Abstract
Objective
Assessment of intravascular volume status to ensure optimization before hospital discharge could significantly reduce readmissions. It is difficult to evaluate congestion on clinical signs during an episode of acute heart failure (ADHF) in elderly patients.
Hypothesis
There is an association between various volume overload parameters in patients older than 75 years.
Methods
We performed a single‐center prospective longitudinal study of patients older than 75 years hospitalized for acute heart failure. We analyzed the association between congestion assessment based on clinical signs, inferior vena cava (IVC) diameter measured by ultrasound, biological evaluation with N terminal pro brain natriuretic peptide (NT‐proBNP), and estimated plasma volume (EPV) during decongestive therapy. We also monitored changes in renal function.
Results
Fifty consecutive ADHF patients (85.2 ± 5.9 years, 68% female) were included in the study. At admission, a dilated, noncompliant IVC was found in all patients. The strongest correlations between different parameters of volume overload estimation were found between IVC and jugular vein distention (r = .8; p < .001), then IVC and oedema (r = .6; p < .001), IVC and crackles (r = .3; p < .036), then IVC and NT‐proBNP (r = .3; p = .02). There was no correlation between EPV and signs of congestion. Patients who had no congestive signs on clinical or IVC examination at Day 2, more often presented with acute renal failure.
Conclusion
In ADHF patients older than 75 years, clinical and IVC evaluation of intravascular congestion correlate well. The concomitant assessment of clinical signs and IVC may prevent depletion‐related renal failure.
Keywords: acute decompensated heart failure, arrhythmia/all, biomarkers, congestive state, management, older patients, ultrasound
Abbreviations
- ACEi
angiotensin‐converting‐enzyme inhibitor
- AF
atrial fibrillation
- AHA
American Heart Association
- ADHF
acute decompensated heart failure
- ALAT
alanine aminotransferase
- APE
acute pulmonary edema
- ARB
angiotensin II receptor blocker
- ARF
acute renal failure
- ASAT
aspartate‐amino‐transférase
- BP
blood pressure
- CHF
chronic heart failure
- CKD
chronic kidney disease
- COPD
chronic obstructive pulmonary disease
- D
diameter
- ECG
electrocardiogram
- ePVS
estimated plasma volume status
- ESC
European Society of Cardiology
- GFR
glomerular filtration rate
- Hb
hemoglobin
- HF‐PEF
heart failure with preserved ejection fraction
- HF‐REF
heart failure with reduced ejection fraction
- HJR
hepatojugular reflux
- HR
heart rate
- HTN
hypertension
- IHD
ischemic heart disease
- IVC
inferior vena cava
- JVD
jugular vein distention
- LLE
lower limb edema
- LV
left ventricle
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- NT‐proBNP
N terminal pro brain natriuretic peptide
- RAP
right atrial pressure
- RV
right ventricle
- RV
respiratory variation
- SEGA
short emergency geriatric assessment
- TTE
transthoracic echocardiogram
1. INTRODUCTION
Heart failure is a common disease, with a prevalence of estimates 1,130,000 people in France, with an incidence of more than 120,000 new cases per year. 1 It is a serious disease, burdened with decompensation, with significant consequences on quality of life and soaring costs of public health. 2 Its incidence and prevalence increase considerably with age, making it the most common cause of hospitalization in the elderly, with more than 50,000 hospitalizations per year in people over 75 in France. 3
The assessment of intravascular congestion is paramount in treating acute decompensated heart failure (ADHF) in elderly patients, as it helps optimize diuretic therapy. Such assessment is based on clinical presentation. Elevated jugular venous pressure is a specific sign of overload, 4 though not a very sensitive one. 5 , 6 Natriuretic peptides and chest X‐ray are difficult to interpret in the elderly. 7 , 8 Transthoracic echocardiography (TTE) remains a method of choice to assess congestion by analyzing left ventricular filling pressures and the inferior vena cava (IVC) variations. 4 However, access to TTE is limited and it is performed less than one in five patients in geriatric departments. 9 , 10
IVC diameter and compliance as assessed by echocardiography is a fast and effective way to obtain the right‐atrial pressure with a threshold of 21 mm and respiratory variation <50% to define hypervolemia. 11 , 12 The IVC can guide the clinician in administering effective decongestive treatment and achieving euvolemia. 13 , 14 In a frail, multimorbid and poly‐medicated patient, an ultra‐portable ultrasound device at bedside is a valuable, easy‐to‐use tool. 14
Other parameters have been proposed for the diagnosis and monitoring of volume overload in ADHF patients. The indirect estimation of plasma volume status (ePVS) by the Duarte formula 15 could be useful in diagnosing ADHF. 16
The occurrence of renal failure under diuretic treatment is frequent and increases the length of hospital stay. The use of a blood volume‐monitoring tool might be helpful to avoid renal failure 17 , 18 , 19 (Appendix A).
