Abstract
Following a request from the European Commission, the FEEDAP Panel was asked to deliver a scientific opinion on the safety and efficacy of l‐methionine ≥ 98.5% or ≥ 90% produced by the combined activities of Corynebacterium glutamicum KCCM 80245 and Escherichia coli KCCM 80246) as nutritional additive for all animal species. The two production strains are genetically modified. l‐Methionine is intended to be used in feed or water for drinking for all animal species. Neither viable cells nor recombinant DNA of the production strains were detected in the final products. The additive does not pose any safety concern associated with the genetic modification of the production strains. The use of both products of l‐methionine produced by C. glutamicum KCCM 80245 and E. coli KCCM 80246 in supplementing feed to compensate for l‐methionine deficiency in feedingstuffs is safe for the target species. The FEEDAP Panel has concerns about the use of amino acids in water for drinking for hygienic reasons, and due to the risk of imbalances when administered simultaneously via feed. The use of both products of l‐methionine produced by C. glutamicum KCCM 80245 and E. coli KCCM 80246 in animal nutrition is considered safe for the consumers and for the environment. The additive, in either product, is not an irritant to skin/eyes and not a dermal sensitiser and shows no toxicity by inhalation. Considering the respiratory exposure to endotoxins, l‐methionine ≥ 90% is a risk for the user. Both products of the additive produced by C. glutamicum KCCM 80245 and E. coli KCCM 80246 are considered as an efficacious source of the essential amino acid l‐methionine for non‐ruminant animal species. For the supplemental l‐methionine to be as efficacious in ruminants as in non‐ruminant species, it would require protection against degradation in the rumen.
Keywords: nutritional additives, amino acids, their salts and analogues, methionine, Corynebacterium glutamicum KCCM 80245, Escherichia coli KCCM 80246, safety, efficacy
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/2003 1 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from CJ Europe GmbH 2 for the authorisation of the additive consisting of l‐methionine produced by fermentation with Corynebacterium glutamicum KCCM 80245 and Escherichia coli KCCM 80246, when used as a feed additive in feed and in water for drinking for all animal species and categories (category: nutritional additives; functional group: amino acids, their salts and analogues).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). The particulars and documents in support of the application were considered valid by EFSA as of 8 June 2021.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the feed additive consisting of l‐methionine produced by fermentation with Corynebacterium glutamicum KCCM 80245 and Escherichia coli KCCM 80246 when used under the proposed conditions of use (see Section 3.1.6).
1.2. Additional information
l‐Methionine produced by fermentation with Corynebacterium glutamicum KCCM 80245 and Escherichia coli KCCM 80246 has not been authorised as feed additive in the European Union.
EFSA issued several opinions on the safety and efficacy of methionine. One opinion related to dl‐methionine produced by chemical synthesis for all animal species (EFSA FEEDAP Panel, 2012a). Another one related to l‐methionine produced by fermentation by E. coli (KCCM 11252P) and E. coli (KCCM 11340P) for all animal species (EFSA FEEDAP Panel, 2013) and one related to l‐methionine produced by fermentation by C. glutamicum (KCCM 80184) and E. coli (KCCM 80096) for all animal species (EFSA FEEDAP Panel, 2019a).
dl‐Methione 3 and l‐methionine 4 , 5 are currently authorised as amino acids. l‐Methionine is currently authorised as a flavouring substance in feed (FLAVIS number: No 17.027). 6
l‐Methionine may be used as a nutritional substance (list of amino acids and other nitrogen compounds) in the manufacture of infant formulae and follow‐on formulae. 7 Methionine (d,l‐Methionine) is registered as an ingredient for use in cosmetics as antistatic and for skin conditioning. 8
The European Pharmacopoeia has a dedicated monograph (01/2017:1027) to l‐methionine (European Pharmacopoeia, 2021).
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier 9 in support of the authorisation request for the use of l‐methionine produced by fermentation with C. glutamicum KCCM 80245 and E. coli KCCM 80246 as a nutritional additive for use in feed and water for drinking for all animal species.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, and other scientific reports, to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the l‐methionine (≥ 90%) and l‐methionine (≥ 98.5%) produced by C. glutamicum KCCM 80245 and E. coli KCCM 80246 in animal feed. The Executive Summary of the EURL report can be found in Annex A. 10
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of l‐methionine is in line with the principles laid down in Regulation (EC) No 429/2008 11 and the relevant guidance documents: Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012b), Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017a), Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEEDAP Panel, 2017b), Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017c), Guidance on the assessment of the efficacy of feed additives (EFSA FEEDAP Panel, 2018a), Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018b), Guidance on the assessment of the safety of feed additives for the environment (EFSA FEEDAP Panel, 2019b).
3. Assessment
The subject of the assessment is the feed additive consisting of l‐methionine produced by fermentation with C. glutamicum KCCM 80245 and E. coli KCCM 80246, intended for use as a nutritional additive (functional group: amino acids, their salts and analogues) in feed and in water for drinking for all animal species and categories. Authorisation is sought for two products based on the amino acid l‐methionine: one with a minimum of 98.5% l‐methionine; and the other with a minimum of 90% l‐methionine.
3.1. Characterisation
3.1.1. Characterisation of the production organisms
l‐methionine is produced by fermentation by the combined activities of two genetically modified strains, C. glutamium KCCM 80245 and E. coli KCCM 80246.
3.1.1.1. C. glutamicum KCCM 80245
The C. glutamicum production strain is deposited in the Korean Centre of Microorganisms (KCCM) under the accession number KCCM 80245. 12
The production strain KCCM 80245 was properly identified as C. glutamicum on the basis of whole genome sequencing (WGS) analysis. ■■■■■ 13
The antibiotic susceptibility of the production strain C. glutamicum KCCM 80245 was evaluated by determining the minimal inhibitory concentration (MICs) values against the antimicrobials listed for ‘Corynebacterium and other Gram‐positive’ in the FEEDAP guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018b). 14 ■■■■■ All MICs were below or equal to the corresponding cut‐off values, and therefore, the strain is considered susceptible to these antimicrobials.
The WGS data of C. glutamicum KCCM 80245 were interrogated for the presence of antimicrobial resistance genes ■■■■■ 15 and ■■■■■ 16 No hits of concern were identified.
The genome was also searched for the presence of genes encoding for virulence and pathogenicity factors ■■■■■ ■■■■■ 17 Genes involved in pathogenicity and virulence were not found.
