Abstract
Second messengers are small rapidly diffusing molecules or ions that relay signals between receptors and effector proteins to produce a physiological effect. Lipid messengers constitute one of the four major classes of second messengers. The hydrolysis of two main classes of lipids, glycerophospholipids and sphingolipids, generate parallel profiles of lipid second messengers: phosphatidic acid (PA), diacylglycerol (DAG), and lysophosphatidic acid versus ceramide, ceramide-1-phosphate, sphingosine, and sphingosine-1-phosphate, respectively. In this review, we examine the mechanisms by which these lipid second messengers modulate aldosterone production at multiple levels. Aldosterone is a mineralocorticoid hormone responsible for maintaining fluid volume, electrolyte balance, and blood pressure homeostasis. Primary aldosteronism is a frequent endocrine cause of secondary hypertension. A thorough understanding of the signaling events regulating aldosterone biosynthesis may lead to the identification of novel therapeutic targets. The cumulative evidence in this literature emphasizes the critical roles of PA, DAG, and sphingolipid metabolites in aldosterone synthesis and secretion. However, it also highlights the gaps in our knowledge, such as the preference for phospholipase D-generated PA or DAG, as well as the need for further investigation to elucidate the precise mechanisms by which these lipid second messengers regulate optimal aldosterone production.
Supplementary key words: adrenal cortex, glycerophospholipids, intracellular signaling, signal transduction, sphingolipids, steroidogenesis, phospholipases, primary aldosteronism
Abbreviations: 3βHSD2, type II 3β-hydroxysteroid dehydrogenase; AA, arachidonic acid; ACSL4, acyl-CoA synthetase long chain family member 4; Ang II, angiotensin II; C1P, ceramide-1-phosphate; CYP11A1, cholesterol side-chain cleavage complex; CYP21, 21-Hydroxylase; DAG, diacylglycerol; DGKθ, DAG kinase θ; IP3, inositol 1,4,5-trisphosphate; LPA, lysophosphatidic acid; LPP, lipid phosphate phosphatase; PA, phosphatidic acid; PLA2, phospholipase A2; PLC, phospholipase C; PEt, phosphatidylethanol; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PKD, protein kinase D; PP1, protein phosphatase 1; PTH, parathyroid hormone; PTP, protein tyrosine phosphatase; PTX, pertussis toxin; RSK, ribosomal S6 kinase; S1P, sphingosine-1-phosphate; SF-1, steroidogenic factor-1; SGPL1, S1P lyase 1; SPC, sphingosylphosphorylcholine; TASK, TWIK-related-acid-sensitive potassium; zG, zona glomerulosa
Extracellular signals, received and transduced by receptors at the cell surface, elicit cellular responses via the generation of small, rapidly diffusing molecules referred to as second messengers. Second messengers are intermediates that link extracellular signals to intracellular responses. Lipid second messengers, produced by the metabolism of lipids, are one of the major classes of second messengers. The purpose of this review is to highlight the role of lipid second messengers in regulating aldosterone production in the adrenal cortex.
Aldosterone biosynthesis
Aldosterone is the principal mineralocorticoid hormone synthesized in and secreted from the zona glomerulosa (zG) layer of the adrenal cortex, primarily in response to angiotensin II (Ang II), elevated serum potassium levels, and adrenocorticotrophic hormone (ACTH). It plays a central role in electrolyte and fluid volume regulation and maintenance of blood pressure homeostasis and is tightly regulated by the renin-angiotensin-aldosterone system. Primary aldosteronism, in which plasma aldosterone levels are normal or elevated relative to suppressed plasma renin levels, is the most frequent cause of secondary hypertension. Primary aldosteronism accounts for 5%–10% of hypertension cases and up to 20% in the case of resistant hypertension (1), with the reported prevalence showing high variability among studies depending upon the population of patients included, the diagnostic criteria, and the severity of hypertension (2, 3, 4). Aldosterone has also been suggested to be one of the causal links between obesity and hypertension (5, 6, 7).
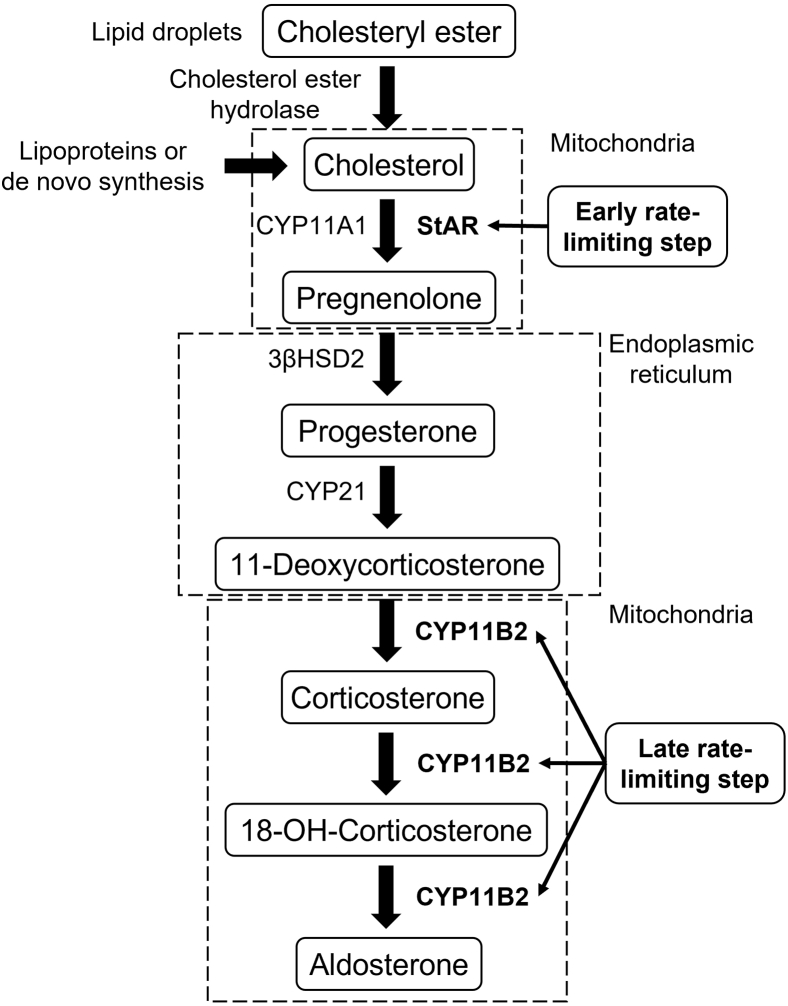
Aldosterone biosynthesis occurs via a series of enzymatic reactions in the mitochondria and the endoplasmic reticulum of the zG cell and involves three cytochrome P450 enzymes and one hydroxysteroid dehydrogenase (Fig. 1). Cholesterol side-chain cleavage complex (CYP11A1) and aldosterone synthase (CYP11B2) are localized in the inner mitochondrial membrane, while 21-hydroxylase (CYP21) and type II 3β-hydroxysteroid dehydrogenase (3βHSD2) are found in the endoplasmic reticulum (8).
Fig. 1.
Aldosterone biosynthesis. This schematic illustrates the enzymatic process through which aldosterone is synthesized in the mitochondria and endoplasmic reticulum of zona glomerulosa cells in the adrenal cortex. The cholesterol precursor can be derived from a combination of sources: mobilization of cholesteryl esters stored in lipid droplets by cholesteryl ester hydrolase, de novo synthesis in the endoplasmic reticulum, and receptor-mediated uptake and internalization of plasma lipoprotein-derived cholesterol. The free cholesterol is transported by the steroidogenic acute regulatory (StAR) protein from the outer to the inner mitochondrial membrane, which is the early rate-limiting step in steroidogenesis. In the inner mitochondrial membrane, steroidogenesis is initiated by the side-chain cleavage of cholesterol catalyzed by CYP11A1 to yield the steroid precursor, pregnenolone. Pregnenolone passively diffuses to the endoplasmic reticulum where it is converted to progesterone by type II 3β-hydroxysteroid dehydrogenase (3βHSD2). Progesterone is then hydroxylated to 11-deoxycorticosterone by CYP17. The final late rate-limiting steps of aldosterone biosynthesis are completed in the mitochondria, where aldosterone synthase (CYP11B2) catalyzes the conversion of 11-deoxycorticosterone to corticosterone and subsequently to aldosterone.
The primary precursor for aldosterone biosynthesis is cholesterol, which can be derived from several sources (9, 10): (1) de novo cholesterol synthesis, (2) circulating lipoprotein-derived cholesteryl esters via either “selective” uptake or receptor-mediated endocytosis, and (3) mobilization of stored cholesteryl esters via the actions of neutral cholesteryl ester hydrolase, also known as hormone-sensitive lipase. The first reaction in aldosterone biosynthesis is the mitochondrial conversion of cholesterol to pregnenolone. This step is tightly regulated by the steroidogenic acute regulatory (StAR) protein, which transports cholesterol from the outer to the inner mitochondrial membrane where CYP11A1 is located (11). Pregnenolone can then passively diffuse to the endoplasmic reticulum where it is converted to progesterone by 3βHSD2. Progesterone is then hydroxylated by CYP21 to 11-deoxycorticosterone. Finally, aldosterone biosynthesis is completed in the mitochondria, where deoxycorticosterone undergoes 11β- and 18-hydroxylation, followed by 18-oxidation. These final reactions are catalyzed by a single enzyme, aldosterone synthase, encoded by CYP11B2. Once synthesized, aldosterone is secreted from the zG cells. Thus, aldosterone biosynthesis involves two key rate-limiting steps: the early (acute) rate-limiting step requires the expression and phosphorylation of StAR protein (12, 13, 14), while the late (chronic) rate-limiting step involves the expression and regulation of CYP11B2 (15, 16).
