Abstract
Malignant melanoma seriously threatens public health and lowers the quality of life of the affected subjects. The present study was designed to explore the effects of tepotinib, a selective tyrosine kinase inhibitor of MET proto-oncogene, receptor tyrosine kinase (MET), on the progression of melanoma. Firstly, MTT assays were used to detect the proliferation of tepotinib-treated WM451 cells. The cell invasive and migratory activities were assessed using Transwell and wound healing assays, respectively. In addition, TUNEL staining was employed to determine cell apoptosis. Western blot analysis was utilized for the evaluation of the expression levels of apoptotic and epithelial-mesenchymal transition-related proteins, as well as of proteins involved in the PI3K/AKT signaling pathway. Subsequently, hepatocyte growth factor (HGF), a natural agonist of MET, was administered to WM451 cells to unravel the detailed mechanism of action of tepotinib in melanoma. The results indicated that the proliferation of WM451 cells was significantly decreased by tepotinib treatment. The inhibitory effects of tepotinib on the proliferation of WM451 cells occurred in a concentration-dependent manner. In addition, the migratory and invasive activities of WM451 cells were significantly suppressed following tepotinib treatment. It was also shown that tepotinib exhibited promotive effects on the induction of apoptosis of WM451 cells. Moreover, activation of MET and PI3K/AKT signaling pathways may be blocked by tepotinib treatment, whereas addition of HGF to the cells reversed the effects of tepotinib treatment on the malignant progression of WM451 cells. In conclusion, the data demonstrated that tepotinib suppressed the proliferation, invasion and migration of melanoma cells, whereas it could also induce their apoptosis. This evidence may provide a new perspective for the improvement of malignant melanoma.
Keywords: tepotinib, malignant melanoma, migration, invasion, PI3K/AKT, MET
Introduction
Malignant melanoma has an age-standardized mortality rate of 0.3 per 100,000 in China (1), and it is considered one of the most challenging cancers to be diagnosed owing to the limited medical knowledge regarding the early-stage development of its lesions (2). It has been proposed that melanoma is produced by the transformation of melanocytes (3,4). According to its general clinical and pathological characteristics, melanoma can be divided into four subtypes, including superficial diffuse, nodular, malignant and acromegaly melanoma (5–7). Despite the fact that numerous primary melanomas can be successfully treated by surgery, the treatment of patients with advanced metastatic melanoma remains challenging (8,9). The exact reasons responsible for the higher incidence of melanoma remain unclear (10). In view of this evidence, the present study was conducted to explore the mechanism of malignant melanoma and to identify optimal therapeutic strategies for its therapy.
Tepotinib (Tepmetko™) is a selective tyrosine kinase inhibitor of MET proto-oncogene, receptor tyrosine kinase (MET) developed by Sigma-Aldrich; Merck KGaA, which is often used to treat solid tumors (11). Due to its high retention in tumors, tepotinib can block MET as well as its downstream pathways and serve as a potent drug for the treatment of various types of cancer, such as gastric and non-small cell lung cancer (12,13). MET is a receptor tyrosine kinase, which exhibits abnormal activity in human cancer. The activity of this enzyme is associated with aggressive cancer phenotypes, metastatic dissemination, and poor disease prognosis (14–16). It has been reported that c-MET is overexpressed in melanoma and contributes to its malignant progression (17). The PI3K/AKT pathway plays a critical role in cell cycle progression, which involves cellular proliferation and cancer formation (18). An increasing number of studies have revealed that the PI3K/AKT pathway can be a therapeutic target for the treatment of various types of cancer, including triple-negative breast cancer and melanoma (19,20).
The purpose of the present study was to investigate the effects of tepotinib on melanoma cells and to examine its specific mechanism of action. The data aimed to provide new insights into the potential treatment of malignant melanoma.
Materials and methods
Cell culture and treatment
The human malignant melanoma cell line WM451 was purchased from the Cell Bank of Central South University. The cells were incubated in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified incubator with 5% CO2. Subsequently, different concentrations of tepotinib (1, 2, 5, 10, 20 and 50 ng/ml; Selleck Chemicals) were used to treat WM451 cells for 48 h (13). For tepotinib + hepatocyte growth factor (HGF; R&D Systems, Inc.)-treated group, 50 ng/ml of HGF (a natural agonist of MET) and 10 ng/ml tepotinib were concurrently administered to WM451 cells for 48 h.
MTT assay
The proliferative capacity of WM451 cells was evaluated using the MTT assay. WM451 cells were incubated in 96-well plates at a density of 5×104 cells/ml/well for 48 h at 37°C. Subsequently, tepotinib was used at different concentrations (1, 2, 5, 10, 20 and 50 ng/ml) to treat WM451 cells. Following exposure to a 10 µl MTT solution for 4 h, the cells were incubated with formazan lysis solution (DMSO) until the purple crystals were completely dissolved. Finally, the absorbance was detected at 570 nm with a spectrophotometer.
