Abstract
Salivary histatins are a family of basic histidine-rich proteins in which therapeutic potential as drugs against oral candidiasis is apparent, considering their potent in vitro antifungal activity and lack of toxicity to humans. Histatin 5 (Hst 5) kills the fungal pathogen Candida albicans via a mechanism that involves binding to specific sites on the yeast cell membrane and subsequent release of cellular ATP in the absence of cytolysis. We explored the killing pathway activated by Hst 5 and compared it to those activated by other antifungal agents. The candidacidal activity of human neutrophil defensin 1 (HNP-1) shared very similar features to Hst 5 cytotoxic action with respect to active concentrations and magnitude of induction of nonlytic ATP efflux, depletion of intracellular ATP pools, and inhibitor profile. Hst 5 and HNP-1 are basic proteins of about 3 kDa; however, they have unique primary sequences and solution structures that cannot explain how these two molecules act so similarly on C. albicans to induce cell death. Our finding that HNP-1 prevented Hst 5 binding to the candidal Hst 5 binding protein suggests that the basis for the overlapping actions of these two naturally occurring antimicrobial proteins may involve interactions with shared yeast components.
Candida albicans is an opportunistic fungal pathogen that is a leading cause for mucosal and systemic candidiasis in people with compromised immune systems (5, 28). Amphotericin B is largely used for treatment of deeply invasive mycoses in severely immunocompromised patients, despite its acute and chronic toxicity. Aggressive use of less toxic antifungal agents such as the azole-based drugs has resulted in emergence of candidal species with drug resistance. The pressing need for improved drug therapies led to the exploration of antimicrobial peptides produced by innate host defense systems of plants and animals as alternative antifungal agents (15).
The parotid and submandibular glands of humans and higher subhuman primates secrete a family of 3- to 4-kDa histidine-rich basic proteins, histatins (Hsts) (29). Hst 1 (38 amino acids), Hst 3 (32 amino acids), and Hst 5 (the N-terminal 24 amino acids of Hst 3 generated by proteolytic cleavage) comprise about 80% of total histatin protein in saliva. Salivary Hsts possess antimicrobial properties and are effective against oral yeast isolates, particularly C. albicans. Hst 5 and Hst 3 are the most potent candidacidal members of the family in vitro, killing yeast and filamentous forms of Candida species at physiological concentrations (15 to 30 μM) (33, 44). Recent clinical studies suggested that Hsts may indeed prevent candidiasis in vivo (17). The therapeutic potential of Hsts as drugs against oral candidiasis is apparent, considering their potent antifungal activity even against azole-resistant candida strains and lack of toxicity to humans (39).
The mammalian defensins, comprising α- and β-subfamilies of trisulfide 29- to 42-amino-acid cationic peptides, possess a broad range of antimicrobial activities in vitro. Classical α-defensins were the first antimicrobial peptide family to be recognized (11). Subsequent to their discovery in neutrophils, α-defensins were found in mouse and human Paneth cells and human reproductive tissue (30, 31). The physiological concentration of α-defensins 1 to 3 within the neutrophils is very high—about 6 mg/ml (1.5 mM). In contrast, the plasma defensin concentration is below 0.1 μM, which is substantially below the levels needed in vitro to mediate antimicrobial effects (6 to 30 μM). α-Defensin levels have been reported to rise significantly with various infections (22).
The physiological activities of many cationic antimicrobial peptides are generally related to their membranolytic properties (10). Defensins contain amphipathic β-sheet structures, a feature that enables formation of ion channels in model lipid membranes (19). Studies of α-defensins did not reveal specificity toward a distinct target membrane, since they were active against bacteria, enveloped viruses, and fungi. Several mammalian α-defensins were also reported to be cytotoxic to mammalian cells in culture (11, 18, 24). Recent data, however, suggested that human α-defensin human neutrophil defensin 2 (HNP-2) discriminates between bacterial and eukaryotic membranes by preferential interaction with anionic phospholipids that are prominent in bacterial membranes (25). It is currently unclear whether α-defensins differentially recognize eukaryotic pathogens like fungi from mammalian cells.
Salivary Hst 5 at physiological concentrations that kill C. albicans is not cytotoxic to human cells, as evidenced by its lack of lytic activity to human erythrocytes and various human cell lines and primary cells (13, 38). In addition, extensive structural and functional studies of Hst 5 argue against its ability to directly lyse target yeast cells by spontaneous insertion into the membranes and pore formation (33, 34, 40). Recent work on the mechanism of Hsts' candidacidal activity revealed three important findings: (i) functional binding sites for salivary Hsts and a 67-kDa Hst 5 binding protein (HstBP) were identified on C. albicans (9); (ii) Hst 3 and Hst 5 binding to the fungal plasma membrane was subsequently proposed to be the first event of a temperature-and ionic strength-dependent multistep killing process involving subsequent internalization of Hsts and interaction with intracellular targets (14, 45); and (iii) exposure of C. albicans to physiological concentrations of Hst 5 caused a drastic reduction of intracellular ATP content, which was a result of efflux of cellular ATP (21). The major characteristic of Hst 5-induced ATP release was that it occurred while C. albicans cells were metabolically active and had polarized membranes, thus precluding cell lysis as a possible route by which ATP was released from the cells (21).
This mechanism for Hst 5-induced yeast killing has not been evaluated for other antifungal agents and antimicrobial proteins. Consequently, it is important to determine whether it represents a common antifungal mechanism or if it is unique for salivary Hsts. The modes of action of currently used drugs in treatment of candidiasis, including polyene antimycotics and azole derivatives, have been attributed to their ability to alter yeast membrane permeability. Polyene antimycotics complex with ergosterol of the plasma membrane, resulting in a release of cellular potassium (3, 4). The azole-based drugs inhibit the biosynthesis of ergosterol (42) and induce release of K+ and 260-nm-wavelength absorbing materials from C. albicans cells (41). Release of cellular ATP was detected following treatment of C. albicans with azole derivatives (2). Similar to Hst 5, miconazole and other imidazole antifungal agents have been reported to cause a rapid depletion of ATP in C. albicans while these nonviable cells (unable to replicate) remained metabolically active (1). However, the mechanism of Hst 5 action appears to be distinct from that of the azole based antifungal drugs, since salivary Hsts were effective in killing azole-resistant Candida species (27, 39). Despite structural differences between Hst 5 and HNP-1, the killing pathways activated display intriguingly similar features. The candidacidal activities of both Hst 5 and HNP-1 were inhibited under anaerobic conditions and by the same pharmacological agents—carbonylcyanide-m-chlorophenylhydrazone (CCCP), dinitrophenol (DNP), and azide (14, 21, 23)—and Ca2+ and Mg2+ have been reported to abolish Hst 5 and HNP-1 killing of C. albicans (23, 44).
