Abstract
Objective:
To identify longitudinal bidirectional associations between unique sleep trajectories and obesity and hypertension among Black, adolescent girls.
Design, setting, and participants:
Longitudinal data were from a randomized controlled trial (2009–2013) implemented in schools serving low-income communities aimed at preventing obesity among adolescent girls (mean age = 12.2 y (SD ± 0.72).
Measures:
Nocturnal sleep data were extracted from accelerometers at T1 (enrollment, n = 470), T2 (6-month, n = 348), and T3 (18-month follow-up, n = 277); height and weight were measured at T1–T3; and systolic/diastolic blood pressure (SDBP) at T1 and T3 using an oscillometric monitor. Multilevel models examined longitudinal associations. Finite mixture models identified sleep trajectory groups. Structural equation models examined whether T1 chronic disease risk predicted sleep profiles, and conversely, if sleep trajectories predicted T3 chronic disease risk. Data were analyzed in 2021.
Results:
For each additional hour of sleep and 1% increase in efficiency there was a 7% lower risk of overweight/obesity at T1 and 6% lower risk at T2, but not at T3. Four sleep trajectories emerged: Worsened, Irregular, Improved, and Regular, with no demographic or metabolic differences between the trajectories. Improved sleep trajectory predicted lower diastolic percentile at T3 (b = −8.81 [95% CI −16.23, −1.40]).
Conclusions:
Group-based trajectories of sleep duration and quality provide information on modifiable factors that can be targeted in interventions to evaluate their impact on reducing chronic diseases and addressing disparities. Additional research is needed on samples beyond those recruited in the context of an intervention study.
Keywords: Adolescent, Sleep, Obesity, Blood Pressure, Poverty, Longitudinal Design, Multi-Group Trajectory
Introduction
Insufficient sleep is common among adolescents, as most middle school-age (60%) and high school-age adolescents (78%) report sleeping less than the recommended 8 hours per night.1, 2 Adolescents experience changes in sleep patterns due to both biological factors, including delay in circadian rhythm with the onset of puberty3, and social factors such as early school start times, after school commitments (i.e. family, academic, vocational, and recreational activities) and increasing autonomy over bedtimes4, all of which may contribute to shorter sleep durations.
However, lack of routines, crowded housing, neighborhood noise and lighting, and crime are among the social and environmental determinants of insufficient sleep duration and poor quality that disproportionally impact adolescents of color and low socioeconomic status.5 Black adolescent girls in the U.S. report shorter sleep durations6, have more fragmented sleep7, and disproportionally higher prevalence of obesity8 than White adolescent girls and are at greatest risk for hypertension in adulthood.9 Sufficient sleep is necessary for optimal daytime functioning, school performance, and wellbeing, thus placing adolescents from low-income backgrounds at higher risk for health disparities. Although most studies investigating health disparities compare outcomes across socioeconomic strata, studies that examine the variability within low-income groups are necessary to avoid perceptions of stereotypic health behavior based on race, age, sex, or socioeconomic status.10
Short sleep duration and low sleep efficiency (i.e., the percentage of time spent awake between sleep onset and offset) are important dimensions of pediatric sleep health11 that may increase the risk of chronic diseases, such as obesity and hypertension.12 It has been suggested that poor sleep disrupts hormone regulation leading to an energy imbalance and changes in body composition.13 In turn, obesity may influence sleep through emotional regulation and stress.14 Stress and depressive symptoms may mediate the association between weight stigma and sleep.15 Furthermore, fat accumulation makes the upper airway vulnerable to collapse during sleep and can contribute to sleep disordered breathing, resulting in shorter and more fragmented sleep.16
A recent cross-sectional study found that sleep duration and efficiency, measured with accelerometry, were inversely associated with blood pressure among adolescents.17 Consistent late bedtimes, paired with longer light exposure, might disrupt the secretion schedule of melatonin and potentially raise average 24-h blood pressure.18 Although reverse-causality may be possible, few studies explore whether high blood pressure can reduce sleep duration or efficiency. Results from a retrospective study showed that children with hypertension reported significantly more difficulty initiating sleep than children with normal blood pressure.19 Although causality cannot be inferred given the design of the referenced study, a possible mechanism for this association is that living with a chronic disease may act as a chronic stressor, thus disrupting sleep homeostasis.20
The directional and mechanistic longitudinal associations between sleep (duration and efficiency) and obesity and hypertension are complex and remain unclear.21 Furthermore, there is heterogeneity in sleep trajectories indicated by intraindividual variability of sleep patterns throughout adolescence.22 However, most research to date investigating changes in sleep over time uses population average trajectories23, assuming a uniform process in the development of sleep behavior and health outcomes.24
Theories of multiple developmental pathways propose dynamic differences in progression to health outcomes (e.g., sleep problems, or chronic risk).25 A person-centered approach with longitudinal data, such as finite mixture modeling, can represent heterogeneity in sleep trajectories by identifying groups of people who may have similar developmental pathways of sleep over time. Although some studies have identified different sleep trajectories among adolescents26, 27, they usually describe the trajectories based on one sleep dimension (e.g., duration) without linking the sleep patterns with health indicators. Identifying distinct sleep patterns, risk factors, and implications for later health outcomes among adolescents would allow programs to design tailored strategies that prevent chronic diseases and address health disparities.
This analysis remedies these gaps by analyzing longitudinal sleep behaviors among Black adolescent girls living in low-income, urban communities to examine the temporal relations among sleep (i.e., duration and efficiency), body mass index z-score (BMIz), overweight/obesity, and systolic and diastolic blood pressure (SDBP). The aims of this study are:
To examine the longitudinal relation between sleep habits (duration and efficiency) and chronic disease risk (BMIz, overweight or obesity, and SDBP).
To identify unique trajectories of sleep patterns during adolescence and determine the bidirectional association between unique trajectories of sleep patterns and chronic disease risk.
Methods
Design.
Longitudinal data were from participants in Challenge! In Middle Schools, a multilevel school-based randomized controlled trial aimed at improving diet and physical activity using a mentorship model in schools serving low-income, urban communities.28 The intervention was implemented over four years (2009–2013). Data collection occurred at three time points within each cohort: Time 1 (enrollment, fall), Time 2 (6-month follow-up, spring), and Time 3 (18-month follow-up, spring).
Study Sample.
The parent study enrolled 22 middle schools in a large urban public school district located within seven miles of a YMCA, with predominantly Black students (70%), and majority (75%) eligible for free/reduced priced meals. Within each school, 20–40 adolescent girls in 6th and 7th grades who were able to participate in a physical education class, and whose parent/caregiver reads English were recruited to participate through mailings or in-person during lunch shifts. A total of 789 girls enrolled in the study. There were no weight inclusion/exclusion criteria. Adolescent girls signed a written informed assent. Adolescents’ primary caregivers signed a written informed consent. The study protocol was approved by the Institutional Review Boards (HP-00040540) at both the University and the city public school system where the study took place.
