Abstract
Background
Intermittent wheezing illnesses, which include viral associated wheeze and asthma, are amongst the most common reasons for children to present urgently to a doctor. Whether parents should commence oral corticosteroids (OCS) for an episode of acute wheeze in their child without waiting for a medical review is an important question, as the potential benefits of early oral corticosteroid intervention have to be weighed against the potential adverse effects of treatment.
Objectives
The objectives were to assess the benefits and harmful effects of parent‐initiated OCS, in the management of intermittent wheezing illnesses in children, based on the results of randomised clinical trials.
Search methods
The Cochrane Airways Group Specialised Register, The Cochrane Controlled Trials Register (CENTRAL), MEDLINE, EMBASE, LILACS, Web of Science and Dissertation Abstracts were combined (all searched November 2008). Manufacturers and researchers in the field were also contacted.
Selection criteria
Only randomised clinical trials studying patients aged between one and eighteen years old, with an intermittent wheezing illness (asthma, viral wheeze, preschool viral wheeze) were included. Interventions encompassed OCS at any dose or duration versus placebo or other drug combination. The trials could be unpublished or published and no language limitations were applied.
Data collection and analysis
Two reviewers independently selected trials for inclusion, assessed trial quality and extracted the data. The statistical package (RevMan) provided by the Cochrane Collaboration was used.
Main results
From 572 original citations, a total of 2 randomised clinical trials (303 randomised participants) were included. The quality of the included trials was high; however, marked clinical heterogeneity precluded a meta‐analysis. The two trials did not find evidence that parent‐initiated OCS are associated with a benefit in terms of hospital admissions, unscheduled medical reviews, symptoms scores, bronchodilator use, parent and patient impressions, physician assessment, or days lost from work or school. Adverse outcomes were inadequately documented.
Authors' conclusions
Limited current evidence is available and it is inconclusive regarding the benefit from parent‐initiated OCS in the treatment of intermittent wheezing illnesses in children. Widespread use of this strategy cannot be recommended until the benefits and harms can be clarified further.
Plain language summary
Parent‐initiated oral corticosteroid therapy for intermittent wheezing illnesses in children
Oral corticosteroids have been shown to be effective in the treatment of acute asthma in children if they are commenced after the child has been assessed by a doctor. This review identified two randomised trials of parent‐initiated oral corticosteroids in the management of an intermittent wheezing illness in children. There was no evidence of more benefit than harm. Widespread use of this strategy cannot be recommended at present. Further randomised clinical trials are required.
Background
Intermittent wheezing illnesses in children, which include viral associated wheeze and asthma, are one of the leading causes of emergency department presentations and hospital admission worldwide (Bousquet 2005). Oral corticosteroids (OCS) commenced following physician review are effective in the treatment of acute asthma in children (Rowe 2001). Accordingly, the four major international asthma management guidelines suggest that OCS should be commenced as soon as possible in an episode of acute asthma (CAG 2005; BTS 2003; GINA 2003; NIH 2002). Each describes a role for parent‐initiated OCS in their treatment algorithms and written asthma management plans, but stops short of explicitly recommending in the text of the guideline that parents be instructed to independently commence OCS without a medical review. To date a systematic review of the role of parent‐initiated OCS in intermittent wheezing illnesses during childhood has not been published.
Many of the clinical benefits of corticosteroids in the setting of an intermittent wheezing illness have been shown to begin within 1‐3 hours of administration (Rachelefsky 2003) and it would seem logical that parent‐initiated treatment may be an effective strategy. However, it is important to recognise two important limitations of this theory. First, only a minority of episodes of acute wheeze result in an unscheduled medical review (Robertson 2004). Hence the group of episodes of wheeze for which parents would be initiating treatment with OCS are likely to be less severe. Second, the strategy relies on the ability of the parent to make an accurate assessment of their child's clinical status, and there are a number of studies which have demonstrated that parents have some difficulty in making this assessment (Levy 2004; Lowe 2004). It is also important to recognise that parent‐initiated treatment with OCS is likely to result in more frequent administration of OCS to children with intermittent wheeze, and this may have important implications for growth, osteoporosis, and other unwanted side effects.
During the first months of life RSV bronchiolitis is the predominant cause of intermittent wheeze. It is contentious that corticosteroids have any role in the management of RSV bronchiolitis (Patel 2004). Therefore parent‐initiated OCS cannot be recommended for this group of patients. Beyond 12 months of age two, quite distinct subgroups of intermittent childhood wheeze have been identified. Younger children tend to suffer from discrete episodes of wheeze in association with viral respiratory tract infections (Mertsola 1991; Kuehni 2001), they generally do not have risk factors for atopic sensitization (Sherrill 1999) and are often asymptomatic by six years of age (Martinez 1995). This disease entity has been described as viral associated wheeze. By comparison older children with intermittent wheeze are characterized by the presence of atopy and increased airway responsiveness to a range of stimuli, in keeping with atopic asthma (Louis 2000; Stein 1997). There is no tool or marker which can reliably distinguish between viral associated wheeze and asthma. As such this review will assess the role of parent‐initiated OCS in intermittent wheezing illnesses in children aged 1 to 18 years of age overall. A subgroup analysis will then be used to assess the role of parent‐initiated OCS in children with those factors that are most strongly associated with the diagnosis of asthma, namely a history of eczema, a family history of asthma in a first degree relative and age (Martinez 1995). Researchers have employed various other clinical and laboratory markers in an attempt an attempt to distinguish asthma from viral associated wheeze, however none of these markers have been shown to have sufficient sensitivity nor specificity to be clinically useful or to form a justifiable basis for a subgroup analysis.
