Abstract
The development of the exposome concept has been one of the hallmarks of environmental and health research for the last decade. The exposome encompasses the life course environmental exposures including lifestyle factors from the prenatal period onwards. It has inspired many research programs and is expected to influence environmental and health research, practices, and policies. Yet, the links bridging toxicology and the exposome concept have not been well developed. In this review, we describe how the exposome framework can interface with and influence the field of toxicology, as well as how the field of toxicology can help advance the exposome field by providing the needed mechanistic understanding of the exposome impacts on health. Indeed, exposome-informed toxicology is expected to emphasize several orientations including (1) developing approaches integrating multiple stressors, in particular chemical mixtures, as well as the interaction of chemicals with other stressors, (2) using mechanistic frameworks such as the adverse outcome pathways to link the different stressors with toxicity outcomes, (3) characterizing the mechanistic basis of long-term effects by distinguishing different patterns of exposures and further exploring the environment-DNA interface through genetic and epigenetic studies, and (4) improving the links between environmental and human health, in particular through a stronger connection between alterations in our ecosystems and human toxicology. The exposome concept provides the linkage between the complex environment and contemporary mechanistic toxicology. What toxicology can bring to exposome characterization is a needed framework for mechanistic understanding and regulatory outcomes in risk assessment.
Keywords: multiple stress, adverse outcome pathways, mixtures, epigenetics, chemical toxicity
Following a number of conceptual and methodological advances during the last decades, toxicology has considerably evolved. A large number of studies have highlighted the relevance of low-dose effects, nonmonotonous dose response curves (Vandenberg et al., 2012), vulnerable developmental stages for the assessment of the link between exposure and effects (Barouki et al., 2012; Grandjean et al., 2015), and the importance of the interplay between intrinsic (genetic, epigenetics, etc.) and extrinsic factors (nutritional, toxic, social, etc.) (McHale et al., 2018). There has been enhanced focus on long-term effects and programming leading to strong connections between toxicology and epigenetics (Barouki et al., 2018; Chung and Herceg, 2020). There was also more interest in the interaction between different types of stressors and, within the scope of chemical toxicology, more work on mixture effects (Bopp et al., 2018; Drakvik et al., 2020). Omics technologies have been increasingly used, leading to a larger scope of effects, and alternative methods to animal testing have been considerably developed (Hartung, 2009; National Research Council, 2007). Toxicology has become increasingly reliant on different types of in silico methods, such as quantitative structure activity relationships (QSAR), physiologically based pharmacokinetic modeling (PBPK), read across (RA), and systems biology approaches (Kongsbak et al., 2014). Naturally, the regulatory arena needs to keep pace with progression in the field, and has developed new methodologies for risk assessment (Krewski et al., 2020; Tralau et al., 2015).
The development of the exposome concept has been one of the hallmarks of environment and health research for the last decade. In 2005, Chris Wild defined the exposome as the life-course environmental exposures (including lifestyle factors), from the prenatal period onwards (Wild, 2005). With this definition, the exposome appears as the complement of the genome, the combination of both accounting for physiological and pathological states (Barouki et al., 2018). There are two critical features in Wild’s definition: first, all exposures are considered, including chemical, physical, biological, psychological, social, and behavioral exposures, even though many of these are outside the traditional scope of toxicology; second, the life-course, including critical periods of vulnerability, is taken into consideration.
Additional and important contributions followed, elaborating what the exposome (potentially) comprises and reflecting different perspectives. Buck Louis highlighted the importance of macro-level and lifestyle factors (Buck Louis et al., 2017), whereas Rappaport and Smith focused on the internal chemical exposome composed of xenobiotics, endogenous metabolites, microbial metabolites, and dietary compounds (Athersuch and Keun, 2015; Rappaport, 2018; Rappaport and Smith, 2010). Following a more toxicological perspective, Miller and Jones defined the exposome as including all environmental influences as well as associated biological responses (Miller and Jones, 2014; Niedzwiecki et al., 2019). A more computational standpoint highlighted the role of modeling and biological connectivity (Sarigiannis, 2017). The “eco-exposome” was defined as the bidirectional influences between ecosystem and human exposures (Committee on Human and Environmental Exposure Science in the 21st Century et al., 2012; Escher et al., 2020), but different definitions are also available(Scholz et al., 2021). More recently, Vermeulen et al. (2020) advocated the characterization of the exposome at a scale similar to that of the genome, in order to meet the health challenges faced by this and future generations.
Currently, these visions of the exposome are expected to lead to studies in which (1) exposures are characterized more extensively, for example, using new tools to monitor or indirectly measure them, such as application of large-scale omics approaches, (2) different types of exposures are combined (chemical, physical, biological, social), (3) the links between exposures and effects are better and more systematically characterized, and (4) the sequence of exposure and developmental events over the life-course are taken into consideration. Although these objectives are ambitious and may not all be met in a single study, they have nevertheless inspired (and will continue to inspire) epidemiological study designs leading to improved exposure characterization and association with health outcomes (Li et al., 2019; Tamayo-Uria et al., 2019; Vineis et al., 2017). Yet, some important issues are currently not sufficiently covered, and these include a better assessment of causality and mechanistic insights of the links between complex exposures and effects. Toxicological studies can address these issues using both traditional and innovative tools and can therefore be an integral component of an exposome approach.
This review considers the consequences of the development of the exposome concept on toxicology and the added value of toxicological approaches and findings and their integration in exposome studies. Often these developments in toxicological research have started before or independently of the exposome studies, but they can be better understood and integrated within the concept. We outline here the features that can characterize exposome-inspired toxicology (Figure 1).
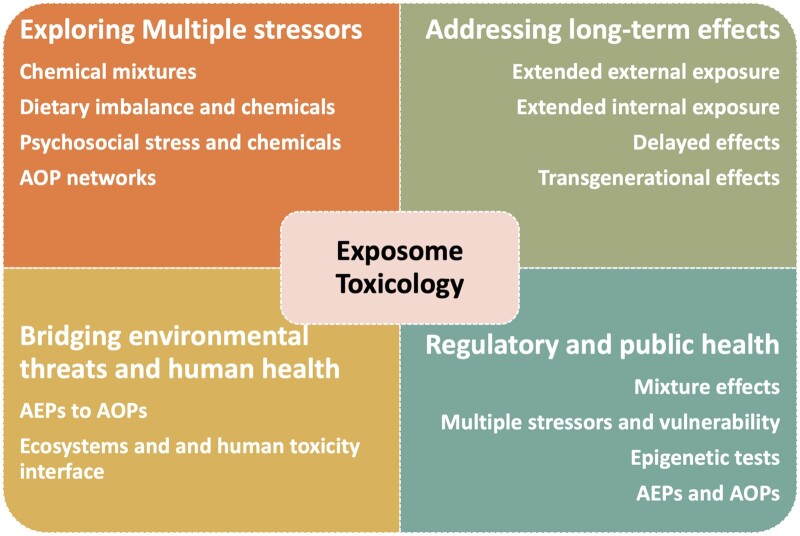
Figure 1.
Major features of exposome toxicology. The exposome concept is essentially multidisciplinary and has influenced several fields. This figure shows the different impact of the exposome concept on toxicology. AEP: aggregated exposure pathways; AOP: adverse outcome pathways.
