Abstract
To find the exact substrate specificities of three species of tripartite efflux systems of Pseudomonas aeruginosa, MexAB-OprM, MexCD-OprJ, and MexXY-OprM, we constructed a series of isogenic mutants, each of which constitutively overproduced one of the three efflux systems and lacked the other two, and their isogenic mutants, which lacked all these systems. Comparison of the susceptibilities of the constructed mutants to 52 antimicrobial agents belonging to various groups suggested the following substrate specificities. All of the efflux systems extrude a wide variety of antimicrobial agent groups, i.e., quinolones, macrolides, tetracyclines, lincomycin, chloramphenicol, most penicillins (all but carbenicillin and sulbenicillin), most cephems (all but cefsulodin and ceftazidime), meropenem, and S-4661, but none of them extrude polymyxin B or imipenem. Extrusion of aminoglycosides is specific to MexXY-OprM, and extrusion of a group of the β-lactams, i.e., carbenicillin, sulbenicillin, ceftazidime, moxalactam, and aztreonam, is specific to MexAB-OprM. Moreover, MexAB-OprM and MexCD-OprJ extrude novobiocin, cefsulodin, and flomoxef, while MexXY-OprM does not. These substrate specificities are distinct from those reported previously.
Several tripartite efflux systems coded on the chromosome play important roles in the intrinsic and acquired resistance in Pseudomonas aeruginosa. Each system consists of a cytoplasmic membrane component of the resistance-nodulation-division family presumed to function as a transporter, an outer membrane component presumed to form channels, and a membrane fusion protein presumed to link the two membrane proteins for reviews, see references (13, 14, and 15). The MexA-MexB-OprM efflux system (7, 16) contributes to both intrinsic and acquired resistance in P. aeruginosa, while the MexC-MexD-OprJ (17) and MexE-MexF-OprN (6) efflux systems contribute only to the acquired resistance in this bacterium. Studies with mutants that overproduce or lack MexAB-OprM demonstrated that this efflux system extrudes quinolones, macrolides, tetracycline, chloramphenicol, novobiocin, and most β-lactams but not imipenem (7, 8, 22). Studies with mutants overproducing MexCD-OprJ demonstrated that this efflux system extrudes quinolones, erythromycin, tetracycline, chloramphenicol, and expanded-spectrum cephems such as cefpirome (9, 10). Furthermore, characterization of mutants lacking the mexA-mexB-oprM region demonstrated that the MexCD-OprJ efflux system extrudes ceftazidime and cefoperazone as well as cefpirome, while it does not extrude carbenicillin and aztreonam (3). Recently, Aires et al. (1) and our group (12) showed that MexX-MexY extrudes aminoglycosides, tetracycline, and erythromycin in cooperation with spontaneously expressed OprM, thereby contributing to the intrinsic resistance to these agents in P. aeruginosa. Our group also showed that no expression of MexXY is observed in wild-type strains but that the expression can be induced by subinhibitory concentrations of its substrates such as tetracycline and gentamicin (12). However, the precise substrate specificity of the MexXY-OprM efflux system is unclear.