Objectives
To determine the effectiveness of parent‐initiated OCS in the management of children with acute wheeze who have a background of:
intermittent wheeze,
intermittent wheeze plus risk factors for atopic asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials in which parent‐initiated (i.e. without preceding physician review) OCS were compared with placebo, or any other drug, or drug combinations, for children aged between 1 and 18 years.
Types of participants
Children aged 1 to 18 years, with intermittent wheeze, treated with OCS or the comparison medication(s) independently initiated by the parent or child. Intermittent wheeze was defined as having previously suffered from discrete wheezing episodes. Trial participants may have been recruited from any setting (emergency room, observation unit, in‐patient, out‐patient, general practice or the community) to take part in a trial of parent‐initiated OCS for subsequent episodes of intermittent wheeze.
Types of interventions
Studies were considered for inclusion if participants were randomised to receive parent‐initiated OCS compared to a comparison medication, or placebo, prior to being seen by clinical staff (e.g. nurse practitioner, nurse, physician). All doses and dosing regimes for OCS were included. Co‐interventions were permitted, therefore we recorded data pertaining to co‐interventions received. If these data were not available within the manuscript, we requested them from the study authors.
Types of outcome measures
Primary outcomes
The primary outcome measure was admission to hospital with a wheezing illness.
Secondary outcomes
Unscheduled medical review;
Changes in symptoms or symptom scores;
Bronchodilator use;
Duration of hospital stay;
Parent and patient perceptions;
Physician assessment;
Lost time from work (parents), or school (children);
Asthma free days;
Adverse effects.
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see the Airways Group Module for further details). All records in the Specialised Register coded as 'asthma' were searched using the following terms:
(prednisolone OR prednisone OR methyl‐prednisolone OR methylprednisolone OR MP OR corticosteroid* OR glucocorticoid* OR *steroid* OR solucortef OR solu‐cortef OR solumedrol OR dexamethasone) AND (parent* OR mother* OR father* OR famil* OR carer* OR caregiver* OR guardian* OR home* OR ambulat*)
The most recent search of the Specialised Register was conducted in November 2008.
Additonal searches were also conducted on the following bibliographic databases: Cochrane Central Register of Controlled Trials (CENTRAL Issue 4/2005), MEDLINE (1966‐Nov 2005), EMBASE (1980 to Nov 2005), LILACS, Web of Science 1981‐Nov 2005 and Dissertation Abstracts. (See Appendix 1,Appendix 2 and Appendix 3 for the full MEDLINE, EMBASE and WOS strategies).
No language restrictions were applied to any of the searches.
Searching other resources
The reference lists of all selected articles, primary studies and review articles were examined for relevant studies. Primary authors were contacted for information on additional trials (published and unpublished). Clinicians, colleagues, collaborators and researchers were contacted to identify potentially relevant studies. Abstracts and proceedings of relevant scientific meetings were reviewed.
Data collection and analysis
Selection of studies
From the title, abstract, or descriptors, two reviewers (PV, MS) independently reviewed literature searches to identify potentially relevant trials for full review. Searches of bibliographies and texts were conducted to identify additional studies. From the full text using specific criteria, the same two reviewers (PV, MS) independently selected trials for inclusion. Agreement was to be measured using kappa statistics and disagreement was to be resolved by consensus or third party adjudication (CR); however, this was not needed as agreement was 100%.
Data extraction and management
Data were extracted independently by two reviewers (PV, MS). Unpublished data was requested from the primary authors when necessary. A standard form was used that described the following: characteristics of the study (design, methods of randomisation, withdrawals/dropouts); participants (age, gender); intervention (type, dose, route of administration, timing and duration of therapy, co‐interventions); control (agent and dose); outcomes (types of outcome measures, timing of outcomes, adverse events); and results. These data were entered into Review Manager (RevMan) by a third party (CR) who also served to identify and resolve dissimilarities.
Assessment of risk of bias in included studies
Assessment of methodological quality: Assessments of quality were completed independently by two reviewers (PV, MS). Two different methods of assessment were used. Firstly, using the Cochrane approach to assess the allocation of concealment, all trials were scored and entered using the following scale:
Grade A: Adequate concealment.
Grade B: Uncertain concealment.
Grade C: Clearly inadequate concealment.
In addition, each study was assessed using a 0‐5 scale (Jadad 1996) summarised as follows:
Was the study described as randomised (1=yes; 0=no)?
Was the study described as double‐blind (1=yes;0=no)?
Was there a description of withdrawal and dropouts (1=yes;0=no)?
Was the method of randomisation well described and appropriate (1=yes;0=no)?
Was the method of double blinding well described and appropriate (1=yes;0=no)?
Deduct 1 point if methods for randomisation OR blinding were inappropriate.
We planned to measure inter‐rater reliability by using kappa, and weighted kappa statistics for each of the two methodological criteria sets. In addition, whether the study used intention‐to‐treat analysis was recorded along with source(s) of funding.
