algorithms assuming these values are missing at random. The number of participants with missing data for each of the covariates studied along with summary statistics is presented in Supplemental Table 2.
The assumptions of the proportional hazards were tested using Schoenfeld residuals. Subsidiary analyses were performed in 4,915 HIV+ veterans and 4,915 propensity-matched HIV− controls; matching on age, sex, race, and index date of HF; VA medical center; and an extensive least of covariates (please refer to Supplemental Table 3 for baseline characteristics of propensity matched-cohort demonstrating successful matching). Analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, North Carolina). A 2-sided p value <0.05 was considered statistically significant.
RESULTS
BASELINE CHARACTERISTICS.
The study involved data on 5,747 HIV+ veterans with HF (including 2006 inpatient HF cases) and 33,497 HIV− veterans with HF (including 10,987 inpatient HF controls). The baseline characteristics of study participants are detailed in Table 1. The mean age of veterans with HIV was 58 ± 10 years; the corresponding value for the frequency-matched HIV− cohort was 71 ± 12 years. One-third of the participants had inpatient index HF event, whereas the remaining two-thirds had an outpatient index HF event.
TABLE 1.
Baseline Characteristics of Participants Included in the Study
| HIV+ (N = 5,747) | HIV− (N = 33,497) | ||||
|---|---|---|---|---|---|
| N | %/Mean ± SD | N | %/Mean ± SD | STD | |
| Age (yrs) | 5,747 | 58.3 ± 9.8 | 33,463 | 70.1 ± 12.0 | −1.07 |
| Male | 5,747 | 98.0 | 33,497 | 97.6 | −0.05 |
| White | 5,747 | 34.4 | 33,497 | 67.7 | 0.77 |
| Black | 5,747 | 56.4 | 33,497 | 23.7 | |
| Smoker | 5,747 | 51.9 | 33,497 | 32.1 | 0.41 |
| Systolic BP (mm Hg) | 4,417 | 135 ± 25 | 25,778 | 136 ± 24 | −0.04 |
| Diastolic BP (mm Hg) | 4,407 | 80 ± 16 | 25,769 | 75 ± 15 | 0.31 |
| BMI (kg/m2) | 3,303 | 26.6 ± 6.7 | 19,892 | 29.7 ± 7.3 | −0.44 |
| Glucose (mg/dl) | 5,299 | 119 ± 46 | 30,020 | 126 ± 48 | −0.15 |
| LDL-c (mg/dl) | 5,133 | 90 ± 37 | 28,906 | 90 ± 36 | 0.00 |
| HDL-c (mg/dl) | 5,142 | 42 ± 15 | 28,952 | 43 ± 14 | −0.10 |
| Triglycerides (mg/dl) | 5,157 | 163 ±107 | 29,084 | 139 ± 90 | 0.25 |
| BNP (pg/ml) | 2,971 | 765 ± 1,305 | 14,836 | 639 ± 1,169 | 0.00 |
| Ejection fraction (%) | 4,611 | 45 ± 16 | 23,918 | 45 ± 16 | −0.04 |
| Creatinine (mg/dl) | 5,284 | 1.9 ± 2.2 | 29,908 | 1.5 ± 1.2 | 0.25 |
| Hemoglobin (mg/dl) | 5,263 | 12.6 ± 2.3 | 29,453 | 13.0 ± 2.2 | −0.18 |
| Albumin (mg/dl) | 5,265 | 3.5 ± 0.7 | 29,362 | 3.7 ± 0.7 | −0.28 |
| WBC count (cells/μl) | 5,249 | 6.8 ± 3.5 | 29,440 | 8.2 ± 6.4 | −0.28 |
| ALT (U/l) | 5,282 | 35 ± 30 | 29,764 | 28 ± 23 | 0.28 |
| Elixhauser index | 5,747 | 5.10 ± 3.17 | 33,497 | 2.95 ± 2.62 | 0.74 |
| Hypertension | 5,747 | 75.2 | 33,497 | 77.2 | −0.05 |
| DM (no complication) | 5,747 | 30.9 | 33,497 | 37.0 | −0.13 |
| DM (with complication) | 5,747 | 15.2 | 33,497 | 17.8 | −0.07 |
| MI | 5,747 | 20.2 | 33,497 | 22.9 | −0.07 |
| PVD | 5,747 | 12.0 | 33,497 | 17.5 | −0.16 |
| CLD | 5,747 | 33.0 | 33,497 | 28.0 | 0.11 |
| Renal failure | 5,747 | 13.8 | 33,497 | 7.3 | 0.21 |
| Liver failure | 5,747 | 28.7 | 33,497 | 5.7 | 0.64 |
| Obesity | 5,747 | 14.8 | 33,497 | 23.3 | −0.22 |
| Alcohol abuse | 5,747 | 31.7 | 33,497 | 12.7 | 0.47 |
| Drug abuse | 5,747 | 36.6 | 33,497 | 8.3 | 0.72 |
| Depression | 5,747 | 41.2 | 33,497 | 22.9 | 0.40 |
| Beta-blocker | 5,747 | 58.3 | 33,497 | 59.8 | −0.03 |
| ACEI/ARB | 5,747 | 59.3 | 33,497 | 57.8 | −0.04 |
| MRA | 5,747 | 13.5 | 33,497 | 11.8 | 0.05 |
p < 0.05 for all except BNP.
ACEI/ARB = angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; ALT = aspartate transaminase; BMI = body mass index; BNP = brain natriuretic peptide; BP = blood pressure; CLD = chronic lung disease; DM = diabetes; HDL-c = high-density lipoprotein cholesterol; LDL-c = low-density lipoprotein cholesterol; MI = myocardial infarction; MRA = mineralocorticoid receptor antagonist; PVD = peripheral vascular disease; STD = standard difference; WBC = white blood cell.