Here, we explored the killing pathways activated by Hst 5 in C. albicans and compared them to those activated by other antifungal agents. Our studies revealed that Hst-5 and HNP-1 kill C. albicans via mechanisms that involve a nonlytic release of cellular ATP and share very similar features, distinguishable from the cytotoxic actions of miconazole or amphotercin B.
MATERIALS AND METHODS
Materials.
C. albicans strain DS1 was isolated from the palate of a denture stomatitis patient (33), and strain 31531A was obtained from E. Rustashenko and F. Sherman, Department of Biochemistry and Biophysics, University of Rochester. Sabouraud dextrose agar and yeast extract-peptone-dextrose (YPD) media were from Difco (Detroit, Mich.); HNP-1, miconazole, amphotericin B, antimycin A, CCCP and suramin were from Sigma.
Hst 5 synthesis and purification.
Hst 5 (DSHAKRHHGYKRKFHEKHHSHRGY) was synthesized using standard solid-phase synthesis protocols and 9-fluorenylmethoxy carbonyl chemistry and purified by reversed-phase high-performance liquid chromatography as described previously (9). Purity of Hst 5 was assessed by amino acid analysis and mass spectroscopy. Biotinylation of Hst 5 (resulting in biotin-Hst 5) was performed using N-hydroxysuccinimidobiotin (NHS-biotin; Pierce). NHS-biotin (200 mg) was dissolved in 1.5 ml of dimethylformamide and mixed with 400 mg of unprotected Wang resin-Hst 5-NH2 at a molar ratio of 3:1. A coupling reaction was carried out for 4 h at room temperature with stirring. The completion of biotinylation was monitored using a Kaiser test for detection of free amino groups. After filtering, three washes with dimethylformamide-methylene chloride (50:50, vol/vol) and five washes with absolute ethanol, biotin-Hst 5 deprotection, cleavage from the dried resin, and purification by high-performance liquid chromatography were carried out as described above. Purity of biotin-Hst 5 was assessed by sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis (SDS–15% PAGE) using a Tris-Tricine electrode buffer and visualized on a Western blot by ExtrAvidin conjugated to horseradish peroxidase and 4-chloro-1-naphthol (Sigma). Candidacidal bioassays verified that biotin-Hst 5 retained full biological activity.
Candidacidal assay.
C. albicans was maintained on Sabouraud dextrose agar and grown to exponential phase at 37°C in YPD or sucrose-salts-biotin (SSB) yeast synthetic medium as previously described (21). For cell growth under anaerobic conditions, C. albicans cells were inoculated into SSB medium containing Oxyrase (Oxyrase Inc., Mansfield, Ohio) and grown at 25°C according to the manufacturer's instructions. Oxyrase in broth reduces O2 concentration to below 10 ppb within 30 min, removes any reintroduced oxygen, and maintains this level of anaerobiosis for more then 16 days. Antifungal activity of Hst 5 was examined by microdilution plate assay (21), with the following modifications. Briefly, C. albicans cells were washed with 10 mM sodium phosphate buffer (Na2HPO4-NaH2PO4), pH 7.4, and cell suspensions (106 cells) were mixed with Hst 5 (3.9 to 61 μM), HNP-1 (1.1 to 31 μM), miconazole (500 μg/ml), or amphotercin B (2 μg/ml) for 1.5 h (unless indicated otherwise) at 37°C with shaking. For assays using inhibitors of Hst 5 killing, cells were mixed with CCCP (500 μM) for 2 h or suramin (100 μM) for 15 min at 37°C. Cells were then left untreated or treated with Hst 5 (31 μM), HNP-1 (15 μM), miconazole (500 μg/ml), or amphotericin B (2 μg/ml) for additional 1.5 h. Miconazole and CCCP were dissolved in methanol and amphotericin B was dissolved in dimethyl sulfoxide (DMSO) and diluted prior to use (the final concentration of methanol or DMSO did not exceed 1%). Control cultures were incubated with 10 mM phosphate buffer or vehicle (1% DMSO or methanol) alone. Cell suspensions were diluted, and aliquots (500 cells) were spread onto Sabouraud dextrose agar plates and incubated for 24 h at 37°C. The optimal concentrations of pharmacological agents and solvents were determined in preliminary experiments to avoid potential artifacts from toxic effects. Candidacidal assays were performed in duplicate or triplicate. Cell survival was expressed as a percentage of control, and loss of viability was calculated as [1 − (colonies from agent-treated cells/colonies from control cells)] × 100.
ATP bioluminescence assay.
ATP levels in cultures of C. albicans were measured as previously described (2, 7, 21) with the following modifications. C. albicans (106 cells) was mixed with increasing concentrations of Hst 5 or HNP-1 for various times in a final volume of 110 μl, or cells were treated for 1.5 h at 37°C with either Hst 5 (31 μM), HNP-1 (15 μM), miconazole (500 μg/ml), or amphotercin B (2 μg/ml). For extracellular ATP measurements cells were pelleted (5,000 × g, 3 min), and 25 μl of the supernatant was pipetted into 225 μl of boiling TE buffer (50 mM Tris, 2 mM EDTA [pH 7.8]), boiled for an additional 2 min, and stored on ice until assayed for ATP. Cell pellets were then resuspended in 1 ml of TE buffer, 10 μl of the cell suspension was diluted to 1 ml, and 50 μl (500 cells) were plated on agar to assess viability as described above. Intracellular ATP measurements were made on the remaining cells (106), which were washed twice with TE buffer, and cell pellets were submerged in liquid nitrogen followed by the addition of 400 μl of boiling TE. Cells were boiled for 4 min and subjected to another freeze-boil cycle and placed on ice until assayed for ATP. Extracellular and intracellular ATP levels were measured by luminometry using an ATP assay kit (Sigma) according to the manufacturer's instructions. Luciferin-luciferase assay mix (100 μl) was added to 100 μl of cell lysates or 25 μl of extracellular material in 96-well black microtiter plates (Wallac) and light emission was monitored in a 1250 LKB-Wallac luminometer. Results are expressed in bioluminescence relative light units; ATP concentrations were determined from ATP standard curves and were normalized to the number of control CFU (actual cell number present during incubation).