The aim of the study was to determine the relationship between various parameters of blood volume assessment (clinical and laboratory parameters, IVC ultrasound exam, and instantaneous plasma volume) in patients over 75 years of age admitted for ADHF. Then, to determine whether IVC parameters enable identifying patients at risk of functional acute renal failure related to excessive depletion.
2. METHODS
2.1. Study presentation
Our study is an observational, longitudinal, prospective, single‐center cohort study that was conducted in the cardiology department of Bicêtre hospital between January 1, 2019, and May 1, 2020. Consecutive patients older than 75 years, hospitalized for ADHF and discharged alive were eligible for the study. The ADHF diagnosis was based on a combination of clinical signs and symptoms, admission N terminal pro brain natriuretic peptide (NT‐proBNP) >1500 pg/ml and echocardiographic structural abnormalities (left ventricular ejection fraction [LVEF], filling pressures). 4 , 20 The study excluded patients with NT‐proBNP <300 pg/ml, cardiogenic shock, acute MI < 1 week, acute pulmonary edema, acute anemia requiring red blood cell transfusions, severe liver failure not related to heart failure with liver enzymes aspartate‐amino‐transférase and alanine aminotransferase > 4 N, severe chronic kidney disease (CKD) with estimated glomerular filtration rate (eGFR) < 15 ml/min on admission, or on dialysis.
The study complied with the declaration of Helsinki and was approved by the local ethics committee (#20181128163709). All patients gave their informed consent.
2.2. Data collection
On admission, we collected demographic data including gender and age, and the number of hospitalizations during the previous year.
Medical history included screening for ischemic heart disease (IHD), atrial fibrillation (AF), severe aortic stenosis, arterial hypertension (HTN), LVEF if known, severe CKD with Glomerular filtration rate 30 ml/min, chronic obstructive pulmonary disease (COPD), and diabetes. Data were collected concerning the following medications at admission and discharge: angiotensin‐converting‐enzyme inhibitors (ACEi), angiotensin II receptor blockers (ARB), diuretics, beta‐blockers, and mineralocorticoid receptor antagonists (MRA).
Patient frailty was assessed using the Short Emergency Geriatric Assessment (SEGA) grid part A version approved in 2014. 20
2.3. Management
On admission, patients were treated with loop diuretics such as Furosemide according to the DOSE trial‐inspired protocol. 21 Doses were adjusted according to the doctor's clinical judgment and the patient's frailty. 22 The motive for switching to oral diuretic and the cumulative IV dose were recorded.
During hospitalization, a daily clinical examination provided data on weight, heart rate (HR), blood pressure (BP), and the presence of hepatojugular reflux (HJR) or jugular vein distention (JVD) if any. The occurrence of lower limb edema (LLE) was graded according to extent (none, ankles, knees, and thighs), and similarly for lung crackles (none, lung base, half of the lungs, more than 2/3 of the lungs). Biological data collected daily‐included plasma protein levels, hemoglobin, hematocrit, urea and creatinine, with estimated renal function (eGFR according to the CKD‐EPI formula) until discharge or up to Day 8. NT‐proBNP and plasma albumin were measured at admission.
