Summary
5-methyl cytosine is widespread in plant genomes in both CG and non-CG contexts. During replication, hemi-methylation on parental DNA strands guides symmetric CG methylation on nascent strands, but non-CG methylation requires modified histones and small RNA guides. Here, we used immortalized Arabidopsis cell suspensions to sort replicating nuclei and determine genome-wide cytosine methylation dynamics during the plant cell cycle. We find that symmetric mCG and mCHG are selectively retained in actively dividing cells in culture, while mCHH is depleted. mCG becomes transiently asymmetric during S phase but is rapidly restored in G2, while mCHG remains asymmetric throughout the cell cycle. Hundreds of loci gain ectopic CHG methylation, as well as 24-nt small-interfering RNAs and H3K9me2, without gaining CHH methylation. This suggests that spontaneous epialelles that arise in plant cell cultures are stably maintained by siRNA and H3K9me2 independently of the canonical RNA-directed DNA methylation pathway. In contrast, loci that fail to produce siRNA may be targeted for demethylation when cell cycle arrests. Comparative analysis with methylomes of various tissues and cell types suggests that loss of small RNA-directed non-CG methylation during DNA replication promotes germline reprogramming and epigenetic variation in plants propagated as clones.
Graphical Abstract

MET1 activity maintains CG methylation during DNA replication. At this stage, mCHG becomes asymmetric but must be restored by CMT3 at G2, based on the lack of mCHG asymmetry observed in microspores and in the daughter vegetative nucleus (VN). mCHH is lost in cycling cells, and in the two sperm cells (SC) that are arrested in S phase leading to asymmetric mCHG. Interestingly, mCG is symmetric but mCHG is asymmetric, indicating that mCG is restored after replication is arrested while mCHG is not. The VN exits cell cycle and restores CHG and CHH methylation via CMT3, CMT2 and RNA-directed DNA-methylation (RdDM). siRNAs from the VN may also contribute to restoring CHH methylation by RdDM at imprinted loci in sperm cells or only after fertilization in the early embryo when RdDM is restored.
Introduction
Chromatin modifications such as cytosine methylation, nucleosome positioning and histone modification, must be faithfully restored to daughter chromatids as replication of the primary DNA sequence proceeds. But unlike the primary sequence, chromatin modifications undergo major changes in response to cellular differentiation [1]. For this reason, chromatin marks and DNA methylation need to be erased and reset if epigenetic inheritance is to be avoided [2]. Plants and mammals differ in their requirements for DNA methylation reprogramming. Unlike in mammals, symmetric DNA methylation is retained in the plant germline, which allows robust epigenetic inheritance [2]. In plants, asymmetric DNA methylation is mostly erased from the male gametes, only to be restored after fertilization. However, imprinted genes retain RNA-directed DNA methylation (RdDM) and escape germline reprogramming in plants, reminiscent of imprinted genes in mammals [2,3]. Developing cell types in Arabidopsis thaliana pollen, for example, undergo dynamic changes in DNA and histone methylation [4-6]. After meiosis, the haploid microspore divides once to result in the vegetative and generative cells. The vegetative cell then arrests in G1/G0, and undergoes no further division unless stimulated to do so in culture [7]. The generative cell divides again, giving rise to two sperm cells that progress through the cell cycle during pollen tube growth. Quantitative microspectrofluorometry and gene expression analysis indicate that Arabidopsis sperm cells arrest in early- to mid-S phase in mature tricellular pollen, and reach G2 before entry in the embryo sac for fertilization [8,9]. Pollen from other angiosperms arrests at different stages of the cell cycle, and it has been proposed that “heterochronic alterations in cell cycle activity” underlie the diversity of pollen cell types [8].
5-methyl cytosine is abundant in the genomes of most eukaryotes, and can occur in both symmetric and asymmetric contexts [10]. Symmetric DNA methylation at CG dinucleotides leads to hemi-methylated CG after semi-conservative replication [11]. Hemi-methylated CG dinucleotides are recognized by UHRF/VIM methyl cytosine binding proteins, which in turn recruit the CG maintenance methyltransferase DNMT1/MET1 behind the replication fork. UHRF/VIM also recognizes histone H3 lysine di-methylation (H3K9me2) through its PHD domain, assuring that heterochromatin is efficiently methylated each cell division [10]. In plants, as well as in mammalian embryonic stem cells and post-mitotic neurons, non-CG methylation is prevalent and highly asymmetric [12,13], and must somehow be restored on both daughter chromatids, even though only one inherits the asymmetric methylated cytosine. This is likely accomplished through the inheritance of H3K9me2 and small-interfering RNA (siRNA). Both of these “guides” are hallmarks of constitutive heterochromatin inherited from cell to cell. In plants, H3K9me2 is read by the chromodomain methyltransferases CMT2 and CMT3 that methylate cytosines in the CHH and CHG contexts, respectively [14], which appears to inhibit the acquisition of facultative heterochromatin in the form of H3K27me3 [15]. In Arabidopsis, siRNAs can guide non-CG methylation via the RNA-directed DNA methylation (RdDM) pathway, in part through interactions between the siRNA binding protein AGO4 and the de novo methyltransferase DRM2, a rearranged homolog of the mammalian DNMT3 [10]. H3K9me2 can also be guided by siRNA, especially in organisms such as S. pombe, Drosophila and C. elegans that do not have DNA methylation [16].
Somaclonal variation, in which genetically identical plant clones have different phenotypes upon regeneration, has been observed in most if not all examples of micropropagation, and is most pronounced when cells are propagated in culture for longer periods of time. Early studies implicated loss of DNA methylation in somaclonal variation, and led to the hypothesis that the plant growth hormones auxin and cytokinin might be responsible [17]. Interestingly, recurrent somaclonal variants have been observed in many species suggesting specificity of methylation loss especially for transposons which are often activated in culture [18-21]. CHG and CHH methylation are highly enriched in transposons and guided by histone modifications and small RNA, which are thus implicated in this loss. In oil palm, loss of methylation of the Karma transposon occurs at high frequency and is responsible for the recurrent mantled fruit homeotic abnormality [22]. Loss of small RNA, CHG and CHH methylation occur in a programmed way, and can revert in mosaic cultures and regenerated palms.
We have used EdU labeling and fluorescence activated cell sorting (FACS) to purify nuclei from G1, G2, S phase and arrested Arabidopsis cells in culture, and performed whole genome bisulfite sequencing. We compared these methylomes with H3K9me2 and small RNA accumulation in unsynchronized cell cultures and leaves. In dividing cells, we found that symmetric CG methylation is transiently lost during late S phase, but rapidly restored in G2. In contrast, CHG methylation remains asymmetric during cell cycle, and its stability seems to require siRNA after cell cycle arrest. Strikingly, CHH methylation disappears entirely in dividing cells in culture, resembling the methylomes of dividing cells in pollen, seeds and roots. We propose that germline reprogramming and somaclonal variation reflect the loss of small RNA-directed DNA methylation during the cell cycle.
Results
Arabidopsis cells derived from embryonic leaves (cotyledons) can be maintained in culture indefinitely when supplied with plant hormones, sucrose and nutritional supplements [23]. Cells are transferred to fresh medium every 7 days, which perpetuates division. Adding fresh medium stimulates another round of DNA synthesis, which peaks between 12 and 16 h later and provides a time-window for labeling replicating DNA with modified deoxynucleotides such as EdU and BrdU [23,24]. If cells are not transferred to fresh medium after 7 days, they slow down cell division, initially without loss of viability, resembling a form of cellular quiescence [18,23]. DNA and histone methylation, as well as 24-nt small-interfering RNAs (siRNAs) are selectively reduced in unsynchronized cell cultures during exponential growth, based on overall levels of cytosine methylation in 1kb features on tiling microarrays covering chromosome 4 of the Arabidopsis genome [18].