Cell respiration measurements.
Oxygen consumption was measured using a Clark type oxygen electrode (YSI 5300 biological monitor; Yellow Springs Instrument, Yellow Springs, Ohio) as described (21). Briefly, C. albicans cells (2 × 106) were treated for 1.5 h at 37°C in the presence or absence of Hst 5 (31 μM), HNP-1 (15 μM), miconazole (500 μg/ml), or amphotercin B (2 μg/ml). The cells were then transferred to the chamber of the electrode in a final volume of 2 ml of 10 mM phosphate buffer, and oxygen consumption was measured for 10 min with stirring. In some experiments antimycin A (1 μg/ml) was added directly to the cells in the chamber. Oxygen uptake was expressed as nanomoles of O2 per minute per 106 cells.
Overlay assays.
C. albicans (2 × 108 to 5 × 108 cells) was washed with 10 mM phosphate buffer (pH 7.4) and resuspended in 100 μl of cold lysing buffer (10 mM phosphate buffer [pH 7.4], 1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA, 1 μg of aprotinin/ml, 1 μg of pepstatin A/ml 1 μg of leupeptin/ml, and l μg of benzamidine/ml) in tubes containing 100 μl of prechilled 0.5-mm-diameter glass beads. Cell breakage was achieved by vigorous vortexing in five 2-min cycles at 4°C, and lysates were clarified by centrifugation at 12,000 × g at 4°C. Cell lysates were either mixed with 4× boiling Laemmli sample buffer or concentrated by precipitation with 6 volumes of ice-cold acetone, and the dried pellet was solubilized in sample buffer. Solubilized proteins were separated by SDS–8% PAGE and transferred to polyvinylidene difluoride (PVDF) membranes for overlay assay. Membranes were blocked for 2 h with 1% milk in Tris-buffered saline (10 mM Tris-HCl [pH 7.5]–137 mM NaCl containing 0.1% Tween 20), washed, and then incubated for 2 h with 250 nM biotin–Hst 5 in binding buffer (10 mM Tris-HCl, pH 7.5). Where indicated, blots were incubated with an excess of either HNP-1, miconazole, amphotericin B, or suramin prior to the addition of biotin-Hst 5. Blots were extensively washed in binding buffer and then incubated for 1 h with ExtrAvidin-peroxidase, at 1:5,000 in 1% bovine serum albumin in binding buffer to visualize the reactive biotinylated proteins.
RESULTS
Comparison between killing pathways activated by Hst 5 and other candidacidal agents.
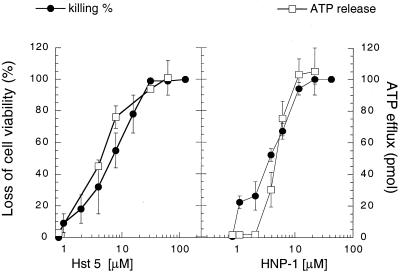
We have previously shown that Hst 5 killing of C. aIbicans is initiated with a nonlytic release of ATP, which coincides with the depletion of cellular ATP. Release of cellular ATP correlated with cell death, since agents that afforded protection against Hst 5 killing (CCCP, DNP, and azide) also prevented Hst 5-induced ATP eflux (21). The same three inhibitors have been shown to protect C. albicans cells from killing by HNP-1 (23). These similarities in cell protection against Hst 5 and HNP-1-induced killing suggested that the candidacidal pathway activated by α-defensins may also involve the efflux of cellular ATP. Therefore, we tested whether release of cellular ATP is specific for the Hst 5 killing pathway or if it represents a common cellular response to other candidacidal molecules such as HNP-1 and the azole-based (miconazole) and polyene (amphotericin B) antifungal drugs. Incubation of the cells for 1.5 h with either Hst 5 (31 μM), HNP-1 (15 μM), miconazole (500 μg/ml) or amphotericin B (2 μg/ml) resulted in a complete loss of cell viability; and with the exception of amphotericin B, all agents caused a significant release of cellular ATP (Table 1). At the concentrations tested, both Hst 5 and HNP-1 produced about a 65-fold increase in extracellular ATP (Table 1). Dose-response curves showed that like that induced by Hst 5, HNP-1-induced efflux of cellular ATP was concentration dependent and correlated with cell killing (Fig. 1). Interestingly, HNP-1 at low concentrations (1.1 to 2.2μM) induced about 20% loss of C. albicans viability, whereas the extracellular ATP level was not significantly increased. Since released ATP was measured after 1.5 h of incubation of the cells with peptide, it is possible that the remaining 80% of cells not affected by HNP-1 possess active ATPases capable of rapid hydrolysis of low levels of extracellular ATP. The amount of extracellular ATP detected in miconazole-treated cultures was about one-third of the ATP that was released from Hst 5 and HNP-1-treated cells (Table 1). Measurements of intracellular ATP revealed a reduction of more than 95% after exposure of the cells to Hst 5, HNP-1, or miconazole, whereas amphotericin B-treated cells contained about 30% of the ATP found in control cells (Table 1).
TABLE 1.