Wound healing assay
WM451 cells were seeded in six-well plates (6×104 cells/well) and cultured to 90–100% confluence. A straight scratch was made in the cell monolayer with a 200-µl pipette tip, followed by PBS washing three times. During the wound healing assay, WM451 cells were cultured in serum-free DMEM in an incubator at 37°C with 5% CO2 for 48 h. The width of the wound was recorded and images were captured at 0 and 48 h time points by using an inverted light microscope (Olympus Corporation). Finally, the area occupied by the migratory cells in the linear scratch was detected by ImageJ Software (version 6.0; National Institutes of Health).
Transwell assay
The invasive ability of WM451 cells was detected using a Transwell plate with an 8-µm pore insert precoated with Matrigel (BD Biosciences) at room temperature for 1 h. The cells were plated at a density of 4×104 cells into the upper compartment of the Transwell chamber with serum-free DMEM. Subsequently, the lower compartment of the Transwell chamber was filled with 500 µl DMEM supplemented with 10% FBS; the cells were incubated for 24 h at 37°C. Subsequently, the invaded cells were fixed with 4% paraformaldehyde for 15 min at room temperature, and stained with 0.1% crystal violet at room temperature for 5 min. Finally, the visualization of the invasive cells was observed with an inverted light microscope (Olympus Corporation).
TUNEL assay
The effects of tepotinib (10 ng/ml) on the induction of WM451 cell apoptosis of were assessed with a TUNEL assay kit (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the manufacturer's protocol. A total of 2×105 cells/well in a 24-well plate were treated with tepotinib for 48 h and subsequently fixed with 4% paraformaldehyde for 15 min at room temperature and then permeabilized with 0.25% Triton X-100 for 2 min at 4°C. Subsequently, the cells were labeled with TUNEL at 37°C for 60 min and counterstained with 0.01 mg/ml DAPI for 10 min in the dark. Slides were mounted using glycerol, and images of the TUNEL-positive apoptotic cells in six randomly selected fields of view were captured with a fluorescence microscope (Nikon Corporation) and quantified by ImageJ Software (version 6.0; National Institutes of Health).
Western blot analysis
Total protein from WM451 cells was isolated using RIPA lysis buffer (Beyotime Institute of Biotechnology) and quantified using the bicinchoninic acid method (Beyotime Institute of Biotechnology). The proteins (40 µg per lane) were separated with 10% SDS-PAGE and transferred on polyvinylidene fluoride membranes. Following blocking with 5% skimmed milk for 2 h at room temperature, specific primary antibodies were incubated with the membranes at 4°C overnight. Following the primary incubation, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:3,000; cat. no. 7074S; Cell Signaling Technology, Inc.) for 2 h at room temperature. Finally, an enhanced chemiluminescence kit (Beyotime Institute of Biotechnology) was applied to determine the expression levels of the protein bands. The intensities of the bands were detected by ImageJ Software (version 6.0; National Institutes of Health). GAPDH was used as an internal control. The following primary antibodies were diluted to 1:1,000 in TBS + 0.1% Tween-20 buffer and used: E-cadherin (cat. no. 3195T), N-cadherin (cat. no. 13116T), vimentin (cat. no. 5741T), Bcl-2 (cat. no. 4223T), Bax (cat. no. 5023T), cleaved caspase 3 (cat. no. 9664T), caspase 3 (cat. no. 14220T), phospho (p)-MET (cat. no. 3077T), MET (cat. no. 4560S), PI3K (cat. no. 4249T), p-AKT (cat. no. 4060T), AKT (cat. no 4685S) GAPDH (cat. no. 5174T; all from Cell Signaling Technology, Inc.) and p-PI3K (cat. no. ab278545; Abcam).
Statistical analysis
All data collected from the experiments are displayed as the mean ± standard deviation and assessed with GraphPad Prism 8.0 (GraphPad Software, Inc.). The comparisons between two groups were conducted with the unpaired Student's t-test. One-way analysis of variance followed by Tukey's post hoc test was applied to determine the statistical significance among multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Tepotinib inhibits the proliferation, migration and invasion of melanoma cells
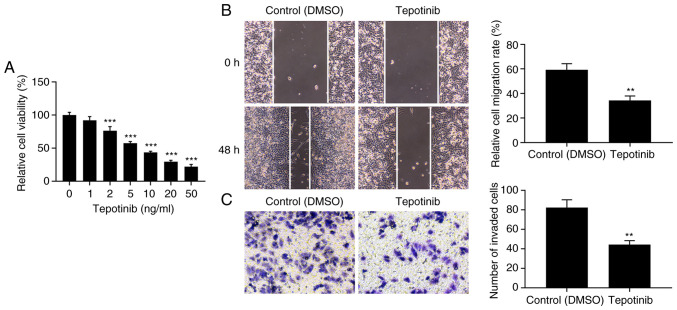
Initially, the effects of tepotinib were investigated on the cell proliferation of WM451 cells. The proliferation of WM451 cells was significantly decreased by tepotinib treatment in comparison with the control group (Fig. 1A). In addition, the inhibitory effects of tepotinib on the proliferation of WM451 cells occurred in a concentration-dependent manner. A total of 10 ng/ml tepotinib presented ~50% inhibitory effect to WM451 cell proliferation, therefore, this concentration was selected to perform the subsequent experiments. The migratory and invasive activities of tepotinib-treated WM451 cells were detected with the application of wound healing and Transwell assays, respectively. The results from Fig. 1B demonstrated that treatment with 10 ng/ml tepotinib significantly diminished the migration of WM451 cells compared with that of the control group. Similarly, the invasive activity of WM451 cells was also reduced by tepotinib (Fig. 1C). These results suggested that tepotinib could suppress the proliferation, migration and invasion of melanoma cells.