IVC was measured with the ultra‐portable VScan Extend device (GE Healthcare) on hospital admission and on Day 2, and then until the intravenous diuretic was stopped (Day 4 on average) and switched to oral diuretic. Measurements were taken by a resident doctor in charge of the study who was not initially experienced. The training given over 5 days (30 patients total) showed excellent agreement between the assessments performed by an intern and by an experienced operator (Kappa = 0.982 (95% confidence interval [CI]: 0.965–0.991)).
IVC status was defined according to the guidelines 23 as five possibilities: “thin,” “non‐dilated with diameter (D) < 21 mm and respiratory variation (RV) > 50%,” “dilated with D < 21 mm or RV < 50%,” “dilated with D > 21 mm and RV < 50%,” “dilated with D > 21 mm and RV < 50% and dilated hepatic veins.”
Estimated plasma volume status was estimated using the Duarte formula:
2.4. Assessment of hypervolemia
Hypervolemia was defined clinically by the presence of at least one clinical sign of congestion (JVD, HJR, LLE associated or not with crackles on lung auscultation).
Hypervolemia was defined by echo by a “dilated IVC > 21 mm and/or RV < 50%.” 24 , 25
The biological definition of hypervolemia was an ePVS score > 5.2 , adapting the results of Chouihed study. 26
To monitor follow‐up and diuretic treatment and its effect on creatinine levels from D0 to D8, four groups were defined by clinical and IVC evaluation: clinical hypervolemia alone, hypervolemia by IVC alone, hypervolemia by clinical and IVC evaluation, and no hypervolemia at D2. A significant increase in blood creatinine level was defined as a 30% increase from baseline.
2.5. Statistical analysis
Continuous variables were expressed as mean (standard deviation ‐SD‐), and categorical variables were expressed as a number or frequency (percentage). Differences in patients' clinical characteristics were compared by the χ2 test (or Fisher test if conditions were not checked) for categorical data and by the Wilcoxon test for continuous data.
A Treemap was used to represent the clinical and ultrasound hypervolemia groups at D1 and D2. A heatmap was used to represent the correlation between clinical, ultrasound, and biological parameters.
A plot over time was used to show the relationships between volume estimations depending on methods and creatinine levels.
A two‐sided p value < .05 was considered statistically significant. All statistical analyses were performed using RStudio 1.4.1103.
3. RESULTS
Fifty patients (85.2 ± 5.9 years old, 68% female) were included. Mean SEGA frailty score was 10.4 ± 2.5, each patient presented with an average of five comorbidities, and 18% of patients lived in nursing homes. The main comorbidities were iron deficiency (66%), diabetes (40%), COPD (16%), undernutrition (22%), and severe chronic renal failure with eGFR < 30 ml/min estimated by CKD‐EPI (10%). Cardiovascular comorbidities included hypertension (94% of patients), Atrial Fibrallation (66%), Iron deficiency (34%), and severe aortic stenosis (12%). The mean ejection fraction was 44 ± 14%, and 48% of patients had an LVEF ≥ 50%. 42% of patients were hospitalized for acute decompensated HF at least once during the previous year (Table 1).
Table 1.
