Abstract
Background
The tumor microenvironment contributes to tumor initiation, growth, invasion, and metastasis. The tumor microenvironment is heterogeneous in cellular and acellular components, particularly structural features and their gene expression at the inter-and intra-tumor levels.
Main text
Single-cell RNA sequencing profiles single-cell transcriptomes to reveal cell proportions and trajectories while spatial information is lacking. Spatially resolved transcriptomics redeems this lack with limited coverage or depth of transcripts. Hence, the integration of single-cell RNA sequencing and spatial data makes the best use of their strengths, having insights into exploring diverse tissue architectures and interactions in a complicated network. We review applications of integrating the two methods, especially in cellular components in the tumor microenvironment, showing each role in cancer initiation and progression, which provides clinical relevance in prognosis, optimal treatment, and potential therapeutic targets.
Conclusion
The integration of two approaches may break the bottlenecks in the spatial resolution of neighboring cell subpopulations in cancer, and help to describe the signaling circuitry about the intercommunication and its exact mechanisms in producing different types and malignant stages of tumors.
Keywords: Integration, Single-cell RNA sequencing, Spatially resolved transcriptomics, Tumor microenvironment
Background
Cancer was viewed as a heterogeneous disease with a succession of genetic changes which led to the conversion of normal cells into malignant cells [1]. In 1889, Paget first came up with the theory of “seed and soil,” postulating the relationship between tumor and tumor microenvironment (TME) [2]. TME comprises cellular and acellular components such as stromal cells, myeloid cells, lymphoid cells, and extracellular matrix (ECM). Now it’s evident that TME plays an essential role in tumorigenesis, having diverse capacities to induce both beneficial and adverse consequences in tumor initiation, growth, invasion, and metastasis [3, 4]. However, there are still many unexplored questions in this field. The emerging problem is how to explore and manage TME diversity, given that both structural features and gene expression in TME are particularly heterogeneous at the inter-and intra-tumor levels [4, 5]. Interactions like intercellular communication need to be investigated forward, as TME is a complex, spatially restricted network [6, 7].
Technological developments have advanced our understanding of tumor biology. Many researchers have utilized RNA-sequencing (RNA-seq) based on next-generation sequencing (NGS) to measure tissue transcriptomes [8, 9]. Traditionally, bulk RNA-sequencing (bulk RNA-seq) is widely used to sequence a mixture of RNA transcripts from the whole tissue profiling averages of cellular expression [10]. Nevertheless, it has lost information about cellular heterogeneity. Single-cell RNA sequencing (scRNA-seq) improves and makes it possible to profile the transcriptome of single cells and infer cell type and trajectory [11, 12]. Whereas, scRNA-seq has failed to acquire spatial information, which is critical to understanding the functionality and pathological changes of tissues that are dissociated in suspension [9, 12, 13]. In addition, spatially resolved transcriptomics (SRT) has been developed to reveal spatial information and study spatial heterogeneity with the drawbacks of coverage or depth of transcripts. Computational developments have enabled the combination of scRNA-seq and spatial transcriptomics data to get through their limitations and make use of their favorable factors.
Developments and limitations of SRT
SRT technologies can be divided into four categories: technologies based on microdissected gene expression, in situ hybridization (ISH) technologies, in situ sequencing (ISS) technologies, and in situ capturing technologies [14].
The typical microdissected method is laser capture microdissection (LCM), which cuts out tissue regions precisely and isolates specific, pure cells from their heterogeneous environments by a laser beam under a microscope [15]. LCM sequencing (LCM-seq) combining LCM with RNA-seq profiles gene expression of selected tissue regions, elucidating cellular heterogeneity and spatial variance with low throughput and requirements in a large number of cells [16]. Geo-seq using scRNA-seq coupled with LCM advances the analysis at a resolution of as few as 10 cells. Yet, it’s still laborious and can’t attain single-cell resolution [17].
ISH technologies are early attempts to visualize gene expression in fixed tissue, as exemplified by single-molecule RNA fluorescence in situ hybridization (smFISH). Many short oligonucleotide probes labeled with fluorophores are hybridized to different regions of the same mRNA transcript [18–20]. SmFISH has high sensitivity and subcellular spatial resolution but a low target throughput of around 1–4 transcripts and up to 100 cells per handle [18, 19, 21, 22]. ISH technologies also include multiplexed error-robust fluorescence in situ hybridization (MERFISH), sequential fluorescence in situ hybridization (seqFISH), and ouroboros smFISH (osmFISH).
ISS technologies are methods for parallel targeted analysis of short RNA fragments in morphologically preserved cells and tissue [23]. ISS technologies encompass ISS using padlock probes, fluorescent in site RNA sequencing (FISSEQ), and spatially resolved transcript amplicon readout mapping (STARmap) [14]. ISS using padlock probes is the first ISS approach that can detect single nucleotide variants (SNV) compared to ISH. In human breast cancers, ISS detected targeted mRNAs and measured 31 genes at a subcellular spatial resolution of about 450 cells per single handle [23]. Some studies have concluded that ISH and ISS technologies are image-based in situ transcriptomics because they are all targeted in situ methods using probes to represent quantitative RNA analysis characterized by great depth and low coverage [11]. The principle of in situ capturing technologies is to capture transcripts in situ, then sequence them ex-situ.
Ståhl et al. first proposed spatial transcriptomics (ST), depositing a customized slide with a diameter of 100 μm microarray features over an area of 6.2 mm by 6.6 mm to capture transcripts. There are over 200 million oligonucleotides used to capture mRNAs in each of the 1007 features. Each microarray feature contains unique DNA-barcoded probes including a cleavage site, a T7 amplification and sequencing handle, a spatial barcode, a unique molecular identifier (UMI), and an oligo (dT) VN-capture region. After capturing and reverse-transcribing mRNA, cDNA synthesis from tissue with arrayed oligonucleotides on a surface is carried out. Then, RNA-seq is used to image gene expression while maintaining positional information [9]. Although this method could provide spatially resolved whole-transcriptome information and be more accessible, its limitations in resolution and depth can’t be ignored.
Integration approaches of scRNA-seq and SRT
Each current method of SRT mentioned above has its strengths and drawbacks. Hence, to meet the demands of exploring spatial patterning of gene expression at a single cell even subcellular resolution in an intricate environment, it’s necessary to integrate scRNA-seq and SRT to maximize the benefits. Two approaches currently exist for the integration of non-spatial scRNA-seq and SRT: (i) experimental improvement strategies, (ii) computing strategies.
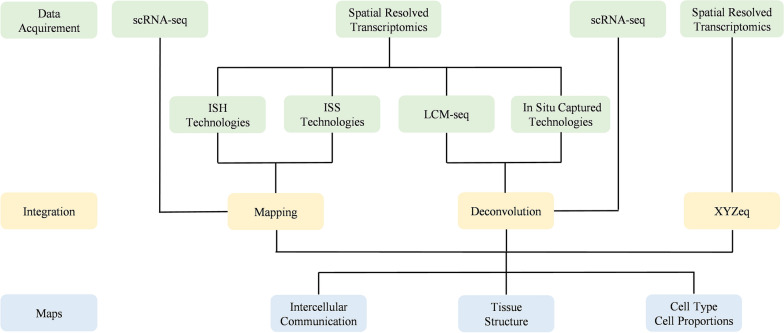
One of the experimental improvement strategies called XYZeq improves experiments by using two rounds of split-pool barcoding to encode the spatial information at single-cell resolution from a sample into scRNA-seq [24–26]. ST is applied to a tissue sample in the first round aiming to get positional information. The innovative step in the second round is to remove intact cells from microarrays, pool them, and amplify indexing with a combinatorial barcode per single cell and sequence. This method maps a single cell’s physical location in the array by spatial barcode [26].