3.1.1.1.1. Origin of the DNA used for the genetic modification
■■■■■
■■■■■
3.1.1.1.2. Genetic modification
■■■■■ ■■■■■ ■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
■■■■■
3.1.1.2. E. coli KCCM 80246
The E. coli production strain is deposited in the Korean Centre of Microorganisms (KCCM) under the accession number KCCM 80246. 19
The production strain KCCM 80246 was identified as an E. coli K‐12 derivative ■■■■■ 20 E. coli K‐12 is well characterised, its safety (non‐pathogenicity) has been documented (Gorbach, 1978) and its ineffectiveness in colonising the human gut is reported (Smith, 1975).
The antibiotic susceptibility of the production strain E. coli KCCM 80246 was determined by estimating the MICs against the antimicrobials listed for Enterobacteriaceae in the FEEDAP guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018b). ■■■■■ All MICs were below or equal to the corresponding cut‐off values, and therefore, the strain is considered susceptible to these antimicrobials.14
The genome of E. coli KCCM 80246 was interrogated for the presence of antimicrobial resistance genes ■■■■■), 21 and ■■■■■). 22 No relevant hits were found in any of the two databases.
The genome was also searched for the presence of genes encoding virulence and pathogenicity factors ■■■■■ 23 All genes identified ■■■■■ are considered of no concern.
3.1.1.2.1. Origin of the DNA used for the genetic modification
■■■■■
3.1.1.2.2. Genetic modification
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
■■■■■
3.1.2. Manufacturing process
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
■■■■■
No remnants of the organic solvent used to disrupt the cells were detected in three batches of each product of the additive. 24
The applicant states that no antimicrobial substances are used during the manufacturing process. 25
The presence of viable cells of the production strains in three batches (triplicate samples) of each product (98.5% and 90% methionine) was investigated. 26 ■■■■■ Viable cells were not detected in any of the six batches analysed.
The presence of recombinant DNA of the production strains in three batches (triplicate samples) of each product (98.5% and 90% methionine) was investigated ■■■■■ 27 ■■■■■ DNA of the production strains was not detected in any of the six batches of the final products tested. ■■■■■
3.1.3. Characterisation of the additive
l‐Methionine (International Union of Pure and Applied Chemistry (IUPAC) name: (S2)‐2‐amino‐4‐(methylthio)butanoic acid; Chemical Abstracts Service (CAS) No 63‐68‐3) has a molecular weight of 149.2 g/mol, the molecular formula is C5H11NO2S and its molecular structure is given in Figure 1.
Figure 1.
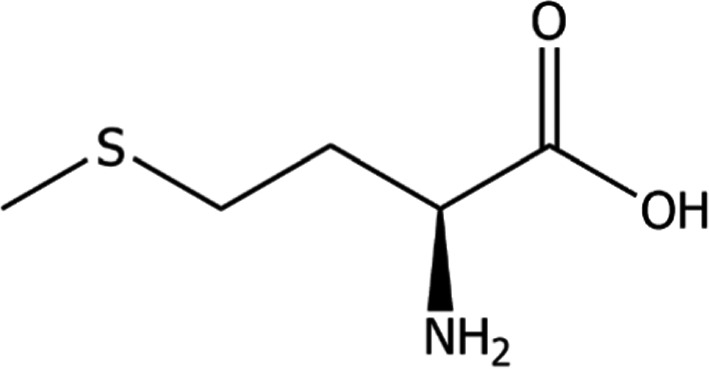
Molecular structure of methionine
3.1.4. Characterisation of the l‐methionine 98.5%
According to specification, this product contains ≥ 98.5% l‐methionine on ‘as is’ basis, ≤ 1.5% water (loss on drying) and ≤ 0.1% of ash. 28 The analysis of five batches of the additive showed an average of 99.0% l‐methionine (range 98.9–99.0%) on a ‘as is’ basis, water (0.21–0.24%) and ash was ≤ 0.1% in all five batches. 29 Other constituents are amino acids other than methionine (phenylalanine, leucine, tyrosine, isoleucine, valine) representing 0.4% (range 0.39–0.4%). Minerals (potassium and sulfate) represented 0.08%. Ammonium did not exceed 0.05%. 30 The amount for unidentified material was < 1% on dry matter basis.
The specific optical rotation determined in five batches of the final product was on average +23.6° which is within the reference range +22.5 to +24.0° specified in the European pharmacopoeia and confirms the l‐enantiomer of methionine. 31
3.1.4.1. Impurities
Three batches of the additive were analysed for impurities. Cadmium, lead, mercury, chromium,nickel, zinc and arsenic concentrations were below the corresponding limit of detection (LOD) of the analytical method. 32
Polychlorinated dibenzodioxins (PCDDs), polychlorinated dibenzofurans (PCDFs) and coplanar dioxin‐like polychlorinated biphenyls (Co‐planar PCBs) and non‐dioxin like PCBs (ndl PCBs) were analysed in three batches and found below the corresponding limit of quantification (LOQ). 33 The calculated (upper bound) levels of dioxins and the sum of dioxins and dioxin‐like‐PCBs were 0.07 ng WHO‐PCDD/F‐TEQ/kg and 0.14 ng WHO‐PCDD/F‐PCB‐TEQ/kg, respectively (in all three batches). The calculated upper bound levels of ndl‐PCBs were 0.6 µg/kg.
The analysis of mycotoxins (aflatoxins, ochratoxin A, zearalenone, deoxynivalenol) showed values below the LOQ. 34
Microbiological contamination was analysed by determination of E . coli, Enterobacteriaceae, filamentous fungi and yeast counts with all values below the LOD. 35 Salmonella spp. was not detected in 25 g samples.
The detected amounts of the above‐described impurities do not raise safety concerns.