Simultaneous expression of plural efflux systems can cause misunderstanding of the substrate specificity of each multidrug efflux system. In this study, to find the exact substrate specificities of the MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux systems, we constructed and compared the susceptibilities of a series of isogenic mutants, each of which constitutively overproduced one of the three efflux systems and lacked the other two, and their isogenic mutants, which lacked all these systems.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacterial cells were grown on Mueller-Hinton II agar (MHA; Becton Dickinson Microbiology Systems, Cockeysville, Md.) or in Mueller-Hinton broth (MHB; Becton Dickinson Microbiology Systems) at 37°C. Minimal agar medium (2) was used for selection of P. aeruginosa. The following antibiotics were added to the media at the indicated concentrations: carbenicillin, 100 μg/ml for Escherichia coli and 200 μg/ml for P. aeruginosa; streptomycin, 30 μg/ml for E. coli and 100 μg/ml for P. aeruginosa; and chloramphenicol, 30 μg/ml for E. coli and MexAB-OprM-deficient P. aeruginosa and 300 μg/ml for MexAB-OprM-producing P. aeruginosa. MHA was supplemented with 10% (wt/vol) sucrose, as required.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| P. aeruginosa strains | ||
| PAO1 | Prototroph | |
| OCR1 | MexAB-OprM-overproducing nalB mutant | 8 |
| KG2259 | ΔmexRAB-oprM of COR6(KG3056), a MexCD-OprJ-overproducing nfxB mutant | 3 |
| N103 | ΔmexXY of KG2239, ΔmexRAB-oprM of PAO1 | 12 |
| N106 | ΔmexXY of KG2507, ampC∷Ω of KG2239 | This study |
| N107 | ΔmexXY of OCR1 | This study |
| N108 | ΔmexXY of KG2259 | This study |
| N116 | ampC∷Ω of N107 | This study |
| N119 | ampC∷Ω of N108 | This study |
| N126 | ΔmexAB of OCR1 | This study |
| N127 | MexXY-overproducing mutant of N126 | This study |
| N128 | ΔmexXY of N127 | This study |
| N133 | ampC∷Ω of N127 | This study |
| N148 | ΔmexAB of N107 | This study |
| N150 | ΔmexCD of N108 | This study |
| N151 | ΔmexAB of N116 | This study |
| N153 | ΔmexCD of N119 | This study |
| N154 | ΔmexXY of N133 | This study |
| E. coli S17-1 | thi pro hsdR recA Tra+ | 21 |
| Plasmids | ||
| pMT5059 | pBend2 derivative carrying multiple-cloning and NotI sites; Cbr | 23 |
| pMT5071 | pMOB3 derivative carrying Ω-Cm instead of Cm; Cmr | 24 |
| pNS003 | pMT5059 with flanking regions of mexXY and Mob cassette; Cbr Cmr | 12 |
| pNS004 | pMT5059 with 0.9-kb PCR fragment with 3′ flanking region of mexB in XhoI-HindIII; Cbr | This study |
| pNS005 | pNS004 with 1.0-kb PCR fragment with 5′ flanking region of mexA in EcoRI-SpeI; Cbr | This study |
| pNS006 | pNS005 with Mob cassette in NotI; Cbr Cmr | This study |
| pKMJ075 | pMT5059 with mexD∷Ω and Mob cassette; Cbr Cmr Smr | 4 |
| pKMB004 | pMT5059 with ampC∷Ω and Mob cassette; Cbr Cmr Smr | K. Okamoto (Kyoto Pharmaceutical University) |
Abbreviations: Cbr, carbenicillin resistant; Cmr, chloramphenicol resistant; Smr, streptomycin resistant.
Susceptibility testing.
MICs were determined by the usual twofold agar dilution technique with MHA with an inoculum size of 104 cells. S-4661 (5) was synthesized at Sankyo Co., Ltd., Tokyo, Japan. Ciprofloxacin, tosufloxacin, and sparfloxacin were synthesized at Ube Industries, Ltd., Tokyo, Japan. The other antimicrobial agents used in this study were obtained from commercial sources.
Molecular biology techniques.
Chromosomal DNA and plasmids were isolated with a DNeasy tissue kit and a QIAfilter plasmid kit (Qiagen K.K., Tokyo, Japan), respectively. PCRs were performed with a Perkin-Elmer 480 thermal cycler with PfuTurb DNA polymerase (Stratagene, La Jolla, Calif.). Restriction endonucleases, alkaline phosphatase, and the DNA-ligation kit were obtained from Takara Shuzo Co., Ltd., Kyoto, Japan. Restriction fragments were isolated, as required, from agarose gels with TaKaRa RECOCHIP (Takara, Shuzo Co., Ltd.). All molecular biology techniques were carried out according to the manufacturer's instructions or as described by Sambrook et al. (19). Transformation of E. coli with plasmid DNA was performed as described previously (3).
Deletion of mexAB by gene replacement.