Genome-wide loss of DNA methylation in Arabidopsis cell cultures occurs in all contexts
To investigate dynamic changes in DNA methylation during the cell cycle, we sorted nuclei according to DNA content and EdU incorporation after a 1-hour pulse at 16 hours after addition of fresh medium, and collected nuclei in the G1, G2, early S, mid S and late S phases of the cell cycle (Figures 1A and S1A). We determined the methylomes by whole genome bisulfite sequencing and found genome-wide loss of DNA methylation in all three sequence contexts, mostly at transposable elements (TEs) (Figures 1B, 1D and S1). The loss of DNA methylation is particularly striking in the CHH context (Figures 1B, 1D and S1), closely resembling germline cells during pollen development [4,5,25,26]. In Arabidopsis sperm cells, CHH methylation is only retained at a few hundred imprinted genes, and is guided by 24-nt siRNAs likely derived from the neighboring vegetative cell [4,27,28]. We validated our observations by sequencing the methylome of a different Arabidopsis cell suspension derived from the T87 cell line, also in Col-0 background [29]. We found a similarly striking reduction of CHH methylation in this cell culture, as compared to CG and CHG that were largely retained and at higher levels than in the cell culture used for nuclei sorting (Figure S3A). Genome sequencing did not reveal any mutations in genes with known functions in DNA methylation (Table S1), and importantly, CG methylation in genes was not affected in either culture (Figures 1B and S3A), indicating that the main DNA methyltransferase MET1 is functional [10]. We therefore conclude that loss of RdDM and CMT2-dependent CHH methylation is an epigenetic signature of actively dividing cells, while variable loss of CG and CHG methylation in independent cell lines may reflect different age, growth dynamics or culture conditions.
Figure 1. Genome-wide loss of DNA methylation in Arabidopsis cell cultures.
(A) Flow cytometric sorting of Arabidopsis Col-0 suspension cell cultures grown for 7 days post subculture, followed by another 16 hours after adding an equal volume of fresh medium. EdU incorporation into DNA was conjugated to Alexa fluor-488, and nuclei were counter stained with DAPI prior to sorting by flow cytometry. Bivariate plot of DNA content and EdU incorporation is shown. Black rectangles indicate the sorting gates used to collect nuclei for each sample. (B) Average CG, CHG and CHH methylation levels for 100-bp intervals were plotted over chromosome 3 in pairwise comparisons between wild type leaf and cell cultures (G1, S and G2 samples). Loss of DNA methylation in cell cultures occurs in all three sequence contexts (CG, CHG and CHH), as compared to leaf methylome. DNA methylation was also plotted over protein-coding genes (bottom left panel) and transposable elements (bottom right panel) annotated according to the TAIR10 reference genome, and aligned at the 5' and 3' ends (dashed lines). (C) Strand asymmetry in CG methylation at the Watson (x) and Crick (y) strand is visualized using a scatter of points with kernel density estimations in two dimensions, down-sampling all datasets to the number of filtered cytosines in the sample with lowest coverage (Early S). CG methylation is mostly symmetric in G1, but becomes transiently asymmetric throughout S phase, only to be restored in G2. The highest level of mCG asymmetry is observed in late S, as most CG methylation is present in pericentromeric heterochromatin that replicates late in S phase. Top left numbers show methylation values with highest density of cytosines, and the bottom right numbers represent the number of filtered cytosines analyzed in each plot. (D) A genome browser snapshot of pericentromeric heterochromatin in chromosome 1 shows that cell cultures are devoid of CHH methylation, while CG and CHG methylation, and siRNA biogenesis are selectively retained. ES - Early S phase, MS - Mid S phase, LS - Late S phase. See also Figures S1 and S3, and Data S2.
Loss of non-CG methylation during replication
We then assessed strand asymmetry in DNA methylation by plotting the level of methylation at each symmetric CG or CHG (CAG and CTG) dinucleotide on the Watson (x) and Crick (y) strands, and displayed the results as a 2-dimensional heatmap [30,31]. As expected, this analysis showed that the level of strand asymmetry at CG sites was higher in S phase nuclei than in G1 and G2 (Figures 1C and 2A), clearly reflecting the accumulation of hemi-methylated DNA during replication. Furthermore, mCG asymmetry seems to be most pronounced in late S phase (Figure 1C), when heterochromatin undergoes replication [24,32]. The majority of CG methylation occurs in pericentromeric heterochromatin, which is consistent with this result. In contrast, we observed mCHG asymmetry at CAG and CTG sites throughout the cell cycle, suggesting that mCHG maintenance following DNA replication is much slower than mCG (Figures 2A and S3B). CHG methylation in Arabidopsis is essentially confined to heterochromatin, and requires H3K9me2 deposition through the histone methyltransferases KYP/SUVH4, SUVH5 and SUVH6 [14,33]. This is consistent with the fact that most CHG methylation occurs in the symmetric CAG and CTG contexts, and requires KYP activity, while asymmetric CCG methylation is maintained by MET1 [34]. Similarly, in cell cultures, we found that CAG and CTG levels over TEs are higher than that of CCG methylation (Figure S1D), indicating that the pathways responsible for H3K9me2 deposition are functional. A high level of asymmetry in CHG context was also observed in the asynchronous T87 cell line (Figure S3B), while mCG is fully symmetric. If we consider that the majority of cells in these cultures is in G1 (Figure S1A), this result supports the idea that CHG methylation is kept asymmetric during active cell divisions in culture.
Figure 2. Strand asymmetry in CG and CHG methylation.
Methylation at the Watson (x) and Crick (y) strand is visualized using a scatter of points with kernel density estimations in two dimensions. (A) In cell cultures, CG methylation becomes transiently asymmetric during S phase, but is rapidly restored in G2, while CHG methylation remains asymmetric throughout the entire cell cycle. (B) DNA methylation in mitotic nuclei during pollen development shows a pattern of CG symmetry and CHG asymmetry in sperm cells that is consistent with their cell cycle stage (arrested in early S phase). In contrast, symmetric mCHG is retained in microspores (G2/M) and the vegetative nucleus (G1/G0). Top left numbers show methylation values with highest density of cytosines, and the bottom right numbers represent the number of filtered cytosines analyzed in each plot. See also Figure S2 and S3B.
We then performed strand asymmetry analysis with the methylomes of Arabidopsis nuclei during pollen development [4,5], which represent distinct and well synchronized cell cycle stages. In mature wild-type pollen, the vegetative nucleus is arrested in G1/G0, while the two mature sperm cells are arrested in early S phase [7,8]. We observed that strand asymmetry at mCHG is much more pronounced in sperm cells than in the vegetative nucleus or the precursor microspore at the G2/M transition (Figure 2B), thus indicating that mCHG asymmetry occurs during DNA replication. CG methylation is mostly symmetric in sperm cells, consistent with arrest in early to mid S phase [8]. As the vegetative nucleus (and microspore to some extent) restore CHG symmetry completely, we conclude that CHG asymmetry in G1 and G2 cells represent a specific epigenetic feature of rapidly dividing cells in culture, perhaps explaining the genome-wide loss of DNA methylation.
Previous studies have shown that CHH methylation is highly variable depending on the developmental stage of the seed [35-37], or cell type in the root apical meristem [38]. We hypothesized that these differences would correlate with mCHG asymmetry and active cell division, as it does in pollen nuclei. Indeed, we found variable levels of mCHG asymmetry in all tissues and cell types (Figure S2), closely following the levels of CHH methylation previously reported [35-38]. For example, in the seed, CHH methylation increases progressively during embryogenesis as cells stop dividing, being the most abundant in the mature embryo [35], when mCHG symmetry is relatively higher than in all other seed tissues (Figure S2A). In contrast, mCHG symmetry is low in the endosperm, where average mCHH levels are also at their lowest [39,40]. mCHG symmetry is higher in differentiated cells from the columella root cap (Figure S2B), where mCHH levels are the highest detected in the Arabidopsis root [38]. We conclude that mCHG asymmetry is an unexpected but widespread feature of the plant epigenome that seems to be coupled to DNA replication.
