Abstract
Higher vitamin K intakes have been associated with better cognitive function, suggestive of a vitamin K mechanistic effect or simply reflective of a healthy diet. To test the hypothesis that brain vitamin K is linked to cognitive decline and dementia, vitamin K concentrations were measured in four brain regions, and their associations with cognitive and neuropathological outcomes were estimated in 325 decedents of the Rush Memory and Aging Project. Menaquinone‐4 (MK4) was the main vitamin K form in the brain regions evaluated. Higher brain MK4 concentrations were associated with a 17% to 20% lower odds of dementia or mild cognitive impairment (MCI) (P‐value < .014), with a 14% to 16% lower odds of Braak stage ≥IV (P‐value < 0.045), with lower Alzheimer's disease global pathology scores and fewer neuronal neurofibrillary tangles (P‐value < 0.012). These findings provide new and compelling evidence implicating vitamin K in neuropathology underlying cognitive decline and dementia.
Keywords: aging, Alzheimer's disease, cognitive decline, dementia, neuropathology, nutrition, vitamin K
1. NARRATIVE
To develop effective strategies that reduce cognitive decline and dementia, it is critical to develop a better understanding of the pathophysiology underlying dementia including Alzheimer's disease (AD). Accumulating evidence implicates dietary factors in reducing cognitive decline and dementia risk, 1 including vitamin K. 2 , 3 , 4 There is a high prevalence of vitamin K insufficiency among older adults. 5 Because vitamin K is safe and readily available in the diet, a solid mechanistic framework that supports its role in cognitive decline and dementia risk would have potential and sustainable public‐health impact. Such a framework requires well‐designed studies to link the vitamin K content of human brains with cognitive function prior to death and with post‐mortem neuropathologically defined outcomes.
In community‐based studies of older adults, higher vitamin K intakes are associated with slower cognitive decline 2 and higher circulating vitamin K is associated with better cognitive function, 3 , 4 together suggesting that vitamin K could be involved in the pathophysiology underlying cognitive decline. However, vitamin K is abundant in green, leafy vegetables so an alternative interpretation is that circulating vitamin K is simply a marker of a healthy lifestyle independent of any underlying mechanism related to vitamin K. The available evidence has relied on circulating biomarkers and estimates of dietary vitamin K, whereas little is known about the forms and amount of vitamin K in the human brain and their relevance to cognitive function and neuropathology of dementia. 6
To address this knowledge gap and test the hypothesis that higher vitamin K levels are associated with specific changes that lower risk of dementia and cognitive decline, we measured human brain concentrations of vitamin K and related metabolites and determined their associations with ante‐mortem measures of cognitive function and post‐mortem neuropathologic outcomes in 325 participants of the Rush Memory and Aging Project (MAP). Circulating vitamin K concentrations from ante‐mortem blood collection were also evaluated for associations with cognitive function prior to death and with post‐mortem neuropathologic outcomes.
Higher post‐mortem brain concentrations of menaquinone‐4 (MK4), the predominant brain vitamin K metabolite, were associated with better cognitive function prior to death. Higher plasma phylloquinone (vitamin K1) concentrations were also associated with better cognitive function and a slower rate of cognitive decline. Further investigation of neuropathologically‐defined outcomes revealed that higher brain MK4 concentrations were also associated with a lower AD global pathology, lower neurofibrillary tangle density, and a lower odds of having a high Braak stage and Lewy bodies present. These findings were consistent across the mid‐temporal and mid‐frontal cortexes, anterior watershed, and cerebellum. These findings contribute to the growing body of literature that intake of a vitamin K‐rich diet has a protective association with cognitive change during aging. 2
The only established function for vitamin K is as a cofactor for the enzyme gamma‐glutamyl carboxylase, which is expressed ubiquitously, including in the nervous system. 7 Vitamin K–dependent proteins, such as Protein S and Gas‐6, are present in cerebral cortex and other brain regions. 8 Several mechanisms related to neuronal apoptosis have been attributed to Protein S and Gas‐6, but it is not currently known if the vitamin K–dependent carboxylation of these proteins is essential to their purported function(s) in the brain. There is also the conundrum that phylloquinone and MK4 have similar efficacy as a cofactor for the gamma‐glutamyl carboxylase. Phylloquinone is the predominant form in the diet, yet mammalian brain tissue preferentially contains MK4, as demonstrated in this study. This conversion of phylloquinone to MK4 in vivo suggests that MK4 may have roles in the brain that are unrelated to vitamin K–dependent protein carboxylation. 8 , 9 One possibility is in its established role in sphingolipid metabolism. 10 The brain is enriched with sphingolipids, which are important membrane constituents that have a role in cognition. 11 , 12 In this study, higher MK4 concentrations in the brain were associated with lower odds of a high Braak stage, which may indicate a mechanism that directly involves protection against AD via neurofibrillary tangles. Braak stage, which reflects neurofibrillary tangle density and burden, was associated with cognitive decline in MAP 13 , 14 and other studies. 15 , 16 In contrast, MK4 concentrations in the brain were not associated with amyloid beta (Aβ), the other central protein of AD. Of interest, MK4 was also associated with a lower odds of Lewy bodies, another common intracellular proteinopathy in aging, and related to dementia and parkinsonism.