To construct isogenic mutants lacking the mexA-mexB region, PCR primers for amplification of the mexA-mexB region and its flanking regions were synthesized on the basis of the nucleotide sequences of the Pseudomonas genome sequencing project database (http://www.pseudomonas.com/). After amplification of a 0.9-kb region downstream of mexB on PAO1 chromosomal DNA as a template with AB3 (5′-TTTCTCGAGCTGGCGATCTTCTGGGTACC-3′) and AB4 (5′-TTTAAGCTTACTTCGGTCAGCAGGGTCTG-3′), a primer pair containing a newly added cutting site (underlined) for restriction nucleases, the region was ligated into the XhoI-HindIII site in a multicloning site of pMT5059 (23) to yield pNS004. Next, a 1.0-kb region upstream of the mexA gene amplified by PCR with the primer pair AB1 (5′-TTTGAATTCGGTGATCAGTGCCTTGTCGC-3′) and AB2 (5′-TTACTAGTCGACAGCACCTTGGTGTAGC-3′) was ligated into the EcoRI-SpeI site of pNS004 to yield pNS005. (A 12-bp sequence derived from a multicloning site of pMT5059 was still left between the two DNA fragments inserted on pNS005.) After addition of a NotI-flanked Mob cassette from pMT5071 (24), the resulting plasmid, pNS006, was mobilized from the E. coli strain S17-1 (21), to the P. aeruginosa strains to introduce deletion of the mexA-mexB region into the recipient chromosomes by allelic exchange, as described previously (12). Deletion of mexAB was confirmed by PCR with the primer pair AB5 (5′-CTCATGAGGACAACGCTATGCAACGAACG-3′) and AB6 (5′-TGGGTCAGGTCGAAACTCTTCTGGTAGGTG-3′). The sizes of the amplified DNA fragments obtained with these primers were 4.9 kb for the wild-type strain and 1.2 kb for the mexAB-deficient strains (data not shown).
Insertional deletion of ampC by gene replacement.
Plasmid pKMB004 residing in S17-1 was conjugationally mobilized to P. aeruginosa cells. After mating on MHA at 37°C overnight, the cell mixture was suspended in saline. Aliquots of the suspensions were spread on minimal agar plates supplemented with streptomycin, and the plates were incubated at 30°C for 2 days. The transconjugants were plated onto MHA supplemented with 10% sucrose and streptomycin. Clones showing hypersusceptibilities to amoxicillin were used in subsequent experiments.
Production of polyclonal antisera specific to MexA, MexC, and MexX.
To obtain antibodies specific to MexA, MexC, and MexX, the oligopeptides (C)YQIDPATYEADYQSA (amino acids 92 to 106 of MexA), (C)AQARVRRYEPLVKIQ (amino acids 120 to 134 of MexC), and (C)EDSPTPLTRVEQID (amino acids 197 to 210 of MexX) were synthesized and conjugated to keyhole limpet hemocyanin by Chiron Technologies Pty., Ltd. (Clayton, Victoria, Australia). A cysteine residue was added to each N terminus for conjugation. Rabbit antiserum raised against each antigen was prepared by Takara Shuzo Co., Ltd.
Isolation of total and outer membranes, SDS-PAGE, and immunoblot assay.
Exponentially growing cells in MHB were harvested as described previously (9). MHB was supplemented with tetracycline, as required. Total membranes (3) and outer membranes (9) were prepared as described previously. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretic transfer were performed as described previously (10). Production levels of MexA, MexC, and MexX were tested by immunoblot assay with rabbit polyclonal antisera specific for MexA, MexC, and MexX, respectively (see above), and production levels of OprM and OprJ were tested with murine monoclonal antibodies specific for OprM (TM001 [3]) and OprJ (HJ001 [4]), respectively. Binding of the primary antibodies was detected as described previously (3), with alkaline phosphatase-conjugated goat antibodies to rabbit or mouse immunoglobulins (Biosource International, Camarillo, Calif.) used as the secondary antibodies and an Alkaline Phosphatase Conjugate Substrate kit (Bio-Rad Laboratories, Hercules, Calif.) used for color development.
RESULTS
Construction of mutants overproducing one of three efflux systems.