To investigate the signaling mechanisms involved in aldosterone biosynthesis, several glomerulosa cell models are routinely used. These include primary cultures derived from different species (e.g., human, bovine, murine) and a few adrenocortical carcinoma cell lines (e.g., human H295R, an H295R clone, HAC15 (17), and Y1 mouse cells). (18, 19, 20, 21)
Lipid second messengers
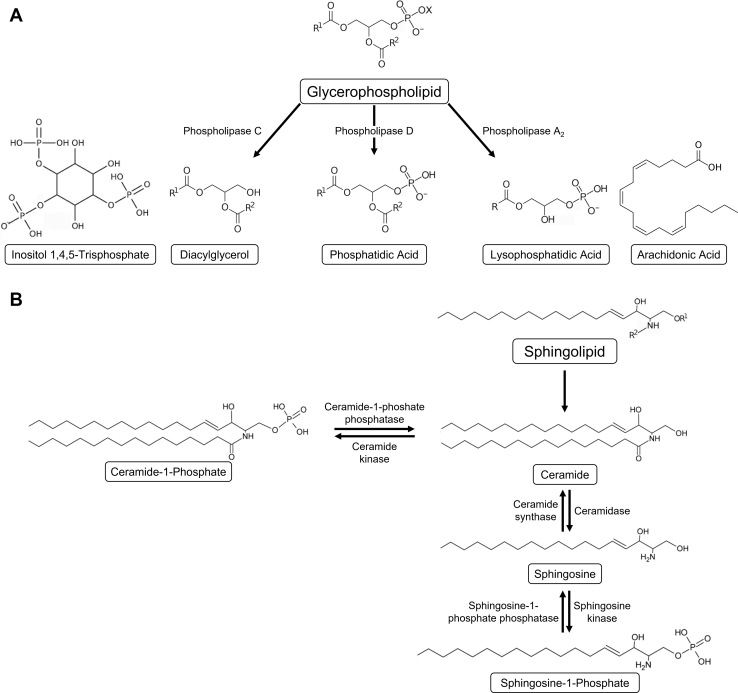
Phospholipids serve as integral structural components of cell membranes by spontaneously forming a lipid bilayer that maintains cell integrity. In addition, phospholipids serve as a reservoir of bioactive lipids involved in important signaling processes. Extracellular signals elicit the hydrolysis of two main classes of lipids to generate lipid second messengers: glycerophospholipids and sphingolipids. In the former class are included the phosphoinositides and phosphatidylcholine, with diacylglycerol (DAG) as the hydrophobic backbone, and the latter includes sphingomyelin, for which the hydrophobic backbone is ceramide. Signaling-induced hydrolysis of glycerophospholipids and sphingolipids generates parallel series of lipid second messengers (Fig. 2): lysophosphatidic acid (LPA), phosphatidic acid (PA), and DAG (and certain free fatty acids that can serve as cell signals themselves or as precursors to signaling molecules) versus sphingosylphosphorylcholine (SPC), ceramide-1-phosphate (C1P), ceramide, sphingosine, and sphingosine-1-phosphate (S1P). Lipid second messengers that retain two acyl chains, such as DAG, PA, C1P and ceramide, remain associated with the membrane while those that have only one acyl chain, such as LPA, SPC, S1P and sphingosine, are hydrophobic but can dissociate from membranes (22).
Fig. 2.
Lipid-derived second messengers. Hydrolysis of two classes of lipids, glycerophospholipids and sphingolipids, generates parallel series of lipid second messengers. A: Hydrolysis of glycerophospholipids yields diacylglycerol (and inositol 1,4,5-trisphosphate), phosphatidic acid, and lysophosphatidic acid (and free fatty acids such as arachidonic acid). B: Hydrolysis of sphingolipids results in the production of ceramide, ceramide-1-phosphate, sphingosine, and sphingosine-1-phosphate.
Glycerophospholipid-derived second messengers
Phosphoinositide signaling system: DAG and IP3
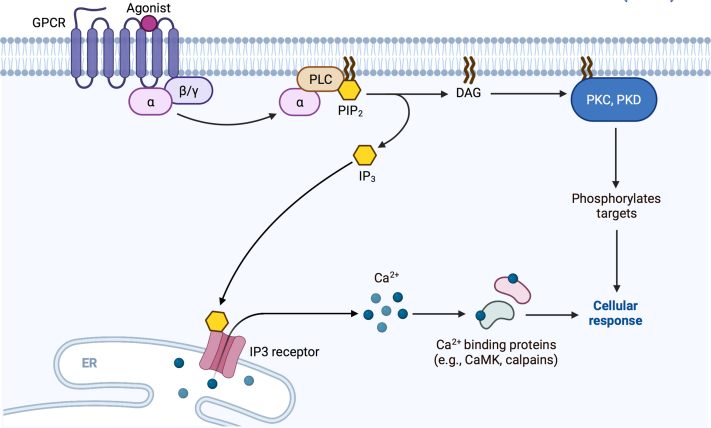
The phosphoinositide signaling system, illustrated in Figure 3, is important in regulating steroidogenesis in adrenocortical cells in response to agonists. Ang II and elevated serum potassium levels are the primary physiological regulators of aldosterone production in the zG of the adrenal gland. Serum potassium signals through changes in zG membrane potential. Adrenal glomerulosa cells are usually hyperpolarized and maintain a negative resting membrane potential (−80 mV) close to the Nernst potential for potassium, which suggests that the membrane potential is determined mainly by the membrane potassium permeability. The potassium channels involved include the two pore-domain potassium channels, TWIK-related-acid-sensitive potassium family (TASK-1, TASK-3) and TWIK-related potassium channel 1, and the G protein-coupled, inwardly rectifying potassium channel Kir3.4 (23, 24, 25, 26, 27, 28). Elevated extracellular potassium levels depolarize the plasma membrane and activate the voltage-dependent T-type and L-type calcium channels (29, 30, 31, 32, 33, 34), leading to calcium influx and triggering signaling mechanisms described below for Ang II, including the activation of phospholipase D (PLD) (35).
Fig. 3.
Phosphoinositide signaling system. Binding of agonists to G protein-coupled receptors (GPCRs) promotes the exchange of GTP for GDP on Gα subunits, which then bind and activate phospholipase C (PLC). PLC hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to generate two second messengers, inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 binds to IP3 receptors on the endoplasmic reticulum (ER) causing the release of calcium into the cytosol. The elevated cytosolic calcium levels can bind to and activate calcium/calmodulin-dependent protein kinase (CaMK), calpains, and classical (in conjunction with calcium) protein kinase C (PKC) isoenzymes. DAG remains in the membrane and activates proteins such as novel PKC isoenzymes and protein kinase D (PKD) isoenzymes. Figure adapted from “Activation of Protein Kinase C (PKC)”, created with BioRender.com (2021). Retrieved from https://app.biorender.com/biorender-templates.
The adrenal gland expresses two receptors for Ang II: Ang II receptor type 1 and Ang II receptor type 2; Ang II receptor type 1 is the primary receptor involved in Ang II-induced stimulation of aldosterone production (36). The primary event following ligand binding and receptor activation is the generation of DAG directly by phospholipase C (PLC). PLC cleaves the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) to yield DAG and inositol 1,4,5-trisphosphate (IP3). Interestingly, DAG can directly inhibit TASK-1 and TASK-3 channels causing a strong decrease of the fractional potassium conductance and depolarization of the membrane (37). Therefore, Ang II-induced depolarization, through inhibition of the two pore-domain potassium as well as G protein-coupled, inwardly rectifying potassium channels (23, 27, 33, 38, 39, 40), also activates voltage-gated T-type and L-type calcium channels resulting in calcium influx and increasing cytosolic calcium concentration (28, 29, 30, 31, 32, 41). Further, upon binding to its receptors expressed in the endoplasmic reticulum (42), IP3 is thought to initiate aldosterone production by inducing calcium release from the intracellular stores and eliciting a transient increase in cytosolic calcium concentration (43). This increase activates calcium/calmodulin-dependent protein kinases, which increase the transcription of steroidogenic enzymes involved in aldosterone biosynthesis (44, 45, 46). DAG remains in the membrane to activate DAG-responsive enzymes, such as protein kinase C (PKC) and protein kinase D (PKD) (47, 48, 49). Signals transduced through PIP2 hydrolysis are terminated by the metabolism of IP3 and DAG into derivatives lacking second messenger capabilities. DAG can also be phosphorylated to PA, which itself has signaling properties or can be deacylated by an arachidonate-preferring DAG lipase, releasing arachidonic acid (AA) for conversion to eicosanoids, such as the aldosterone-promoting 12-HETE (50, 51, 52, 53, 54) (refer to section LPA and AA). Inositol tetrakisphosphate formed by phosphorylation of IP3 may act as a second messenger to further regulate calcium homeostasis (55).