Data synthesis
All data were entered into Review Manager. We planned to conduct separate analysis for the different intervention combinations (i.e. OCS alone, or OCS in combination with a short course of inhaled corticosteroids) versus the different control groups (i.e. inhaled β2‐agonist or placebo); however, pooling was not possible due to heterogeneity. For dichotomous variables, both individual statistics were expressed as odds ratios (OR) and relative risks (RR) with 95% confidence intervals (CI). For continuous data, individual data were reported as mean differences (MD) with 95% CI. Data which were only available as a mean difference and 95% CI or standard error or P value, were entered as generic inverse variance data. This method was added post‐protocol.
Pooled dichotomous variables were to be expressed as odds ratios (OR) and relative risks (RR) with 95% confidence intervals (CI). The number needed to treat (NNT) was to be derived to help clarify the degree of clinical benefit for a range of baseline rates and was to be calculated from the pooled OR. Pooled continuous variable were to be reported as the weighted mean difference (WMD) or standardized mean differences (SMD) with 95% CI. Time‐to‐event data were to be summarized by the log hazards ratio (Parmar 1998) and an overall log hazards ratio was to be calculated.
Subgroup analysis and investigation of heterogeneity
We planned to measure heterogeneity using the I2 test and pooled results were to be calculated using both random and fixed‐effects models. Possible sources of heterogeneity were to be assessed by subgroup and sensitivity analyses.
Subgroup analysis: We anticipated subgroup analyses to examine the effects of:
Age: Less than 6‐years‐old versus 6‐years of age or older;
The presence of a history of eczema and or a family history of asthma in first degree relative (suggestive of the diagnosis of asthma as distinct from viral associated wheeze).
Co‐interventions: With a short course of inhaled corticosteroids versus none; with maintenance inhaled corticosteroids versus none.
Severity of the intermittent wheezing illness: mild and moderate versus severe;
Baseline pattern of wheeze: chronic persistent versus episodic and frequent episodic.
In future review versions, when sufficient numbers of trials and the necessary data are available, these subgroup analyses will be performed.
Assessment of clinical heterogeneity: The influence of trial characteristics that may influence the observed treatment effect were to be explored according to:
Methodological quality of included trials;
Intention‐to‐treat status;
Recruitment setting (hospital or Emergency Department versus primary care or community setting).
In future review versions, when sufficient numbers of trials and the necessary data are available, clinical heterogeneity will be investigated.
Sensitivity analysis
Sensitivity analysis: Sensitivity analyses provide an approach for testing how robust the results of a review are relative to key decisions and assumptions that have been made in process of conducting the review. We planned to investigate three factors:
Publication bias ‐ The existence of publication bias using a funnel plot;
Quality ‐The effects of overall quality on the pooled result was examined using both the Cochrane approach and that of Jadad (Jadad 1996);
Statistical testing: random versus fixed effects.
In future review versions, when sufficient numbers of trials and the necessary data are available, these sensitivity analyses will be performed.
Results
Description of studies
Results of the search
The initial search strategy identified 596 citations. Of these, 594 citations were excluded for one or more of the following reasons: (1) duplicate references (n=9), (2) not a randomised controlled trial (n=299), (3) ongoing trials (n=1), (4) the subjects did not suffer from an intermittent wheezing illness of childhood (n=248), (5) intervention tested was not an OCS (n=565), (6) the intervention tested doctor‐initiated OCS rather than parent‐initiated OCS (n=9). Due to the large number of citations considered, the references and reasons for exclusion are provided only for full‐text randomised controlled trials involving children with an intermittent wheezing illness treated with an OCS. An update search run in November 2008 did not identify any additional studies.
Included studies
A total of 2 studies (303 children) met the criteria for inclusion (Grant 1995; Oommen 2003).
The first study (Grant 1995) comprised a randomised placebo controlled cross‐over trial involving 86 children 2 to 14 years of age with a diagnosis of 'asthma' who were identified from clinic records. The intervention consisted of a single 2 mg per kg dose of prednisolone. The patients were observed for a pre‐determined 12‐month period: 6 months of prednisolone vs. 6 months placebo. The primary outcome measure was the number of unscheduled medical reviews for acute asthma. The analysis was paired i.e. each of the 78 participants who completed the study was compared with his or herself. All participants who completed the study were included in the analysis, regardless whether or not they suffered from zero, a single or multiple episodes of acute wheezing. A secondary analysis was conducted which was restricted to participants who actually suffered one or more episodes of acute asthma treated with the study medication. However, we were unable to determine the method of this analysis or obtain the raw data.
The second study (Oommen 2003), a randomised placebo controlled trial, involved 217 children 1 to 5 years old who had been admitted to hospital with a diagnosis of viral wheeze. The intervention consisted of 20 mg of prednisolone administered once daily for 5 days. The period of observation was not pre‐determined and ranged from several months to more than three years. The primary outcomes measures were the mean 7‐day day‐time and 7‐day night‐time symptom scores. The analysis comprised a parallel comparison restricted to only those participants who suffered an episode of wheeze resulting in parent‐initiated treatment with the study medication and successfully completed the symptom diary.
Risk of bias in included studies
Both trials had high methodological quality, and received a Jadad score of five. The authors of the first study (Grant 1995) failed to adequately report their methods of randomisation and allocation concealment; however, the adequacy of these processes was confirmed following correspondence with the authors. The second study (Oommen 2003) had high methodological quality without requiring correspondence. Neither study involved an intention to treat analysis.