Hst 5 and HNP-1 killing of C. albicans is characterized with similar effects on cellular ATP and is inhibited by the same pharmacological agentsa
| Treatment | ATP (pmol)
|
% Loss of cell viability with treatment:
|
|||
|---|---|---|---|---|---|
| Extracellular | Intracellular | None | Suramin | CCCP | |
| Control | 1.6 ± 1 | 60 ± 8 | 0 | 0 | 0 |
| Hst 5 | 100 ± 20b | 1.5 ± 0.2b | 97 ± 4 | 18 ± 2 | 38 ± 5 |
| HNP-1 | 110 ± 30b | 1 ± 1b | 96 ± 5 | 16 ± 1 | 34 ± 7 |
| Miconazole | 39 ± 3b | 0.3 ± 0b | 100 | 100 | 100 |
| Amphotericin B | 2 ± 1 | 21 ± 7b | 100 | 100 | 100 |
C. albicans strain DS1 (106 cells) was incubated for 1.5 h at 37°C with Hst 5 (3.1 μM), HNP-1 (15 μM), miconazole (500 μg/ml), or amphotericin B (2 μg/ml), or cells were mixed with CCCP (500 μM) for 2 h or suramin (100 μM) for 15 min at 37°C and then treated with Hst 5, HNP-1, miconazole, or amphotericin B for an additional 1.5 h. Cell supernatants were assayed for released ATP by luminometry; cell pellets were diluted and aliquots were plated on agar to assess viability, whereas the remainder of the cells were used for determination of intracellular ATP. ATP was measured in bioluminescence relative light units and ATP concentrations (picomoles per 106 cells) determined from ATP standard curves. Loss of cell viability is expressed as [1 − (CFU treated cells/CFU control] × 100. Control CFU (480 ± 27) was the average of CFU from cells incubated in a solution of 10 mM phosphate buffer, 1% methanol (vehicle for miconazole and CCCP), and 1% DMSO (vehicle for amphotericin B). Results are means ± standard deviations from duplicates from three to five independent experiments. Statistical significance was calculated using Student's t test from data from control cells compared with extracellular ATP released by Hst 5, HNP-1, or miconazole and data from control cells compared with intracellular ATP in Hst 5-, HNP-1-, miconazole-, or amphotericin B-treated cells.
P < 0.005.
FIG. 1.
Dose-dependent ATP efflux characterizes Hst 5- and HNP-1-induced killing of C. albicans. C. albicans strain DS1 (106 cells) was incubated for 1.5 h at 37°C in the presence or absence of the indicated concentrations of Hst 5 or HNP-1. Cells were then plated on agar to assess viability, and supernatants were used for extracellular ATP measurements. Extracellular ATP (picomoles released from 106 cells) and loss of viability were assayed as described in Table 1. Fifty percent lethal dose values are calculated from the dose-response curves as 7.0 ± 2.1 μM (Hst 5) and 4.7 ± 0.6 μM (HNP-1). Each data point is the mean ± standard deviation (error bar) of duplicate determination from five (Hst 5) and two (HNP-1) independent experiments.
The difference between the maximum level of extracellular ATP detected following incubation with Hst 5 or HNP-1 (about 0.1 fmol of ATP released/cell) and the amount of intracellular ATP measured in control cells (approximately 0.06 fmol/cell) (Table 1) may be due to a lower recovery of intracellular ATP as a result of incomplete disruption of yeast cells or hydrolysis of ATP during the breakage of the cells. However, it is possible that the detected levels of extracellular ATP after Hst 5 or HNP-1 treatment represent an efflux of continuously synthesized ATP in the cells, at least in part, via the mitochondrial oxidative phosphorylation (see Table 2).
TABLE 2.
Effect of Hst 5, HNP-1, miconazole, and amphotericin B on C. albicans respirationa
| Treatment | Oxygen uptake (nmol of O2/min/106 cells) |
|---|---|
| Control | 11.1 ± 0.3 |
| Hst 5 | 7.7 ± 0.5 (<0.001) |
| HNP-1 | 8.2 ± 0.1 (<0.001) |
| Miconazole | 1.6 ± 0.4 (<0.005) |
| Amphotericin B | 1.7 ± 0.4 (<0.005) |
| Antimycin A | 0.5 ± 0 (<0.005) |
C. albicans strain DS1 (106 cells) was incubated for 1.5 h at 37°C in the presence or absence of Hst 5 (31 μM), HNP-1 (15 μM), miconazole (500 μg/ml), or amphotercin B (2 μg/ml), or cells were treated with the respiratory inhibitor antimycin A (1 μg/ml), and oxygen consumption was measured for 10 min as described in Materials and Methods. Results are means ± standard deviations from three independent experiments. Statistical significance was calculated using Student's t test from data from control cells compared to the rate of respiration in Hst 5-, HNP-1-, miconazole-, amphotericin B-, or antimycin A-treated cells. Parenthetical data are P values for treated cells compared with control cells.
The finding that Hst 5, HNP-1, and miconazole induced marked increases in extracellular ATP and caused depletion of intracellular ATP suggested that they may kill C. albicans via overlapping mechanisms. To explore this possibility we tested whether known inhibitors of Hst 5 killing also protect C. albicans from HNP-1, miconazole, and amphotericin B-induced yeast killing. For these experiments we utilized the P2 antagonist suramin and the proton ionophore CCCP, which were previously shown to prevent Hst 5 killing of C. albicans and at the concentrations used did not affect cell viability (21). Preincubation of the cells with suramin inhibited approximately 80% of Hst 5- and HNP-1-induced cell killing (Table 1). Similarly, pretreatment of the cells with CCCP before the addition of Hst 5 or HNP-1 resulted in increased survival. In contrast, neither suramin nor CCCP protected C. albicans from miconazole- or amphotericin B-induced killing (Table 1). Thus, a direct comparison of effects on cellular ATP, together with the use of inhibitors of cell killing demonstrated that the killing pathways activated by Hst 5 and HNP-1 share similar features, distinguishable from the killing mechanisms for miconazole or amphotericin B.
Effect of Hst 5, HNP-1, and the antifungal drugs on cellular respiration.
Hst 5-induced ATP release from C. albicans represented an efflux from structurally intact and metabolically active cells. This was supported by the findings that C. albicans cells treated with Hst 5 for 10 to 30 min (ATP release) or 1.5 h (complete cell killing measured by inability to form colonies) were actively respiring and the cell membranes remained polarized (21). Therefore, we examined whether C. albicans cells maintained physiological function following exposure to candidacidal agents that induced release of cellular ATP. For these experiments we measured C. albicans endogenous respiration (in the absence of exogenous substrates for the mitochondria). C. albicans cells incubated for 1.5 h with Hst 5 or HNP-1 were metabolically active, with a substantial rate of oxygen consumption (about 70% of the rate measured in untreated cells), whereas respiration was completely blocked by antimycin A, an inhibitor of the classical respiratory chain (Table 2). In contrast, exposure of the cells for 1.5 h to miconazole (which caused extracellular ATP release) or treatment with amphotericin B greatly decreased cellular respiration (about 85% reduction compared to control cells) (Table 2). The fact that Hst 5- or HNP-1-treated cells were respiring at the time of plating but could not subsequently form colonies after 24 h suggests that similar to that of Hst 5, HNP-1's effect on cell viability during the 1.5 h of treatment may not be a result of a direct lytic action. Altogether, the oxygen consumption measurements revealed another similarity in C. albicans response to Hst 5 and HNP-1.