DEVELOPING APPROACHES INTEGRATING MULTIPLE STRESSORS
Multidisciplinary approaches
A major feature of the exposome approach is to carry out a comprehensive assessment of exposure and to assess the impact of the combination of multiple stressors (Figure 2). This requires a multidisciplinary approach including a comprehensive exposure assessment, epidemiological studies, toxicokinetics, and a variety of computational methods (Manrai et al., 2017; Wu et al., 2020). In some projects, a toxicological approach is also included to support causality and to identify mechanistic pathways. The EU exposome project Health and Environment-wide Associations based on Large population Surveys (HEALS) illustrates such an approach and uses a framework which consists of gathering detailed exposure data through global satellite information and local or personal sensors (Asimina et al., 2018; Loh et al., 2017), environmental and food monitoring (Garí et al., 2013), human biomonitoring (Pino et al., 2017; Steckling et al., 2018), questionnaires, and integrated exposure modeling (Sarigiannis et al., 2016). Individuals included in cohort studies are extensively characterized through omics analyses, ideally including genomics, transcriptomics, proteomics, and metabolomics. This enables large-scale associations between cumulative exposure and effect (for Exposome-Wide Association Studies), and interactions between gene and environment (for Gene–Environment-Wide Association Studies) to be investigated (Steckling et al., 2018). With such a framework or similar ones, it is possible to identify exposure to a variety of stressors and to investigate the interactions between these stressors. A toxicological approach can complement such studies by providing evidence for causality and suggesting possible mechanisms.
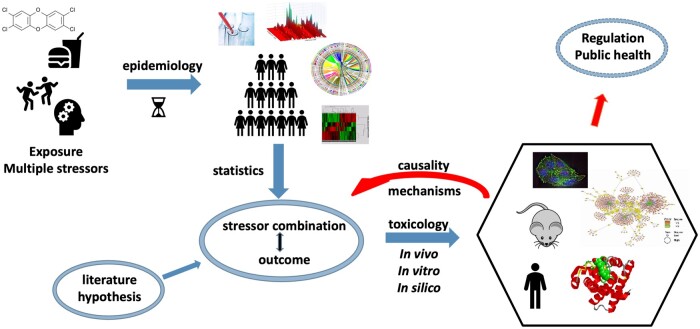
Figure 2.
Multidisciplinary characterization of multiple stressor exposure and impacts. Exposure and epidemiological studies (including molecular epidemiology) can reveal associations between certain combinations of stressors and health outcomes. Based on these data, toxicological studies can analyze the involved mechanism and support causality. These studies can also be triggered by hypothesis put forward based on the scientific literature (eg, involvement of a common receptor for different stressors). Toxicological data together with those of population studies can support regulation and public health decisions.
Using exposome data to inform chemical mixture studies
In many environmental health epidemiological studies, exposure to chemicals has been widely characterized using either targeted biomonitoring approaches or, more recently, untargeted and/or suspect screening approaches (Pourchet et al., 2020; Vermeulen et al., 2020). There are different approaches to study mixture effects of chemicals through their mode of action (Drakvik et al., 2020). For example, if several chemicals display a similar mode of action or target the same outcome, dose addition is the most likely approach to reflect their combined effect (Martin et al., 2021). In addition to mixture composition, another point to consider is the sequential dosing pattern of different chemicals, for example, priming cells/organism with one agent prior to application of another; different exposure possibility to identify realistic mixtures which have been associated to an adverse outcome in humans sequences could lead to different outcomes (Ashauer et al., 2017). Data from epidemiological studies offer the using various statistical tools. Based on such data, these mixtures could be tested experimentally to verify the causal relationship. For example, in the HEALS project, epidemiological studies suggested that exposure to a combination of phthalates and metals during pregnancy was associated with both changes in mitochondrial metabolism and neuromotor deficits in children (Polańska et al., 2011). In functional experiments (cell culture studies), it was shown that this mixture was indeed able to alter the mitochondrial metabolism indicating that this mechanism may be responsible for the neuromotor deficits (Papaioannou et al., 2021). In another elegant study on the Selma birth cohort in Sweden, a mixture of chemicals was statistically associated with neurobehavioral outcomes in children and this was supported in at least two experimental models using cell cultures and Xenopus developmental models (Birgersson et al., 2017; Repouskou et al., 2020).
An exposome approach to explore the interaction of chemical toxicants with other stressors
Comprehensive human exposome studies can reveal interactions between exposure to chemicals and other stressors that can be confirmed using toxicological approaches. The interaction between unbalanced diets, such as high-fat or high-calorie diets, and chemical contaminants has been studied for several years, particularly when metabolic outcomes such as diabetes were investigated (Thayer et al., 2012). High-carbohydrate and/or high-lipid diets can modulate toxic effects of contaminants and can lead to outcomes that are not clearly observed with the chemicals alone (Thayer et al., 2012). Several studies have shown such interactions. For example, a high-fat diet combined with exposure to dioxin leads to a synergistic increase of liver fibrosis and steatosis, associated with increased expression of related biomarkers (Duval et al., 2017). The metabolic effects of low-dose mixtures of contaminants was shown to be dependent on the type of diets in rodents (Naville et al., 2015). In the case of bisphenol A, several developmental outcomes including metabolic diseases were shown to be dependent on the type of diets and it was also shown that epigenetic marker profiles were influenced by the combination of prenatal exposure to bisphenol A and various diets in mice (Kochmanski et al., 2017; Wei et al., 2011). There is ample evidence that dietary components share common molecular targets with chemical toxicants, for example the aryl hydrocarbon receptor (AhR; a xenobiotic receptor) and several members of the nuclear receptors family, for example, the PPAR (Peroxisome Proliferator-Activated Receptor) receptors (Denison and Nagy, 2003; Francis et al., 2003), Keap1/Nrf2 which is influenced by the cellular content in reactive oxygen species or oxidative stress if such content reaches high levels, the PI3K (phosphoinositide-3-kinase)/AKT/mTOR (mechanistic Target Of Rapamycin) pathway which depends on the activities of the insulin receptors, or FOXO proteins whose expression is influenced by dietary compounds (eg, compounds of fish oil) but which could also interact with xenobiotic receptors (eg, CAR) and therefore with xenobiotics (Kodama et al., 2004; Liu et al., 2013; Wan et al., 2021). This could lead to interactions in the activation or inhibition of biological pathways as discussed below. For example, nutrients such as the short-chain fatty acids act as protectors against oxidative and mitochondrial stress (influencing the Keap1/Nrf2 pathway), whereas xenobiotics such as perfluorooctane sulfonate may alter metabolic pathways reducing the expression of the insulin receptor and of members of the PI3K/Akt-mTOR pathway (González-Bosch et al., 2021; Wan et al., 2021). Direct interaction between metabolic regulators (FOXO proteins such as FOXO1) and xenobiotic receptors (CAR) has been shown to regulate both gluconeogenesis and xenobiotic metabolism (Kodama et al., 2004; Konno et al., 2008). Both types of metabolisms share common substrates, such as NADPH and H+, and a cross-regulation of these pathways could help channel the use of those substrates toward the most relevant pathway according to the current pathophysiological or exposure state. This interaction with metabolism could be even more complex: for example, AhR ligands influence tryptophan metabolism which could itself lead to the production of AhR ligands and some dietary components can directly act on xenobiotic metabolizing enzymes (Murray, 2006; Pallotta et al., 2014). The interactions between dietary components and chemical toxicants are diverse, some leading to increased toxic outcomes while others are protective. Clearly, the combinations of various diets and chemical contaminants will have to be taken into account at a larger scale in both human and experimental studies.
In several epidemiological studies, it was shown that psychosocial stress and chemical stress interact particularly when neurodevelopment or neuroendocrine outcomes are explored (Schreier et al., 2015; Tamayo y Ortiz et al., 2017; Vesterinen et al., 2017). In addition, a significant body of literature has been gathered relating air pollution, racial injustice, socioeconomic status and cardiovascular disease, highlighting the role of interactions between multiple stresses in leading to chronic diseases (Fuller et al., 2017; Hajat et al., 2021). Psychosocial stress has been more difficult to explore experimentally, yet there are in vivo models in which such investigation is possible, for example, social defeat models or chronic intermittent stress models (Bouvier et al., 2017; Mach et al., 2008; Wright et al., 2017). Thus, there is already some evidence for toxic outcomes of the combination of psychosocial stress and chemical stress supporting the findings in human studies. With the developing exposome approaches, more data on such interactions will be gathered and it is expected that a major objective of toxicological studies, despite their limitations, will be to increase knowledge on the interactions of psychosocial stress and other types of stress.