Larson et al. first demonstrated the incorporation of radiolabeled phosphate into phosphatidylinositol and polyphosphoinositides in the zG by treating rat adrenal capsules in vitro with Ang II and elevated potassium levels (56). Subsequent studies by Rasmussen et al. showed that Ang II induced a rapid hydrolysis of phosphatidylinositol 4-phosphate and PIP2 resulting in sustained production of inositol bisphosphate, IP3, and DAG in adrenal glomerulosa cells (43). Ang II was also shown to induce an increase in absolute DAG levels in freshly isolated and cultured bovine adrenal glomerulosa cells (57, 58, 59) This Ang II-induced DAG increase is biphasic, with an initial peak followed by a transient decrease; the second increase is higher in magnitude than the first increase (57). Moreover, the DAG produced by Ang II stimulation consists of multiple species, for example, those containing the fatty acids arachidonate or myristate. Following the increase in DAG formation upon Ang II binding, the subsequent addition of an Ang II antagonist results in a rapid decline in arachidonate-DAG, but a slower decline in myristate-DAG, suggesting that the species of DAG generated might play a role in the speed with which signaling is terminated (58).
In addition to conventional aldosterone secretagogues, VLDL, a nonconventional aldosterone secretagogue (60), induces an increase in radiolabeled DAG levels, and a PLC inhibitor, U-73122, blocks this increase in HAC15 cells. In addition, an IP3 receptor inhibitor decreases VLDL-induced aldosterone production in HAC15 cells (48). U-71322 also inhibits the parathyroid hormone (PTH)- and PTH-related peptide-induced elevation in radiolabeled IP3 release and aldosterone secretion from dispersed human adrenocortical cells (61). PTH and PTH-related peptide, members of the vasoactive intestinal peptide-secretin-glucagon family of peptides, are well-known paracrine modulators of the secretory activity of the adrenal cortex (62, 63). Yet, another nonconventional aldosterone secretagogue is S1P (64). Sewer et al. showed that S1P-induced StAR transcription requires PLC activation and results in the accumulation of cytoplasmic IP3 in H295R cells (65).
Together, these data suggest the importance of phosphoinositide turnover to aldosterone synthesis.
DAG and PA
In addition to being directly produced by PLC-mediated cleavage of phospholipids, DAG can be generated indirectly by the action of PLD. PLD is an enzyme that hydrolyzes phosphatidylcholine to produce PA (phosphorylated DAG), also a lipid second messenger. PA can then be dephosphorylated by lipid phosphate phosphatases (LPPs; also known as phosphatidate phosphatases or the lipin family) to yield DAG (66). The phospholipid source of the DAG dictates the species of DAG generated. Since phosphatidylcholine is enriched in myristate, Ang II-induced increases in myristate-DAG in adrenal glomerulosa cells support the role of PLD in Ang II-elicited DAG generation (58). In turn, numerous reports demonstrate the importance of PLD activity in the aldosterone secretory response to Ang II and other secretagogues (64, 66, 67, 68, 69).
PA has been proposed to function as a slow-release reservoir of DAG for sustained aldosterone production (66, 70, 71). Bollag et al. demonstrated that Ang II elicits increases in the levels of radiolabeled PA and phosphatidylethanol (PEt; in the presence of ethanol) in cultured bovine adrenal glomerulosa cells (66). Phosphatidylcholine undergoes transphosphatidylation in the presence of ethanol to yield PEt, rather than hydrolysis to generate PA. PEt is not readily metabolized to DAG. The ability of ethanol to divert product formation away from PA (and DAG) in response to hormonal stimulation indicates that hormone-elevated DAG, in part, originates from the combined activity of PLD and LPP. Other primary alcohols can be used as well; PLD can utilize 1-butanol in place of water to form phosphatidylbutanol instead of PA. Phosphatidylbutanol also is not readily metabolized to DAG. Therefore, 1-butanol can be used to inhibit PLD-mediated lipid signal generation. Bollag et al. showed that 1-butanol inhibited Ang II-induced increases in PA and DAG levels as well as aldosterone secretion in bovine adrenal glomerulosa cells (67). S1P-stimulated aldosterone secretion in zG cells is also inhibited by primary alcohols (64). Combined PLD/LPP activity underlying sustained DAG production has also been observed using propranolol in terms of both S1P-induced aldosterone production in bovine adrenal glomerulosa cells (64) and Ang II-induced cortisol production in zona fasciculata cells of bovine adrenal glands (72). Propranolol, in addition to being a β-blocker, inhibits LPP (73, 74).
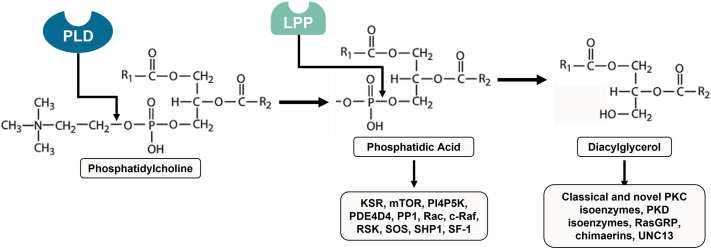
The involvement of a particular PLD-generated lipid signal, PA or DAG, in mediating aldosterone production is not entirely clear (Fig. 4). PLD-generated DAG clearly contributes to Ang II- and S1P-induced aldosterone production in zG cell models (64, 66, 67, 71). DAG can, in turn, activate PKC isoforms, the PKD family of protein kinases, and Ras guanine nucleotide-releasing protein 1-3 (Ras guanine nucleotide exchange factors). DAG is an allosteric activator of PKC (75). In bovine adrenal glomerulosa cells, a PKC-activating phorbol ester, phorbol 12-myristate 13-acetate or PMA, is able to activate PLD while selective PKC inhibitors partially block PLD activation by Ang II and S1P. Moreover, PMA does not enhance PLD activation by Ang II or S1P; this lack of an additive effect with PMA suggests that both Ang II and S1P function to stimulate PLD activity through PKC. In addition, these results suggest that not only is PKC sufficient to activate PLD but also that it is necessary for agonist-induced PLD activation (64, 67). The PLC/DAG/PKC pathway can also activate PKD (as shown in Swiss 3T3 cells) (76), and Ang II-mediated PKD phosphorylation/activation is mediated, in part, by PLD activity (68), as well as by PKC and Src family kinases (49, 77) in primary bovine adrenal glomerulosa and H295R cells. PKD in turn phosphorylates and activates activating transcription factor (ATF)/cAMP response element binding (CREB) protein transcription factors to induce the transcription of StAR and aldosterone synthase and mediate acute Ang II-aldosterone secretion, as demonstrated in H295R cells and primary bovine adrenal glomerulosa cells (47, 77, 78).
Fig. 4.
Downstream effectors of phospholipase D-generated lipid second messengers. Phospholipase D (PLD) hydrolyzes phosphatidylcholine to produce choline and phosphatidic acid (PA). PA can interact and modulate the activity of various downstream effectors such as the kinase suppressor of Ras (KSR), mammalian target of rapamycin (mTOR), phosphatidylinositol 4-phosphate 5-kinase (PI4P5K), phosphodiesterase 4D3 (PDE4D3), protein serine/threonine phosphatase 1 (PP1), the small GTP-binding proteins Rac and c-Raf, ribosomal S6 kinase (RSK), son of sevenless (SOS), Src homology region 2 domain-containing phosphatase-1 (SHP1), and nuclear hormone receptor steroidogenic factor-1 (SF-1). PA can be dephosphorylated by lipid phosphate phosphatases (LPPs) to yield diacylglycerol (DAG). DAG effectors include the classical and novel protein kinase C (PKC) isoenzymes, protein kinase D (PKD) family of protein kinases, the Ras guanine nucleotide release proteins (RasGRP), the Rac GTPase-activating proteins chimaerins, and UNC13 proteins.
However, PA is itself a lipid second messenger with downstream effectors, such as mammalian target of rapamycin, phosphodiesterase, phosphoinositide-synthesizing enzymes, protein phosphatase 1 (PP1), ribosomal S6 kinase (RSK), and steroidogenic factor-1 (SF-1). Elevated potassium levels and Ang II have been shown to dose-dependently increase the concentrations of PA in rat adrenal capsules in vitro (56). In addition to being generated by PLD, PA can also be produced by DAG kinase θ (DGKθ), which phosphorylates DAG formed by phosphoinositide turnover. ACTH/cAMP signaling stimulates DGKθ-catalyzed nuclear PA production in H295R cells (79, 80). Silencing DGKθ represses both basal and cAMP-dependent expression of genes (and proteins) involved in cholesterol mobilization (StAR, scavenger receptor type B class 1, low density lipoprotein receptor, hormone-sensitive lipase) and steroidogenesis (CYP11A1, 3βHSD2). In contrast, CYP21 gene and protein expression are increased in these DGKθ knockdown cells (81). PA acts as an endogenous ligand for SF-1 (79), a nuclear hormone receptor important in steroidogenesis (82). In line with the DGKθ knockdown results, PA has been shown to activate multiple SF-1 steroidogenic targets including CYP11A1, 3βHSD, CYP21, and CYP11B1/2 (79). SF-1 is also known to positively regulate StAR gene expression (83, 84), whereas it appears to negatively regulate CYP21 and CYP11B2 expression as well as aldosterone production (82, 85). However, a threshold level of SF-1 is required for basal CYP11B2 expression, with an elevation of SF-1 above this baseline leading to repression of CYP11B2 expression (82).