Intervention.
In the multi-level intervention, schools were randomized to a school–wide intervention vs. control (1:1 randomization ratio). The school-wide intervention developed strategies to encourage physical activity and healthy eating throughout the school. Participating 6th and 7th grade girls in both intervention and control schools were randomized to small groups that addressed either physical activity and nutrition (small-group intervention) or stress-reduction (control). Thus, there were four-level intervention groups with approximately 25% of sample in each: school-wide only, small group only, both school-wide and small group, and neither. Sleep was not incorporated into either the school-wide or small group interventions. At the conclusion of the trial, there were no significant changes in BMIz, the prevalence of obesity, or in body composition attributed to either the school-wide or small group interventions.
Measures
Overweight and Obesity.
Height and weight were measured at three time points using a portable stadiometer (Shorr Productions, Olney, MD) and a weight scale (Tanita TBF-410, Arlington Heights, IL) to the nearest 0.1 cm and 0.1 kg in triplicate and then averaged. Participants were measured with their shoes removed and light clothing. BMI-for-age and -sex specific z-scores were calculated and compared to the CDC growth charts. Overweight or obesity were defined as BMI-for-age > 1 z-score.
Systolic and Diastolic Blood Pressure.
Blood pressure (systolic and diastolic) was objectively measured at two time points (Time 1 and 3) using a validated digital oscillometric blood pressure monitor (Omron, HEM-907XL, Bannockburn, IL). Blood pressure was measured at least twice, between a three-minute rest, and then averaged with the participant in a seated position and right arm in an extended position resting at heart level with palm facing up. Age-, gender- and height-specific SDBP percentile were calculated following national standards normalized for U.S. adolescents.29 Prehypertension was defined as having systolic or diastolic blood pressure at or above the 90th percentile.
Accelerometry.
A subset of participants was selected using a randomization procedure to wear an Actical accelerometer (n=556), a widely used and validated proxy for sleep.30 Adolescent girls wore an omnidirectional accelerometer (Actical; Respironics, Inc.; Bend, OR) for at least seven days on their non-dominant ankle with a non-removable hospital band (i.e., no nonwear time) at three time points (Times 1–3).31 Data from Actical were collected in 60-second epochs (time-sampling intervals) detecting movement in the 0.5- to 3.0-Hz range.
Sleep Metrics.
Sleep and wake measures were estimated from accelerometer data using the validated American Sleep Association (ASA) Sadeh algorithm for adolescents32 based on a wake threshold of 40 (medium sensitivity). Only complete days (i.e., full 24-hr periods) with a daily average of 80 counts/minute, and at least three nights/assessment33 were included in the analysis. Data were truncated after seven days (totaling maximum of 6 nights).
Sleep onset was defined as the first of ten continuous minutes scored as sleep based on the algorithm occurring between 7:00 p.m. and 5:00 a.m. next day to capture delayed sleep schedules. Sleep offset was the last minute of sleep followed by ten continuous minutes of awake34, and could occur anytime between 5:00 a.m. and 1:00 p.m. Nocturnal sleep was the sum of minutes scored as sleep from sleep onset to sleep offset, converted into hours. Wake time after sleep onset (WASO) was the number of wake minutes between sleep onset and offset. Sleep efficiency was the percentage of nocturnal sleep minutes out of the total sleep period. Supplemental Figure 1 illustrates the participant flow from the baseline sample, visual inspection, reasons for nights excluded, and final analytical sample. Only 3 adolescents had missing sleep onset/offset, thus cut-offs were deemed plausible.
Covariates.
Sociodemographic characteristics were collected at Time 1. Age was calculated in years using dates of birth and interview and treated as a continuous variable. Caregivers reported household size and annual household income (US$) via a self-administered questionnaire sent by mail. The income-to-poverty ratio was calculated using the weighted average poverty thresholds by the U.S. Census Bureau specific to each cohort (2009–2013). A poverty ratio below 1 indicates that the average income for each respective family size is below the federal poverty threshold.
At every time point (T1–3), the two-item Tanner scale was used to assess puberty development. Girls reported sexual maturation on a scale from 1–4 for breast and pubic. Higher scores denoted more maturation. The average of the two-item Tanner scale was used as a time-varying covariate.
Moderate-to-vigorous physical activity (MVPA) data were extracted using Actical accelerometer and reduced using Actiware 9.0. Validated thresholds for MVPA were applied35, yielding minutes of MVPA per day.
Statistical Analysis.
All analyses were conducted using Stata SE 16.1 software (StataCorp, College Station, TX, USA). For all analyses, statistical significance was defined by a p-value of <0.05.
Multilevel models assessed the longitudinal associations between sleep parameter (time-variant, independent variable) and chronic disease risk factors (i.e., BMIz, overweight/obesity, and SDBP), using full-information maximum likelihood estimation with a random intercept to account for repeated measures over time (ICCBMIz = 0.96; ICCoverweight/obese=0.61; ICCsystolic = 0.48, ICCdiastolic = 0.47) and clustering of students within school (ICCBMIz = 0.006; ICCoverweight/obese=0.008; ICCsystolic = 0.09, ICCdiastolic = 0.11). To assess whether the association varied over time, an interaction term between time of assessment (T1–T3) and sleep parameters were included in all models. For the categorical outcome (overweight/obesity), multilevel models with Poisson distribution were conducted. Cohen’s f2 effect size was calculated to estimate the magnitude of the longitudinal association between sleep and chronic disease risk factors, allowing for a direct comparison of the two regressors.
Adolescents’ sleep trajectories were identified using a group-based trajectory model (finite mixture model) based on two adolescent-level mean sleep parameters (i.e., nocturnal sleep hours and sleep efficiency), assessed longitudinally. Akaike information criterion (AIC) statistic for model fit, the Bayesian information criteria (BIC), entropy, interpretability of the trajectories, and percentage of sample in the smallest profile were used to identify the number of trajectories. A restriction requiring at least 5% per trajectory was implemented to avoid small group sizes. Then, adolescents were assigned to a mutually exclusive trajectory based on the individual’s maximum posterior probability. To assess the potential bidirectionality of the association between sleep trajectories and chronic disease risk factors, structural equation models with linear response variables (or Poisson for categorical outcome) were employed to examine if chronic disease risk factors at T1 were associated with sleep trajectory at T3 and conversely, generalized structural equation modeling with multinomial logistic models examined whether sleep trajectory at T1 was associated with later chronic disease risk factors at T3 (BMIz, SDBP).