Baseline characteristics
| Groups | Overall (D1) |
|---|---|
| N (%) | 50 (100) |
| Demographic and medical factors | |
| Age, mean (SD) | 85.2 (5.9) |
| Age groups, n (%) | |
| 75‐85 years | 25 (50) |
| >85 years | 25 (50) |
| Female sex, n (%) | 34 (68) |
| SEGA, mean (SD) | 10.4 (2.5) |
| Iron deficiency, n (%) | 33 (66) |
| Coronary heart disease, n (%) | 17 (34) |
| Hypertension, n (%) | 47 (94) |
| eGFR (ml/min), mean (SD) | 46 (15) |
| Chronic kidney disease, n (%) eGFR<30 | 5 (10) |
| COPD, n (%) | 8 (16) |
| Atrial fibrillation, n (%) | 33 (66) |
| Diabetes mellitus, n (%) | 20 (40) |
| LVEF (%), mean (SD) | 44 (14) |
| LVEF ≥ 50%, n(%) | 24 (48) |
| Biological factors | |
| C reactive protein (ng/ml), mean (SD) | 32 (62) |
| Albuminemia (g/dl), mean (SD) | 34 (5) |
| NT‐pro BNP (ng/l), mean (SD) | 11516 (12695) |
| Treatments in hospital | |
| Delay of PO relay, mean (SD) | 4.8 (1.7) |
| PO relay reason | |
| Euvolemia | 38 (76) |
| Creatinine increase > 30 µmol/l | 4 (8) |
| Other reason | 7 (14) |
| Diuretics cumulative dose, mean (SD) | 775 (1132) |
| Treatments at discharge | |
| ACEi, n (%) | 19 (38) |
| ARBs, n (%) | 8 (16) |
| Diuretic, n (%) | 46 (92) |
| Diuretic dose, mean (SD) | 117 (172) |
| Beta‐blocker, n (%) | 34 (68) |
| MRA, n (%) | 2 (4) |
Abbreviations: ACEi, angiotensin‐converting‐enzyme inhibitors; ARB, angiotensin II receptor blockers; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; PO, per os; SEGA, Short Emergency Geriatric Assessment.
In the acute phase, the mean duration of intravenous Furosemide treatment was 4.8 ± 1.7 days with a mean dose of 775 ± 1132 mg. Acute renal failure (>30% increase from baseline creatinine) was found in 8% of patients.
98% of patients had ePVS > 5.2 on admission. Mean NT‐proBNP was 11 516 (±12 695) ng/L (Table 2; Figure 1).
Table 2.
Clinical, biological, and ultrasound assessment of congestion, Day 1 and Day 2
| Days | Day 1 | Day 2 |
|---|---|---|
| N (%) | 50 (100) | 47 (94) |
| Clinical signs | ||
| Weight (kg), mean (SD) | 76 (17) | 74 (24) |
| Systolic blod pressure (mmHg), mean (SD) | 133 (20) | ‐ |
| Diastolic blod pressure (mmHg), mean (SD) | 70 (14) | ‐ |
| Heart rate (bpm), mean (SD) | 84 (18) | 83 (20) |
| LLE, n (%) | 45 (90) | 41 (87) |
| None, n (%) | 5 (10) | 9 (19) |
| Wrist, n (%) | 14 (28) | 16 (34) |
| Leg, n (%) | 18 (36) | 11 (23) |
| Thigh, n (%) | 13 (26) | 11 (23) |
| JVD or HJR, n (%) | 22 (44) | 18 (38) |
| Crackles, n (%) | 47 (94) | 42 (89) |
| None, n (%) | 3 (6) | 8 (17) |
| Lung base, n (%) | 11 (22) | 11 (23) |
| Half lungs, n (%) | 29 (58) | 24 (51) |
| More than 2/3 lungs, n (%) | 7 (14) | 4 (9) |
| ePVS, mean (SD) | 8.9 (1.4) | 9 (1.5) |
| IVC | ||
| Collapsed and thin, n (%) | 0 (0) | 1 (2) |
| D < 21 mm and RV > 50%, n (%) | 0 (0) | 8 (17) |
| D > 21 or RV < 50%, n (%) | 19 (38) | 11 (23) |
| D > 21 mm and RV < 50%, n (%) | 18 (36) | 19 (40) |
| Dilated hepatic veins, n (%) | 13 (26) | 8 (17) |
| Hypervolemia | ||
| Clinical hypervolemia, n (%) | 46 (92) | 40 (85) |
| Hypervolemia by IVC type 1, n (%) | 50 (100) | 38 (81) |
| Hypervolemia by ePVS, n (%) | 49 (98) | 43 (91) |
| Biological factors | ||
| Baseline Creatinin (µmol/l), mean (SD) | 136 (58) | 135 (53) |
| Increase > 30%, n (%) | ‐ | 3 (6) |
| Plasma protein levels (mg/l), mean (SD) | 66 (7) | 64 (6) |
| Hemoglobin (g/dl), mean (SD) | 11.4 (1.6) | 11.4 (1.7) |
| Hematocrit (%), mean (SD) | 36 (4.5) | 35 (5.4) |
| NT‐pro BNP (ng/l), mean (SD) | 11 516 (12695) |
Abbreviations: ePVS, estimation of plasma volume status; HJR, hepatojugular reflux; IVC, inferior vena cava; JVD, jugular vein distention; LLE, lower limb edema; RV, respiratory variation.