Effects of interventions
The degree of clinical heterogeneity between the included trials precluded a meta‐analysis. In addition, it was not possible to extract or obtain the required data from the first paper (Grant 1995) in order express outcomes in the manner that had been planned.
Admission to hospital
In one study (Grant 1995), there were nine admissions to hospital during the period that the children were in the prednisolone group, and seven admissions when the children were in the placebo group (P=0.73). In the second study (Oommen 2003) 6 of 52 children treated with prednisolone, and 2 of the 69 treated with placebo, were admitted to hospital (OR: 4.4; 95% CI: 0.8 to 22.6).
Unscheduled medical reviews
In one study (Grant 1995) a significantly greater number of unscheduled medical reviews occurred during the period that the participants' study medication was prednisolone as compared to placebo (P=0.004). Data on unscheduled medical reviews overall were not recorded during the other study (Oommen 2003).
Changes in symptoms or symptom scores
Symptom data were not recorded in one study (Grant 1995). The authors of the other study (Oommen 2003) reported that the mean daytime symptom score recorded over 7 days did not differ between children treated with prednisolone vs. placebo (MD: ‐0.01; 95% CI: ‐0.22 to 0.2). They also found no difference in the mean night‐time symptom score recorded over 7 nights (MD: ‐0.12; 95% CI: ‐0.12 to 0.32)
Bronchodilator use
Data on bronchodilator use were not recorded during the first study (Grant 1995). The researchers responsible for the other study (Oommen 2003) reported that the geometric mean number of salbutamol actuations was similar between groups (ratio of the geometric means: 0.93; 95% CI: 0.65 to 1.32).
Duration of hospital stay
In one study (Grant 1995) there was no difference in the mean number of inpatient days per participant during the period that children were in the prednisolone group as compared to the placebo group (0.31 days vs. 0.35 days, P=0.82). Data on duration of hospital stay were not recorded in the other study (Oommen 2003).
Parent and patient perceptions
In one study (Grant 1995) data regarding parent or patient perceptions of treatment effectiveness were not recorded. In the other study (Oommen 2003) there was no difference in the proportion of parents who considered treatment effective between treatment groups (OR: 1.2; 95% CI: 0.5 to 2.7).
Physician assessment
Data on outcome measures reflecting physician assessment were not recorded during one study (Grant 1995). In the other study (Oommen 2003) the researchers recorded the number of occasions that the trial medication was substituted with oral prednisolone following physician assessment, which can be considered one measure of physician assessment. There was no difference in the rate of substitution of the trial medication between treatment groups (OR: 1.8; 95% CI: 0.6 to 5.0).
Lost time from work (parents) or school (children)
Data regarding time lost from work or school were not recorded during either study (Grant 1995; Oommen 2003).
Asthma free days
Data regarding asthma free days were not obtained during either study (Grant 1995; Oommen 2003).
Adverse effects
One study (Grant 1995) included data on a range of reported side effects associated with the administration of prednisolone vs. placebo (nausea, vomiting, headache, bad behaviour, laughing or crying and skin rash). The frequency of each of these was similar during both periods. Data regarding potential side effects were not recorded during the other study (Oommen 2003).
Subgroup analyses
The authors of the first paper (Grant 1995) repeated the analysis of unscheduled medical reviews separately for children five‐years of age and younger, and for those six‐years of age or older. The number of children within each of these two age groups was not reported/obtained. For children five‐years of age and younger significantly more episodes of wheeze resulted in an unscheduled medical review during the six‐month period that the study medication was prednisolone vs. placebo (P=0.009). Similarly, for those five years of age and younger there was a greater proportion of medicated episodes resulting in an unscheduled medical review during the six‐month period that the study medication was prednisolone versus placebo (P=0.04). For those six‐years of age or older there was no difference in the number or proportion of episodes, or medicated episodes resulting in an unscheduled medical review.
Discussion
There is a sound theoretical basis for the hypothesis that parent‐initiated OCS may be effective in the management of intermittent wheezing illnesses in children. However, this systematic review demonstrates that the limited amount of published data available do not show a benefit in terms of hospital admissions, unscheduled medical reviews, symptom scores, parent perceptions or the use of bronchodilators in children treated with parent‐initiated prednisolone compared with placebo. Indeed the data from the first paper (Grant 1995) suggests children treated with prednisolone may be more likely to require and unscheduled medical review. Moreover, there is evidence from at least one trial, that such an intervention actually increases medical visits, at least in the younger age groups.
The included data were insufficient to perform any of the planned subgroup analyses. The authors of the earliest study (Grant 1995) reported a subgroup analysis of children five years of age and younger and those six years of age and older. The younger children were more likely to require an unscheduled medical review during the six‐month period that their study medication was prednisolone (in keeping with the study's findings overall). Amongst the older children there was no difference detected between treatment groups. The proportion of children within these two age brackets was not reported, nor obtained following correspondence with the authors. Asthma prevalence falls with age (Martinez 1995), and therefore it is likely that this review contains little data relevant to children beyond the primary school years.
There are a number of limitations in the existing literature, in addition to the insufficient volume of data. The disease severity of the trial participants in the included studies is difficult to quantify. It is also difficult to know if parents in the included studies commenced study medication for an appropriate indication. Neither of the included papers described the process by which parents were educated about the protocol for commencing treatment, nor measurement of the ability of the parents to make this assessment accurately. Adverse effects of OCS were inadequately examined in the included trials. It is intriguing that the data from the earliest study (Grant 1995) suggested the parent‐initiated OCS may be associated with an increase in unscheduled medical reviews. This may relate to emotional and behavioural changes that have been well documented in children treated with 2 mg/kg of prednisolone (Kayani 2002). A more complete measurement of adverse effects would facilitate the assessment of benefit and harm.