RESEARCH IN CONTEXT
Systematic review: Observational studies report that higher circulating vitamin K concentrations were associated with better cognitive function in older adults. Rodent experiments report that vitamin K is present in brain tissue. Little is known about vitamin K in the human brain. The goal of this study was to analyze post‐mortem human brain concentrations of vitamin K and related metabolites and determine their association with cognitive function. We also evaluated the association of brain vitamin K concentrations with dementia‐related neuropathologies.
Interpretation: In this study of Rush Memory and Aging Project participants, higher post‐mortem brain concentrations of the vitamin K metabolite, menaquinone‐4 (MK4), were associated with better cognitive function prior to death. Brain MK4 concentrations were inversely associated with Alzheimer's disease (AD) global pathology, neurofibrillary tangle density, Braak stage, and Lewy body presence.
Future directions: Additional research is needed to clarify the mechanisms by which vitamin K has a neuroprotective effect.
Most studies of nutrients and AD rely on limited circulating biomarkers to estimate nutrient status of the brain. A protective association between circulating phylloquinone and various measures of cognitive function has been reported in several observational studies of older adults without cognitive impairment. 3 , 17 We found that higher plasma phylloquinone concentrations measured at the most recent clinic visit prior to death were associated with overall better cognitive function, but not with any post‐mortem neuropathological outcome. Furthermore, plasma phylloquinone concentrations were not correlated with post‐mortem brain MK4 concentrations. The food supply contains at least 10 forms of vitamin K, including phylloquinone and MK4. Whereas phylloquinone is plant based, most menaquinones, of which there are 11 known forms, are found in fermented and animal‐based foods, including dairy and meats. 18 , 19 Only phylloquinone is detectable in the circulation following ingestion, and our knowledge of phylloquinone forms the basis of the current dietary recommendations for vitamin K. 20 It is now emerging that all forms of ingested vitamin K can be converted to MK4 found in brain tissue. 21 , 22 However, little is known about how or what form of vitamin K is transported across the blood‐brain barrier and into the brain. It is plausible that MK4 brain concentrations reflect the total contribution of all vitamin K forms to dietary intakes, whereas the plasma phylloquinone concentrations reflect only intake of plant‐based phylloquinone, thereby attenuating the association of circulating vitamin K with the neuropathology outcomes. Currently, the food composition data for menaquinones are limited, which precludes the assessment of total vitamin K intake to confirm this hypothesis. Alternatively, plasma phylloquinone concentrations at the last clinic visit before death may simply be a biomarker of green leafy vegetable intake or a healthy lifestyle, which is associated with better cognition. 2 We adjusted for the Dietary Approaches to Stop Hypertension (DASH) diet score, an indicator of a healthy diet, since plasma phylloquinone may be a marker of healthy diets, including leafy green vegetables. 23 However, some residual confounding may remain.
The strengths of this study include the unique application of ante‐mortem biomarker and cognition measures combined with post‐mortem measures, including neuropathologically defined outcomes, obtained from decedents of a well‐characterized community‐based cohort. Guided by our prior findings, 24 we included decedents whose brains were stored ≤8 years to be confident the measures reflected the MK4 concentrations at time of death. In a small study of 48 centenarians, 4 serum phylloquinone concentrations were similarly positively associated with cognitive function but post‐mortem MK4 concentrations in the frontal and temporal cortexes were not associated with ante‐mortem cognitive function. In that study, the brain tissue samples used were stored for >10 years before analysis, so it is possible that the brain MK4 degraded during the storage time, which may have affected the results. 4 , 24 Unfortunately, there were no neuropathologically defined AD outcomes reported in the centenarian study, which limits the comparison of the two studies.
Our findings should be interpreted in light of the following limitations. The observational design precludes inferring causation. Reverse causation is possible, although time ordering of pre‐mortem exposures mitigates this limitation for some of our analyses. The cohort was almost exclusively White, so generalizability to other race‐ethnic groups is uncertain. We also excluded decedents who regularly used warfarin prior to death, so the findings may not pertain to warfarin users. Patients with chronic warfarin use are reported to have greater cognitive decline compared to those taking non‐vitamin K‐dependent oral anticoagulants, although the data are not consistent. 25 , 26 , 27 To the best of our knowledge, there are currently no data available evaluating the role of oral anticoagulant therapies, vitamin K–dependent or otherwise, and neuropathologically defined AD outcomes, which represents an important gap in the research. The associations of brain MK4 concentrations with cognitive status remained statistically significant after correcting for multiple testing, but the associations of brain MK4 concentrations with the neuropathology outcomes did not. Additional studies are needed to replicate our findings and to reduce the possibility that they are due to chance.
The findings of this unique study suggest that vitamin K is involved in dementia and cognitive decline, which is important given the increasing public health burden of dementia, and the encouraging reality that low vitamin K status can be easily remedied through adherence to the Dietary Guidelines for Americans, which encourages intake of green vegetables. Clinical trials are essential to confirm this hypothesis. The findings of this study also emphasize the need for preclinical research to elucidate the mechanism(s) by which vitamin K has a neuroprotective effect.
2. CONSOLIDATED RESULTS AND STUDY DESIGN
The Rush MAP is an ongoing community‐based longitudinal study designed to identify risk factors for AD and related dementias (ADRD) and cognitive decline. 28 At enrollment, MAP participants are free of known dementia and agree to participate in detailed clinical evaluations annually and organ donation upon death. Concentrations of phylloquinone and MK4 were measured in four brain regions (the mid‐temporal and mid‐frontal cortices, anterior watershed white matter, and cerebellar cortex) in brain tissues samples obtained from 499 MAP decedents who died between 2005 and 2019 24 , 29 (Figure S1).