Inverse probability weighting (IPW) was used to address potential bias due to loss to follow-up and to correct for the effects of missing data using the pweight option in Stata. For all analyses, there was no significant effect by intervention group on the association between sleep and chronic disease risk, nor was there baseline differences in sleep between the 4-level intervention groups, thus intervention group was treated as a covariate in all models. For the most parsimonious model, the following covariates were selected: adolescent’s school grade (categorical), Tanner score (continuous, time-varying), cohorts of data collection (2009–2013), moderate-to-vigorous physical activity (minutes), and intervention group (time invariant). Covariates were chosen based on associations reported previously and significant associations with sleep and chronic disease risk factors.
Results
On average, at Time 1, adolescent girls were 12.15 years old (± 0.72) and most were at tanner stage of 3 (mean 3.2 points ± 0.96) (Table 1). Two percent were prehypertensive (systolic or diastolic blood pressure at or above 90th percentile) and 47.8% had overweight or obesity. On average, adolescent girls had 4.8 nights of valid sleep data (min: 3, max: 6), slept for 7.9 hours, average WASO of 67 minutes, and sleep efficiency at 87.9%. Sleep efficiency decreased by 1.45% and WASO increased by six minutes over time (p = 0.005) in unadjusted growth mixture models (Table 1). There were no statistically significant differences between the analytical sample and all available data (Supplemental Table 1).
Table 1.
Descriptive information on adolescent characteristics, cardiometabolic outcomes, and sleep measures of analytical sample.
| Time 1 | Time 2 | Time 3 | Change over time (p-value)6 | ||||
|---|---|---|---|---|---|---|---|
| Adolescent characteristics | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Age (years) | 464 | 12.1 (0.7) | 348 | 12.6 (0.7) | 276 | 13.2 (0.7) | <0.001 |
| School grade | 464 | 6.4 (0.5) | 348 | 6.5 (0.5) | 276 | 7.4 (0.5) | <0.001 |
| Tanner score1 | 420 | 3.2 (0.9) | 326 | 3.5 (0.9) | 264 | 3.6 (0.9) | <0.001 |
| Race Black (%) | 470 | 100.0 | |||||
| Poverty ratio (<1.0) (%)2 | 113 | 51.1 | |||||
| Chronic disease risk outcomes | |||||||
| Body mass index (z-score)3 | 464 | 0.9 (1.0) | 348 | 1.0 (1.0) | 276 | 0.9 (1.1) | 0.827 |
| Systolic blood pressure (mmHg, Average) | 449 | 104.4 (9.8) | -- | -- | 276 | 106.5 (9.2) | 0.275 |
| Diastolic blood pressure (mmHg, Average) | 449 | 59.7 (7.9) | -- | -- | 276 | 60.1 (8.0) | 0.673 |
| Systolic blood pressure (percentile) | 449 | 39.9 (23.3) | 276 | 38.1 (23.2) | 0.902 | ||
| Diastolic blood pressure (percentile) | 449 | 44.9 (27.8) | -- | -- | 276 | 46.2 (25.9) | 0.933 |
| Overweight or obese (%) | 222 | 47.8 | 172 | 49.4 | 131 | 47.5 | 0.371 |
| Prehypertensive4 (%) | 7 | 1.6 | -- | -- | 5 | 1.8 | 0.913 |
| Sleep parameters | 470 | 349 | 276 | ||||
| Total number of nights | 4.8 (0.7) | 4.7 (0.6) | 4.7 (0.5) | 0.010 | |||
| Nighttime sleep (hours) | 7.9 (1.2) | 7.6 (1.5) | 7.8 (1.6) | 0.092 | |||
| WASO (minutes) | 66.8 (49.1) | 76.3 (46.6) | 80.5 (50.8) | 0.005 | |||
| Sleep efficiency (%)5 | 87.9 (8.6) | 85.4 (10.9) | 84.9 (10.9) | 0.002 | |||
Abbreviations: SD (standard deviation); WASO (wake after sleep onset)
-- Not measured at Time 2 assessment.
Time 1 (enrollment), Time 2 (6-month follow-up), Time 3 (18-month follow-up)
Mean score of the two Tanner items breast and pubic
The income-to-poverty ratio was calculated according to the U.S. Census Bureau, Weighted Average Poverty Thresholds, 2009–2012. Annual household income was reported by adolescent’s caregiver (n = 261)
Body mass index was calculated for age- and sex-specific z-score according to the CDC Child Growth Reference for girls.
90th percentile for either SBP or DBP
% of time asleep during sleep period
P-value for change over time was derived from multilevel growth models (unadjusted)
Longitudinal associations between sleep and chronic disease risk factors
The magnitude of the longitudinal associations between sleep and chronic disease risk factors was larger in models treating sleep parameters as predictors of health outcomes (Supplemental Table 2). Longer sleep duration and higher efficiency were associated with lower risk for having overweight/obesity at Time 1 and 2, but not at Time 3 (Table 2). For each one-minute increase in nocturnal sleep duration, there was a 7% lower risk for having overweight or obesity at Time 1 (RR = 0.93 [95% CI 0.89, 0.97]) and 6% lower risk at Time 2 (RR = 0.94 [95% CI 0.91, 0.96]), but not at Time 3 (RR = 0.99 [95% CI 0.96, 1.03]). Similarly, for each one-percentage increase in sleep efficiency, there was a 1% lower risk for having overweight/obesity at Time 1 and 2 (RR = 0.99 [95% CI 0.98, 0.99]), but not at Time 3 (RR = 0.99 [95% CI 0.99, 1.01]). Longer sleep duration was cross-sectionally associated with lower BMIz (b = −0.03 [95% CI −0.06, −0.01]), and systolic percentile (b = −1.42 [95% CI −2.75, −0.10]), but not longitudinally. Similarly, greater sleep efficiency was associated with lower BMIz (b = −0.003 [95% CI −0.006, −0.001]) at Time 1, but not at Time 2 and 3. WASO did not predict any chronic disease risk outcomes. Sleep duration, WASO, nor efficiency predicted diastolic percentile in the person-centered models.
Table 2.
