Abstract
Introduction:
Chemotherapy plus radiation (Cis-RT+CP) did not demonstrate superiority in prolonging relapse-free survival compared to chemotherapy alone in patients with stage III or IVA endometrial carcinoma. The impact of treatment on quality of life (QOL), neurotoxicity (NTX) and psychometric properties of the gastrointestinal (GI) symptoms subscale during treatment and up to 1 year are described herein.
Methods:
QOL assessments were scheduled at baseline, 6 weeks (post completion of RT (Cis-RT+CP) or prior to cycle 3 (CP)), then 18 weeks (end of treatment) and 70 weeks (1 year after the end of treatment) after starting treatment. QOL instruments included the FACT-En TOI, FACT/GOG-neurotoxicity (Ntx) subscale (short), and the gastrointestinal (GI) symptoms subscale.
Results:
At the end of treatment, patients receiving Cis-RT+CP reported a statistically significant decreased QOL when compared to CP. The decline in QOL was reflected in physical well-being, functional well-being, and endometrial cancer specific concerns, but the minimally important differences (MID) were not considered clinically meaningful. Patients in both groups reported increased chemotherapy-induced Ntx symptoms with the CP group having worse scores and reaching peak symptoms at the time of chemotherapy completion. Patients on Cis-RT+CP reported statistically significantly worse GI symptoms after radiation therapy compared to patients on CP, this occurred across assessment intervals, though the MID was not meaningful. Psychometric evaluations indicated that the GI symptom scale is reliable, valid, and responsive to change.
Conclusions:
PROs indicate that the chemoradiotherapy group experienced worse HRQoL and GI toxicity compared to patients randomized to chemotherapy alone for locally advanced endometrial cancer though based on the MID, these were not clinically meaningful differences. The GI symptom subscale was a reliable and valid scale that has value for future trials.
Keywords: endometrial cancer, quality of life, patient reported outcomes, chemotherapy, combined radiation therapy, chemotherapy
INTRODUCTION
Endometrial cancer is the most commonly diagnosed gynecologic cancer in the United States with an annual incidence of 61,880 cases in 2019, is the 6th most common cause of cancer-related death, and is increasing in incidence worldwide (1, 2). Most endometrial cancer cases are diagnosed at an early stage, but 20% of patients are diagnosed with stage III or IV disease that carries a substantial recurrence risk (1, 3). Different treatment modalities including chemotherapy, radiation therapy, and brachytherapy have been studied post-surgery (4–13) in an effort to improve outcomes. The phase 3 study GOG-0122 randomized women with newly diagnosed advanced endometrial cancer to either whole abdominal radiotherapy versus doxorubicin and cisplatin post-surgery (4); both progression-free survival (PFS) and overall survival (OS) were better in the chemotherapy group, but acute toxicities and local recurrence risk were higher in the chemotherapy group. PORTEC3 tested pelvic radiation therapy alone compared to radiotherapy with radiosensitizing chemotherapy followed by chemotherapy in women with high-risk endometrial cancer (5) demonstrating improved failure-free survival and OS with combined modality treatment (5, 6).
GOG-0258 tested combined modality therapy Cis-RT+CP versus 6 cycles of CP in women with stage III or IVA endometrial cancer; the primary endpoint was to determine if Cis-RT+CP improved investigator-assessed relapse-free survival (RFS) compared to CP (14). Findings from this study showed that Cis-RT+CP did not improve relapse-free survival in stage III/IVA endometrial carcinoma, and the type and extent of recurrences differed based on treatment; Cis-RT+CP had reduced 5-year incidence of vaginal (2% vs. 7%, HR = 0.36, 95% CI 0.16 to 0.82) and pelvic and para-aortic lymph node recurrences compared to CP (11% vs. 20%, HR=0.43, 95% CI 0.28 to 0.66) (14). Distant recurrences were higher with Cis-RT+CP (27% vs. 21%, HR 1.36, 95% CI 1.00 to 1.86). Grade 3 to 5 adverse events were reported in 202 (58%) and 227 (63%) patients in the Cis-RT+CP group and CP arm, respectively (14). Given the lack of superiority of the combined therapy arm and the fact that a large percentage of patients with stage III/IVA endometrial cancer will be long term survivors, QOL measures will be highly informative for decision making in this population. Here, we report the PROs of the full study population which assessed overall QOL and neurotoxicity as well as an exploratory endpoint of gastrointestinal toxicity and the psychometric properties of the developed GI symptom subscale.
METHODS
Trial design.
GOG-0258 was a phase III study that randomized treatment of women with newly diagnosed endometrial cancer stages III or IVA to either Cis-RT+CP consisting of cisplatin 50 mg/m2 given intravenously (IV) on days 1 and 29 together with volume-directed external beam radiation therapy followed by carboplatin area under the concentration time curve (AUC) 5 or 6 plus paclitaxel 175 mg/m2 every 21 days for 4 cycles with myeloid growth factor support (CisRT+CP) or chemotherapy (CP) which consisted of carboplatin AUC 6 and paclitaxel 175 mg/m2 every 21 days for 6 cycles with equal allocation (14). The primary endpoint was to determine if treatment with Cis-RT+CP for 4 cycles (experimental arm) reduced the rate of recurrence or death (i.e. increases RFS) when compared to CP for 6 cycles (control arm) in patients with Stages III-IVA endometrial carcinoma (<2 cm residual disease). Secondary endpoints included OS, toxicities, and QOL. Eligibility and exclusion criteria are described in the original publication (14). The primary PRO objective was to determine the impact of treatments on patient-reported QOL during and following treatment for up to 1 year with the two treatment regimens. This study was funded by the National Cancer Institute through the NRG cooperative group and was registered on ClinicalTrials.gov (NCT00942357). Local or central Institutional Review Board/Independent Ethics Committee study, and patients provided written informed consent before enrollment.
PRO endpoint design and assessments.