Global cognitive function was determined using scores from a battery of 19 cognitive tests administered at each visit. 30 The estimated person‐specific rate of change in the global cognition variable over time was determined using mixed‐effects models. 31 At the time of death, a final cognitive diagnosis was made based on all available clinical data reviewed by a neurologist with expertise in dementia, and classified as dementia, mild cognitive impairment (MCI), or no cognitive impairment (NCI), as described. 32 , 33 After death, brains were removed and dissected using following established protocols 34 and evaluated histologically for AD pathology, 34 , 35 neurofibrillary tangle pathology, 34 neuritic plaques, 34 Aβ protein, 35 neuronal paired helical filaments (PHF)‐tau tangle density and burden, 35 microscopic cerebral infarctions, 36 , 37 and Lewy bodies. 38
Linear and logistic regressions were used to estimate the associations of brain MK4 and plasma phylloquinone concentrations with continuous and categorical cognitive and neuropathological outcomes. Clinical cognitive diagnosis and final cognitive diagnosis were analyzed with ordinal logistic regression using dementia, MCI, and NCI categories. Participants who had MCI or AD diagnosis with another condition contributing to cognitive impairment were included in the MCI and AD groups, respectively; participants with other primary cause of dementia were excluded.
Participants were, on average ± SD, 92 ± 6 years old at the time of death. Seventy‐five percent were female and 72% had at least 12 years of education (Table 1). MK4 was the main form of vitamin K in all human brain regions evaluated, and because phylloquinone was not detected in brain tissue of >85% of participants, statistical analyses of the brain regions focused on MK4.
TABLE 1.
Participant characteristics (n = 325) a
| Age at death, mean (SD) years | 92 (6) |
| Female, n (%) | 245 (75%) |
| Education, n (%) | |
| ≤12 years | 92 (28%) |
| >12‐≤16 years | 168 (52%) |
| >16 years | 65 (20%) |
| APOE ε4 allele, n (%) | |
| 1 or more | 71 (22%) |
| No alleles | 254 (78%) |
| Post‐mortem interval, mean (SD) hours | 8.8 (5.1) |
| Triglycerides, mean (SD) mg/dL b | 117 (55) |
| DASH diet score, mean (SD) | 3.8 (1.2) |
| Brain MK4, mean (SD) pmol/g b | |
| Mid‐frontal and mid‐temporal cortex c | 1.51 (5.5) |
| Anterior watershed | 0.73 (4.6) |
| Cerebellum | 1.61 (7.2) |
| Plasma phylloquinone, mean (SD) nmol/L b | 0.97 (0.7) |
| Global cognitive function score (last visit), mean (SD) | −1.00 (1.09) |
| Clinical diagnosis at last clinic visit, n (%) | |
| Dementia | 130 (41%) |
| MCI | 78 (25%) |
| NCI | 109 (34%) |
| Final cognitive diagnosis, n (%) | |
| Dementia | 136 (42%) |
| MCI | 81 (25%) |
| NCI | 105 (33%) |
| Global AD pathology, mean (SD) | 0.81 (0.6) |
| Braak stage, n (%) | |
| IV‐VI | 217 (67%) |
| 0‐III | 108 (33%) |
| CERAD neuritic plaque score, n (%) | |
| Moderate‐Frequent | 239 (74%) |
| None or sparse | 86 (26%) |
| NIA‐Reagan diagnosis, n (%) | |
| AD | 229 (70%) |
| No AD | 96 (30%) |
| Amyloid beta, mean (SD) % area | 5.1 (4.5) |
| Gross chronic cerebral infarcts, n (%) | |
| 1 or more | 128 (39%) |
| None | 197 (61%) |
| Chronic microinfarcts, n (%) | |
| 1 or more | 114 (35%) |
| None | 211 (65%) |
| Lewy body disease, n (%) | |
| Present | 83 (26%) |
| Absent | 234 (74%) |
| Diffuse plaques, mean (SD) | 0.74 (0.7) |
| Neuritic plaques, mean (SD) | 0.95 (0.8) |
| Neurofibrillary tangle burden, mean (SD) | 0.73 (0.8) |
| PHF‐tau tangle density, mean (SD) count per mm2 | 8.64 (9.3) |
Abbreviations: AD, Alzheimer's disease; CERAD, Consortium to Establish a Registry for Alzheimer's disease; DASH, Dietary Approaches to Stop Hypertension; MK4, menaquinone‐4; NIA, National Institute on Aging; PHF, paired helical filaments.
Triglycerides, DASH diet score, and plasma phylloquinone n = 296; global cognitive function n = 324; slope of global cognition n = 320; clinical diagnosis at last clinic visit n = 317; final cognitive diagnosis n = 322; Lewy body disease n = 317.
Geometric mean reported.
Geometric mean of the mean across the midfrontal and midtemporal cortical regions.