Figure 1.
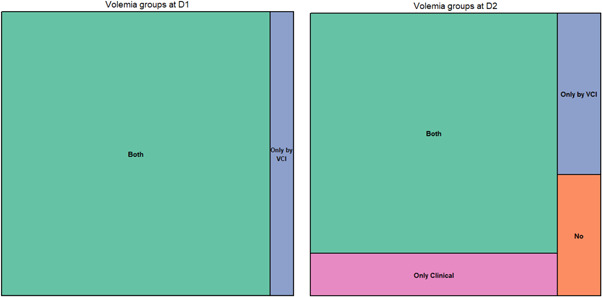
Volemia groups, Day 1 (D1) and Day 2 (D2) (Treemap)
On admission, at Day 1 (D1) a dilated, noncompliant IVC correlated well with signs of right heart failure (IVC and JVD/HJR with r = .8; p < .001, 95% CI: 0.67–0.88; IVC and LLE with r = .6; p < .001, 95% CI: 0.36–0.74) but correlated slightly with signs of left heart failure (IVC and crackles r = .3; p = .036).
This result was the same on day 2 (D2). The ePVS did not correlate with either clinical signs or IVC.
There was a correlation between NT‐proBNP level at D1 and lung crackles (r = .28; p = .03) as well as with IVC (r = .3; p = .02) (Figure 2). Hematocrit and plasma protein levels between D1 and D2 showed no significant variation. Plasma protein levels correlated slightly with LLE and NT‐proBNP.
Figure 2.
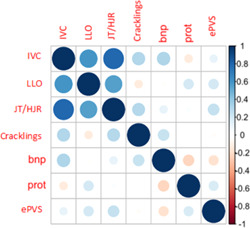
Correlation matrix on D1
By combining the different means of monitoring volume status at D2 in four groups (clinical hypervolemia alone, hypervolemia by IVC alone, hypervolemia by clinical evaluation and IVC, no hypervolemia), changes in creatinine over the duration of diuretic therapy could be analyzed between D0 and D7 (Figure 3). In the group combining the two parameters (clinical and IVC), a variation in creatinine was found. The six patients who presented functional acute renal failure were those who had a thin, compliant IVC as of Day 2.
Figure 3.
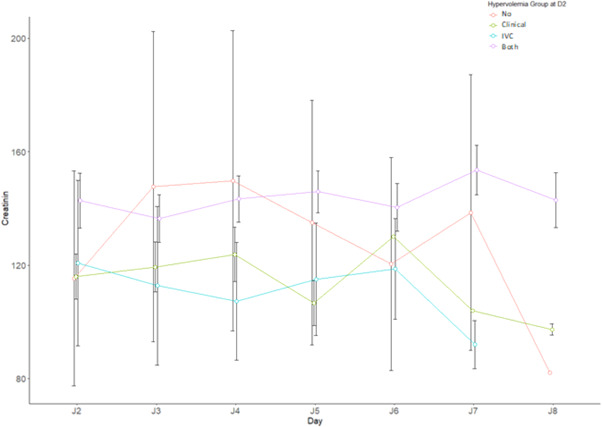
Creatinine variation by Volemia groups at D2
4. DISCUSSION
Our study shows that clinical and ultrasound assessment of the intravascular congestive states of elderly patients with acute heart failure is reliable. There is a significant correlation between JVD/HJR and IVC measurements in the context of hypervolemia. Daily clinical examination, including JVD and IVC assessment, provides a reliable assessment of volume status, and helps quickly adapt the diuretic treatment to avoid functional renal failure.