PRO and QOL assessments were taken at: 1) baseline; (within 14 days prior to starting treatment), 2) 6 weeks from start of protocol treatment (1 week post completion of radiation therapy for the Cis-RT+CP) and 3 weeks post completion of 2 cycles (prior to cycle 3) of chemotherapy for the CP group), and 3) for all patients: 18 weeks (end of study treatment), and 4) 70 weeks (1 year post the end of study treatment). The following QOL and PRO measures were used (described in Supplementary data, sections I–II): 1) Functional Assessment of Cancer Therapy (FACT)-Endometrial (En) Trial Outcome Index (TOI) (30 items) for QOL endpoint, 2) FACT/Gynecologic Oncology Group (GOG)Neurotoxicity (Ntx) subscale (short) for chemotherapy-induced peripheral neuropathy, and 3) gastrointestinal (GI) symptoms subscale (exploratory) which included six items: C3 (I have control of my bowels), C5 (I have diarrhea) in the FACT-C in combination with En1 (I have trouble digesting food), O1 (I have swelling in my stomach area), O3 (I have cramps in my stomach area), and Cx6 (I am bothered by constipation) in TOI of FACT-En. The FACT-En TOI consists of three subscales: Physical Well Being (PWB) (7 items), Functional Well Being (FWB) (7 items), and Endometrial Cancer subscale (ECS) (16 items). Each item in the FACT-En TOI, the FACT/GOG-Ntx subscale, and GI symptom subscale were scored using a 5-point scale (0=not at all; 1=a little bit; 2=somewhat; 3=quite a bit; 4=very much). For the negative statements (or questions), reversal was performed prior to score calculation. According to the FACIT measurement system, a subscale score was the summation of the individual item scores if more than 50% of subscale items were answered. When unanswered items existed, a subscale score was prorated by multiplying the mean of the answered item scores by the number of items in the subscale. The total FACT-En TOI score is calculated as the sum of the subscale scores if more than 80% of the FACT-En TOI items provide valid answers and all of the component subscales have valid scores. The total scores range 0–120 for FACT-En TOI, 0–16 for the FACT/GOG-Ntx subscale, 0–24 for the GI symptoms subscale. A higher score indicates better QOL or less symptoms/concerns. The Minimal Important Difference (MID) is 6 points for the FACT-En TOI and 1.2 points for the FACT/GOG-Ntx subscale (15).
PRO Statistical Analysis.
For testing the FACT-En TOI and the FACT/GOG-Ntx subscale, the type I error was set at 0.025 for each of the two PROs measures to ensure the overall type I error was 0.05 (two-sided). The analyses on the three subscales of the FACT-En TOI and GI symptoms subscale were considered as exploratory and were tested at 5% significance level. The p-values for comparisons at each time point were also adjusted with the Hochberg Step-up method for multiple time points. The treatment differences in PROs were assessed with a linear mixed model adjusting for patient’s pretreatment score, treatment assignment, and age at enrollment and stratified for gross residual disease status. The assessment time points were treated as categorical since they were not equally spaced. The covariance matrix among the repeated PRO scores reported by the same patient was assumed to be unstructured. To reflect the observed covariance pattern of the PROs scores, the ‘empirical’ variance was used in estimating the precision of parameter estimates. First, the interactions between assessment time points and treatment assignments were tested for the constant differential effects of treatments over time. If the interaction effect was not statistically significant, an overall treatment effect was estimated by a weighted average of estimates from each time point. If the testing for interaction was rejected, treatment comparison was performed for each assessment time. In this case, Hochberg’s step-up method was used to adjust for the p values for testing the least-squares means differences between treatment groups obtained from the fitted mixed model over the assessment time points. The final p-values were then further adjusted with Sidak method for multiple measures.
The GI subscale was designed to assess patient-reported gastrointestinal (GI) symptoms that might be associated with the radiation therapy and was developed under the FACIT (Functional Assessment of Chronic Illness Therapy) measurement system. The psychometric properties of the exploratory GI subscale were evaluated using the data collected in patients randomized to Cis-RT +CP at 6 weeks and 18 weeks post the start of treatment (end of radiation therapy) when the radiation-related GI symptoms were most likely observed and reported. Reliability was assessed with the standardized Cronbach’s coefficient alpha. Since the GI subscale was designed to assess possible symptoms or side effects on the gastrointestinal system (not measuring a single symptom), we postulated that a moderate reliability of 0.5 would be acceptable. The Pearson correlation coefficients of the GI subscale score with the FACT-En TOI and the FACT/GOG-Ntx subscale were used to assess the convergent validity and discriminant validity. It was expected that the GI subscale score would correlate with the FACT-En TOI (less GI symptoms associated with better QOL) but would be more weakly associated with the FACT/GOG-Ntx scale given the different organ systems involved. The criterion validity was assessed by examining the association between the GI subscale score and the maximum CTCAE grade for GI symptoms assessed after radiation therapy. Responsiveness to change was assessed by examining the change of the GI subscale from baseline to 6 as well as 18 weeks post treatment initiation. The sensitivity to treatment was evaluated by examining between-group treatment differences.
RESULTS
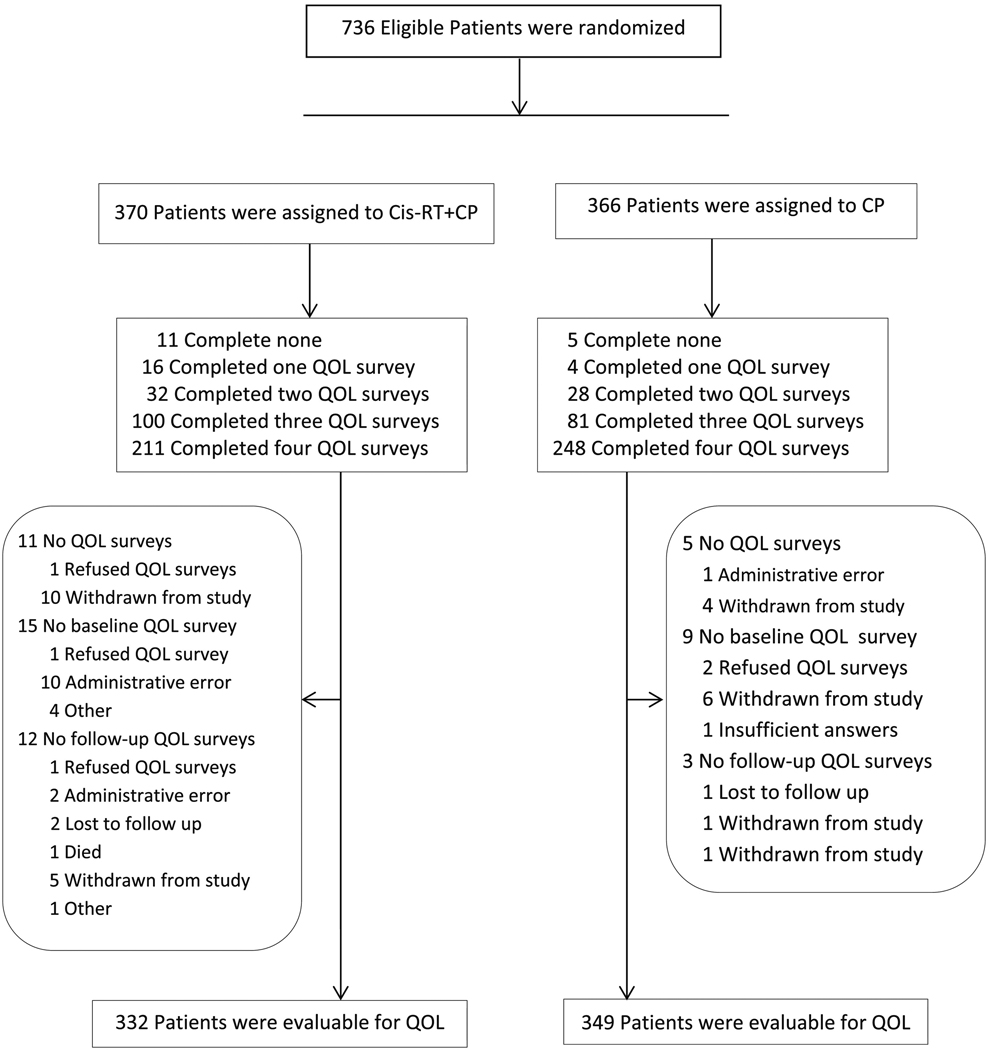
Between June 29, 2009 and July 28, 2014, 736 eligible patients (370 on the Cis-RT+CP arm and 366 on the CP arm) were enrolled to GOG-0258, and 95% of patients completed the baseline assessment. After the initiation of study treatment, compliance was 90% at 6 weeks, 87% at 18 weeks, and 78% at 70 weeks. More patients on the CP arm compared to those on the CisRT+CP arm completed PRO assessments (p=0.001). Figure 1 represents the Consort Diagram for the PRO data. There were 16 patients (11 on Cis-RT+CP and 5 on CP) who did not participate in the QOL surveys, 15 patients (12 on Cis-RT+CP and 3 on CP) dropped off for follow-up assessments, and 24 patients (15 on Cis-RT+CP and 9 on CP) missed their baseline assessment. All of these patients were not evaluable for PRO assessment and were excluded from the PRO analysis (Figure 1). Reasons for not participating in the QOL surveys are detailed in Supplementary section III. The demographic and disease characteristics of the 681 evaluable patients (332 on Cis-RT+CP and 349 on CP) are found in Supplementary section IV and in the original manuscript (14).