The odds of having dementia or MCI at the last cognitive assessment before death were 17% to 20% lower per doubling of MK4 in all four brain regions measured. These odds were generally consistent with the odds for dementia or MCI at the final cognitive diagnosis. Higher brain MK4 concentrations in the mid‐frontal and mid‐temporal cortexes and the anterior watershed were also associated with better ante‐mortem global cognitive function scores and a slower rate of cognitive decline. Higher brain MK4 concentrations in the cerebellum was associated with better ante‐mortem global cognitive function but not with the rate of cognitive decline (Table 2). For neuropathologically defined outcomes, the odds of Braak stage ≥IV were 14% to 16% lower per doubling of MK4 in the mid‐frontal and mid‐temporal cortexes, anterior watershed, and cerebellum (Table 3). The odds of having Lewy bodies were 13% to 18% lower per doubling of MK4 in the mid‐frontal and mid‐temporal cortexes and cerebellum. Higher MK4 in the mid‐frontal and mid‐temporal cortical and anterior watershed regions was also associated with lower global AD pathology scores and a lower neurofibrillary tangle burden (Table 3). Higher plasma phylloquinone at the last clinic visit was associated with better cognitive function, a slower rate of cognitive decline, and with a better clinical cognitive diagnosis at the final clinic visit and death.
TABLE 2.
Associations of brain menaquinone‐4 (MK4) concentrations with cognitive function
| MT & MF cortex | AWS | CR | ||||
|---|---|---|---|---|---|---|
| β (SE) | P‐value | β (SE) | P‐value | β (SE) | P‐value | |
| Cognitive diagnosis at last clinic visit before death (n = 317) | −0.212 (0.073) | .003 | −0.229 (0.064) | <.001 | −0.183 (0.074) | .014 |
| Final cognitive diagnosis (n = 322) | −0.167 (0.070) | .017 | −0.170 (0.061) | .005 | −0.136 (0.072) | .06 |
| Global cognitive function (n = 324) | 0.088 (0.037) | .018 | 0.088 (0.032) | .007 | 0.082 (0.039) | .036 |
| Slope of cognitive function (n = 320) | 0.006 (0.003) | .040 | 0.006 (0.003) | .018 | 0.005 (0.003) | .09 |
Abbreviation: MT, mid‐temporal cortex; MF, mid‐frontal cortex; AWS, anterior watershed; CR, cerebellum.
Regression coefficients are reported from ordinal logistic regression for cognitive diagnosis outcomes (categorized as AD, MCI, or NCI) and multiple linear regression (for global cognitive function and slope of cognitive function). Models are adjusted for age at death, sex, education, APOE ε4 status, and time between last clinic visit and death. Regression coefficients from ordinal logistic regression indicate cumulative log odds of AD.
TABLE 3.
Associations of brain menaquinone‐4 (MK4) concentrations with brain pathology (n = 325)
| MT & MF cortex | AWS | CR | ||||
|---|---|---|---|---|---|---|
| β (SE) | P‐value | β (SE) | P‐value | β (SE) | P‐value | |
| Dichotomous outcomes: | ||||||
| Braak ≥IV | −0.173 (0.083) | .038 | −0.157 (0.071) | .027 | −0.172 (0.085) | .044 |
| CERAD neuritic plaque score | −0.133 (0.085) | .12 | −0.141 (0.074) | .06 | −0.156 (0.088) | .07 |
| AD based on NIA Reagan | −0.109 (0.083) | .19 | −0.112 (0.071) | .12 | −0.120 (0.085) | .16 |
| Presence of gross chronic infarcts | 0.041 (0.074) | .58 | 0.0004 (0.065) | .99 | −0.037 (0.077) | .63 |
| Presence of chronic microinfarcts | 0.046 (0.075) | .54 | 0.072 (0.066) | .27 | 0.014 (0.078) | .86 |
| Presence of Lewy body disease a | −0.168 (0.084) | .045 | −0.135 (0.074) | .07 | −0.193 (0.090) | .032 |
| Continuous outcomes: | ||||||
| Global AD pathology | −0.025 (0.013) | .05 | −0.026 (0.011) | .020 | −0.021 (0.013) | .11 |
| Amyloid beta | −0.017 (0.040) | .67 | −0.028 (0.035) | .42 | −0.019 (0.041) | .65 |
| Diffuse plaques | −0.021 (0.016) | .20 | −0.017 (0.014) | .23 | −0.020 (0.017) | .23 |
| Neuritic plaques | −0.022 (0.018) | .21 | −0.021 (0.015) | .17 | −0.019 (0.018) | .30 |
| PHF‐tau tangle density | −0.023 (0.015) | .12 | −0.025 (0.013) | .05 | −0.019 (0.015) | .23 |
| Neurofibrillary tangle burden | −0.017 (0.008) | .041 | −0.022 (0.007) | .003 | −0.015 (0.009) | .10 |
Abbreviations: MT, mid‐temporal cortex; MF, mid‐frontal cortex; AWS, anterior watershed; CR, cerebellum; AD, Alzheimer's disease; CERAD Consortium to Establish a Registry for Alzheimer's Disease; NIA National Institute on Aging; PHF paired helical filaments.
Regression coefficients are reported from multiple logistic regression (for dichotomous outcomes) and multiple linear regression (for continuous outcomes). Continuous outcomes are square root transformed, except for tangle density and neurofibrillary tangle burden, which are quartic root transformed. Models are adjusted for age at death, sex, education, APOE genotype, and post‐mortem interval.
n = 317 for analysis of Lewy body due to missing values.
