Abstract
Several endogenous cytokines, including granulocyte-macrophage colony-stimulating factor (GM-CSF), are necessary for eliminating Histoplasma capsulatum from tissues. In this study, we explored the efficacy of recombinant murine GM-CSF in the treatment of pulmonary histoplasmosis. This cytokine significantly reduced fungal burden in a dose-dependent manner. Pretreatment did not consistently produce a better result than treatment started after infection. The biological effectiveness of GM-CSF was not associated with modulation of lung cytokine production or alteration in lung inflammation, but it directly activated a nonadherent lung cell population to exert anti-Histoplasma activity. GM-CSF improved survival of T-cell-depleted mice exposed to H. capsulatum. When combined with a suboptimal amount of amphotericin B, GM-CSF enhanced survival of normal or T-cell-depleted mice given a lethal challenge. These results suggest that this cytokine may be useful as an adjunctive treatment for histoplasmosis.
The dimorphic pathogenic fungus Histoplasma capsulatum causes a wide spectrum of illness that ranges from a mild respiratory illness to a progressive infection that may involve multiple organ systems (12). The host's elimination of the pathogen is critically dependent on the interaction between T cells, in particular CD4+ cells, and professional phagocytes (4, 11, 18, 35). In addition to the cellular mediators of resistance, a major determinant of efficient clearance of the fungus is the production and release of cytokines by various cell types. Among the soluble factors that are key in controlling intracellular growth of H. capsulatum in mice are interleukin-12 (IL-12), gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and granulocyte-macrophage colony-stimulating factor (GM-CSF) (1, 3, 4, 13, 34). These cytokines are necessary for survival of naive mice upon exposure to the fungus, and the latter three contribute to clearance in secondary infection.
GM-CSF has many biological activities, including increased hematopoiesis, antimicrobial or tumoricidal activities by phagocytes, and expression of class II major histocompatibility complex (MHC) molecules. The recombinant form of this endogenous cytokine has been used therapeutically to elevate the number of circulating neutrophils and to enhance their bactericidal activity (16, 19, 23, 31). A previous publication indicated that human monocytes, but not macrophages, exposed in vitro to recombinant GM-CSF (rGM-CSF) inhibited the growth of H. capsulatum (29). Our laboratory has reported that in murine histoplasmosis, endogenous GM-CSF is required for resolution of primary infection, and blockade of endogenous GM-CSF leads to perturbations in release of IFN-γ, TNF-α, and nitric oxide (NO) (13). These two studies provide sufficient evidence that GM-CSF contributes to host resistance against H. capsulatum. In this study, we hypothesized that exogenous administration of recombinant murine GM-CSF (rmGM-CSF) might impact the course of histoplasmosis in immunocompetent and immunodeficient mice. Mice infected via the intranasal (i.n.) route with yeast cells were administered rmGM-CSF and assessed for their ability to control infection.
MATERIALS AND METHODS
Animals.
Male C57BL/6 mice, 5 weeks old, were purchased from the National Cancer Institute (Frederick, Md.). All animal experiments were done in accordance with the Animal Welfare Act guidelines of the National Institutes of Health.
Preparation of H. capsulatum and infection of mice.
H. capsulatum (strain G217B) yeast cells were prepared as described previously (1). Animals were infected i.n. with either 2.5 × 106 (sublethal challenge) or 1.25 × 107 (lethal challenge) yeast cells in a 30-μl volume.
Organ culture for H. capsulatum.
Recovery of H. capsulatum was performed as described previously (18). Fungal burden was expressed as mean CFU per whole organ ± standard error. The limit of detection was 102 CFU.
Administration of rmGM-CSF or amphotericin B to mice.
rmGM-CSF (specific activity, 7.42 × 107 U/kg) was kindly provided by Elaine Thomas, Immunex Corp. It was diluted in phosphate-buffered saline (pH 7.4) containing 1% bovine serum albumin. rmGM-CSF was injected intraperitoneally (i.p.) into mice on a daily basis. No toxicity was observed in normal mice given 50 μg/kg/day (3.71 × 106 U/kg/day) i.p. for 21 days. Amphotericin B sodium desoxycholate (Sigma Chemical Co., St. Louis, Mo.) was dissolved in 5% glucose in water. In some experiments, mice were treated with 0.125 mg/kg of body weight i.p. three times a week.
Depletion of T cells by treatment with MAbs.
Monoclonal antibodies (MAbs) were ascites fluid derived or generated via tissue culture. Rat anti-mouse CD8+ (clone 2.43; rat immunoglobulin 2b [IgG2b]) and anti-CD4+ (GK 1.5; rat IgG2b) MAbs were used to deplete CD8+ and CD4+ T cells. The concentration of MAb was assessed by enzyme-linked immunosorbent assay (ELISA) and calculated by linear regression from an IgG (Organon Teknika, Durham, N.C.) standard curve. All MAbs contained <5ng of endotoxin per ml as determined by Limulus amebocyte lysate test (BioWhittaker, Walkersville, Md.). To eliminate both CD4+ and CD8+ T cells, 300 and 100 μg were given i.p. concomitantly. The efficiency of depletion, as assessed by flow cytometry, was >96%. Injection of each dose of MAb was scheduled for days −7 and −3 and at the time of i.n. challenge. MAbs were given each week thereafter. Control animals received an equal amount of rat IgG.
Cytokine measurement.