Figure 1:
Consort Diagram describing all 736 patients enrolled and randomized in GOG258
I. FACT-En TOI Score and subscales.
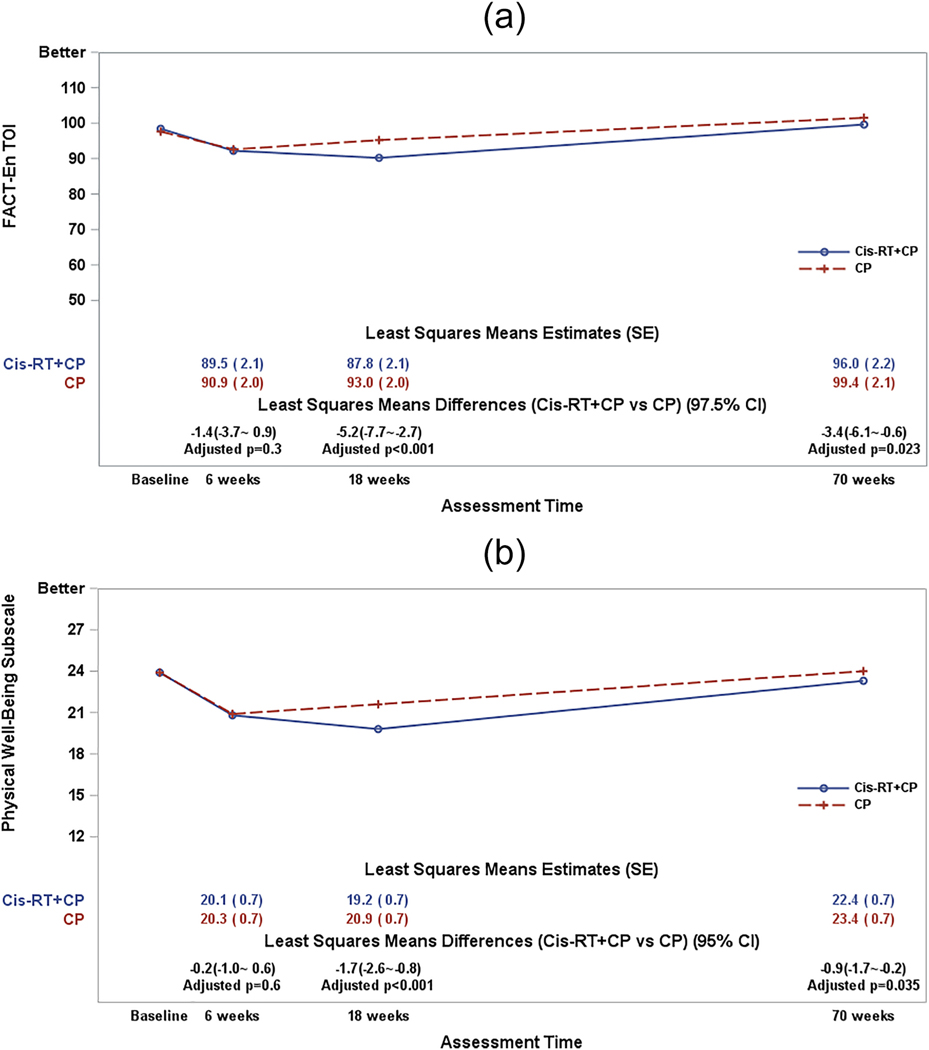
In the primary analysis, patients in both groups reported similar TOI scores at baseline. Since the start of protocol treatment, the treatment differences in the TOI score varied significantly over assessment time (p-value=0.003 for the interaction between time and treatment groups). After adjustment for patient’s age and baseline score, the patients receiving the Cis-RT+CP treatment reported a 5.2 point (97.5% CI: 2.7~7.8; adjusted p<0.001) lower (worse) QOL score at 18 weeks (end of treatment) as compared to those on CP. The treatment-induced difference remained statistically significant at 1 year of follow-up post treatment completion (3.4 points lower on the combined modality arm; 97.5% CI: 0.7~6.2; adjusted p=0.022) (Figure 2a). However, these differences in the TOI scores which are less than the MID of 6 points, are not considered clinically meaningful.
Figure 2:
Figure 2a:The plot lines present the patient-reported FACT-En TOI scores. CP: Carboplatin/Paclitaxel (6 cycles); Cis-RT+CP: Cisplatin /RT+ CP (4 cycles). The least-squares means estimates were obtained from a fitted mixed model adjusting for pre-treatment score (baseline score) and patient’s age at the enrollment. A larger score indicates favorable or better QOL. The least squares means differences (Cis-RT+CP vs CP) were estimated from the fitted mixed models. The p-values for the least squares means differences were adjusted with Hochberg step-up method first for multiple time points and then adjusted with Bonferroni method for multiple measures.