3. DETAILED METHODS AND RESULTS
3.1. Participants
All participants provided written informed consent and signed the Uniform Anatomic Gift Act. The institutional review boards of Rush University Medical Center and Tufts University approved this study. Brain vitamin K concentrations were measured in brain tissues obtained from 499 MAP decedents who died between 2005 and 2019. We previously reported that prolonged freezer storage time reduced brain vitamin K concentrations 24 so we excluded decedents whose brains were stored > 8 years (n = 123). We additionally excluded decedents who reported taking the vitamin K antagonist warfarin (Coumadin) at the two clinic visits prior to death (n = 49) or who were missing apolipoprotein E (APOE) genotype data (n = 2), leaving 325 decedents available for statistical analysis. Plasma phylloquinone was measured in 296 of these participants (Figure S1).
3.2. Vitamin K measurements
Phylloquinone is the main form of vitamin K detected in circulation, whereas MK4 is the predominant form in mammalian brain. 39 , 40 Phylloquinone and MK4 concentrations were measured in the mid‐temporal and mid‐frontal cortices, anterior watershed white matter, and cerebellar cortex using high‐performance liquid chromatography (HPLC). 24 The lower limit of assay detection for brain phylloquinone and MK4 was 0.1 pmol/g. The inter‐assay precision values for phylloquinone and MK4 were 9.6% and 10.4%, respectively. Plasma phylloquinone was measured using HPLC 29 in fasted samples obtained at the last clinic visit before death and stored at −80°C until analysis in 297 decedents. The lower limit of assay detection was 0.1 nmol/L. No MK4 was detected in circulation. All vitamin K measurements were conducted at the Vitamin K Laboratory at the United States Department of Agriculture (USDA) Human Nutrition Research Center on Aging at Tufts University. This laboratory participates in the Vitamin K External Quality Assurance (KEQAS) program. 41 In >15 years of KEQAS participation, the laboratory consistently generates serum/plasma phylloquinone data within the acceptable range of expected values (analyses occur every 4 months, for >30 cycles of verification). Low and high control specimens had average values of 1.1 and 4.5 nmol/L, with inter‐assay coefficients of variation (CVs) of 8.1% and 7.7%, respectively.
3.3. Outcomes
3.3.1. Cognitive function
Rush MAP participants are enrolled without known dementia and followed annually. At each visit, global cognitive function was determined using scores from a battery of 19 cognitive tests. 30 The estimated person‐specific rate of change in the global cognition variable over time was determined using mixed‐effects models. 31 At the time of death and blinded to the results of autopsy, a final cognitive diagnosis was made based on all available clinical data reviewed by a neurologist with expertise in dementia, and classified as dementia, MCI, or NCI. 32 , 33
3.3.2. Neuropathologic evaluation
After death, brains were dissected during rapid autopsy following established protocols as described 34 and evaluated histologically by examiners blinded to clinical information. The mean (SD) post‐mortem interval was 8.8 (5.1) hours. A quantitative summary of global AD pathology was derived from counts of neuritic plaques, diffuse plaques, and neurofibrillary tangles in the Bielschowski‐stained sections of the mid‐frontal cortex, mid‐temporal cortex, inferior parietal cortex, entorhinal cortex, and hippocampus. 35 Braak stages were based on the distribution and severity of neurofibrillary tangle pathology. 34 Consortium to Establish a Registry for Alzheimer's Disease (CERAD) scores were based on neuritic plaques. 34 The Braak stages for neurofibrillary pathology and the CERAD estimate of neuritic plaques was used to derive the National Institute on Aging (NIA)–Reagan diagnosis of AD. 34 The percent area occupied by Aβ protein in eight cortical regions was identified by molecularly specific immunohistochemistry and calculated as described. 35 Neuronal PHF‐tau tangle density and burden were identified by immunohistochemistry in eight regions and quantified as described. 35 The age, volume (mm3), side, and location of macroscopic and microscopic cerebral infarctions were identified as described. 36 , 37 Lewy bodies were identified using immunohistochemistry. 38
3.4. Statistical approach
Clinical cognitive diagnosis and final cognitive diagnosis were analyzed with ordinal logistic regression using dementia, MCI, and NCI categories. Ordinal logistic regression with proportional odds was used for ordered categories, as we saw no evidence for non‐proportional odds. Global cognitive function and person‐specific rate of change in cognitive function (slope of global cognition) were analyzed as continuous outcomes. AD neuropathology was considered as present or absent based on NIA‐Reagan criteria and CERAD scores. 34 , 42 Braak stage of illness, which captures neurofibrillary tangle pathology, was categorized as III or less or IV or greater due to the small number of cases in individual Braak stages II, III, and VI. 43 Lewy Body disease and infarcts were considered present or absent. 36 Global pathology, amyloid burden, diffuse and neuritic plaques, and neurofibrillary tangle density and burden were analyzed as continuous outcomes. Appropriate variable transformations were applied to continuous neuropathology outcomes as indicated by Box‐Cox transformations and visual inspection of residuals. Square root transformation was used for global AD pathology, A, and plaque burden outcomes; quartic root transformation was used for neurofibrillary tangle outcomes. Brain MK4 and plasma phylloquinone concentrations were log2‐transformed for the analysis to satisfy linearity assumptions, and non‐detectable values were analyzed using half the value of the limit of detection. The MK4 concentrations in the mid‐temporal and mid‐frontal cortexes were averaged, and the anterior watershed and cerebellum were analyzed as separate regions. Co‐variates included age at death, sex, education (≤12 years, >12 to ≤16 years, >16 years), APOE ε4 status (ε4 present/absent) and post‐mortem interval. Models for clinical cognitive diagnosis and global cognitive function are adjusted for time between last cognitive assessment and death. Plasma phylloquinone models were additionally adjusted for triglycerides (because phylloquinone is transported on triglyceride‐rich lipoproteins 44 ), DASH diet score (because plasma phylloquinone can reflect a healthy diet 23 ), and time between plasma sample collection and death/last cognitive assessment. One participant with a plasma phylloquinone measurement of 30.8 nmol/L was excluded from the statistical analysis. Estimated associations are reported as beta coefficients or odds ratios (OR = exp(β)). MK4 concentrations between brain regions were compared using repeated‐measures analysis of variance (ANOVA). Spearman rank coefficients were reported for pairwise correlations. Analyses were performed in R v4.0 (R Core Team, 2020) and the VGAM package. 45 A level of α = 0.05 was considered statistically significant. Adjusted P‐ values (q‐values) were calculated to evaluate significance of associations after multiple testing correction.
