Abstract
Purpose
We report a first case of bilateral occult macular dystrophy (OMD) with a c.133C>T (p.Arg45Trp) pathogenic variant in the retinitis pigmentosa 1-like 1 (RP1L1) gene in a patient of Caucasian Swiss decent.
Observations
A 34-year-old man presented with decreased visual acuity known since childhood. Fundus examination of both eyes revealed no pathology other than mildly increased granularity of the foveal retinal pigment epithelium. The full-field electroretinogram (ffERG) presented with normal findings while the multifocal electroretinogram (mfERG) showed severely reduced amplitudes of the foveal response. Optical coherence tomography (OCT) showed foveal outer retinal atrophy. Fundus autofluorescence (FAF) imaging demonstrated near-normal findings with minimal mottling at the posterior pole. The genetic analysis revealed a heterozygous pathogenic variant (c.133C>T, p.Arg45Trp) in the RP1L1 gene.
Conclusion and importance
Our present case suggests that OMD shows a wide range of clinical presentations with a variety of ophthalmological findings, age of disease onset, visual acuity, and genetic diversity.
Keywords: Pathogenic variant, Molecular diagnosis, Occult macular dystrophy, RP1L1 gene, Switzerland
1. Introduction
Occult macular dystrophy (OMD) is an inherited retinal disease (IRD) marked by a progressive loss of central vision with preserved paracentral and peripheral retinal function.1,2 It is mostly inherited in an autosomal dominant manner and is caused by a pathogenic variant in the RP1L1 gene with incomplete penetrance.3 To date, it has been assumed that these pathogenic variants are mostly predominant in patients of East Asian heritage.3,4 However, with the implementation of new generation sequencing (NGS) into daily clinical practice of IRDs these rare pathogenic variants are more likely to be found also within other ethnical groups.5 Here, we present a case of OMD caused by a Arg45Trp pathogenic gene variant described in a 34- year-old Caucasian patient of Swiss descent.3,4
2. Case report
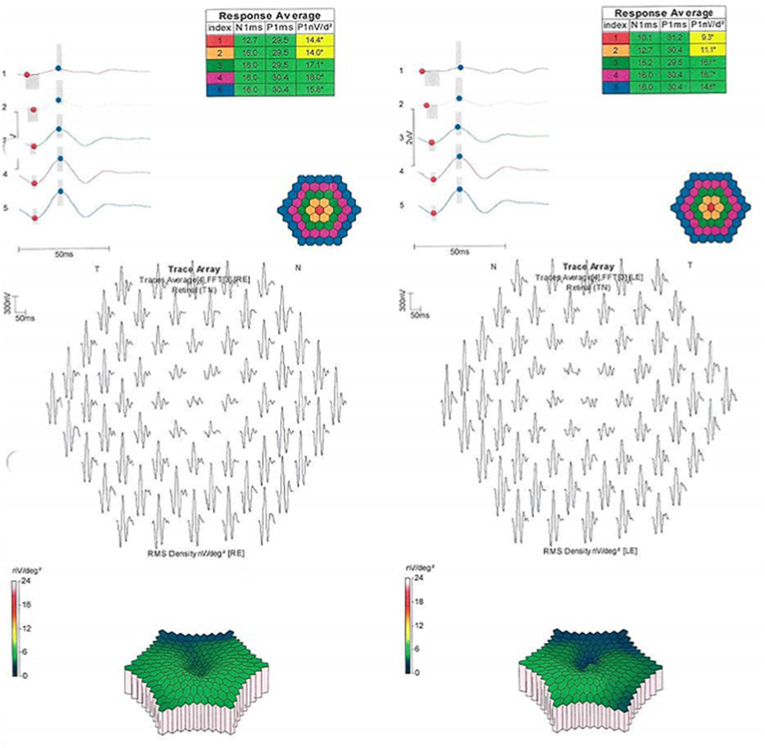
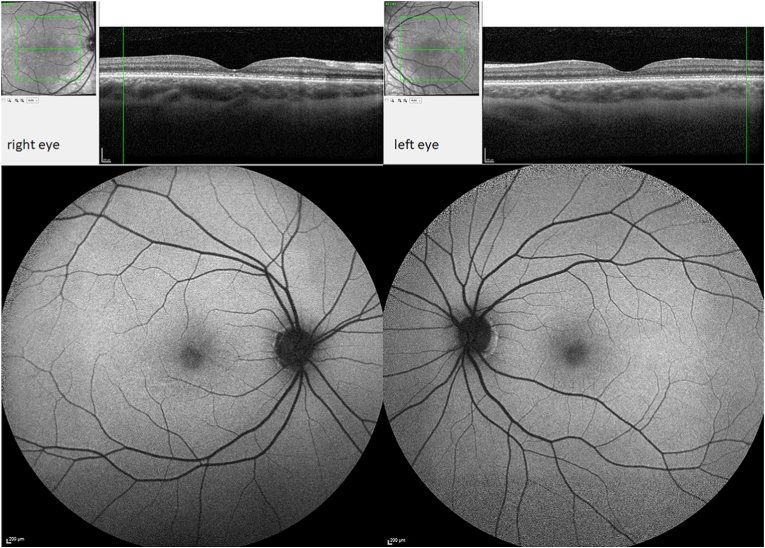
A 34-year-old gentleman was referred to our department with suspected central cone dystrophy, where he reported about visual acuity deterioration since childhood. Best-corrected visual acuity measured under standardized Early Treatment Diabetic Retinopathy Study (ETDRS) conditions6 was 20/125 (ETDRS letter score: 45) and 20/160 (ETDRS letter score: 40) in his right and left eye, respectively. Intraocular pressure was normal with a normal anterior segment. Fundus examination of both eyes revealed minimal changes in the foveal retinal pigment epithelium (RPE). The full-field electroretinogram (ffERG, Diagnosys, LCC Espion system; ISCEV standard7) presented with normal findings of the scotopic, photopic and 30Hz flicker responses (Fig. 1). However, the multifocal electroretinogram (mfERG, Diagnosys, LCC Espion system; ISCEV standard8) presented with severely