Authors' conclusions
Implications for practice.
Limited research evidence is currently available upon which to base clinical conclusions.
This evidence is unable to demonstrate any benefit from parent‐initiated OCS in the treatment of intermittent wheezing illnesses in children. Widespread use of this strategy cannot be recommended until the benefits and harms can be clarified further.
There is no evidence to show benefits in the rate of hospitalisation or unscheduled medical reviews, changes in symptoms scores, bronchodilator use, duration of hospital stay, parent and perceptions, physician assessment, lost time from work or school or asthma free days. There are no adequate data on the potential adverse effects of this practice
Implications for research.
Large trials will be required to clarify the role of parent‐initiated OCS in intermittent wheezing illnesses in children. The optimal steroid type, dose and duration remain unknown.
Sufficient sample sizes will be required to determine the benefits in subgroups who may respond more favourably to steroid therapy. For example, children with risk factors for atopic asthma, children with more severe disease and school‐aged children should be clearly identified in future research.
It will be important to document the severity of disease amongst study participants and to optimise and measure the ability of parents to commence treatment for an appropriate indication.
Lastly, the potential adverse effects of OCS, in particular, emotional and behavioural changes, must be adequately measured and recorded.
What's new
| Date | Event | Description |
|---|---|---|
| 10 November 2008 | New search has been performed | Literature search re‐run; no new studies found |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 15 August 2008 | Amended | Converted to new review format. |
| 20 November 2007 | New search has been performed | New studies sought but none found: 20/11/07 |
| 1 November 2006 | New search has been performed | New studies found and included or excluded: 01/11/06 |
| 22 May 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The assistance of Brian Rowe, Toby Lasserson , Chris Cates and Elizabeth Arnold from the Cochrane Airways Group is gratefully acknowledged. We would like to thank Sharon Brennan for her help in processing the identified citations.
Appendices
Appendix 1. MEDLINE strategy
1. exp Asthma/ 2. asthma$.mp
3. exp Respiratory Sounds/ 4. wheez$.mp. 5. exp Respiratory Hypersensitivity/ 6. exp Bronchial Hyperreactivity/ 7. bronchospas$.mp.
8. bronchoconstric$.mp. 9. (bronch$ adj5 spas$).mp. 10. exp Bronchial Spasm/ 11. (airway$ adj5 obstruct$).mp. 12. or/1‐11 13. exp adrenal cortex hormones/ 14. exp steroids/ 15. exp glucocorticoids/ 16. (prednisolone or prednisone or methyl‐prednisolone or methylprednisolone or MP or corticosteroid$ or glucocorticoid$ or steroid$ or solucortef or solu‐cortef or solumedrol or dexamethasone).mp. 17. or/13‐16 18. exp parents/ or exp family/ 19. ((parent$ or mother$ or father$ or famil$ or carer$ or caregiver$ or guardian$ or home$) adj5 (initiat$ or suggest$ or instigat$ or originat$ or request$ or propose$ or encourage$ or advocate$)).mp. 20. exp Caregivers/ 21. or/18‐20 22. 12 and 17 and 21
RCT FILTER
1. (clinical trial or controlled clinical trial or randomized controlled trial).pt.
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
Appendix 2. EMBASE Strategy
1. exp Asthma/
2. asthma$.mp.
3. exp Respiratory Sounds/
4. wheez$.mp.
5. exp Respiratory Hypersensitivity/
6. exp Bronchial Hyperreactivity/
7. bronchospas$.mp.
8. bronchoconstric$.mp.
9. (bronch$ adj5 spas$).mp.
10. exp Bronchial Spasm/
11. (airway$ adj5 obstruct$).mp.
12. or/1‐11
13. exp adrenal cortex hormones/
14. exp steroids/
15. exp glucocorticoids/
16. (prednisolone or prednisone or methyl‐prednisolone or methylprednisolone or MP or corticosteroid$ or glucocorticoid$ or steroid$ or solucortef or solu‐cortef or solumedrol or dexamethasone).mp.
17. or/13‐16
18. exp parent/ or exp family/
19. ((parent$ or mother$ or father$ or famil$ or carer$ or caregiver$ or guardian$ or home$) adj5 (initiat$ or suggest$ or instigat$ or originat$ or request$ or propose$ or encourage$ or advocate$ or prefer$ or influence$)).mp.
20. exp Caregivers/
21. or/18‐20
22. 12 and 17 and 21
RCT FILTER
1. Randomized Controlled Trial/
2. Controlled Study/
3. randomization/
4. Double Blind Procedure/
5. Single Blind Procedure/
6. Clinical Trial/
7. Crossover Procedure/
8. follow up/
9. exp prospective study/
10. or/1‐9
11. (clinica$ adj3 trial$).mp.
12. ((singl$ or doubl$ or trebl$ or tripl$) adj5 (mask$ or blind$ or method$)).mp.
13. exp Placebo/
14. placebo$.mp.
15. random$.mp.
16. (latin adj3 square$).mp.
17. exp Comparative Study/
18. ((control$ or prospectiv$ or volunteer$) adj3 (trial$ or method$ or stud$)).mp.