Ectopic CHG methylation in cell cultures is selectively retained during replication independently of the canonical RdDM pathway
Despite the widespread loss of DNA methylation in cell cultures, close to one hundred loci gain CHG methylation, along with concomitant siRNAs that are not normally found in leaf tissue of wild-type Arabidopsis (Figure 3) [18]. While DNA hypomethylation in cultured cells is found mostly at TEs (Figures 1B and S1), hypermethylated mCHG DMRs are enriched for genic regions (Data S1), resembling spontaneous epialleles that arise in mutants impaired in DNA and histone methylation and demethylation pathways such as met1, ddm1, ros1/dml2/dml3 (rdd) and ibm1 [41], We compared the methylomes of cells with leaf tissue from these mutants [41] (Figure 3D), and found 37 DMRs that overlap with hypermethylated loci in ibm1 and ddm1 (Figures 3A, 3B and 3E), and 45 DMRs overlap between the two independent cell cultures (Figure S3D), indicating that particular genes are prone to gain ectopic CHG methylation, H3K9me2 and siRNA in cell cultures. DNA methylation at the large intron of IBM1 has been previously associated with ectopic CHG methylation in met1 mutants [42]. Therefore, we profiled DNA methylation at the IBM1 large intron in the two independent cell cultures (Figures S3E and S3F), but did not find a significant loss of CG methylation that could be indicative of IBM1 loss-of-function.
Figure 3. Ectopic CHG methylation in cell cultures.
(A) Heatmap representation of CHG methylation levels at 95 hypermethylated mCHG DMRs in cells (G1 Cells vs WT Leaf), which partially overlap with hypermethylation in ibm1 and ddm1 mutant leaves. The majority of hyper mCHG DMRs is specific to cell cultures, and corresponds to gains in H3K9me2 and 24-nt siRNAs. H3K9me2 levels are plotted as a heatmap showing enrichment over normalized H3 levels, and siRNA bar plot shows the number of reads per million mapping to each DMR. In cells, 62 hypermethylated mCHG DMRs show enriched levels of H3K9me2 (H3K9me2/H3 >= 1.5) and 74 DMRs accumulate 24-nt siRNA (RPM>=5), while only 8 DMRs show enriched levels of H3K9me2 and 5 DMRs accumulate siRNAs in WT leaf. (B) Genome browser tracks show ectopic CHG methylation at four Arabidopsis genes in cells (G1 sample), as compared to WT, met1, ddm1, ibm1 and rdd mutant leaf. Gains of CHG methylation in cell cultures matches H3K9me2 and siRNA peaks. (C) Small RNA profiling shows preferential accumulation of siRNAs of 24-nt in length. RPM - Reads Per Million. (D) Average methylation over the 95 DMRs is plotted in CG, CHG and CHH contexts, and (E) Venn diagram is used to show that hypermethylated DMRs in cells overlap partially with ectopic mCHG DMRs observed in ddm1 and ibm1 mutants. See also Figure S3, and Data S1 and S2.
Ectopic methylation in ros1 mutant leaves occurs in all sequence contexts and requires Pol IV-dependent 24-nt siRNA [43]. In contrast, ectopic methylation in ddm1 mutants only occurs in the mCHG context, and requires 21-nt small RNA that guide ectopic H3K9me2 in the absence of RdDM [44], while ectopic CHG methylation in ibm1 mutants has not been directly linked to small RNA activity [45]. We found that hypermethylated loci in cell cultures gain mCHG, H3K9me2 (Figures 3A and 3B) and 24-nt siRNAs (Figure 3C), but do not acquire mCHH (Figure 3D). This strongly suggests that ectopic CHG methylation in cells is dependent on small RNA, resembling ddm1 mutants in this respect. Unlike in ros1, the absence of CHH methylation suggests a mechanism for RNAi-guided histone modification during DNA replication that is potentially independent of the canonical RdDM pathway. However, this interesting possibility awaits further investigation.
Loss of siRNA during replication results in targeted demethylation after cell cycle arrest
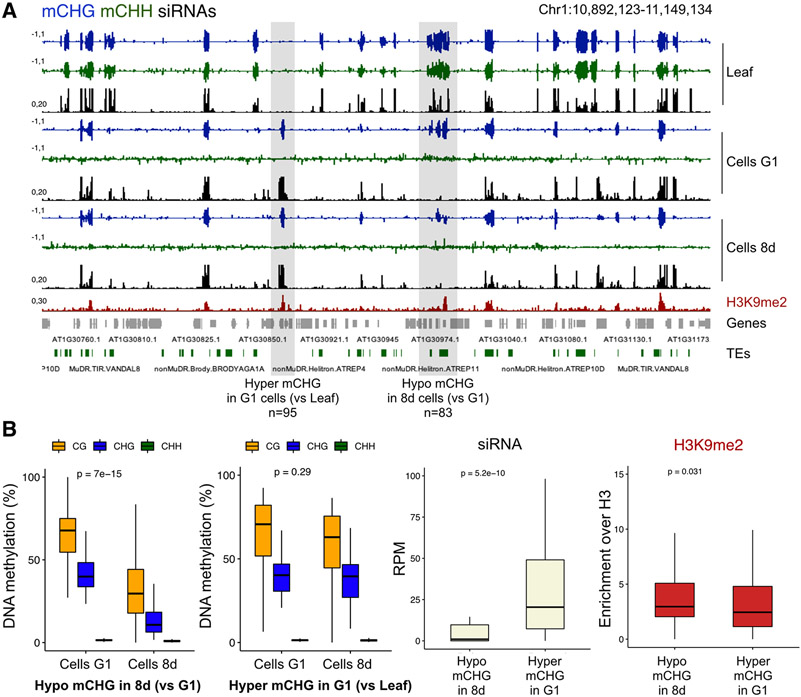
Our results indicate that CHH methylation is lost in cycling cells, although siRNA biogenesis is largely maintained (Figures 1D and S4B) [18]. Previous studies have shown that DNA and histone methylation increase in Arabidopsis cell suspensions, but only several days after they stop proliferation [46]. We sequenced the methylome of cells after 8 days in culture (8d) when cell divisions cease without loss of viability [23]. We found that CHH methylation remained low in 8d cells both at G1 and G2 (Figure S1B and Data S2), suggesting that recovery of RdDM and CMT2 activities after cell cycle arrest requires longer periods in quiescence. Instead, we observed a significant loss of CG and CHG methylation in 8d cells, which is particularly pronounced at TEs (Figure S1B and Data S1). Methylation loss without cell division may result from active DNA demethylation by the DNA glycosylases DME and ROS1 that remove DNA methylation by a base excision and repair mechanism [13]. ROS1 activity requires a functional RdDM pathway [47], and is down-regulated in actively dividing cells as a result [18]. DME and ROS1 become active in the vegetative nucleus of pollen upon cell cycle arrest, leading to hypomethylation of thousands of DNA transposons [4,5]. This DNA demethylation is counteracted to some extent by RdDM guided by siRNA [4,5], which prevents genome-wide hypomethylation that is observed when ROS1 activity is uncoupled from RdDM [48]. We compared 24-nt siRNA and H3K9me2 levels at the 95 ectopic mCHG DMRs in actively dividing cells, and at the 83 DMRs that lost mCHG in 8d arrested cells (Figures 4A and S4A). Strikingly, while the levels of H3K9me2 were identical between the two types of DMRs, 24-nt siRNA were significantly depleted at loci that lost methylation in 8d arrested cells (Figure 4B). Therefore, loci that fail to produce sufficient amounts of siRNA during rapid cell divisions in culture may be targeted for demethylation when cell cycle arrests during subculture and regeneration, potentially leading to somaclonal variation.