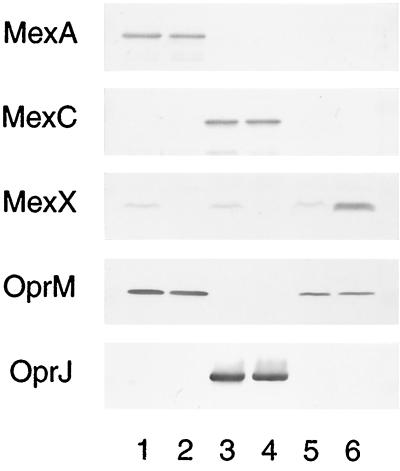
To confirm the precise substrate specificity of the MexXY-OprM efflux system, we constructed a mutant that overproduced MexXY-OprM. mexXY and oprM are located at different places on the chromosome, and their expressions are regulated independently. Our first step in constructing the MexXY-OprM-overproducing mutant was to perform an in-frame deletion of mexAB from MexAB-OprM-overproducing strain OCR1. To avoid affecting the expression of OprM in the strain obtained (designated strain N126), we conserved the point mutation in mexR in OCR1 (18), together with the presumed second promoter responsible for OprM expression identified upstream of oprM (27). The amount of OprM produced in N126 was lower than that produced in OCR1 but was almost the same as that produced in PAO1 (Fig. 1, lanes 1 and 5). The reduction in the level of OprM expression in N126 might be a polar effect caused by deletion of mexAB. Next, we isolated a MexXY-overproducing mutant, N127, from N126 by selection on a plate containing 4 μg of gentamicin and 0.5 μg of ofloxacin per ml (Fig. 1, lane 6). Furthermore, we reexamined the substrate specificities of MexAB-OprM and MexCD-OprJ to compare them with the substrate specificity of MexXY-OprM. Chimeric MexAB-OprJ and MexCD-OprM pumps can function in the efflux of antimicrobial agents (4, 22, 26). Given the presence of the mexX-mexY operon in the mutants constructed previously, the functioning of these chimeric pumps might suggest that MexXY influences the previously observed specificities of MexAB-OprM and MexCD-OprJ. For instance, MexXY may be associated with OprJ and may affect the susceptibility even in KG2259, a mutant that produces large amounts of MexCD-OprJ and lacks mexAB-oprM. Therefore, we isolated mutants lacking the mexXY region from OCR1 and KG2259 using pNS003, as described previously (12), and designated these mutants N107 and N108, respectively. The overproduction or lack of each efflux pump component in N107, N108, and N127 was confirmed by immunoblot assay (Fig. 1). We have reported that no production of MexX is observed in wild-type strains but that the MexX production is induced by antimicrobial agents such as tetracycline (12). Thus, loss of MexX in N107 and N108 was confirmed by culture with subinhibitory concentrations of tetracycline. Moreover, we deleted each overexpressed efflux system from N107, N108, and N127 by gene replacement and designated them N148, N150, and N128, respectively. Insertional deletion of mexD was performed with pKMJ075, as described previously (4). Deletions of mexAB and mexXY were performed as described above. Disruption of each gene was confirmed by PCR (data not shown).
FIG. 1.
Detection of MexA, MexC, and MexX with antisera directed against synthetic oligopeptides containing part of the amino acid sequences of MexA, MexC, and MexX and detection of OprM and OprJ with monoclonal antibodies specific to OprM or OprJ. Each lane contains 5 μg (MexA and MexC) or 25 μg (MexX) of total membrane protein or 10 μg (OprM) or 1 μg (OprJ) of outer membrane protein. OCR1 (lane 1), N107 (lane 2), KG2259 (lane 3), N108 (lane 4), N126 (lane 5), and N127 (lane 6) were grown in MHB (MexA, MexC, OprM, and OprJ) or MHB containing a subinhibitory concentration of tetracycline (MexX). MHB containing tetracycline at 16 μg/ml (OCR1), 8 μg/ml (N107, KG2259, and N108), or 4 μg/ml (N126 and N127) and approximately 108 CFU of cells per ml was incubated for 2 h at 37°C with shaking.
Substrate specificities of three efflux systems to non-β-lactam agents.
Table 2 presents the MICs of all of the antimicrobial agents tested except the β-lactams for N107, N108, N127, and the respective efflux system-deficient mutants, N148, N150, and N128. N127 showed lower levels of susceptibility to quinolones, macrolides, tetracyclines, lincomycin, chloramphenicol, and aminoglycosides than N128, suggesting that MexXY-OprM extrudes these agents. In contrast, N107 and N108 showed lower levels of susceptibility to quinolones, macrolides, tetracyclines, lincomycin, chloramphenicol, and novobiocin than N148 and N150, respectively, suggesting that MexAB-OprM and MexCD-OprJ extrude these agents.
TABLE 2.