Lungs from infected mice given diluent or rmGM-CSF were removed on day 7 of infection. Tissue was homogenized in 10 ml of RPMI 1640, centrifuged at 1,500 × g, filter sterilized, and stored at −70°C until assayed. The protein concentration of homogenates among the groups ranged from 3.4 mg/ml to 6.3 mg/ml. There were no significant differences (P > 0.05) in protein content among the groups. Commercially available ELISA kits were used to measure IFN-γ, IL-4, IL-10, and TNF-α (Endogen, Cambridge, Mass.). An ELISA for IL-18 was purchased from R&D Systems, Minneapolis, Minn.
Single-cell suspension from lungs.
To isolate leukocytes from lungs, mice were sacrificed and lungs were flushed with 10 ml of Hanks' balanced salt solution (HBSS) by inserting a catheter into the right heart. The lungs were excised and teased apart with forceps and homogenized by sequential passage through 16-, 18-, and 20-gauge needles. Leukocytes were isolated by separation on a 40 to 70% Percoll (Pharmacia, Piscataway, N.J.) gradient (9). This population is ≥95% CD45+ (common leukocyte antigen).
Flow cytometry analysis of lung cells.
Leukocytes were adjusted to 5 × 105/200 μl in HBSS containing 10% fetal bovine serum (FBS) and 0.02% sodium azide and stained with 0.5 μg of one of the following fluorescein isothiocyanate (FITC)-labeled MAbs (PharMingen, San Diego, Calif.): anti-CD4 (clone RM4-5), anti-CD8 (clone 53-6.7), anti-Ly-6G (Gr-1), clone RB6-8C5, which recognizes polymorphonuclear cells (PMN), anti-I-Ab (clone 25-9-17), Mac-3 (clone M3/84, detects tissue MΦ), or isotype-matched rat IgG MAb. The samples were washed and fixed in 2% paraformaldehyde until analyzed on a flow cytometer.
Assay of lung leukocyte fungistatic activity against H. capsulatum.
Intracellular growth of H. capsulatum in lung leukocytes was quantified by the incorporation of [3H]leucine into viable yeast cells (15). Leukocytes from pools of lungs (n = 8 to 12 mice per group) of rmGM-CSF-treated mice and controls were suspended in Dulbecco's modified Eagle's medium (DMEM) containing 10% heat-inactivated FBS and 10 μg of gentamicin per ml. One million cells were added in a volume of 100 μl to wells of a 96-well microtiter plate for 1 h at 37°C in 5% CO, and the nonadherent cells were removed by vigorous washing. The nonadherent cells from the wells were pooled and recounted, and 106 cells in 100 μl were added to each well of a microtiter plate. The adherent cells were >95% MΦ. Viable H. capsulatum yeast cells (5 × 103) suspended in 0.1 ml of DMEM containing 10% FBS and 10 μg of gentamicin per ml were added to each well. After incubation for 24 h at 37°C in 5% CO2, the plates were centrifuged at 1,000 × g, and the supernatants were aspirated through a 27-gauge needle. Fifty microliters of [3H]leucine in sterile water (1 μCi) and 5 μl of a 10× yeast nitrogen broth (Difco Laboratories, Detroit, Mich.) were added to each well, and plates were incubated for an additional 24 h at 37°C in 5% CO2. To each well were then added 50 μl of l-leucine (10 mg/ml) and 50 μl of sodium hypochlorite, and cell contents were harvested onto glass fiber filters by using an automated harvester (Skatron, Sterling, Va.). All experimental procedures were performed in triplicate or quadruplicate. Data are expressed as mean cpm ± standard errors.
NO assay.
Supernatants from nonadherent cells exposed to H. capsulatum were removed after 48 h and filtered, and nitrite was measured by Griess reaction with Cayman's nitrate/nitrite assay kit (Alexis Corp., San Diego, Calif.).
In some experiments, the NO synthase inhibitor N(G)-monomethyl-l-arginine (l-NMMA) (Calbiochem, San Diego, Calif.) was dissolved in methanol and added to cultures at a concentration of 1 mM.
Statistical analyses.
The log rank test was used to analyze differences in survival; Student's t test was employed to analyze differences in cytokine production and fungal burden of organs. If the data were not normally distributed, the Mann-Whitney test was used.
RESULTS
Treatment with rmGM-CSF reduces fungal burden.
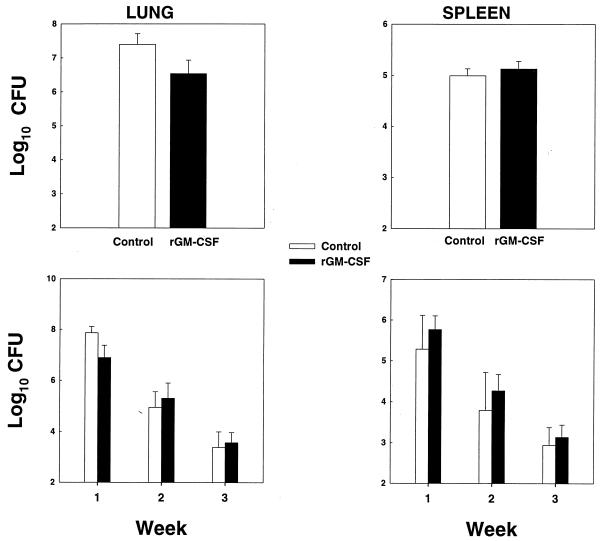
Mice were challenged i.n. with 2.5 × 106 H. capsulatum yeast cells and treated with 50 μg of rmGM-CSF/kg/day i.p., beginning 8 h after infection. The dosage was selected based on previous studies in the literature (6, 24). In the first experiment, the mean log10 CFU in lungs of rmGM-CSF-treated mice (6.44 ± 0.16 CFU) was a significantly lower burden (P < 0.01) than that in lungs from infected controls (7.40 ± 0.13 CFU). There was no difference in CFU in spleens between groups (Fig. 1, top panels).