reduced amplitudes of the foveal response with preserved parafoveal signals (Fig. 2). Optical coherence tomography (OCT, Heidelberg Engineering Spectralis®) imaging showed a slightly discontinued ellipsoid zone line and foveal outer nuclear layer thinning (Fig. 3). Fundus autofluorescence (FAF) imaging demonstrated almost normal findings with minimal mottling of the posterior pole (Fig. 3). Kinetic visual field testing (V4e, III4e, I4e, III3e isopters tested with Octopus 900®, Haag-Streit AG Bern, Switzerland) revealed normal outer boarders (Fig. 4). A more detailed examination with microperimetry (Nidek, MP-3, Nidek Co, Japan, 10-2 pattern) revealed stable central fixation (shown in Fig. 4), however, with a diminished sensitivity of the foveal region while other retinal regions showed a normal sensitivity.
Fig. 1.
Full-field electroretinogram (ffERG) on both eyes: dark-adapted (scotopic) 0.01 ERG presents normal rod-driven response on bipolar cells (b-wave); dark-adapted 3.0 ERG showing combined responses arising from photoreceptors (a-wave) and bipolar cells of both the rod and cone systems within normal limits; normal oscillatory potentials showing three main positive peaks followed by fourth smaller one; light-adapted (photopic) 3.0 ERG demonstrates regular single flash cone response as well as the activity of the cone system as seen in the 31Hz flicker ERG. The green boxes indicate normal values for implicit time and amplitude of age-matched controls. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Multifocal electroretinogram (mfERG) presenting with a lower amplitude of the foveal response with preserved parafoveal signals.
Fig. 3.
Optical coherence tomography (OCT; top) showing disruptions of the ellipsoid zone line on both eyes. Fundus autofluorescence (FAF) imaging (bottom) demonstrates near-normal findings with minimal mottling at the posterior pole on both eyes.
Fig. 4.
Kinetic visual field testing (V4e, III4e, I4e, III3e isopters tested with Octopus 900®, Haag-Streit AG Bern, Switzerland; top) revealed normal outer boarders. A more detailed examination with microperimetry (Nidek, MP-3, Nidek Co, Japan, 10-2 pattern; bottom) revealed stable central fixation, however, with a diminished sensitivity of the foveal region while the other retinal regions showed a normal sensitivity.
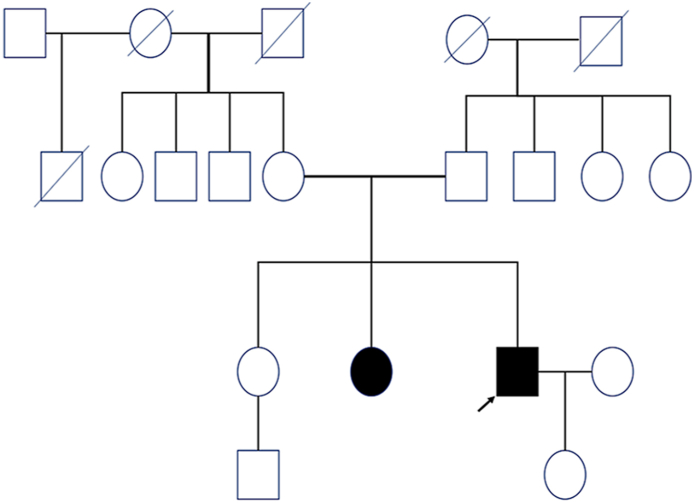
Molecular genetic testing with next-generation sequencing (whole exome sequencing, WES; Ophthalmogenetics, Department of Ophthalmology, University Hospital Bern) and pedigree analysis detected a heterozygous pathogenic variant in the RP1L1 gene (c.133C>T; p.Arg45Trp).3,4 Taking all clinical findings into account we diagnosed OMD. Our patient had no Asian ancestors, also no consanguinity was known in the family. In agreement with the incomplete penetrance in OMD,9 the patient presents with a positive family history with similar symptoms of his sister who was not genetically tested yet but unaffected parents (Fig. 5).