Veterans with HIV and HF were more likely to be black (56% vs. 14%), have lower BMI (27 kg/m2 vs. 30 kg/m2), have higher creatinine (1.9 mg/dl vs. 1.5 mg/dl), have liver failure (29% vs. 6%), smoke (52% vs. 29%), abuse alcohol (32% vs. 13%), abuse illicit drugs (37% vs. 8%), or be depressed (41% vs. 23%). Overall, HIV+ veterans had higher comorbidity burden compared with HIV− veterans (Elixhauser comorbidity index 5.1 vs. 2.6). HIV+ veterans tended to have lower prevalence of hypertension, diabetes, myocardial infarction, and peripheral vascular disease, but the magnitude was smaller. The mean ejection fraction (EF) was comparable between HIV+ and HIV− veterans (EF 45 ± 16%). The use of HF medications (i.e., angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, beta-blocker, and mineralocorticoid receptor antagonist) was comparable between HIV+ and HIV− veterans (Table 1). There was a similar pattern of differences between HIV+ and HIV− veterans for inpatient HF cases (data not shown). The prevalence of EF <40%, EF 40% to 49%, and EF ≥50% were 29%, 15% and 37%, respectively, for HIV+ veterans. The corresponding values for HIV− veterans were 25%, 14%, and 33%, respectively. EF data were more likely to be missing for HIV− veterans compared with HIV+ veterans (29% vs. 20%). The prevalence of veterans with a CD4 count <200 cells/μl and viral load ≥75 copies/ml was 21% and 31%, respectively. A total of 79% HIV+ veterans were receiving ART.
INCIDENCE OF CLINICAL OUTCOMES.
Kaplan-Meier plots for all-cause mortality, all-cause readmissions, and HF readmissions are shown in Figure 1. HIV+ veterans with HF, compared with HIV− veterans with HF, had higher 1-year adjusted all-cause mortality (30.7% vs. 20.3%), all-cause hospital admission (50.2% vs. 38.5%), and HF hospital admission (21.2% vs. 18.0%) (Supplemental Table 4A). The incidence of outcomes was higher but followed similar pattern for veterans with inpatient index HF (Supplemental Table 4B).
FIGURE 1. Kaplan-Meier Survival Curves of Veterans With HF by HIV Status.
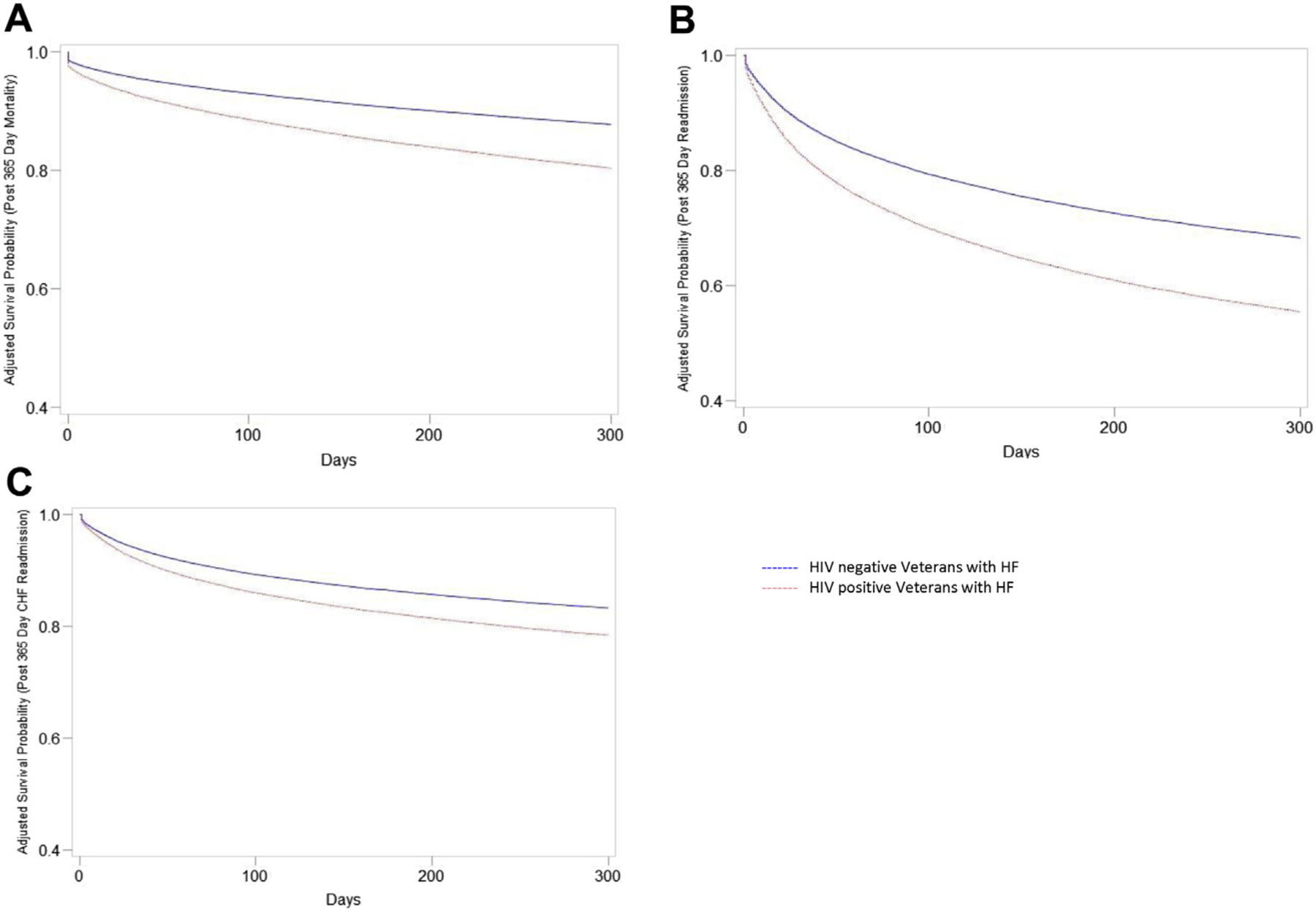
(A) All-cause mortality, (B) all-cause readmission, and (C) HF readmission. Kaplan-Meier pots were adjusted for age, sex, race. p < 0.05 for comparisons in A to C. HF = heart failure; HIV = human immunodeficiency virus.