FIG. 1.
Effect of rmGM-CSF on fungal burden of mice infected with H. capsulatum. Mice (n = 6) were infected i.n. with 2.5 × 106 yeast cells and treated with 50 μg of rmGM-CSF/kg/day. Organ burden was determined for week 1 postinfection (top two panels) and on weeks 1, 2, and 3 postinfection (bottom two panels). Data are expressed as means ± standard errors. One of two experiments is shown.
Subsequently, we determined if continued treatment with rmGM-CSF would modify fungal recovery beyond week 1. Mice were treated with 50 μg/kg/day 8 h after infection, and fungal burden was assessed each week for 3 weeks. rmGM-CSF reduced the number of CFU at week 1 in lungs only (7.87 ± 0.12 log10 CFU with diluent versus 6.89 ± 0.20 log10 CFU with rmGM-CSF) (P < 0.01), but not thereafter (Fig. 1, bottom panels).
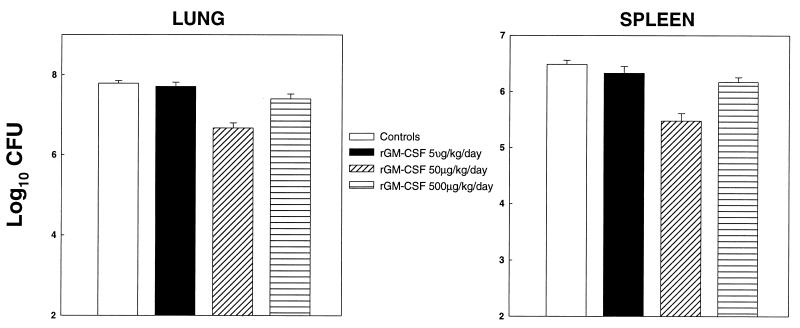
Dose response profile of rmGM-CSF.
Mice were treated with rmGM-CSF at 5, 50, or 500 μg/kg/day (3.71 × 105, 3.71 × 106, or 3.71 × 107 U/kg/day) beginning 8 h after infection. One week later, mice were sacrificed and tested for the number of yeast CFU (Fig. 2). Doses of 50 and 500 μg/kg/day significantly reduced CFU in lungs and spleens (P < 0.01) in mice compared to infected controls, and the effect of a 50-μg/kg/day dosage was greater (P < 0.01) than the 500-μg/kg/day dosage. The amounts of CFU in lungs and spleens of mice given 50 μg/kg/day also were significantly smaller (P < 0.01) than in organs of mice administered 5 or 500 μg/kg/day.
FIG. 2.
Dose response of rmGM-CSF. Mice (n = 6) infected i.n. were given 5, 50, or 500 μg of rmGM-CSF/kg/day beginning 8 h after infection. Results for week 1 postinfection are displayed. Data are expressed as means ± standard errors. One of two experiments is shown.
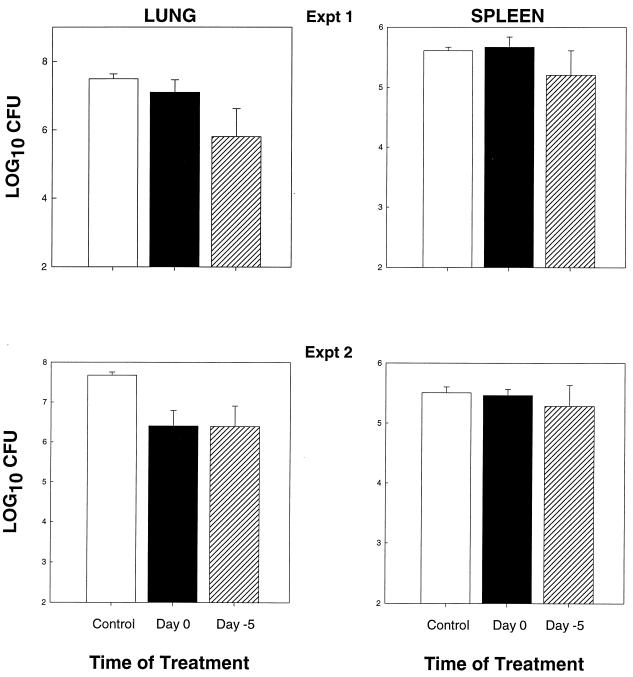
Does pretreatment with rmGM-CSF enhance the host's anti-Histoplasma activity?
Mice were treated with rmGM-CSF beginning 5 days before infection, or 8 h after infection, and the impact of cytokine treatment was assessed. In the first experiment, the lungs and spleens of mice given rmGM-CSF on day −5 contained significantly less CFU (P < 0.01) in lungs, but not spleens, of infected controls and those animals in which rmGM-CSF was started on day 0 (Fig. 3, experiment 1). Furthermore, fewer CFU were recovered from the lungs of mice given cytokine on day 0 than infected controls (P < 0.03). In experiment 2, treatment on either day 0 or day −5 produced a considerable reduction (P < 0.01) in fungal burden in lungs compared to controls, but there was no statistical difference between day 0 and day −5 treatment. A third experiment demonstrated that there were was no statistically significant (P > 0.05) difference between treatment started on day −5 and that started on the day of infection (data not shown).
FIG. 3.
Pretreatment with rmGM-CSF does not necessarily improve the efficacy of response to H. capsulatum. Mice (n = 6) were treated with 50 μg of rmGM-CSF/kg/day i.p. either 5 days before infection or 8 h after exposure to H. capsulatum, and fungal burden was determined at 1 week of infection. Data are expressed as means ± standard errors.