Fig. 5.
The patient's pedigree suggests an autosomal-dominant mode of inheritance with incomplete penetrance (black arrow: propositus; colored black: affected phenotype, but not genetically tested yet).
3. Discussion
First described by Y. Miyake in 1989, OMD is characterized by central cone dysfunction and in some cases with rod dysfunction, resulting in a deterioration of the visual acuity despite having a nearly normal ophthalmoscopic appearance, normal fluorescein angiography, and normal ffERG1,2,10,11 The average age of symptom onset is 25–30 years, with a range from 2 to 74 years.5,9,12 Brockhurst and Sandberg11 reported about four out of eight OMD patients with age of onset over 65 years, similar findings could be observed in previous cases.
So far, for OMD patients there is no evidence-based therapeutic option available. The rarity of the disease and the unknown function of the RP1L1 gene are two major difficulties in developing a gene therapy for OMD. However, unlike many other IRDs, patients with OMD often do not become symptomatic until adulthood. Also, OMD seems to present with a slow and predictable disease progression. Therefore, OMD appears to offer a large window of opportunities for therapeutic intervention and thus could become an interesting target for gene therapy in the future.
So far, pathogenic variants in RP1L1 gene were likely to be found predominantly in people of East Asian descent.4 To the best of our knowledge, this is the first published case of a molecular genetically confirmed OMD case with a pathogenic variant in the RP1L1 gene in Switzerland. This postulates how rare, diverse and often unrecognized this disease is in daily clinical practice. Physicians should consider OMD as a differential diagnosis of other retinal degenerations in patients with normal-appearing fundus and otherwise unexplained deterioration of visual acuity. An accurate investigation performed by ophthalmologists specialized in IRDs and the collaboration with an experienced laboratory for molecular genetics of the retina allows to establish the diagnosis of OMD.
Patient consent
Written informed consent was obtained from patient for publication of this case report and any accompanying images.
Financial disclosure statement
Dr. Scholl is member of the Scientific Advisory Board of: Apellis Switzerland GmbH, ARCTOS medical AG; Astellas Pharma Global Development, Inc./Astellas Institute for Regenerative Medicine; Biogen MA Inc.; Boehringer Ingelheim Pharma GmbH & Co; Gyroscope Therapeutics Ltd.; Janssen Research & Development, LLC (Johnson & Johnson); Novartis Pharma AG (CORE); Okuvision GmbH; Pharma Research & Early Development (pRED) of F. Hoffmann-La Roche Ltd; ReVision Therapeutics, Inc.; and Stargazer Pharmaceuticals, Inc. Dr. Scholl is paid consultant of: Gerson Lehrman Group; Guidepoint Global, LLC; Tenpoint Therapeutics Limited; and Third Rock Ventures, LLC. Dr. Scholl is member of the Data Monitoring and Safety Board/Committee of Belite Bio and ReNeuron Group Plc/Ora Inc. and member of the Steering Committee of Novo Nordisk (FOCUS trial). Dr. Scholl is co-director of the Institute of Molecular and Clinical Ophthal Basel (IOB) which is constituted as a non-profit foundation and receives funding from the University of Basel, the University Hospital Basel, Novartis, and the government of Basel-Stadt. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Johns Hopkins University and Bayer Pharma AG have an active research collaboration and option agreement. These arrangements have also been reviewed and approved by the University of Basel (Universitätsspital Basel, USB) in accordance with its conflict of interest policies. Dr. Hendrik Scholl is principal investigator of grants at the USB sponsored by the following entity: IVERIC bio (Ophthotech Corporation); Kinarus AG; and Novartis Pharma AG. Grants at USB are negotiated and administered by the institution (USB) which receives them on its proper accounts. Individual investigators who participate in the sponsored project(s) are not directly compensated by the sponsor but may receive salary or other support from the institution to support their effort on the project(s). Dr. Gyorgy holds equity and is a paid consultant of Sphere Therapeutics Inc. (Cambridge, MA, USA). Dr Gyorgy receives research funding from Beam Therapeutics Inc. (Cambrdige, MA, USA) and GenScript Inc. (Piscataway, New Jersey, USA).