Culture anaerobiosis protects C. albicans from Hst 5 and HNP-1 but not from miconazole or amphotericin B killing.
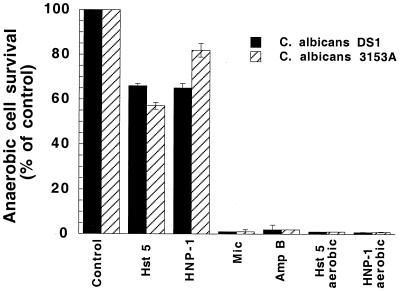
Active mitochondrial metabolism has been suggested to sensitize C. albicans cells to both Hst-5 and HNP-1 (12, 14, 23). We therefore tested whether anaerobically grown cells that do not have functioning mitochondria were susceptible to Hst 5. We employed the enzyme preparation Oxyrase to remove oxygen and create anaerobic conditions for C. albicans cell growth. Measurements of cellular endogenous respiration confirmed culture anaerobiosis. Cells grown for 2 days in standard medium containing Oxyrase did not consume oxygen and respiration was not detected for at least 30 min after cells were washed free of Oxyrase and resuspended in air-saturated medium. C. albicans cells grown anaerobically exhibited 65% (strain DS1) and 55% (strain 3153A) reduction in killing when exposed for 1.5 h to 31 μM Hst 5, compared to cells grown in air-saturated medium (Fig. 2). We next examined whether similar conditions would affect the candidacidal activities of HNP-1 and the antifungal drugs miconazole and amphotericin B. As expected, the susceptibility to HNP-1 was greatly reduced in anaerobically grown C. albicans cells (about 65 and 82% increase in survival for strains DS1 and 3153A, respectively). In contrast, anaerobic growth did not protect the cells from either miconazole or amphotericin B killing (Fig. 2). Thus, experiments using anaerobically grown cells provided additional evidence that the killing pathways activated by Hst 5 and HNP-1 may be similar.
FIG. 2.
Culture anaerobiosis protects C. albicans from Hst 5 and HNP-1 killing. C. albicans cells (strains DS1 and 3153A) were grown anaerobically in medium containing Oxyrase. Cells were washed and resuspended in anaerobic buffer (10 mM phosphate buffer [pH 7.4] with Oxyrase) and then treated for 1.5 h at 37°C with Hst 5 (31 μM), HNP-1 (15 μM), miconazole (500 μg/ml), or amphotercin B (2 μg/ml). Cell survival is expressed as percentage of control, and values are means ± standard deviations [error bars] from duplicates from four independent experiments. Abbreviations: Mic, miconazole; Amp B, amphotericin B.
Hst 5 and HNP-1 share a binding protein in C. albicans.
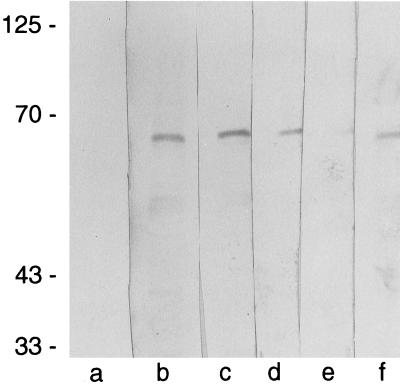
We have previously reported that C. albicans expresses functional binding sites for salivary Hsts and identified a 67-kDa candidal HstBP. HstBPs were also detected on susceptible Saccharomyces cerevisiae cells (9). The similarities of the Hst 5 killing pathway to that activated by HNP-1 raised the intriguing possibility that HNP-1 and Hst 5 may share yeast binding components. To test this possibility, we examined whether HNP-1 as well as the antifungal drugs miconazole and amphotericin B interact with HstBP by using an overlay assay. For these experiments, proteins from C. albicans cell lysates were separated by electrophoresis and analyzed on PVDF membranes with biotin-Hst 5. Biotin-Hst 5 bound to a candidal 67-kDa protein (Fig. 3, lane f). This was the only protein consistently observed to bind biotin-Hst 5 (n = 20), and its apparent molecular weight corresponded to the size of the protein previously recognized by 125I-Hst 5 in overlay and cross-linking experiments (9). HstBP was not detected when membranes were incubated only with ExtrAvidin conjugated to peroxidase, confirming that the detected band did not represent an endogenously biotinylated C. albicans protein (Fig. 3, lane a). Preincubation of the blots with a 60-fold excess of HNP-1 (Fig. 3, lane e) or unlabeled Hst 5 (data not shown) inhibited biotin-Hst 5 binding to HstBP. In contrast, neither of the antifungal drugs tested—miconazole or amphotericin B—prevented the interaction of biotin-Hst 5 with its binding protein (Fig. 3, lanes c and d). Thus, the ability of HNP-1 to compete with biotin-Hst 5 for HstBP suggests a basis for the similarity in the killing pathways activated by these naturally occurring candidacidal proteins. Although miconazole induced a marked increase in extracellular ATP, it differed from Hst 5 and HNP-1 in that its candidacidal activity was not inhibited by culture anaerobiosis or by the pharmacological agents suramin and CCCP and it did not interact with the C. albicans HstBP. The exact mechanism by which CCCP and suramin induce protection is currently unclear. CCCP dissipated C. albicans membrane potential (21) and is also known to uncouple respiratory chain phosphorylation and induce endogenous fermentation in yeast (6, 26). The effects of suramin on yeast are not clear, but in other systems it acts as a P2 receptor antagonist and also as a less-specific growth factor receptor antagonist (43). The anionic nature of suramin, however, suggested that the inhibition of Hst 5 yeast killing may not be due to P2 antagonism or other cellular effects, but rather to an interaction with the cationic Hst 5 in solution, thereby decreasing the amount of available Hst 5 to access the cells. Therefore, we used an overlay assay to test whether suramin inhibits Hst 5 binding to the candidal HstBP. Although inactivation of Hst 5 due to electrostatic interactions with suramin in solution was considered as a possibility, we found no evidence for it, since a 1,200-fold molar excess of suramin did not decrease or prevent the binding of biotin-Hst 5 to HstBP (Fig. 3, lane b).