Linking the exposome and biological impacts through adverse outcome pathway networks
An adverse outcome pathway (AOP) is a description and organization of the evidence-based succession of multilevel key events (KEs) from a molecular initiating event (MIE) to an adverse outcome (AO). KEs can be shared by different individual AOPs and/or can be connected to several outcomes, thus constituting AOP networks. Although AOPs are stressor agnostic, it is extremely relevant to link stressors to AOP events in order to predict or support the relationship between a stressor and a toxic effect, or support links between up- and down-stream events (key event relationships). When several stressors are considered in the context of an exposome study, it is possible to connect them to AOP events in order to assess the likelihood of converging toxicity and possible interactions. Therefore, the AOP framework is a useful approach to decipher the biological mechanisms of chemicals (Aguayo-Orozco et al., 2019), or to predict potential AOs for priority chemicals lacking toxicological data (Bajard et al., 2019). Methods combining text mining, through artificial intelligence tools and systems biology approaches, have been developed to link chemical stressors to AOP events taking advantage of the available literature and databases (Carvaillo et al., 2019; Jornod et al., 2020, 2021; Rugard et al., 2020). Furthermore, these computational methods can be used to connect any type of stressor to KEs from an AOP, thereby meeting the multiple stressor scope of the exposome. It is highly anticipated that an AO may be triggered by different MIEs (linked to multiple stressors) that could converge into a common KE and result in amplified biological responses, which in some cases are described as effects beyond additivity. This is especially relevant for exposome studies; the assessment of the biological responses as a follow-up of exposure is where AOPs integrate into the exposome, whereas the consideration of endogenous and nonchemical stress as well as mixture effects is where the exposome contributes within the AOP concept (Escher et al., 2017). In line with these considerations, and in order to prioritize the most relevant exposome fraction for health and disease, Chung et al. defined the functional exposome as the totality of biologically active exposures which can be identified through targeted biological tests (Chung et al., 2021). A more general definition of functional exposomics describing the systematic study of exposure–phenotype interaction has been put forward by Price et al. (2021), in line with the definition of functional genomics. Both definitions perfectly illustrate the close links between the exposome and toxicology.
DELINEATING TOXICITY MECHANISMS OF EXPOSOME LIFE-COURSE EVENTS
One of the most difficult tasks in toxicology is to delineate the mechanisms of long-term effects leading to chronic diseases and to find the right models to study them. Long term means years, decades, and perhaps generations (transgenerational effects) and this is a challenge to both mechanistic and regulatory toxicology. It is also important to distinguish the impact of different exposure patterns, in particular effects related to continuous long-term exposure and those related to delayed response following an acute exposure because the mechanisms involved may be quite distinct. Exposome research can reveal such patterns and toxicology should be able to develop a better understanding of the mechanisms involved and provide predictive models and tools that may be valuable in a regulatory perspective.
Toxicological examination of extended exposures captured by the exposome
Long-term effects linked to prolonged exposure, for example, air pollution and smoking or intake of ubiquitous food contaminants, are numerous and have been studied in epidemiological and toxicological studies. Toxicity mechanisms of long-term extended exposures are complex and they actually display paradoxical characteristics that, concerning chemicals, can be related to cumulative direct toxicity, but also to induced adaptive pathways; for example, the metabolism of polyaromatic hydrocarbons by cytochromes P450 monooxygenases is the first step leading to the excretion of these toxicants but these same enzymes also generate reactive intermediates and oxidative stress (Vogel et al., 2020). Thus these metabolic pathways are protective in the short term, however, if they are repeatedly induced or remain chronically activated for an extended period of time, they also lead to toxicity because of the repeated transient production of reactive toxicants (Barouki, 2010). Thus, the same pathway could be adaptive or toxic depending on the time scale considered.
The exposome captures extended exposure from internal compartments
Long-term effects could also be due to the internal persistence of chemicals. This is the case of persistent organic pollutants (POPs) which are poorly metabolized and eliminated and accumulate in the adipose tissue, which in turn becomes an internal source of low-level prolonged exposure (La Merrill et al., 2013). This tissue has a paradoxical effect on POPs exposure: by storing these pollutants, it protects other sensitive organs such as the brain or the gonads from acute exposure to POPs, yet, in the long run, it constitutes an internal source of chronic exposure (Joffin et al., 2018). This pattern is also relevant for other persistent chemicals that are poorly stored in fatty tissues such as per- and polyfluoroalkyl substances and metals (McGowan, 1996; Pizzurro et al., 2019).
Mechanisms of delayed toxic outcomes elicited by the exposome
There is growing interest in toxicity mechanisms, whereby a short exposure to a stressor leads to health manifestations many years later. This is obviously the case for genotoxic compounds (which will not be discussed further here); however, a similar pattern is also observed with nongenotoxic substances. Both experimental and epidemiological studies have shown that exposure to certain nongenotoxic chemicals (particularly endocrine disruptors) during certain stages of development was associated with an increased risk of disease later in life (Barouki et al., 2012; Grandjean et al., 2015). Exposure can be limited in time, but it can have an impact particularly when the target organism is in a state of vulnerability. Developmental vulnerability is believed to be due to the remodeling of tissues and organs during certain stages of development and to limited defense capabilities. Although several mechanisms are possible, the most likely one for nongenotoxic substances is epigenetic regulation such as DNA methylation, modifications of histones, and certain noncoding RNAs (Walker, 2016). Such regulations are influenced by environmental conditions and are inheritable, at least at the somatic level (Skinner, 2011). Therefore, such alterations can persist for a long time and could lead to subtle changes in the physiology of different organs, which may increase the risk of developing the disease later in life. Despite considerable recent progress, we still need more data linking epigenetic regulations to health effects and more mechanistic insights. We also need to strengthen the evidence showing that developmental epigenetic remodeling is a major cause of vulnerability and provide more evidence to support epigenetic toxicity (Barouki et al., 2018; Chung and Herceg, 2020). Additional research is also needed to investigate the role of the microbiome in long-term toxicity of chemicals (Barouki et al., 2018; Liu et al., 2020).
The exposome and transgenerational effects
Concomitantly with the development of the exposome concept taking into consideration life-long exposures, a new field emerged in experimental toxicology highlighting possible transgenerational effects of ancestral exposures to chemicals, referred to as generational toxicology (Skinner, 2011). Although more evidence in humans is still required, mechanistic evidence is currently being produced in experimental models. Indeed, although genomic changes in germ cells during the exposures cannot be excluded, epigenetic changes in germ cells (including male and female germ cells) have been demonstrated and may account for transmission of effects across generations (Hanson and Skinner, 2016).
The different mechanisms described above are not exclusive. For example, in the case of smoking, there is clear evidence for the impact of continuous exposure and genotoxicity, for long-term storage and for lasting changes in DNA methylation likely altering relevant biological pathways (DeMarini, 2004; Sikdar et al., 2019). Because the exposome integrates multiple life-course exposures, toxicological studies should not be limited to a series of separate exposure/stress components, but rather cover a comprehensive set of biological pathways involved in long-term effects, for example, multiple omics with a particular focus on epigenomics which can integrate molecular impacts of multiple stressors.
BRIDGING ENVIRONMENTAL INSULTS, HEALTH OUTCOMES, AND MECHANISTIC APPROACHES
Exposome-based network integration of exposures and effects using AEPs and AOPs
Targeted and untargeted approaches in epidemiological studies can provide realistic data on the chemical exposures faced by the human population (Pourchet et al., 2020). The concept of aggregate exposure pathways (AEPs) provides an environmental correlate of the AOP and there have been proposals to connect these two frameworks (Price et al., 2020). AEP models also integrate the aspect of temporality during individual ontogenies (windows of high susceptibility such as pre-natal early development) and consider susceptibility among different populations. Connecting AEPs and AOPs is a relevant approach to connect realistic exposures to outcomes in a risk assessment framework (Escher et al., 2017; Hines et al., 2018). These links can be supported using biokinetic models and the toxicological framework of absorption, distribution, metabolism, excretion (Figure 3). Connecting exposure with health outcome using large-scale metabolomics has been carried out for a variety of drugs (Liu et al., 2020), nutrients (Posma et al., 2020), and for environmental xenobiotics (Dennis et al., 2016; Vermeulen et al., 2020). A single technological approach based on coupling gas chromatography and/or Liquid Chromatography (LC) to high-resolution mass spectrometry (HRMS) allows simultaneous measurement of xenobiotic toxicants, representing exposure, and endogenous metabolites that may represent the response of the organism to such exposure. An additional relevant approach is to combine nontargeted chemical analyses with bioassays to delineate complex mixture effects (Escher et al., 2020).