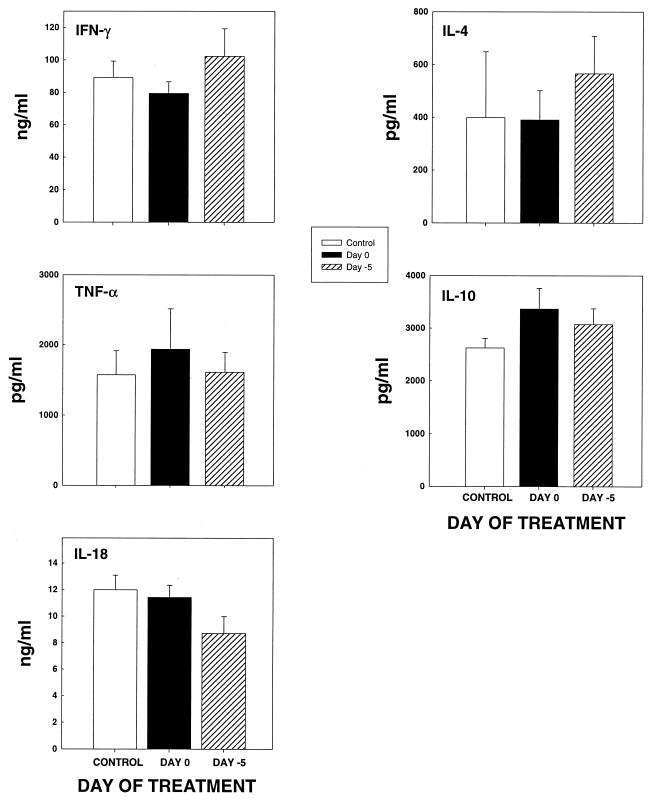
Cytokine responses in mice treated with rmGM-CSF.
Lungs of mice given diluent or rmGM-CSF either on day −5 or day 0 were assayed for the presence of IFN-γ, TNF-α, IL-4, IL-10, and IL-18 at week 1 of infection. The levels of cytokine did not differ (P > 0.05) substantially among the groups (Fig. 4).
FIG. 4.
Cytokine levels in lungs of mice (n = 6) infected with H. capsulatum and treated with diluent or rmGM-CSF. Data are expressed as means ± standard errors. No significant differences were observed between controls and treated mice (P > 0.05).
Flow cytometric analysis of the lungs of mice given rmGM-CSF.
Since GM-CSF is involved in hematopoiesis (20), we assessed if treatment with this cytokine altered the nature of the inflammatory response. Mice treated with rmGM-CSF or infected controls were analyzed at week 1 of infection for the absolute numbers of neutrophils (Gr-1+), macrophages (Mac-3+), or CD4+, CD8+, and I-A+ cells. The lungs of mice given rmGM-CSF had fewer inflammatory cells than those given diluent, although none of the differences achieved statistical significance (P > 0.05) (Table 1).
TABLE 1.
Phenotype of cells in lungs of H. capsulatum-infected mice given rmGM-CSF
| Cell populationa | Mean ± SE cell no. (105)
|
|
|---|---|---|
| Diluent | rmGM-CSF | |
| Total | 34.7 ± 10.9 | 26.2 ± 16.1 |
| CD4+ | 3.2 ± 1.3 | 1.5 ± 0.8 |
| CD8+ | 1.9 ± 0.7 | 1.1 ± 0.6 |
| Gr-1+ | 14.8 ± 5.8 | 15.8 ± 11.4 |
| Mac-3+ | 11.3 ± 3.9 | 8.9 ± 5.1 |
| I-A+ | 14.6 ± 5.1 | 10.0 ± 6.1 |
n = 6 mice per group. There was no statistical difference between groups (P < 0.0.5).
Ex vivo influence of rmGM-CSF on anti-Histoplasma activity of lung leukocytes.
To determine if treatment with rmGM-CSF directly armed lung leukocytes to inhibit the growth of H. capsulatum, mice were treated for 1 week with 50 μg of rmGM-CSF/kg/day or an equal volume of diluent, and lung cells were recovered from each group. Cells were fractionated into adherent and nonadherent populations and tested for anti-Histoplasma activity. In two experiments, the nonadherent fraction from rmGM-CSF-treated mice inhibited the growth of yeasts by 51 and 66% compared to nonadherent cells from diluent-treated animals. In contrast, yeast cells incorporated more [3H]leucine in adherent lung cells from rmGM-CSF-treated mice (Table 2).
TABLE 2.
Anti-Histoplasma activity of lung leukocytes isolated from rmGM-CSF-treated micea
| Treatment | Cell population | Mean ± SE no. of cpm (103)b | % Inhibitionc |
|---|---|---|---|
| Expt 1 | |||
| Diluent | Adherent | 29.5 ± 1.4 | |
| Nonadherent | 49.7 ± 7.7 | ||
| rmGM-CSF | Adherent | 37.9 ± 2.6 | −28 |
| Nonadherent | 20.9 ± 4.1 | 59 | |
| Expt 2 | |||
| Diluent | Adherent | 10.8 ± 1.3 | |
| Nonadherent | 8.9 ± 2.1 | ||
| rmGM-CSF | Adherent | 13.2 ± 1.8 | −22 |
| Nonadherent | 3.0 ± 0.3 | 66 |
Mice were treated with 50 μg of rmGM-CSF/kg/day or an equal volume of diluent for 1 week, and lung leukocytes were harvested.
Mean cpm of [3H]leucine incorporation of triplicate or quadruplicate wells.
Percent inhibition calculated as [1 − (experimental/control)] × 100.