Susceptibilities of constructed mutants to various antimicrobial agents
| Antimicrobial agent | MIC (μg/ml)a
|
|||||
|---|---|---|---|---|---|---|
| N107 | N148 | N108 | N150 | N127 | N128 | |
| Quinolones | ||||||
| Nalidixic acid | 512 | 2 | 256 | 1 | 32 | 2 |
| Piromidic acid | >4,096 | 8 | >4,096 | 2 | 128 | 4 |
| Pipemidic acid | 32 | 1 | 64 | 0.5 | 64 | 1 |
| Cinoxacin | >2,048 | 4 | 128 | 4 | 32 | 2 |
| Norfloxacin | 2 | 0.06 | 16 | 0.016 | 2 | 0.06 |
| Ofloxacin | 4 | 0.06 | 8 | 0.016 | 4 | 0.06 |
| Enoxacin | 2 | 0.06 | 8 | 0.03 | 4 | 0.06 |
| Ciprofloxacin | 0.25 | 0.008 | 2 | 0.002 | 0.5 | 0.008 |
| Tosufloxacin | 0.5 | 0.016 | 2 | 0.002 | 1 | 0.016 |
| Sparfloxacin | 1 | 0.03 | 16 | 0.008 | 2 | 0.03 |
| Macrolides | ||||||
| Erythromycin | 256 | 64 | 4,096 | 8 | 512 | 64 |
| Oleandomycin | >8,192 | 2,048 | >8,192 | 32 | >8,192 | 2,048 |
| Spiramycin | 2,048 | 512 | 8,192 | 64 | 4,096 | 512 |
| Tetracyclines | ||||||
| Tetracycline | 64 | 1 | 64 | 0.13 | 32 | 1 |
| Chlortetracycline | >2,048 | 16 | 2,048 | 4 | >2,048 | 16 |
| Oxytetracycline | 16 | 1 | 16 | 0.13 | 16 | 1 |
| Aminoglycosides | ||||||
| Streptomycin | 2 | 1 | 2 | 256 | 128 | 1 |
| Neomycin | 2 | 1 | 0.5 | 1 | 128 | 1 |
| Kanamycin | 16 | 16 | 8 | 8 | 256 | 8 |
| Gentamicin | 0.13 | 0.06 | 0.06 | 0.13 | 8 | 0.06 |
| Amikacin | 0.25 | 0.13 | 0.13 | 0.25 | 16 | 0.13 |
| Tobramycin | 0.13 | 0.06 | 0.13 | 0.13 | 2 | 0.06 |
| Others | ||||||
| Lincomycin | >4,096 | 512 | >4,096 | 64 | >4,096 | 512 |
| Chloramphenicol | 512 | 4 | 256 | 1 | 8 | 2 |
| Novobiocin | 4,096 | 32 | 256 | 32 | 32 | 32 |
| Polymyxin B | 1 | 1 | 1 | 1 | 1 | 1 |
N107 produced MexAB-OprM, N108 produced MexCD-OprJ, N127 produced MexXY-OprM, and N148, N150, and N128 were deficient in the three efflux systems.
Substrate specificities of three efflux systems to β-lactams.
The presence of chromosomal AmpC β-lactamase makes it quite difficult to interpret data on MICs because some β-lactams are removed by both the β-lactamase and the efflux system(s) (11). Thus, we introduced ampC∷Ω into the chromosomes of N107, N108, and N127 by gene replacement and designated them N116, N119, and N133, respectively. Deletion of AmpC was confirmed by spectrophotometric assay, as described previously (8). Moreover, we deleted each overexpressed efflux system from N116, N119, and N133 by gene replacement as described above and designated them N151, N153, and N154, respectively. Table 3 presents the MICs of various β-lactams for N116, N119, N133, and the respective efflux system-deficient mutants, N151, N153, and N154. First, N133 was less susceptible than N154 to meropenem, S-4661, all of the penicillins except carbenicillin and sulbenicillin, and all of the cephems except cefsulodin and ceftazidime, suggesting that MexXY-OprM also extrudes these β-lactams. Next, N119 was less susceptible than N153 to flomoxef, meropenem, S-4661, all of the penicillins except carbenicillin and sulbenicillin, and all of the cephems except ceftazidime, suggesting that MexCD-OprJ extrudes not only cephems (except for ceftazidime) but also flomoxef, meropenem, S-4661, and all of the penicillins except carbenicillin and sulbenicillin. Finally, N116 was less susceptible than N151 to all of the β-lactams tested except imipenem, suggesting that MexAB-OprM extrudes all of the β-lactams except imipenem.
TABLE 3.