Advances in computing techniques have made copy number inference and fusion transcription recognition possible [27]. Mapping and deconvolution are two key steps to achieving this goal. The mapping process is to distribute cell types and states based on scRNA-seq to each cell spatially resolved by image-based in situ transcriptomics. It also predicts the locations of spatially confined and dispersed subpopulations [10, 28, 29]. Deconvolution is the process of estimating cell-type proportions in spatial data at a microdissected or in situ capturing spot, informed by single-cell data [30]. Here is a workflow for integration (Fig. 1). The first step is to establish discrete cell subtypes through scRNA-seq and investigate tissue structures of interest from spatial data, which comes from the same or different biopsy as well as reference databases. Then, mapping or deconvolution as in silico strategies is applied to scRNA-seq and SRT data to understand the architecture of the cell-type distribution and the putative mechanisms of intercellular communication based on this architecture [10]. Computing strategies have overcome drawbacks of each technology and provided spatially resolved whole-transcriptome information at single-cell resolution yielding greater coverage and depth. Mapping could create spatially resolved maps at single-cell resolution. Deconvolution strategies enable estimating cell-type proportions and characterizing specific gene expression and other biological processes. However, the integration of scRNA-seq and image-based in situ transcriptomics has not been specifically addressed by most mapping models. Besides, since ST is a newly emerging technology, only a few models are applied in this field and its efficacy needs to be developed [10]. Deconvoluted tissues are limited in the spatially resolved at the original scale of ST arrays and ignore the adjacent tissues [31]. With the developments of SRT, more and more integrating models may be explored to face the challenges of mapping and deconvolution.
Fig. 1.
A workflow for integration
Application of integration in TME
Recent work in combining scRNA-seq with spatial technologies has focused on tissue homeostasis, tissue development, disease, and tumor microenvironment. The integration of two approaches may break the bottlenecks in the spatial resolution of neighboring cell subpopulations in cancer, and help to describe the signaling circuitry about the intercommunication and its exact mechanisms in producing different types and malignant stages of tumors. The TME is the most widely used area in SRT, and it is closely related to cancer malignant behaviors, which are governed by crosstalk within and across all cellular compartments [32–35]. In addition, a modern (though not exhaustive) list of key molecular markers of cell populations discussed below in the TME with their characteristics is given in Table 1. A deeper study of intricate spatial patterning of single cells brings biological insight into cellular and spatial heterogeneity between and within tumors in a complex environment. Moreover, it also has the potential to explain how TME affects cellular infiltration and interaction, which represent an attractive target for treatment and prognosis.
Table 1.
Key molecular markers of each cell type in the TME
| Population | Subtypes | Marker | References |
|---|---|---|---|
| CAFs | – | α-SMA, vimentin, FAP, PDPN, PDGFRα/β, FSP1, DDR2, S100A4, CD10, GPR77 | [36–41] |
| MSCs | – | CD105+, CD73+, CD70+, CD13+, CD29+, CD44+, CD10+, CD45−, CD34−, CD14− or CD11b−, CD79a−, HLA-DR− | [42–44] |
| TAMs | M1 | CD68, CD11b, CD80, CD86 | [45, 46] |
| M2 | CD68, CD11b, CD163, CD206 | ||
| DCs | cDC1 | XCR1, CD45, CADM1, CLEC9A, CD141 | [47, 48] |
| cDC2 | CD45, CD1C, FcεR1A, CD172A | ||
| pDCs | CD45RA, CD123, CD2 | ||
| Endothelial cells | – | PECAM1, CD31, CD34, CD13, CD29 | [49, 50] |
| CD4+ T cells | – | CD3+CD4+CD8− | [51–54] |
| Th1 cells | CXCR3 | ||
| Th2 cells | CCR4 | ||
| Th17 cells | CCR6 | ||
| Th22 cells | CCR10 | ||
| Treg cells | CD4+CD25+Foxp3+ | ||
| CD8+ T cells | - | CD3+ CD8+CD4− | |
| Tc1 cells | CRCX3, IRF4 | ||
| Tc2 cells | CCR4, CRTH2, GATA3 | ||
| Tc9 cells | CRCX3, IRF4, IL9, IL10 | ||
| Tc17 cells | CCR6, IL23R, IRF4, IL17 | ||
| MDSCs | PMN-MDSCs | CD11b+CD33+HLA−DR−/CD14−CD15+ | [55, 56] |
| M-MDSCs | CD11b+CD33+HLA−DR−/CD14+CD15− |
CAFs cancer-associated fibroblasts, MSCs mesenchymal stem cells, TAMs tumor-associated macrophages, DCs dendritic cells, cDCs conventional DCs, pDCs plasmacytoid DCs, MDSCs myeloid-derived suppressor cells, PMN-MDSCs granulocyte-like MDSCs, M-MDSCs monocytic MDSCs
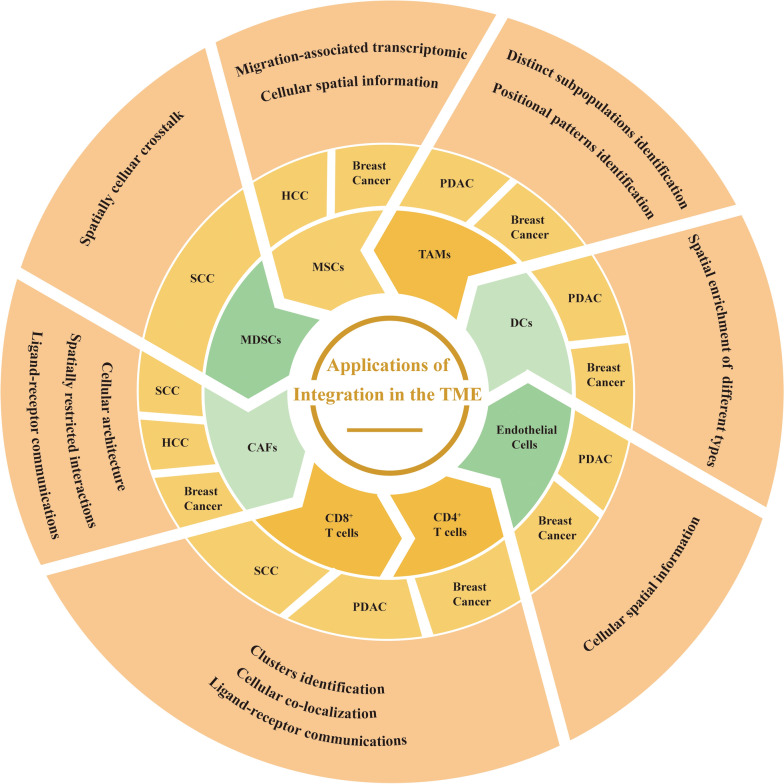
Here, we show applications of integrating scRNA-seq with SRT in cellular components in TME. Combining datasets of scRNA-seq and ST could map signaling between adjacent tumor and TME cells at the leading edge, suggesting advances in mapping cellular crosstalk at leading-edge niches [35]. Furthermore, we give a schematic overview of the applications of integration methods on the different populations present within the microenvironment (Fig. 2).
Fig. 2.