PA is also a potent and selective inhibitor of PP1 (86). PP1 is expressed in whole adrenals as well as the capsule, and its inhibition by nonselective inhibitors significantly reduces ACTH-stimulated aldosterone production in zG cells (87). Finally, in patients with primary aldosteronism (idiopathic hyperaldosteronism and aldosterone-producing adenomas), enhanced phosphorylation of mammalian target of rapamycin and RSK, downstream effectors of PA, correlates with plasma aldosterone levels (88). Therefore, whether PA or DAG or both of these lipid second messengers are important for aldosterone production remains to be determined.
LPA and AA
PA can also be deacylated by phospholipase A2 (PLA2) to yield LPA and a free fatty acid such as AA. Bovine adrenal glomerulosa cells express G protein-coupled receptors for LPA that are predominantly coupled to Gi (89). LPA has been shown to have a mitogenic effect in bovine adrenal glomerulosa cells (similar to Ang II), which is completely prevented by pertussis toxin (PTX) (90). PTX ADP ribosylates and inhibits Gi in the bovine adrenal glomerulosa cell preparation (91, 92). Shah et al. showed that LPA causes proliferation of these cells through activation of Src and phosphoinositide-3-kinase (89) A selective Src family inhibitor, PP2, has been shown to inhibit Ang II-, potassium-, and dibutyryl-cAMP-stimulated aldosterone production through the induction of CYP17 in H295R cells (49, 93). LPA can also stimulate aldosterone secretion (64), and this ability is partially dependent upon epidermal growth factor receptor transactivation (89). LPA induces the activation of ERK1/2 and its downstream protein, RSK-1, and this activation is blocked by PTX (89). Further, PKC depletion by PMA-mediated downregulation in rat adrenal glomerulosa cells (94) partly attenuates LPA responses and a pan-PKC inhibitor, Gö6983, reduces LPA-induced ERK1/2 activation. These effects indicate that LPA exerts its action through two distinct signaling cascades: the epidermal growth factor receptor and PKC (89). LPA has also been shown to inhibit the TASK-1 channel, a two pore-domain potassium channel, in oocytes (39); Barrett et al. have demonstrated that zG-specific deletion of this TASK-1 channel in mice can produce mild autonomous hyperaldosteronism that is independent of the renin-angiotensin system and can result in chronic blood pressure elevation (25).
The other product of PLA2 activity, free fatty acids like AA, can also function as second messengers. AA can also be released upon DAG hydrolysis by DAG lipase (51, 53). AA specifically inhibits Ang II type 1 receptors but exerts no effect on Ang II type 2 receptors in bovine adrenal glomerulosa cells (95). Exogenous AA stimulates both radiocalcium efflux and aldosterone secretion in bovine adrenal glomerulosa cells (96). A trifluoromethylketone analog of AA and inhibitor of cytosolic PLA2, AACOCF3, raises basal aldosterone secretion in dispersed rat zG and human adrenocortical cells (97). AA can be subsequently metabolized by the lipoxygenase and the cyclooxygenase pathways in zG cells (98). It seems that the lipoxygenase, but not the cyclooxygenase, products of AA metabolism, play a role in aldosterone secretion as positive feed forward mediators (52, 96, 99, 100, 101, 102). Isolated rat adrenal glomerulosa cells and normal human adrenal glomerulosa cells can produce the lipoxygenase products, 12- and 15-HETE, in the basal state. Ang II, but not ACTH or potassium, selectively stimulates 12-HETE production. Selective and nonselective lipoxygenase inhibitors block Ang II-mediated intracellular calcium increases and aldosterone secretion as well as the formation of 12-HETE, and these responses can be restored by the addition of exogenous 12-HETE in isolated rat adrenal glomerulosa cells and cultured aldosterone-producing adenoma cells (50, 52, 54). 12-HETE increases aldosterone production, in part, through activation of CREB/ATF-1 and the p38 mitogen-activated protein kinase pathway in H295R cells (103). Ang II-mediated aldosterone secretion is unaltered by 5-lipoxygenase inhibitors (50). Nadler et al. showed a similar effect in aldosterone-producing adenomas (52). Podestá et al. highlighted the role of the generation and export of intramitochondrial AA in regulating the induction of StAR protein in H295R cells (104). In this mechanism, acyl-CoA synthetase long chain family member 4 (ACSL4) esterified free AA to yield AA-CoA, which can be delivered to mitochondrial acyl-CoA thioesterase 2 to release AA into the mitochondria upon hormone treatment. Ang II and potassium regulate the expression of ACSL4 and acyl-CoA thioesterase 2, with the induction of ACSL4 dependent on protein tyrosine phosphatases (PTPs). The site of action of PTPs precedes activation of cholesterol transport into the mitochondria. Therefore, aldosterone synthesis by Ang II can be linked to the sequential actions of PTP, ACSL4, and StAR protein. However, additional studies are needed to elucidate the role of LPA and AA metabolites in the regulatory mechanisms of aldosterone synthesis.
Sphingolipid-derived second messengers
Sphingolipid metabolism and steroidogenesis have a reciprocal relationship in many respects. In H295R cells, both ACTH and dibutyryl-cAMP stimulate sphingolipid metabolism by rapidly promoting the catabolism of sphingomyelin, ceramide, and sphingosine, with a concomitant increase in S1P secretion (105).
Brizuela et al. showed in bovine adrenal glomerulosa cells that short-chain cell permeable ceramides such as C6-ceramide, which are potent inhibitors of PLD (106, 107), not only blocked PLD stimulation by S1P but also completely abolished S1P-induced aldosterone secretion (64). Ceramide has no effect on cAMP production but has been shown to abrogate StAR mRNA and protein expression in 8Br-cAMP-treated MA-10 Leydig cells and in human chorionic gonadotropin-stimulated adult Sprague Dawley rats (108, 109, 110). However, the precise molecular mechanism(s) by which ceramide regulates aldosterone secretion is unclear.
Ceramide can be phosphorylated to C1P by ceramide kinase. C1P has been reported to be a specific and potent inducer of AA release and since AA plays a role in steroidogenesis (refer to LPA and AA), C1P may be a regulator of steroidogenesis (111). However, this sphingolipid has been little studied in the zG.
Ceramide can also be hydrolyzed to sphingosine by ceramidases. Sphingosine serves as an antagonist of SF-1 to modulate steroidogenic gene transcription. Under basal conditions, sphingosine is bound to SF-1, and this binding antagonizes cAMP activation of CYP17 gene transcription in H295R cells (112). PA has been demonstrated to be an endogenous activating ligand for SF-1 (79), with cAMP promoting the displacement of sphingosine (and SPC; see below) from and the binding of PA to the SF-1 ligand-binding pocket (112). The sphingosine-generating enzyme, acid ceramidase (ASAH1), directly regulates the intracellular balance of ceramide, sphingosine, and S1P. In H295R cells, suppression of ASAH1 can induce the expression of steroidogenic genes CYP17A1, CYP11A1, CYP21A2, CYP11B1, CYP11B2, StAR, and hormone-sensitive lipase (113). Silencing ASAH1 mimics cAMP-stimulated CYP17A1 transcription, supporting ASAH1’s role in regulating the function of SF-1 and therefore, steroidogenic gene expression. SPC (i.e., lysosphingomyelin) can also bind to SF-1 but with a lesser affinity than sphingosine under basal conditions, and cAMP treatment also promotes its dissociation from the receptor. However, SPC has no effect on the ability of cAMP or SF-1 to promote CYP17 expression (112).
Sphingosine kinases can phosphorylate sphingosine to form S1P. S1P can exert its signaling functions both extracellularly and within the cell (114, 115). Extracellularly, S1P binds to various G protein-coupled S1P receptors (S1PR1-5) through which it serves as a stimulator of aldosterone secretion and PLD activity in bovine adrenal glomerulosa cells (64). Protein kinase B (also known as Akt) and ERK1/2 have been identified as S1P targets. Brizuela et al. proposed a working model in bovine adrenal glomerulosa cells wherein S1P stimulates phosphoinositide-3-kinase/protein kinase B and mitogen-activated kinase kinase/ERK pathways, leading to PLD activation. S1P also causes an influx of calcium and activation of PKCα and PKCδ, which are upstream of PLD (116).
ACTH and cAMP can stimulate an increase in intracellular S1P in H295R cells (105). S1P, in turn, can increase CYP17 mRNA expression by promoting the cleavage and nuclear localization of sterol regulatory element binding protein 1 (SREBP1) (117). Intracellular S1P pools are mainly regulated by S1P lyase 1 (SGPL1); SGPL1 executes the final decisive step in sphingolipid catabolism by initiating the irreversible breakdown of S1P into ethanolamine phosphate and hexadecenal. Loss-of-function mutations in SGPL1 have been associated with primary adrenal insufficiency and steroid-resistant nephrotic syndrome in humans (118, 119, 120). SGPL1 is expressed in normal human adrenals while adrenals from Sgpl-/- mice shows compromised cortical zonation with less definition between the zG and zona fasciculata. Sgpl-/- mouse adrenals also show lower expression of CYP11A1, while the characteristic patchy expression of aldosterone synthase (CYP11B2) in wild-type mice is replaced by a more continuous pattern of expression (119).
These studies highlight the importance of sphingolipid metabolites in aldosterone synthesis and secretion.
Conclusion
In conclusion, cumulative evidence points toward an important role of lipid second messengers in the regulation of aldosterone production. These bioactive lipids serve as potential links between the agonists binding to their receptors and the synthesis and secretion of aldosterone. The studies discussed in this review also highlight the need for further investigation to elucidate the role of DAG, PA, LPA, and sphingolipid metabolites in aldosterone production.