Associations between sleep behavior and chronic disease risk factors among adolescents across three time points.
| Body mass index z-score (BMIz) as chronic disease risk outcome | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modela | Metabolic outcome | Difference in beta between time 1 and time 2 | Difference in beta between time 1 and time 3 | Difference in beta between time 2 and time 3 | Cohen’s f2 effect size | |||||||||||||||||||||||||||
| Time-variant predictors | b (95% CI) | p-value | b (95% CI) | p-value | b (95% CI) | p-value | b (95% CI) | p-value | ||||||||||||||||||||||||
| Sleep hours | ||||||||||||||||||||||||||||||||
| Time 1 | −0.033 (−0.054, −0.012) | 0.002 | Reference | Reference | 25.80 | |||||||||||||||||||||||||||
| Time 2 | −0.002 (−0.017, 0.012) | 0.751 | 0.031 (0.011, 0.050) | 0.002 | Reference | |||||||||||||||||||||||||||
| Time 3 | 0.004 (−0.025, 0.032) | 0.808 | 0.036 (0.012, 0.061) | 0.004 | 0.006 (−0.020, 0.032) | 0.656 | ||||||||||||||||||||||||||
| WASO | ||||||||||||||||||||||||||||||||
| Time 1 | 0.000 (−0.000, 0.001) | 0.143 | Reference | Reference | 25.54 | |||||||||||||||||||||||||||
| Time 2 | 0.000 (−0.000, 0.001) | 0.358 | −0.000 (−0.001, 0.001) | 0.687 | Reference | |||||||||||||||||||||||||||
| Time 3 | 0.000 (−0.001, 0.001) | 0.599 | −0.001 (−0.001, 0.000) | 0.080 | −0.000 (−0.001, 0.000) | 0.247 | ||||||||||||||||||||||||||
| Sleep efficiency | ||||||||||||||||||||||||||||||||
| Time 1 | −0.003 (−0.006, −0.001) | 0.012 | Reference | Reference | 25.67 | |||||||||||||||||||||||||||
| Time 2 | −0.001 (−0.003, 0.001) | 0.307 | 0.002 (−0.001, 0.005) | 0.123 | Reference | |||||||||||||||||||||||||||
| Time 3 | 0.001 (−0.003, 0.004) | 0.769 | 0.004 (0.001, 0.007) | 0.025 | 0.002 (−0.002, 0.005) | 0.304 | ||||||||||||||||||||||||||
| Overweight or obesity as chronic disease risk outcome | ||||||||||||||||||||||||||||||||
| RR (95 % CI) | p-value | RR (95% CI) | p-value | RR (95% CI) | p-value | RR (95% CI) | p-value | |||||||||||||||||||||||||
| Sleep hours | ||||||||||||||||||||||||||||||||
| Time 1 | 0.931 (0.897, 0.966) | <0.001 | Reference | Reference | 0.001 | |||||||||||||||||||||||||||
| Time 2 | 0.941 (0.919, 0.964) | <0.001 | 1.020 (0.921, 1.119) | 0.689 | Reference | |||||||||||||||||||||||||||
| Time 3 | 0.996 (0.957, 1.036) | 0.839 | 1.140 (1.009, 1.271) | 0.031 | 1.118 (1.020, 1.216) | 0.013 | ||||||||||||||||||||||||||
| WASO | ||||||||||||||||||||||||||||||||
| Time 1 | 1.001 (1.000, 1.002) | 0.117 | Reference | Reference | −0.001 | |||||||||||||||||||||||||||
| Time 2 | 1.001 (1.000, 1.002) | 0.059 | 1.001 (0.998, 1.003) | 0.674 | Reference | |||||||||||||||||||||||||||
| Time 3 | 1.001 (1.000, 1.002) | 0.146 | 1.000 (0.998, 1.002) | 0.605 | 1.000 (0.998, 1.002) | 0.786 | ||||||||||||||||||||||||||
| Sleep efficiency | ||||||||||||||||||||||||||||||||
| Time 1 | 0.994 (0.989, 0.998) | 0.010 | Reference | Reference | 0.001 | |||||||||||||||||||||||||||
| Time 2 | 0.993 (0.989, 0.997) | 0.001 | 0.999 (0.986, 1.013) | 0.936 | Reference | |||||||||||||||||||||||||||
| Time 3 | 0.997 (0.990, 1.003) | 0.313 | 1.005 (0.991, 1.020) | 0.470 | 1.006 (0.993, 1.018) | 0.355 | ||||||||||||||||||||||||||
| Systolic blood pressure percentile as chronic disease risk outcome | ||||||||||||||||||||||||||||||||
| b (95 % CI) | p-value | b (95% CI) | p-value | |||||||||||||||||||||||||||||
| Sleep hours | ||||||||||||||||||||||||||||||||
| Time 1 | −1.424 (−2.748, −0.101) | 0.035 | Reference | 0.26 | ||||||||||||||||||||||||||||
| Time 3 | 1.099 (−1.059, 3.256) | 0.318 | 2.630 (−0.180, 5.440) | 0.067 | ||||||||||||||||||||||||||||
| WASO | ||||||||||||||||||||||||||||||||
| Time 1 | 0.008 (−0.027, 0.042) | 0.667 | Reference | 0.25 | ||||||||||||||||||||||||||||
| Time 3 | −0.038 (−0.092, 0.016) | 0.166 | −0.046 (−0.099, 0.007) | 0.089 | ||||||||||||||||||||||||||||
| Sleep efficiency | ||||||||||||||||||||||||||||||||
| Time 1 | −0.085 (−0.295, 0.124) | 0.423 | Reference | 0.24 | ||||||||||||||||||||||||||||
| Time 3 | 0.137 (−0.204, 0.477) | 0.431 | 0.222 (−0.159, 0.603) | 0.253 | ||||||||||||||||||||||||||||
| Diastolic blood pressure percentile as chronic disease risk outcome | ||||||||||||||||||||||||||||||||
| b (95 % CI) | p-value | b (95% CI) | p-value | |||||||||||||||||||||||||||||
| Sleep hours | ||||||||||||||||||||||||||||||||
| Time 1 | 0.091 (−1.399, 1.582) | 0.904 | Reference | 0.25 | ||||||||||||||||||||||||||||
| Time 3 | −0.589 (−2.642, 1.464) | 0.574 | −0.680 (−3.229, 1.868) | 0.601 | ||||||||||||||||||||||||||||
| WASO | ||||||||||||||||||||||||||||||||
| Time 1 | 0.022 (−0.024, 0.068) | 0.345 | Reference | 0.27 | ||||||||||||||||||||||||||||
| Time 3 | 0.036 (−0.007, 0.079) | 0.102 | 0.014 (−0.045, 0.073) | 0.646 | ||||||||||||||||||||||||||||
| Sleep efficiency | ||||||||||||||||||||||||||||||||
| Time 1 | −0.103 (−0.364, 0.158) | 0.439 | Reference | 0.27 | ||||||||||||||||||||||||||||
| Time 3 | −0.196 (−0.439, 0.047) | 0.113 | −0.093 (−0.403, 0.217) | 0.556 | ||||||||||||||||||||||||||||
Abbreviations: CI (confidence interval), WASO (wake after sleep onset) b represents the average marginal effect of change in BMIz that is produced by a 1-unit increase in sleep at a given time point.