FIG. 3.
HNP-1 prevents Hst 5 from binding to its binding protein in C. albicans in an overlay assay. Proteins from whole-cell lysates (108 C. albicans cells, strain DS1) were separated by SDS-PAGE and transferred onto a PVDF membrane. The membrane was cut into strips containing equal amounts of total protein and incubated for 2 h in the presence (lane f) or absence (lane a) of 250 nM biotinylated Hst 5, or the strips were preincubated with 300 μM suramin (lane b), 100 μg of amphotericin B per ml (lane c), 500 μg of miconazole per ml (lane d), or 15 μM HNP-1 (lane e) before the addition of biotin-Hst 5. Reactive biotinylated proteins were visualized with the ExtrAvidin-peroxidase system. The molecular sizes of protein standards (in thousands) are indicated to the left.
DISCUSSION
The principal finding of the present work is the remarkable similarity in the response of C. albicans to Hst 5 and HNP-1. The most direct interpretation of our results is that these two naturally occurring antimicrobial proteins act on C. albicans via shared killing pathways.
The current understanding of the mechanism of Hst yeast killing is that it involves at least three steps: transient binding to the yeast plasma membrane, intracellular uptake, and interaction with cellular targets (9, 14, 45). Furthermore, Hst 5-induced killing correlated with a nonlytic release of cellular ATP (21). Transmembrane ATP efflux through ATP-specific channels in the absence of cytolysis has been described in other cell systems and ATP binding cassette proteins have been implicated in conductive transport of ATP (36, 37). It remains to be determined whether induction of ATP release results from Hst 5 binding to C. albicans membrane or if it is initiated after Hst 5 internalization upon interaction with intracellular targets.
Here, we showed that HNP-1 kills C. albicans via a mechanism that shares very similar features to Hst-5 cytotoxic action. First, the effects of Hst 5 and HNP-1 on cellular ATP were essentially indistinguishable with respect to active concentrations, time and magnitude of induction of ATP efflux, and depletion of intracellular ATP (Fig. 1; Table 1). Second, HNP-1 and Hst 5 displayed parallel inhibitor profiles; i.e., pharmacological agents or conditions (CCCP, suramin, or culture anaerobiosis) that protected C. albicans from Hst 5 killing also afforded protection against HNP-1 killing (Table 1; Fig. 2). Third, HNP-1 prevented the interaction of Hst 5 with its candidal 67-kDa binding protein (Fig. 3). Finally, the major characteristic of Hst 5 candidacidal action is the induction of ATP efflux while the cells were respiring and had polarized membranes (21). Similarly, HNP-1 induced ATP release from C. albicans cells that maintained physiologic function, as evidenced by their substantial rate of oxygen consumption (Table 2). Early studies have shown that rabbit neutrophil defensin NP-1 killed C. albicans in vitro within minutes, causing rapid cessation of oxygen consumption (32). However, the killing ability of NP-1 was not affected by inhibitors of HNP-1 candidacidal activity (CCCP, DNP, azide, anaerobic conditions, or Ca2+ and Mg2+) (23). Based on these differences, mammalian α-defensins were divided into two functional classes—type I rabbit defensins (NP-1 and NP-2), which do not require an active metabolic microbial target for their killing activity, and type II human defensins (HNP-1 and HNP-2), which act against metabolically active target (11). In this respect, the candidacidal action of HNP-1 (type II defensin) appears to be more similar to the killing induced by salivary Hst 5 than to its close relative NP-1.
The polyene and azole antifungal drugs target either ergosterol on the yeast plasma membrane or inhibit its biosynthesis and exert fungicidal action related to direct membrane damage (2, 4, 42). Molecular modeling studies predicted similarity between dipeptide segments in the Hst molecule and the azole moiety and possibly a common mechanism of cell killing (35); however, Hst 5's ability to kill azole-resistant C. albicans strains did not support these observations (27, 39). Our results further demonstrate that the responses of C. albicans to peptide antibiotics such as Hst 5 and HNP-1 are distinct from those to the antifungal drugs miconazole and amphotericin B. Conditions that provided protection to C. albicans from Hst 5 and HNP-1 killing did not affect miconazole- and amphotericin B-induced cell death (Table 1; Fig. 2). In contrast to Hst 5- and HNP-treated cultures, cells exposed to miconazole or amphotericin B displayed very little respiratory activity (Table 2). In addition, neither of the drugs prevented the interaction of Hst 5 with the candidal HstBP (Fig. 3). Miconazole and amphotericin B also differed in their effects on cellular ATP. The detected extracellular ATP following minconazole treatment may represent leakage from lysed cells as previously reported for other azoles (2). However, the reduction in intracellular ATP seen in cultures exposed to amphotericin B or miconazole may also be due to inhibition of the ATP synthetic process as suggested elsewhere (1, 4).
Our findings raise many questions concerning the similarity between Hst 5 and HNP-1 candidacidal action. Hst 5 and HNP-1 are of similar size (24 and 30 amino acids, respectively), and both are highly basic (pI > 10); however, each molecule possesses unique structural features. The weak amphiphilic nature of Hst 5 precludes spontaneous insertion into microbial membranes and formation of ion channels (34). In contrast, the amphiphilic molecules of defensins permit insertion into model phospholipid bilayers (18). It is not yet known whether HNP-1-induced channel formation is in itself sufficient to kill microorganisms or if other cellular events are required for killing (11). Regardless, the structural features of Hst 5 and HNP-1 are unsatisfyingly different to explain how these two molecules act so similarly on C. albicans to induce cell death. A distant but relevant precedent is the number of structurally different and broadly acting cytokines and neurokines that have overlapping activities in cells expressing appropriate shared receptor components (16, 20). Perhaps the host defense strategies also include production of a variety of antimicrobial peptides that function in a redundant manner; different peptides can act on the same type of microbial cell to mediate similar effects leading to death. The killing pathways of Hst 5 and HNP-1 may converge through interactions with shared components. HstBP in C. albicans is one possible candidate for such shared components.