Figure 4. Targeted demethylation at loci that loss siRNA.
(A) Genome browser tracks showing mCHG, mCHH and siRNA levels in actively dividing cells in culture (Cells G1), and in cells after 8 days in culture when cell cycle arrests (Cells 8d), as well as H3K9me2 levels detected in actively dividing cell cultures. Shaded regions show examples of hypermethylated mCHG DMR in G1 cells that remain methylated after cell cycle arrest (n=95), and hypomethylated mCHG DMRs in 8d cells (n=83). (B) Average methylation levels plotted over the two types of DMRs shows that hypomethylation in 8d cells occurs in CG and CHG contexts and corresponds to loss of siRNA biogenesis in cell cultures, while H3K9me2 levels at the two types of DMRs remains identical. siRNA boxplot shows distribution of normalized 24-nt siRNAs mapping to each set of DMRs (RPM - reads per million), and H3K9me2 enrichment over H3 was normalized based on the number of ChIP-seq reads per million in each dataset. P-values were calculated based on the Wilcoxon test. See also Figure S4 and Data S1.
Discussion
Plant cell suspension cultures undergo dramatic changes in DNA methylation, many of which are inherited when clonal progeny are regenerated from callus [18,20,49]. We have used EdU labeling and flow sorting of nuclei isolated from Arabidopsis suspension cell cultures to examine the basis for this loss of methylation. We found that symmetric CG methylation is largely maintained in gene bodies (Figure 1B), and becomes transiently lost from one strand in late S phase, only to be fully restored in G2 cells (Figures 1C and 2A). As S phase lasts for 90-100 minutes under these conditions [50], and most methylated DNA replicates late in S phase [32], this result indicates rapid restoration of CG methylation following DNA synthesis in less than 30 minutes. A recent study in mammalian cells, using bisulfite sequencing of nascent strands, made very similar conclusions [51].
In Arabidopsis cell cultures, DNA methylation is lost from pericentromeric regions in all contexts, particularly non-CG methylation that is associated with heterochromatin formation and transcriptional silencing of TEs. We found that CHG methylation and H3K9me2 is selectively retained at particular loci, but mCHG is kept asymmetric throughout the cell cycle (Figures 2A and S3B), suggesting that maintenance is slow or inefficient, or simply not coupled to DNA replication as previously suggested [33]. Most CHG methylation in the Arabidopsis genome is found in late replicating heterochromatin, and requires the chromomethylase CMT3 that is recruited by H3K9me2 [33]. The DNA binding SRA domain of H3K9 methyltransferase KYP/SUVH4 recognizes hemi-methylated CHG before methylating H3K9 and thereby recruiting CMT3 [14,33]. This self-reinforcing loop might delay CHG methylation, if H3K9 modifications occurs in G2 or even later, as it does in mammalian cells [52]. We found that microspores (G2/M) and the VN (G1) are the only cell types to have symmetric mCHG (Figure 2B), consistent with methylation at the end of G2.
Asymmetric CHH methylation, in the absence of methylation on the parental strand, depends on three de novo methyltransferases, namely CMT2, DRM1 and DRM2, and was completely lost from dividing cells (Figures 1B, 1D and S3). The CMT2 chromomethylase is likely guided via H3K9me2 although the binding affinity is much lower than that of CMT3 [14]. High levels of CMT2-dependent DNA methylation in the VN (G0/G1) but very low levels in the microspore (G2/M) [4], suggest that CMT2 must act later in the cell cycle than CMT3, most likely only after cell divisions arrest. In contrast, the DRM1 and DRM2 methyltransferases are guided by 24-nt siRNA to maintain mCHH by RdDM.
Taken together, we propose a model for cell cycle regulation of DNA methylation in Arabidopsis. CG and CCG methylation are rapidly restored during S phase by MET1, while CAG and CTG methylation requires H3K9me2 deposition and must occur in G2. The K9 methyltransferases require asymmetric CHG methylation after S phase to guide H3K9me2. H3K9me2 then recruits CMT3 to restore mCHG levels at hemi-methylated DNA, albeit inefficiently [33], in late G2, resulting in a substantial fraction of asymmetric mCHG in most cells, and a sharp reduction of mCHG from rapidly dividing cells. CHH methylation requires both H3K9me2 and 24-nt siRNA, which also guides H3K9 methylation independent of RdDM. Thus, loss of 24-nt [18] and poor binding affinity of CMT2 to H3K9me2 [14] might all contribute to the loss of CHH methylation in rapidly dividing cells.
We compared the cell cycle methylomes to those found in pollen [4,5,25], seed [35-37,53] and root [38] cell types. Mature pollen grains arise from haploid microspores via two rounds of cell division. The first gives rise to the vegetative nucleus, which enter quiescence and fails to divide further. The second division gives rise to the two sperm cells, which enter a third cell cycle and slowly progress through S phase during pollen tube growth [8]. Sperm cells resemble dividing cells in culture, in that they lose mCHH and have asymmetric mCHG (Figure 2B). In contrast, both are restored in the vegetative cell nuclei via DRM2, CMT2 and CMT3 activities [25]. If CMT3 acts in G2, this would account for CHG asymmetry especially in endoreduplicating cells, such as leaf cells, in which M phase is lost and G2 is truncated. Conversely, if CMT2 and RdDM act in prolonged G1/G0, this would account for loss of CHH methylation in all germline cells in pollen, but not in the VN. Strikingly, this pattern is also observed in seeds and roots, as tissues/cells with low CHH methylation levels show higher CHG asymmetry (Figure S2). Following this logic, CHH methylation in mature embryos and columella cells is very high and CHG more symmetric (Figure S2), because these tissues have a low mitotic index [35-38].
A few hundred genes become ectopically methylated in cell culture in the CHG context (Figure 3 and Data S1). Many of these hypermethylated mCHG DMRs overlap with those in ibm1 and ddm1 mutants, but most are specific to cell culture (Figure 3A). These DMRs overlap with H3K9me2 and 24-nt siRNAs (Figures 3B and C). In contrast, loci that retain mCHG and H3K9me2 but have lost siRNA, are targeted for demethylation when cell cycle arrests (Figure 4). This suggests that siRNAs are required for stable maintenance of newly acquired epialleles, to prevent demethylation by DNA glycosylases. Intriguingly, this siRNA pathway guides CHG methylation independent of RdDM, and is mediated instead by H3K9me2. A similar pathway was recently found in ddm1 mutants, guided in this case by 21/22nt easiRNA [44].
24-nt small RNAs are depleted in suspension cells after 4 days in fresh media and are replaced by 21/22-nt easiRNAs from transcriptionally activated retrotransposons [18]. In pollen, 21/22-nt easiRNA also accumulate in a manner reminiscent of dividing cells in culture [18,28], and depend on Pol IV for their biogenesis [54-56]. As in cultured cells, replicating sperm cells are relatively depleted in 24-nt siRNA, RdDM activity and CHH methylation. After fertilization, CHH methylation of the paternal genome is restored in embryos [35-37,57], guided by 24-nt siRNA provided by the ovule, or transported into sperm cells from the VN [28,58,59]. In this sense, cells in culture depart from dividing cells in planta by not having an external source of small RNA, which could help restore epigenetic marks after DNA replication. Somatic small RNA are thought to be made in the meristem, where quiescent stem cells reside [60], and Arabidopsis suspension cell cultures, like most plant cell cultures, are derived from somatic tissues that have been separated from the meristem. Cell cycle reprogramming, and separation from small RNA sources in the meristem could thus account for somaclonal variation in plants propagated as clones, with major economic and environmental consequences. In micropropagated oil palm clones, for example, loss of CHG and CHH methylation occurs from the Karma non-LTR retrotransposon, resulting in floral abnormalities in “mantled” clones [22]. Remarkably, oil palm tissue cultures also accumulate 21/22 easiRNA, and also progressively lose non-CG methylation [22].