Conflict of interest
No author has an actual or perceived conflict of interest with the content of this article.
Acknowledgments
This project was supported by an intramural grant award from Augusta University and the Augusta University Adrenal Center.
Author contributions
S. C. S. writing–original draft; W. B. B. writing–review and editing.
Funding and additional information
W. B. B is supported by a VA Research Career Scientist Award (#BX005691). The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Jr., Collins K.J., Dennison Himmelfarb C., DePalma S.M., Gidding S., Jamerson K.A., Jones D.W., MacLaughlin E.J., Muntner P., Ovbiagele B., Smith S.C., Jr., Spencer C.C., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 2.Fagugli R.M., Taglioni C. Changes in the perceived epidemiology of primary hyperaldosteronism. Int. J. Hypertens. 2011;2011:162804. doi: 10.4061/2011/162804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hannemann A., Wallaschofski H. Prevalence of primary aldosteronism in patient's cohorts and in population-based studies--a review of the current literature. Horm. Metab. Res. 2012;44:157–162. doi: 10.1055/s-0031-1295438. [DOI] [PubMed] [Google Scholar]
- 4.Käyser S.C., Dekkers T., Groenewoud H.J., van der Wilt G.J., Carel Bakx J., van der Wel M.C., Hermus A.R., Lenders J.W., Deinum J. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J. Clin. Endocrinol. Metab. 2016;101:2826–2835. doi: 10.1210/jc.2016-1472. [DOI] [PubMed] [Google Scholar]
- 5.Briet M., Schiffrin E.L. The role of aldosterone in the metabolic syndrome. Curr. Hypertens. Rep. 2011;13:163–172. doi: 10.1007/s11906-011-0182-2. [DOI] [PubMed] [Google Scholar]
- 6.Kawarazaki W., Fujita T. The role of aldosterone in obesity-related hypertension. Am. J. Hypertens. 2016;29:415–423. doi: 10.1093/ajh/hpw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie D., Bollag W.B. Obesity, hypertension and aldosterone: is leptin the link? J. Endocrinol. 2016;230:F7–F11. doi: 10.1530/JOE-16-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rainey W.E. Adrenal zonation: clues from 11beta-hydroxylase and aldosterone synthase. Mol. Cell Endocrinol. 1999;151:151–160. doi: 10.1016/s0303-7207(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 9.Gwynne J.T., Strauss J.F., 3rd. The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr. Rev. 1982;3:299–329. doi: 10.1210/edrv-3-3-299. [DOI] [PubMed] [Google Scholar]
- 10.Azhar S., Reaven E. Scavenger receptor class BI and selective cholesteryl ester uptake: partners in the regulation of steroidogenesis. Mol. Cell Endocrinol. 2002;195:1–26. doi: 10.1016/s0303-7207(02)00222-8. [DOI] [PubMed] [Google Scholar]
- 11.Capponi A.M. The control by angiotensin II of cholesterol supply for aldosterone biosynthesis. Mol. Cell Endocrinol. 2004;217:113–118. doi: 10.1016/j.mce.2003.10.055. [DOI] [PubMed] [Google Scholar]
- 12.Arakane F., King S.R., Du Y., Kallen C.B., Walsh L.P., Watari H., Strauss J.F., 3rd. Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J. Biol. Chem. 1997;272:32656–32662. doi: 10.1074/jbc.272.51.32656. [DOI] [PubMed] [Google Scholar]
- 13.Cherradi N., Brandenburger Y., Capponi A.M. Mitochondrial regulation of mineralocorticoid biosynthesis by calcium and the StAR protein. Eur. J. Endocrinol. 1998;139:249–256. doi: 10.1530/eje.0.1390249. [DOI] [PubMed] [Google Scholar]
- 14.Fleury A., Mathieu A.P., Ducharme L., Hales D.B., LeHoux J.G. Phosphorylation and function of the hamster adrenal steroidogenic acute regulatory protein (StAR) J. Steroid Biochem. Mol. Biol. 2004;91:259–271. doi: 10.1016/j.jsbmb.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Müller J. Regulation of aldosterone biosynthesis: the end of the road? Clin. Exp. Pharmacol. Physiol. Suppl. 1998;25:S79–S85. doi: 10.1111/j.1440-1681.1998.tb02306.x. [DOI] [PubMed] [Google Scholar]
- 16.Bassett M.H., White P.C., Rainey W.E. The regulation of aldosterone synthase expression. Mol. Cell Endocrinol. 2004;217:67–74. doi: 10.1016/j.mce.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Parmar J., Key R.E., Rainey W.E. Development of an adrenocorticotropin-responsive human adrenocortical carcinoma cell line. J. Clin. Endocrinol. Metab. 2008;93:4542–4546. doi: 10.1210/jc.2008-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T., Rainey W.E. Human adrenocortical carcinoma cell lines. Mol. Cell Endocrinol. 2012;351:58–65. doi: 10.1016/j.mce.2011.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bollag W.B. Regulation of aldosterone synthesis and secretion. Compr. Physiol. 2014;4:1017–1055. doi: 10.1002/cphy.c130037. [DOI] [PubMed] [Google Scholar]
- 20.Nanba K., Blinder A.R., Rainey W.E. Primary cultures and cell lines for in vitro modeling of the human adrenal cortex. Tohoku J. Exp. Med. 2021;253:217–232. doi: 10.1620/tjem.253.217. [DOI] [PubMed] [Google Scholar]
- 21.Rainey W.E., Saner K., Schimmer B.P. Adrenocortical cell lines. Mol. Cell Endocrinol. 2004;228:23–38. doi: 10.1016/j.mce.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Newton A.C., Bootman M.D., Scott J.D. Second messengers. Cold Spring Harb. Perspect. Biol. 2016;8 doi: 10.1101/cshperspect.a005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enyeart J.J., Enyeart J.A. Human adrenal glomerulosa cells express K2P and GIRK potassium channels that are inhibited by ANG II and ACTH. Am. J. Physiol. Cell Physiol. 2021;321:C158–C175. doi: 10.1152/ajpcell.00118.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies L.A., Hu C., Guagliardo N.A., Sen N., Chen X., Talley E.M., Carey R.M., Bayliss D.A., Barrett P.Q. TASK channel deletion in mice causes primary hyperaldosteronism. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2203. doi: 10.1073/pnas.0712000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guagliardo N.A., Yao J., Stipes E.J., Cechova S., Le T.H., Bayliss D.A., Breault D.T., Barrett P.Q. Adrenal tissue-specific deletion of TASK channels causes aldosterone-driven angiotensin IIindependent hypertension. Hypertension. 2019;73:407–414. doi: 10.1161/HYPERTENSIONAHA.118.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czirják G., Enyedi P. TASK-3 dominates the background potassium conductance in rat adrenal glomerulosa cells. Mol. Endocrinol. 2002;16:621–629. doi: 10.1210/mend.16.3.0788. [DOI] [PubMed] [Google Scholar]
- 27.Czirják G., Fischer T., Spät A., Lesage F., Enyedi P. TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol. Endocrinol. 2000;14:863–874. doi: 10.1210/mend.14.6.0466. [DOI] [PubMed] [Google Scholar]
- 28.Lotshaw D.P. Effects of K+ channel blockers on K+ channels, membrane potential, and aldosterone secretion in rat adrenal zona glomerulosa cells. Endocrinology. 1997;138:4167–4175. doi: 10.1210/endo.138.10.5463. [DOI] [PubMed] [Google Scholar]
- 29.Lotshaw D.P. Role of membrane depolarization and T-type Ca2+ channels in angiotensin II and K+ stimulated aldosterone secretion. Mol. Cell Endocrinol. 2001;175:157–171. doi: 10.1016/s0303-7207(01)00384-7. [DOI] [PubMed] [Google Scholar]
- 30.Chen X.L., Bayliss D.A., Fern R.J., Barrett P.Q. A role for T-type Ca2+ channels in the synergistic control of aldosterone production by ANG II and K+ Am. J. Physiol. 1992;276:F674–F683. doi: 10.1152/ajprenal.1999.276.5.F674. [DOI] [PubMed] [Google Scholar]
- 31.Yang T., He M., Zhang H., Barrett P.Q., Hu C. L- and T-type calcium channels control aldosterone production from human adrenals. J. Endocrinol. 2020;244:237–247. doi: 10.1530/JOE-19-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen C.J., McCarthy R.T., Barrett P.Q., Rasmussen H. Ca channels in adrenal glomerulosa cells: K+ and angiotensin II increase T-type Ca channel current. Proc. Natl. Acad. Sci. U. S. A. 1988;85:2412. doi: 10.1073/pnas.85.7.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nogueira E.F., Gerry D., Mantero F., Mariniello B., Rainey W.E. The role of TASK1 in aldosterone production and its expression in normal adrenal and aldosterone-producing adenomas. Clin. Endocrinol. (Oxf) 2010;73:22–29. doi: 10.1111/j.1365-2265.2009.03738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uebele V.N., Nuss C.E., Renger J.J., Connolly T.M. Role of voltage-gated calcium channels in potassium-stimulated aldosterone secretion from rat adrenal zona glomerulosa cells. J. Steroid Biochem. Mol. Biol. 2004;92:209–218. doi: 10.1016/j.jsbmb.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Betancourt-Calle S., Jung E.M., White S., Ray S., Zheng X., Calle R.A., Bollag W.B. Elevated K(+) induces myristoylated alanine-rich C-kinase substrate phosphorylation and phospholipase D activation in glomerulosa cells. Mol. Cell Endocrinol. 2001;184:65–76. doi: 10.1016/s0303-7207(01)00642-6. [DOI] [PubMed] [Google Scholar]