19. (crossover$ or cross‐over$).mp.
20. or/11‐19
21. 10 or 20
22. exp ANIMAL/
23. Nonhuman/
24. Human/
25. 22 or 23
26. 25 not 24
27. 21 not 26
Appendix 3. Web of Science Strategy
#6 #4 and #5
#5 TS=(random* OR placebo* or double‐blind* or double blind* or single‐blind* or single blind* or crossover* or cross‐over* or controlled*) or TI=(RANDOM* OR PLACEBO* or double‐blind* or double blind* or single‐blind* or single blind* or crossover* or cross‐over* or controlled*)
#4 #1 AND #2 AND #3
#3 TS=(prednisolone or prednisone or methyl‐prednisolone or methylprednisolone or MP or corticosteroid* or glucocorticoid* or steroid* or solucortef or solu‐cortef or solumedrol or dexamethasone) OR TI=(prednisolone or prednisone or methyl‐prednisolone or methylprednisolone or MP or corticosteroid* or glucocorticoid* or steroid* or solucortef or solu‐cortef or solumedrol or dexamethasone)
#2 TS=(parent* or mother* or father* or family or carer* or caregiver* or home* or ambulat*) OR TI=(parent* or mother* or father* or family or carer* or caregiver* or home* or ambulat*)
#1 TS=(wheez* or asthma*) or TI=(wheez* or asthma*)
Data and analyses
Comparison 1. Prednisolone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Unscheduled medical review | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Admission to hospital | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Day‐time symptom score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Night‐time symptom score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Parent preferred treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Bronchodilator use | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Study medication substitution | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Number of attacks resulting in outpatient visit for acute asthma | 1 | Attacks/pt (Fixed, 95% CI) | Totals not selected | |
| 9 Proportion of attacks resulting in outpatient visit for acute asthma | 1 | Attacks/pt (Fixed, 95% CI) | Totals not selected | |
| 10 Number of medicated attacks resulting in outpatient visits for acute asthma | 1 | Attacks/pt (Fixed, 95% CI) | Totals not selected | |
| 11 Proportion of medicated attacks resulting in outpatient visits for acute asthma | 1 | Attacks/pt (Fixed, 95% CI) | Totals not selected |
1.2. Analysis.

Comparison 1 Prednisolone versus placebo, Outcome 2 Admission to hospital.
1.3. Analysis.
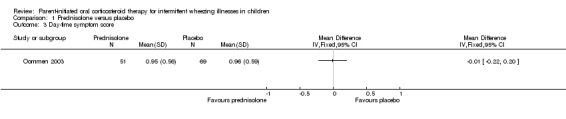
Comparison 1 Prednisolone versus placebo, Outcome 3 Day‐time symptom score.
1.4. Analysis.
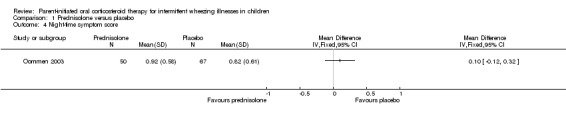
Comparison 1 Prednisolone versus placebo, Outcome 4 Night‐time symptom score.
1.5. Analysis.
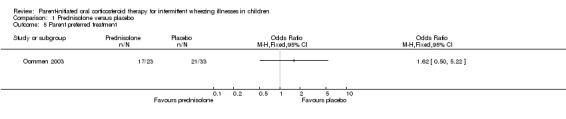
Comparison 1 Prednisolone versus placebo, Outcome 5 Parent preferred treatment.
1.6. Analysis.

Comparison 1 Prednisolone versus placebo, Outcome 6 Bronchodilator use.
1.7. Analysis.
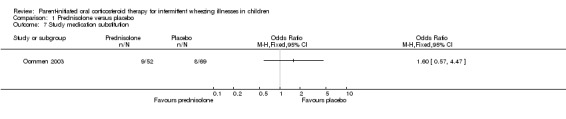
Comparison 1 Prednisolone versus placebo, Outcome 7 Study medication substitution.
1.8. Analysis.

Comparison 1 Prednisolone versus placebo, Outcome 8 Number of attacks resulting in outpatient visit for acute asthma.
1.9. Analysis.

Comparison 1 Prednisolone versus placebo, Outcome 9 Proportion of attacks resulting in outpatient visit for acute asthma.
1.10. Analysis.

Comparison 1 Prednisolone versus placebo, Outcome 10 Number of medicated attacks resulting in outpatient visits for acute asthma.
1.11. Analysis.

Comparison 1 Prednisolone versus placebo, Outcome 11 Proportion of medicated attacks resulting in outpatient visits for acute asthma.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Grant 1995.
| Methods | Cross‐over study: participants observed for two consecutive six month periods. | |
| Participants | 86 children (described as having asthma) 2 to 14 years of age who had made 2 or more unscheduled outpatient (emergency department or primary‐care clinic) visits in the preceding year. Mean age and gender balance was unclear. | |
| Interventions | A single 2mg per Kg dose of prednisolone. | |
| Outcomes | Unscheduled medical review. | |
| Notes | An intention to treat analysis was not conducted. The primary analysis comprised a paired T‐test in which each participant who completed the 12 month trial was compared with his or herself (6 month period with prednisolone vs. 6 month period with placebo). The reported data did not facilitate raw data extraction and the authors were unable to provide clarifications. Data restricted to episodes af wheeze that resulted in treatment with the study medication were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Study investigators unaware as to order of treatment group assignment |
Oommen 2003.