RELATIVE RISK OF CLINICAL OUTCOMES.
Compared with HIV− HF patients, those with HIV had age-, sex-, and race-adjusted HRs (95% CIs) of 1.63 (1.52 to 1.75), 1.43 (1.36 to 1.49), and 1.20 (1.12 to 1.28) for 1-year all-cause mortality, all-cause hospital admissions, and HF hospital admissions, respectively (Table 2). These associations were attenuated but remained statistically significant in stepwise Cox regression models adjusting for extensive list of covariates, with corresponding HR (95% CI) of 1.17 (1.08 to 1.26), 1.19 (1.14 to 1.25), and 1.13 (1.06 to 1.22), respectively. For all-cause mortality the major attenuation in association was with adjustment in Model 3 (i.e., adjusting for diabetes, hypertension, BMI, and renal failure), and with adjustment in Model 6 (adjusting for Elixhauser comorbidities) (Table 2). For hospital admissions, the major attenuation was with adjustment in Model 6. Similar patterns of association were observed when restricting analyses to inpatient HF cases (Table 2). The hazard ratios of 365-day mortality, all-cause readmission, and HF admission were comparable in subsidiary analyses of propensity matched cohort involving 4,915 HIV+ veterans and 4,915 HIV− veterans (Supplemental Table 5).
TABLE 2.
Association of HIV Status With 1-Year Clinical Outcomes
| Adjustment | HR (95% CI) HIV+ vs. HIV− Veterans With HF | ||
|---|---|---|---|
| 365-Day Mortality | 365-Day All-Cause Readmission | 365-Day HF Readmission | |
| All index HF cases | |||
| Model 1 | 1.63 (1.52–1.75) | 1.43 (1.36–1.49) | 1.20 (1.12–1.28) |
| Model 2 | 1.59 (1.49–1.72) | 1.42 (1.36–1.49) | 1.21 (1.13–1.29) |
| Model 3 | 1.38 (1.28–1.48) | 1.39 (1.33–1.46) | 1.21 (1.13–1.29) |
| Model 4 | 1.32 (1.23–1.42) | 1.33 (1.27–1.39) | 1.17 (1.10–1.25) |
| Model 5 | 1.32 (1.23–1.42) | 1.27 (1.21–1.34) | 1.15 (1.08–1.23) |
| Model 6 | 1.17 (1.08–1.26) | 1.19 (1.14–1.25) | 1.13 (1.06–1.22) |
| Inpatient index HF cases only* | |||
| Model 1 | 1.54 (1.40–1.71) | 1.24 (1.16–1.34) | 1.17 (1.07–1.29) |
| Model 2 | 1.51 (1.36–1.67) | 1.26 (1.17–1.35) | 1.20 (1.08–1.32) |
| Model 3 | 1.33 (1.20–1.48) | 1.25 (1.16–1.34) | 1.21 (1.10–1.34) |
| Model 4 | 1.32 (1.19–1.47) | 1.23 (1.14–1.32) | 1.20 (1.08–1.32) |
| Model 5 | 1.36 (1.22–1.51) | 1.16 (1.08–1.25) | 1.16 (1.04-,1.28) |
| Model 6 | 1.26 (1.13–1.40) | 1.11 (1.03–1.20) | 1.13 (1.02–1.26) |
Model 1: age, sex, race; Model 2: model 1 + diabetes, hypertension, renal failure; Model 3: Model 2 + BMI, SBP, glucose, creatinine; Model 4: Model 3 + smoking and alcohol abuse; Model 5: Model 4 + drug abuse, depression, and homelessness; Model 6: Model 5 + Elixhauser comorbidity.
HIV = human immunodeficiency virus; SBP = systolic blood pressure; other abbreviations as in Table 1.
HIV MARKERS AND CLINICAL OUTCOMES.
Among patient with HIV, those with low CD4 count and increased HIV viral load had worse clinical outcomes (Figure 2). Veterans with CD4 count <200 cells/μl had age-, sex-, and race-adjusted hazard ratio of 2.36 (95% CI: 2.04 to 2.74) for all-cause mortality compared with veterans with CD4 count of ≥200 cells/μl (Table 3). The association was only mildly attenuated with adjustment for an extensive list of covariates. Veterans with HIV and a viral load of ≥75 copies/ml had an age-, sex-, and race-adjusted HR of 1.33 (95% CI: 1.14 to 1.54) for all-cause mortality compared with those with a viral load of <75 copies/ml. The association did not change with adjustment in the full model. There were similar patterns but weaker associations between CD4 count or viral load and all-cause or HF-specific hospital admission outcomes (Table 3). Individuals with missing data on CD4 count or viral load had the worst clinical outcomes.