In summary, the changes in DNA methylation we describe in Arabidopsis cell cultures may account for somaclonal variation after micropropagation in crop plants, and may also contribute to germline reprogramming. In both cases, 24-nt siRNAs and non-CG methylation are depleted from actively dividing cells. Intriguingly, successful clonal propagation of mammals by nuclear transfer also seems to depend on the cell cycle, requiring that nuclear donor cells are in quiescence [61]. It is likely that reprogramming underlies this cell cycle requirement in mammalian cells as well.
STAR Methods
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Robert A. Martienssen (martiens@cshl.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
Raw sequencing data generated in this study is accessible through NCBI’s Gene Expression Omnibus (GSE117318). R script used to generate asymmetry plots is publicly available in GitHub (https://github.com/fsborges/mC_strand_asymmetry).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell cultures
Arabidopsis suspension cells were cultured and EdU-labeled as previously described [23]. Briefly, cells were grown in 50 mL medium and propagated every 7 days by transferring a 6 mL aliquot to a fresh 50 mL fresh and pre-warmed medium. EdU incorporation was performed in the logarithmic phase of the growth curve, by diluting 25 mL of 7-day cells in an equal volume of fresh, prewarmed medium and grown for 16 h before labeling for 1 h with a final concentration of 10 μM EdU. Similarly, G1 and G2 nuclei were also sorted from 8-day cultures (Figure S1A) without addition of fresh medium, when cell cycle arrest occurs and the cells enter quiescence. T87 derived cell suspension culture has been maintained in Jouanneau and Péaud-Lenoël (JPL) medium [29] under continuous light at 22°C and subcultured at two-week intervals.
METHOD DETAILS
Bisulfite sequencing
Genomic DNA was isolated from sorted nuclei using phenol/chlorophorm and fragmented by Covaris. Library preparation of genomic DNA fragments was performed using the NEXTflex Bisulfite Sequencing kit (Bioo Scientific) and the EZ DNA Methylation-Gold Kit (Zymo) following manufacturer instructions. Amplified fragments of 340-360 bp were size selected by gel extraction and sequenced on a Illumina HiSeq 2500 instrument. Whole genome bisulfite sequencing of the unsynchronized cell culture derived from the T87 cell line was performed by BGI Genomics (Hong Kong). Genomic DNA was fragmented to 100-300 bp by sonication, end-repaired, and ligated to methylated adaptors. Bisulfite treatment was then performed using the EZ DNA Methylation-Gold kit (Zymo), and the bisulfite-treated fragments were PCR amplified and sequenced as paired-end 150 bp reads (PE150) with Illumina technology.
Small RNA sequencing
Total RNA was extracted from cycling cells (7 days in culture + 16h after addition of fresh medium), arrested cells (8 days in culture without addition of fresh medium) and wild-type leaf tissue using the Direct-zol RNA Microprep kit (Zymo Research). Library construction and sequencing was performed at BGI Genomics (Hong Kong).
Chromatin immunoprecipiation of histone marks
Cells were cultured in 1x B5 medium with minimal organics supplemented with 3% sucrose and 1.1 mg/l 2,4-D with a 1:1 and 1:9 final dilution for 4 days. An approximate volume of 5 mL of sedimented cells from cell suspension culture were cross-linked by treating with 1% paraformaldehyde for 15 min. After adding 2 M glycine (100 mM final concentration) and incubating for 5 min to stop the cross-linking, the cells were washed three times with PBS by centrifuging at 1,500 rpm for 5 min. The fixed cells were snap-frozen in liquid nitrogen and stored at 80°C. Chromatin isolation and immunoprecipitation with antibodies against specific histone modifications (anti-H3 and anti-H3K9me2) were done according to (Tanurdžić et al., 2008) with minor modifications (an extra wash at each washing step). ChIP DNA was prepared for Illumina sequencing using NEBNext ChIP Seq Library Prep mix (NEB). A summary of all ChIP sequencing data generated in this study is presented in Data S2.
QUANTIFICATION AND STATISTICAL ANALYSIS
Genome analysis of cultured cells
A high coverage, whole genome input library from the A. thaliana suspension cell line Col-0 was used to detect deleterious polymorphisms impacting epigenetic regulators (ChIP-seq input library SRR6078572 accessible through the NCBI’s Gene Expression Omnibus). The library was sequenced on an Illumina HiSeq 2000 instrument, resulting in 97.4 million 150nt paired-end reads. Reads were processed with Trimmomatic [62] and FastQC [63] prior to alignment with BWA-MEM [64] to the TAIR10 reference genome. All alignments were filtered on the basis of mapping quality (mapQ ≥ 30), and optical duplicates were removed with Samtools [65]. Remaining reads represented a cumulative coverage of 81X, with 99.7 percent of the genome covered to greater than 10X depth. Variants were called using Freebayes [66], and all putative polymorphisms were filtered using standard quality metrics and read depth (≥10 supporting reads). Passing variants were classified and annotated using SnpEff [67] in order to compile a list of affected protein-coding genes (Table S1).
DNA methylation analysis
Pre-processed high quality reads were mapped to the TAIR10 genome using Bismark with default settings for paired-end libraries [68], and all downstream analysis were performed using custom R scripts. Datasets of WT leaf, pollen, seed and root methylomes used for comparisons were previously published [4,5,25,35-38,41,53]. A summary of all bisulfite sequencing data used in this study is presented in Data S2.
Analysis of Differentially Methylated Regions (DMRs)
Differentially methylated regions (DMRs) were defined as 100-bp bins containing at least 4 or 5 differentially methylated CGs or CHGs, with an absolute methylation difference of 0.25. DMRs localizing within 200 bp of each other were merged. Annotations for all mCG and mCHG DMRs discussed in this study are presented in Data S1.
CG/CHG asymmetry analysis
Strand asymmetry analysis was performed by pairing cytosines in the CG or CHG (CAG and CTG) context and on each strand, and calculating the methylation level (#C/(#C+#T)) for cytosines covered by at least 10 reads and 25% of methylation. Methylation at the Watson (x) and Crick (y) strand was visualized using a scatter of points with kernel density estimations in two dimensions, which was calculated using the R function kde2d. A loess regression model was computed using the ‘loess’ function, and the model was applied to the original data by using the R function ‘predict’.
Analysis of small RNA sequencing data
Single-end 50-nt reads were pre-processed by filtering collapsed reads according to length and quality. Filtered reads were mapped to the Arabidopsis TAIR10 genome annotation with Bowtie, reporting all multi-mappers. Only perfect-match reads were used for all downstream analysis. Reads were normalized by dividing non-redundant read counts by the number of genomic hits, and subsequently calculating the number of reads per million of filtered (18-30 nt) and perfectly mapped reads. Additional downstream analyses and plots were done with custom R scripts. Pearson's correlation coefficient (r) was calculated to show that the two samples (Cells 7d + 16h and Cells 8d) are identical (Figure S4B), and were therefore used as replicates in downstream analysis. A summary of all small-RNA sequencing data generated in this study is presented in Data S2.
Supplementary Material
Table S1. SNP calling analysis. Single nucleotide polymorphism (SNP) calling was based on the following ChIP-seq input library SRR6078572 accessible through the NCBI’s Gene Expression Omnibus (GEO). We found 9,825 high quality polymorphisms. Of these, the majority had an allele balance of less than 1 (meaning they are heterozygous or represent a sub-population within the bulk cell culture). All putative polymorphisms were annotated on the basis of biological effect (i.e. missense, frame-shift, etc) and took only those scored as moderate or high. Total polymorphisms with moderate effect: 367. Total with high/severe effect: 125. Polymorphisms scored as moderate to high effect mapped to ~800 protein-coding genes.