Figure 1.
Tepotinib inhibits proliferation, migration and invasion of melanoma cells. (A) The proliferation of WM451 cells treated with different concentrations of tepotinib (1, 2, 5, 10, 20 and 50 ng/ml) was detected using the MTT assay. ***P<0.001 vs. 0 ng/ml tepotinib. (B) The migratory ability of WM451 cells treated with or without 10 ng/ml tepotinib was assessed by the wound healing assay. Magnification, ×100. (C) The invasion of 10 ng/ml tepotinib-treated WM451 cells was detected using the Transwell assay. Magnification, ×100. **P<0.01 and ***P<0.001 vs. control (DMSO).
Tepotinib suppresses the epithelial-mesenchymal transition (EMT) process of melanoma cells
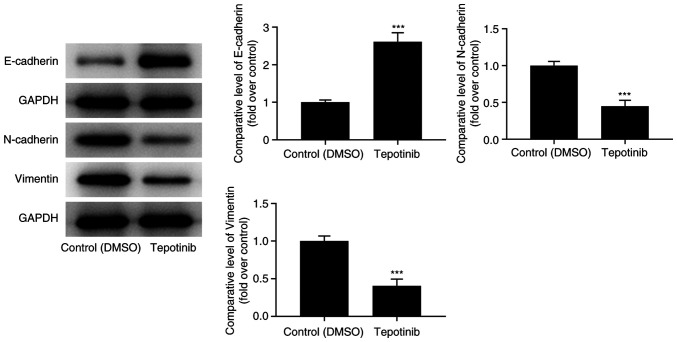
The expression levels of EMT-related proteins were assessed with western blot analysis. Tepotinib treatment caused an apparent upregulation of E-cadherin expression levels, while downregulating N-cadherin and vimentin expression levels compared with those of the control group (Fig. 2). In summary, the aforementioned results suggested that tepotinib exerted inhibitory effects on the EMT process of malignant melanoma cells.
Figure 2.
Tepotinib suppresses the EMT process of melanoma cells. The expression levels of EMT-related proteins (E-cadherin, N-cadherin and vimentin) in WM451 cells treated with or without 10 ng/ml tepotinib were evaluated with western blot analysis. ***P<0.001 vs. control (DMSO) group. EMT, epithelial-mesenchymal transition.
Tepotinib promotes the apoptosis of melanoma cells
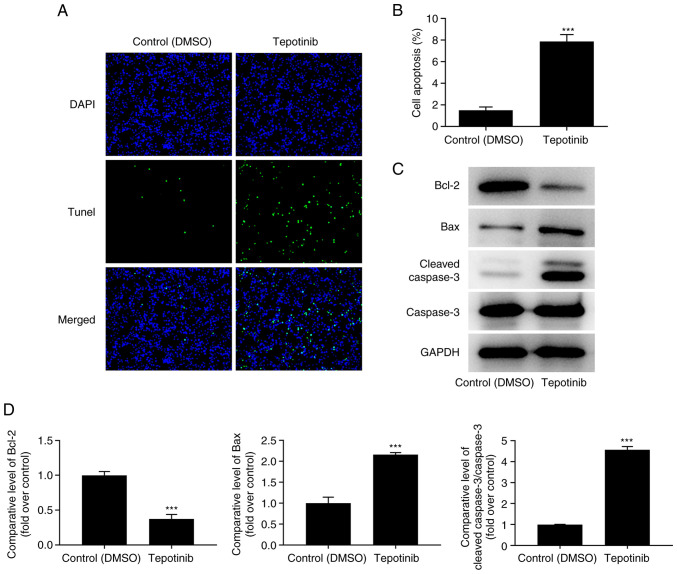
The induction of apoptosis of tepotinib-treated WM451 cells was evaluated using TUNEL staining. The induction of apoptosis of WM451 cells was significantly increased following tepotinib (10 ng/ml) treatment compared with that of the control group, revealing that this compound could induce apoptosis of WM451 cells (Fig. 3A and B). In addition, the expression levels of the apoptosis-related proteins were detected by western blot analysis and the results indicated that tepotinib significantly downregulated Bcl-2 expression, while upregulating the expression levels of Bax and cleaved caspase 3 in comparison with the corresponding effects noted in the control group (Fig. 3C and D). In conclusion, the data indicated that tepotinib induced apoptosis of melanoma cells.