RR represents the exponentiated Poison coefficient (relative risk) for 1-unit increase in sleep at a given time point. Time 1 (enrollment), Time 2 (6-month follow-up), Time 3 (18-mo follow-up)
Hierarchical models controlled for adolescent’s school grade (6th to 7th), minutes of moderate-to-vigorous physical activity, Tanner score, cohort of data collection (2009–2012), and intervention group. (n=547)
Adolescent Sleep Trajectory
Results of the finite mixture model following the statistical criteria illustrated in the Supplemental Table 3 indicated a four-sleep trajectory solution: 1) worsened sleep pattern with decrease in sleep duration and efficiency over 18-months of assessment (Worsened, 12.3% of the sample); 2) irregular sleep pattern with high variability in sleep duration and efficiency over time (Irregular, 10.0%); 3) improved sleep pattern with increase in sleep duration and efficiency over time (Improved, 6.5%); 4) regular sleep pattern over time with low variability in sleep duration and efficiency (Regular, 71.2% of the sample) (Figure 1). Girls in the Improved trajectory had a greater mean Tanner score at T1 (3.4 ± 1.1) compared to girls in the Worsened trajectory (mean = 3.2 ± 0.9) (p-value for mixed level model < 0.001) (Supplemental Table 4).
Figure 1.
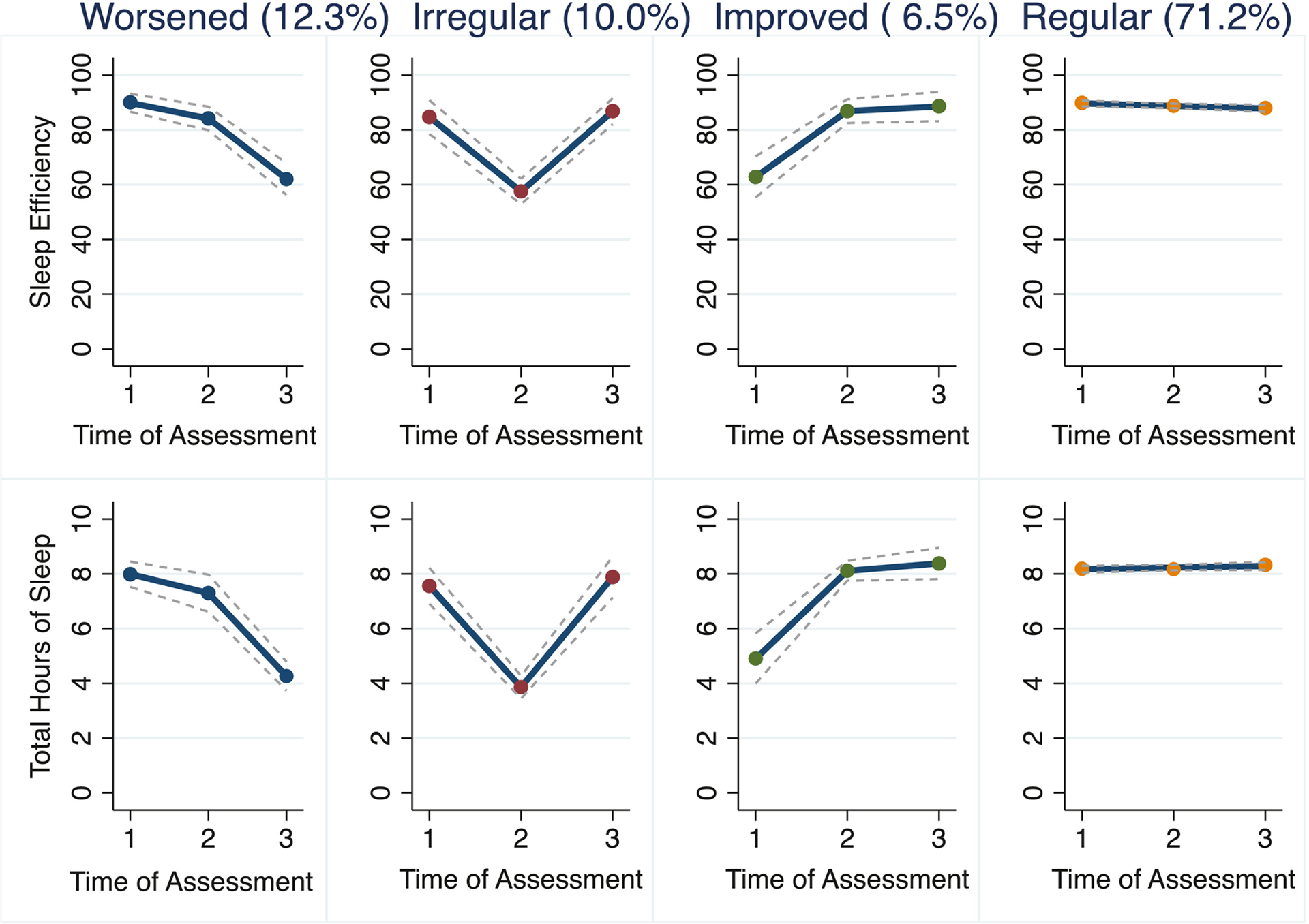
Four profile trajectory solution of the group-based trajectory model of quality (efficiency) and duration (hours) of nocturnal sleep.
Associations between chronic disease risk factors with sleep trajectory
Girls in the Improved sleep trajectory had the lowest sleep duration (M = 5.1 ± 1.3) at Time 1 and maintained an eight-hour average sleep duration for Times 2 and 3 (Supplemental Table 5). Chronic disease risk at Time 1 did not predict sleep trajectory at Time 3. Adolescents in the Improved sleep trajectory presented reduction in diastolic-for-age by 8.8 percentile (95% CI: −16.23, −1.40) at Time 3, after controlling for demographics, intervention group, BMIz, and diastolic percentile at enrollment (Table 3). Statistical significance remained after Bonferroni corrections for multiple testing. Sleep trajectories at time 1 did not predict BMIz, overweight/obesity, nor systolic percentile at Time 3.
Table 3.