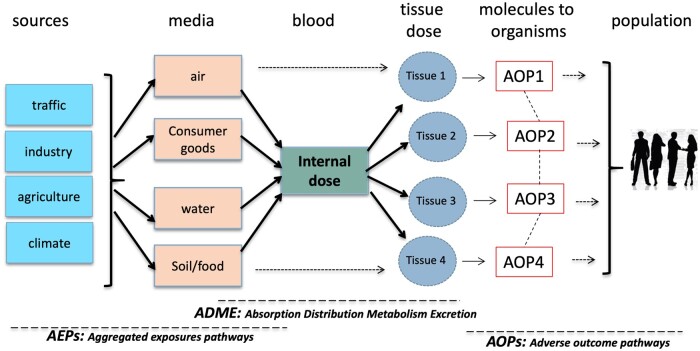
Figure 3.
Linking exposures and effects: the triple A framework (AEP, absorption, distribution, metabolism, excretion, AOP). A major objective of environment and health studies is to link exposure and effect. The figure presents a framework connecting aggregated exposure pathways and AOP. The connection is at least partially mediated by the internal dose of contaminants which is governed by the ADME framework.
The exposome allows evaluation of the ecosystem–human–toxicology interface
The eco-exposome concept, together with corresponding global visions (Calistri et al., 2013; Whitmee et al., 2015), has put forward a more integrated approach where human and ecosystems toxicology meet in many different instances (Gao, 2021). Although classical ecotoxicological approaches still focus on apical endpoints, such as survival, growth, or reproduction impairment, in recent years, the advance and maturity attained by mechanistic approaches, such as omics technologies, computational toxicology, in silico, QSAR, and RA methods, have opened new research horizons at different levels of biological organization. Correspondingly, advanced (quantitative) AOPs, and their networks are being developed and utilized in the context of the environmental risk assessment (Kramer et al., 2011; Perkins et al., 2019). AOPs support Integrated Approaches to Testing and Assessment of environmental toxicants (Tollefsen et al., 2014), and allow using of mechanistic information across different species (Leist et al., 2017; Rivetti et al., 2020). Recently, generalized—species agnostic—bioenergetics AOPs have been proposed that mechanistically link the energy availability and its allocation in the organisms with the overall growth and fitness predicting their success of reproduction (Goodchild et al., 2019). The integration of environmental exposures mechanistic toxicology including AOPs, and ecosystem services with human health and societal impacts, resulted in some promising applications such as using the mechanistic effect models for environmental risk assessment (Hommen et al., 2016) and exploitation of AOPs within the climate change context (Hooper et al., 2013).
A more detailed assessment of the impact of the exposome can be achieved using recently developed technologies such as single-cell and spatial phenotyping. These approaches are vital to understand cellular heterogeneity and the variability in distributions of exposure agents and localized/cell-specific effect and response. The finer resolution of analysis can provide a greater understanding of uptake and metabolism (Pedro and Rudewicz, 2020), increase the spatial and temporal resolution of toxicity pathway/PBPK models (Thurber et al., 2013; Zhang et al., 2019), and so could aid the organization of AOP networks and integration of toxicokinetics within quantitative AOP frameworks. There are several examples illustrating the relevance of such approaches. Mass spectrometry imaging of the kidney following bisphenol S (BPS) exposure demonstrated spatially organized, concentration-dependent BPS-induced lipid changes (Zhao et al., 2018). Greater BPS accumulation and disturbance of lipid distribution was observed in the renal cortex, compared with the renal medulla and pelvis. Furthermore, 2 lipid-localized substructures were identified as indicative of BPS-induced tissue heterogeneity with potential as candidate markers for the early detection of nephrotoxicity. Single cell transcriptomics following exposure to di(2-ethylhexyl) phthalate (DEHP) during maternal perinatal and postnatal period showed that DEHP caused decreased expression of genes essential for primordial follicle (PF) assembly (Wang et al., 2021). Moreover, single-cell analysis provided more detail about the mechanisms of reproductive toxicity than possible to parse in multicellular analysis, indicating that germline cyst breakdown and PF formation may be caused by DEHP-induced oxidative stress in germ cells.
CONCLUSIONS AND FUTURE DIRECTIONS
Exposomics works at the interface of exposure science and toxicology. By providing information on internal dose along with the corresponding relationships with biological pathways, exposomics serves as a scientific bridge between the external exposures that are subject to regulations and the internal effects that are linked to health outcomes. For example, if a particular chemical was suspected of increased risk of dementia exposure, scientists would measure that chemical in the environment and toxicologists would test that chemical in the laboratory. The exposome scientist provides information on internal dose of the chemical, including potential bioactive metabolites, and on the biological pathways or systems that are impacted by the chemical. Exposomics connects the dots which could improve characterization of the causal pathways and strengthen translation to policy. Furthermore, because exposomics takes an agnostic and unbiased approach it is harder for a particular industry to claim that they are being unfairly targeted. Exposomics lets the science decide which chemical should be the focus of policy intervention.
Linking exposomics to toxicology also addresses some highly relevant topics such as the impacts of climate change and vulnerabilities. Alterations in environmental conditions due to climate change must be translated into biological signals to directly impact health outcomes. Exposomic approaches, such as HRMS, offer an opportunity to measure changes in environmental constituents and biological perturbations that may occur from an altered climate. The increased susceptibility to disease in vulnerable populations will also have molecular underpinnings that reveal the disease-associated biological consequences of disparate environmental exposures.
Exposomic-based approaches should enhance our ability to predict future disease vulnerabilities. Similar to how polygenic risk scores aim to predict increased disease incidence, an analogous exposomic-based score could be used independently or in concert with the polygenic risk scores to better predict disease outcomes(He et al., 2021, 2019).
There are, however, some significant challenges and limitations to be overcome in order to fully benefit from exposome-inspired toxicology. Indeed, although the combination of stressors can be studied experimentally, the time dimension is often more difficult to address. Intermediate biomarkers and mechanistic approaches can obviously help but this remains to be fully developed. Furthermore, interaction between multiple stressors can be studied in epidemiology but only with large cohorts, so the comparison with toxicological approaches may prove to be difficult. Another challenge will be our ability to fully develop the AOP concept, and in particular the quantitative AOPs.
The holistic approaches represented by the exposome concept will have significant impact on toxicology but also on public health measures and regulatory decisions. The major outcomes that are likely to result from these approaches are presented below.
Current regulatory toxicology is founded on the one product one regulation principle. This is likely to change with the developing studies on mixture effects. The regulatory uptake of these studies will occur in several stages and could start with the application of a mixture assessment factor, or use of a default approach such as dose addition with equivalency factors (Bopp et al., 2018; Drakvik et al., 2020).
The exposome concept highlights the importance of multiple exposures and the interaction between multiple stressors, as seen earlier for diet and chemicals. The same is also valid for psychosocial stress and chemicals. Thus, the context of exposure to a chemical is highly relevant. In fact, this is related to vulnerability, for example, individuals with poor social conditions or unbalanced diets are at a higher risk for chemical toxicity. In the long run toxicity tests could be revisited in order to better take into account multi-stress conditions.