Figure 2b: Figure 2b shows the patient-reported physical well-being subscale scores. CP: Carboplatin/Paclitaxel (6 cycles); Cis-RT+CP: Cisplatin /RT+ CP (4 cycles). The least-squares means estimates were obtained from a fitted mixed model adjusting for pre-treatment score (baseline score) and patient’s age at the enrollment. A larger score indicates favorable or better QOL. The least squares means differences (Cis-RT+CP vs CP) were estimated from the fitted mixed models. The p-values for the least squares means differences were adjusted with Hochberg step-up method first for multiple time points.
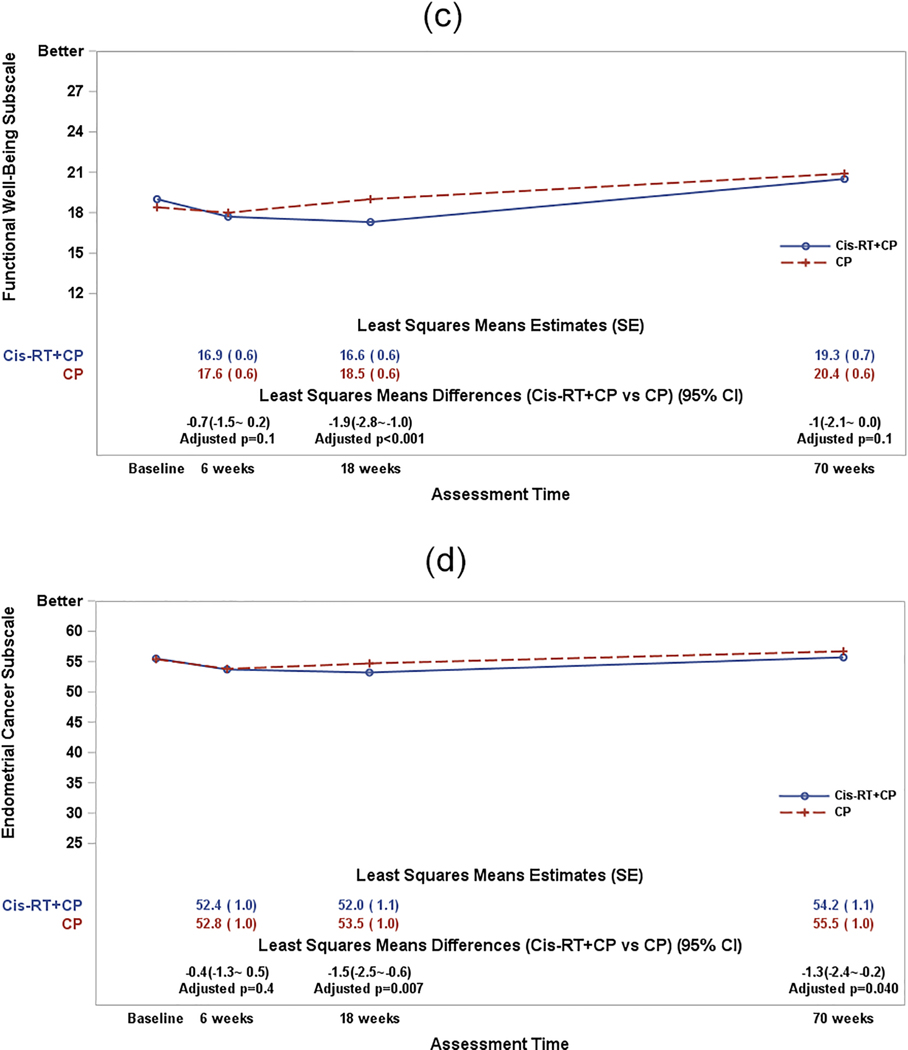
Figure 2c: The plot lines in Figure 2c present the patient-reported functional well-being subscale scores.
CP: Carboplatin/Paclitaxel (6 cycles); Cis-RT+CP: Cisplatin /RT+ CP (4 cycles). The least-squares means estimates were obtained from a fitted mixed model adjusting for pre-treatment score (baseline score) and patient’s age at the enrollment. A larger score indicates favorable or better QOL. The least squares means differences (Cis-RT+CP vs CP) were estimated from the fitted mixed models. The p-values for the least squares means differences were adjusted with Hochberg step-up method first for multiple time points.
Figure 2d: The plot lines present the patient-reported endometrial cancer subscale scores.
CP: Carboplatin/Paclitaxel (6 cycles); Cis-RT+CP: Cisplatin /RT+ CP (4 cycles). The least-squares means estimates were obtained from a fitted mixed model adjusting for pre-treatment score (baseline score) and patient’s age at the enrollment. A lager score indicates favorable or better QOL. The least squares means differences (Cis-RT+CP vs CP) were estimated from the fitted mixed models. The p-values for the least squares mean differences were adjusted with Hochberg step-up method first for multiple time points.
The Physical Well-Being (PWB) Subscale Score.
At baseline, the patient-reported physical well-being subscale score was 23.9 for patients on both CP and Cis-RT+CP arms respectively. After starting treatment, treatment-induced differences in the PWB subscale score varied significantly over assessment time (p-value=0.003 for the interaction between time and treatment groups). After adjustment for patient’s age and baseline score, the patients receiving Cis-RT+CP reported 1.7 points lower/worse (95% CI: 0.9~2.6; adjusted p<0.001) physical well-being at 18 weeks (end of treatment) when compared to those on CP; however, the differences in the PWB scores are not clinically meaningful. The treatment difference continued to be statistically significant at the 1-year post-treatment evaluation/follow-up visit (0.9 points lower on CisRT+CP group; 95% CI: 0.2~1.7; adjusted p=0.035) (Figure 2b).
The Functional Well-Being (FWB) Subscale Score.
At baseline, the patient-reported functional well-being subscale scores were 18.4 and 19.0 by patients on CP and Cis-RT+CP respectively. Since initiating protocol treatment, the treatment differences in the FWB subscale scores varied significantly over assessment time (p-value=0.036 for the interaction between time and treatment groups). After adjustment for patient’s age and baseline score, the patients receiving Cis-RT+CP reported 1.9 points lower/worse (95% CI: 1.0~2.8; adjusted p<0.001) functional well-being at 18 weeks (end of treatment) as compared to those on CP. (Figure 2c); however, this difference is not considered clinically meaningful.
The Endometrial Cancer Subscale (ECS) Score.
At baseline, the patient-reported endometrial cancer subscale scores were 55.4 and 55.5 by patients on both CP and Cis-RT+CP respectively. Following protocol treatment initiation, treatment differences in the ECS subscale score did not vary significantly over assessment time (p-value=0.07 for the interaction between time and treatment groups). After adjustment for patient’s age and baseline score, the patients receiving Cis-RT+CP reported a non-clinically meaningful 1.0 points lower/worse (95% CI: 0.2~1.8; p=0.011) endometrial cancer concerns or symptoms on average across assessment time as compared to those on CP (Figure 2d).