The endotoxin activity in the final product was tested in three batches of the additive via the European Pharmacopoeia method and ranged from 991 to 1,811 EU/g. 36
3.1.4.2. Physical properties
l‐Methionine 98.5% appears as a white or yellowish crystalline powder, with a bulk density of 550–700 kg/m3, a solubility in water at 25°C of 56.6 g/L, and a melting point of 284°C. 37
The dusting potential of three batches was determined using the Stauber‐Heubach method and showed values that ranged from365 to 620 mg/m3. 38
The particle size distribution of three batches was measured by sieving and the fraction of particles < 75 µm ranged from 2.4% to 3.8%; that < 106 µm ranged from 8% to 11% and the fraction > 106 µm ranged from 86% to 89%. 39
3.1.4.3. Stability and homogeneity
The studies on the shelf‐life; on the stability in premixtures, compound feed and water for drinking; and on the capacity to homogeneously distribute in feed have been performed testing an l‐methionine 98.5% produced by fermentation using different production strains. These studies were assessed in a previous opinion (EFSA FEEDAP Panel, 2013). Since the physical characteristics, the purity and the production process of the two l‐methionine products are similar, the FEEDAP Panel considers that the results of the previous studies are applicable to the product containing ≥ 98.5% methionine of the additive under assessment.
3.1.5. Characterisation of the l‐methionine 90%
According to specification, this product contains ≥ 90% l‐methionine on DM basis, ≤ 1.5% water and ≤ 1% of ash. 40 The analysis of five batches of the additive showed an average of 92.1% methionine (range 92.1–92.4%) on a DM basis, water 0.5% (range 0.4–0.7%) and ash 0.2% (range 0.2–0.4%).
The compositional analysis indicated that other constituents are amino acids other than methionine (alanine, glutamic acid, isoleucine, leucine, n‐acetyl‐L‐homoserine, phenylalanine, threonine, tyrosine, valine) representing 0.63% (range 0.61–0.66%); minerals (calcium, chloride, magnesium, phosphate, potassium, sodium and sulfate) representing 1.6%; ammonium was on average 0.6%; and organic acids (citric and acetic acid) represented 0.5%. Carrier (e.g. corncob, starch, dextrin) was on average 4.7%. The amount of unidentified material was < 1% on dry matter basis. 41
3.1.5.1. Impurities
Three batches were analysed for impurities. Concentrations of cadmium, lead, mercury and arsenic were all below LOQ, 42 except for lead that ranged from 0.04 to 0.06 mg/kg in two batches; and one batch of arsenic that showed a value of 0.05 mg/kg.
Polychlorinated dibenzodioxins (PCDDs), polychlorinated dibenzofurans (PCDFs) and coplanar dioxin‐like polychlorinated biphenyls (Co‐planar PCBs) were analysed in three batches and found below the corresponding limit of quantification (LOQ). 43 The calculated (upper bound) levels of dioxins and the sum of dioxins and dioxin‐like‐PCBs were 0.7 ng WHO‐PCDD/F‐TEQ/kg and 0.14 ng WHO‐PCDD/F‐PCB‐TEQ/kg, respectively (in all three batches).Non‐dioxin‐like PCBs were0.6 μg/kg additive.
The analysis of mycotoxins (ochratoxin A, zearalenone, deoxynivalenol and aflatoxins), showed values below the LOQ. 44
Microbiological contamination was analysed by determination of Escherichia coli, Enterobacteriaceae, filamentous fungi and yeasts. Counts of all these microbial populations were < 10 CFU/g of product. 45 Salmonella spp. was not detected in the 25 g samples analysed.
The detected amounts of the above‐described impurities do not raise safety concerns.
The endotoxin activity in the final product was tested in three batches of the additive via the European Pharmacopoeia method and ranged from 1,196 to 1,638 EU/g. 46
3.1.5.2. Physical properties
l‐Methionine > 90% appears as a yellowish or brown powder with a slight sulfurous smell, bulk density was analysed in three batches and ranged from 580 to 600 kg/m3, 47 and solubility of the additive in water at 25°C was 56.6 g/L and a melting point of 284°C. 48
The dusting potential of three batches was determined using the Stauber‐Heubach method and showed values that ranged from115 to 1,035 mg/m³. 49
The particle size distribution of three batches of the additive was measured by sieving and the fraction of particles < 75 µm ranged from 2% to 7%; that < 106 µm ranged from 7.4% to 11.5%; and the fraction > 106 µm ranged from 89% to 94%. 50
3.1.5.3. Stability and homogeneity
The shelf‐life of l‐methionine ≥90% (three batches) was studied when stored at 25°C in sealed plastic bags for 6 months. In all cases, losses were < 1%. 51
The stability of l‐methionine ≥90% (three batches) in a vitamin–mineral premixture (containing 40 mg choline chloride) was studied when supplemented at 11.1%, stored at 25°C in sealed foil bags for 6 months. Losses at the end of the storage period were 10%. 52
The stability of l‐methionine ≥ 90% (three batches) in complete feed (meal and pelleted form) for chickens for fattening was studied when supplemented at 0.28%, stored at 25°C in sealed foil bags for 3 months. The basal diet consisted of soybean meal, maize and wheat and contained a background concentration of 0.26% methionine. The pelleting process was performed at 70–75°C and caused an average loss of 7%. No losses were observed in the meal at the end of the storage period, while in pelleted feed losses ranged from 6% to 12%. 53
The stability of l‐methionine ≥ 90% (three batches) in water for drinking was studied when supplemented at 30 mg/L. Samples were stored at 25°C and at 40°C in sealed foil bags for 24 h. No losses were observed at the end of the storage period except for one batch kept at 40°C which showed a loss of 1%. 54
The capacity for homogeneous distribution of l‐methionine ≥ 90% was studied in 10 subsamples of premixture, meal and pelleted feed for chickens for fattening. Free methionine was analysed. The coefficient of variation was 4% in premixture, 8% in meal and 4% in pelleted feed. 55
3.1.6. Conditions of use
The additive l‐methionine is proposed to be used in feed in order to achieve the adequate amino acid profile and meet the requirements of l‐methionine for all animal species. It can be added directly to the feed or via premixture. The use of l‐methionine in water for drinking is also proposed. No inclusion levels are recommended as the requirements in quantitative terms depend on the species, the physiological state of the animal, the performance level and the environmental conditions, as well as the amino acid content of the unsupplemented diet.
3.2. Safety
3.2.1. Safety of the production microorganisms
The production strain KCCM 80245 belongs to a species, C. glutamicum, that qualifies for the QPS approach to safety assessment (EFSA, 2007) when used for production purposes (EFSA BIOHAZ Panel, 2021). The production strain was unambiguously identified as C. glutamicum, it was shown to be susceptible to all relevant antibiotics, does not contain antimicrobial resistance genes, and the genetic modification raised no safety concerns.