Additional studies were performed to ascertain if NO was involved in the growth inhibition. Nonadherent cells were incubated with H. capsulatum yeast cells in the presence or absence of 1 mM of l-NMMA. In two experiments, this inhibitor failed to reverse the effect of nonadherent cells. As an example, [3H]leucine incorporation by yeasts in the presence of l-NMMA (4,885 ± 530 cpm) was similar to that in the absence of this compound (4,068 ± 477 cpm). The amount of NO also was measured in supernatants from lung nonadherent cells from rmGM-CSF-treated mice and from mice given diluent. Supernatants from lung cells of rmGM-CSF-treated mice and controls exposed to H. capsulatum contained similar amounts of NO (3.1 ± 0.2 μM versus 2.8 ± 0.4 μM, respectively).
To determine if treatment with rmGM-CSF altered the composition of nonadherent cells, we analyzed the proportion of cells expressing CD4+, CD8+, Gr-1+, and Mac-3+. Nonadherent lung cells from diluent- and rmGM-CSF-treated mice contained a similar proportion and absolute number of each population (Table 3).
TABLE 3.
Enumeration of nonadherent cells from lungs of mice given diluent or rmGM-CSF
| Cell population | No. (105) of cells (% positive)a
|
|
|---|---|---|
| Diluent | rmGM-CSF | |
| Expt 1 | ||
| CD4+ | 2.4 (17.7) | 3.0 (18.4) |
| CD8+ | 1.8 (12.8) | 2.1 (14.2) |
| Gr-1+ | 2.3 (16.3) | 2.7 (17.0) |
| Mac-3+ | 2.0 (8.0) | 1.9 (11.3) |
| Expt 2 | ||
| CD4+ | 5.2 (19.2) | 6.0 (16.8) |
| CD8+ | 3.6 (13.2) | 3.0 (13.0) |
| Gr-1+ | 4.0 (14.6) | 4.3 (16.5) |
| Mac-3+ | 3.9 (14.0) | 3.5 (11.0) |
Nonadherent cells were collected from pools (n = 5) of lung leukocytes isolated from mice given rmGM-CSF or diluent. A portion of the total cells were subjected to fluorescence-activated cell sorter analysis. The numbers in parentheses indicate the percentage of positive cells of the total cell recovery.
Does rmGM-CSF alter the course of overwhelming histoplasmosis?
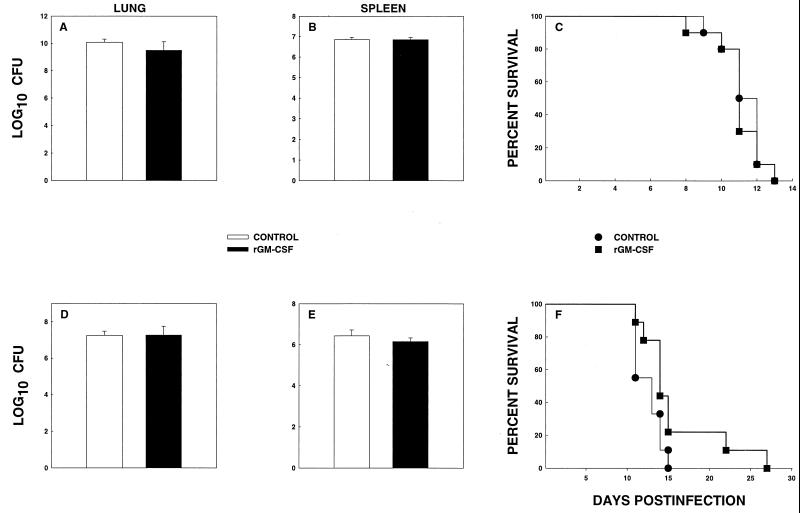
We tested the ability of rmGM-CSF to rescue mice from overwhelming histoplasmosis resulting from either a high inoculum or T-cell depletion. Two models were analyzed. In the first model, naive mice were exposed i.n. to lethal inocula of H. capsulatum (1.25 × 107 yeast cells) and treated with either 50 μg of rmGM-CSF/kg or an equal volume of diluent daily (group 1). In the second model, mice were depleted of both CD4+ and CD8+ cells and inoculated i.n. with 2.5 × 106 yeast cells (group 2).
Group 1 mice were challenged with the 1.25 × 107 organisms i.n. and administered rmGM-CSF or diluent. At week 1, groups of mice were sacrificed, and CFU in lungs and spleens were determined. The remaining mice were observed for survival. Administration of rmGM-CSF did not alter CFU at week 1 of infection, nor did it improve survival (Fig. 5A-C).
FIG. 5.
Fungal recovery and survival of mice treated with rmGM-CSF. Groups of immunocompetent mice (n = 6) were exposed to 1.25 × 107 organisms and treated with rmGM-CSF or diluent. (A and B) Fungal recovery from lungs (A) and spleens (B). Survival of mice (n = 10) is shown in panel C. (D and E) Fungal burden in lungs (D) and spleens (E) of CD4+ CD8+ cell-deficient mice (n = 6) challenged with 2.5 × 106 organisms. Survival of mice (n = 10) is illustrated in panel F. Data for fungal burden are expressed as means ± standard errors. The data are pooled results from two independent experiments.
In the second experiment, we tested if rmGM-CSF could alter the course of infection in T-cell-depleted mice. Following depletion of CD4+ and CD8+ cells, mice were infected with 2.5 × 106 yeast cells, and treatment with diluent or rmGM-CSF was initiated 8 h later. At week 1, some of the mice (n = 6) were sacrificed, and the level of fungal CFU was assessed in lungs and spleens. No differences in fungal burden were observed between the two groups (Fig. 5D and E). rmGM-CSF significantly (P = 0.01) delayed mortality, although the biological effect was modest (Fig. 5F). The levels of CFU in lungs and spleens of surviving mice were <200 CFU.