II. FACT/GOG-Ntx subscale:
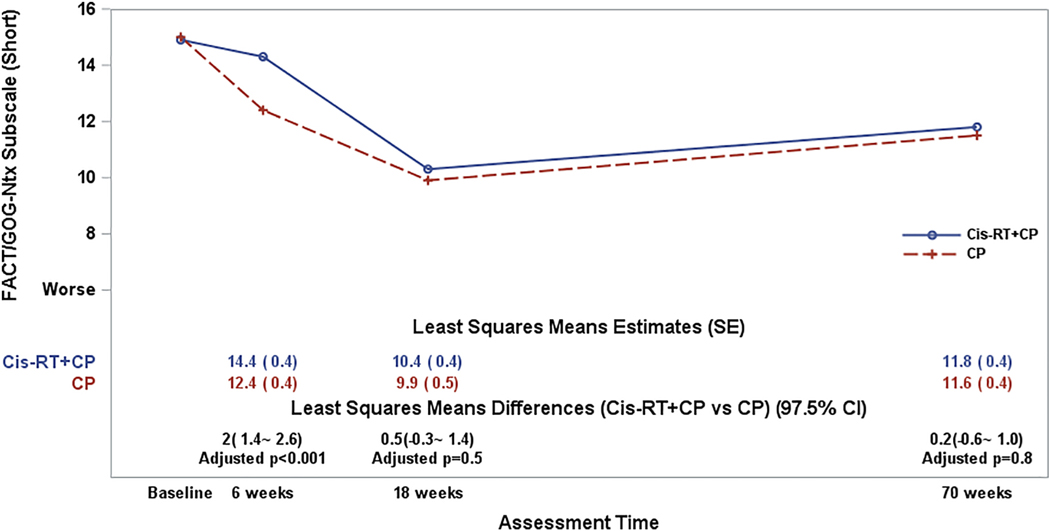
Patients in both regimens, especially in the CP group, reported increased chemotherapy-induced sensory neuropathy symptoms upon starting study treatment. Neurotoxicity was most severe at the end of chemotherapy in both groups. After adjustment for patient’s age at enrollment and baseline Ntx subscale score, the fitted mixed model estimate suggested that the treatment differences in the FACT/GOG-Ntx subscale scores varied significantly over the assessment times (p<0.001 for the interaction between assessment times and treatment groups). The largest treatment difference was observed at 6 weeks when patients who received two cycles of chemotherapy reported 2.0 points (97.5% CI: 1.4~2.6; adjusted p-value<0.001) lower or worse neurotoxicity symptoms in the Ntx subscale score when compared to the combined therapy group (Supplementary section II). Furthermore, this treatment difference exceeded the minimal clinically important difference (MCID) of 1.2 points, which is considered clinically meaningful. However, with further follow-up, neuropathy was not significantly different between the 2 treatment groups at 18 weeks and 70 weeks (Figure 3).
Figure 3:
The plot lines present the patient-reported FACT/GOG-Ntx Subscale (short) scores. CP: Carboplatin/Paclitaxel (6 cycles); Cis-RT+CP: Cisplatin /RT+ CP (4 cycles). The least-squares means estimates were obtained from a fitted mixed model adjusting for pre-treatment score (baseline score) and patient’s age at the enrollment. A lager score indicates favorable or less NTX symptoms. The least squares means differences (CisRT+CP vs CP) were estimated from the fitted mixed models. The p-values for the least squares means differences were adjusted with Hochberg step-up method first for multiple time points and then adjusted with Bonferroni method for multiple measures.
III. Psychometric properties of the Gastrointestinal Symptoms Subscale.
Reliability and construct validity
The internal reliability of the GI subscale as measured with standardized Cronbach’s coefficient alpha was 0.56 at 6 weeks and 0.6 at 18 weeks. The Pearson correlation coefficients of the GI subscale score with other scales are presented in Table 1. The GI subscale demonstrated a moderate correlation with QOL as measured by the FACT-En TOI (convergent validity) and was least correlated with the neurotoxicity subscale (discriminant validity).
Table 1.
The Pearson correlation coefficients of the GI subscale score compared with other scales. The GI subscale demonstrated a moderate correlation with the QOL as FACT-En TOI (convergent validity) and least correlation with the Ntx subscale (discriminant validity).
| PRO Scales | 6 weeks | 18 weeks |
|---|---|---|
| Physical Well Being | 0.62 | 0.49 |
| Functional Well Being | 0.42 | 0.39 |
| Endometrial Cancer Subscale | 0.65 | 0.68 |
| The FACT-En TOI | 0.67 | 0.64 |
| FACT/GOG-Ntx subscale | 0.13 | 0.29 |
Criterion validity
The criterion validity of the GI subscale score was assessed against the highest CTCAE grade of GI disorders, graded by clinical physicians or staff at 6 weeks and 18 weeks (Table 2). After adjustment for baseline score, patient’s age, and assessment group, the patients with CTC grade 1 of GI disorders reported 1.0 point lower on average (95% CI: 0.3~1.7; p=0.0035) in GI subscale and the patients with CTC grade 2 reported 2.2 points lower (95% CI: 0.7~3.8; p=0.005) when compared to those with CTC grade 0 of GI disorder.
Table 2.
The criterion validity of the GI subscale score was assessed against the highest CTCAE grade of GI disorders, graded by clinical physicians or staff at 6 weeks and 18 weeks.
| Assessment time | 0 | 1 | 2+ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Std | N | Mean | Std | N | Mean | Std | |
| 6 Weeks | 190 | 19.57 | 3.36 | 93 | 18.88 | 3.32 | 29 | 16.80 | 4.29 |
| 18 Weeks | 218 | 20.12 | 3.28 | 61 | 18.64 | 3.14 | 19 | 18.74 | 4.69 |
Responsiveness to change over time
At 6 weeks when patients completed their radiation therapy, the GI subscale score declined 1.5 points from baseline (95 CI: 1.1~2.0; p<0.001). The decrement in the GI subscale score was 0.8 points (95% CI: 0.4~ 1.2; p<0.001) at 18 weeks; demonstrating the responsiveness of the GI subscale to patients’ experienced GI toxicities during treatment.