Figure 3.
Tepotinib induces apoptosis of melanoma cells. (A and B) The apoptosis of 10 ng/ml tepotinib-treated WM451 cells was examined by TUNEL staining. Magnification, ×200. (C and D) The expression levels of apoptosis-related proteins in WM451 cells treated with or without 10 ng/ml tepotinib were detected using western blot analysis. ***P<0.001 vs. control (DMSO) group.
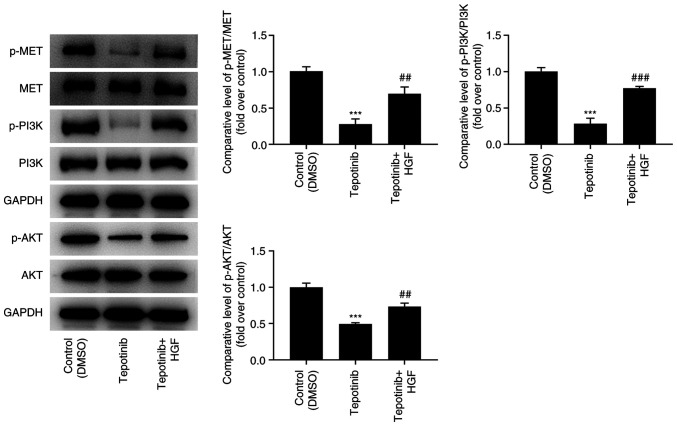
Tepotinib reduces activation of MET and PI3K/AKT signaling pathways
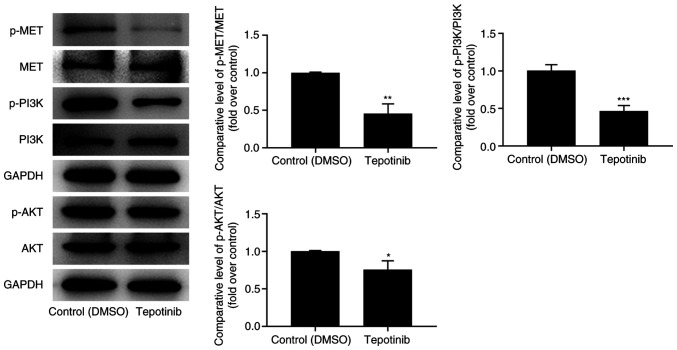
To investigate the effects of tepotinib on MET and PI3K/AKT signaling pathways, western blot analysis was utilized to measure the expression levels of MET, phosphorylated (p)-MET, PI3K, p-PI3K, AKT and p-AKT. Tepotinib diminished the expression levels of MET, p-MET, p-PI3K and p-AKT compared with those noted in the control group, suggesting that it exhibited inhibitory effects on activation of MET and PI3K/AKT signaling pathways (Fig. 4).
Figure 4.
Tepotinib restrains activation of MET and PI3K/AKT signaling pathways in melanoma cells. The expression levels of p-MET, MET, p-PI3K, PI3K, p-AKT and AKT in WM451 cells treated with or without 10 ng/ml tepotinib were determined using western blot analysis. *P<0.05, **P<0.01 and ***P<0.001 vs. control (DMSO) group. MET, MET proto-oncogene, receptor tyrosine kinase; p-, phosphorylated.