Association between chronic disease risk factors (body mass index z-score, overweight/obesity, systolic and diastolic percentile) with sleep trajectory
| BMIz | Overweight or obesity | Systolic percentile | Diastolic percentile | |||||
|---|---|---|---|---|---|---|---|---|
| Cardiometabolic (Time 1) → sleep trajectorya | ||||||||
| RR (95 % CI) | p-value | RR (95 % CI) | p-value | RR (95% CI) | p-value | RR (95% CI) | p-value | |
| Regular | Reference | Reference | Reference | Reference | ||||
| Worsened | 0.93 (0.67, 1.30) | 0.689 | 0.99 (0.97, 1.01) | 0.160 | 0.89 (0.45, 1.78) | 0.763 | 0.99 (0.98, 1.01) | 0.259 |
| Irregular | 0.75 (0.53, 1.05) | 0.098 | 1.01 (0.99, 1.01) | 0.445 | 1.01 (0.40, 1.68) | 0.591 | 1.01 (0.99, 1.01) | 0.599 |
| Improved | 1.05 (0.69, 1.61) | 0.795 | 1.01 (0.98, 1.01) | 0.591 | 0.99 (0.66, 3.56) | 0.320 | 0.99 (0.98, 1.01) | 0.737 |
| Sleep trajectory → cardiometabolic (Time 3)b | ||||||||
| b (95% CI) | p-value | RR (95% CI) | p-value | b (95% CI) | p-value | b (95% CI) | p-value | |
| Regular | Reference | Reference | Reference | Reference | ||||
| Worsened | −0.04 (−0.20, 0.10) | 0.564 | 0.96 (0.69, 1.32) | 0.795 | −4.70 (−13.43, 4.02) | 0.291 | −0.33 (−7.55, 6.88) | 0.928 |
| Irregular | −0.01 (−0.12, 0.11) | 0.970 | 0.96 (0.75, 1.22) | 0.736 | 1.94 (−6.08, 9.97) | 0.634 | −2.67 (−11.56, 6.21) | 0.555 |
| Improved | −0.01 (−0.13, 0.12) | 0.867 | 0.95 (0.72, 1.26) | 0.742 | −4.80 (−17.26, 7.65) | 0.450 | −8.81 (−16.23, −1.40) | 0.020 |
Abbreviations: BMIz (body mass index z-score); RR (relative risk ratio); b (regression coefficient); CI (confidence interval);
RR represents the exponentiated multinomial or Poison coefficient (relative risk) for one unit increase in cardiometabolic variable for the worsened or irregular trajectory relative to the regular trajectory (reference)
b represents the change in the metabolic outcome that is produced by a sleep profile compared to regular trajectory (reference)
Regular = Regular sleep trajectory; Worsened = Worsened sleep profile trajectory; Irregular = Irregular sleep trajectory; Improved = Improved sleep trajectory
Generalized structural equation with multinomial logistic models with full information maximum likelihood estimation corrected missing data using the inverse probability weighted method controlled for adolescent’s age at baseline, Tanner score (baseline), moderate-to-vigorous physical activity (baseline), and intervention group; n = 464. Systolic and Diastolic Percentile models were further controlled for body mass index z-score at baseline.
Structural equation with linear response variables (or Poisson for categorical outcome) and full information maximum likelihood estimation controlled for adolescent’s cardiometabolic factor at baseline, age at baseline, Tanner score (baseline), moderate-to-vigorous physical activity (baseline), and intervention group; n = 464. Systolic and Diastolic Percentile models were further controlled for body mass index z-score at baseline.
Discussion
Three major findings emerged from this longitudinal investigation of associations between sleep parameters and chronic disease risk factors among Black adolescent girls from a predominantly low-income background. First, greater sleep duration was associated with lower chronic disease risk cross-sectionally (Time 1), and greater sleep duration and efficiency were associated with lower risk for having overweight/obesity over time. Second, four trajectories of sleep were identified. Third, chronic disease risk did not predict sleep trajectories, although Improved sleep trajectory (sleep duration and efficiency) predicted reduction in diastolic-for-age percentile at Time 3, after accounting for diastolic measures and BMIz at enrollment.
Both variable-centered and person-centered approaches consistently indicated that sleep predicted chronic disease risk, but not the other way round. Despite the increased importance of sleep on cardiovascular health, clinical and public health guidelines (e.g., American Heart Association Life’s Simple 7®) do not routinely promote sleep as a protective behavioral factor. In this study, longer and more efficient sleep was cross-sectionally associated with lower BMIz and SDBP and longitudinally with lower risk for having overweight/obesity, even after accounting for physical activity. The findings from this investigation are consistent with many reviews and meta-analysis demonstrating the protective role of sleep on chronic disease risk in adolescents.36
The Improved sleep trajectory was associated with lower diastolic blood pressure percentile at Time 3, independent of age, puberty stage, intervention group, BMIZ, and physical activity level. This is an encouraging finding, meaning that interventions to improve sleep behaviors during adolescence may have a positive influence on cardiovascular health. Although the underlying mechanism for the association between short sleep duration or efficiency and high blood pressure is not fully understood, it has been hypothesized that short sleep duration may influence cardiometabolic factors through inflammatory mechanisms.37 Habitual short sleep duration has been shown to increase free fatty acids in plasma, which would in turn increase mitochondrial uncoupling, causing a reduction in high-energy phosphate level, manifested as diastolic dysfunction.38 Thus, a longer duration of nocturnal sleep with greater efficiency could decrease free fatty acids in plasma and influence average blood pressure, especially diastolic blood pressure.38 More clinical studies are needed to confirm the potential biological mechanism of sleep on blood pressure.
Other studies evaluating sleep duration trajectories among adolescents classified most individuals as having a reduction in sleep duration over time.27 However, in this study, the majority was classified into the Regular trajectory; perhaps the 18-month follow-up period did not capture the sleep reduction that often occurs with pubertal onset and psychosocial alterations.3, 4 Conversely, results from the variable-centered approach indicated that efficiency, as opposed to duration of sleep, decreased over time. In the present study, girls who had a Worsened and Irregular sleep trajectories had a significant variation in sleep duration and efficiency over time, possibly due to lack of sleep routines (e.g., irregular bedtimes). Consistent bedtime is an important protective factor to prevent weight gain, especially in the context of poverty.39
Both sleep duration and efficiency were longitudinally associated with lower risk for having overweight/obesity. Most studies on sleep among adolescents have been cross-sectional34 and used self-reported sleep.27 However, meta-analyses of prospective studies have consistently shown the inverse association between sleep duration and weight gain among children and adolescents.40 Of note, sleep duration nor efficiency were associated with chronic disease risk at the 18-month assessment (Time 3). The lack of the long-term association could be due to the loss to follow-up, leading to a reduced statistical power to detect true differences. Other studies could replicate these findings in other samples of adolescents that are followed up over 18 months, while examining potential moderators (e.g., individual-level factors such as stress and environmental-level factors such as school start time). Contrary to what was observed in the variable-centered approach, the trajectory of sleep duration and efficiency over time did not predict chronic disease risk, like overweight/obesity, but predicted lower diastolic percentile. Girls in the Worsened trajectory had a significant decrease in sleep duration and efficiency between the two follow-ups, which may not have been enough time to influence chronic disease risk.