A schematic overview: application of integration for cell types in the TME. CAFs: cancer-associated fibroblasts; MDSCs: myeloid-derived suppressor cells; MSCs: mesenchymal stem cells; TAMs: tumor-associated macrophages; DCs: dendritic cells; SCC: squamous cell carcinoma; HCC: hepatocellular carcinoma; PDAC: pancreatic ductal adenocarcinoma
Stromal cells
TME is composed of tumor cells and nearby endogenous stromal cells [57, 58]. Stromal cells recruited from the local host stroma range in types and include CAFs (cancer-associated fibroblasts) and MSCs (mesenchymal stem cells) that promote extracellular matrix remodeling, cellular migration, angiogenesis, and evasion of immunosurveillance in tumor growth and development [58, 59]. Reactive stroma can be regarded as an emerging hallmark of cancer initiation and progression. Berglund et al. revealed an unexplored landscape of heterogeneity through spatial maps of prostate cancer. It enabled de-novo characterization and delineation of reactive stroma in the proximity of cancer and inflammation, uncovering high levels of oxidative stress and ILK signaling within the reactive stroma. It indicated that cancer depended on stroma to release energy to support tumor growth and survival [60].
Cancer-associated fibroblasts
CAFs are one of the dominant entities in the stroma of many cancers, including breast cancer, hepatic cell carcinoma, human squamous cell carcinoma, and lung cancers. CAFs are a heterogeneous population of irreversibly activated fibroblasts that serve distinct, critical functions in tumor metabolism, immunity, drug resistance, negative regulation, tumorigenesis, and metastasis [36, 61–63]. Besides, numerous studies have shown the metastasis potential of cancer cells depending on CAFs, like in lung cancer, squamous cell carcinoma lung metastasis, and colorectal cancer [38, 64–66]. It has distinct subsets of functional fibroblasts differentiated from resting fibroblasts, such as tumor-restraining (F1), tumor-promoting (F2), secretory (F3), and ECM-remodelling (F4) subtypes detected and identified by various means based on the expression of a limited set of cell surface markers, such as α-SMA, vimentin, FAP, PDPN, PDGFRα/β, FSP1, DDR2, and S100A4 [36–39]. Recently, some studies have identified CD10 and GPR77 as specific fibroblast surface markers which facilitate live-cell sorting of CAFs in breast cancer [40, 41].
In breast cancer, four subpopulations of CAFs, such as vascular CAFs, matrix CAFs, cycling CAFs, and developmental CAFs, and their distinct gene programs were revealed by scRNA-seq with high resolution [67]. A single-cell and spatially resolved atlas of human breast cancers described the cellular architecture and spatially restricted interactions with the immune system. Stereoscope belongs to deconvolution as a strategy of combining scRNA-seq and ST, of which two datasets came from different breast cancer tissue samples. Stereoscope identified spatially distinct subpopulations of CAFs, with myofibroblast-like enriched in invasive cancer regions, while inflammatory CAFs were scattered in invasive cancer, stroma, and TIL-aggregate regions in different clinical subtype samples. In addition, this study also explored the top ligand-receptor interactions between most CAFs and CD4+/CD8+ T cells, suggesting that CAFs may directly regulate immune cells [49]. Moreover, inflammatory CAFs were enriched in the stress-response region of pancreatic ductal adenocarcinoma (PDAC) [32].
As for the human liver microenvironment, this study applied scRNA-seq, LCM-seq, and smFISH in cholangiocarcinoma, colorectal liver metastasis, and benign tissue samples. A developed approach named AutoGeneS deconvoluted scRNA-seq with transcriptomics of LCM tissue to obtain zonation patterns of human hepatocytes when prior knowledge of landmark genes was lacking [68, 69]. This study revealed the far distance between CAFs and endothelial cells, and the interactions between CAFs and scar-associated macrophages (SAMs). CAFs, which were most abundant in the fibrotic zones, produced most of the collagen and lamina proteins, interacting with integrin receptors on tumor cells [70].
In a study of human squamous cell carcinoma, CAFs were modulated by an immunosuppressive tumor-specific keratinocyte subpopulation that expressed immunotherapy resistance genes in a fibrovascular niche at the tumor borders through ligand-receptor communications [10, 35]. By using the deconvolution strategy, this study indicated the contribution of CAFs to tumor progression, immunosuppression, and heterogeneity.
Thus, researchers propose a cellular, molecular, and spatial functional taxonomy of CAFs by using the integration of scRNA-seq and ST, opening up the possibility for the development of novel targeted drugs aiming to block CAFs-immune or CAFs-tumor cell signaling [49, 67, 70].
Mesenchymal stem cells
MSCs are multipotent stromal cells that can differentiate into cells of the mesodermal lineage providing structural support to organs, synthesizing and remodeling the ECM, and regulating development [71]. MSCs play an important role in tumor development at various stages of progression, which modifies several effector functions [72, 73]. MSCs generally express CD105, CD73, CD70, CD13, CD29, CD44, and CD10, and lack expression of CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA-DR [42–44]. Recent studies have found that MSCs may differentiate at the site of the tumor and interact with tumor cells through paracrine signaling. Cross-talk between tumor cells and MSCs has been shown to increase metastatic potential. In colorectal cancer, MSCs have been found to increase tumor migration and invasion through IL-6/JAK2/STAT3 signaling, providing a novel therapeutic or preventive target [74].
Lee et al. used XYZeq in hepatocellular carcinoma (HCC) and found that MSCs have differentially expressed genes regulating ECM. It suggested that hepatocellular carcinoma cells may induce a local gene expression program in MSCs nearby that could contribute to malignant remodeling of the ECM. The location of the tumor cells and non-tumor cells may determine heterogeneous gene expression in MSCs. Transcriptionally variable genes within MSCs were driven by their location within the complex tissue architecture [26]. Observations in breast cancer showed MSCs were often spatially segregated underscoring the role of TME in their differentiation and migration. These findings suggested it was possible to block stromal signaling or differentiation as therapeutic strategies [49]. It has an advantage for joint analysis of spatial and single-cell transcriptomic to reveal not only local information but also migration-associated transcriptomic programs in MSCs.
Tumor-associated macrophages (TAMs)
Many supporting cells having distinct functions during tumorigenesis are derived particularly from the myeloid lineage especially TAMs (tumor-associated macrophages) [4, 75]. TAMs polarize into two functional phenotypes: the M1 state (pro-inflammatory and anti-tumor) and the M2 state (anti-inflammatory and pro-tumor) [34]. The distinct two phenotypes may influence cancer progression and overall survival [76]. CD68 and CD11b are co-markers for M1 and M2 macrophages [45]. CD80, CD86 are specific for M1 subtypes, while CD163, CD206 are specific for M2 subtypes [45, 46]. The existing problems with TAMs are that this classification is oversimplified because it does not fully represent the complexity of macrophage activation and its positional patterns [4, 77]. Macrophages have a leading position in pathophysiological responses, such as TME, which paves the way to tumorigenesis [78, 79]. In progressive cancer, TAMs recruited to TME are fast becoming a key instrument in cancer cell proliferation, immunosuppression, and angiogenesis in support of tumor growth and metastasis [79, 80]. In colorectal cancer, Yu et al. have found that TAMs play a key role in cancer proliferation depending on MMP1 via accelerating cell cycle transition [81]. Wei et al. have shown the crosstalk between TAMs and colorectal cancer cells which is associated with cancer migration, invasion, and circulating tumor cell-mediated metastasis [82]. Dora et al. have characterized TAMs in neuroendocrine-high and -low small cell lung cancer [76]. Furthermore, spatial density and distribution and gene expression of TAM phenotypes have been shown prognostic value in non-small-cell lung carcinoma (NSCLC) [83].