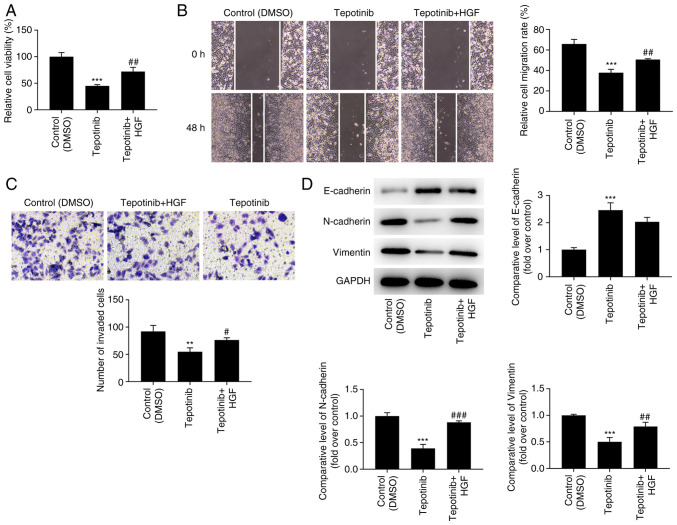
Tepotinib inhibits the proliferation, migration and invasion of WM451 cells by reducing activation of MET and PI3K/AKT signaling pathways
In order to assess the detailed mechanism of action of tepotinib, HGF (50 ng/ml), which is a natural agonist of MET, was administered to WM451 cells for 48 h. As exhibited in Fig. 5, the addition of HGF significantly upregulated the expression of p-MET, p-PI3K and p-AKT when compared with the tepotinib group. Moreover, tepotinib inhibited the proliferative ability of WM451 cells, which was partially reversed by HGF, indicating the beneficial effects of this factor on the proliferation of tepotinib-treated WM451 cells (Fig. 6A). Tepotinib considerably diminished the relative migration rate of WM451 cells, while HGF administration partially abolished the suppressive effects of tepotinib, as demonstrated by the increased migration of the tepotinib + HGF cell group in comparison with that noted in the tepotinib cell group (Fig. 6B). Similarly, the declined invasive activity of tepotinib-treated WM451 cells was subsequently enhanced following HGF administration (Fig. 6C). Besides, tepotinib increased E-cadherin expression, while reducing the expression levels of N-cadherin and vimentin, whereas HGF administration exhibited the opposite effects, as determined by the decreased expression levels of E-cadherin and the increased expression levels of N-cadherin and vimentin in the tepotinib + HGF group (Fig. 6D).
Figure 5.
HGF activates MET and PI3K/AKT signaling pathways in melanoma cells. The expression levels of p-MET, MET, p-PI3K, PI3K, p-AKT and AKT in WM451 cells treated with or without 10 ng/ml tepotinib and 50 ng/ml HGF were evaluated using western blot analysis. ***P<0.001 vs. control (DMSO) group; ##P<0.01 and ###P<0.001 vs. tepotinib. MET, MET proto-oncogene, receptor tyrosine kinase; p-, phosphorylated; HGF, hepatocyte growth factor.
Figure 6.
Tepotinib inhibits proliferation, migration, and invasion of WM451 cells by restraining the activation of MET and PI3K/AKT signaling pathways. (A) The proliferation of WM451 cells treated with or without 10 ng/ml tepotinib and 50 ng/ml HGF was detected using the MTT assay. (B) The migratory activity of WM451 cells treated with or without 10 ng/ml tepotinib and 50 ng/ml HGF was assessed using the wound healing assay. Magnification, ×100. (C) The invasive activity of WM451 cells treated with or without 10 ng/ml tepotinib and 50 ng/ml HGF was detected using the Transwell assay. Magnification, ×100. (D) The expression levels of the EMT-related proteins in WM451 cells treated with or without 10 ng/ml tepotinib and 50 ng/ml HGF were detected using western blot analysis. **P<0.01 and ***P<0.001 vs. control (DMSO) group; #P<0.05, ##P<0.01 and ###P<0.001 vs. tepotinib. MET, MET proto-oncogene, receptor tyrosine kinase; EMT, epithelial-mesenchymal transition; HGF, hepatocyte growth factor.
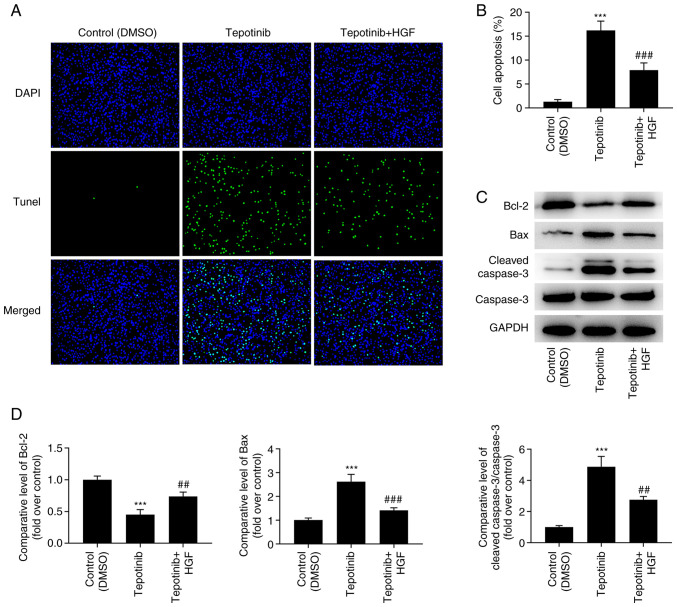
Tepotinib promotes the induction of apoptosis of WM451 cells by reducing the activation of the MET pathway and inhibiting the PI3K/AKT signaling pathway
According to the results obtained from the TUNEL assay, the increased apoptotic levels caused by tepotinib treatment were significantly diminished by HGF administration (Fig. 7A and B). In addition, the expression levels of the apoptosis-related proteins were measured by western blot analysis and the results revealed that tepotinib significantly downregulated Bcl-2 expression levels, while causing a significant upregulation in the expression levels of Bax and cleaved caspase 3. Nevertheless, HGF administration exerted the opposite effects on these proteins, as determined by the increased Bcl-2 expression levels as well as the decreased expression levels of Bax and cleaved caspase 3 in the tepotinib + HGF group (Fig. 7C and D).