- 36.T Tanabe A., Naruse M., Arai K., Naruse K., Yoshimoto T., Seki T., Imaki T., Kobayashi M., Miyazaki H., Demura H. Angiotensin II stimulates both aldosterone secretion and DNA synthesis via type 1 but not type 2 receptors in bovine adrenocortical cells. J. Endocrinol. Invest. 1998;21:668–672. doi: 10.1007/BF03350796. [DOI] [PubMed] [Google Scholar]
- 37.Wilke B.U., Lindner M., Greifenberg L., Albus A., Kronimus Y., Bünemann M., Leitner M.G., Oliver D. Diacylglycerol mediates regulation of TASK potassium channels by Gq-coupled receptors. Nat. Commun. 2014;5:5540. doi: 10.1038/ncomms6540. [DOI] [PubMed] [Google Scholar]
- 38.Oki K., Plonczynski M.W., Lam M.L., Gomez-Sanchez E.P., Gomez-Sanchez C.E. The potassium channel, Kir3.4 participates in angiotensin II-stimulated aldosterone production by a human adrenocortical cell line. Endocrinology. 2012;153:4328–4335. doi: 10.1210/en.2012-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czirják G., Petheo G.L., Spät A., Enyedi P. Inhibition of TASK-1 potassium channel by phospholipase C. Am. J. Physiol. Cell Physiol. 2001;281:C700–C708. doi: 10.1152/ajpcell.2001.281.2.C700. [DOI] [PubMed] [Google Scholar]
- 40.Enyeart J.A., Danthi S.J., Enyeart J.J. TREK-1 K+ channels couple angiotensin II receptors to membrane depolarization and aldosterone secretion in bovine adrenal glomerulosa cells. Am. J. Physiol. Endocrinol. Metab. 2004;287:E1154–E1165. doi: 10.1152/ajpendo.00223.2004. [DOI] [PubMed] [Google Scholar]
- 41.McCarthy R.T., Isales C., Rasmussen H. T-type calcium channels in adrenal glomerulosa cells: GTP-dependent modulation by angiotensin II. Proc. Natl. Acad. Sci. U. S. A. 1993;90:3260–3264. doi: 10.1073/pnas.90.8.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enyedi P., Szabadkai G., Horváth A., Szilágyi L., Gráf L., Spät A. Inositol 1,4,5-trisphosphate receptor subtypes in adrenal glomerulosa cells. Endocrinology. 1994;134:2354–2359. doi: 10.1210/endo.134.6.8194461. [DOI] [PubMed] [Google Scholar]
- 43.Kojima I., Kojima K., Kreutter D., Rasmussen H. The temporal integration of the aldosterone secretory response to angiotensin occurs via two intracellular pathways. J. Biol. Chem. 1984;259:14448–14457. [PubMed] [Google Scholar]
- 44.Pezzi V., Clark B.J., Ando S., Stocco D.M., Rainey W.E. Role of calmodulin-dependent protein kinase II in the acute stimulation of aldosterone production. J. Steroid Biochem. Mol. Biol. 1996;58:417–424. doi: 10.1016/0960-0760(96)00052-0. [DOI] [PubMed] [Google Scholar]
- 45.Condon J.C., Pezzi V., Drummond B.M., Yin S., Rainey W.E. Calmodulin-dependent kinase I regulates adrenal cell expression of aldosterone synthase. Endocrinology. 2002;143:3651–3657. doi: 10.1210/en.2001-211359. [DOI] [PubMed] [Google Scholar]
- 46.Nanba K., Chen A., Nishimoto K., Rainey W.E. Role of Ca(2+)/calmodulin-dependent protein kinase kinase in adrenal aldosterone production. Endocrinology. 2015;156:1750–1756. doi: 10.1210/en.2014-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shapiro B.A., Olala L., Arun S.N., Parker P.M., George M.V., Bollag W.B. Angiotensin II-activated protein kinase D mediates acute aldosterone secretion. Mol. Cell Endocrinol. 2010;317:99–105. doi: 10.1016/j.mce.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai Y.Y., Rainey W.E., Johnson M.H., Bollag W.B. VLDL-activated cell signaling pathways that stimulate adrenal cell aldosterone production. Mol. Cell Endocrinol. 2016;433:138–146. doi: 10.1016/j.mce.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olala L.O., Shapiro B.A., Merchen T.C., Wynn J.J., Bollag W.B. Protein kinase C and Src family kinases mediate angiotensin II-induced protein kinase D activation and acute aldosterone production. Mol. Cell Endocrinol. 2014;392:173–181. doi: 10.1016/j.mce.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nadler J.L., Natarajan R., Stern N. Specific action of the lipoxygenase pathway in mediating angiotensin II-induced aldosterone synthesis in isolated adrenal glomerulosa cells. J. Clin. Invest. 1987;80:1763–1769. doi: 10.1172/JCI113269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Natarajan R., Dunn W.D., Stern N., Nadler J. Key role of diacylglycerol-mediated 12-lipoxygenase product formation in angiotensin II-induced aldosterone synthesis. Mol. Cell Endocrinol. 1990;72:73–80. doi: 10.1016/0303-7207(90)90096-q. [DOI] [PubMed] [Google Scholar]
- 52.Natarajan R., Stern N., Hsueh W., Do Y., Nadler J. Role of the lipoxygenase pathway in angiotensin II-mediated aldosterone biosynthesis in human adrenal glomerulosa cells. J. Clin. Endocrinol. Metab. 1988;67:584–591. doi: 10.1210/jcem-67-3-584. [DOI] [PubMed] [Google Scholar]
- 53.Natarajan R., Stern N., Nadler J. Diacylglycerol provides arachidonic acid for lipoxygenase products that mediate angiotensin II-induced aldosterone synthesis. Biochem. Biophys. Res. Commun. 1988;56:717–724. doi: 10.1016/s0006-291x(88)80902-1. [DOI] [PubMed] [Google Scholar]
- 54.Stern N., Yanagawa N., Saito F., Hori M., Natarajan R., Nadler J., Tuck M. Potential role of 12 hydroxyeicosatetraenoic acid in angiotensin II-induced calcium signal in rat glomerulosa cells. Endocrinology. 1993;133:843–847. doi: 10.1210/endo.133.2.8344221. [DOI] [PubMed] [Google Scholar]
- 55.Sanborn B.B., Schneider A.S. Muscarininc receptor-mediated inositol tetrakisphosphate response in bovine adrenal chromaffin cells. Life Sci. 1990;47:1447–1452. doi: 10.1016/0024-3205(90)90523-t. [DOI] [PubMed] [Google Scholar]
- 56.Farese R.V., Sabir M.A., Larson R.E. Potassium and angiotensin II increase the concentrations of phosphatidic acid, phosphatidylinositol, and polyphosphoinositides in rat adrenal capsules in vitro. J. Clin. Invest. 1980;66:1428–1431. doi: 10.1172/JCI109997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hunyady L., Baukal A.J., Bor M., Ely J.A., Catt K.J. Regulation of 1,2-diacylglycerol production by angiotensin-II in bovine adrenal glomerulosa cells. Endocrinology. 1990;126:1001–1008. doi: 10.1210/endo-126-2-1001. [DOI] [PubMed] [Google Scholar]
- 58.Bollag W.B., Barrett P.Q., Isales C.M., Rasmussen H. Angiotensin-II-induced changes in diacylglycerol levels and their potential role in modulating the steroidogenic response. Endocrinology. 1991;128:231–241. doi: 10.1210/endo-128-1-231. [DOI] [PubMed] [Google Scholar]
- 59.Isales C.M., Bollag W.B., Kiernan L.C., Barrett P.Q. Effect of ANP on sustained aldosterone secretion stimulated by angiotensin II. Am. J. Physiol. 1989;256:C89–C95. doi: 10.1152/ajpcell.1989.256.1.C89. [DOI] [PubMed] [Google Scholar]
- 60.Xing Y., Rainey W.E., Apolzan J.W., Francone O.L., Harris R.B., Bollag W.B. Adrenal cell aldosterone production is stimulated by very-low-density lipoprotein (VLDL) Endocrinology. 2012;153:721–731. doi: 10.1210/en.2011-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazzocchi G., Aragona F., Malendowicz L.K., Nussdorfer G.G. PTH and PTH-related peptide enhance steroid secretion from human adrenocortical cells. Am. J. Physiol. Endocrinol. Metab. 2001;280:E209–E213. doi: 10.1152/ajpendo.2001.280.2.E209. [DOI] [PubMed] [Google Scholar]
- 62.Nussdorfer G.G., Malendowicz L.K. Role of VIP, PACAP, and related peptides in the regulation of the hypothalamo-pituitary-adrenal axis. Peptides. 1998;19:1443–1467. doi: 10.1016/s0196-9781(98)00102-8. [DOI] [PubMed] [Google Scholar]