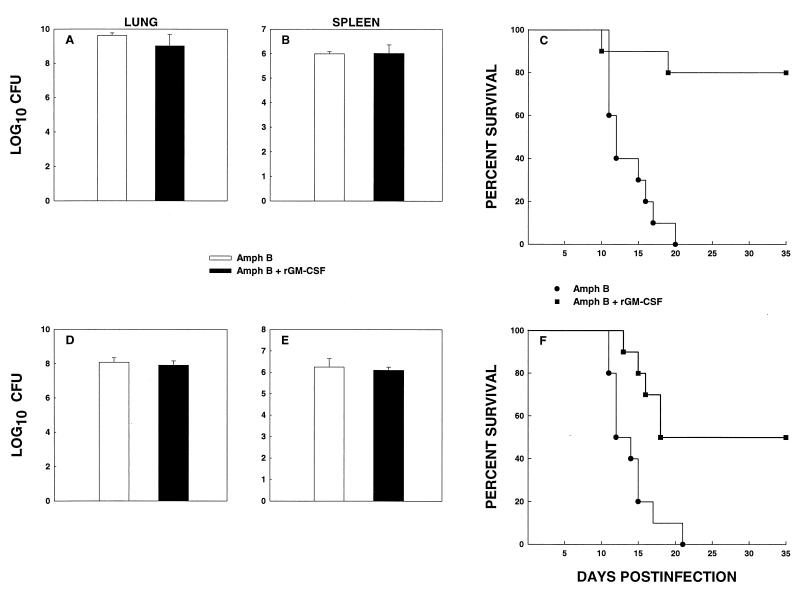
Can rmGM-CSF augment the antifungal activity of amphotericin B?
Mice were infected i.n. with 1.25 × 107 yeast cells and received either amphotericin B at 0.125 mg/kg three times a week i.p. or amphotericin B plus rmGM-CSF, given daily. This dosage of amphotericin B reduced CFU in lungs and spleens by two- to fourfold and was much less effective than dosages ranging from 0.25 to 1 mg/kg (data not shown). A suboptimal dose of amphotericin B was selected to assess if rmGM-CSF acted in concert with this antifungal agent. The levels of CFU of lungs and spleens were quantified at week 1, and survival of the remaining mice was monitored. CFU at week 1 did not differ (P > 0.05) between the two groups in either the lungs or spleens (Fig. 6A and B). Survival of mice treated with a suboptimal dose of amphotericin B was prolonged by the addition of rmGM-CSF (Fig. 6C).
FIG. 6.
rmGM-CSF augments the protective effect of low-dose amphotericin B. Fungal recovery from lungs (A) and spleens (B) of mice (n = 6) challenged with 1.25 × 107 yeast cells and treated with amphotericin B (0.125 mg/kg/day) plus diluent or amphotericin B plus rmGM-CSF. Survival (n = 10) is depicted in panel C. (D and E) CFU in lungs (D) and spleens (E) of CD4+ CD8+ cell-deficient mice treated with amphotericin B plus diluent or amphotericin B plus rmGM-CSF. Survival of the mice (n = 10) is exhibited in panel F. Data for CFU are expressed as means ± standard errors.
In parallel studies, treatment of CD4+ CD8+-deficient mice with rmGM-CSF plus amphotericin B did not produce a decrement in the fungal burden of lungs and spleens at week 1 of infection, but did result in a marked improvement in survival (P < 0.01) (Fig. 6D to F). Lungs and spleens of survivors were assessed for fungal burden at the termination of the study. In each mouse, the number of CFU was <200.
DISCUSSION
Although treatment of most forms of histoplasmosis continues to be successful, there are a subgroup of patients who manifest far-advanced infection either because of rapid progression or because of the failure of physicians to establish a diagnosis in a timely manner. In those individuals, the institution of polyenes or azoles alone may not suffice to combat the infectious process, and adjunctive therapies could potentially enhance therapy or reduce the duration of antifungal therapy. In this study, we sought to determine in a murine model of pulmonary histoplasmosis if the immune modulator rmGM-CSF enhanced the protective immune response.
GM-CSF demonstrates a number of salutary effects upon the host immune system. Among the many biological properties, it mobilizes hematopoietic cells from the bone marrow, stimulates production of toxic oxygen and nitrogen intermediates in phagocytes, induces class II MHC, promotes dendritic cell maturation and migration, induces tumoricidal activity by MΦ, and modulates production of IFN-γ and TNF-α (5, 7, 16, 17, 19, 20, 23, 27, 31–33). Given the plethora of effects on the immune system, it is not surprising that rGM-CSF has been used not only to increase the number of circulating phagocytes, but also to augment host resistance to several pathogens. In mouse models of leishmaniasis, pneumocystis, or Mycobacterium avium infection, rGM-CSF either alone or in combination with antimicrobial agents has enhanced elimination of the invading pathogen (6, 24, 28).
Administration of rmGM-CSF to naive mice infected i.n. with a sublethal inoculum of H. capsulatum lessened the fungal burden by approximately 1 log10 at week 1 of infection in three separate experiments. Although not a dramatic reduction, the results were consistent in three separate experiments. The efficacy of this cytokine was not detected beyond week 1 even if treatment was continued. The activity of rmGM-CSF corresponds to the period when innate rather than acquired immunity is critically important (1). Thus, the most likely reason that rmGM-CSF did not augment host resistance mechanisms after week 1 of infection is that the host T-cell-mediated response is maximally activated by week 1. We also examined if pretreatment with rmGM-CSF added to the beneficial effects of this cytokine. Treatment beginning 5 days prior to infection was variably superior to that begun after infection. Thus, preactivation of phagocytes did not necessarily augment the effect of rmGM-CSF.