Figure 7.
Tepotinib promotes apoptosis of WM451 cells by restraining the activation of MET and the PI3K/AKT signaling pathways. (A and B) Apoptosis of WM451 cells treated with or without 10 ng/ml tepotinib and 50 ng/ml HGF was detected using TUNEL staining. Magnification, ×200. (C and D) The expression levels of the apoptosis-related proteins in WM451 cells treated with or without 10 ng/ml tepotinib and 50 ng/ml HGF were detected using western blotting. ***P<0.001 vs. control (DMSO) group; ##P<0.01 and ###P<0.001 vs. tepotinib. MET, MET proto-oncogene, receptor tyrosine kinase; HGF, hepatocyte growth factor.
Discussion
To the best of our knowledge, the present study is the first to explore the effects of tepotinib on melanoma. The effects of this compound on the proliferation of WM451 cells were assessed and the results indicated that it may suppress the proliferative ability of WM451 cells in a concentration-dependent manner. Subsequently, a series of cellular experiments were conducted to examine the effects of tepotinib on melanoma cells. The data indicated that tepotinib exerted suppressive effects on the migration, invasion, and the EMT, while promoting the induction of apoptosis of WM451 cells. It was also shown that tepotinib diminished the expression levels of p-MET, p-PI3K and p-AKT, indicating the inhibitory effects of this compound on the activation of MET and PI3K/AKT signaling pathways. To further assess the mechanism of action of tepotinib, HGF, an agonist of MET, was applied to treat WM451 cells. The data indicated that the effects of tepotinib on the proliferation, migration, invasion and apoptosis of WM451 cells were partially reversed by the activation of MET and PI3K/AKT signaling pathways.
Melanoma is the most devastating form of skin cancer, contributing to the largest number of skin cancer-related deaths globally which was estimated as 57,000 deaths in 2020 (21). One of the main characteristics of cancer cells is the abnormal and uncontrolled proliferation (22). In addition, migration and invasion are considered to contribute to cancer-related death (22). Moreover, the alteration of the rate of apoptosis participates in the advancement and progression of tumors (23). Based on this finding, it can be concluded that the underlying mechanism of melanoma involves proliferation, migration, invasion and apoptosis. Since tepotinib is a highly selective oral MET inhibitor, it has shown promising prospect in the treatment of MET-driven tumors (24). In the present study, it was found that tepotinib suppressed the proliferation of WM451 cells in a concentration-dependent manner. In addition, it was also revealed that tepotinib suppressed migration and invasion and promoted the apoptosis of WM451 cells. Furthermore, tepotinib reduced the expression levels of N-cadherin and vimentin in WM451 cells, indicating its suppressive effects on the EMT of melanoma cells.
It has been shown that the expression of the HGF receptor MET is associated with the malignant stage of melanoma (25). In preclinical studies, the increase in HGF/c-MET activity was shown to activate the proliferation of melanoma cells (26), increase their invasive ability (27), and protect them from the induction of apoptosis (28). The protein kinase signaling pathways are involved in promoting tumor proliferation, migration and invasion. The abnormal activation of the PI3K/AKT signaling is related to the occurrence and development of a variety of cancer types and plays crucial roles in regulating cell migration, invasion and other biological processes (29,30). Previous studies have demonstrated that the inhibition of the PI3K/AKT pathway contributes to the suppression of melanoma progression (31–33). In addition, the activation of MET can promote the proliferation, migration and invasion of tumor cells by regulating intracellular signaling pathways, such as the PI3K/AKT signaling pathway (34). In the present study, it was shown that tepotinib suppressed the activation of MET and PI3K/AKT signaling.
To further investigate the underlying mechanism of action of MET and PI3K/AKT signaling on the proliferation, invasion, migration and apoptosis of melanoma cells, WM451 cells were treated with tepotinib and the activation of MET and PI3K/AKT signaling was assessed by using HGF, which is an agonist of MET. The present study indicated that the diminished proliferation, migration and invasion of WM451 cells caused by tepotinib treatment was improved by HGF, implying that the activation of MET and PI3K/AKT signaling abolished the protective effects of tepotinib on melanoma cells. Moreover, the increased levels of apoptosis and the expression levels of the pro-apoptotic proteins Bax and cleaved caspase 3 were decreased in tepotinib-treated WM451 cells following HGF administration. These findings indicated that tepotinib protected against malignant melanoma cell proliferation by blocking the activation of MET and PI3K/AKT signaling pathways.