Exposome studies also highlight long-term effects. These effects are particularly challenging for regulatory toxicology. Epigenetic mechanisms are among the most likely mechanisms to account for these effects. Furthermore, targeted or large-scale epigenetic tests can be developed and possibly validated and used as regulatory tests. It would be too speculative at this stage to define the actual testing framework, but at least work in this direction should be encouraged.
In line with the exposome concept, the uptake of AEPs and AOPs by environmental and public health regulations should be further supported.
Exposure sciences and epidemiology have embraced some of the approaches developed in light of the exposome concept. During the last years, toxicology has also benefited from new technologies, such as omics and computational methods in addition to new models systems. The exposome provides a framework for further significant development of toxicology taking into account the impact of different types of stressors and their interactions, the importance of long-term delayed effects, as well as closer coordination between human and environmental toxicology and incorporation of ideas and tools put forward by holistic visions (Figure 1). What toxicology can bring to exposome characterization is a needed framework for mechanistic understanding and regulatory outcomes in risk assessment.
FUNDING
U.S. National Institutes of Health (U2C ES030163 to G.W.M.); Czech Operational Programme Research, Development and Education—Project Postdoc@MUNI (CZ.02.2.69/0.0/0.0/16_027/0008360); MSCAfellow4@MUNI (CZ.02.2.69/0.0/0.0/20_079/0017045 to E.J.P.); Inserm and Université de Paris (unit 1124 to R.B., K.A., X.C.); Institut National de la Santé et de la Recherche Médicale (unit 1124).
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Robert Barouki, Inserm UMR S-1124, Université de Paris, T3S, Paris F-75006, France; Service de Biochimie métabolomique et protéomique, Hôpital Necker enfants malades, AP-HP, Paris, France.
Karine Audouze, Inserm UMR S-1124, Université de Paris, T3S, Paris F-75006, France.
Christel Becker, Inserm UMR S-1124, Université de Paris, T3S, Paris F-75006, France.
Ludek Blaha, RECETOX, Faculty of Science, Masaryk University, Brno 60200, Czech Republic.
Xavier Coumoul, Inserm UMR S-1124, Université de Paris, T3S, Paris F-75006, France.
Spyros Karakitsios, Center for Interdisciplinary Research and Innovation, HERACLES Research Center on the Exposome and Health, Aristotle University of Thessaloniki, Thessaloniki 57001, Greece; Enve.X, Thessaloniki 55133, Greece.
Jana Klanova, RECETOX, Faculty of Science, Masaryk University, Brno 60200, Czech Republic.
Gary W Miller, Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, New York, USA.
Elliott J Price, RECETOX, Faculty of Science, Masaryk University, Brno 60200, Czech Republic; Faculty of Sports Studies, Masaryk University, Brno 62500, Czech Republic.
Denis Sarigiannis, Center for Interdisciplinary Research and Innovation, HERACLES Research Center on the Exposome and Health, Aristotle University of Thessaloniki, Thessaloniki 57001, Greece; Enve.X, Thessaloniki 55133, Greece.
REFERENCES
- Aguayo-Orozco A., Audouze K., Siggaard T., Barouki R., Brunak S., Taboureau O. (2019). sAOP: Linking chemical stressors to adverse outcomes pathway networks. Bioinforma. Oxf. Engl 35, 5391–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashauer R., O'Connor I., Escher B. I. (2017). Toxic mixtures in time—The sequence makes the poison. Environ. Sci. Technol. 51, 3084–3092. [DOI] [PubMed] [Google Scholar]
- Asimina S., Chapizanis D., Karakitsios S., Kontoroupis P., Asimakopoulos D. N., Maggos T., Sarigiannis D. (2018). Assessing and enhancing the utility of low-cost activity and location sensors for exposure studies. Environ. Monit. Assess. 190, 155. [DOI] [PubMed] [Google Scholar]
- Athersuch T. J., Keun H. C. (2015). Metabolic profiling in human exposome studies. Mutagenesis 30, 755–762. [DOI] [PubMed] [Google Scholar]
- Bajard L., Melymuk L., Blaha L. (2019). Prioritization of hazards of novel flame retardants using the mechanistic toxicology information from ToxCast and adverse outcome pathways. Environ. Sci. Eur. 31, Article number 14. [Google Scholar]
- Barouki R. (2010). Linking long-term toxicity of xeno-chemicals with short-term biological adaptation. Biochimie 92, 1222–1226. [DOI] [PubMed] [Google Scholar]
- Barouki R., Audouze K., Coumoul X., Demenais F., Gauguier D. (2018). Integration of the human exposome with the human genome to advance medicine. Biochimie 152, 155–158. [DOI] [PubMed] [Google Scholar]
- Barouki R., Gluckman P. D., Grandjean P., Hanson M., Heindel J. J. (2012). Developmental origins of non-communicable disease: Implications for research and public health. Environ. Health Glob. Access Sci. Source 11, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouki R., Melén E., Herceg Z., Beckers J., Chen J., Karagas M., Puga A., Xia Y., Chadwick L., Yan W., et al. (2018). Epigenetics as a mechanism linking developmental exposures to long-term toxicity. Environ. Int. 114, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgersson L., Borbély G., Caporale N., Germain P.-L., Leemans M., Rendel F., D’Agostino G. A., Bressan R. B., Cavallo F., Chorev N. E., et al. (2017). From cohorts to molecules: Adverse impacts of endocrine disrupting mixtures. Mol. Biol. PREPRINT. [DOI] [PubMed] [Google Scholar]
- Bopp S. K., Barouki R., Brack W., Dalla Costa S., Dorne J.-L. C. M., Drakvik P. E., Faust M., Karjalainen T. K., Kephalopoulos S., van Klaveren J., et al. (2018). Current EU research activities on combined exposure to multiple chemicals. Environ. Int. 120, 544–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier E., Brouillard F., Molet J., Claverie D., Cabungcal J.-H., Cresto N., Doligez N., Rivat C., Do K. Q., Bernard C., et al. (2017). Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol. Psychiatry 22, 1701–1713. [DOI] [PubMed] [Google Scholar]
- Buck Louis G. M., Smarr M. M., Patel C. J. (2017). The exposome research paradigm: An opportunity to understand the environmental basis for human health and disease. Curr. Environ. Health Rep. 4, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calistri P., Iannetti S., L. Danzetta M., Narcisi V., Cito F., Di Sabatino D., Bruno R., Sauro F., Atzeni M., Carvelli A., et al. (2013). The components of ‘one world - one health’ approach. Transbound. Emerg. Dis. 60, 4–13. [DOI] [PubMed] [Google Scholar]
- Carvaillo J.-C., Barouki R., Coumoul X., Audouze K. (2019). Linking bisphenol S to adverse outcome pathways using a combined text mining and systems biology approach. Environ. Health Perspect. 127, 47005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung F. F.-L., Herceg Z. (2020). The promises and challenges of toxico-epigenomics: Environmental chemicals and their impacts on the epigenome. Environ. Health Perspect. 128, 15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M. K., Rappaport S. M., Wheelock C. E., Nguyen V. K., van der Meer T. P., Miller G. W., Vermeulen R., Patel C. J. (2021). Utilizing a biology-driven approach to map the exposome in health and disease: An essential investment to drive the next generation of environmental discovery. Environ. Health Perspect. 129, 85001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Human and Environmental Exposure Science in the 21st Century, Board on Environmental Studies and Toxicology, Division on Earth and Life Studies, National Research Council. (2012). Exposure Science in the 21st Century: A Vision and a Strategy. National Academies Press (US; ), Washington, DC. [PubMed] [Google Scholar]
- DeMarini D. M. (2004). Genotoxicity of tobacco smoke and tobacco smoke condensate: A review. Mutat. Res. 567, 447–474. [DOI] [PubMed] [Google Scholar]