The preponderance of studies herein demonstrated that rmGM-CSF ameliorated the early course (≤1 week) of pulmonary histoplasmosis. The effect was not pronounced and was observed in lungs predominantly. In only one set of two experiments, spleen CFU were reduced in mice given rmGM-CSF, whereas it was consistently observed in lungs. These results suggest that, in general, rmGM-CSF does not reliably control infection in lymphoid tissue.
In this study, the optimal dosage was found to be 50 μg/kg/day, and it was more effective than the higher one of 500 μg/kg/day. In fact, the latter dosage exerted a modest biological effect. The reason for the decreased efficacy of the larger amount is not readily apparent. One possibility is that the higher dosage increased the activation of macrophages to the extent that the immune response was dampened rather than enhanced.
To explore the mechanism(s) by which rmGM-CSF promoted host resistance to H. capsulatum, we analyzed its effects on cytokine generation in lungs, modulation of the inflammatory response, and the capacity of this cytokine to arm lung leukocytes to inhibit the growth of the fungus. GM-CSF interacts with a number of cytokines, including IFN-γ and TNF-α, both of which are vital in host resistance to H. capsulatum (1, 3–5, 24, 26). The capacity of GM-CSF to promote host resistance in other models has been attributed in part, to altering production of other necessary cytokines (32). Treatment with rmGM-CSF did not modify the levels of either of these cytokines, nor did it cause a decrement in the levels of IL-4 or IL-10, both of which are known to exacerbate pulmonary histoplasmosis (1, 3, 32). Levels of IL-18, a cytokine that interacts with GM-CSF (15) and influences the course of murine cryptococcosis (21), were similar in rmGM-CSF-treated animals to those in controls. The biological efficacy of rmGM-CSF was not ascribed to modulation of cytokines that may influence protective immunity. Moreover, the beneficial effect of rmGM-CSF was not associated with a significant alteration in either the number or the proportion of inflammatory cells in lungs of infected mice.
Treatment with rmGM-CSF induced anti-Histoplasma activity in a nonadherent cell population from the lungs of mice. Although CD4+ and CD8+ cells were present in this fraction, these cells were unlikely to be the target of rmGM-CSF, since it prolonged survival of mice depleted of both T-cell subsets. On the other hand, the likely cell population that exerted antifungal activity was neutrophils. Human and murine neutrophils are known to inhibit the growth of this fungus (22, 29). The fact that adherent cells which were largely macrophages failed to express anti-Histoplasma activity is not surprising, since previous studies indicated that rmGM-CSF arms monocytes but not macrophages to inhibit intracellular growth of this fungus (29). GM-CSF is known to enhance release of reactive oxygen intermediates (23, 31), but this effect is unlikely to explain its biological activity, since H. capsulatum survives the release of toxic oxygen radicals (8). Moreover, the inhibitory activity of nonadherent cells was not associated with NO production. Although defensins express anti-Histoplasma activity (10), these molecules are not present in murine neutrophils (14).
rmGM-CSF treatment, when used alone, prolonged survival in mice lacking CD4+ and CD8+ cells and infected with a sublethal inoculum of yeast cells. The efficacy of rmGM-CSF was independent of the presence of T cells. These results contrast with that reported for murine visceral leishmaniasis, in which the therapeutic activity of rmGM-CSF was highly dependent on the presence of T cells (28). Interestingly, rmGM-CSF did not exert the same effect in mice exposed to a lethal challenge. The reasons for this failure are not known. One possible explanation is that the massive exposure overwhelms the capacity of this cytokine to activate the immune system. Limitations may exist upon the capacity of a cytokine or any therapy to contain infection, especially when the host is exposed to large numbers of organisms.
In the two models of overwhelming histoplasmosis, rmGM-CSF did not promote clearance of the fungus within the first 7 days of therapy, yet delayed mortality when given alone to T-cell-depleted mice and improved survival when combined with subeffective dosages of amphotericin B. These results indicate that the effect on fungal elimination required prolonged exposure to stimulate the protective immune response. However, rmGM-CSF did effect clearance, since the lung and splenic tissues of surviving mice that received amphotericin B plus rmGM-CSF contained <200 CFU in at the conclusion of the experiments.
The combination of low-dose amphotericin B and rmGM-CSF produced a dramatic effect on survival of either mice exposed to a large number of yeast cells or mice depleted of CD4+ and CD8+ cells. The intention of these studies was to determine if GM-CSF could contribute to the efficacy of amphotericin B. We chose a dosage of this polyene antifungal that reduced the fungal burden only by twofold rather than a dosage that was curative. The rationale for the selection of such a suboptimal amount of amphotericin B was to determine if the efficacy of rmGM-CSF was additive with amphotericin. Animals injected amphotericin B alone failed to control infection, whereas those given both amphotericin B and GM-CSF manifested a higher rate of survival. Thus, rmGM-CSF could be coupled with amphotericin B to promote clearance.
In summary, we have demonstrated that rmGM-CSF augments the host's immune response to contain infection with H. capsulatum. In these studies, innate immunity against H. capsulatum appears to be more responsible for protection than does acquired immunity. The mechanism of its protective activity was identified as a direct effect on a nonadherent cell population that consisted chiefly of neutrophils. rmGM-CSF improved survival in T-cell-depleted mice in the absence or presence of suboptimal amounts of amphotericin B, suggesting a role for GM-CSF in the treatment of patients with immune dysfunction, such as AIDS, or immunosuppressed individuals. This cytokine also promoted survival in amphotericin B-treated mice challenged with a lethal inoculum. Thus, rmGM-CSF may be a useful adjunct to the treatment of histoplasmosis.