10 × Chromium (scRNA-seq), 10 × Genomics (ST), and their deconvolution method were used to identify two large TREM2-high lipid-associated macrophages (LAMs) that were similar to the PD-L1+ macrophage population associated with high clinical grade and exhausted T cells in breast cancers [49, 84]. Additionally, LAMs and CXCL10hi macrophages were relevant to immunosuppression and were paratactic to PD-1+ lymphocytes. As TAMs were associated with poor prognosis and are emerging targets for cancer immunotherapy, nine ecotypes driven by cells spanning the major lineages in primary breast cancers were defined. The cellular composition and tumor biology of each ecotype were similar [49, 84–86].
Elosua-Bayes et al. developed SPOTlight, a deconvolution that enabled the integration of ST with scRNA-seq data in PDAC publicly available reference, finding a remarkable enrichment in the tumor region of pro-inflammatory M1 TAMs while anti-inflammatory M2 TAMs were enriched in normal pancreas tissue, endothelial, and endocrine cells in different tissue regions [87]. In another PDAC study, M2 TAMs were most enriched in the ducts, while inflammatory M1 TAMs expressing IL1B were more enriched in the stroma and cancer regions, which was consistent with Bayes’s study [32, 88]. It illustrated opposite positional patterns of enrichment in two subtypes of macrophages through MIA. The multimodal intersection analysis (MIA) approach integrated scRNA-seq and spatially barcoded oligo-deoxythymidine microarrays. Moncada et al. used two melanoma ST samples to validate MIA, in which macrophages were restricted to the melanoma region periphery or a particular region within the larger annotated melanoma area, indicating that macrophages were in spatially restricted regions in melanoma [32].
Therefore, a detailed subpopulation of TAMs with expression levels for genes and their specific relationships in positional patterns can be revealed by integrating methods, which may have the potential to refine classification.
Dendritic cells
Dendritic cells (DCs) are potent antigen-presenting cells, which can present antigen to T cells and activate these cells to enhance the immune response [89]. Previous studies have distinguished two types of DCs: one is conventional DCs (cDCs), while another is plasmacytoid DCs (pDCs) [90]. Human cell surface markers of pDCs are CD45RA, CD123, and CD2 [47]. Besides, cDCs have two subsets, cDC1 and cDC2, with distinct cell surface markers and functions [48]. cDC1 are generally defined by XCR1, CD45, CADM1, CLEC9A, and CD141, while cDC2 are described by CD45, CD1C, FcεR1A, CD172A [47]. DCs are a promising therapy in cancer treatment. In early-stage PDAC, overcoming cDCs deficiency has led to disease restraint. Otherwise, in advanced PDAC, restoration of cDC function has led to restoring tumor-restraining immunity [91]. In addition, functional DCs in tumor regions were excluded from lung cancers dynamically, which may support malignant progression [92].
In transcriptional profiling of human breast cancer, Wu et al. identified three types of DCs, cDCs, pCDs, and LAMP3 high DC population, which was not previously detected in single-cell studies of breast cancer [49]. In PDAC via high throughput single-cell sequencing and MIA, two subpopulations of DCs, A and B, were identified. Subpopulation A was enriched in pancreatic tissue, while subpopulation B was enriched in the ducts of the tissue [32].
Endothelial cells
Tumor endothelial cells play a critical role in cancer cell metastasis and dormancy exhibiting unique phenotypic and functional characteristics when compared to normal endothelial cells [93]. Many studies have shown that the proliferation and motility of tumor endothelial cells are associated with several pathological processes for tumor progression and metastasis, such as microvessel sprout formation and angiogenesis [94]. PECAM1, CD13, CD29, CD31 and CD 34 are main markers for endothelial identification [49, 50]. In breast cancer, Ma et al. have identified the heterogeneity of endothelial cells and indicated its potential role in contributing to cancer metastasis [95]. Besides, lung cancer cells have been shown to promote endothelial cell tube formation which changes the TME to facilitate tumor growth [94]. In colorectal cancer, TME-dependent heterogeneity of tumor endothelial cells regulated by SPARCL1 has promoted tumor cell dormancy and vessel homeostasis [96]. Furthermore, Meng et al. have shown the role and mechanism of Hsp90β in tumor endothelial cell-dependent angiogenesis and its therapeutic value in hepatocellular carcinoma [97].
Three endothelial states (s1, s2, and s3) were identified through a single-cell and spatially resolved atlas of human breast cancers. These three states mainly in the normal, stroma, and lymphocytes areas were dynamic and interconvertible, suggesting that these endothelial cells may serve as resident cell types in the TME [49]. Otherwise, in one PDAC sample, endothelial cells were significantly enriched in the interstitium using integration of two advanced techniques [32]. These insights, such as revealing spatial information of different subpopulations of endothelial cells, may provide a deeper understanding of cancer metastasis and dormancy.
CD4+ and CD8+ T cells
Tumor-infiltrating lymphocytes (TIL) play a crucial role in TME, which is associated with cancer progression, response to therapy, and clinical outcomes [98]. In addition, studies of TIL mainly focus on T cells. T cell infiltration formed in human cancer is a regulator of natural disease progression and also determines the probability of clinical response to cancer immunotherapy, which may provide potential prognostic value [99]. There are three main types of T cells: helper T cells (TH cells/CD4+ T cells), cytotoxic T cells (TC cells/CD8+ T cells), as well as regulatory T cells (Treg cells). CD4+ T cells generally express CD3+CD4+CD8−, while CD8+ T cells generally express CD3+ CD8+CD4− [53]. CD4+CD25+Foxp3+ are used to describe Treg cells [51, 52]. In addition, different subsets of CD4+ and CD8+ T cells have their specific markers, such as Th1 cells (CXCR3), Th2 cells (CCR4), Th17 cells (CCR6), Th22 cells (CCR10), Tc1 cells (CRCX3 and IRF4), Tc2 cells (CCR4, CRTH2, and GATA3), Tc9 cells (CRCX3, IRF4, IL9, and IL10), Tc17 cells (CCR6, IL23R, IRF4, and IL17) [53, 54]. CD8+ T cells encounter dysfunction and exhaustion due to immunosuppression within the TME during tumor development and progression [100]. CD4+ T cells play a key role in the adapted immune system, which can variously target tumors either directly by eliminating tumor cells through cytolytic mechanisms or indirectly by modulating TME [101–103]. Hiraoka et al. have indicated that deeper infiltration by both CD8+ and CD4+ T cells presents a better prognosis for patients with NSCLC [104]. Another study has mapped the heterogeneity of TILs in NSCLC, which may attribute to cancer immunotherapy [105]. Yang et al. have shown the association between CD8+ and CD4+ T cell-related genes and colon cancer prognosis [106]. In addition, high infiltration of lymphocytes has been observed in one subpopulation characterized by low peroxisome and high TIM3 of colorectal cancer [107].