Sensitivity to treatment difference
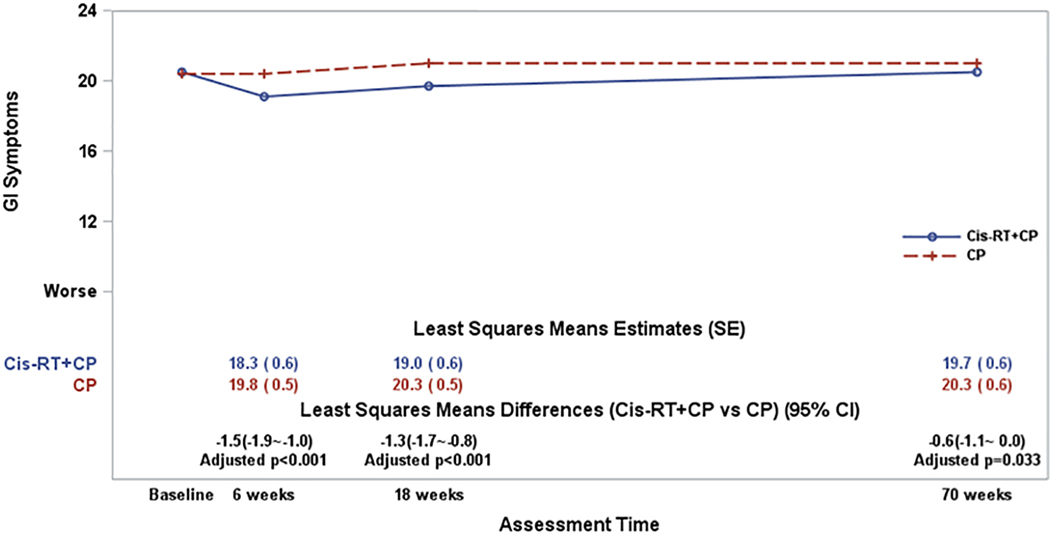
Patients receiving combined treatment reported lower (worse) GI symptoms after radiation therapy. After adjusting for patient’s age at enrollment and the baseline GI score, the fitted mixed model estimate suggested that the treatment differences varied significantly over the assessment times (p=0.023 for the interaction between assessment times and treatment groups). Patients on the combined therapy arm reported significantly lower or worse GI symptoms across the assessment times compared to those on CP (Figure 4), providing evidence for sensitivity to treatment effects.
Figure 4:
The plot lines present the patient-reported Gastrointestinal Symptoms scores. CP: Carboplatin/Paclitaxel (6 cycles); Cis-RT+CP: Cisplatin /RT+ CP (4 cycles). The least-squares means estimates were obtained from a fitted mixed model adjusting for pre-treatment score (baseline score) and patient’s age at the enrollment. A lager score indicates favorable or better QOL. The least squares means differences (Cis-RT+CP vs CP) were estimated from the fitted mixed models. The p-values for the least squares means differences were adjusted with Hochberg step-up method for multiple time points.
DISCUSSION
The PRO results from GOG-0258 demonstrate that patients treated with the combined modality approach that included combined chemoradiation experienced overall statistically significant worse QOL and GI toxicity compared to patients receiving CP; however, the differences in the FACT-En/TOI scale and GI symptoms subscale were not clinically meaningful in terms of the MID’s.
Patients on both treatment arms in this trial experienced neuropathy induced by carboplatin and paclitaxel chemotherapy that reached the worst point by the end of chemotherapy and did not return to baseline by one year for both groups. Even though the Cis-RT+CP arm included 4 paclitaxel-containing cycles compared to 6 cycles which were administered in the CP group, significant neuropathy still occurred and was sustained for patients treated in the Cis-RT+CP arm. By one year after the end of study treatment, there were still about 30% patients reporting ‘quite a bit’ or ‘very much’ tingling/numbness in their hands or feet. PORTEC3 which compared radiotherapy versus combined modality therapy in high-risk advanced endometrial cancer also demonstrated the persistence of neuropathy, with 25% of patients at 24 months post treatment who received chemotherapy rating their neuropathy as “quite a bit” or “very much” at 24 months post-treatment (16).
Additionally, patients on the combined modality arm in GOG-0258 reported significantly worse GI symptoms across the assessment points compared to those receiving chemotherapy alone. To establish this measure as a valid and fit for use in future clinical trials, we evaluated relationships between this “new” subscale and the FACT-EN-TOI, in addition to its subscales. Psychometric analyses were performed demonstrating that this subscale reliably and validly assesses GI toxicity in advanced endometrial cancer patients. As part of this validation, we noted a relationship between the GI scores and the CTCAE GI toxicities of grades 1 and 2 and recognize the consistent proportional difference between patient and clinician reporting of these toxicities. This is consistent with a growing literature recognizing that patients are more likely to report more serious toxicities and more adverse events across symptoms compared to clinicians thereby underscoring the importance of utilizing a PRO specific to GI toxicity (17, 18).
Differences in QOL and GI toxicity can be used to inform decision making for clinicians and patients given the lack of superiority observed with the combined regimen. Interestingly, the PORTEC3 PRO data did not demonstrate any additional long-term toxicities, except neuropathy with no long-term GI toxicities being detected with the combined modality arm. This may be because of the focus on overall QOL evaluated in PORTEC3, while GOG-258 PROs piloted several GI-specific questions, and these pointed questions likely identified long-term toxicities, also lending support to a potentially new, brief measure of GI toxicity.
The QOL differences observed between groups in this trial provide information that could be utilized in decision making for treatment planning in this still contentious clinical setting. As previously reported in Matei et al, the primary endpoint of this study was to compare the RFS between Cis-RT+CP treatment versus CP (14). Although there were no significant differences between the 2 arms with respect to RFS; in the Cis-RT+CP arm, 59% (95% CI 53%−65%) were alive and progression-free compared to 58% (95% CI 53%−64%) of patients on the CP alone arm (HR 0.9; 90% CI 0.74 to 1.10), questions still linger regarding the potential use of RT in this patient population. This controversy is particularly related to the finding that the cumulative incidence of local recurrences was lower in the Cis-RT+CP compared to the CP arm, specifically the 5-year cumulative incidence of vaginal recurrence was 2% vs. 7%, (HR = 0.36, 95% CI 0.16 to 0.82) and of pelvic or para-aortic lymph node recurrence 11% vs. 20%, (HR=0.43, 95% CI 0.28 to 0.66).
Additionally, specific toxicity profiles differed between the arms, providing additional decision-making points to assist clinicians in counseling patients in determining an optimal treatment regimen. Grade 3 or higher toxicities were slightly more frequent in the CP arm compared to Cis-RT+CP (63% versus 58%, respectively); similarly, grade 4 or higher adverse events were more frequently observed in the CP arm (30%) versus Cis-RT+CP (14%) (14). However, fatigue, gastrointestinal, renal/genitourinary and musculoskeletal adverse events were more common in the combined modality arm, whereas hematologic toxicities were observed more in the chemotherapy arm though myeloid growth factor was part of the Cis-RT+CP arm. These findings were reflected in PROs, as the FACT-En subscales showed that physical well-being and functional well-being differed between the arms and in favor of the chemotherapy arm whereas the Endometrial Cancer Subscale results were quite similar between the groups except when patient’s age and baseline score were considered, at which point, the Cis-RT+CP -treated patients reported worse scores.