In summary, the present study indicated that tepotinib inhibited proliferation, invasion, and migration of melanoma cells, while inducing apoptosis of melanoma cells by blocking the MET and the PI3K/AKT signaling pathways. However, the side effects of tepotinib on melanoma cells were not explored in the present study. Therefore, additional studies need to be carried out. Additionally, the usage of only one melanoma cell line to explore the effects of tepotinib on the malignant biological behaviors of melanoma is another limitation of the present study; more melanoma cell lines will be recruited in the future experiments to support the present conclusions.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
GJ and FY designed the study and analyzed the data. HX performed the experiments. GJ and FY drafted the manuscript and interpreted the data. FY and HX confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bai R, Huang H, Li M, Chu M. Temporal trends in the incidence and mortality of skin malignant melanoma in China from 1990 to 2019. J Oncol. 2021;24:9989824. doi: 10.1155/2021/9989824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magro CM, Crowson AN, Mihm MC. Unusual variants of malignant melanoma. Mod Pathol. 2006;19((Suppl 2)):S41–S70. doi: 10.1038/modpathol.3800516. [DOI] [PubMed] [Google Scholar]
- 3.Koh HK. Cutaneous melanoma. N Engl J Med. 1991;325:171–182. doi: 10.1056/NEJM199107183250306. [DOI] [PubMed] [Google Scholar]
- 4.Miller AJ, Mihm MC., Jr Melanoma. N Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 5.Arrington JH, III, Reed RJ, Ichinose H, Krementz ET. Plantar lentiginous melanoma: A distinctive variant of human cutaneous malignant melanoma. Am J Surg Pathol. 1977;1:131–143. doi: 10.1097/00000478-197706000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Clark WH, Jr, From L, Bernardino EA, Mihm MC. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705–727. [PubMed] [Google Scholar]
- 7.McGovern VJ. The classification of melanoma and its relationship with prognosis. Pathology. 1970;2:85–98. doi: 10.3109/00313027009077330. [DOI] [PubMed] [Google Scholar]
- 8.de Souza CF, Morais AS, Jasiulionis MG. Biomarkers as key contributors in treating malignant melanoma metastases. Dermatol Res Pract. 2012;2012:156068. doi: 10.1155/2012/156068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fecher LA, Cummings SD, Keefe MJ, Alani RM. Toward a molecular classification of melanoma. J Clin Oncol. 2007;25:1606–1620. doi: 10.1200/JCO.2006.06.0442. [DOI] [PubMed] [Google Scholar]
- 10.Somasundaram R, Villanueva J, Herlyn M. Intratumoral heterogeneity as a therapy resistance mechanism: Role of melanoma subpopulations. Adv Pharmacol. 2012;65:335–359. doi: 10.1016/B978-0-12-397927-8.00011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falchook GS, Kurzrock R, Amin HM, Xiong W, Fu S, Piha-Paul SA, Janku F, Eskandari G, Catenacci DV, Klevesath M, et al. First-in-man phase I trial of the selective MET inhibitor tepotinib in patients with advanced solid tumors. Clin Cancer Res. 2020;26:1237–1246. doi: 10.1158/1078-0432.CCR-19-2860. [DOI] [PubMed] [Google Scholar]
- 12.Friese-Hamim M, Bladt F, Locatelli G, Stammberger U, Blaukat A. The selective c-Met inhibitor tepotinib can overcome epidermal growth factor receptor inhibitor resistance mediated by aberrant c-Met activation in NSCLC models. Am J Cancer Res. 2017;7:962–972. [PMC free article] [PubMed] [Google Scholar]
- 13.Sohn SH, Sul HJ, Kim B, Kim BJ, Kim HS, Zang DY. Tepotinib inhibits the epithelial-mesenchymal transition and tumor growth of gastric cancers by increasing GSK3beta, E-cadherin, and mucin 5AC and 6 levels. Int J Mol Sci. 2020;21:6027. doi: 10.3390/ijms21176027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bladt F, Faden B, Friese-Hamim M, Knuehl C, Wilm C, Fittschen C, Grädler G, Meyring M, Dorsch D, Jaehrling F, et al. EMD 1214063 and EMD 1204831 constitute a new class of potent and highly selective c-Met inhibitors. Clin Cancer Res. 2013;19:2941–2951. doi: 10.1158/1078-0432.CCR-12-3247. [DOI] [PubMed] [Google Scholar]
- 15.Bladt F, Friese-Hamim M, Ihling C, Wilm C, Blaukat A. The c-met inhibitor MSC2156119J effectively inhibits tumor growth in liver cancer models. Cancers (Basel) 2014;6:1736–1752. doi: 10.3390/cancers6031736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Silva DM, Roy A, Kato T, Cecchi F, Lee YH, Matsumoto K, Bottaro DP. Targeting the hepatocyte growth factor/Met pathway in cancer. Biochem Soc Trans. 2017;45:855–870. doi: 10.1042/BST20160132. [DOI] [PubMed] [Google Scholar]