SPOTlight applied to PDAC samples annotated 12 T cells and predicted the proportion within each capture spot. Recently activated CD4+, pre-exhausted CD8+, and proliferative CD8+ T cells significantly increased in tumor regions, while most transitional memory CD4+ T cells were in normal tissue. Intriguingly, recently activated CD4+ T cells co-localized with pre-exhausted CD8+ T cells in tumor areas and could not be detected through their presence alone, indicating a possible target for precise pathology assessments [87]. In breast tumor samples, 18 T-cell and innate lymphoid clusters were identified. One subset of exhausted CD8+ T cells named LAG3/c8 in triple-negative breast cancer (TNBC) had higher expression of PD-1, LAG3, and the ligand-receptor pair of CD27 and CD70, known to enhance T cell cytotoxicity [49, 108]. In human squamous cell carcinoma, CD8+ T cells were observed to co-localize with Treg cells in the compartmentalized tumor stroma, which showed a feature of potential immunosuppression [35]. Such visualization underlined interactions between T cells that mediate the tumor immune environment and can shed new light on the peculiarities of tumor microenvironments [10, 87].
Myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells with immunosuppressive functions. When in pathological conditions, especially cancer, the differentiation, and maturation of immature myeloid are stopped leading to the expansion of MDSCs in vivo [109]. MDSCs consist of two large groups of cells: granulocyte-like MDSCs (PMN-MDSC or G-MDSC) and monocytic MDSCs (M-MDSCs) [110, 111]. PMN-MDSCs can be described as CD11b+CD33+HLA−DR−/CD14−CD15+, while M-MDSCs can be defined as CD11b+CD33+HLA−DR−/CD14+CD15− in human [55, 56]. MDSCs play an important role in immune surveillance in TME via immunosuppressive mechanisms, such as metabolic mechanisms, STAT signaling pathway, and endoplasmic reticulum stress in lung cancers [112]. Besides, high levels of MDSCs have been associated with resistance to several therapeutic strategies, like chemotherapy and immunotherapy with a poor prognosis [112]. Huang et al. have found modulating MDSCs in TME may improve the efficacy of EZH2 inhibitors to suppress antitumor immunity [113]. Another study also showed the potential therapeutic target of PMN-MDSC to overcome resistance to immune checkpoint inhibition in NSCLC [114]. In colorectal cancer, reprogramming MDSCs may have the potential to enhance the efficacy of therapeutic strategies [115].
Multimodel profiling of cutaneous squamous cell carcinoma, MDSCs were identified and highly expressed several potential mediators of Treg recruitment, such as CCXL9/10/11, CCL4, and CCL20, via the integration of scRNA-seq and ST [35]. In addition, extensive autocrine and paracrine interactions between MDSCs and tumor-specific keratinocytes revealed cellular crosstalk at leading-edge niches [35]. However, most studies have just identified myeloid cells without identifying MDSCs when using scRNA-seq and ST techniques. MDSCs have emerged as an important contributor to tumor progression, so it’s quite important to reveal the spatial information and cellular interactions of MDSCs, which may benefit cancer therapeutic strategies.
Conclusions
Integrating scRNA-seq with SRT is beneficial in understanding cell-type proportions to the proximity of tissue architecture. It also helps to study intercellular communications through expressions of ligands and receptors in TME, which may be beneficial to define disease subtypes, provide potential therapeutic targets, and predict prognosis. Additionally, integrating methods can be used to describe the atlas at the single-cell resolution of healthy or diseased tissues and explore normal tissue homeostasis and tissue ontogeny at key points. Nowadays, SRT is growing at a rapid pace with improvements in resolution, sensitivity, throughput, as well as accessibility. Despite deconvolution and mapping algorithms, new learning algorithms are exploited to define the most relevant features of biological function in SRT. However, it’s costly when applying scRNA-seq and SRT using matched samples or publicly available references. Considering the original scale of the ST technology, deconvolved mixtures are still only spatially resolved and the proximity structure of cell types cannot be recovered. Recently, real-time cell tracking based on SRT at single-cell resolution has been developed to monitor spatially resolved intercellular tissue dynamics in real-time elucidating metastatic progression and immune cell dynamics in disease, which has an extensive prospect in cancer research.
Acknowledgements
Not applicable.
Abbreviations
- TME
Tumor microenvironment
- ECM
Extracellular matrix
- RNA-seq
RNA-sequencing
- NGS
Next-generation sequencing
- bulk RNA-seq
Bulk RNA-sequencing
- scRNA-seq
Single-cell RNA sequencing
- SRT
Spatially resolved transcriptomics
- ISH
In situ hybridization
- ISS
In situ sequencing
- LCM
Laser capture microdissection
- LCM-seq
LCM sequencing
- smFISH
Single-molecule RNA fluorescence in situ hybridization
- MERFISH
Multiplexed error-robust fluorescence in situ hybridization
- seqFISH
Sequential fluorescence in situ hybridization
- osmFISH
Ouroboros smFISH
- FISSEQ
Fluorescent in site RNA sequencing
- STARmap
Spatially resolved transcript amplicon readout mapping
- SNV
Single nucleotide variants
- ST
Spatial transcriptomics
- UMI
Unique molecular identifier
- CAFs
Cancer-associated fibroblasts
- MSCs
Mesenchymal stem cells
- HCC
Hepatocellular carcinoma
- PDAC
Pancreatic ductal adenocarcinoma
- SAM
Scar-associated macrophages
- TAMs
Tumor-associated macrophages
- LAMs
Lipid-associated macrophages
- NSCLC
Non-small-cell lung carcinoma
- MIA
Multimodal intersection analysis
- DCs
Dendritic cells
- cDCs
Conventional DCs
- pDCs
Plasmacytoid DCs
- TIL
Tumor-infiltrating lymphocytes
- TH cells
Helper T cells
- TC cells
Cytotoxic T cells
- Treg cells
Regulatory T cells
- TNBC
Triple-negative breast cancer
- MDSCs
Myeloid-derived suppressor cells
- PMN-MDSCs
Granulocyte-like MDSCs
- G-MDSCs
Granulocyte-like MDSCs
- M-MDSCs
Monocytic MDSCs
Author contributions
JHL and HLY designed the paper; HLY and JHS wrote the paper; YD and XYL revised the paper; YSW and JZ collected and manage the references; ZYG and CYZ made the figures and the table. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82071628).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1889;8(2):98–101. [PubMed] [Google Scholar]
- 3.Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5(1):166. doi: 10.1038/s41392-020-00280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanta G, Bonin S. Overview on clinical relevance of intra-tumor heterogeneity. Front Med (Lausanne) 2018;5:85. doi: 10.3389/fmed.2018.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125(Pt 23):5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 7.Fan J, Slowikowski K, Zhang F. Single-cell transcriptomics in cancer: computational challenges and opportunities. Exp Mol Med. 2020;52(9):1452–1465. doi: 10.1038/s12276-020-0422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(1):57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stahl PL, Salmen F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, Giacomello S, Asp M, Westholm JO, Huss M, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science. 2016;353(6294):78–82. doi: 10.1126/science.aaf2403. [DOI] [PubMed] [Google Scholar]