To our knowledge, our study is one of the first to analyze the association between clinical, biological, and IVC variations, as well as to use both parameters to monitor diuretic treatment of acute heart failure in an elderly patients.
4.1. Blood volume analysis
Our findings are consistent with previous studies. The clinical evaluation of blood volume showed a good performance of the examination of JVD, which has a class IB recommendation according to American Heart Association (AHA) guidelines. 27 In Miller JB's 2012 study, 28 the inspiratory collapse of IVC < 33% has a Se of 80% and Sp of 81% to define hypervolemia. In our study, an IVC > 21 mm and an RV < 50% on admission is superior to clinical evaluation alone and identifies 100% of acutely decompensated patients.
The estimation of plasma volume as a parameter guiding ADHF management has inspired several studies following K. Duarte's 2015 publication 15 of the results on the EPHESUS study database. 29 A study by Chouihed 26 suggests that an ePVS value above 5.12 × 10−4 dL/g at the first blood test in patients presenting with acute dyspnea at the emergency department could help diagnose ADHF. In Kobayashi's study, ePVS is associated with hemodynamic markers of congestion. 29 In our study, we did not find any correlation between the selected ePVS value and clinical or ultrasound (IVC) signs of congestion. As for the change in ePVS after the onset of depletion, the results lack differential value. A possible explanation may be that the ePVS may not be as reliable in elderly patients and that the threshold needs adjusting.
In our study, NT‐proBNP correlated only slightly with other criteria for hypervolemia. This is consistent with previous studies 27 and invites us to retain the use of NT‐proBNP for ADHF diagnosis and prognosis only, but not for monitoring patients' congestive states.
Other criteria such as plasma protein levels and hematocrit vary little in the first few days and are poorly correlated with other signs of hypervolemia. This has been shown in other studies. 28
4.2. Managing diuretic therapy
In the study by Yavaşi et al, the authors show that the analysis of IVC collapsibility is useful to manage diuretic therapy. 28
In our study, we investigated four ways to monitor patients' blood volume during diuretic therapy, and we found that the combined evaluation of daily clinical signs (JVD) and the ultrasound assessment of IVC was the best method. The combined practice of clinical and ultrasound assessment of congestion allows for early detection of patients whose renal function is likely to deteriorate due to excess depletion on diuretics. This method may be useful in geriatric departments with limited access to cardiac ultrasound (lack of technical facilities, demented patients difficult to evaluate by standard examination, and long waiting time) for frail patients at risk of developing functional acute renal failure.
4.3. Limitations of the study
Our study represents a descriptive analysis of the volume status of elderly subjects hospitalized for decompensated HF and explores the combination of different monitoring parameters. The limitations of the study are a small number of patients and the absence of a control group. Therefore, we could not measure diagnostic performance markers (Se, Sp, positive predictive value, negative predictive value).
5. CONCLUSION
In ADHF patients older than 75 years, clinical evaluation of congestion and the ultrasound examination of IVC correlate well. Evaluation of IVC measurement with an ultraportable device is feasible by a geriatrician. The correlation between JVD/HJR and IVC measurement is good. The combined clinical and ultrasound analysis identifies patients at risk of functional renal failure and should facilitate the adjustment of diuretic doses in elderly patients with acute decompensated HF.
CONFLICT OF INTERESTS
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Xenia Leahova‐Cerchez collected data and wrote the main manuscript. Emmanuelle Berthelot Had the idea of the study, reviewed all the data, wrote and edit the main manuscript. Bastien Genet did the statistical analysis, tables and figures, and reviewed and commented the article. Olivier Hanon edit the main manuscript. Patrick Jourdain complete the idea of the study, edit the main manuscript.