- 63.Isales C.M., Barrett P.Q., Brines M., Bollag W., Rasmussen H. Parathyroid hormone modulates angiotensin II-induced aldosterone secretion from the adrenal glomerulosa cell. Endocrinology. 1991;129:489–495. doi: 10.1210/endo-129-1-489. [DOI] [PubMed] [Google Scholar]
- 64.Brizuela L., Rabano M., Pena A., Gangoiti P., Macarulla J.M., Trueba M., Gomez-Munoz A. Sphingosine 1-phosphate: a novel stimulator of aldosterone secretion. J. Lipid Res. 2006;47:1238–1249. doi: 10.1194/jlr.M500510-JLR200. [DOI] [PubMed] [Google Scholar]
- 65.Lucki N.C., Li D., Sewer M.B. Sphingosine-1-phosphate rapidly increases cortisol biosynthesis and the expression of genes involved in cholesterol uptake and transport in H295R adrenocortical cells. Mol. Cell Endocrinol. 2012;348:165–175. doi: 10.1016/j.mce.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bollag W.B., Barrett P.Q., Isales C.M., Liscovitch M., Rasmussen H. A potential role for phospholipase-D in the angiotensin-II-induced stimulation of aldosterone secretion from bovine adrenal glomerulosa cells. Endocrinology. 1990;127:1436–1443. doi: 10.1210/endo-127-3-1436. [DOI] [PubMed] [Google Scholar]
- 67.Bollag W.B., Jung E., Calle R.A. Mechanism of angiotensin II-induced phospholipase D activation in bovine adrenal glomerulosa cells. Mol. Cell Endocrinol. 2002;192:7–16. doi: 10.1016/s0303-7207(02)00134-x. [DOI] [PubMed] [Google Scholar]
- 68.Olala L.O., Seremwe M., Tsai Y.Y., Bollag W.B. A role for phospholipase D in angiotensin II-induced protein kinase D activation in adrenal glomerulosa cell models. Mol. Cell Endocrinol. 2013;366:31–37. doi: 10.1016/j.mce.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsai Y.Y., Rainey W.E., Pan Z.Q., Frohman M.A., Choudhary V., Bollag W.B. Phospholipase D activity underlies very-low-density lipoprotein (VLDL)-induced aldosterone production in adrenal glomerulosa cells. Endocrinology. 2014;155:3550–3560. doi: 10.1210/en.2014-1159. [DOI] [PubMed] [Google Scholar]
- 70.Bollag W.B., Kent P., White S., Malinova M., Isales C.M., Calle R.A. Characterization and phospholipase D mediation of the angiotensin II priming response in adrenal glomerulosa cells. Endocrinology. 2007;148:585–593. doi: 10.1210/en.2006-0898. [DOI] [PubMed] [Google Scholar]
- 71.Qin H., Frohman M.A., Bollag W.B. Phospholipase D2 mediates acute aldosterone secretion in response to angiotensin II in adrenal glomerulosa cells. Endocrinology. 2010;151:2162–2170. doi: 10.1210/en.2009-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rábano M., Peña A., Brizuela L., Macarulla J.M.a., Gómez-Muñoz A., Trueba M. Angiotensin II-stimulated cortisol secretion is mediated by phospholipase D. Mol. Cell Endocrinol. 2004;222:9–20. doi: 10.1016/j.mce.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 73.Gomez-Muñoz A., Hamza E.H., Brindley D.N. Effects of sphingosine, albumin and unsaturated fatty acids on the activation and translocation of phosphatidate phosphohydrolases in rat hepatocytes. Biochim. Biophys. Acta. 1992;1127:49–56. doi: 10.1016/0005-2760(92)90200-f. [DOI] [PubMed] [Google Scholar]
- 74.Jamal Z., Martin A., Gomez-Muñoz A., Brindley D.N. Plasma membrane fractions from rat liver contain a phosphatidate phosphohydrolase distinct from that in the endoplasmic reticulum and cytosol. J. Biol. Chem. 1991;266:2988–2996. [PubMed] [Google Scholar]
- 75.Huang K.P. The mechanism of protein kinase C activation. Trends Neurosci. 1989;12:425–432. doi: 10.1016/0166-2236(89)90091-x. [DOI] [PubMed] [Google Scholar]
- 76.Zugaza J.L., Sinnett-Smith J., Van Lint J., Rozengurt E. Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. EMBO J. 1996;15:6220–6230. [PMC free article] [PubMed] [Google Scholar]
- 77.Romero D.G., Welsh B.L., Gomez-Sanchez E.P., Yanes L.L., Rilli S., Gomez-Sanchez C.E. Angiotensin II-mediated protein kinase D activation stimulates aldosterone and cortisol secretion in H295R human adrenocortical cells. Endocrinology. 2006;147:6046–6055. doi: 10.1210/en.2006-0794. [DOI] [PubMed] [Google Scholar]
- 78.Olala L.O., Choudhary V., Johnson M.H., Bollag W.B. Angiotensin II-induced protein kinase D activates the ATF/CREB family of transcription factors and promotes StAR mRNA expression. Endocrinology. 2014;155:2524–2533. doi: 10.1210/en.2013-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li D., Urs A.N., Allegood J., Leon A., Merrill A.H., Jr., Sewer M.B. Cyclic AMP-stimulated interaction between steroidogenic factor 1 and diacylglycerol kinase theta facilitates induction of CYP17. Mol. Cell Biol. 2007;27:6669–6685. doi: 10.1128/MCB.00355-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cai K., Sewer M.B. cAMP-stimulated transcription of DGKθ requires steroidogenic factor 1 and sterol regulatory element binding protein 1. J. Lipid Res. 2013;54:2121–2132. doi: 10.1194/jlr.M035634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cai K., Lucki N.C., Sewer M.B. Silencing diacylglycerol kinase-theta expression reduces steroid hormone biosynthesis and cholesterol metabolism in human adrenocortical cells. Biochim. Biophys. Acta. 2014;1841:552–562. doi: 10.1016/j.bbalip.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ye P., Nakamura Y., Lalli E., Rainey W.E. Differential effects of high and low steroidogenic factor-1 expression on CYP11B2 expression and aldosterone production in adrenocortical cells. Endocrinology. 2009;150:1303–1309. doi: 10.1210/en.2008-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clark B.J., Soo S.C., Caron K.M., Ikeda Y., Parker K.L., Stocco D.M. Hormonal and developmental regulation of the steroidogenic acute regulatory protein. Mol. Endocrinol. 1995;9:1346–1355. doi: 10.1210/mend.9.10.8544843. [DOI] [PubMed] [Google Scholar]
- 84.Caron K.M., Ikeda Y., Soo S.C., Stocco D.M., Parker K.L., Clark B.J. Characterization of the promoter region of the mouse gene encoding the steroidogenic acute regulatory protein. Mol. Endocrinol. 1997;11:138–147. doi: 10.1210/mend.11.2.9880. [DOI] [PubMed] [Google Scholar]
- 85.Bassett M.H., Zhang Y., Clyne C., White P.C., Rainey W.E. Differential regulation of aldosterone synthase and 11beta-hydroxylase transcription by steroidogenic factor-1. J. Mol. Endocrinol. 2002;28:125–135. doi: 10.1677/jme.0.0280125. [DOI] [PubMed] [Google Scholar]
- 86.Kishikawa K., Chalfant C.E., Perry D.K., Bielawska A., Hannun Y.A. Phosphatidic acid is a potent and selective inhibitor of protein phosphatase 1 and an inhibitor of ceramide-mediated responses. J. Biol. Chem. 1999;274:21335–21341. doi: 10.1074/jbc.274.30.21335. [DOI] [PubMed] [Google Scholar]
- 87.Sayed S.B., Whitehouse B.J., Jones P.M. Phosphoserine/threonine phosphatases in the rat adrenal cortex: a role in the control of steroidogenesis? J. Endocrinol. 1997;154:449–458. doi: 10.1677/joe.0.1540449. [DOI] [PubMed] [Google Scholar]
- 88.Su H., Gu Y., Li F., Wang Q., Huang B., Jin X., Ning G., Sun F. The PI3K/AKT/mTOR signaling pathway is overactivated in primary aldosteronism. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shah B.H., Baukal A.J., Shah F.B., Catt K.J. Mechanisms of extracellularly regulated kinases 1/2 activation in adrenal glomerulosa cells by lysophosphatidic acid and epidermal growth factor. Mol. Endocrinol. 2005;19:2535–2548. doi: 10.1210/me.2005-0082. [DOI] [PubMed] [Google Scholar]
- 90.Tian Y., Balla T., Baukal A.J., Catt K.J. Growth responses to angiotensin II in bovine adrenal glomerulosa cells. Am. J. Physiol. 1995;268:E135–144. doi: 10.1152/ajpendo.1995.268.1.E135. [DOI] [PubMed] [Google Scholar]
- 91.Burns D.L. Subunit structure and enzymic activity of pertussis toxin. Microbiol. Sci. 1988;5:285–287. [PubMed] [Google Scholar]
- 92.Ambroz C., Catt K.J. Angiotensin II receptor-mediated calcium influx in bovine adrenal glomerulosa cells. Endocrinology. 1992;131:408–414. doi: 10.1210/endo.131.1.1377126. [DOI] [PubMed] [Google Scholar]