The parental strain of E. coli KCCM 80246 is ■■■■■ which is considered safe. The production strain was identified as an Escherichia coli K‐12 derivative. The genetic modification introduced a partial ampicillin resistance gene; however, since the strain was susceptible to all relevant antibiotics, and the interrogation of the WGS data did not identify complete antimicrobial resistance genes, it can be concluded that the genetic modification raised no safety concerns.
Further, no viable cells or recombinant DNA of any of the two production strains (C. glutamicum KCCM 80245 and E. coli KCCM 80246) were detected in the final products. Therefore, the FEEDAP Panel concludes that the use of C. glutamicum KCCM 80245 and E. coli KCCM 80246 in the production of l‐methionine is safe.
3.2.2. Safety for the target species, consumers and the environment
Safety concerns from the additive may derive either from the amino acid or from the residues of the fermentation process/production strain remaining in the final product. The two products under assessment have a high purity (≥ 98.5% methionine and < 1% unidentified substances; and ≥ 90% and < 1% unidentified substances). Neither the production strains (C. glutamicum KCCM 80245 and E. coli KCCM 80246) nor their recombinant DNA was detected in the final product; the safety assessment of the production strains did not raise elements of concern.
The requirements of l‐methionine in different animal species (non‐ruminants and ruminants) and categories and the tolerance to L‐methionine excess in the diet have been described in a previous opinion of the FEEDAP Panel (EFSA FEEDAP Panel, 2013).
The FEEDAP Panel considers that safety concerns for target species are highly unlikely to arise from the l‐methionine products under application. Regarding the use in water, the FEEDAP Panel has concerns about the use of amino acids in water for drinking for hygienic reasons, and due to the risk of imbalances when administered simultaneously via feed (EFSA FEEDAP Panel, 2010). The level of endotoxins (LPS) in the product (up to 1.8 IU/mg in the product containing 98.5% l‐methionine and up to 1.6 IU/mg in the product containing 90% l‐methionine) is about three orders of magnitude lower than that commonly observed in feedingstuffs (1,000 IU/mg; Cort et al., 1990) and is therefore of no concern for the target species.
The amino acid l‐methionine, supplemented to feed, will be incorporated into proteins of tissues and/or products of animal origin and any potential excess will be catabolised and excreted as urea/uric acid, sulfate and carbon dioxide. Therefore, the composition of tissues and products of animal origin will not be affected by the use of l‐methionine under assessment in animal nutrition.
None of the products of the additive poses any environmental concern associated with the production strains. The amino acid l‐methionine is a physiological and natural component in plants and animals. The use of amino acids in water for drinking, when given in addition to complete diets with a well‐balanced amino acid profile, would disturb the nitrogen balance and increase nitrogen excretion via urine. The use of l‐methionine in animal nutrition would not expectedly lead to any localised increase in its concentration in the environment.
3.2.2.1. Conclusions on safety for the target species, consumers and the environment
The use of both products of the additive produced by C. glutamicum KCCM 80245 and E. coli KCCM 80246 as nutritional additive in supplementing feed to compensate for methionine deficiency in feedingstuffs is safe for the target species. The FEEDAP Panel has concerns on the use of amino acids in water for drinking for hygienic reasons, and due to the risk of imbalances when administered simultaneously via feed.
The use of both products of the additive produced by C. glutamicum KCCM 80245 and E. coli KCCM 80246 in animal nutrition is considered safe for the consumer and for the environment.
3.2.3. Safety for the user
3.2.3.1. l‐methionine 98.5%
The applicant submitted an acute inhalation toxicity study (according to OECD Testing Guideline (TG) 403), an acute dermal irritation/corrosion study (according to OECD TG 404), an eye irritation study (according to OECD TG 405), a skin sensitisation study (according to OECD TG 406, Buehler method) performed using the L‐methionine obtained from a different production strain as test item. The studies were assessed in a previous opinion (EFSA FEEDAP Panel, 2013). As the product characteristics and the manufacturing process were similar, the FEEDAP Panel considers that the results obtained in those studies are applicable to the additive under assessment. Consequently, the additive can be considered not toxic by inhalation, not irritant to skin and eyes and not a dermal sensitiser.
Users can suffer from occupational respiratory disease depending on the level of endotoxins in air and dust (Rylander et al., 1999; Thorn, 2001). The product under assessment showed bacterial endotoxin activity (analysed in three batches) which ranged from 991 to 1,811 IU/g and has a dusting potential up to 620 mg/m3. The health‐based recommended threshold for the quantity of inhaled endotoxins per working day is 900 IU, derived from provisional occupational exposure limits given by the Dutch Expert Committee on Occupational Safety (DECOS) (HCN, 2010) and the UK Health and Safety Executive (HSE, 2013). Based upon the calculation of the potential endotoxin content in dust (highest reported measurement), the inhalation exposure is calculated as 624 endotoxin IU per working day, indicating no risk of exposure by inhalation to endotoxins for persons handling the additive.
3.2.3.2. l‐methionine 90%
The applicant submitted an acute inhalation toxicity study, a skin and an eye acute irritation study and a skin sensitisation study performed with the l‐methionine ≥ 90% under assessment.
3.2.3.2.1. Effect on respiratory system
The acute inhalation study was performed following the GLP principles and in accordance with the OECD TG 403 (nose only exposure). 56 The results indicate that under the conditions of the study, the acute inhalation median lethal concentration (LC50) of l‐methionine (≥ 90%) was greater than 2.75 mg/L.
The bacterial endotoxin activity (analysed in three batches) ranged from 1,196 to 1,638 IU/g and has a dusting potential up to 1,035 mg/m3. Based upon the calculation of the potential endotoxin content in dust, the inhalation exposure is calculated as 942 endotoxin IU per working day, indicating that inhalation exposure to endotoxins for persons handling the additive is above the recommended threshold (900 IU; HCN, 2010; HSE, 2013).
3.2.3.2.2. Effect on skin and eyes
The acute skin irritation study was performed following the GLP principles and in accordance with the OECD TG 404. 57 Based on the results of this test, the additive is not considered a skin irritant.
The acute eye irritation study was performed following the GLP principles and according to the OECD TG 405. 58 Iridial inflammation was observed in one rabbit 1 h after administration. Moderate conjunctival irritation was observed in two rabbits, 1 and 24 h after treatment that had disappeared at the end of the observation period (72 h). Based on the results of this test, the additive is not considered an eye irritant.