ACKNOWLEDGMENT
Not applicable.
APPENDIX A. DIURETIC TREATMENT PROTOCOL
A.1. Patient naive to loop diuretics
40 mg Furosemide intravenous bolus injection with re‐evaluation at 6 h (diuresis and clinical) and continuation at a dose of 40 mg × 2 per 24 h intravenously with a 6 h interval if signs of congestion persist.
A.2. Patient on long‐term loop diuretics
-
–
Intravenous bolus dose of Furosemide equivalent to the usual dose with re‐evaluation at 6 h (diuresis and clinical), continuation of usual daily dose doubled divided into two intravenous injections with a 6‐h interval over 24 h.
-
–
60 mg bolus dose of Furosemide then continuation of the double of the usual daily dose by continuous intravenous infusion if quantity exceeds 120 mg.
Leahova‐Cerchez X, Berthelot E, Genet B, Hanon O, Jourdain P. Estimation of the plasma volume status of elderly patients with acute decompensated heart failure using bedside clinical, biological, and ultrasound parameters. Clin Cardiol. 2022;45:379‐385. 10.1002/clc.23791
DATA AVAILABILITY STATEMENT
Data and materials are not available.
REFERENCES
- 1. De Peretti C, Pérel C, Tuppin P, Iliou MC, Juillière Y, Gabet A. Prévalences et statut fonctionnel des cardiopathies ischémiques et de l'insuffisance cardiaque dans la population adulte en France: apports des enquêtes déclaratives « Handicap‐Santé ». BEH. 2014;(no. 9–10):172‐181. [Google Scholar]
- 2. Haute Autorité de Santé HAS. Guide parcours de soins insuffisance cardiaque. Saint‐Denis La Plaine. 2014. https://www.has-sante.fr/jcms/c_1242988/fr/guide-parcours-de-soins-insuffisance-cardiaque [Google Scholar]
- 3. Tuppin P, Cuerq A, de Peretti C, et al. First hospitalization for heart failure in France in 2009: patient characteristics and 30‐day follow‐up. Arch Cardiovasc Dis. 2013;106(11):570‐585. [DOI] [PubMed] [Google Scholar]
- 4. McDonagh TA, Metra M, Adamo M, Gardner RS, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599‐3726. [DOI] [PubMed] [Google Scholar]
- 5. Andres E, Mecili M, Zulfiqar AA, Keller O, Mourot‐Cottet R, Bourgarit‐Durand A. Projet d'étude sur la SÉmiologie CArdiaque dans l'Insuffisance Cardiaque en médecine interne (projet SECAIC). La Revue de Médecine Interne. 2016;37:A141. [Google Scholar]
- 6. Oudejans I, Mosterd A, Bloemen JA, et al. Clinical evaluation of geriatric outpatients with suspected heart failure: value of symptoms, signs, and additional tests. Eur J Heart Fail. 2011;13(5):518‐527. [DOI] [PubMed] [Google Scholar]
- 7. Chenevier‐Gobeaux C, Delerme S, Allo JC, et al. B‐type natriuretic peptides for the diagnosis of congestive heart failure in dyspneic oldest‐old patients. Clin Biochem. 2008;41(13):1049‐1054. [DOI] [PubMed] [Google Scholar]
- 8. Berthelot E, Nouhaud C, Lafuente‐Lafuente C, Assayag P, Hittinger L. Insuffisance cardiaque chez les sujets âgés de plus de 80 ans. Presse Med. 2019;48(2):143‐153. [DOI] [PubMed] [Google Scholar]
- 9. Berthelot E, Broussier A, Damy T, et al. Good performance in the management of acute heart failure in cardiogeriatric departments: the ICREX‐94 experience. BMC Geriatr. 2021;21(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parmar KR, Xiu PY, Chowdhury MR, Patel E, Cohen M. In‐hospital treatment and outcomes of heart failure in specialist and non‐specialist services: a retrospective cohort study in the elderly. Open Heart. 2015;2(1):e000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Price S, Platz E, Cullen L, et al. Expert consensus document: echocardiography and lung ultrasonography for the assessment and management of acute heart failure. Nat Rev Cardiol. 