3.5. Results
The last assessment of global cognitive function before death occurred an average of 1.3 (SD = 1.5) years before death. Plasma phylloquinone was sampled an average of 2.1 (SD = 1.9) and 3.3 (SD = 2.1) years before the last assessment of global cognitive function and death, respectively.
Brain MK4 concentrations were variable (Figure 1). Although MK4 concentrations were lower in the anterior watershed compared to the other three regions (all pairwise comparisons with AWS P‐values < .001, Figure 1), MK4 concentrations were highly correlated across the four regions (intra‐class correlation coefficient = 0.86). Plasma phylloquinone measured at the last visit before death was not correlated with brain MK4 or phylloquinone concentrations in any region (partial correlation adjusted for triglycerides, all r <0.10, all P‐values > .05), with the exception of correlation with brain phylloquinone in the mid‐frontal region (partial correlation adjusted for triglycerides, r = 0.14, P‐value = .02).
FIGURE 1.
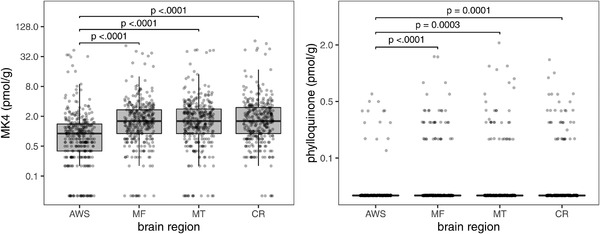
Boxplots of menaquinone‐4 (MK4) and phylloquinone concentrations in four human brain regions (AWS, anterior watershed; MF, mid‐frontal cortex; MT, mid‐temporal cortex; CR, cerebellum) (n = 325). Boxplot indicates the median (middle line of the box), first quartile (lower boundary of the box), and third quartile (upper boundary of the box) of MK4 and phylloquinone concentrations in each brain region. Points at the bottom of each plot indicate values below detection limits (0.1 pmol/g). Brackets indicate post hoc pairwise comparison tests with Tukey adjustment that have significant P‐values
Overall, the mean rate of decline in global cognitive scores was −0.008 units per year. The odds of having dementia or MCI at the last cognitive assessment before death were 17% to 20% lower per doubling of MK4 in all four brain regions measured (ORs 0.80 to 0.83, all P‐values < .014.) (Table 2). These odds were generally consistent with the odds for dementia or MCI at the final cognitive diagnosis. Higher brain MK4 concentrations in the mid‐frontal and mid‐temporal cortexes and the anterior watershed were also associated with better ante‐ mortem global cognitive function scores and a slower rate of cognitive decline (all P‐values < .040). Higher brain MK4 concentrations in the cerebellum was associated with better ante‐mortem global cognitive function (P‐value = .036), but not with the rate of cognitive decline (P‐value = .09) (Table 2). Associations remained statistically significant after multiple testing correction (Table S1).
For neuropathologically defined outcomes, the odds of Braak stage ≥IV were 14% to 16% lower per doubling of MK4 in the mid‐frontal and mid‐temporal cortexes, anterior watershed, and cerebellum (ORs 0.84 to 0.86, all P‐values < .045) (Table 3). The odds of having Lewy bodies were 13% to 18% lower per doubling of MK4 in the mid‐frontal and mid‐temporal cortexes and cerebellum (ORs 0.82 to 0.87, P < .045). Higher MK4 in the mid‐frontal and mid‐temporal cortical and anterior watershed regions was also associated with lower global AD pathology scores (although statistical significance was borderline for the mid‐frontal and mid‐temporal cortexes) and a lower neurofibrillary tangle burden (Table 3). Brain MK4 concentrations were not associated with neuritic plaques (CERAD score), density of neuritic or diffuse plaques, a pathologic diagnosis of AD defined using NIA–Reagan criteria, amyloid load, or with infarcts. Significant associations with neuropathological outcomes were no longer statistically significant after multiple testing correction (Table S2).
Among ante‐mortem measures, higher plasma phylloquinone at the last clinic visit was associated with better cognitive function (global cognitive function β [SE] = 0.229 [0.072], P‐value = .002, n = 295), a slower rate of cognitive decline (slope of cognitive function β [SE] = 0.017 [0.006], P‐value = .004, n = 295), and with a better clinical cognitive diagnosis at the final clinic visit and death (cognitive diagnosis at final visit β[SE] = −0.379 [0.137], P‐value = .006, n = 288; cognitive diagnosis at death visit β [SE] = −0.338 [0.137], P = .013, n = 293). In contrast, circulating plasma phylloquinone was not associated with any neuropathologically defined outcome evaluated (all P‐values ≥ .10).