The purification of HstBP and molecular identification of this protein would be a crucial step in understanding its role in the yeast cell killing. In addition, it is important to determine whether HNP-1 binding to HstBP is sufficient to account for the observed similarities between Hst 5 and HNP-1 killing (e.g., nonlytic ATP release and inhibitor sensitivity) or whether HNP-1 is further translocated into C. albicans cells. Finally, an issue that requires consideration is the physiological significance of the induction of ATP release by antimicrobial proteins like Hst 5 and HNP-1 in yeast killing. In a higher eukaryotic model, released ATP in the absence of cytolysis can act as a cytotoxic mediator by binding to membrane nucleotide P2 receptors (8). Consistent with this hypothesis, we have shown that P2 agonists (ATP analogues) induced loss of C. albicans cell viability and that the P2 antagonists suramin and pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) prevented Hst 5 killing (21). Consequently, this model of ATP-mediated cytotoxicity represents a new focus for further elaboration of the molecular mechanisms of cell death induced by Hst 5 and HNP-1.
Collectively, the results presented here support the concept that the mechanism of candidacidal action of salivary Hst 5 may not be unique but rather appears to be shared by another innate host defense protein, HNP-1.
ACKNOWLEDGMENTS
We thank Molakala Reddy and Hakimuddin Sojar for insightful scientific input, Philip Loverde and Arvind Thakur for the use of their luminometer, and Tracy Lloyd for expert technical assistance.
This work was supported by U.S. Public Health grants DE10641, DE00406, and DE12159 from the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Abbot A B, Odds F C. Abrogation by glucose of the ATP suppression induced by miconazole in C. albicans. Antimicrob Agents Chemother. 1989;24:905–919. doi: 10.1093/jac/24.6.905. [DOI] [PubMed] [Google Scholar]
- 2.Ansehn S, Nilsson L. Direct membrane-damaging effect of ketoconazole and tioconazole on Candida albicans demonstrated by bioluminescent assay of ATP. Antimicrob Agents Chemother. 1984;26:22–25. doi: 10.1128/aac.26.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beggs W G. Physiochemical cell damage in relation to lethal amphotericin B action. Antimicrob Agents Chemother. 1994;38:363–364. doi: 10.1128/aac.38.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolard J. Mechanism of action of an anti-Candida drug: amphotericin B and its derivatives. In: Prasad R, editor. Candida albicans: cellular and molecular biology. Berlin, Germany: Springer-Verlag; 1991. pp. 215–238. [Google Scholar]
- 5.Cannon R D, Holmes A R, Mason A B, Monk B C. Oral candida: clearance, colonization or candidiasis? J Dent Res. 1995;74:1152–1161. doi: 10.1177/00220345950740050301. [DOI] [PubMed] [Google Scholar]
- 6.Clark F C, Parkinson T, Hitchcock C A, Gow N A R. Correlation between rhodamine 123 accumulation and azole sensitivity in Candida species: possible role for drug efflux in drug resistance. Antimicrob Agents Chemother. 1996;40:419–425. doi: 10.1128/aac.40.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cockayne A, Odds F C. Interactions of Candida albicans yeast cells, germ tubes and hyphae with human polymorphonuclear leukocytes in vitro. J Gen Microbiol. 1984;130:465–471. doi: 10.1099/00221287-130-3-465. [DOI] [PubMed] [Google Scholar]
- 8.Di Virgilio F, Chiozzi P, Falzoni S, Ferrari D, Sanz J M, Venketaraman V, Baricordi O R. Cytolytic P2X purinoceptors. Cell Death Differ. 1998;5:191–199. doi: 10.1038/sj.cdd.4400341. [DOI] [PubMed] [Google Scholar]
- 9.Edgerton M, Koshlukova S E, Lo T E, Chrzan B G, Straubinger R M, Raj P A. Candidacidal activity of salivary histatins: identification of a histatin 5-binding protein on Candida albicans. J Biol Chem. 1998;273:20438–20447. doi: 10.1074/jbc.273.32.20438. [DOI] [PubMed] [Google Scholar]
- 10.Gabay J E. Ubiquitous natural antibiotics. Science. 1994;264:373–374. doi: 10.1126/science.8153623. [DOI] [PubMed] [Google Scholar]
- 11.Ganz T, Selsted M, Lehrer R I. Defensins. Eur J Haematol. 1990;44:1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 12.Gyurko C, Lendenmann U, Troxler R, Oppenheim F. Candida albicans mutants deficient in respiration are resistant to the small cationic salivary antimicrobial peptide histatin 5. Antimicrob Agents Chemother. 2000;44:348–354. doi: 10.1128/aac.44.2.348-354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helmerhorst E J, Reijnders I M, van't Hof W, Veerman E C, Nieuw Amerongen A V. A critical comparison of the hemolytic and fungicidal activities of cationic antimicrobial peptides. FEBS Lett. 1999;23:105–110. doi: 10.1016/s0014-5793(99)00411-1. [DOI] [PubMed] [Google Scholar]
- 14.Helmerhorst E J, Breeuwer P, van't Hof W, Walgreen-Weterings E, Oomen L C, Veerman E C, Amerongen A V, Abee T. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J Biol Chem. 1999;274:7286–7291. doi: 10.1074/jbc.274.11.7286. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann J A, Kafatos F C, Janeway C A, Ezekowitz R A B. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 16.Ip N Y, Nye S H, Boulton T G, Davis S, Taga T, Li Y, Birren S J, Yasukawa K, Kishimoto T, Anderson D J, et al. CNTF and LIF act on neuronal cells via shared signaling pathways that involve the IL-6 signal transducing receptor component gp130. Cell. 1992;69:1121–1132. doi: 10.1016/0092-8674(92)90634-o. [DOI] [PubMed] [Google Scholar]
- 17.Jainkittivong A, Johnson D A, Yeh C K. The relationship between salivary histatin levels and oral yeast carriage. Oral Microbiol Immunol. 1998;13:181–187. doi: 10.1111/j.1399-302x.1998.tb00730.x. [DOI] [PubMed] [Google Scholar]