The skin sensitisation study was performed following the GLP principles and in accordance with the OECD TG 429 59 and the Method B42 Skin sensitization of Commission Regulation (EC) No 440/2008. 60 Based on the results of the test, the additive was not considered a dermal sensitiser.
3.2.3.3. Conclusions on safety for the user
The additive, in either product, is not an irritant to skin/eyes and not a dermal sensitiser and shows no toxicity by inhalation. Considering the exposure to endotoxins by inhalation, the product l‐methionine ≥ 90% is a risk for the user.
3.3. Efficacy
Efficacy studies are not required for amino acids naturally occurring in proteins of plants and animals. The nutritional role of the amino acid l‐methionine is well established in the scientific literature. Both products (≥ 98.5% l‐methionine and ≥ 90% l‐methionine) are regarded as an effective source of methionine for non‐ruminant animal species. For the supplemental l‐methionine to be as efficacious in ruminants as in non‐ruminant species, it would require protection against degradation in the rumen.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation 61 and Good Manufacturing Practice.
4. Conclusions
Viable cells of the production strains, C. glutamicum KCCM 80245 and E. coli KCCM 80246, and their recombinant DNA were not detected in the products under assessment. The additive does not pose any safety concern associated with the production strains.
The use of both products of l‐methionine produced by C. glutamicum KCCM 80245 and E. coli KCCM 80246 in supplementing feed to compensate for L‐methionine deficiency in feedingstuffs is safe for the target species. The FEEDAP Panel has concerns on the use of amino acids in water for drinking for hygienic reasons, and due to the risk of imbalances when administered simultaneously via feed.
The use of both products of L‐methionine produced by C. glutamicum KCCM 80245 and E. coli KCCM 80246 in animal nutrition is considered safe for the consumers and for the environment.
The additive, in either product, is not an irritant to skin/eyes and not a dermal sensitiser and shows no toxicity by inhalation. Considering the respiratory exposure to endotoxins, l‐methionine ≥ 90% is a risk for the user.
Both products of the additive produced by C. glutamicum KCCM 80245 and E. coli KCCM 80246 are considered as an efficacious source of the essential amino acid l‐methionine for non‐ruminant animal species. For the supplemental l‐methionine to be as efficacious in ruminants as in non‐ruminant species, it would require protection against degradation in the rumen.
5. Documentation provided to EFSA/Chronology
| Date | Event |
|---|---|
| 03/03/2021 | Dossier received by EFSA. l‐methionine (min. 98.5%) and l‐methionine (min. 90%) produced by fermentation with Corynebacterium glutamicum KCCM 80245 and Escherichia coli KCCM 80246. Submitted by Cheil Jedang Europe GmbH. |
| 09/03/2021 | Reception mandate from the European Commission |
| 08/06/2021 | Application validated by EFSA – Start of the scientific assessment |
| 07/07/2021 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: manufacturing process, characterisation of the additive. |
| 02/09/2021 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 10/09/2021 | Comments received from Member States |
| 28/09/2021 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterisation of the additive. |
| 08/10/2021 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 25/11/2021 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 23/03/2022 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- ANI
Average nucleotide identity
- BLAST
Basic local alignment search tool
- CAS
Chemical Abstracts Service
- CFU
colony‐forming unit
- CV
coefficient of variation
- DM
dry matter
- EINECS
European Inventory of Existing Chemical Substances
- EURL
European Union Reference Laboratory
- FEEDAP
EFSA Scientific Panel on Additives and Products or Substances used in Animal Feed
- FLAVIS
The EU Flavour Information System
- FL‐no
FLAVIS number
- IUPAC
International Union of Pure and Applied Chemistry
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- LOD
limit of detection
- LOQ
limit of quantification
- MIC
minimum inhibitory concentration
- OECD
Organisation for Economic Co‐operation and Development
- VFDB
Virulence factors database
Appendix A – Safety for the user
The effects of endotoxin inhalation and the exposure limits have been described in a previous opinion (EFSA FEEDAP Panel, 2015).
Calculation of maximum acceptable levels of exposure from feed additives
The probable exposure time according to EFSA guidance (EFSA FEEDAP Panel, 2012a) for additives added in premixtures assumes a maximum of 40 periods of exposure per day, each comprising 20 s = 40 × 20 = 800 s/day. With an uncertainty factor of 2, maximum inhalation exposure would occur for 2 × 800 = 1,600 s = 0.444 h/day. Again, assuming a respiration volume of 1.25 m3/h, the inhalation volume providing exposure to potentially endotoxin‐containing dust would be 0.444 × 1.25 = 0.556 m3/day. This volume should contain no more than 900 IU endotoxin, so the dust formed from the product should contain no more than 900/0.556 = 1,619 IU/m3.
Calculation of endotoxin content of dust
Two key measurements are required to evaluate the potential respiratory hazard associated with the endotoxin content of the product (the dusting potential of the product, expressed in g/m3, and the endotoxin activity of the dust, determined by the Limulus amoebocyte lysate assay (expressed in IU/g)). If data for the dust are not available, the content of endotoxins of the product can be taken instead. If the content of endotoxins of the relevant additive is a IU/g and the dusting potential is b g/m3, then the content of endotoxins of the dust, c IU/m3, is obtained by simple multiplication, a × b. This resulting value is further used for calculation of the potential inhalatory exposure of users to endotoxins from the additive under assessment (Table A.1 and A.2) (EFSA FEEDAP Panel, 2012a).
Table A.1.
Estimation of user exposure to endotoxins from the additive l‐methionine 98.5% produced by Corynebacterium glutamicum KCCM 80245 and Escherichia coli 80246
| Calculation | Identifier | Description | Amount | Source |
|---|---|---|---|---|
| a | Endotoxin content IU/g product | 1,811 | Technical dossier | |
| b | Dusting potential (g/m3) | 0.62 | Technical dossier | |
| a × b | c | Endotoxin content in the air (IU/m3) | 1,122.82 | |
| d | No of premixture batches made/working day | 40 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012a) | |
| e | Time of exposure (s) per production of one batch | 20 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012a) | |
| d × e | f | Total duration of daily exposure/worker (s) | 800 | |
| g | Uncertainty factor | 2 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012a) | |
| f × g | h | Refined total duration of daily exposure/worker (s) | 1,600 | |
| h/3,600 | i | Refined total duration of daily exposure (h) | 0.44 | |
| j | Inhaled air (m3) per 8‐hour working day | 10 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012a) | |
| j/8 × i | k | Inhaled air during exposure (m3) | 0.56 | |
| c × k | l | Endotoxin inhaled (IU) during exposure per 8‐hour working day | 623.79 | |
| m | Health‐based recommended exposure limit of endotoxin (IU/m3) per 8‐hour working day | 90 | HCN (2010) | |
| m × j | n | Health‐based recommended exposure limit of total endotoxin exposure (IU) per 8‐hour working day | 900 |
Table A.2.