FIGURE 2. Kaplan-Meier Survivor Curves of Veterans With HF and HIV by Viral Load and CD4 Count for All-Cause Mortality and All-Cause Readmission Outcomes.
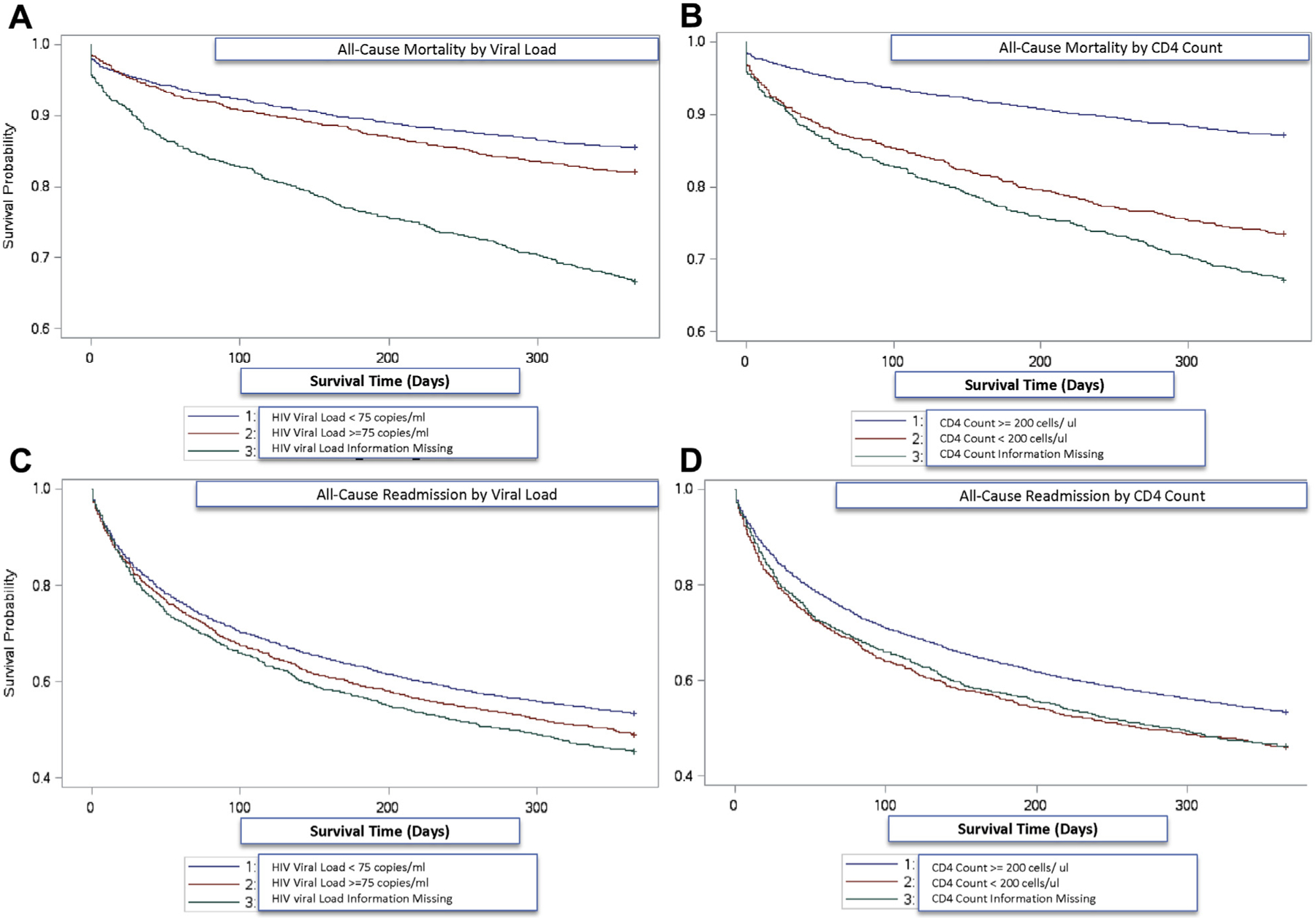
Kaplan-Meier plots were adjusted for age, sex, race. p < 0.05 for comparisons in A to D. Event-free survival curves for all-cause mortality by (A) HIV viral load and (B) CD4 count, and for all-cause readmissions by (C) HIV viral load and (D) CD4 count. Abbreviations as in Figure 1.
TABLE 3.
HR of Clinical Outcomes for People With HIV and HF by CD4 Count or by HIV Viral Load
| Adjustment* | HR (95% CI) | ||
|---|---|---|---|
| CD4 Count <200 vs. ≥200 Cells/μl (n = 4,683) | Viral Load ≥75 vs. <75 Copies/ml (n = 4,593) | ||
| 365-day mortality | Model 1 | 2.36 (2.04–2.74) | 1.33 (1.14–1.54) |
| Full model | 1.83 (1.57–2.13) | 1.37 (1.18–1.61) | |
| 365-day all-cause readmission | Model 1 | 1.24 (1.13–1.36) | 1.09 (1.00–1.19) |
| Full model | 1.17 (1.06–1.29) | 1.14 (1.04–1.25) | |
| 365-day HF readmission | Model 1 | 1.19 (1.04–1.36) | 1.14 (1.07–1.29) |
| Full model | 1.16 (1.01–1.33) | 1.15 (1.01–1.31) | |
Model 1: age, sex, race adjusted; Full model: adjusted for: age, sex, race, SBP, HTN history, BMI, total cholesterol, HDL-cholesterol, prior smoking, prior alcohol abuse, prior drug use, DM, CKD, MI, serum creatinine, depression, homeless, Elixhauser comorbidity index adjusted.