- 18.Kagan B L, Ganz T, Lehrer R I. Defensins: a family of antimicrobial and cytotoxic peptides. Toxicology. 1994;87:131–149. doi: 10.1016/0300-483x(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 19.Kagan B L, Selsted M, Ganz T, Lehrer R I. Antimicrobial defensin peptides form voltage-dependent ion permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci USA. 1990;87:210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kishimoto T, Taga T, Akira S. Cytokine signal transduction. Cell. 1994;76:253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 21.Koshlukova S E, Lloyd T L, Araujo M W B, Edgerton M. Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J Biol Chem. 1999;274:18872–18879. doi: 10.1074/jbc.274.27.18872. [DOI] [PubMed] [Google Scholar]
- 22.Lehrer R I. Editorial response: questions and answers about defensins. Clin Infect Dis. 1997;25:1141–1142. doi: 10.1086/516082. [DOI] [PubMed] [Google Scholar]
- 23.Lehrer R I, Ganz T, Szklarek D, Selsted M E. Modulation of the in vitro candidacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J Clin Investig. 1988;81:1829–1835. doi: 10.1172/JCI113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichtenstein A, Ganz T, Nguyen T, Selsted M, Lehrer R I. Mechanism of target cytolysis by peptide defensins. J Immunol. 1988;140:2686–2694. [PubMed] [Google Scholar]
- 25.Lohner K, Latal A, Lehrer R I, Ganz T. Differential scanning microcalorimetry indicates that human defensin, HNP-2, interacts specifically with biomembrane mimetic systems. Biochemistry. 1997;36:1525–1531. doi: 10.1021/bi961300p. [DOI] [PubMed] [Google Scholar]
- 26.Noshiro A, Purwin C, Laux M, Nicolay K, Scheffers W A, Holzer H. Mechanisms of stimulation of endogenous fermentation in yeast by carbonyl cyanide m-chlorophenylhydrazone. J Biol Chem. 1987;262:14154–14157. [PubMed] [Google Scholar]
- 27.O'Connel B C, Xu T, Walsh T J, Sein T, Mastrangeli A, Crystal R G, Oppenheim F G, Baum B J. Transfer of a gene encoding the anticandidacidal protein histatin 3 to salivary glands. Hum Gene Ther. 1996;7:2255–2261. doi: 10.1089/hum.1996.7.18-2255. [DOI] [PubMed] [Google Scholar]
- 28.Odds F C. Candida infections: an overview. Crit Rev Microbiol. 1987;15:1–5. doi: 10.3109/10408418709104444. [DOI] [PubMed] [Google Scholar]
- 29.Oppenheim F G, Xu T, McMillian F M, Levitz S M, Diamond R D, Offner G D, Troxler R F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. J Biol Chem. 1988;263:7472–7477. [PubMed] [Google Scholar]
- 30.Ouellette A. Paneth cells and innate immunity in the crypt microenvironment. Gastroenterology. 1997;113:1779–1784. doi: 10.1053/gast.1997.v113.pm9352884. [DOI] [PubMed] [Google Scholar]
- 31.Ouellette A. Mucosal immunity and inflammation. Paneth cell antimicrobial peptides and their biology of the mucosal barrier. Am J Physiol. 1999;277:G257–261. doi: 10.1152/ajpgi.1999.277.2.G257. [DOI] [PubMed] [Google Scholar]
- 32.Paterson-Delafield J, Szklarek D, Martinez R J, Lehrer R I. Microbicidal cationic proteins of rabbit aveolar macrophages: amino acid composition and functional attributes. Infect Immun. 1981;31:723–731. doi: 10.1128/iai.31.2.723-731.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raj P A, Edgerton M, Levine M J. Salivary histatin 5- dependence of sequence, chain length and helical conformation for candidacidal activity. J Biol Chem. 1990;265:3898–3905. [PubMed] [Google Scholar]
- 34.Raj P A, Marcus E, Sukumaran D K. Structure of human salivary histatin 5 in aqueous and nonaqueous solutions. Biopolymers. 1998;45:51–67. doi: 10.1002/(SICI)1097-0282(199801)45:1<51::AID-BIP5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 35.Ramalingam K, Gururaja T L, Ramasubbu N, Levine M J. Stabilization of helix by side-chain interactions in histatin-derived peptides: role in candidacidal activity. Biochem Biophys Res Commun. 1996;225:47–53. doi: 10.1006/bbrc.1996.1129. [DOI] [PubMed] [Google Scholar]
- 36.Roman R M, Wang Y, Lidofsky S D, Feranchak A P, Lomri N, Scharschmidt B F, Fitz J G. Hepatocellular ATP-binding cassette protein expression enhances ATP release and autocrine regulation of cell volume. J Biol Chem. 1997;272:21970–21976. doi: 10.1074/jbc.272.35.21970. [DOI] [PubMed] [Google Scholar]
- 37.Schwiebert E M, Egan M E, Hwang T H, Fulmer S B, Allen S S, Cutting G R, Guggino W B. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- 38.Situ H, Bobek L A. In vitro assessment of antifungal therapeutic potential of salivary histatin-5, two variants of histatin-5, and salivary mucin (MUC7) domain 1. Antimicrob Agents Chemother. 2000;44:1485–1493. doi: 10.1128/aac.44.6.1485-1493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai H, Bobek L A. Studies of the mechanism of human salivary histatin-5 candidacidal activity with histatin-5 variants and azole-sensitive and -resistant Candida species. Antimicrob Agents Chemother. 1997;41:2224–2228. doi: 10.1128/aac.41.10.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai H, Raj P A, Bobek L A. Candidacidal activity of recombinant salivary histatin-5 and variants. Infect Immun. 1996;64:5000–5007. doi: 10.1128/iai.64.12.5000-5007.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uno J, Shigematsu M, Arai T. Primary site of action of ketoconazole on C. albicans. Antimicrob Agents Chemother. 1982;21:912–918. doi: 10.1128/aac.21.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanden Bossche H. Ergosterol biosynthesis inhibitors. In: Prasad R, editor. Candida albicans: cellular and molecular biology. Berlin, Germany: Springer-Verlag; 1991. pp. 239–257. [Google Scholar]
- 43.Voogd T, Vansterkenburg E, Wilting J, Janssen L. Recent research on the biological activity of suramin. Pharmacol Rev. 1993;45:177–203. [PubMed] [Google Scholar]
- 44.Xu T S, Levitz M, Diamond R D, Oppenheim F G. Anticandidal activity of major salivary histatins. Infect Immun. 1991;70:2549–2554. doi: 10.1128/iai.59.8.2549-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Y, Ambudkar I, Yamagushi H, Swam W, Walsh T, O'Connell B. Histatin 3-mediated killing of Candida albicans: effect of extracellular salt concentration on binding and internalization. Antimicrob Agents Chemother. 1999;43:2256–2262. doi: 10.1128/aac.43.9.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]