Susceptibilities of constructed mutants to β-lactams
| Antimicrobial agent | MIC (μg/ml)a
|
|||||
|---|---|---|---|---|---|---|
| N116 | N151 | N119 | N153 | N133 | N154 | |
| Penicillins | ||||||
| Penicillin G | 512 | 0.13 | 16 | 0.06 | 1 | 0.13 |
| Cloxacillin | >4,096 | 64 | 4,096 | 4 | 512 | 64 |
| Nafcillin | 1,024 | 8 | 256 | 0.25 | 32 | 8 |
| Amoxicillin | 64 | 0.13 | 1 | 0.13 | 0.5 | 0.13 |
| Piperacillin | 8 | 0.06 | 1 | 0.06 | 0.5 | 0.06 |
| Carbenicillin | 128 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Sulbenicillin | 64 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Cephems | ||||||
| Cefamandole | 8,192 | 2 | 256 | 0.5 | 32 | 2 |
| Cefuroxime | 4,096 | 2 | 256 | 0.25 | 16 | 2 |
| Cefoperazone | 16 | 0.13 | 2 | 0.06 | 1 | 0.13 |
| Cefotaxime | 64 | 0.06 | 2 | 0.06 | 1 | 0.06 |
| Ceftizoxime | 128 | 0.06 | 2 | 0.03 | 2 | 0.06 |
| Ceftriaxone | 16 | 0.25 | 1 | 0.13 | 1 | 0.13 |
| Cefsulodin | 4 | 0.25 | 0.5 | 0.13 | 0.5 | 0.25 |
| Ceftazidime | 2 | 0.25 | 0.25 | 0.25 | 0.5 | 0.25 |
| Cefpirome | 4 | 0.25 | 16 | 0.06 | 8 | 0.25 |
| Cefepime | 2 | 0.06 | 2 | 0.03 | 2 | 0.06 |
| Cefozopran | 2 | 0.25 | 8 | 0.13 | 4 | 0.25 |
| Cefoselis | 2 | 0.13 | 4 | 0.03 | 4 | 0.13 |
| Cefoxitin | 512 | 0.5 | 8 | 0.13 | 2 | 0.5 |
| Oxacephems | ||||||
| Moxalactam | 32 | 0.5 | 0.5 | 0.5 | 1 | 1 |
| Flomoxef | 128 | 0.25 | 2 | 0.25 | 0.5 | 0.25 |
| Carbapenems | ||||||
| Imipenem | 0.25 | 0.5 | 0.13 | 0.25 | 0.5 | 0.5 |
| Meropenem | 1 | 0.016 | 0.13 | 0.016 | 0.06 | 0.016 |
| S-4661 | 0.5 | 0.03 | 0.13 | 0.03 | 0.25 | 0.03 |
| Monobactam, Aztreonam | 32 | 0.13 | 0.13 | 0.13 | 0.25 | 0.25 |
N116 produced MexAB-OprM, N119 produced MexCD-OprJ, N133 produced MexXY-OprM, and N151, N153, and N154 were deficient in the three efflux systems.
DISCUSSION
In this study, we found that MexXY-OprM also extrudes several β-lactams. However, deletion of MexXY from PAO1 produced no significant change in susceptibility to any of the four substrates of MexXY-OprM, cefuroxime, cefpirome, cefepime, and cefozopran (unpublished data). In addition, exposure to cefpirome did not induce MexX in PAO1 (unpublished data). These results suggest that MexXY-OprM does not contribute to the intrinsic resistance to β-lactams, despite the potency of MexXY-OprM in the extrusion of several β-lactams. This is reminiscent of our previous result (12), which showed that while ofloxacin is a substrate of MexXY-OprM, it induces no production of MexXY in the wild-type strain, thereby confirming that MexXY-OprM does not contribute to the intrinsic resistance to ofloxacin. Furthermore, cefpirome induced production of MexX in a mutant lacking both MexAB and AmpC (unpublished data), just as ofloxacin was shown to induce production of MexX in the N126 lacking MexAB in our earlier study (12). Thus, MexAB-OprM and AmpC are primary systems for removal of cefpirome in the wild-type strain, whereas MexXY-OprM is a compensatory system for removal of cefpirome in the mutant lacking the primary systems.