- 10.Longo SK, Guo MG, Ji AL, Khavari PA. Integrating single-cell and spatial transcriptomics to elucidate intercellular tissue dynamics. Nat Rev Genet. 2021;22(10):627–644. doi: 10.1038/s41576-021-00370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao J, Lu X, Shao X, Zhu L, Fan X. Uncovering an organ's molecular architecture at single-cell resolution by spatially resolved transcriptomics. Trends Biotechnol. 2021;39(1):43–58. doi: 10.1016/j.tibtech.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Kiselev VY, Andrews TS, Hemberg M. Challenges in unsupervised clustering of single-cell RNA-seq data. Nat Rev Genet. 2019;20(5):273–282. doi: 10.1038/s41576-018-0088-9. [DOI] [PubMed] [Google Scholar]
- 13.Klein AM, Mazutis L, Akartuna I, Tallapragada N, Veres A, Li V, Peshkin L, Weitz DA, Kirschner MW. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell. 2015;161(5):1187–1201. doi: 10.1016/j.cell.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asp M, Bergenstrahle J, Lundeberg J. Spatially resolved transcriptomes-next generation tools for tissue exploration. BioEssays. 2020;42(10):e1900221. doi: 10.1002/bies.201900221. [DOI] [PubMed] [Google Scholar]
- 15.Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, Zhuang Z, Goldstein SR, Weiss RA, Liotta LA. Laser capture microdissection. Science. 1996;274(5289):998–1001. doi: 10.1126/science.274.5289.998. [DOI] [PubMed] [Google Scholar]
- 16.Nichterwitz S, Chen G, Aguila Benitez J, Yilmaz M, Storvall H, Cao M, Sandberg R, Deng Q, Hedlund E. Laser capture microscopy coupled with smart-seq2 for precise spatial transcriptomic profiling. Nat Commun. 2016;7:12139. doi: 10.1038/ncomms12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Suo S, Tam PP, Han JJ, Peng G, Jing N. Spatial transcriptomic analysis of cryosectioned tissue samples with Geo-seq. Nat Protoc. 2017;12(3):566–580. doi: 10.1038/nprot.2017.003. [DOI] [PubMed] [Google Scholar]
- 18.Femino AM, Fay FS, Fogarty K, Singer RH. Visualization of single RNA transcripts in situ. Science. 1998;280(5363):585–590. doi: 10.1126/science.280.5363.585. [DOI] [PubMed] [Google Scholar]
- 19.Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods. 2008;5(10):877–879. doi: 10.1038/nmeth.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji N, van Oudenaarden A. Single molecule fluorescent in situ hybridization (smFISH) of C. elegans worms and embryos. WormBook. 2012 doi: 10.1895/wormbook.1.153.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gall JG, Pardue ML. Formation and detection of RNA–DNA hybrid molecules in cytological preparations. Proc Natl Acad Sci USA. 1969;63(2):378–383. doi: 10.1073/pnas.63.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan Y, Braut SA, Lin Q, Singer RH, Skoultchi AI. Determination of transgenic loci by expression FISH. Genomics. 2001;71(1):66–69. doi: 10.1006/geno.2000.6403. [DOI] [PubMed] [Google Scholar]
- 23.Ke R, Mignardi M, Pacureanu A, Svedlund J, Botling J, Wahlby C, Nilsson M. In situ sequencing for RNA analysis in preserved tissue and cells. Nat Methods. 2013;10(9):857–860. doi: 10.1038/nmeth.2563. [DOI] [PubMed] [Google Scholar]
- 24.Cao J, Packer JS, Ramani V, Cusanovich DA, Huynh C, Daza R, Qiu X, Lee C, Furlan SN, Steemers FJ, et al. Comprehensive single-cell transcriptional profiling of a multicellular organism. Science. 2017;357(6352):661–667. doi: 10.1126/science.aam8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg AB, Roco CM, Muscat RA, Kuchina A, Sample P, Yao Z, Graybuck LT, Peeler DJ, Mukherjee S, Chen W, et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science. 2018;360(6385):176–182. doi: 10.1126/science.aam8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Y, Bogdanoff D, Wang Y, Hartoularos GC, Woo JM, Mowery CT, Nisonoff HM, Lee DS, Sun Y, Lee J, et al. XYZeq: Spatially resolved single-cell RNA sequencing reveals expression heterogeneity in the tumor microenvironment. Sci Adv. 2021 doi: 10.1126/sciadv.abg4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maniatis S, Petrescu J, Phatnani H. Spatially resolved transcriptomics and its applications in cancer. Curr Opin Genet Dev. 2021;66:70–77. doi: 10.1016/j.gde.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camp JG, Platt R, Treutlein B. Mapping human cell phenotypes to genotypes with single-cell genomics. Science. 2019;365(6460):1401–1405. doi: 10.1126/science.aax6648. [DOI] [PubMed] [Google Scholar]
- 29.Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol. 2015;33(5):495–502. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Park J, Susztak K, Zhang NR, Li M. Bulk tissue cell type deconvolution with multi-subject single-cell expression reference. Nat Commun. 2019;10(1):380. doi: 10.1038/s41467-018-08023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao E, Stone MR, Ren X, Guenthoer J, Smythe KS, Pulliam T, Williams SR, Uytingco CR, Taylor SEB, Nghiem P, et al. Spatial transcriptomics at subspot resolution with BayesSpace. Nat Biotechnol. 2021;39(11):1375–1384. doi: 10.1038/s41587-021-00935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moncada R, Barkley D, Wagner F, Chiodin M, Devlin JC, Baron M, Hajdu CH, Simeone DM, Yanai I. Integrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas. Nat Biotechnol. 2020;38(3):333–342. doi: 10.1038/s41587-019-0392-8. [DOI] [PubMed] [Google Scholar]
- 33.Thrane K, Eriksson H, Maaskola J, Hansson J, Lundeberg J. Spatially resolved transcriptomics enables dissection of genetic heterogeneity in stage III cutaneous malignant melanoma. Cancer Res. 2018;78(20):5970–5979. doi: 10.1158/0008-5472.CAN-18-0747. [DOI] [PubMed] [Google Scholar]
- 34.Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019;30(1):36–50. doi: 10.1016/j.cmet.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Ji AL, Rubin AJ, Thrane K, Jiang S, Reynolds DL, Meyers RM, Guo MG, George BM, Mollbrink A, Bergenstrahle J, et al. Multimodal analysis of composition and spatial architecture in human squamous cell carcinoma. Cell. 2020;182(6):1661–1662. doi: 10.1016/j.cell.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 37.Irvine AF, Waise S, Green EW, Stuart B, Thomas GJ. Characterising cancer-associated fibroblast heterogeneity in non-small cell lung cancer: a systematic review and meta-analysis. Sci Rep. 2021;11(1):3727. doi: 10.1038/s41598-021-81796-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asif PJ, Longobardi C, Hahne M, Medema JP. The role of cancer-associated fibroblasts in cancer invasion and metastasis. Cancers (Basel) 2021 doi: 10.3390/cancers13184720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han C, Liu T, Yin R. Biomarkers for cancer-associated fibroblasts. Biomark Res. 2020;8(1):64. doi: 10.1186/s40364-020-00245-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su S, Chen J, Yao H, Liu J, Yu S, Lao L, Wang M, Luo M, Xing Y, Chen F, et al. CD10 (+) GPR77 (+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172(4):841–856 e816. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18(2):99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 42.Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 43.Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, Markham AF, Jack A, Emery P, McGonagle D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46(12):3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 44.Buhring HJ, Battula VL, Treml S, Schewe B, Kanz L, Vogel W. Novel markers for the prospective isolation of human MSC. Ann NY Acad Sci. 2007;1106:262–271. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- 45.Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216. doi: 10.1186/1479-5876-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bertani FR, Mozetic P, Fioramonti M, Iuliani M, Ribelli G, Pantano F, Santini D, Tonini G, Trombetta M, Businaro L, et al. Classification of M1/M2-polarized human macrophages by label-free hyperspectral reflectance confocal microscopy and multivariate analysis. Sci Rep. 2017;7(1):8965. doi: 10.1038/s41598-017-08121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson DA, 3rd, Dutertre CA, Ginhoux F, Murphy KM. Genetic models of human and mouse dendritic cell development and function. Nat Rev Immunol. 2021;21(2):101–115. doi: 10.1038/s41577-020-00413-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Breton G, Lee J, Zhou YJ, Schreiber JJ, Keler T, Puhr S, Anandasabapathy N, Schlesinger S, Caskey M, Liu K, et al. Circulating precursors of human CD1c+ and CD141+ dendritic cells. J Exp Med. 2015;212(3):401–413. doi: 10.1084/jem.20141441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu SZ, Al-Eryani G, Roden DL, Junankar S, Harvey K, Andersson A, Thennavan A, Wang C, Torpy JR, Bartonicek N, et al. A single-cell and spatially resolved atlas of human breast cancers. Nat Genet. 2021;53(9):1334–1347. doi: 10.1038/s41588-021-00911-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goncharov NV, Popova PI, Avdonin PP, Kudryavtsev IV, Serebryakova MK, Korf EA, Avdonin PV. Markers of endothelial cells in normal and pathological conditions. Biochem (Mosc) Suppl Ser A Membr Cell Biol. 2020;14(3):167–183. doi: 10.1134/S1990747819030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savage PA, Klawon DEJ, Miller CH. Regulatory T cell development. Annu Rev Immunol. 2020;38:421–453. doi: 10.1146/annurev-immunol-100219-020937. [DOI] [PubMed] [Google Scholar]
- 52.Mousset CM, Hobo W, Woestenenk R, Preijers F, Dolstra H, van der Waart AB. Comprehensive phenotyping of T cells using flow cytometry. Cytometry A. 2019;95(6):647–654. doi: 10.1002/cyto.a.23724. [DOI] [PubMed] [Google Scholar]
- 53.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12(3):191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74(1):5–17. doi: 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Law AMK, Valdes-Mora F, Gallego-Ortega D. Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells. 2020 doi: 10.3390/cells9030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bronte V, Brandau S, Chen S-H, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kidd S, Spaeth E, Watson K, Burks J, Lu H, Klopp A, Andreeff M, Marini FC. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS ONE. 2012;7(2):e30563. doi: 10.1371/journal.pone.0030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, Marini FC. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016;18(1):84. doi: 10.1186/s13058-016-0740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 60.Berglund E, Maaskola J, Schultz N, Friedrich S, Marklund M, Bergenstråhle J, Tarish F, Tanoglidi A, Vickovic S, Larsson L, et al. Spatial maps of prostate cancer transcriptomes reveal an unexplored landscape of heterogeneity. Nat Commun. 2018;9(1):2419. doi: 10.1038/s41467-018-04724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316(8):1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 62.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5(12):1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 63.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2015;28(6):831–833. doi: 10.1016/j.ccell.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Wang L, Cao L, Wang H, Liu B, Zhang Q, Meng Z, Wu X, Zhou Q, Xu K. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget. 2017;8(44):76116–76128. doi: 10.18632/oncotarget.18814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi X, Luo J, Weigel KJ, Hall SC, Du D, Wu F, Rudolph MC, Zhou H, Young CD, Wang XJ. Cancer-associated fibroblasts facilitate squamous cell carcinoma lung metastasis in mice by providing TGFbeta-mediated cancer stem cell niche. Front Cell Dev Biol. 2021;9:668164. doi: 10.3389/fcell.2021.668164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kamali Zonouzi S, Pezeshki PS, Razi S, Rezaei N. Cancer-associated fibroblasts in colorectal cancer. Clin Transl Oncol. 2021 doi: 10.1007/s12094-021-02734-2. [DOI] [PubMed] [Google Scholar]
- 67.Bartoschek M, Oskolkov N, Bocci M, Lövrot J, Larsson C, Sommarin M, Madsen CD, Lindgren D, Pekar G, Karlsson G, et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat Commun. 2018;9(1):5150. doi: 10.1038/s41467-018-07582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bahar Halpern K, Massalha H, Zwick RK, Moor AE, Castillo-Azofeifa D, Rozenberg M, Farack L, Egozi A, Miller DR, Averbukh I, et al. Lgr5+ telocytes are a signaling source at the intestinal villus tip. Nat Commun. 2020;11(1):1936. doi: 10.1038/s41467-020-15714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moor AE, Harnik Y, Ben-Moshe S, Massasa EE, Rozenberg M, Eilam R, Bahar Halpern K, Itzkovitz S. Spatial reconstruction of single enterocytes uncovers broad zonation along the intestinal villus axis. Cell. 2018;175(4):1156–1167 e1115. doi: 10.1016/j.cell.2018.08.063. [DOI] [PubMed] [Google Scholar]
- 70.Massalha H, Bahar Halpern K, Abu-Gazala S, Jana T, Massasa EE, Moor AE, Buchauer L, Rozenberg M, Pikarsky E, Amit I, et al. A single cell atlas of the human liver tumor microenvironment. Mol Syst Biol. 2020;16(12):e9682. doi: 10.15252/msb.20209682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koliaraki V, Prados A, Armaka M, Kollias G. The mesenchymal context in inflammation, immunity and cancer. Nat Immunol. 2020;21(9):974–982. doi: 10.1038/s41590-020-0741-2. [DOI] [PubMed] [Google Scholar]
- 72.Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: key players in cancer progression. Mol Cancer. 2017;16(1):31. doi: 10.1186/s12943-017-0597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 74.Zhang X, Hu F, Li G, Li G, Yang X, Liu L, Zhang R, Zhang B, Feng Y. Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling. Cell Death Dis. 2018;9(2):25. doi: 10.1038/s41419-017-0176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dora D, Rivard C, Yu H, Pickard SL, Laszlo V, Harko T, Megyesfalvi Z, Dinya E, Gerdan C, Szegvari G, et al. Characterization of tumor-associated macrophages and the immune microenvironment in limited-stage neuroendocrine-high and -low small cell lung cancer. Biology (Basel) 2021 doi: 10.3390/biology10060502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cortes M, Sanchez-Moral L, de Barrios O, Fernandez-Acenero MJ, Martinez-Campanario MC, Esteve-Codina A, Darling DS, Gyorffy B, Lawrence T, Dean DC, et al. Tumor-associated macrophages (TAMs) depend on ZEB1 for their cancer-promoting roles. EMBO J. 2017;36(22):3336–3355. doi: 10.15252/embj.201797345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Q, Guo N, Zhou Y, Chen J, Wei Q, Han M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm Sin B. 2020;10(11):2156–2170. doi: 10.1016/j.apsb.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang Y, Guo J, Huang L. Tackling TAMs for cancer immunotherapy: it's nano time. Trends Pharmacol Sci. 2020;41(10):701–714. doi: 10.1016/j.tips.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu J, Xu Z, Guo J, Yang K, Zheng J, Sun X. Tumor-associated macrophages (TAMs) depend on MMP1 for their cancer-promoting role. Cell Death Discov. 2021;7(1):343. doi: 10.1038/s41420-021-00730-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, Liu Q, Dou R, Xiong B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18(1):64. doi: 10.1186/s12943-019-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zheng X, Weigert A, Reu S, Guenther S, Mansouri S, Bassaly B, Gattenlohner S, Grimminger F, Pullamsetti S, Seeger W, et al. Spatial density and distribution of tumor-associated macrophages predict survival in non-small cell lung carcinoma. Cancer Res. 2020;80(20):4414–4425. doi: 10.1158/0008-5472.CAN-20-0069. [DOI] [PubMed] [Google Scholar]