CARDIAC FUNCTION AND CLINICAL OUTCOMES.
The association of EF with the 3 clinical outcomes was variable (Table 4). In a multivariable adjusted Cox model, HIV+ veterans with EF ≥45% had significantly lower 1-year HF admission (HR: 0.48; 95% CI: 0.43 to 0.54), but not all-cause readmission (HR: 0.96; 95% CI: 0.88 to 1.04), compared with those with EF <45%. The corresponding association with all-cause mortality was weaker than that with HF admissions but was statistically significant (HR: 0.84; 95% CI: 0.73 to 0.97). Restricting the analyses to inpatient index HF cases only yielded comparable results for HF readmission, whereas the association was not statistically significant for all-cause mortality (Table 4). Subsidiary analyses based on propensity-matched cohort yielded comparable results (Supplemental Table 6). The findings were also consistent when we analyzed data on HIV− veterans with HF (Supplemental Table 7).
TABLE 4.
HR of Clinical Outcomes for HIV+ Veterans With HF, by EF (≥45% vs. <45%)
| Outcome | Adjustment | HR (95% CI) EF ≥45% vs. <45% |
|---|---|---|
| All index HF cases | ||
| 365-day mortality | Model 1 | 0.91 (0.80–1.05) |
| Full Model | 0.84 (0.73–0.97) | |
| 365-day all-cause readmission | Model 1 | 1.04 (0.96–1.13) |
| Full Model | 0.96 (0.88–1.04) | |
| 365-day HF readmission | Model 1 | 0.52 (0.46–0.58) |
| Full Model | 0.48 (0.43–0.54) | |
| Inpatient index HF cases only* | ||
| 365-day mortality | Model 1 | 0.88 (0.73–1.07) |
| Full Model | 0.86 (0.70–1.05) | |
| 365-day all-cause readmission | Model 1 | 1.14 (1.01–1.30) |
| Full Model | 1.08 (0.94–1.23) | |
| 365-day HF readmission | Model 1 | 0.66 (0.55–0.78) |
| Full Model | 0.62 (0.52–0.74) | |
Model 1: age, sex, race adjusted; Full Model: adjusted for: adjusted for: age, sex, race, SBP, HTN history, BMI, total cholesterol, HDL-cholesterol, prior smoking, prior alcohol abuse, prior drug use, DM, CKD, MI, serum creatinine, depression, homeless, Elixhauser comorbidity index adjusted.
DISCUSSION
Although epidemiological studies have shown that HIV+ veterans have significantly higher risk of HF compared with HIV− veterans, data on prognosis of HF and associated factors in PLHIV are limited. We performed a retrospective cohort study of VHA data involving more than 5,700 HIV+ veterans with HF and 33,500 HIV− veteran controls with HF, we found that HIV status is associated with significantly worse clinical outcomes including all-cause mortality, all-cause hospital admission, and HF hospital admission at 1-year follow-up (Central Illustration). The association was similar when analyses were restricted to inpatient index HF cases. We also found that, among those with HIV, low CD4 count and unsuppressed viral load were independently associated with poor outcomes. HIV+ veterans with preserved EF had lower incidence of HF readmissions and all-cause mortality.
CENTRAL ILLUSTRATION. Kaplan-Meier Survivor Curves of Veterans With HF by HIV Status, and by HIV Viral Load and CD4 Count (for the HIV+ Subgroup).
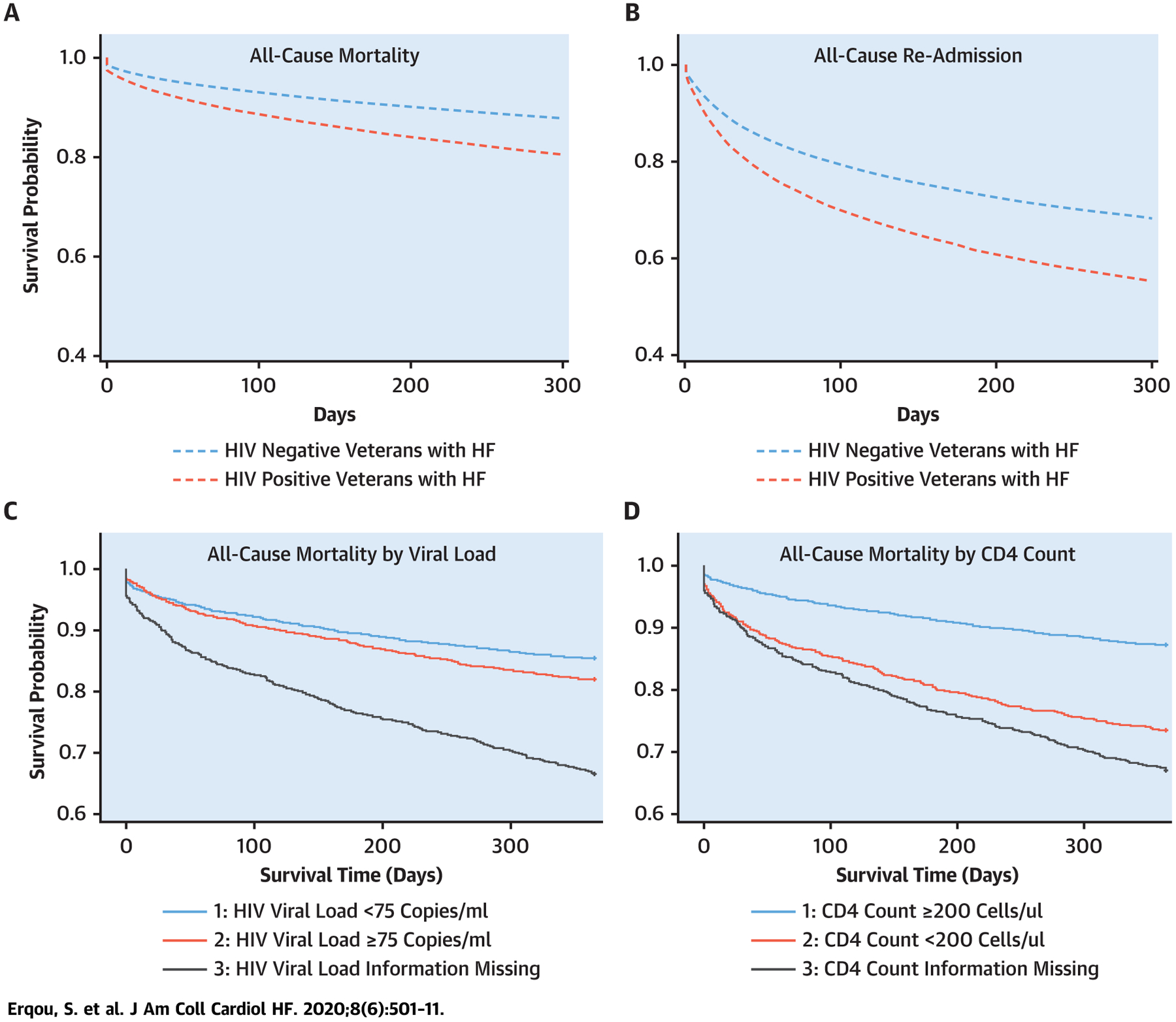
Kaplan Meier plots were adjusted for age, sex, and race. p < 0.05 for comparisons in A to D.