In the present study, we elucidate the exact substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM (listed in Table 4). Although most antimicrobial agents are substrates of all efflux systems, the three systems have slight but significant differences in substrate specificities. MexAB-OprM-overproducing mutants such as OCR1 show no significant change in susceptibility to gentamicin (8, 9), whereas MexAB-OprM-deficient mutants show lower levels of susceptibility to gentamicin than the wild-type strains (20, 25). This discrepancy is expected since MexXY-OprM contributes to the resistance to gentamicin, while MexAB-OprM does not. MexAB-OprM extrudes the broadest kinds of β-lactams. MexCD-OprJ and MexXY-OprM have substrate specificities similar to those of β-lactams, the exception being that the former extrudes cefsulodin and flomoxef, while the latter does not. MexCD-OprJ and MexXY-OprM extrude most penicillins tested but not carbenicillin and sulbenicillin. Carbenicillin and sulbenicillin possess a negatively charged substitution at position C-6 that the other penicillins tested lack. This may explain the differences in the substrate specificities to penicillins.
TABLE 4.
Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM
| Transporter | Linker | Channel | Substrates |
|---|---|---|---|
| MexB | MexA | OprM | Quinolones, macrolides, tetracyclines, lincomycin, chloramphenicol, novobiocin, β-lactams except imipenem |
| MexD | MexC | OprJ | Quinolones, macrolides, tetracyclines, lincomycin, chloramphenicol, novobiocin, penicillins except carbenicillin and sulbenicillin, cephems except ceftazidime, flomoxef, meropenem, S-4661 |
| MexY | MexX | OprM | Quinolones, macrolides, tetracyclines, lincomycin, chloramphenicol, aminoglycosides, penicillins except carbenicillin and sulbenicillin, cephems except cefsulodin and ceftazidime, meropenem, S-4661 |
N150 was more susceptible to macrolides, tetracyclines, some of the quinolones, and lincomycin than N148 and N128, although all three strains lack the three efflux systems (Table 2). N153 was also more susceptible to cloxacillin, nafcillin, cefamandole, cefuroxime, cefpirome, cefoselis, and cefoxitin than N151 and N154 (Table 3). N103 (12), a strain which lacks MexAB-OprM and MexXY, showed lower levels of susceptibility than N150 and higher levels of susceptibility than N148 and N128 (data not shown). N106, an ampC∷Ω strain of N103, also showed a lower level of susceptibility than N153 and a higher level of susceptibility than N151 and N154 (data not shown). The reason for the discrepancy in susceptibilities is unclear, but there are several possibilities. The first is that the OprM overexpressed in N148, N128, N151, and N154 is associated with unknown periplasmic and inner membrane components and contributes to the resistance to these agents. A second possibility is that the mutation of nfxB suppresses an efflux system(s) other than MexAB-OprM and MexXY-OprM. A third possibility, that OprJ enhances the permeability of the P. aeruginosa outer membrane to these agents as a porin, cannot be excluded immediately, but we can rule it out by the finding that the susceptibilities of N103 and N106 to the agents were not affected by plasmid-bone OprJ expression (data not shown).
Bacterial genome sequencing projects have elucidated that each bacterium has several transporter genes. Multiple-knockout experiments such as those performed in this study might be necessary to elucidate the actual substrate specificities of other efflux pumps.
ACKNOWLEDGMENTS
This research was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan and by a grant from the Ministry of Health and Welfare of Japan.
We are grateful to K. Okamoto for providing pKMB004.