- 93.Sirianni R., Sirianni R., Carr B.R., Pezzi V., Rainey W.E. A role for src tyrosine kinase in regulating adrenal aldosterone production. J. Mol. Endocrinol. 2001;26:207–215. doi: 10.1677/jme.0.0260207. [DOI] [PubMed] [Google Scholar]
- 94.Hajnóczky G., Várnai P., Buday L., Faragó A., Spät A. The role of protein kinase-C in control of aldosterone production by rat adrenal glomerulosa cells: activation of protein kinase-C by stimulation with potassium. Endocrinology. 1992;130:2230–2236. doi: 10.1210/endo.130.4.1547736. [DOI] [PubMed] [Google Scholar]
- 95.Goodfriend T.L., Lee W.M., Ball D.L., Elliott M.E. Specificity and mechanism of fatty acid inhibition of aldosterone secretion. Prostaglandins Leukot. Essent. Fatty Acids. 1995;52:145–149. doi: 10.1016/0952-3278(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 96.Kojima I., Kojima K., Rasmussen H. Possible role of phospholipase A2 action and arachidonic acid metabolism in angiotensin II–mediated aldosterone secretion. Endocrinology. 1985;117:1057–1066. doi: 10.1210/endo-117-3-1057. [DOI] [PubMed] [Google Scholar]
- 97.Andreis P.G., Buttazzi P., Tortorella C., De Caro R., Aragona F., Neri G., Nussdorfer G.G. The inhibitor of phospholipase-A2, AACOCF3, stimulates steroid secretion by dispersed human and rat adrenocortical cells. Life Sci. 1999;64:1287–1294. doi: 10.1016/s0024-3205(99)00063-6. [DOI] [PubMed] [Google Scholar]
- 98.Campbell W.B., Brady M.T., Rosolowsky L.J., Falck J.R. Metabolism of arachidonic acid by rat adrenal glomerulosa cells: synthesis of hydroxyeicosatetraenoic acids and epoxyeicosatrienoic acids. Endocrinology. 1991;128:2183–2194. doi: 10.1210/endo-128-4-2183. [DOI] [PubMed] [Google Scholar]
- 99.Matsuoka H., Tan S.Y., Mulrow P.J. Effects of prostaglandins on adrenal steroidogenesis in the rat. Prostaglandins. 1980;19:291–298. doi: 10.1016/0090-6980(80)90027-1. [DOI] [PubMed] [Google Scholar]
- 100.Miller R.T., Douglas J.G., Dunn M.J. Dissociation of aldosterone and prostaglandin biosynthesis in the rat adrenal glomerulosa. Prostaglandins. 1980;20:449–462. doi: 10.1016/0090-6980(80)90032-5. [DOI] [PubMed] [Google Scholar]
- 101.Enyedi P., Spät A., Antoni F.A. Role of prostaglandins in the control of the function of adrenal glomerulosa cells. J. Endocrinol. 1981;91:427–437. doi: 10.1677/joe.0.0910427. [DOI] [PubMed] [Google Scholar]
- 102.Swartz S.L., Williams G.H. Role of prostaglandins in adrenal steroidogenesis. Endocrinology. 1983;113:992–996. doi: 10.1210/endo-113-3-992. [DOI] [PubMed] [Google Scholar]
- 103.Gu J., Wen Y., Mison A., Nadler J.L. 12-lipoxygenase pathway increases aldosterone production, 3',5'-cyclic adenosine monophosphate response element-binding protein phosphorylation, and p38 mitogen-activated protein kinase activation in H295R human adrenocortical cells. Endocrinology. 2003;144:534–543. doi: 10.1210/en.2002-220580. [DOI] [PubMed] [Google Scholar]
- 104.Mele P.G., Duarte A., Paz C., Capponi A., Podestá E.J. Role of intramitochondrial arachidonic acid and acyl-CoA synthetase 4 in angiotensin II-regulated aldosterone synthesis in NCI-H295R adrenocortical cell line. Endocrinology. 2012;153:3284–3294. doi: 10.1210/en.2011-2108. [DOI] [PubMed] [Google Scholar]
- 105.Ozbay T., Merrill A.H., Sewer M.B. ACTH regulates steroidogenic gene expression and cortisol biosynthesis in the human adrenal cortex via sphingolipid metabolism. Endocr. Res. 2004;30:787–794. doi: 10.1081/erc-200044040. [DOI] [PubMed] [Google Scholar]
- 106.Gomez-Muñoz A., Martin A., O'Brien L., Brindley D.N. Cell-permeable ceramides inhibit the stimulation of DNA synthesis and phospholipase D activity by phosphatidate and lysophosphatidate in rat fibroblasts. J. Biol. Chem. 1994;269:8937–8943. [PubMed] [Google Scholar]
- 107.Gómez-Muñoz A., Waggoner D.W., O'Brien L., Brindley D.N. Interaction of ceramides, sphingosine, and sphingosine 1-phosphate in regulating DNA synthesis and phospholipase D activity. J. Biol. Chem. 1995;270:26318–26325. doi: 10.1074/jbc.270.44.26318. [DOI] [PubMed] [Google Scholar]
- 108.Kwun C., Patel A., Pletcher S., Lyons B., Abdelrahim M., Nicholson D., Morris E., Salata K., Francis G.L. Ceramide increases steroid hormone production in MA-10 leydig cells. Steroids. 1999;64:499–509. doi: 10.1016/s0039-128x(99)00013-6. [DOI] [PubMed] [Google Scholar]
- 109.Budnik L.T., Jähner D., Mukhopadhyay A.K. Inhibitory effects of TNF alpha on mouse tumor Leydig cells: possible role of ceramide in the mechanism of action. Mol. Cell Endocrinol. 1999;150:39–46. doi: 10.1016/s0303-7207(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 110.Morales V., Santana P., Díaz R., Tabraue C., Gallardo G., López Blanco F., Hernández I., Fanjul L.F., Ruiz de Galarreta C.M. Intratesticular delivery of tumor necrosis factor-alpha and ceramide directly abrogates steroidogenic acute regulatory protein expression and leydig cell steroidogenesis in adult rats. Endocrinology. 2003;144:4763–4772. doi: 10.1210/en.2003-0569. [DOI] [PubMed] [Google Scholar]
- 111.Pettus B.J., Bielawska A., Spiegel S., Roddy P., Hannun Y.A., Chalfant C.E. Ceramide kinase mediates cytokine- and calcium ionophore-induced arachidonic acid release. J. Biol. Chem. 2003;278:38206–38213. doi: 10.1074/jbc.M304816200. [DOI] [PubMed] [Google Scholar]
- 112.Urs A.N., Dammer E., Sewer M.B. Sphingosine regulates the transcription of CYP17 by binding to steroidogenic factor-1. Endocrinology. 2006;147:5249–5258. doi: 10.1210/en.2006-0355. [DOI] [PubMed] [Google Scholar]
- 113.Lucki N.C., Bandyopadhyay S., Wang E., Merrill A.H., Sewer M.B. Acid ceramidase (ASAH1) is a global regulator of steroidogenic capacity and adrenocortical gene expression. Mol. Endocrinol. 2012;26:228–243. doi: 10.1210/me.2011-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Strub G.M., Maceyka M., Hait N.C., Milstien S., Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv. Exp. Med. Biol. 2010;688:141–155. doi: 10.1007/978-1-4419-6741-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Green C.D., Maceyka M., Cowart L.A., Spiegel S. Sphingolipids in metabolic disease: the good, the bad, and the unknown. Cell Metab. 2021;33:1293–1306. doi: 10.1016/j.cmet.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brizuela L., Rábano M., Gangoiti P., Narbona N., Macarulla J.M., Trueba M., Gómez-Muñoz A. Sphingosine 1-phosphate stimulates aldosterone secretion through a mechanism involving the PI3-kinase/PKB and MEK/ERK 1/2 pathways. J. Lipid Res. 2007;48:2264–2274. doi: 10.1194/jlr.M700291-JLR200. [DOI] [PubMed] [Google Scholar]
- 117.Ozbay T., Rowan A., Leon A., Patel P., Sewer M.B. Cyclic adenosine 5'-monophosphate-dependent sphingosine-1-phosphate biosynthesis induces human CYP17 gene transcription by activating cleavage of sterol regulatory element binding protein 1. Endocrinology. 2006;147:1427–1437. doi: 10.1210/en.2005-1091. [DOI] [PubMed] [Google Scholar]
- 118.Janecke A.R., Xu R., Steichen-Gersdorf E., Waldegger S., Entenmann A., Giner T., Krainer I., Huber L.A., Hess M.W., Frishberg Y., Barash H., Tzur S., Schreyer-Shafir N., Sukenik-Halevy R., Zehavi T., et al. Deficiency of the sphingosine-1-phosphate lyase SGPL1 is associated with congenital nephrotic syndrome and congenital adrenal calcifications. Hum. Mutat. 2017;38:365–372. doi: 10.1002/humu.23192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prasad R., Hadjidemetriou I., Maharaj A., Meimaridou E., Buonocore F., Saleem M., Hurcombe J., Bierzynska A., Barbagelata E., Bergadá I., Cassinelli H., Das U., Krone R., Hacihamdioglu B., Sari E., et al. Sphingosine-1-phosphate lyase mutations cause primary adrenal insufficiency and steroid-resistant nephrotic syndrome. J. Clin. Invest. 2017;127:942–953. doi: 10.1172/JCI90171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maharaj A., Theodorou D., Banerjee I., Metherell L.A., Prasad R., Wallace D. A sphingosine-1-phosphate lyase mutation associated with congenital nephrotic syndrome and multiple endocrinopathy. Front. Pediatr. 2020;8:151. doi: 10.3389/fped.2020.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]