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors would like to acknowledge the contribution of the late Dr. Martha Clare Morris in the development of this project. Sarah. L Booth and M. Kyla Shea are supported by National Institutes of Health (NIH) grants (to Tufts University). Kathryn Barger is a member of the American Society of Nutrition Statistical Review Board, served on the NIH Study Section Review Committee, and receives support from the Bill and Melinda Gates Foundation, the Edward N. and Della L. Thome Memorial Foundation, and the NIH (all to Tufts University). Sue E. Leurgans is a Biostatistics Editor of Neurology, served on the NIH Study Section Review Committees and is supported by NIH grants (to Rush University). Bryan D. James is a consultant for the Alzheimer's Association. Thomas M. Holland is a member of the U.S. against Alzheimer's non‐profit risk reduction working group. Puja Agarwal receives support from the Michael J Fox Foundation, the Physicians Committee for Responsible Medicine and is a member of the US against Alzheimer's non‐profit risk reduction working group. Julie A. Schneider receives support from Alnylam, Eli Lily, and for expert legal testimony; serves on External Advisory Committees for Kansas University, Boston University, University of California Irvine, University of Washington, and New York University and Observational Monitoring Boards for Framingham Heart study and National Institute of Neurological Disorders and Stroke (NINDS) Discovery at Boston University and is supported by NIH grants (to Rush University). All other co‐authors have nothing to disclose. Study supported by National Institute on Aging R01AG051641 and R01AG17917 and the US Department of Agriculture (USDA) Agricultural Research Service under Cooperative Agreement No. 58‐1950‐7‐707. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA.
Booth SL, Shea MK, Barger K, et al. Association of vitamin K with cognitive decline and neuropathology in community‐dwelling older persons. Alzheimer's Dement. 2022;8:e12255. 10.1002/trc2.12255
REFERENCES
- 1. Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17(11):1006‐1015. [DOI] [PubMed] [Google Scholar]
- 2. Morris MC, Wang Y, Barnes LL, Bennett DA, Dawson‐Hughes B, Booth SL. Nutrients and bioactives in green leafy vegetables and cognitive decline: prospective study. Neurology. 2018;90(3):e214‐e222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kiely A, Ferland G, Ouliass B, O'Toole PW, Purtill H, O'Connor EM. Vitamin K status and inflammation are associated with cognition in older Irish adults. Nutr Neurosci. 2020;23(8):591‐599. [DOI] [PubMed] [Google Scholar]
- 4. Tanprasertsuk J, Ferland G, Johnson MA, et al. Concentrations of circulating phylloquinone, but not cerebral menaquinone‐4, are positively correlated with a wide range of cognitive measures: exploratory findings in centenarians. J Nutr. 2020;150(1):82‐90. [DOI] [PubMed] [Google Scholar]
- 5. Harshman SG, Finnan EG, Barger KJ, et al. Vegetables and mixed dishes are top contributors to phylloquinone intake in US adults: data from the 2011‐2012 NHANES. J Nutr. 2017;147(7):1308‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thijssen HH, Drittij‐Reijnders MJ. Vitamin K status in human tissues: tissue‐specific accumulation of phylloquinone and menaquinone‐4. Br J Nutr. 1996;75(1):121‐127. [DOI] [PubMed] [Google Scholar]
- 7. Shea MK, Berkner KL, Ferland G, Fu X, Holden RM, Booth SL. Perspective: evidence before enthusiasm‐a critical review of the potential cardiovascular benefits of Vitamin K. Adv Nutr. 2021;12(3):632‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferland G. Vitamin K and the nervous system: an overview of its actions. Adv Nutr. 2012;3(2):204‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li J, Lin JC, Wang H, et al. Novel role of vitamin K in preventing oxidative injury to developing oligodendrocytes and neurons. J Neurosci. 2003;23(13):5816‐5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sundaram KS, Fan JH, Engelke JA, Foley AL, Suttie JW, Lev M. Vitamin K status influences brain sulfatide metabolism in young mice and rats. J Nutr. 1996;126(11):2746‐2751. [DOI] [PubMed] [Google Scholar]
- 11. Gonzalez de San Roman E, Manuel I, Giralt MT, Ferrer I, Rodriguez‐Puertas R. Imaging mass spectrometry (IMS) of cortical lipids from preclinical to severe stages of Alzheimer's disease. Biochim Biophys Acta Biomembr. 2017;1859:1604‐1614. 9 Pt B. [DOI] [PubMed] [Google Scholar]
- 12. Czubowicz K, Jesko H, Wencel P, Lukiw WJ, Strosznajder RP. The role of ceramide and sphingosine‐1‐phosphate in Alzheimer's disease and other neurodegenerative disorders. Mol Neurobiol. 2019;56(8):5436‐5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jansen WJ, Wilson RS, Visser PJ, et al. Age and the association of dementia‐related pathology with trajectories of cognitive decline. Neurobiol Aging. 2018;61:138‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Munoz E, Filshtein T, Bettcher BM, et al. Cognitive function and neuropathological outcomes: a forward‐looking approach. J Neurol. 2019;266(12):2920‐2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age‐related cognitive decline. Neurology. 2010;75(12):1070‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nguyen MT, Mattek N, Woltjer R, et al. Pathologies underlying longitudinal cognitive decline in the oldest old. Alzheimer Dis Assoc Disord. 2018;32(4):265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Presse N, Belleville S, Gaudreau P, et al. Vitamin K status and cognitive function in healthy older adults. Neurobiol Aging. 2013;34(12):2777‐2783. [DOI] [PubMed] [Google Scholar]