Estimation of user exposure to endotoxins from the additive l‐methionine 90% produced by Corynebacterium glutamicum 80245 and Escherichia coli 80246
| Calculation | Identifier | Description | Amount | Source |
|---|---|---|---|---|
| a | Endotoxin content IU/g product | 1,638 | Technical dossier | |
| b | Dusting potential (g/m3) | 1.035 | Technical dossier | |
| a × b | c | Endotoxin content in the air (IU/m3) | 1,695.33 | |
| d | No of premixture batches made/working day | 40 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012a) | |
| e | Time of exposure (s) per production of one batch | 20 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012a) | |
| d × e | f | Total duration of daily exposure/worker (s) | 800 | |
| g | Uncertainty factor | 2 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012a) | |
| f × g | h | Refined total duration of daily exposure/worker (s) | 1,600 | |
| h/3,600 | i | Refined total duration of daily exposure (h) | 0.44 | |
| j | Inhaled air (m3) per 8‐hour working day | 10 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012a) | |
| j/8 × i | k | Inhaled air during exposure (m3) | 0.56 | |
| c × k | l | Endotoxin inhaled (IU) during exposure per 8‐hour working day | 941.85 | |
| m | Health‐based recommended exposure limit of endotoxin (IU/m3) per 8‐hour working day | 90 | Health Council of the Netherlands, 2010 | |
| m × j | n | Health‐based recommended exposure limit of total endotoxin exposure (IU) per 8‐hour working day | 900 |
References
EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. https://doi.org/10.2903/j.efsa.2012.2539
EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2015. Scientific Opinion on the safety and efficacy of L‐lysine monohydrochloride produced by fermentation with Escherichia coli for all animal species based on a dossier submitted by HELM AG on behalf of Meihua Holdings Group Co. Ltd. EFSA Journal 2015;13(3):4052, 16 pp. https://doi.org/10.2903/j.efsa.2015.4052
HCN (Health Council of the Netherlands), 2010. Endotoxins. Health‐based recommended occupational exposure limit. Publication no 2010/04OSH, 100 pp.
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Methods of the Analysis for l‐methionine (min. 98.5%) and l‐methionine (min. 90%) produced by fermentation with Corynebacterium glutamicum KCCM 80245 and Escherichia coli KCCM 80246
In the current application, authorisation is sought under Article 4(1) for l‐methionine (min. 98.5%) and l‐methionine (min. 90%) produced by fermentation with Corynebacterium glutamicum KCCM 80245 and Escherichia coli KCCM 80246, under the category/functional group 3(c) ‘nutritional additives'/‘amino acids, their salts and analogues', according to Annex I of Regulation (EC) No 1831/2003. The authorisation is sought for all animal species.
According to the Applicant, the feed additive has a minimum purity (mass fraction on dry matter basis) of 98.5% and 90% with respect to the L‐methionine content, respectively. The feed additive is intended to be added directly into feedingstuffs or through complementary feed, premixtures and water for drinking. However, the Applicant did not propose any minimum or maximum content of l‐methionine in feedingstuffs.
For the quantification of l‐methionine in the feed additive, the Applicant submitted (i) the method based on titration described in the corresponding European Pharmacopoeia monograph and (ii) a single‐laboratory validated method based on high‐performance liquid chromatography (HPLC) coupled to spectrophotometric detection at 210 nm (UV).
For the quantification of methionine in the feed additive, the EURL identified instead, the ring‐trial validated method EN ISO 17180:2013 – ‘Animal feeding stuffs – Determination of lysine, methionine and threonine in commercial amino acid products and premixtures’ based on ion‐exchange chromatography coupled to visible or fluorescence detection (IECVIS/FLD). This standard method does not distinguish between the salts of amino acids and cannot differentiate between enantiomers. It applies for products containing more than 10% of amino acid. The following performance characteristics were reported: a relative standard deviation for repeatability (RSDr) ranging from 0.5 to 1.6% and a relative standard deviation for reproducibility (RSDR) ranging from 1.5 to 2.6%. In addition, the EURL identified the ‘l‐methionine monograph’ of the Food Chemical Codex (FCC) for the identification of l‐methionine in the feed additive.
For the quantification of l‐methionine in premixtures and feedingstuffs, the Applicant submitted the ring‐trial validated European Union method (Commission Regulation (EC) No 152/2009) based on IEC coupled to photometric detection (IEC‐VIS). This method, designed only for the analysis of amino acids in premixtures and feedingstuffs, does not distinguish between the salts and the amino acid enantiomers. The European Union method was ring‐trial validated using four different matrices. This method was further ring‐trial validated by 23 laboratories, resulting in the EN ISO 13903:2005 method. The following performance characteristics were reported for the quantification of total methionine in premixtures and feedingstuffs: RSDr ranging from 1.1 to 5.6% and RSDR ranging from 6.9 to 13%.
The Applicant did not provide experimental data to determine l‐methionine in water. Nevertheless, for the quantification of methionine in water, as concluded in the previous EURL reports and further specified in the corresponding legislation, the EURL recommended for official control the European Union method.
In the frame of this authorisation, the EURL recommends for official control (i) the ‘l‐methionine monograph’ of the Food Chemical Codex (FCC) based on infrared absorption for the identification of l‐methionine in the feed additive; (ii) the ring‐trial validated method EN ISO 17180:2013 based on ion‐exchange chromatography coupled to visible or fluorescence detection (IEC‐VIS/FLD) to quantify free methionine in the feed additive and premixtures (containing more than 10% methionine); and (iii) the European Union method based on IECVIS for the quantification of methionine in premixtures, feedingstuffs and water.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005), as last amended by Regulation (EU) 2015/1761) is not considered necessary.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Fašmon Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Anguita M, Galobart J, Pettenati E and Tarrés‐Call J, 2022. Scientific Opinion on the safety and efficacy of a feed additive consisting of l‐methionine produced by the combined activities of Corynebacterium glutamicum KCCM 80245 and Escherichia coli KCCM 80246 for all animal species (CJ Europe GmbH). EFSA Journal 2022;20(4):7247, 20 pp. 10.2903/j.efsa.2022.7247
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00154
Panel members: Vasileios Bampidis, Giovanna Azimonti, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Fašmon Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Commission. The full output has been shared with the European Commission, EU Member States and the applicant. The blackening will be subject to review once the decision on the confidentiality requests is adopted by the European Commission.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: The Panel wishes to thank the following for the support provided to this scientific output: Angelica Amaduzzi, Rosella Brozzi, Fabiola Pizzo, Martina Reitano and the members of the Microbiology Working Group.