A major strength of this study was the use of objectively measured sleep quantity (duration), efficiency, and chronic disease risk factors (BMIz and blood pressure) analyzed longitudinally. Further, a person-centered approach was used to identify groups of adolescent girls sharing similar developmental pathways of sleep longitudinally. This is a relatively novel method in sleep research and is typically characterized by higher levels of statistical power than comparable traditional methods applied to the same data.
The use of group-based trajectory modeling comes with some limitations. Misclassification can occur in assigning trajectory membership. Irregular and Improved trajectories represented a small percentage of the population, although many studies identifying trajectories typically require at least 5% of the population per group. This was a secondary analysis with various exposures being tested and it is possible that type I error inflation was present. Although this study examined important domains of pediatric sleep such as duration and efficiency, future studies should consider additional domains such as satisfaction, napping, timing, and sleep routines.11 Furthermore, the presented analysis incorporated weekend sleep into sleep measurements, given that most sleep data available were collected during weekdays (65%). Future studies should examine potential differences in sleep between weekday versus weekend among adolescents. Accelerometers are commonly used in population-based studies; however, they are less precise than the gold standard (PSG). Actical, placed on the wrist or ankle, yields similar or high intraclass correlation compared to PSG in sleep latency, total sleep time, and sleep efficiency. Environmental factors known to influence sleep such as housing conditions and temperature control, room-sharing, and screen use, as well as psychological factors such as stress, depression, and anxiety, were not evaluated. Obstructive sleep apnea syndrome is also known to impact sleep duration and efficiency and it should be measured in future studies. Seasonality is unlikely to have played a role in our study, as Actical data were collected when school was in session and not during periods of extreme weather conditions (Summer or Winter) or holiday breaks. Participant loss is a limitation, although attrition rate was typical of expectations in community- and school-based intervention trials. To address potential selection bias, IPW and full-information likelihood estimation models were employed in all analyses. Lastly, although the parent study recruited adolescent from schools serving low-income communities, almost half of the sample had a family income-to-needs ratio > 1.0. However, data on household income were limited and self-reported by only 56% of the adult caregivers. Thus, poverty level may be underestimated. Additional clinical research is needed on samples of color and of low-income backgrounds, beyond those recruited in the context of an intervention study.
Conclusions
In summary, greater sleep duration and efficiency were longitudinally associated with lower risk of having overweight/obesity among a sample of Black adolescent girls. Girls followed four unique sleep trajectories through adolescence: Worsened, Decreased, Improved, and Regular. When linking sleep trajectories with health indicators, girls who improved sleep duration and efficiency over time also improved their blood pressure profile. Determining the sources of vulnerability in health behaviors experienced within each of these groups, such as short sleep duration and low sleep efficiency, provides opportunities to intervene to disrupt chronic disease risk and address disparities. Future interventions could aim to promote both sleep duration and sleep consolidation by employing multilevel preventive strategies to improve both individual-level factors (i.e., sleep hygiene) and social and environmental factors (i.e., noise, light, school start time) and test the effect on weight gain and blood pressure in adolescents.
Supplementary Material
Acknowledgements
The presented work had financial support from the American Heart Association (20POST35210825, PI: Trude) and the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) at the National Institute of Health (NIH) (5R01HD054727-02, PI: Black).
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration
Challenge! A Health Promotion/Obesity Prevention Program for Teens. https://clinicaltrials.gov/ct2/show/NCT03103269, NCT03103269.
Disclosure of Conflicts of Interest
None.
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Boards (HP-00040540) at both the University and the city public school system where the study took place. Informed assent and consent were gathered from the adolescent and caregiver, respectively.
References
- 1.Wheaton AG, Jones SE, Cooper AC, Croft JB. Short sleep duration among middle school and high school students - United States, 2015. MMWR Morb Mortal Wkly Rep. Jan 26 2018;67(3):85–90. doi: 10.15585/mmwr.mm6703a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. Jun 15 2016;12(6):785–6. doi: 10.5664/jcsm.5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol (Lausanne). 2018;9:364. doi: 10.3389/fendo.2018.00364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owens J, Adolescent Sleep Working Group, Committee on Adolescence. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014;134(3):e921–e932. doi: 10.1542/peds.2014-1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkins SS, Takeuchi DT. Social determinants of inadequate sleep in US children and adolescents. Public Health. Sep 2016;138:119–26. doi: 10.1016/j.puhe.2016.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maslowsky J, Ozer EJ. Developmental trends in sleep duration in adolescence and young adulthood: evidence from a national United States sample. J Adolesc Health. 2014/June/01/2014;54(6):691–697. doi: 10.1016/j.jadohealth.2013.10.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews KA, Hall M, Dahl RE. Sleep in healthy black and white adolescents. Pediatrics. May 2014;133(5):e1189–96. doi: 10.1542/peds.2013-2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315(21):2292–2299. doi: 10.1001/jama.2016.6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Dyke M, Greer S, Odom E, et al. Heart disease death rates among Blacks and Whites aged >/=35 years - United States, 1968–2015. MMWR Surveill Summ. Mar 30 2018;67(5):1–11. doi: 10.15585/mmwr.ss6705a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okoro ON, Hillman LA, Cernasev A. “We get double slammed!”: Healthcare experiences of perceived discrimination among low-income African-American women. Womens Health (Lond). Jan-Dec 2020;16:1745506520953348. doi: 10.1177/1745506520953348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meltzer LJ, Williamson AA, Mindell JA. Pediatric sleep health: It matters, and so does how we define it. Sleep Medicine Reviews. 2021/June/01/ 2021;57:101425. doi: 10.1016/j.smrv.2021.101425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews KA, Pantesco EJ. Sleep characteristics and cardiovascular risk in children and adolescents: an enumerative review. Sleep Med. Feb 2016;18:36–49. doi: 10.1016/j.sleep.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. Jan 2015;3(1):52–62. doi: 10.1016/S2213-8587(14)70012-9 [DOI] [PubMed] [Google Scholar]