- 84.Wagner J, Rapsomaniki MA, Chevrier S, Anzeneder T, Langwieder C, Dykgers A, Rees M, Ramaswamy A, Muenst S, Soysal SD, et al. A single-cell atlas of the tumor and immune ecosystem of human breast cancer. Cell. 2019;177(5):1330–1345 e1318. doi: 10.1016/j.cell.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ali HR, Jackson HW, Zanotelli VRT, Danenberg E, Fischer JR, Bardwell H, Provenzano E, Rueda OM, Chin S-F, Aparicio S, et al. Imaging mass cytometry and multiplatform genomics define the phenogenomic landscape of breast cancer. Nature Cancer. 2020;1(2):163–175. doi: 10.1038/s43018-020-0026-6. [DOI] [PubMed] [Google Scholar]
- 86.Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, Soong DYH, Cotechini T, Anur P, Lin EY, et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell. 2019;35(4):588–602 e510. doi: 10.1016/j.ccell.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Elosua-Bayes M, Nieto P, Mereu E, Gut I, Heyn H. SPOTlight: seeded NMF regression to deconvolute spatial transcriptomics spots with single-cell transcriptomes. Nucleic Acids Res. 2021;49(9):e50. doi: 10.1093/nar/gkab043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sadeghzadeh M, Bornehdeli S, Mohahammadrezakhani H, Abolghasemi M, Poursaei E, Asadi M, Zafari V, Aghebati-Maleki L, Shanehbandi D. Dendritic cell therapy in cancer treatment; the state-of-the-art. Life Sci. 2020;254:117580. doi: 10.1016/j.lfs.2020.117580. [DOI] [PubMed] [Google Scholar]
- 90.Worbs T, Hammerschmidt SI, Forster R. Dendritic cell migration in health and disease. Nat Rev Immunol. 2017;17(1):30–48. doi: 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- 91.Hegde S, Krisnawan VE, Herzog BH, Zuo C, Breden MA, Knolhoff BL, Hogg GD, Tang JP, Baer JM, Mpoy C, et al. Dendritic cell paucity leads to dysfunctional immune surveillance in pancreatic cancer. Cancer Cell. 2020;37(3):289–307 e289. doi: 10.1016/j.ccell.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang JB, Huang X, Li FR. Impaired dendritic cell functions in lung cancer: a review of recent advances and future perspectives. Cancer Commun (Lond) 2019;39(1):43. doi: 10.1186/s40880-019-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nagl L, Horvath L, Pircher A, Wolf D. Tumor endothelial cells (TECs) as potential immune directors of the tumor microenvironment—new findings and future perspectives. Front Cell Dev Biol. 2020;8:766. doi: 10.3389/fcell.2020.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheng HW, Chen YF, Wong JM, Weng CW, Chen HY, Yu SL, Chen HW, Yuan A, Chen JJ. Cancer cells increase endothelial cell tube formation and survival by activating the PI3K/Akt signalling pathway. J Exp Clin Cancer Res. 2017;36(1):27. doi: 10.1186/s13046-017-0495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma Y, Li Y, Guo P, Zhao J, Qin Q, Wang J, Liang Z, Wei D, Wang Z, Shen J, et al. Endothelial cells potentially participate in the metastasis of triple-negative breast cancer. J Immunol Res. 2022;2022:5412007. doi: 10.1155/2022/5412007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Naschberger E, Liebl A, Schellerer VS, Schutz M, Britzen-Laurent N, Kolbel P, Schaal U, Haep L, Regensburger D, Wittmann T, et al. Matricellular protein SPARCL1 regulates tumor microenvironment-dependent endothelial cell heterogeneity in colorectal carcinoma. J Clin Invest. 2016;126(11):4187–4204. doi: 10.1172/JCI78260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meng J, Liu Y, Han J, Tan Q, Chen S, Qiao K, Zhou H, Sun T, Yang C. Hsp90beta promoted endothelial cell-dependent tumor angiogenesis in hepatocellular carcinoma. Mol Cancer. 2017;16(1):72. doi: 10.1186/s12943-017-0640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59. doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van der Leun AM, Thommen DS, Schumacher TN. CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer. 2020;20(4):218–232. doi: 10.1038/s41568-019-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234(6):8509–8521. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 101.Melssen M, Slingluff CL., Jr Vaccines targeting helper T cells for cancer immunotherapy. Curr Opin Immunol. 2017;47:85–92. doi: 10.1016/j.coi.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129–144. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 103.Borst J, Ahrends T, Babala N, Melief CJM, Kastenmuller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18(10):635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 104.Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, Itoh T, Ohbuchi T, Kondo S, Katoh H. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94(2):275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oja AE, Piet B, van der Zwan D, Blaauwgeers H, Mensink M, de Kivit S, Borst J, Nolte MA, van Lier RAW, Stark R, et al. Functional heterogeneity of CD4(+) tumor-infiltrating lymphocytes with a resident memory phenotype in NSCLC. Front Immunol. 2018;9:2654. doi: 10.3389/fimmu.2018.02654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang X, Wu W, Pan Y, Zhou Q, Xu J, Han S. Immune-related genes in tumor-specific CD4(+) and CD8(+) T cells in colon cancer. BMC Cancer. 2020;20(1):585. doi: 10.1186/s12885-020-07075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yin J, Wang H, Hong Y, Ren A, Wang H, Liu L, Zhao Q. Identification of an at-risk subpopulation with high immune infiltration based on the peroxisome pathway and TIM3 in colorectal cancer. BMC Cancer. 2022;22(1):44. doi: 10.1186/s12885-021-09085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamada S, Shinozaki K, Agematsu K. Involvement of CD27/CD70 interactions in antigen-specific cytotoxic T-lymphocyte (CTL) activity by perforin-mediated cytotoxicity. Clin Exp Immunol. 2002;130(3):424–430. doi: 10.1046/j.1365-2249.2002.02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tian X, Shen H, Li Z, Wang T, Wang S. Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment. J Hematol Oncol. 2019;12(1):84. doi: 10.1186/s13045-019-0772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hegde S, Leader AM, Merad M. MDSC: markers, development, states, and unaddressed complexity. Immunity. 2021;54(5):875–884. doi: 10.1016/j.immuni.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19(2):108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang Z, Guo J, Weng L, Tang W, Jin S, Ma W. Myeloid-derived suppressor cells-new and exciting players in lung cancer. J Hematol Oncol. 2020;13(1):10. doi: 10.1186/s13045-020-0843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang S, Wang Z, Zhou J, Huang J, Zhou L, Luo J, Wan YY, Long H, Zhu B. EZH2 inhibitor GSK126 suppresses antitumor immunity by driving production of myeloid-derived suppressor cells. Cancer Res. 2019;79(8):2009–2020. doi: 10.1158/0008-5472.CAN-18-2395. [DOI] [PubMed] [Google Scholar]
- 114.Li R, Salehi-Rad R, Crosson W, Momcilovic M, Lim RJ, Ong SL, Huang ZL, Zhang T, Abascal J, Dumitras C, et al. Inhibition of granulocytic myeloid-derived suppressor cells overcomes resistance to immune checkpoint inhibition in LKB1-deficient non-small cell lung cancer. Cancer Res. 2021;81(12):3295–3308. doi: 10.1158/0008-5472.CAN-20-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu W, Yu W, He J, Liu W, Yang J, Lin X, Zhang Y, Wang X, Jiang W, Luo J, et al. Reprogramming immunosuppressive myeloid cells facilitates immunotherapy for colorectal cancer. EMBO Mol Med. 2021;13(1):e12798. doi: 10.15252/emmm.202012798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.