REFERENCES
- 1.Aires J R, Köhler T, Nikaido H, Plésiat P. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother. 1999;43:2624–2628. doi: 10.1128/aac.43.11.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukuoka T, Masuda N, Takenouchi T, Sekine N, Iijima M, Ohya S. Increase in susceptibility of Pseudomonas aeruginosa to carbapenem antibiotics in low-amino-acid media. Antimicrob Agents Chemother. 1991;35:529–532. doi: 10.1128/aac.35.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gotoh N, Tsujimoto H, Tsuda M, Okamoto K, Nomura A, Wada T, Nakahashi M, Nishino T. Characterization of the MexC-MexD-OprJ multidrug efflux system in ▵mexA-mexB-oprM mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:1938–1943. doi: 10.1128/aac.42.8.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gotoh N, Tsujimoto H, Nomura A, Okamoto K, Tsuda M, Nishino T. Functional replacement of OprJ by OprM in the MexCD-OprJ multidrug efflux system of Pseudomonas aeruginosa. FEMS Microbiol Lett. 1998;165:21–27. doi: 10.1111/j.1574-6968.1998.tb13122.x. [DOI] [PubMed] [Google Scholar]
- 5.Iso Y, Irie T, Nishino Y, Motokawa K, Nishitani Y. A novel 1 beta-methylcarbapenem antibiotic, S-4661. Synthesis and structure-activity relationships of 2-(5-substituted pyrrolidin-3-ylthio)-1-beta-methylcarbapenems. J Antibiot (Tokyo) 1996;49:199–209. doi: 10.7164/antibiotics.49.199. [DOI] [PubMed] [Google Scholar]
- 6.Köhler T, Michéa-Hamzepour M, Henze U, Gotoh N, Curty L K, Pechère J-C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 7.Li X-Z, Nikaido H, Poole K. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–1953. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masuda N, Ohya S. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:1847–1851. doi: 10.1128/aac.36.9.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuda N, Sakagawa E, Ohya S. Outer membrane proteins responsible for multiple drug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:645–649. doi: 10.1128/AAC.39.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masuda N, Gotoh N, Ohya S, Nishino T. Quantitative correlation between susceptibility and OprJ production in NfxB mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996;40:909–913. doi: 10.1128/aac.40.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masuda N, Gotoh N, Ishii C, Sakagawa E, Ohya S, Nishino T. Interplay between chromosomal β-lactamase and the MexAB-OprM efflux system in intrinsic resistance to β-lactams in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:400–402. doi: 10.1128/aac.43.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masuda N, Sakagawa E, Ohya S, Gotoh N, Tsujimoto H, Nishino T. Contribution of the MexX-MexY-OprM system to intrinsic resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2000;44:2242–2246. doi: 10.1128/aac.44.9.2242-2246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikaido H. Antibiotic resistance caused by gram-negative multidrug efflux pumps. Clin Infect Dis. 1998;27:S32–S41. doi: 10.1086/514920. [DOI] [PubMed] [Google Scholar]
- 15.Nikaido H. Multiple antibiotic resistance and efflux. Curr Opin Microbiol. 1998;1:516–523. doi: 10.1016/s1369-5274(98)80083-0. [DOI] [PubMed] [Google Scholar]
- 16.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J, Li X-Z, Nishino T. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 18.Poole K, Tetro K, Zhao Q, Neshat S, Heinrichs D, Bianco N. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob Agents Chemother. 1996;40:2021–2028. doi: 10.1128/aac.40.9.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 20.Schweizer H P. Intrinsic resistance to inhibitors of fatty acid biosynthesis in Pseudomonas aeruginosa is due to efflux: application of a novel technique for generation of unmarked chromosomal mutations for the study of efflux systems. Antimicrob Agents Chemother. 1998;42:394–398. doi: 10.1128/aac.42.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology. 1983;1:784–790. [Google Scholar]
- 22.Srikumar R, Li X-Z, Poole K. Inner membrane efflux components are responsible for β-lactam specificity of multidrug efflux pumps in Pseudomonas aeruginosa. J Bacteriol. 1997;179:7875–7881. doi: 10.1128/jb.179.24.7875-7881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuda M, Miyazaki H, Nakazawa T. Genetic and physical mapping of genes involved in pyoverdine production in Pseudomonas aeruginosa PAO. J Bacteriol. 1995;177:423–431. doi: 10.1128/jb.177.2.423-431.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuda M. Use of a transposon-encoded site-specific resolution system for construction of large and defined deletion mutations in bacterial chromosome. Gene. 1998;207:33–41. doi: 10.1016/s0378-1119(97)00601-x. [DOI] [PubMed] [Google Scholar]
- 25.Yoneyama H, Ocaktan A, Tsuda M, Nakae T. The role of the mex-gene products in antibiotic extrusion in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1997;233:611–618. doi: 10.1006/bbrc.1997.6506. [DOI] [PubMed] [Google Scholar]
- 26.Yoneyama H, Ocaktan A, Gotoh N, Nishino T, Nakae T. Subunit swapping in the mex-extrusion pumps in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1998;244:898–902. doi: 10.1006/bbrc.1998.8351. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Q, Li X-Z, Srikumar R, Poole K. Contribution of outer membrane efflux protein OprM to antibiotic resistance in Pseudomonas aeruginosa independent of MexAB. Antimicrob Agents Chemother. 1998;42:1682–1688. doi: 10.1128/aac.42.7.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]