- 18. Fu X, Harshman SG, Shen X, et al. Multiple vitamin K forms exist in dairy foods. Curr Dev Nutr. 2017;1(6):e000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fu X, Shen X, Finnan EG, Haytowitz DB, Booth SL. Measurement of multiple vitamin K forms in processed and fresh‐cut pork products in the U.S. food supply. J Agric Food Chem. 2016;64(22):4531‐4535. [DOI] [PubMed] [Google Scholar]
- 20. Institute of Medicine . Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington DC: Institute of Medicine; 2001. [Google Scholar]
- 21. Nakagawa K, Hirota Y, Sawada N, et al. Identification of UBIAD1 as a novel human menaquinone‐4 biosynthetic enzyme. Nature. 2010;468(7320):117‐121. [DOI] [PubMed] [Google Scholar]
- 22. Ellis JL, Fu X, Karl JP, et al. Multiple dietary vitamin K forms are converted to tissue menaquinone‐4 in mice. J Nutr. 2021. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shea MK, Booth SL. Concepts and controversies in evaluating vitamin K status in population‐based studies. Nutrients. 2016;8(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fu X, Shea MK, Dolnikowski GG, et al. Vitamin D and Vitamin K concentrations in human brain tissue are influenced by freezer storage time: the Memory and Aging Project. J Nutr. 2021;151(1):104‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brangier A, Ferland G, Rolland Y, Gautier J, Feart C, Annweiler C. Vitamin K antagonists and cognitive decline in older adults: a 24‐month follow‐up. Nutrients. 2018;10(6):666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee ZX, Ang E, Lim XT, Arain SJ. Association of risk of dementia with direct oral anticoagulants versus warfarin use in patients with non‐valvular atrial fibrillation: a systematic review and meta‐analysis. J Cardiovasc Pharmacol. 2021;77(1):22‐31. [DOI] [PubMed] [Google Scholar]
- 27. Mongkhon P, Fanning L, Lau WCY, et al. Oral anticoagulant and reduced risk of dementia in patients with atrial fibrillation: a population‐based cohort study. Heart Rhythm. 2020;17:706‐713. 5 Pt A. [DOI] [PubMed] [Google Scholar]
- 28. Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9(6):646‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davidson KW, Sadowski JA. Determination of vitamin K compounds in plasma or serum by high‐performance liquid chromatography using postcolumn chemical reduction and fluorimetric detection. Methods Enzymol. 1997;282:408‐421. [DOI] [PubMed] [Google Scholar]
- 30. Wilson RS, Boyle PA, Yu L, et al. Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology. 2015;85(11):984‐991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Jager PL, Shulman JM, Chibnik LB, et al. A genome‐wide scan for common variants affecting the rate of age‐related cognitive decline. Neurobiol Aging. 2012;33(5):1017. e1011‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community‐dwelling older persons. Neurology. 2007;69(24):2197‐2204. [DOI] [PubMed] [Google Scholar]
- 33. Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community‐based cohort studies compared to standard practice in a clinic‐based cohort study. Neuroepidemiology. 2006;27(3):169‐176. [DOI] [PubMed] [Google Scholar]
- 34. Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community‐based studies. Neurology. 2006;66(12):1837‐1844. [DOI] [PubMed] [Google Scholar]
- 35. Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious orders study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64(s1):S161‐S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42(3):722‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schneider JA, Bienias JL, Wilson RS, Berry‐Kravis E, Evans DA, Bennett DA. The apolipoprotein E epsilon4 allele increases the odds of chronic cerebral infarction [corrected] detected at autopsy in older persons. Stroke. 2005;36(5):954‐959. [DOI] [PubMed] [Google Scholar]
- 38. Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community‐dwelling subjects with Lewy bodies. Brain. 2012;135:3005‐3014. Pt 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Okano T, Shimomura Y, Yamane M, et al. Conversion of phylloquinone (Vitamin K1) into menaquinone‐4 (Vitamin K2) in mice: two possible routes for menaquinone‐4 accumulation in cerebra of mice. J Biol Chem. 2008;283(17):11270‐11279. [DOI] [PubMed] [Google Scholar]
- 40. Al‐Rajabi A, Booth SL, Peterson JW, et al. Deuterium‐labeled phylloquinone has tissue‐specific conversion to menaquinone‐4 among Fischer 344 male rats. J Nutr. 2012;142(5):841‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Card DJ, Shearer MJ, Schurgers LJ, Harrington DJ. The external quality assurance of phylloquinone (vitamin K(1)) analysis in human serum. Biomed Chromatogr. 2009;23(12):1276‐1282. [DOI] [PubMed] [Google Scholar]
- 42. Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64(5):834‐841. [DOI] [PubMed] [Google Scholar]
- 43. Nag S, Yu L, VanderHorst VG, et al. Neocortical Lewy bodies are associated with impaired odor identification in community‐dwelling elders without clinical PD. J Neurol. 2019;266(12):3108‐3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ellis JL, Fu X, Al Rajabi A, et al. Plasma response to deuterium‐labeled vitamin K intake varies by TG response, but not age or vitamin K status, in older and younger adults. J Nutr. 2019;149(1):18‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yee TW. The VGAM package for categorical data analysis. J Stat Softw. 2010;32(10):1‐34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