| Methods | Parallel randomised controlled trial. | |
| Participants | 217 children aged 1 to 5 years old who had been admitted to hospital with an episode of viral wheeze. Mean age 26 months, 68% male. | |
| Interventions | 20 mg prednisolone once daily for 5 days. | |
| Outcomes | Day‐time symptom score. Night‐time symptom score. Mean salbutamol actuations per day. Parents considered the treatment effective. Substitution of trial medication with oral prednisolone. Need for hospitalization. | |
| Notes | The analysis was only undertaken for those children who had an episode of wheeze for which the parents commenced treatment with the study medication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Study investigators unaware as to order of treatment group assignment |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agrawal 1999 | Treatment with OCS was only commenced after the participants had been reviewed by a doctor. |
| Gries 2000 | Treatment with OCS was only commenced after the participants had been reviewed by a doctor. |
| Harris 1987 | Treatment with OCS was only commenced after the participants had been reviewed by a doctor. |
| Horowitz 1994 | Treatment with OCS was only commenced after the participants had been reviewed by a doctor. |
| Klig 1997 | Treatment with OCS was only commenced after the participants had been reviewed by a doctor. |
| Qureshi 2001 | Treatment with OCS was only commenced after the participants had been reviewed by a doctor. |
| Scarfone 1993 | Treatment with OCS was only commenced after the participants had been reviewed by a doctor. |
| Scarfone 1995 | Treatment with OCS was only commenced after the participants had been reviewed by a doctor. |
| Webb 1986 | Treatment with OCS was only commenced after the participants had been reviewed by a doctor. |
Characteristics of ongoing studies [ordered by study ID]
Vuillermin.
| Trial name or title | Parent‐initiated prednisolone in asthma: randomised controlled trial |
| Methods | |
| Participants | Children 5 to 12 years of age. |
| Interventions | 3‐5 days of 1mg per kg of oral prednisolone |
| Outcomes | Daytime symptom score, night‐time symptom score, unscheduled medical reviews, hospital admission, substitution of the study medication, asthma‐free days, days of school missed, days of parent work missed. |
| Starting date | March 2005. |
| Contact information | Dr Peter Vuillermin. Email: peterv@barwonhealth.org.au Ph: +61 03 5260 3044 |
| Notes |
Contributions of authors
Dr Peter Vuillermin: Principal reviewer. Involved all aspects of the project's design and implementation.
Prof Mike South: Participated in project design. Selected studies for inclusion. Entered data on data extraction forms. Reviewed draft manuscripts.
Prof Colin Robertson: Pariciparted in project design. Reviewed list of included studies from PV and MS. Reviewed data extraction forms from PV and MS. Entered data into RevMan. Reviewed draft manuscipts.
Sources of support
Internal sources
Murdoch Children's Research Institute, Australia.
University of Melbourne, Australia.
External sources
No sources of support supplied
Declarations of interest
Dr. Peter Vuillermin, Professor Mike South and Associate Professor Colin Robertson are currently involved in a clinical trial to assess the effectiveness of parent‐initiated oral prednisolone in primary school aged children with asthma.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Grant 1995 {published data only}
- Grant CC, Dugan AK, DeAngelis C. Independent parental administration of prednisone in acute asthma: a double‐blind, placebo‐controlled, crossover study. Pediatrics 1995;96:224‐9. [PubMed] [Google Scholar]
Oommen 2003 {published data only}
- Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent‐initiated oral prednisolone for viral wheeze in children aged 1‐5 years: randomised controlled trial. Lancet 2003;362(9394):1433‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agrawal 1999 {published data only}
- Agrawal M, French L. Oral versus i.v. steroids for children hospitalized with asthma. Journal of Family Practice 1999;48(8):578‐9. [PubMed] [Google Scholar]
Gries 2000 {published data only}
- Gries DM, Moffitt DR, Pulos E, Carter ER. A single dose of intramuscularly administered dexamethasone acetate is as effective as oral prednisone to treat asthma exacerbations in young children. Journal of Pediatrics 2000;136(3):298‐303. [DOI] [PubMed] [Google Scholar]
Harris 1987 {published data only}
- Harris JB, Weinberger MM, Nassif E, Smith G, Milavetz G, Stillerman A. Early intervention with short courses of prednisone to prevent progression of asthma in ambulatory patients incompletely responsive to bronchodilators. Journal of Pediatrics 1987;110(4):627‐33. [DOI] [PubMed] [Google Scholar]
Horowitz 1994 {published data only}
- Horowitz L, Zafrir O, Gilboa S, Berger I, Wolach B. Acute asthma. Single dose oral steroids in paediatric community clinics. European Journal of Pediatrics 1994;153(7):526‐30. [DOI] [PubMed] [Google Scholar]
Klig 1997 {published data only}
- Klig JE, Hodge D 3rd, Rutherford MW. Symptomatic improvement following emergency department management of asthma: a pilot study of intramuscular dexamethasone versus oral prednisone. Journal of Asthma 1997;34(5):419‐25. [DOI] [PubMed] [Google Scholar]
Qureshi 2001 {published data only}
- Qureshi F, Zaritsky A, Poirier MP. Comparative efficacy of oral dexamethasone versus oral prednisone in acute pediatric asthma. Journal of Pediatrics 2001;139(1):20‐6. [DOI] [PubMed] [Google Scholar]
Scarfone 1993 {published data only}
- Scarfone RJ, Fuchs SM, Nager AL, Shane SA. Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics 1993;92(4):513‐8. [PubMed] [Google Scholar]
Scarfone 1995 {published data only}
- Scarfone RJ, Loiselle JM, Wiley JF 2nd, Decker JM, Henretig FM, Joffe MD. Nebulized dexamethasone versus oral prednisone in the emergency treatment of asthmatic children. Annals of Emergency Medicine 1995;26(4):480‐6. [DOI] [PubMed] [Google Scholar]
Webb 1986 {published data only}
- Webb MS, Henry RL, Milner AD. Oral corticosteroids for wheezing attacks under 18 months. Archive of Disease in Childhood 1986;61(1):15‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Vuillermin {unpublished data only}
- Parent‐initiated prednisolone in asthma: randomised controlled trial. Ongoing study March 2005..