Data S2. Summary of bisulfite, ChIP and small RNA libraries, Related to Figures 1 and 3. Summary of small RNA, Chromatic immunoprecipitation (ChIP) sequencing and whole genome bisulfite sequencing. For small RNA analysis, trimmed reads (18-30 nucleotides) were mapped to TAIR10 genome using bowtie (-v 1). For the bisulfite-seq libraries, mean genomic coverage and methylation percentages for CG, CHG and CHH context is presented. Cytosines covered by 3 or more reads were used to calculate methylation percentages. Single-end 36 nucleotide ChIP-seq reads were mapped to TAIR10 with bowtie2, using theq --very-sensitive preset option.
Data S1. Differentially Methylated Regions (DMRs), Related to Figures 3 and 4. DMRs were defined as 100-bp bins containing at least 4 or 5 differentially methylated mCGs or mCHGs, respectively, and with an absolute methylation difference of 0.25. DMRs localizing within 200 bp of each other were merged.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-H3 | Upstate/Millipore | 06-755 |
| anti-H3K9me2 | Upstate/Millipore | 07-212 |
| Biological Samples | ||
| Arabidopsis Col-0 cell suspension | [23] | N/A |
| Arabidopsis Col-0 cell suspension derived from T87 cell line | [29] | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| 5-Ethynyl-2′-deoxyuridine (EdU) | Thermo Fisher Scientific | A10044 |
| Critical Commercial Assays | ||
| NEBNext ChIP Seq Library Prep mix | New England Biolabs | E6240 |
| EZ DNA Methylation-Gold Kit | Zymo Research | D5005 |
| Direct-zol RNA Microprep | Zymo Research | R2061 |
| Deposited Data | ||
| Cells G1 | This manuscript | GSE117318 |
| Cells early S | This manuscript | GSE117318 |
| Cells mid S | This manuscript | GSE117318 |
| Cells late S | This manuscript | GSE117318 |
| Cells G2 | This manuscript | GSE117318 |
| Cells G1 - 8 days | This manuscript | GSE117318 |
| Cells G2 - 8 days | This manuscript | GSE117318 |
| Cells T87 | This manuscript | GSE117318 |
| Cells 7d Split + 16h | This manuscript | GSE117318 |
| Cells 8d | This manuscript | GSE117318 |
| WT leaf | This manuscript | GSE117318 |
| H3 leaf | This manuscript | GSE117318 |
| H3K9me2 leaf | This manuscript | GSE117318 |
| H3 cells | This manuscript | GSE117318 |
| H3K9me2 cells | This manuscript | GSE117318 |
| WT leaf | [41] | GSE39901 |
| met1 leaf | [41] | GSE39901 |
| ddm1 leaf | [41] | GSE39901 |
| ibm1 leaf | [41] | GSE39901 |
| rdd leaf | [41] | GSE39901 |
| Micropore | [4] | GSE40501 |
| Sperm cells | [4,5,25] | GSE40501, GSE38935, GSE87170 |
| Vegetative nucleus | [4,5,25] | GSE40501, GSE38935, GSE87170 |
| Seed globular | [36] | GSE68132 |
| Seed linear cotyledon | [36] | GSE68132 |
| Seed mature green | [36] | GSE68132 |
| Seed post mature green | [36] | GSE68132 |
| Seed dry | [36] | GSE68132 |
| Embryo | [53] | GSE53642 |
| Endosperm | [53] | GSE53642 |
| Root epidermis | [38] | GSE79708 |
| Root cortex | [38] | GSE79708 |
| Root endodermis-1 | [38] | GSE79708 |
| Root endodermis-2 | [38] | GSE79708 |
| Root stele | [38] | GSE79708 |
| Root columella | [38] | GSE79708 |
| Root lower columella | [38] | GSE79708 |
| Experimental Models: Cell Lines | ||
| Arabidopsis Col-0 cell suspension | [23] | N/A |
| Arabidopsis Col-0 cells derived from T87 cell suspension | [29] | N/A |
| Software and Algorithms | ||
| Trimmomatic | [62] | http://www.usadellab.org/cms/?page=trimmomatic |
| FastQC | [63] | bioinformatics.babraham.ac.uk/projects/fastqc/ |
| BWA-MEM | [64] | http://bio-bwa.sourceforge.net/bwa.shtml |
| Samtools | [65] | http://www.htslib.org/ |
| Freebayes | [66] | https://github.com/ekg/freebayes |
| SnpEff | [67] | https://pcingola.github.io/SnpEff/ |
| Bismark | [68] | https://www.bioinformatics.babraham.ac.uk/projects/bismark/ |
| Bowtie | [69] | http://bowtie-bio.sourceforge.net/index.shtml |
| BEDTools | [70] | https://bedtools.readthedocs.io/en/latest/# |
| R | [71] | https://www.r-project.org/ |
| mC strand asymmetry | This manuscript | https://github.com/fsborges/mC_strand_asymmetry |
Acknowledgments
We thank all members of our laboratories for discussions. We thank Isabelle Debeaujon for providing the cell culture originally derived from the T87 cell suspension. Research in the Martienssen laboratory is supported by the Howard Hughes Medical Institute. This work was supported by the Plant Genome Research Program of the National Science Foundation (grant IOS-1025830 to L.H.-B., W.F.T. and R.A.M.). F.B. acknowledges an installation grant provided by the BAP department of INRAE. The authors acknowledge assistance from the Cold Spring Harbor Laboratory Shared Resources, which are funded in part by the Cancer Center (Support Grant 5PP30CA045508).
Footnotes
Declaration of Interests
The authors declare no competing financial interests.