Funding sources
Giacomo Calzetti was supported by a Diana Davis Spencer Clinical Research Fellowship Award from the Foundation Fighting Blindness. Hendrik P. N. Scholl was supported by the Swiss National Science Foundation, National Center of Competence in Research Molecular Systems Engineering “Molecular Systems Engineering” and project funding in biology and medicine (“Developing novel outcomes for clinical trials in Stargardt disease using structure/function relationship and deep learning” #310030_201165), the Wellcome Trust (PINNACLE study), the Translational Research Acceleration Program Award by the Foundation Fighting Blindness (“Cone-based optogenetics for vision restoration” #TA-NMT-0621-0805-TRAP), and the Foundation Fighting Blindness Clinical Research Institute (ProgStar study). Maria della Volpe Waizel was supported by the SAMW (Schweizerische Akademie der Medizinischen Wissenschaften), the Bangerter Foundation and the SNF (Swiss National Science Foundation) with ad personam grants. The sponsor had no role in the design or conduct of this research. Bence Gyorgy was supported by the Swiss National Science Foundation (NZX2080), the Foundation Fighting Blindness (#TA-GT-0620-0786-IOB) and the European Joint Programme on Rare Diseases (grant agreement 825575, GET-READY consortium).
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
All other authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Acknowledgements
We thank Mr. Corrado della Volpe, MSc for editing the manuscript.
References
- 1.Miyake Y., Ichikawa K., Shiose Y., Kawase Y. Hereditary macular dystrophy without visible fundus abnormality. Am J Ophthalmol. 1989;108:292–299. doi: 10.1016/0002-9394(89)90120-7. [DOI] [PubMed] [Google Scholar]
- 2.Miyake Y., Horiguchi M., Tomita N., Kondo M., Tanikawa A. Occult macular dystrophy. Am J Ophthalmol. 1996;122:644–653. doi: 10.1016/s0002-9394(14)70482-9. [DOI] [PubMed] [Google Scholar]
- 3.Akahori M., Tsunoda K., Miyake Y., Fukuda Y., Ishiura H. Dominant mutations in RP1L1 are responsible for occult macular dystrophy. Am J Hum Genet. 2010;87 doi: 10.1016/j.ajhg.2010.08.009. 429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujinami K., Yang L., Joo K., Tsunoda K., Kameya S. Clinical and genetic characteristics of East Asian patients with occult macular dystrophy (Miyake disease): East Asia occult macular dystrophy studies report number 1. Ophthalmology. 2019;126:1432–1444. doi: 10.1016/j.ophtha.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Zobor D., Zobor G., Hipp S., Baumann B., Weisschuh N. Phenotype variations caused by mutations in the RP1L1 gene in a large mainly German cohort. Invest Ophthalmol Vis Sci. 2018;59:3041–3052. doi: 10.1167/iovs.18-24033. [DOI] [PubMed] [Google Scholar]
- 6.Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report numer 7. Ophthalmology. 1991;98:741–756. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 7.McCulloch D.L., Marmor M.F., Brigell M.G., Hamilton R., Holder G.E. ISCEV Standard for full-field clinical electroretinography (2015 update) Doc Ophthalmol. 2015;130:1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann M.B., Bach M., Kondo M., et al. ISCEV standard for clinical multifocal electroretinography (mfERG) (2021 update) Doc Ophthalmol. 2021;142:5–16. doi: 10.1007/s10633-020-09812-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsunoda K., Usui T., Hatase T., Yamai H., Fujinami K. Clinical characteristics of occult macular dystrophy in family with mutation of RP1L1 gene. Retina-J Retinal Vitreous Dis. 2012;32:1135–1147. doi: 10.1097/IAE.0b013e318232c32e. [DOI] [PubMed] [Google Scholar]
- 10.Wildberger H., Niemeyer G., Junghardt A. Multifocal electroretinogram (mfERG) in a family with occult macular dystrophy (OMD) Klin Monatsbl Augenheilkd. 2003;220:111–115. doi: 10.1055/s-2003-38161. [DOI] [PubMed] [Google Scholar]
- 11.Brockhurst R.J., Sandberg M.A. Optical coherence tomography findings in occult macular dystrophy. Am J Ophthalmol. 2007;143:516–518. doi: 10.1016/j.ajo.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura N., Tsunoda K., Mizuno Y., Usui T., Hatase T. Clinical stages of occult macular dystrophy based on optical coherence tomographic findings. Invest Ophthalmol Vis Sci. 2019;60:4691–4700. doi: 10.1167/iovs.19-27486. [DOI] [PubMed] [Google Scholar]