- Denison M. S., Nagy S. R. (2003). Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 43, 309–334. [DOI] [PubMed] [Google Scholar]
- Dennis K. K., Auerbach S. S., Balshaw D. M., Cui Y., Fallin M. D., Smith M. T., Spira A., Sumner S., Miller G. W. (2016). The importance of the biological impact of exposure to the concept of the exposome. Environ. Health Perspect. 124, 1504–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakvik E., Altenburger R., Aoki Y., Backhaus T., Bahadori T., Barouki R., Brack W., Cronin M. T. D., Demeneix B., Hougaard Bennekou S., et al. (2020). Statement on advancing the assessment of chemical mixtures and their risks for human health and the environment. Environ. Int. 134, 105267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval C., Teixeira-Clerc F., Leblanc A. F., Touch S., Emond C., Guerre-Millo M., Lotersztajn S., Barouki R., Aggerbeck M., Coumoul X. (2017). Chronic exposure to low doses of dioxin promotes liver fibrosis development in the C57BL/6J diet-induced obesity mouse model. Environ. Health Perspect. 125, 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher B. I., Hackermüller J., Polte T., Scholz S., Aigner A., Altenburger R., Böhme A., Bopp S. K., Brack W., Busch W., et al. (2017). From the exposome to mechanistic understanding of chemical-induced adverse effects. Environ. Int. 99, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher B. I., Stapleton H. M., Schymanski E. L. (2020). Tracking complex mixtures of chemicals in our changing environment. Science 367, 388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G. A., Fayard E., Picard F., Auwerx J. (2003). Nuclear receptors and the control of metabolism. Annu. Rev. Physiol. 65, 261–311. [DOI] [PubMed] [Google Scholar]
- Fuller C. H., Feeser K. R., Sarnat J. A., O'Neill M. S. (2017). Air pollution, cardiovascular endpoints and susceptibility by stress and material resources: A systematic review of the evidence. Environ. Health 16, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P. (2021). The exposome in the era of one health. Environ. Sci. Technol. 55, 2790–2799. [DOI] [PubMed] [Google Scholar]
- Garí M., Grimalt J. O., Torrent M., Sunyer J. (2013). Influence of socio-demographic and diet determinants on the levels of mercury in preschool children from a Mediterranean island. Environ. Pollut. 182, 291–298. [DOI] [PubMed] [Google Scholar]
- González-Bosch C., Boorman E., Zunszain P. A., Mann G. E. (2021). Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 47, 102165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild C. G., Simpson A. M., Minghetti M., DuRant S. E. (2019). Bioenergetics-adverse outcome pathway: Linking organismal and suborganismal energetic endpoints to adverse outcomes. Environ. Toxicol. Chem. 38, 27–45. [DOI] [PubMed] [Google Scholar]
- Grandjean P., Barouki R., Bellinger D. C., Casteleyn L., Chadwick L. H., Cordier S., Etzel R. A., Gray K. A., Ha E.-H., Junien C., et al. (2015). Life-long implications of developmental exposure to environmental stressors: New perspectives. Endocrinology 156, 3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A., MacLehose R. F., Rosofsky A., Walker K. D., Clougherty J. E. (2021). Confounding by socioeconomic status in epidemiological studies of air pollution and health: Challenges and opportunities. Environ. Health Perspect. 129, 65001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. A., Skinner M. K. (2016). Developmental origins of epigenetic transgenerational inheritance. Environ. Epigenetics 2, dvw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung T. (2009). Toxicology for the twenty-first century. Nature 460, 208–212. [DOI] [PubMed] [Google Scholar]
- He Y., Lakhani C. M., Manrai A. K., Patel C. J. (2019). Poly-exposure and poly-genomic scores implicate prominent roles of non-genetic and demographic factors in four common diseases in the UK. Bioinformatics. PREPRINT. [Google Scholar]
- He Y., Lakhani C. M., Rasooly D., Manrai A. K., Tzoulaki I., Patel C. J. (2021). Comparisons of polyexposure, polygenic, and clinical risk scores in risk prediction of type 2 diabetes. Diabetes Care 44, 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines D. E., Edwards S. W., Conolly R. B., Jarabek A. M. (2018). A case study application of the aggregate exposure pathway (AEP) and adverse outcome pathway (AOP) frameworks to facilitate the integration of human health and ecological end points for cumulative risk assessment (CRA). Environ. Sci. Technol. 52, 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommen U., Forbes V., Grimm V., Preuss T. G., Thorbek P., Ducrot V. (2016). How to use mechanistic effect models in environmental risk assessment of pesticides: Case studies and recommendations from the SETAC workshop MODELINK: MODELINK workshop summary. Integr. Environ. Assess. Manage. 12, 21–31. [DOI] [PubMed] [Google Scholar]
- Hooper M. J., Ankley G. T., Cristol D. A., Maryoung L. A., Noyes P. D., Pinkerton K. E. (2013). Interactions between chemical and climate stressors: A role for mechanistic toxicology in assessing climate change risks. Environ. Toxicol. Chem. 32, 32–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffin N., Noirez P., Antignac J.-P., Kim M.-J., Marchand P., Falabregue M., Le Bizec B., Forest C., Emond C., Barouki R., et al. (2018). Release and toxicity of adipose tissue-stored TCDD: Direct evidence from a xenografted fat model. Environ. Int. 121, 1113–1120. [DOI] [PubMed] [Google Scholar]
- Jornod F., Jaylet T., Blaha L., Sarigiannis D., Tamisier L., Audouze K. (2021). AOP-helpFinder webserver: A tool for comprehensive analysis of the literature to support adverse outcome pathways development. Bioinforma. Oxf. Engl btab750. 10.1093/bioinformatics/btab750. Accessed May 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jornod F., Rugard M., Tamisier L., Coumoul X., Andersen H. R., Barouki R., Audouze K. (2020). AOP4EUpest: Mapping of pesticides in adverse outcome pathways using a text mining tool. Bioinformatics 36, 4379–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochmanski J., Marchlewicz E. H., Savidge M., Montrose L., Faulk C., Dolinoy D. C. (2017). Longitudinal effects of developmental bisphenol A and variable diet exposures on epigenetic drift in mice. Reprod. Toxicol. Elmsford N. 68, 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S., Koike C., Negishi M., Yamamoto Y. (2004). Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol. Cell. Biol. 24, 7931–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsbak K., Hadrup N., Audouze K., Vinggaard A. M. (2014). Applicability of computational systems biology in toxicology. Basic Clin. Pharmacol. Toxicol. 115, 45–49. [DOI] [PubMed] [Google Scholar]
- Konno Y., Negishi M., Kodama S. (2008). The roles of nuclear receptors CAR and PXR in hepatic energy metabolism. Drug Metab. Pharmacokinet. 23, 8–13. [DOI] [PubMed] [Google Scholar]
- Kramer V. J., Etterson M. A., Hecker M., Murphy C. A., Roesijadi G., Spade D. J., Spromberg J. A., Wang M., Ankley G. T. (2011). Adverse outcome pathways and ecological risk assessment: Bridging to population-level effects. Environ. Toxicol. Chem. 30, 64–76. [DOI] [PubMed] [Google Scholar]
- Krewski D., Andersen M. E., Tyshenko M. G., Krishnan K., Hartung T., Boekelheide K., Wambaugh J. F., Jones D., Whelan M., Thomas R., et al. (2020). Toxicity testing in the 21st century: Progress in the past decade and future perspectives. Arch. Toxicol. 94, 1–58. [DOI] [PubMed] [Google Scholar]
- La Merrill M., Emond C., Kim M. J., Antignac J.-P., Le Bizec B., Clément K., Birnbaum L. S., Barouki R. (2013). Toxicological function of adipose tissue: Focus on persistent organic pollutants. Environ. Health Perspect. 121, 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist M., Ghallab A., Graepel R., Marchan R., Hassan R., Bennekou S. H., Limonciel A., Vinken M., Schildknecht S., Waldmann T., et al. (2017). Adverse outcome pathways: Opportunities, limitations and open questions. Arch. Toxicol. 91, 3477–3505. [DOI] [PubMed] [Google Scholar]