Adopted: 23 March 2022
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the council of 22 September 2003 on the additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
CJ Europe GmbH, Unterschweinstiege 2‐14, 60549 Frankfurt am Main, Germany.
Commission implementing regulation (EU) No 469/2013 of 22 May 2013 concerning the authorisation of DL‐methionine, DL‐methionine sodium salt, hydroxy analogue of methionine, calcium salt of hydroxy analogue of methionine, isopropyl ester of hydroxy analogue of methionine, DL‐methionine protected with copolymer vinylpyridine/styrene and DL‐methionine protected with ethylcellulose as feed additives. OJ L 136, 23.5.2013.
Commission implementing regulation (EU) No 852/2014 of 5 August 2014 concerning the authorisation of L‐methionine as a feed additive for all animal species. OJ L 233/22, 6.8.2014.
Commission implementing regulation (EU) 2020/1497 of 15 October 2020 concerning the authorisation of L‐methionine produced by Corynebacterium glutamicum KCCM 80 184 and Escherichia coli KCCM 80 096 as a feed additive for all animal species. OJ L 342, 16.10.2020.
Commission Implementing Regulation (EU) 2018/249 of 15 February 2018 concerning the authorisation of taurine, beta‐alanine, l‐alanine, l‐arginine, l‐aspartic acid, l‐histidine, d,l‐isoleucine, l‐leucine, l‐phenylalanine, l‐proline, d,l‐serine, l‐tyrosine, l‐methionine, l‐valine, l‐cysteine, glycine, monosodium glutamate and l‐glutamic acid as feed additives for all animal species and l‐cysteine hydrochloride monohydrate for all species except cats and dogs. OJ L 53, 23.2.2018, p. 134.
Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow‐on formulae and amending Directive 1999/21/EC. OJ L 401, 30.12.2006, p. 1.
Commission Decision of 9 February 2006 amending Decision 96/335/EC establishing an inventory and a common nomenclature of ingredients employed in cosmetic products. OJ L 97, 5.4.2006, p. 1.
FEED dossier reference: FAD‐2021‐0018.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/default/files/finrep_fad‐2021‐0018‐l‐methionine.pdf
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical dossier/Section II/Annex CONFID II.2.1.
Technical dossier/Section II/Annex CONFID II.2.4.
Technical dossier/Section II/Annex CONFID II.2.6.
Technical dossier/Section II/Annex_CONFID_II_2_04 WGS analysis.
Technical dossier/Supplementary information November 2021/Annex SIN CONF 01 CJ MET.
Technical dosser/Section II/Annex CONFID II.2.4.
Technical dossier. Section II.B.1A.1.
Technical dossier/Section II/Annex CONFID II.2.8.
Technical dossier/Section II/Annex CONFID II.2.9.
Technical dossier/Section II/Annexes CONFID II.2.9 and II.2.10.
Technical dossier/Supplementary information November 2021/Annex SIN CONF 02 CJ Met.
Technical dossier/Section II/Annex CONFID II.2.4 WGS analysis_KCCM 80245
Technical dossier/Supplementary information September 2021/Annex SIN CONF O1 ■■■■■
Technical dossier/Section II/Subsections II.3.1.1 and II.3.1.2.
Technical dossier/Section II/Annex CONFID II.2.3.
Technical dossier/Section II/Annex CONFID II.2.11.
Technical dossier/Section II/Annex II.1.1.
Technical dossier/Section II/Annex II.1.5 and Supplementary information September 2021/0.3 SIN CJ Met. The analytical method for methionine was HPLC with UV detector and potassium phosphate as mobile phase.
Technical dossier/Section II/Annex II.1.2.
The FEEDAP Panel notes that the specifications for optical rotation proposed by the applicant do not meet the specifications for L‐methionine established in the European Pharmacopoeia which are +22.5° to +24.0°
Technical dossier/Section II/Annex II.1.7. LOD in mg/kg was 0.01 for cadmium and mercury; 0.015 for lead; 0.04 for arsenic; 0.2 for nickel and chromium; and 0.5 for zinc.
Technical dossier/Section II/Annex II.1.7. LOQ in ng/kg ranged from 0.01 to 1 for PCDD/F; from 0.5 to 10 ng/kg for non‐ortho PCBs. LOQ in µg/kg were 0.1 for ndl PCBs.
Technical dossier/Section II/Annex II.1.7. LOQ were 0.2 µg/kg for aflatoxins; 0.5 µg/kg for ochratoxin A; 5 µg/kg for zearalenone; 10 µg/kg for deoxynivalenol.
Technical dossier/Section II/Annex II.1.7. LOD was 10 CFU/g for E. coli, Enterobacteriaceae, filamentous fungi and yeasts; not detectable for Salmonella spp.
Technical dossier/Section II/Annex II.1.13.
Technical dossier/Section II/Annex II.5.1.
Technical dossier/Section II/Annex II.1.11.
Technical dossier/Section II/Annex II.1.09.
Technical dossier/Section II/Annex II.1.04 and Supplementary information September 2021/0.3 SIN CJ Met. The analytical method for methionine was HPLC with UV detector and potassium phosphate as mobile phase.
Technical dossier/Section II/Annex II.1.06.
Technical dossier/Section II/Annex II.1.08. LOQ in mg/kg were 0.015 for lead; 0.01 for cadmium; 0.01 for mercury; 0.04 for arsenic.
Technical dossier/Section II/Annex II.1.08. LOQ in ng/kg ranged from 0.01 to 1 for PCDD/F; from 0.5 to 10 ng/kg for non‐ortho PCBs. LOQ in µg/kg were 0.1 for ndl PCBs.
Technical dossier/Section II/Annex II.1.8. LOQ were 0.2 µg/kg for aflatoxins; 0.5 µg/kg for Ochratoxin A; 5 µg/kg for Zearalenon; 10 µg/kg for Deoxynivalenol.