Ongoing phase 3 studies in this high-risk patient population are currently focused on the addition of immune checkpoint blockade to carboplatin and paclitaxel chemotherapy; ongoing trials are testing pembrolizumab (NCT03914612), TSR-042 (NCT 03981796 PROs included), as well as atezolizumab (NCT03603184). These studies are testing the addition of immune checkpoint blockade to carboplatin and paclitaxel chemotherapy which will be given either as primary treatment or after radiation therapy; thus, GI toxicities may be particularly significant in patients who receive radiation therapy and go on to receive chemotherapy and immune checkpoint blockade (19). PRO measurement with a focus on GI toxicities along with other immune-related toxicities will thus be critical to measure, and as illustrated herein, the addition of PROs measurement can augment knowledge used in treatment decision-making.
Supplementary Material
RESEARCH HIGHLIGHTS.
Radiation/chemotherapy caused more QOL and GI symptoms versus chemotherapy; the differences were not clinically meaningful.
Both treatment groups experienced neuropathy, especially in the chemotherapy group.
The differing toxicities of the 2 arms can assist clinicians in counseling patients given the efficacy equivalence.
ACKNOWLEDGEMENTS
This study was funded by the National Cancer Institute awards to NRG Oncology SDMC (1U10 CA180822), NRG Operations (U10CA180868), and NCORP (UG1CA189867).
The following NRG Oncology/Gynecologic Oncology Group institutions participated in this study: Seoul National University Hospital, Women and Infants Hospital, University of Oklahoma Health Sciences Center, Ohio State University Comprehensive Cancer Center, Washington University School of Medicine, Women’s cancer Center of Nevada, University of California Medical Center at Irvine-Orange Campus, Georgia Center for Oncology Research and Education (CORE), Case Western Reserve University, Cancer Trials Support Unit, University of Colorado Cancer Center-Anschutz Cancer Pavilion, University of North Carolina at Chapel Hill, Cancer Research Consortium of West Michigan NCORP, University of Iowa Hospitals and Clinics, Abington Memorial Hospital, University of Kentucky, Cooper Hospital University Medical Center Stony Brook University Medical Center, Yale University, Cancer Research for the Ozarks NCORP, Metro-Minnesota CCOP, University of New Mexico, MD Anderson Cancer Center, Fox Chase Cancer Center, University of Chicago, Walter Reed National Military Medical Center, Rush University Medical Center, State University of New York Downstate Medical Center, Memorial Sloan Kettering Cancer Center, The Hospital of Central Connecticut, Wake Forest University Health Sciences, Cleveland Clinic Foundation, Aurora Women’s Pavilion of Aurora West Allis Medical Center, University of Hawaii, Mayo Clinic, Wayne State University/Karmanos Cancer Institute, University of Cincinnati, University of Texas Southwestern Medical Center, Indiana University Hospital/Melvin and Bren Simon Cancer Center, University of Wisconsin Hospitals and Clinics, Kaiser Permanente-Vallejo, Iowa-Wide Oncology Research Coalition NCORP, Duke University Medical Center, University of California at Los Angeles Health System, Fred Hutchinson Cancer Research Center, University of Pittsburgh Cancer Institute (UPCI), Saint Joseph’s Hospital and Medical Center, Carolinas Medical Center/Levine Cancer Institute, Lewis Cancer and Research Pavilion at St. Joseph’s/Candler, Kalamazoo CCOP, University of Alabama at Birmingham, University of Mississippi Medical Center, Abramson Cancer Center of The University of Pennsylvania, Penn State Milton S Hershey Medical Center, Gynecologic Oncology of West Michigan PLLC, UCSF-Mount Zion, Froedtert and the Medical College of Wisconsin, Geisinger Medical Center, Saint Vincent Hospital, Wichita CCOP, Sanford NCI Community Oncology Research Program of the North Central Plains, Columbus NCI Community Oncology Research Program, Southeast Cancer Control Consortium CCOP, Sanford NCI Community Oncology Research Program of the North Central Plains, Columbus NCI Community Oncology Research Program, Southeast Cancer Control Consortium CCOP, University of Virginia, University of Texas - Galveston, Baystate Medical Center, Evanston CCOP-NorthShore University Health System, Greenville Health System Cancer Institute/Greenville CCOP, Florida Hospital Cancer Institute CCOP, University of Minnesota Medical Center - Fairview, Henry Ford Hospital, Rutgers Cancer Institute of New Jersey, Thomas Jefferson University Hospital, Huntsman Cancer Institute/University of Utah, Emory University School of Medicine, Johns Hopkins University/Sidney Kimmel Cancer Center, Allegheny General Hospital, Wisconsin NCI Community Oncology Research Program, Central Illinois CCOP, Northern Indiana Cancer Research Consortium, Virginia Mason CCOP, Nevada Cancer Research Foundation CCOP, Sanford Roger Maris Cancer Center, Montana Cancer Consortium-CCOP, University of New Mexico and John H. Stroger Jr. Hospital of Cook County.
CONFLICTS OF INTEREST
Dr. Matulonis reports receiving consulting fees received from Merck, Novartis, Blueprint Medicine and Next Cure as well as participating on a Data Safety Monitoring Board or Advisory Board for Symphogen and Advaxis.
Ms. Helen Huang, Dr. Marcus Randall, Dr. Paul DiSilvestro, Dr. Fowler, Dr. Powell, Dr. Dr. Spirtos, Dr. Tewari, Dr. Nakayama, Dr. Mutch have no conflicts of interest to disclose.
Dr. Virginia L. Filiaci reports receiving support for the present manuscript from NCTN and NCORP SDMC grant funding from the NIH/NCI. She also reports grants from GOG Foundation, Inc. for contracts with institution for clinical trial work and NCI/NIH for IOTN, BIQSFP, MP2PRT and miscellaneous other subcontracts. Dr. Filiaci also reports receiving support for attending IDMC meeting from VBL Therapeutics as well as participating on Advisory Board for Tesaro. Monitoring Board.
Dr. Katherine Moxley reports P20 grant to University of Oklahoma for Drug Resistance Core from the NIH as well as support for travel to attend NRG Oncology 2019 Winter and Summer meetings. Additionally, Dr. Moxley reports serving in a Leadership role for the SGO Program Committee.