- Li N., Friedrich R., Maesano C. N., Medda E., Brescianini S., Stazi M. A., Sabel C. E., Sarigiannis D., Annesi-Maesano I. (2019). Lifelong exposure to multiple stressors through different environmental pathways for European populations. Environ. Res. 179, 108744. [DOI] [PubMed] [Google Scholar]
- Liu J., Lahousse L., Nivard M. G., Bot M., Chen L., van Klinken J. B., Thesing C. S., Beekman M., van den Akker E. B., Slieker R. C., et al. (2020). Integration of epidemiologic, pharmacologic, genetic and gut microbiome data in a drug–metabolite atlas. Nat. Med. 26, 110–117. [DOI] [PubMed] [Google Scholar]
- Liu Y., Chen F., Odle J., Lin X., Zhu H., Shi H., Hou Y., Yin J. (2013). Fish oil increases muscle protein mass and modulates Akt/FOXO, TLR4, and NOD signaling in weanling piglets after lipopolysaccharide challenge. J. Nutr. 143, 1331–1339. [DOI] [PubMed] [Google Scholar]
- Loh M., Sarigiannis D., Gotti A., Karakitsios S., Pronk A., Kuijpers E., Annesi-Maesano I., Baiz N., Madureira J., Oliveira Fernandes E., et al. (2017). How sensors might help define the external exposome. Int. J. Environ. Res. Public. Health 14, 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach M., Grubbs R. D., Price W. A., Nagaoka M., Dubovický M., Lucot J. B. (2008). Delayed behavioral and endocrine effects of sarin and stress exposure in mice. J. Appl. Toxicol. 28, 132–139. [DOI] [PubMed] [Google Scholar]
- Manrai A. K., Cui Y., Bushel P. R., Hall M., Karakitsios S., Mattingly C. J., Ritchie M., Schmitt C., Sarigiannis D. A., Thomas D. C., et al. (2017). Informatics and data analytics to support exposome-based discovery for public health. Annu. Rev. Public Health 38, 279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin O., Scholze M., Ermler S., McPhie J., Bopp S. K., Kienzler A., Parissis N., Kortenkamp A. (2021). Ten years of research on synergisms and antagonisms in chemical mixtures: A systematic review and quantitative reappraisal of mixture studies. Environ. Int. 146, 106206. [DOI] [PubMed] [Google Scholar]
- McGowan J. A. (1996). Bone: Target and source of environmental pollutant exposure. Otolaryngol. Head Neck Surg. 114, 220–223. [DOI] [PubMed] [Google Scholar]
- McHale C. M., Osborne G., Morello-Frosch R., Salmon A. G., Sandy M. S., Solomon G., Zhang L., Smith M. T., Zeise L. (2018). Assessing health risks from multiple environmental stressors: Moving from G×E to I×E. Mutat. Res. 775, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. W., Jones D. P. (2014). The nature of nurture: Refining the definition of the exposome. Toxicol. Sci. 137, 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. (2006). Altered CYP expression and function in response to dietary factors: Potential roles in disease pathogenesis. Curr. Drug Metab. 7, 67–81. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.). (Ed.) (2007). Toxicity Testing in the 21st Century: A Vision and a Strategy. National Academies Press, Washington, DC. [Google Scholar]
- Naville D., Labaronne E., Vega N., Pinteur C., Canet-Soulas E., Vidal H., Le Magueresse-Battistoni B. (2015). Metabolic outcome of female mice exposed to a mixture of low-dose pollutants in a diet-induced obesity model. PLoS One 10, e0124015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiecki M. M., Walker D. I., Vermeulen R., Chadeau-Hyam M., Jones D. P., Miller G. W. (2019). The exposome: Molecules to populations. Annu. Rev. Pharmacol. Toxicol. 59, 107–127. [DOI] [PubMed] [Google Scholar]
- Pallotta M. T., Fallarino F., Matino D., Macchiarulo A., Orabona C. (2014). AhR-mediated, non-genomic modulation of IDO1 function. Front. Immunol. 5, 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou N., Distel E., de Oliveira E., Gabriel C., Frydas I. S., Anesti O., Attignon E. A., Odena A., Díaz R., Aggerbeck Μ., et al. (2021). Multi-omics analysis reveals that co-exposure to phthalates and metals disturbs urea cycle and choline metabolism. Environ. Res. 192, 110041. [DOI] [PubMed] [Google Scholar]
- Pedro L., Rudewicz P. J. (2020). Analysis of live single cells by confocal microscopy and high-resolution mass spectrometry to study drug uptake, metabolism, and drug-induced phospholipidosis. Anal. Chem. 92, 16005–16015. [DOI] [PubMed] [Google Scholar]
- Perkins E. J., Ashauer R., Burgoon L., Conolly R., Landesmann B., Mackay C., Murphy C. A., Pollesch N., Wheeler J. R., Zupanic A., et al. (2019). Building and applying quantitative adverse outcome pathway models for chemical hazard and risk assessment. Environ. Toxicol. Chem. 38, 1850–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino A., Chiarotti F., Calamandrei G., Gotti A., Karakitsios S., Handakas E., Bocca B., Sarigiannis D., Alimonti A. (2017). Human biomonitoring data analysis for metals in an Italian adolescents cohort: An exposome approach. Environ. Res. 159, 344–354. [DOI] [PubMed] [Google Scholar]
- Pizzurro D. M., Seeley M., Kerper L. E., Beck B. D. (2019). Interspecies differences in perfluoroalkyl substances (PFAS) toxicokinetics and application to health-based criteria. Regul. Toxicol. Pharmacol. 106, 239–250. [DOI] [PubMed] [Google Scholar]
- Polańska K., Hanke W., Jurewicz J., Sobala W., Madsen C., Nafstad P., Magnus P. (2011). Polish mother and child cohort study (REPRO_PL)–methodology of follow-up of the children. Int. J. Occup. Med. Environ. Health 24, 391–398. [DOI] [PubMed] [Google Scholar]
- Posma J. M., Garcia-Perez I., Frost G., Aljuraiban G. S., Chan Q., Van Horn L., Daviglus M., Stamler J., Holmes E., Elliott P., et al. (2020). Nutriome–metabolome relationships provide insights into dietary intake and metabolism. Nat. Food 1, 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourchet M., Debrauwer L., Klanova J., Price E. J., Covaci A., Caballero-Casero N., Oberacher H., Lamoree M., Damont A., Fenaille F., et al. (2020). Suspect and non-targeted screening of chemicals of emerging concern for human biomonitoring, environmental health studies and support to risk assessment: From promises to challenges and harmonisation issues. Environ. Int. 139, 105545. [DOI] [PubMed] [Google Scholar]
- Price E. J., Vitale C. M., Miller G. W., David A., Barouki R., Audouze K., Walker D. I., Antignac J.-P., Coumoul X., Bessonneau V., et al. (2021). Merging the exposome in an integrated framework for “omic” sciences. 10.5281/ZENODO.5363305. Accessed March 24, 2020. [DOI] [PMC free article] [PubMed]
- Price P. S., Jarabek A. M., Burgoon L. D. (2020). Organizing mechanism-related information on chemical interactions using a framework based on the aggregate exposure and adverse outcome pathways. Environ. Int. 138, 105673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport S. M. (2018). Redefining environmental exposure for disease etiology. NPJ Syst. Biol. Appl. 4, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport S. M., Smith M. T. (2010). Epidemiology. Environment and disease risks. Science 330, 460–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repouskou A., Papadopoulou A.-K., Panagiotidou E., Trichas P., Lindh C., Bergman Å., Gennings C., Bornehag C.-G., Rüegg J., Kitraki E., et al. (2020). Long term transcriptional and behavioral effects in mice developmentally exposed to a mixture of endocrine disruptors associated with delayed human neurodevelopment. Sci. Rep. 10, 9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivetti C., Allen T. E. H., Brown J. B., Butler E., Carmichael P. L., Colbourne J. K., Dent M., Falciani F., Gunnarsson L., Gutsell S., et al. (2020). Vision of a near future: Bridging the human health–environment divide. Toward an integrated strategy to understand mechanisms across species for chemical safety assessment. Toxicol. In Vitro 62, 104692. [DOI] [PubMed] [Google Scholar]