Although few prior small-scale observational studies have suggested that HIV is associated with poor HF outcomes (12,13,21), this study provided a large comprehensive data on the topic allowing more precise and detailed characterization. Prior reports did not make adjustment for covariates (12,13,21), whereas 1 study (22) that adjusted for confounding using propensity matching only looked at rehospitalization outcome. In this study, we were able to take into account an extensive list of covariates to explore the relationship between HIV status and multiple HF outcomes. Our findings demonstrate that HIV patients with HF have worse prognosis even in the ART era, highlighting the importance of exploring primary and secondary preventive strategies. The present study adds novel data to the findings from a prior smaller study (23) reporting that PLHIV hospitalized with HF have increased risk of sudden cardiac death and another study (24) reporting that elevated concentrations of biomarkers of myocardial strain and fibrosis predict CV mortality among PLHIV. (Table 5 provides summary of innovation of study.)
TABLE 5.
Summary of Innovation of the Present Study
| What Is Known | What Is Not Known | What This Study Adds |
|---|---|---|
| HIV increases risk of heart failure | Outcome of heart failure in people with HIV is not well studied | A large and comprehensive data on multiple heart failure outcomes (hospitalization, death) in people with HIV (comprising >5,700 HIV patients with heart failure) allowed precise and detailed characterization than has been possible before |
| People with HIV and heart failure have increased risk of sudden cardiac death | There are limited data on all-cause mortality outcome in people with HIV and heart failure (prior studies comprise only ~400 HIV patients with heart failure) | The study was able to account for extensive list of risk factors |
| People with HIV and heart failure have higher risk of hospitalization | Studies on mortality outcome in people with HIV did not make adjustment for risk factors | The study explored factors that may contribute to worse clinical outcome of heart failure in those with HIV |
| Biomarkers of myocardial strain and fibrosis (ST2 and GDF-15) are associated with cardiovascular disease mortality in HIV | The association of systolic cardiac function with heart failure outcomes in people with HIV is not known | Reduced ejection fraction, lower CD4 count and detectable viral load were associated with increased risk of heart failure hospitalization and all-cause mortality |
Abbreviation as in Table 2.
Despite differences in population characteristics of HIV+ and HIV− veterans with HF, the association between HIV status and clinical outcomes remained statistically significant after adjusting for extensive list of covariates. We made adjustment for covariates in stepwise models to explore factors that may be mediating and/or confounding the association. For all-cause mortality, the major portion of the attenuation in the association was noted when adjusting for 2 groups of covariates: 1) blood pressure, BMI, and serum glucose and creatinine; and 2) Elixhauser comorbidities. This suggests the potential role of traditional CV risk factors and comorbidity in accounting for poor HF outcomes that is observed in PLHIV. Some of these factors including blood pressure and blood glucose levels may be studied as potential targets for improving clinical outcomes in this population. HIV+ veterans were more likely to be ethnic minority, and were by far more likely to smoke, use drugs or alcohol, or have depression or psychoses, highlighting the importance of social factors in affecting the prognosis of HF in this population.