2017;14(7):427‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Biais M, Carrié C, Delaunay F, Morel N, Revel P, Janvier G. Evaluation of a new pocket echoscopic device for focused cardiac ultrasonography in an emergency setting. Crit Care. 2012;16(3):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mjolstad OC, Dalen H, Graven T, Kleinau JO, Salvesen O, Haugen BO. Routinely adding ultrasound examinations by pocket‐sized ultrasound devices improves inpatient diagnostics in a medical department. Eur J Intern Med. 2012;23(2):185‐191. [DOI] [PubMed] [Google Scholar]
- 14. Yavaşi Ö, Ünlüer EE, Kayayurt K, et al. Monitoring the response to treatment of acute heart failure patients by ultrasonographic inferior vena cava collapsibility index. Am J Emerg Med. 2014;32(5):403‐407. [DOI] [PubMed] [Google Scholar]
- 15. Duarte K, Monnez J‐M, Albuisson E, Pitt B, Zannad F, Rossignol P. Prognostic value of estimated plasma volume in heart failure. JACC Heart Fail. 2015;3(11):886‐893. [DOI] [PubMed] [Google Scholar]
- 16. Yoshihisa A, Abe S, Sato Y, et al. Plasma volume status predicts prognosis in patients with acute heart failure syndromes. Eur Heart J Acute Cardiovasc Care. 2018. Jun;7(4):330‐338. [DOI] [PubMed] [Google Scholar]
- 17. Kobayashi M, Rossignol P, Ferreira JP, et al. Prognostic value of estimated plasma volume in acute heart failure in three cohort studies. Clin Res Cardiol. 2019;108(5):549‐561. [DOI] [PubMed] [Google Scholar]
- 18. Valente MA, Voors AA, Damman K, et al. Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J. 2014;35:1284‐1293. [DOI] [PubMed] [Google Scholar]
- 19. Hasselblad V, Gattis Stough W, Shah MR, et al. Relation between dose of loop diuretics and outcomes in heart failure population: results of the ESCAPE trial. Eur J Heart Fail. 2007;9(10):1064‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. http://www.geronto-sud-lorraine.com/docs/Grille_SEGA_A_validee.pdf
- 21. Lala A, McNulty SE, Mentz RJ, et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE‐AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS‐HF). Circ Heart Fail. 2015;8(4):741‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There's got to be a happy medium”. JAMA. 2010;304(14):1592‐1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography: endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685‐713. [DOI] [PubMed] [Google Scholar]
- 24. Neskovic AN, Edvardsen T, Galderisi M, et al. Focus cardiac ultrasound: the European Association of Cardiovascular Imaging viewpoint. Eur Heart J–Cardiovasc Imaging. 2014;15(9):956‐960. [DOI] [PubMed] [Google Scholar]
- 25. Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66(4):493‐496. [DOI] [PubMed] [Google Scholar]
- 26. Chouihed T. Identification des profils congestifs de l'insuffisance cardiaque aiguë pour guider les stratégies diagnostiques et thérapeutiques de prise en charge en urgence. Doctoral dissertation. Université de Lorraine; 2018.
- 27. Kobayashi M, Huttin O, Donal E, et al. Association of estimated plasma volume status with hemodynamic and echocardiographic parameters. Clin Res Cardiol. 2020;109:1‐10. [DOI] [PubMed] [Google Scholar]
- 28. Carubelli V, Lombardi C, Lazzarini V, et al. N‐terminal pro‐B‐type natriuretic peptide‐guided therapy in patients hospitalized for acute heart failure. J Cardiovasc Med. 2016;17(11):828‐839. [DOI] [PubMed] [Google Scholar]
- 29. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309‐1321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials are not available.