Additional references
Bousquet 2005
- Bousquet J, Bousquet PJ, Godard P, Daures J. The public health implications of asthma. Bulletin of the World Health Organization 2005;83(7):548‐54. [PMC free article] [PubMed] [Google Scholar]
BTS 2003
- British Thoracic Society, Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax 2003;58(Suppl 1):i1‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
CAG 2005
- Becker A, Lemiere C, Berube D, Boulet LP, Ducharme FM, FitzGerald M, et al. Summary of recommendations from the Canadian Asthma Consensus Guidelines, 2003. Canadian Medical Association Journal 2005;173(6 Suppl):S3‐11. [PMC free article] [PubMed] [Google Scholar]
GINA 2003
- GINA. Global Strategy for Asthma Management and Prevention. NIH publication No. 02‐3659. 2003.
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Kayani 2002
- Kayani S, Shannon DC. Adverse behavioral effects of treatment for acute exacerbation of asthma in children: a comparison of two doses of oral steroids. Chest 2002;122(2):624‐8. [DOI] [PubMed] [Google Scholar]
Kuehni 2001
- Kuehni CE, Davis A, Brooke AM, Silverman M. Are all wheezing disorders in very young (preschool) children increasing in prevalence?. Lancet 2001;357(9271):1821‐5. [DOI] [PubMed] [Google Scholar]
Levy 2004
- Levy ML, Godfrey S, Irving CS, Sheikh A, Hanekom W, Bush A, et al. Wheeze detection: recordings vs. assessment of physician and parent. Journal of Asthma 2004;41(8):845‐53. [DOI] [PubMed] [Google Scholar]
Louis 2000
- Louis R, Lau LC, Bron AO, Roldaan AC, Radermecker M, Djukanovic R. The relationship between airways inflammation and asthma severity. American Journal of Respiratory and Critical Care Medicine 2000;161(1):9‐16. [DOI] [PubMed] [Google Scholar]
Lowe 2004
- Lowe L, Murray CS, Martin L, Deas J, Cashin E, Poletti G, at al. Reported versus confirmed wheeze and lung function in early life. Archives of Disease in Childhood 2004;89(6):540‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 1995
- Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. New England Journal of Medicine 1995;332(3):133‐8. [DOI] [PubMed] [Google Scholar]
Mertsola 1991
- Mertsola J, Ziegler T, Ruuskanen O, Vanto T, Koivikko A, Halonen P. Recurrent wheezy bronchitis and viral respiratory infections. Archives of Disease of Childhood 1991;66(1):124‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
NIH 2002
- NIH. National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the diagnosis and management of asthma update on selected topics‐‐2002. Journal of Allergy & Clinical Immunology 2002;110(5 Suppl):S141‐219. [PubMed] [Google Scholar]
Patel 2004
- Patel H, Platt R, Lozano JM, Wang EE. Glucocorticoids for acute viral bronchiolitis in infants and young children (Cochrane review). Cochrane Database of Systematic Reviews 2004, Issue 3. [Art. No.: CD004878. DOI: 10.1002/14651858.CD004878.] [DOI] [PubMed] [Google Scholar]
Rachelefsky 2003
- Rachelefsky G. Treating exacerbations of asthma in children: the role of systemic corticosteroids. Pediatrics 2003;112(2):382‐97. [DOI] [PubMed] [Google Scholar]
Robertson 2004
- Robertson CF, Roberts MF, Kappers JH. Asthma prevalence in Melbourne schoolchildren: have we reached the peak?. Medical Journal of Australia 2004;180(6):273‐6. [DOI] [PubMed] [Google Scholar]
Rowe 2001
- Rowe BH, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids (Cochrane review). Cochrane Database of Systematic Reviews 2001, Issue 1. [Art. No.: CD002178. DOI: 10.1002/14651858.CD002178.] [DOI] [PubMed] [Google Scholar]
Sherrill 1999
- Sherrill DL, Stein R, Halonen M, Holberg CJ, Wright A, Martinez FD. Total serum IgE and its association with asthma symptoms and allergic sensitization among children. Journal of Allergy and Clinical Immunology 1999;104(1):28‐36. [DOI] [PubMed] [Google Scholar]
Stein 1997
- Stein RT, Holberg CJ, Morgan WJ, Wright AL, Lombardi E, Taussig L, et al. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax 1997;52(11):946‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]