- Rugard M., Coumoul X., Carvaillo J.-C., Barouki R., Audouze K. (2020). Deciphering adverse outcome pathway network linked to bisphenol f using text mining and systems toxicology approaches. Toxicol. Sci. 173, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarigiannis D. A. (2017). Assessing the impact of hazardous waste on children’s health: The exposome paradigm. Environ. Res. 158, 531–541. [DOI] [PubMed] [Google Scholar]
- Sarigiannis D. A., Karakitsios S. P., Handakas E., Simou K., Solomou E., Gotti A. (2016). Integrated exposure and risk characterization of bisphenol-A in Europe. Food Chem. Toxicol. 98, 134–147. [DOI] [PubMed] [Google Scholar]
- Scholz S., Nichols J. W., Escher B. I., Ankley G. T., Altenburger R., Blackwell B., Brack W., Burkhard L., Collette T. W., Doering J. A., et al. (2021). The Eco‐Exposome concept: Supporting an integrated assessment of mixtures of environmental chemicals. Environ. Toxicol. Chem. 10.1002/etc.5242. Accessed October 29, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier H. M., Hsu H.-H., Amarasiriwardena C., Coull B. A., Schnaas L., Téllez-Rojo M. M., Tamayo y Ortiz M., Wright R. J., Wright R. O. (2015). Mercury and psychosocial stress exposure interact to predict maternal diurnal cortisol during pregnancy. Environ. Health 14, 10.1186/s12940-015-0016-9. Accessed March 27, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikdar S., Joehanes R., Joubert B. R., Xu C.-J., Vives-Usano M., Rezwan F. I., Felix J. F., Ward J. M., Guan W., Richmond R. C., et al. (2019). Comparison of smoking-related DNA methylation between newborns from prenatal exposure and adults from personal smoking. Epigenomics 11, 1487–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. K. (2011). Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics 6, 838–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckling N., Gotti A., Bose-O'Reilly S., Chapizanis D., Costopoulou D., De Vocht F., Garí M., Grimalt J. O., Heath E., Hiscock R., et al. (2018). Biomarkers of exposure in environment-wide association studies - Opportunities to decode the exposome using human biomonitoring data. Environ. Res. 164, 597–624. [DOI] [PubMed] [Google Scholar]
- Tamayo y Ortiz M., Téllez-Rojo M. M., Trejo-Valdivia B., Schnaas L., Osorio-Valencia E., Coull B., Bellinger D., Wright R. J., Wright R. O. (2017). Maternal stress modifies the effect of exposure to lead during pregnancy and 24-month old children’s neurodevelopment. Environ. Int. 98, 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo-Uria I., Maitre L., Thomsen C., Nieuwenhuijsen M. J., Chatzi L., Siroux V., Aasvang G. M., Agier L., Andrusaityte S., Casas M., et al. (2019). The early-life exposome: Description and patterns in six European countries. Environ. Int. 123, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer K. A., Heindel J. J., Bucher J. R., Gallo M. A. (2012). Role of environmental chemicals in diabetes and obesity: A National Toxicology Program workshop review. Environ. Health Perspect. 120, 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurber G. M., Yang K. S., Reiner T., Kohler R. H., Sorger P., Mitchison T., Weissleder R. (2013). Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat. Commun. 4, 1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen K. E., Scholz S., Cronin M. T., Edwards S. W., de Knecht J., Crofton K., Garcia-Reyero N., Hartung T., Worth A., Patlewicz G. (2014). Applying adverse outcome pathways (AOPs) to support integrated approaches to testing and assessment (IATA). Regul. Toxicol. Pharmacol. 70, 629–640. [DOI] [PubMed] [Google Scholar]
- Tralau T., Oelgeschläger M., Gürtler R., Heinemeyer G., Herzler M., Höfer T., Itter H., Kuhl T., Lange N., Lorenz N., et al. (2015). Regulatory toxicology in the twenty-first century: Challenges, perspectives and possible solutions. Arch. Toxicol. 89, 823–850. [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N., Colborn T., Hayes T. B., Heindel J. J., Jacobs D. R., Lee D.-H., Shioda T., Soto A. M., vom Saal F. S., Welshons W. V., et al. (2012). Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 33, 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen R., Schymanski E. L., Barabási A.-L., Miller G. W. (2020). The exposome and health: Where chemistry meets biology. Science 367, 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterinen H. M., Morello-Frosch R., Sen S., Zeise L., Woodruff T. J. (2017). Cumulative effects of prenatal-exposure to exogenous chemicals and psychosocial stress on fetal growth: Systematic-review of the human and animal evidence. PLoS One 12, e0176331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vineis P., Chadeau-Hyam M., Gmuender H., Gulliver J., Herceg Z., Kleinjans J., Kogevinas M., Kyrtopoulos S., Nieuwenhuijsen M., Phillips D. H., et al. (2017). The exposome in practice: Design of the EXPOsOMICS project. Int. J. Hyg. Environ. Health 220, 142–151., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C. F. A., Van Winkle L. S., Esser C., Haarmann-Stemmann T. (2020). The aryl hydrocarbon receptor as a target of environmental stressors - Implications for pollution mediated stress and inflammatory responses. Redox Biol. 34, 101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. L. (2016). Minireview: Epigenomic plasticity and vulnerability to EDC exposures. Mol. Endocrinol. 30, 848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H. T., Cheung L. Y., Chan T. F., Li M., Lai K. P., Wong C. K. C. (2021). Characterization of PFOS toxicity on in-vivo and ex-vivo mouse pancreatic islets. Environ. Pollut. 289, 117857. [DOI] [PubMed] [Google Scholar]
- Wang J.-J., Tian Y., Li M.-H., Feng Y.-Q., Kong L., Zhang F.-L., Shen W. (2021). Single-cell transcriptome dissection of the toxic impact of di (2-ethylhexyl) phthalate on primordial follicle assembly. Theranostics 11, 4992–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Lin Y., Li Y., Ying C., Chen J., Song L., Zhou Z., Lv Z., Xia W., Chen X., et al. (2011). Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology 152, 3049–3061. [DOI] [PubMed] [Google Scholar]
- Whitmee S., Haines A., Beyrer C., Boltz F., Capon A. G., de Souza Dias B. F., Ezeh A., Frumkin H., Gong P., Head P., et al. (2015). Safeguarding human health in the Anthropocene epoch: Report of The Rockefeller Foundation-Lancet Commission on planetary health. Lancet 386, 1973–2028. [DOI] [PubMed] [Google Scholar]
- Wild C. P. (2005). Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomark. Prev. 14, 1847–1850. [DOI] [PubMed] [Google Scholar]
- Wright E. C., Johnson S. A., Hao R., Kowalczyk A. S., Greenberg G. D., Ordoñes Sanchez E., Laman-Maharg A., Trainor B. C., Rosenfeld C. S. (2017). Exposure to extrinsic stressors, social defeat or bisphenol A, eliminates sex differences in DNA methyltransferase expression in the amygdala. J. Neuroendocrinol. 29. 10.1111/jne.12475. Accessed April 12, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Achebouche R., Audouze K. (2020). Computational systems biology as an animal-free approach to characterize toxicological effects of persistent organic pollutants. ALTEX 37, 287–299. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Caudle W. M., Pi J., Bhattacharya S., Andersen M. E., Kaminski N. E., Conolly R. B. (2019). Embracing systems toxicology at single-cell resolution. Curr. Opin. Toxicol. 16, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Xie P., Yong T., Wang H., Chung A. C. K., Cai Z. (2018). MALDI-MS imaging reveals asymmetric spatial distribution of lipid metabolites from bisphenol S-induced nephrotoxicity. Anal. Chem. 90, 3196–3204. [DOI] [PubMed] [Google Scholar]