Dr. David Miller reports grants received from EMD Serono Research and Development Institute to him as well as grants to his Institution from the following entities: US Biotest, Advenchen Laboratories, Tesaro, Xenetic Biosciences, Advaxis, Janssen, Aeterna Zentaris, TRACON Pharma, Pfizer, Immunogen, Mateon Therapeutics, Merck Sharp & Dohme, AstraZeneca, Millenium Pharmaceuticals, Aprea AB, Regeneron, NVision, Leap Therapeutics, Novartis, Syros Pharmaceuticals, Karyopharm Therapeutics, Agenus and Akesobio. Dr. Miller also personally received consulting fees from the following: Genentech, Tesaro, Eisai, AstraZeneca, Guardant Health, Janssen Oncology, Alexion Pharmaceuticals, Karyopharm Therapeutics, Incyte, Guardant Health, Janssen, Alexion Pharmaceuticals, Clovis Oncology, Asymmetric Therapeutics, LLC, Boston Biomedical Research Institute, Tarveda Therapeutics, Myriad Genetic Laboratories Inc., GlaxoSmithKline LLC, AbbVie Inc., Incyte, EMD Serono Inc. and Seager Inc. as well as consulting fees paid to his Institution from Merck Sharp & Dohme. Dr. Miller also received honoraria from Clovis Oncology and Genentech. He also reports participating on an Advisory Board for Incyte.
Dr. Lari Wenzel reports serving as Co-Chair of the NRG Oncology PCOR Committee.
Dr. Daniela Matei reports NCI NRG Support grant received.
Due to his unfortunate passing before publication, no statement is available for Dr. William Richards.
Footnotes
Dr. William Richards is deceased.
Dr. John Nakayama’s current affiliation is Allegheny Health Network, Division of Gynecologic,Oncology, Pittsburgh, PA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics 2021: CA Cancer J Clin. 71 (2021) 7–33. 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- [2].Lortent-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978–2013. J Natl Cancer Inst. 110 (2018) 354–361. 10.1093/jnci/djx214. [DOI] [PubMed] [Google Scholar]
- [3].Cowan M, Strauss JB, Barber EL, Matei D. Updates on adjuvant chemotherapy and radiation therapy for endometrial cancer. Curr Opin Obstet Gynecol 31 (2019) 31–37. 10.1097/GCO.0000000000000506. [DOI] [PubMed] [Google Scholar]
- [4].Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, Thigpen JT, Benda JA. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma; A Gynecologic Oncology Group Study. J Clin Oncol 24 (2006) 36–44. 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- [5].Katsaros D, Bessette P, Haie-Meder C, Ottevanger PB, Ledermann JA, Khaw P, Colombo A, Fyles A, Baron MH, Jürgenliemk-Schulz IM, Kitchener HC, Nijman HW, Wilson G, Brooks S, Carinelli S, Provencher D, Hanzen C, Lutgens LCHW, Smit VTHBM, Singh N, Do V, D’Amico R, Nout RA, Feeney A, Verhoeven-Adema KW, Putter H, Creutzberg CL Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 19 (2018) 295–309. 10.1016/S1470-2045(18)30079-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, Ottevanger PB, Ledermann JA, Khaw P, D’Amico R, Fyles A, Baron MH, Jürgenliemk-Schulz IM, Kitchener HC, Nijman HW, Wilson G, Brooks S, Gribaudo S, Provencher D, Hanzen C, Kruitwagen RF, Smit VTHBM, Singh N, Do V, Lissoni A, Nout RA, Feeney A, Verhoeven-Adema KW, Putter H, Creutzberg CL Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 20 (2019) 1273–1285. 10.1016/S1470-2045(19)30395-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maggi R LA, Fossati R. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. British J Cancer 95 (2006) 266–271. 10.1038/sj.bjc.6603279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, Kudo R. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 108 (2008) 226–333. 10.1016/j.ygyno.2007.09.029. [DOI] [PubMed] [Google Scholar]
- [9].Hogberg T, Signorelli M, de Oliveira CF, Fossati R, Lissoni AA, Sorbe B, Andersson H, Grenman S, Lundgren C, Rosenberg P, Boman K, Tholander B, Scambia G, Reed N, Cormio G, Tognon G, Clarke J, Sawicki T, Zola P, Kristensen G. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer--results from two randomised studies. Eur J Cancer. 46 (2010) 2422–2431. 10.1016/j.ejca.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bingham B, Orton A, Boothe D, Stoddard G, Huang YJ, Gaffney DK, Poppe MM. Brachytherapy improves survival in stage III endometrial cancer with cervical involvement. Int J Radiat Oncol Biol Phys. 97 (2017) 1040–1050. 10.1016/j.ijrobp.2016.12.035. [DOI] [PubMed] [Google Scholar]
- [11].Lu SM, Chang-Halpenny C, Hwang-Graziano J. Sequential versus “sandwich” sequencing of adjuvant chemoradiation for the treatment of stage III uterine endometrioid adenocarcinoma. Gynecol Oncol. 137 (2015) 28–33. 10.1016/j.ygyno.2015.01.546. [DOI] [PubMed] [Google Scholar]
- [12].Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Final analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol. 103 (2006) 155–159. 10.1016/j.ygyno.2006.02.007. [DOI] [PubMed] [Google Scholar]
- [13].Homesley HD, Filiaci V, Gibbons SK, Long HJ, Cella D, Spirtos NM, Morris RT, DeGeest K, Lee R, Montag A. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: A Gynecologic Oncology Group study. Gynecol Oncol. 112 (2009) 543–552. 10.1016/j.ygyno.2008.11.014. E [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Matei D, Filiaci V, Randall ME, Mutch D, Steinhoff MM, DiSilvestro PA, Moxley KM, Kim YM, Powell MA, O’Malley DM, Spirtos NM, Small W Jr, Tewari KS, Richards WE, Nakayama J, Matulonis UA, Huang HQ, Miller DS. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 380 (2019) 2317–2326. 10.1056/NEJMoa1813181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, Ottevanger PB, Ledermann JA, Khaw P, Colombo A, Fyles A, Baron MH, Kitchener HC, Nijman HW, Kruitwagen RF, Nout RA, Verhoeven-Adema KW, Smit VT, Putter H, Creutzberg CL. Toxicity and quality of life after adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 17 (2016) 1114–1126. 10.1016/S1470-2045(16)30120-6. [DOI] [PubMed] [Google Scholar]
- [16].Basch E, Iasonos A, McDonough T, Barz A, Culkin A, Kris MG, Scher HI, Schrag D. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol 7 (2006) 903–909. 10.1016/S1470-2045(06)70910-X. [DOI] [PubMed] [Google Scholar]
- [17].Basch E, Dueck AC, Rogak LJ, Minasian LM, Kelly WK, O’Mara AM, Denicoff AM, Seisler D, Atherton PJ, Paskett E, Carey L, Dickler M, Heist RS, Himelstein A, Rugo HS, Sikov WM, Socinski MA, Venook AP, Weckstein DJ, Lake DE, Biggs DD, Freedman RA, Kuzma C, Kirshner JJ, Schrag D. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol. 3 (2017) 1043–1050. 10.1001/jamaoncol.2016.6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hwang WL, Pike LRG, Royce TJ, Mahal BA, Loeffler JS. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol. 15 (2018) 477–494. 10.1038/s41571-018-0046-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.