References
- 1.Chen T, and Dent SYR (2014). Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat. Rev. Genet 15, 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heard E, and Martienssen RA (2014). Transgenerational epigenetic inheritance: myths and mechanisms. Cell 157, 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg MVC, and Bourc’his D (2019). The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol 20, 590–607. [DOI] [PubMed] [Google Scholar]
- 4.Calarco JP, Borges F, Donoghue MTA, Van Ex F, Jullien PE, Lopes T, Gardner R, Berger F, Feijó JA, Becker JD, et al. (2012). Reprogramming of DNA methylation in pollen guides epigenetic inheritance via small RNA. Cell 151, 194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibarra CA, Feng X, Schoft VK, Hsieh T-F, Uzawa R, Rodrigues JA, Zemach A, Chumak N, Machlicova A, Nishimura T, et al. (2012). Active DNA Demethylation in Plant Companion Cells Reinforces Transposon Methylation in Gametes. 337, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borg M, Jacob Y, Susaki D, LeBlanc C, Buendía D, Axelsson E, Kawashima T, Voigt P, Boavida L, Becker J, et al. (2020). Targeted reprogramming of H3K27me3 resets epigenetic memory in plant paternal chromatin. Nat. Cell Biol Available at: http://www.nature.com/articles/s41556-020-0515-y [Accessed May 12, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borg M, Brownfield L, and Twell D (2009). Male gametophyte development: a molecular perspective. J. Exp. Bot 60, 1465–1478. [DOI] [PubMed] [Google Scholar]
- 8.Friedman WE (1999). Expression of the cell cycle in sperm of Arabidopsis: implications for understanding patterns of gametogenesis and fertilization in plants and other eukaryotes. Dev. Camb. Engl 126, 1065–1075. [DOI] [PubMed] [Google Scholar]
- 9.Borges F, Gomes G, Gardner R, Moreno N, McCormick S, Feijó JA, and Becker JD (2008). Comparative transcriptomics of Arabidopsis sperm cells. Plant Physiol. 148, 1168–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Law JA, and Jacobsen SE (2010). Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet 11, 204–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wigler MH (1981). The inheritance of methylation patterns in vertebrates. Cell 24, 285–286. [DOI] [PubMed] [Google Scholar]
- 12.Luo C, Keown CL, Kurihara L, Zhou J, He Y, Li J, Castanon R, Lucero J, Nery JR, Sandoval JP, et al. (2017). Single-cell methylomes identify neuronal subtypes and regulatory elements in mammalian cortex. Science 357, 600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Lang Z, and Zhu J-K (2018). Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol 19, 489–506. [DOI] [PubMed] [Google Scholar]
- 14.Stroud H, Do T, Du J, Zhong X, Feng S, Johnson L, Patel DJ, and Jacobsen SE (2014). Non-CG methylation patterns shape the epigenetic landscape in Arabidopsis. Nat. Struct. Mol. Biol 21, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deleris A, Stroud H, Bernatavichute Y, Johnson E, Klein G, Schubert D, and Jacobsen SE (2012). Loss of the DNA methyltransferase MET1 Induces H3K9 hypermethylation at PcG target genes and redistribution of H3K27 trimethylation to transposons in Arabidopsis thaliana. PLoS Genet. 8, e1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castel SE, and Martienssen RA (2013). RNA interference in the nucleus: roles for small RNAs in transcription, epigenetics and beyond. Nat. Rev. Genet 14, 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamuro C, Zhu J-K, and Yang Z (2016). Epigenetic Modifications and Plant Hormone Action. Mol. Plant 9, 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanurdžić M, Vaughn MW, Jiang H, Lee T-J, Slotkin RK, Sosinski B, Thompson WF, Doerge RW, and Martienssen RA (2008). Epigenomic consequences of immortalized plant cell suspension culture. PLoS Biol. 6, 2880–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cubas P, Vincent C, and Coen E (1999). An epigenetic mutation responsible for natural variation in oral symmetry. 401, 5. [DOI] [PubMed] [Google Scholar]
- 20.Kaeppler SM, and Phillips RL (1993). Tissue culture-induced DNA methylation variation in maize. Proc. Natl. Acad. Sci. U. S. A 90, 8773–8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martienssen R, Barkan A, Taylor WC, and Freeling M (1990). Somatically heritable switches in the DNA modification of Mu transposable elements monitored with a suppressible mutant in maize. Genes Dev. 4, 331–343. [DOI] [PubMed] [Google Scholar]
- 22.Ong-Abdullah M, Ordway JM, Jiang N, Ooi S-E, Kok S-Y, Sarpan N, Azimi N, Hashim AT, Ishak Z, Rosli SK, et al. (2015). Loss of Karma transposon methylation underlies the mantled somaclonal variant of oil palm. Nature 525, 533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wear EE, Concia L, Brooks AM, Markham EA, Lee T-J, Allen GC, Thompson WF, and Hanley-Bowdoin L (2016). Isolation of Plant Nuclei at Defined Cell Cycle Stages Using EdU Labeling and Flow Cytometry. Methods Mol. Biol. Clifton NJ 1370, 69–86. [DOI] [PubMed] [Google Scholar]
- 24.Lee T-J, Pascuzzi PE, Settlage SB, Shultz RW, Tanurdzic M, Rabinowicz PD, Menges M, Zheng P, Main D, Murray JAH, et al. (2010). Arabidopsis thaliana Chromosome 4 Replicates in Two Phases That Correlate with Chromatin State. PLoS Genet. 6, e1000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh P-H, He S, Buttress T, Gao H, Couchman M, Fischer RL, Zilberman D, and Feng X (2016). Arabidopsis male sexual lineage exhibits more robust maintenance of CG methylation than somatic tissues. Proc. Natl. Acad. Sci 113, 15132–15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker J, Gao H, Zhang J, Aldridge B, Vickers M, Higgins JD, and Feng X (2018). Sexual-lineage-specific DNA methylation regulates meiosis in Arabidopsis. Nat. Genet 50, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martínez G, Panda K, Köhler C, and Slotkin RK (2016). Silencing in sperm cells is directed by RNA movement from the surrounding nurse cell. Nat. Plants 2, 16030. [DOI] [PubMed] [Google Scholar]
- 28.Slotkin RK, Vaughn M, Borges F, Tanurdžić M, Becker JD, Feijó JA, and Martienssen RA (2009). Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136, 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Axelos M, Curie C, Mazzolini L, Bardet C, and Lescure B (1992). A protocol for transient gene expression in Arabidopsis thaliana protoplasts isolated from cell suspension cultures. Plant Physiol Biochem, 123–128. [Google Scholar]
- 30.Willing E-M, Rawat V, Mandáková T, Maumus F, James GV, Nordström KJV, Becker C, Warthmann N, Chica C, Szarzynska B, et al. (2015). Genome expansion of Arabis alpina linked with retrotransposition and reduced symmetric DNA methylation. Nat. Plants 1, 14023. [DOI] [PubMed] [Google Scholar]
- 31.Niederhuth CE, Bewick AJ, Ji L, Alabady MS, Kim KD, Li Q, Rohr NA, Rambani A, Burke JM, Udall JA, et al. (2016). Widespread natural variation of DNA methylation within angiosperms. Genome Biol. 17, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Concia L, Brooks AM, Wheeler E, Zynda GJ, Wear EE, LeBlanc C, Song J, Lee T-J, Pascuzzi PE, Martienssen RA, et al. (2018). Genome-Wide Analysis of the Arabidopsis Replication Timing Program. Plant Physiol. 176, 2166–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du J, Zhong X, Bernatavichute YV, Stroud H, Feng S, Caro E, Vashisht AA, Terragni J, Chin HG, Tu A, et al. (2012). Dual binding of chromomethylase domains to H3K9me2-containing nucleosomes directs DNA methylation in plants. Cell 151, 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gouil Q, and Baulcombe DC (2016). DNA Methylation Signatures of the Plant Chromomethyltransferases. PLOS Genet. 12, e1006526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouyer D, Kramdi A, Kassam M, Heese M, Schnittger A, Roudier F, and Colot V (2017). DNA methylation dynamics during early plant life. Genome Biol. 18, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawakatsu T, Nery JR, Castanon R, and Ecker JR (2017). Dynamic DNA methylation reconfiguration during seed development and germination. Genome Biol. 18, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narsai R, Gouil Q, Secco D, Srivastava A, Karpievitch YV, Liew LC, Lister R, Lewsey MG, and Whelan J (2017). Extensive transcriptomic and epigenomic remodelling occurs during Arabidopsis thaliana germination. Genome Biol. 18, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawakatsu T, Stuart T, Valdes M, Breakfield N, Schmitz RJ, Nery JR, Urich MA, Han X, Lister R, Benfey PN, et al. (2016). Unique cell-type-specific patterns of DNA methylation in the root meristem. Nat. Plants 2, 16058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsieh T-F, Ibarra CA, Silva P, Zemach A, Eshed-Williams L, Fischer RL, and Zilberman D (2009). Genome-Wide Demethylation of Arabidopsis Endosperm. Science 324, 1451–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gehring M, Bubb KL, and Henikoff S (2009). Extensive Demethylation of Repetitive Elements During Seed Development Underlies Gene Imprinting. Science 324, 1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stroud H, Greenberg MVC, Feng S, Bernatavichute YV, and Jacobsen SE (2013). Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell 152, 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rigal M, Kevei Z, Pélissier T, and Mathieu O (2012). DNA methylation in an intron of the IBM1 histone demethylase gene stabilizes chromatin modification patterns. EMBO J. 31, 2981–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang K, Lang Z, Zhang H, and Zhu J-K (2016). The DNA demethylase ROS1 targets genomic regions with distinct chromatin modifications. Nat. Plants 2. Available at: http://www.nature.com/articles/nplants2016169 [Accessed September 6, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SC, Ernst E, Berube B, Borges F, Parent J-S, Ledon P, Schorn A, and Martienssen RA (2020). Arabidopsis retrotransposon virus-like particles and their regulation by epigenetically activated small RNA. Genome Res., genome;gr.259044.119v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inagaki S, Miura-Kamio A, Nakamura Y, Lu F, Cui X, Cao X, Kimura H, Saze H, and Kakutani T (2010). Autocatalytic differentiation of epigenetic modifications within the Arabidopsis genome. EMBO J. 29, 3496–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwiatkowska A, Zebrowski J, Oklejewicz B, Czarnik J, Halibart-Puzio J, and Wnuk M (2014). The age-dependent epigenetic and physiological changes in an Arabidopsis T87 cell suspension culture during long-term cultivation. Biochem. Biophys. Res. Commun 447, 285–291. [DOI] [PubMed] [Google Scholar]
- 47.Williams BP, Pignatta D, Henikoff S, and Gehring M (2015). Methylation-sensitive expression of a DNA demethylase gene serves as an epigenetic rheostat. PLoS Genet. 11, e1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams BP, and Gehring M (2017). Stable transgenerational epigenetic inheritance requires a DNA methylation-sensing circuit. Nat. Commun 8, 2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stroud H, Ding B, Simon SA, Feng S, Bellizzi M, Pellegrini M, Wang G-L, Meyers BC, and Jacobsen SE (2013). Plants regenerated from tissue culture contain stable epigenome changes in rice. eLife 2, e00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mickelson-Young L, Wear E, Mulvaney P, Lee T-J, Szymanski ES, Allen G, Hanley-Bowdoin L, and Thompson W (2016). A flow cytometric method for estimating S-phase duration in plants. J. Exp. Bot 67, 6077–6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu C, and Corces VG (2018). Nascent DNA methylome mapping reveals inheritance of hemimethylation at CTCF/cohesin sites. Science 359, 1166–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alabert C, Bukowski-Wills J-C, Lee S-B, Kustatscher G, Nakamura K, de Lima Alves F, Menard P, Mejlvang J, Rappsilber J, and Groth A (2014). Nascent chromatin capture proteomics determines chromatin dynamics during DNA replication and identifies unknown fork components. Nat. Cell Biol 16, 281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schatlowski N, Wolff P, Santos-González J, Schoft V, Siretskiy A, Scott R, Tamaru H, and Köhler C (2014). Hypomethylated pollen bypasses the interploidy hybridization barrier in Arabidopsis. Plant Cell 26, 3556–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borges F, Parent J-S, van Ex F, Wolff P, Martinez G, Kohler C, and Martienssen RA (2018). Transposon-derived small RNAs triggered by miR845 mediate genome dosage response in Arabidopsis. Nat. Genet 50, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinez G, Wolff P, Wang Z, Moreno-Romero J, Santos-González J, Conze LL, DeFraia C, Slotkin RK, and Köhler C (2018). Paternal easiRNAs regulate parental genome dosage in Arabidopsis. Nat. Genet 50, 193–198. [DOI] [PubMed] [Google Scholar]
- 56.Wang Z, Butel N, Santos-Gonzalez J, Borges F, Yi J, Martienssen RA, Martinez G, and Kohler C (2019). Functional role of Polymerase IV during pollen development in Capsella (Plant Biology) Available at: http://biorxiv.org/lookup/doi/10.1101/863522 [Accessed February 6, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vu TM, Nakamura M, Calarco JP, Susaki D, Lim PQ, Kinoshita T, Higashiyama T, Martienssen RA, and Berger F (2013). RNA-directed DNA methylation regulates parental genomic imprinting at several loci in Arabidopsis. Dev. Camb. Engl 140, 2953–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erdmann RM, Hoffmann A, Walter H-K, Wagenknecht H-A, Groß-Hardt R, and Gehring M (2017). Molecular movement in the Arabidopsis thaliana female gametophyte. Plant Reprod. 30, 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mosher RA, Melnyk CW, Kelly KA, Dunn RM, Studholme DJ, and Baulcombe DC (2009). Uniparental expression of PolIV-dependent siRNAs in developing endosperm of Arabidopsis. Nature 460, 283–286. [DOI] [PubMed] [Google Scholar]
- 60.Baubec T, Finke A, Mittelsten Scheid O, and Pecinka A (2014). Meristem-specific expression of epigenetic regulators safeguards transposon silencing in Arabidopsis. EMBO Rep. 15, 446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilmut I, and Campbell KH (1998). Quiescence in nuclear transfer. Science 281, 1611. [DOI] [PubMed] [Google Scholar]
- 62.Bolger AM, Lohse M, and Usadel B (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinforma. Oxf. Engl 30, 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andrews SR (2010). FastQC: a quality control tool for high throughput sequence data. Available at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/. [Google Scholar]
- 64.Li H (2013). Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. ArXiv13033997 Q-Bio. Available at: http://arxiv.org/abs/1303.3997 [Accessed July 10, 2018] [Google Scholar]
- 65.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, and 1000 Genome Project Data Processing Subgroup (2009). The Sequence Alignment/Map format and SAMtools. Bioinforma. Oxf. Engl 25, 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garrison E, and Marth G (2012). Haplotype-based variant detection from short-read sequencing. ArXiv12073907 Q-Bio. Available at: http://arxiv.org/abs/1207.3907 [Accessed July 10, 2018] [Google Scholar]
- 67.Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, Land SJ, Lu X, and Ruden DM (2012). A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6, 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krueger F, and Andrews SR (2011). Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinforma. Oxf. Engl 27, 1571–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Langmead B, Trapnell C, Pop M, and Salzberg SL (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quinlan AR, and Hall IM (2010). BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.R: a language and environment for statistical computing Available at: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing [Accessed October 15, 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. SNP calling analysis. Single nucleotide polymorphism (SNP) calling was based on the following ChIP-seq input library SRR6078572 accessible through the NCBI’s Gene Expression Omnibus (GEO). We found 9,825 high quality polymorphisms. Of these, the majority had an allele balance of less than 1 (meaning they are heterozygous or represent a sub-population within the bulk cell culture). All putative polymorphisms were annotated on the basis of biological effect (i.e. missense, frame-shift, etc) and took only those scored as moderate or high. Total polymorphisms with moderate effect: 367. Total with high/severe effect: 125. Polymorphisms scored as moderate to high effect mapped to ~800 protein-coding genes.
Data S2. Summary of bisulfite, ChIP and small RNA libraries, Related to Figures 1 and 3. Summary of small RNA, Chromatic immunoprecipitation (ChIP) sequencing and whole genome bisulfite sequencing. For small RNA analysis, trimmed reads (18-30 nucleotides) were mapped to TAIR10 genome using bowtie (-v 1). For the bisulfite-seq libraries, mean genomic coverage and methylation percentages for CG, CHG and CHH context is presented. Cytosines covered by 3 or more reads were used to calculate methylation percentages. Single-end 36 nucleotide ChIP-seq reads were mapped to TAIR10 with bowtie2, using theq --very-sensitive preset option.
Data S1. Differentially Methylated Regions (DMRs), Related to Figures 3 and 4. DMRs were defined as 100-bp bins containing at least 4 or 5 differentially methylated mCGs or mCHGs, respectively, and with an absolute methylation difference of 0.25. DMRs localizing within 200 bp of each other were merged.
Data Availability Statement
Raw sequencing data generated in this study is accessible through NCBI’s Gene Expression Omnibus (GSE117318). R script used to generate asymmetry plots is publicly available in GitHub (https://github.com/fsborges/mC_strand_asymmetry).