Technical dossier/Section II/Annex II.1.8. LOD were <10 CFU/g for E. coli, Enterobacteriaceae, filamentous fungi and yeast; not detectable for Salmonella spp.
Technical dossier/Section II/Annex II.1.14.
Technical dossier/Section II/Annex II.4.4.
Technical dossier/Section II/Annex II.5.2.
Technical dossier/Section II/Annex II.1.12.
Technical dossier/Section II/Annex II.1.10.
Technical dossier/Section II/Annex II.4.02.
Technical dossier/Section II/Annex II.4.04.
Technical dossier/Section II/Annex II.4.06.
Technical dossier/Section II/Annex II.4.09 and supplementary information September 2021/Annex SIN 01 CJ Met stab in water.
Technical dossier/Section II/Annex II.4.13 and Annex II.4.14.
Technical dossier/Section III/Annex III.3.4.
Technical dossier/Section III/Annex III.3.8.
Technical dossier/Section III/Annex III.3.6.
Technical dossier/Section III/Annex III.3.10.
Available online: https://www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev04/English/ST‐SG‐AC10‐30‐Rev4e.pdf
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
References
- Cort N, Fredriksson G, Kindahl H, Edqvist L‐E and Rylander R, 1990. A clinical and endocrine study on the effect of orally administered bacterial endotoxin in adult pigs and goats. Journal of Veterinary Medicine Series A, 37, 130–137. 10.1111/j.1439-0442.1990.tb00884.x [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2007. Opinion of the Scientific Committee on a request from EFSA on the introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA. EFSA Journal 2007;5(12):587, 16 pp. 10.2903/j.efsa.2007.587 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Cocconcelli PS, Fernández Escámez PS, Maradona MP, Querol A, Sijtsma L, Suarez JE, Sundh I, Vlak J, Barizzone F, Hempen M and Herman L, 2021. Update of the list of QPS‐recommended biological agents intentionally added to food or feed as notified to EFSA 13: suitability of taxonomic units notified to EFSA until September 2020. EFSA Journal 2021;19(2):6377, 35 pp. 10.2903/j.efsa.2021.6377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (Panel on Additives and Products or Substances Used in Animal Feed) , 2010. Scientific Opinion on the use of feed additives authorised/applied for use in feed when supplied via water. EFSA Journal 2010;8(12):1956, 9 pp. 10.2903/j.efsa.2010.1956 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2012a. Scientific Opinion on DL‐methionine, DL‐methionine sodium salt, the hydroxy analogue of methionine and the calcium salt of methionine hydroxy analogue in all animal species; on the isopropyl ester of methionine hydroxy analogue and DL‐methionine technically pure protected with copolymer vinylpyridine/styrene in dairy cows; and on DL‐methionine technically pure protected with ethylcellulose in ruminants. EFSA Journal 2012;10(3):2623, 42 pp. 10.2903/j.efsa.2012.2623 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2012b. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2013. Scientific Opinion on the safety and efficacy of L‐methionine produced by Escherichia coli (KCCM 11252P) and Escherichia coli (KCCM 11340P) for all animal species. EFSA Journal 2013;11(10):3428, 16 pp. 10.2903/j.efsa.2013.3428 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017a. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(2):5022, 45 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J and Innocenti ML, 2017b. Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal 2017;15(2):5023, 35 pp. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017c. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 45 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2018a. Guidance on the assessment of the efficacy of feed additives. EFSA Journal, 2018;16(2):5274, 45 pp. 10.2903/j.efsa.2018.5274 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Glandorf B, Herman L, Karenlampi S, Aguilera J, Anguita M, Brozzi R and Galobart J, 2018b. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal, 2018;16(3):5206, 13 pp. 10.2903/j.efsa.2018.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Cocconcelli PS, Glandorf B, Herman L, Prieto Maradona M, Saarela M, Brozzi R, Galobart J, Gregoretti L, Innocenti ML, Sofianidis K, Vettori MV and López‐Gálvez G, 2019a. Scientific Opinion on the safety and efficacy of l‐methionine produced by fermentation with Corynebacterium glutamicum KCCM 80184 and Escherichia coli KCCM 80096 for all animal species. EFSA Journal 2019;17(12):5917, 15 pp. 10.2903/j.efsa.2019.5917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Bastos M, Christensen H, Dusemund B, Kouba M, Kos Durjava M, Lopez‐Alonso M, Lopez Puente S, Marcon F, Mayo B, Pechova A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brock T, de Knecht J, Kolar B, van Beelen P, Padovani L, Tarres‐Call J, Vettori MV and Azimonti G, 2019b. Guidance on the assessment of the safety of feed additives for the environment. EFSA Journal 2019;17(3):5648, 45 pp. 10.2903/j.efsa.2019.5648 [DOI] [Google Scholar]
- European Pharmacopoeia , 2021. European Directorate for the quality of medicines and health care. Council of Europe. Monograph 01/2007/1027 L‐Methionine [63‐68‐3], p. 3223. NetISTM E‐Publishing Platform. 10th Edition.
- Gorbach SL, 1978. Risk assessment of recombinant DNA experimentation with Escherichia coli K12. Proceedings from a workshop at Falomouth, Massachusetts. Journal of Infectious Diseases, 137, 613–714. [Google Scholar]
- HCN (Health Council of the Netherlands) , 2010. Endotoxins. Health‐based recommended occupational exposure limit. Publication no 2010/04OSH, 100 pp.
- HSE (Health and Safety Executive) , 2013. Occupational Hygiene implications of processing waste at Materials Recycling Facilities (MRFs). RR977 Research Report, UK, 41 pp. [Google Scholar]
- Rylander R, Thorn J and Attefors R, 1999. Airways inflammation among workers in a paper industry. European Respiratory Journal, 13, 1151–1157. 10.1034/j.1399-3003.1999.13e35.x [DOI] [PubMed] [Google Scholar]
- Smith HW, 1975. Survival of orally administered E. coli K‐12 in alimentary tract of man. Nature, 255, 500–502. [DOI] [PubMed] [Google Scholar]
- Thorn J and Kerekes E, 2001. Health effects among employees in sewage treatment plants: a literature survey. American Journal of Industrial Medicine, 40, 170–179. 10.1002/ajim.1085 [DOI] [PubMed] [Google Scholar]