- 17.Cao HH, Cheng CY, Su T, Fu XQ, Guo H, Li T, Tse AKW, Kwan HY, Yu H, Yu ZL. Quercetin inhibits HGF/c-Met signaling and HGF-stimulated melanoma cell migration and invasion. Mol Cancer. 2015;14:103. doi: 10.1186/s12943-015-0367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y, Shi X, Sheng K, Han G, Li W, Zhao Q, Jiang B, Feng J, Li J, Gu Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review) Mol Med Rep. 2019;19:783–791. doi: 10.3892/mmr.2018.9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan AC. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC) Thorac Cancer. 2020;11:511–518. doi: 10.1111/1759-7714.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies MA. The role of the PI3K-AKT pathway in melanoma. Cancer J. 2012;18:142–147. doi: 10.1097/PPO.0b013e31824d448c. [DOI] [PubMed] [Google Scholar]
- 21.Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021 Apr 5; doi: 10.1002/ijc.33588. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 22.Lu W, Zhang H, Niu Y, Wu Y, Sun W, Li H, Kong J, Ding K, Shen HM, Wu H, et al. Long non-coding RNA linc00673 regulated non-small cell lung cancer proliferation, migration, invasion and epithelial mesenchymal transition by sponging miR-150-5p. Mol Cancer. 2017;16:118. doi: 10.1186/s12943-017-0685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D'Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY) 2016;8:603–619. doi: 10.18632/aging.100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, Mazieres J, Viteri S, Senellart H, Meerbeeck JV, et al. Tepotinib in non-small-cell lung cancer with met exon 14 skipping mutations. N Engl J Med. 2020;383:931–943. doi: 10.1056/NEJMoa2004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YJ, Kim DH, Lee SH, Kim DW, Nam HS, Cho MK. Expression of the c-Met proteins in malignant skin cancers. Ann Dermatol. 2011;23:33–38. doi: 10.5021/ad.2011.23.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Recio JA, Merlino G. Hepatocyte growth factor/scatter factor activates proliferation in melanoma cells through p38 MAPK, ATF-2 and cyclin D1. Oncogene. 2002;21:1000–1008. doi: 10.1038/sj.onc.1205150. [DOI] [PubMed] [Google Scholar]
- 27.Otsuka T, Takayama H, Sharp R, Celli G, LaRochelle WJ, Bottaro DP, Ellmore N, Vieira W, Owens JW, Anver M, Merlino G. c-Met autocrine activation induces development of malignant melanoma and acquisition of the metastatic phenotype. Cancer Res. 1998;58:5157–5167. [PubMed] [Google Scholar]
- 28.Beuret L, Flori E, Denoyelle C, Bille K, Busca R, Picardo M, Bertolotto C, Ballotti R. Up-regulation of MET expression by alpha-melanocyte-stimulating hormone and MITF allows hepatocyte growth factor to protect melanocytes and melanoma cells from apoptosis. J Biol Chem. 2007;282:14140–14147. doi: 10.1074/jbc.M611563200. [DOI] [PubMed] [Google Scholar]
- 29.Cheng JC, Chou CH, Kuo ML, Hsieh CY. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene. 2006;25:7009–7018. doi: 10.1038/sj.onc.1209706. [DOI] [PubMed] [Google Scholar]
- 30.Chen WY, Xu YY, Zhang XY. Targeting GOLM1 by microRNA-200a in melanoma suppresses cell proliferation, invasion and migration via regulating PI3K/Akt signaling pathway and epithelial-mesenchymal transition. Eur Rev Med Pharmacol Sci. 2019;23:6997–7007. doi: 10.26355/eurrev_201908_18740. [DOI] [PubMed] [Google Scholar]
- 31.Dang N, Meng X, Ma S, Zhang Q, Sun X, Wei J, Huang S. MDA-19 suppresses progression of melanoma via inhibiting the PI3K/Akt pathway. Open Med (Wars) 2018;13:416–424. doi: 10.1515/med-2018-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Li L, Liu S, Zhao Y, Wang L, Du G. FOXC1 promotes melanoma by activating MST1R/PI3K/AKT. Oncotarget. 2016;7:84375–84387. doi: 10.18632/oncotarget.11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi EO, Cho EJ, Jeong JW, Park C, Hong SH, Hwang HJ, Moon SK, Son CG, Kim WJ, Choi YH. Baicalein inhibits the migration and invasion of B16F10 mouse melanoma cells through inactivation of the PI3K/Akt signaling pathway. Biomol Ther (Seoul) 2017;25:213–221. doi: 10.4062/biomolther.2016.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma N, Adjei AA. In the clinic: Ongoing clinical trials evaluating c-MET-inhibiting drugs. Ther Adv Med Oncol. 2011;3((Suppl 1)):S37–S50. doi: 10.1177/1758834011423403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.