- 14.Vgontzas AN, Fernandez-Mendoza J, Miksiewicz T, et al. Unveiling the longitudinal association between short sleep duration and the incidence of obesity: the Penn State Cohort. Int J Obes (Lond). 2014;38(6):1476–5497 (Electronic). doi: 10.1038/ijo.2013.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ZX, Dang JJ, Zhang XG, Moore JB, Li R. Assessing the relationship between weight stigma, stress, depression, and sleep in Chinese adolescents. Qual Life Res. Jan 2021;30(1):229–238. doi: 10.1007/s11136-020-02620-4 [DOI] [PubMed] [Google Scholar]
- 16.Xiao Q, Gu F, Caporaso N, Matthews CE. Relationship between sleep characteristics and measures of body size and composition in a nationally-representative sample. BMC Obesity. 2016/November/11 2016;3(1):48. doi: 10.1186/s40608-016-0128-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cespedes Feliciano EM, Quante M, Rifas-Shiman SL, Redline S, Oken E, Taveras EM. Objective sleep characteristics and cardiometabolic health in young adolescents. Pediatrics. 2018;142(1):e20174085. doi: 10.1542/peds.2017-4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. May 2006;47(5):833–9. doi: 10.1161/01.HYP.0000217362.34748.e0 [DOI] [PubMed] [Google Scholar]
- 19.Croix B, Feig DI. Childhood hypertension is not a silent disease. Pediatr Nephrol. 2006;21(4):0931–041X (Print). doi:doi: 10.1007/s00467-006-0013-x [DOI] [PubMed] [Google Scholar]
- 20.Palagini L, Bruno RM, Gemignani A, Baglioni C, Ghiadoni L, Riemann D. Sleep loss and hypertension: a systematic review. Curr Pharm Des. 2013;19(13):2409–19. doi: 10.2174/1381612811319130009 [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Patel SR, Redline S, et al. Weekly sleep trajectories and their associations with obesity and hypertension in the Hispanic/Latino population. Sleep. 2018;41(10)doi: 10.1093/sleep/zsy150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenni OG, Molinari L, Caflisch JA, Largo RH. Sleep duration from ages 1 to 10 years: variability and stability in comparison with growth. Pediatrics. Oct 2007;120(4):e769–76. doi: 10.1542/peds.2006-3300 [DOI] [PubMed] [Google Scholar]
- 23.Herle M, Micali N, Abdulkadir M, et al. Identifying typical trajectories in longitudinal data: modelling strategies and interpretations. Eur J Epidemiol. Mar 2020;35(3):205–222. doi: 10.1007/s10654-020-00615-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beets MW, Foley JT. Comparison of 3 different analytic approaches for determining risk-related active and sedentary behavioral patterns in adolescents. J Phys Act Health. May 2010;7(3):381–92. doi: 10.1123/jpah.7.3.381 [DOI] [PubMed] [Google Scholar]
- 25.Loeber R, Burke JD. Developmental pathways in juvenile externalizing and internalizing problems. J Res Adolesc. 2011;21(1):34–46. doi:doi: 10.1111/j.1532-7795.2010.00713.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranum BM, Wichstrom L, Pallesen S, Falch-Madsen J, Steinsbekk S. Persistent short sleep from childhood to adolescence: child, parent and peer predictors. Nat Sci Sleep. 2021;13:163–175. doi: 10.2147/NSS.S290586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer Fiala Machado A, Wendt A, Baptista Menezes AM, Gonçalves H, Wehrmeister FC. Sleep duration trajectories from adolescence to emerging adulthood: Findings from a population-based birth cohort. https://doi.org/10.1111/jsr.13155. J Sleep Res. 2020/August/17 2020;n/a(n/a):e13155. doi: 10.1111/jsr.13155 [DOI] [PubMed] [Google Scholar]
- 28.Buckingham-Howes S, Armstrong B, Pejsa-Reitz MC, et al. BMI and disordered eating in urban, African American, adolescent girls: The mediating role of body dissatisfaction. Eat Behav. Apr 2018;29:59–63. doi: 10.1016/j.eatbeh.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008;167(6):653–666. doi: 10.1093/aje/kwm348 [DOI] [PubMed] [Google Scholar]
- 30.Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 2012/October/01/ 2012;16(5):463–475. doi: 10.1016/j.smrv.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Black MM, Hager ER, Le K, et al. Challenge! Health promotion/obesity prevention mentorship model among urban, black adolescents. Pediatrics. 2010;126(2):280–8. doi: 10.1542/peds.2009-1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadeh A, Sharkey M, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. doi: 10.1093/sleep/17.3.201 [DOI] [PubMed] [Google Scholar]
- 33.Quante M, Kaplan ER, Rueschman M, Cailler M, Buxton OM, Redline S. Practical considerations in using accelerometers to assess physical activity, sedentary behavior, and sleep. Sleep Health. 2015/December/01/ 2015;1(4):275–284. doi: 10.1016/j.sleh.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 34.Zhou M, Lalani C, Banda JA, Robinson TN. Sleep duration, timing, variability and measures of adiposity among 8- to 12-year-old children with obesity. Obes Sci Pract. Dec 2018;4(6):535–544. doi: 10.1002/osp4.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hager ER, Treuth MS, Gormely C, Epps L, Snitker S, Black MM. Ankle Accelerometry for Assessing Physical Activity Among Adolescent Girls: Threshold Determination, Validity, Reliability, and Feasibility. Res Q Exerc Sport. 2015;86(4):397–405. doi: 10.1080/02701367.2015.1063574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun J, Wang M, Yang L, Zhao M, Bovet P, Xi B. Sleep duration and cardiovascular risk factors in children and adolescents: A systematic review. Sleep Med Rev 2020/October/01/ 2020;53:101338. doi: 10.1016/j.smrv.2020.101338 [DOI] [PubMed] [Google Scholar]
- 37.Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. Apr 2007;5(2):93–102. doi: 10.2174/157016107780368280 [DOI] [PubMed] [Google Scholar]
- 38.Rider OJ, Francis JM, Ali MK, et al. Effects of catecholamine stress on diastolic function and myocardial energetics in obesity. Circulation. Mar 27 2012;125(12):1511–9. doi: 10.1161/CIRCULATIONAHA.111.069518 [DOI] [PubMed] [Google Scholar]
- 39.Covington L, Armstrong B, Trude ACB, Black MM. Longitudinal associations among diet quality, physical activity and sleep onset consistency with body mass index z-score among toddlers in low-income families. Ann Behav Med. Nov 16 2020;kaaa100 doi: 10.1093/abm/kaaa100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng X, He M, He D, Zhu Y, Zhang Z, Niu W. Sleep duration and obesity in children and adolescents: evidence from an updated and dose-response meta-analysis. Sleep Med. Feb 2021;78:169–181. doi: 10.1016/j.sleep.2020.12.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