On analyses restricted to veterans with HIV and HF, lower CD4 count <200 cells/μl had 1.8-fold higher adjusted risk of 1-year all-cause mortality compared with CD4 count ≥200 cells/μl. Similarly, individuals with elevated HIV viral load (≥75 copies/ml) had higher adjusted risk of all-cause mortality compared with those with suppressed viral load (<75 copies/ml), although the HR was lower (~1.4). Similar patterns but weaker associations were observed for the other outcomes. These findings are consistent with the recent report of lower CD4 count and higher viral load being associated with poor HF outcomes in HIV (12). Also, a prior report on veterans without history of HF or baseline cardiovascular disease (CVD) showed that lower CD4 count and higher viral load were associated with increased risk of incident HF (3). Epidemiological studies have also reported that immunosuppression and HIV viremia are associated with other adverse CVD outcomes such as myocardial infarction (17,25). It has been hypothesized that these observed associations may reflect the abnormal immune activation and persistent inflammation observed in uncontrolled HIV infection and/or direct adverse effect of HIV viremia or opportunistic infections on cardiomyocytes in people who do not receive adequate ART (12,17,26–28). Another explanation that may at least partially account for the observe association is that higher CD4 count and lower viral load may reflect the confounding effect of engagement with HIV care and good medical compliance, which are inherently protective factors. Consistent with this, we found that individuals with missing data on CD4 or viral load (which may suggest poor engagement with HIV care) had worse clinical outcomes. Although we attempted to adjust for factors that may be related with engagement with care such as homelessness and substance abuse, these may not fully capture its effects leading to residual confounding. However, that the association was only mildly attenuated and remained strong in fully adjusted models suggests that immune status and viral load are independently linked to these adverse HF outcomes.
With regard to cardiac function, we found that HIV+ veterans with preserved EF (EF ≥45%) had 50% lower incidence of HF readmissions compared with those with reduced EF (EF <45%). These findings were consistent in adjusted models, in analyses restricted to inpatient index HF cases, or in subsidiary analyses using propensity-matched sample. There was similar association in analyses of HIV− veterans. Whether the risk of mortality in HIV− individuals with HF with persevered EF versus those with HF with reduced EF (HFREF) is different is controversial. A recent study of international multiethnic cohort of patients with HF showed that individuals with HF with persevered EF had lower mortality compared with those with HFREF (29). Our study extends these findings to HIV+ individuals with HF. Although it may be postulated that these findings are in part the result of ischemic heart disease being more prevalent in patients with HFREF, that the HRs were adjusted for extensive list of covariates including CVD-related comorbidity, such as diabetes, myocardial infarction, and peripheral vascular disease, makes it less likely. Previous studies have not reported on the association of cardiac function with HF outcomes in PLHIV.
STUDY LIMITATIONS.
First, this was retrospective observational study; therefore, causal inference is limited by residual confounding. The concern of residual confounding maybe less for the associations between CD4 count and HIV viral load with HF outcomes as the estimates were minimally changed with extensive adjustment. On the other hand, the association of HIV status with HF outcomes was attenuated materially with adjustment in multivariate models, raising the concern for residual confounding and whether the observed association represents a causal relationship. However, that the association remained highly statistically significant after extensive adjustment is reassuring. Some of the covariates adjusted for such as depression and renal function may also act as mediators, in which case the models may be partially overadjusted. Nevertheless, understanding the outcomes of HF in patients with HIV, and identifying factors that are related to them (e.g., substance abuse, depression) is still useful in identifying strategies to improving the outcomes in this population. Second, the study was based on record linkage and ICD code of exposure and outcome from electronic medical records as opposed to direct patient inquiry, which can limit the accuracy of the data. However, prior studies and reports have shown the validity of VHA electronic medical record data for using this approach (3,18,30). Furthermore, any misclassification arising from such error may be presumed to be random and hence would be less likely to cause a systematic bias. Third, because the VHA is a national integrated health care system providing care for veterans only, which are approximately 98% male, the generalizability of these data to other populations may not extend to the female population. However, prior smaller scale studies in non-VHA population have not found major discrepancies (12,13). There are also plausible biological and epidemiological mechanisms for the observed associations. Fourth, we did not take into account medical therapy of HF, which is relevant for patients with HF with reduced LVEF. Future analyses taking into account dosage of medications, duration of treatment, and medication adherence in those with HF with reduced LVEF will be useful, as we did find HIV+ veterans with HFREF had worse clinical outcomes compared with those with preserved EF. Finally, differences in the age structures of the underlying populations of those with and without HIV in the VA Healthcare System likely contribute significantly to the observed differences in baseline age of index HF between veterans with and without HIV. On average those without HIV were significantly older in the present study.
CONCLUSIONS
In a retrospective cohort study of large record-linkage data from the VHA, we found that HIV+ veterans with HF had significantly worse clinical outcomes compared with their HIV− counterparts after adjusting for multiple factors. Further investigations are needed to identify strategies to improve HF outcomes among veterans with HIV.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
In a study using national VA data, we found hospitalizations and deaths from HF are higher among veterans with HIV compared with those without HIV. Veterans with HIV and HF had higher burden of CV risk factors and comorbidity. However, the association persisted even after controlling for these factors. These findings should help to raise awareness of clinicians to the impact of HIV status on the prognosis of HF, and prompt a rigorous evaluation and intervention to counteract this increased risk, such as early institution of goal-directed medical therapy and aggressive modification of risk factors.
TRANSLATIONAL OUTLOOK:
Future studies should investigate further the reasons for disparities in HF outcomes by HIV status to help identify potential targets for interventions. For instance, additional analyses into the use of goal-directed medical therapy, taking into account dosage of medications, duration of treatment, and medication adherence, may help to elucidate some of the reasons. In addition, extensive characterization of patients with HIV-associated HF, including genetic, proteomic, and metabolic studies, can form the basis for translational research uncovering biological mechanisms impacting HF outcomes in HIV.
Acknowledgments
The statements and opinions expressed are those of the authors and do not represent the official policy or procedures of the United States Government or the Department of Veterans Affairs. Dr. Erqou is supported by funding from Rhode Island Foundation and Center for AIDS Research at Brown University. Drs. Erqou, Choudhary, Lally, Zullo, Rudolph, and Wu are employees of the Veterans Health Administration. Drs. Rudolph and Wu are funded by the VA Health Services Research and Development Center of Innovation in Long Term Services and Supports (CIN 13-4193). Dr. Bloomfield is supported by R01MD013493. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- ART
antiretroviral therapy
- BMI
body mass index
- CI
confidence interval
- CV
cardiovascular
- CVD
cardiovascular disease
- EF
ejection fraction
- HF
heart failure
- HFREF
heart failure with reduced ejection fraction
- HIV
human immunodeficiency virus
- HR
hazard ratio
- ICD
International Classification of Diseases
- PLHIV
people living with human immunodeficiency virus
- STD
standard differences
- VA
Veterans Affairs
- VHA
Veterans Health Administration
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Heart Failure author instructions page.
APPENDIX For supplemental tables, please see the online version of this paper.
REFERENCES
- 1.HIV Surveillance Report, 2016. 2017. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed September 25, 2018.
- 2.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013;8: e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freiberg MS, Chang CH, Skanderson M, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol 2017;2: 536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Womack JA, Chang CC, So-Armah KA, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc 2014;3:e001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with the human immunodeficiency virus: a systematic review and meta-analysis. Circulation 2018;138:1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erqou S, Lodebo BT, Masri A, et al. Cardiac dysfunction among people living with HIV: a systematic review and meta-analysis. J Am Coll Cardiol HF 2019;7:98–108. [DOI] [PubMed] [Google Scholar]
- 7.Remick J, Georgiopoulou V, Marti C, et al. Heart failure in patients with human immunodeficiency virus infection: epidemiology, pathophysiology, treatment, and future research. Circulation 2014; 129:1781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumsden RH, Bloomfield GS. The causes of HIV-associated cardiomyopathy: a tale of two worlds. Biomed Res Int 2016;2016:8196560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000;342:1077–84. [DOI] [PubMed] [Google Scholar]
- 10.Currie PF, Jacob AJ, Foreman AR, Elton RA, Brettle RP, Boon NA. Heart muscle disease related to HIV infection: prognostic implications. BMJ 1994;309:1605–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler J, Kalogeropoulos AP, Anstrom KJ, et al. Diastolic dysfunction in individuals with human immunodeficiency virus infection: literature review, rationale and design of the Characterizing Heart Function on Antiretroviral Therapy (CHART) Study. J Card Fail 2018;24:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvi RM, Afshar M, Neilan AM, et al. Heart failure and adverse heart failure outcomes among persons living with HIV in a US tertiary medical center. Am Heart J 2019;210:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janjua SA, Triant VA, Addison D, et al. HIV infection and heart failure outcomes in women. J Am Coll Cardiol 2017;69:107–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvi RM, Neilan AM, Tariq N, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV and heart failure. J Am Coll Cardiol 2018; 72:518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvi RM, Neilan AM, Tariq N, et al. Incidence, predictors, and outcomes of implantable cardioverter-defibrillator discharge among people living with HIV. J Am Heart Assoc 2018;7: e009857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloomfield GS, Alenezi F, Barasa FA, Lumsden R, Mayosi BM, Velazquez EJ. Human immunodeficiency virus and heart failure in low- and middle-income countries. JACC Heart Fail 2015;3:579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation 2019;140: e98–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGinnis KA, Fine MJ, Sharma RK, et al. Understanding racial disparities in HIV using data from the veterans aging cohort 3-site study and VA administrative data. Am J Public Health 2003; 93:1728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garvin JH, DuVall SL, South BR, et al. Automated extraction of ejection fraction for quality measurement using regular expressions in unstructured information management architecture (UIMA) for heart failure. J Am Med Inform Assoc 2012;19:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson NR, Fan Y, Dalton JE, et al. A new Elixhauser-based comorbidity summary measure to predict in-hospital mortality. Med Care 2015;53: 374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sliwa K, Davison BA, Mayosi BM, et al. Readmission and death after an acute heart failure event: predictors and outcomes in sub-Saharan Africa: results from the THESUS-HF registry. Eur Heart J 2013;34:3151–9. [DOI] [PubMed] [Google Scholar]
- 22.Brouch D, Tashtish N, Di Felice C, Longenecker CT, Al-Kindi SG. Human immunodeficiency virus infection and risk of heart failure rehospitalizations. Am J Cardiol 2019;124: 1232–8. [DOI] [PubMed] [Google Scholar]
- 23.Alvi RM, Neilan AM, Tariq N, et al. The risk for sudden cardiac death among patients living with heart failure and human immunodeficiency virus. J Am Coll Cardiol HF 2019;7:759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Secemsky EA, Scherzer R, Nitta E, et al. Novel biomarkers of cardiac stress, cardiovascular dysfunction, and outcomes in HIV-infected individuals. J Am Coll Cardiol HF 2015;3:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013;173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steverson AB, Pawlowski AE, Schneider D, et al. Clinical characteristics of HIV-infected patients with adjudicated heart failure. Eur J Prev Cardiol 2017;24:1746–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy WS, Simon GL, Rios JC, Ross AM. Prevalence of cardiac abnormalities in human immunodeficiency virus infection. Am J Cardiol 1989;63: 86–9. [DOI] [PubMed] [Google Scholar]
- 28.De Castro S, d’Amati G, Gallo P, et al. Frequency of development of acute global left ventricular dysfunction in human immunodeficiency virus infection. J Am Coll Cardiol 1994;24: 1018–24. [DOI] [PubMed] [Google Scholar]
- 29.Lam CSP, Gamble GD, Ling LH, et al. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi-ethnic cohort study. Eur Heart J 2018;39:1770–80. [DOI] [PubMed] [Google Scholar]
- 30.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care 2006;44 Suppl 2: S25–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