ACKNOWLEDGMENTS
This work was supported by a VA Merit Review, AI-42747, AI-34361, and a grant from Immunex Corporation.
REFERENCES
- 1.Allendoerfer R, Boivin G P, Deepe G S. Modulation of immune responses in murine pulmonary histoplasmosis. J Infect Dis. 1997;175:905–914. doi: 10.1086/513989. [DOI] [PubMed] [Google Scholar]
- 2.Allendoerfer R, Deepe G S., Jr Intrapulmonary response to Histoplasma capsulatum in gamma interferon knockout mice. Infect Immun. 1997;65:2564–2569. doi: 10.1128/iai.65.7.2564-2569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allendoerfer R, Deepe G S., Jr Blockade of endogenous TNF-α exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J Immunol. 1998;160:6072–6082. [PubMed] [Google Scholar]
- 4.Allendörfer-Fernandez R, Brunner G D, Deepe G S., Jr Complex requirements for nascent and secondary immunity in pulmonary histoplasmosis. J Immunol. 1999;162:7389–7396. [PubMed] [Google Scholar]
- 5.Basu S, Dunn A R, Marino M W, Savoia H, Hodgson G, Lieschke G J, Cebon J. Increased tolerance to endotoxin by granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol. 1997;159:1412–1417. [PubMed] [Google Scholar]
- 6.Bermudez L E, Martinelli J, Petrosky M, Kolonoski P, Young L S. Recombinant granulocyte-macrophage colony-stimulating factor enhances the effects of antibiotics against Mycobacterium avium complex infection in the beige mouse model. J Infect Dis. 1994;169:575–580. doi: 10.1093/infdis/169.3.575. [DOI] [PubMed] [Google Scholar]
- 7.Blau H, Riklis S, Van Iwaarden J F, McCormack F X, Kalina M. Nitric oxide production by rat alveolar macrophages can be modulated in vitro by surfactant protein A. Am J Physiol. 1997;272:L1198–L1204. doi: 10.1152/ajplung.1997.272.6.L1198. [DOI] [PubMed] [Google Scholar]
- 8.Bullock W E, Wright S D. Role of adherence promoting receptors, CR3, LFA-1, and p150, 95 in binding of Histoplasma capsulatum by human macrophages. J Exp Med. 1987;165:195–210. doi: 10.1084/jem.165.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cain J A, Deepe G S., Jr Evolution of the primary immune response to Histoplasma capsulatum in murine lung. Infect Immun. 1998;66:1473–1481. doi: 10.1128/iai.66.4.1473-1481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couto M A, Liu L, Lehrer R I, Ganz T. Inhibition of intracellular Histoplasma capsulatum replication by murine macrophages that produce human defensin. Infect Immun. 1994;62:2375–2378. doi: 10.1128/iai.62.6.2375-2378.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deepe G S., Jr Role of CD8+ T cells in host resistance to systemic infection with Histoplasma capsulatum in mice. J Immunol. 1994;152:3491–3500. [PubMed] [Google Scholar]
- 12.Deepe G S., Jr . Histoplasmosis. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practices of infectious diseases. 5th ed. Vol. 2. Philadelphia, Pa: Churchill Livingstone; 2000. pp. 2718–2733. [Google Scholar]
- 13.Deepe G S, Jr, Gibbons R, Woodward E. Neutralization of endogenous GM-CSF subverts the protective immune response to Histoplasma capsulatum. J Immunol. 1999;163:4985–4993. [PubMed] [Google Scholar]
- 14.Eisenhaur P B, Lehrer R I. Mouse neutrophils lack defensins. Infect Immun. 1992;60:3446–3447. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehninger T A, Shah M H, Turner M J, VanDeusen J B, Whitman S P, Cooper M A, Suzuki K, Wechser M, Goodsaid F, Caligiuri M A. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- 16.Fischer H G, Frosch S, Reske K, Reske-Kunz A B. Granulocyte-macrophage colony-stimulating factor activates macrophages derived from bone marrow cultures to synthesis of MHC class II molecules and to augmented antigen presentation function. J Immunol. 1988;141:3882–3888. [PubMed] [Google Scholar]
- 17.Fontt E O, De Baetselier P, Heirman C, Thielemans K, Lucas R, Vray B. Effects of granulocyte-macrophage colony-stimulating factor and tumor necrosis factor alpha on Trypanosoma cruzi trypomastigotes. Infect Immun. 1998;66:2722–2727. doi: 10.1128/iai.66.6.2722-2727.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez A M, Bullock W E, Taylor C L, Deepe G S., Jr The role of L3T4+ T cells in host defense against Histoplasma capsulatum. Infect Immun. 1988;56:1685–1691. doi: 10.1128/iai.56.7.1685-1691.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grabstein K H, Urdal D L, Tushinski R J, Mochizuki D Y, Price V L, Cantrell M A, Gillis S, Conlon P J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986;232:506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- 20.Hill A D, Naarma H, Shou J, Calvano S E, Daly J M. Antimicrobial effects of granulocyte-macrophage colony-stimulating factor in protein-energy malnutrition. Arch Surg. 1995;130:1273–1277. doi: 10.1001/archsurg.1995.01430120027004. [DOI] [PubMed] [Google Scholar]
- 21.Kawakami K, Qureshi M H, Zhang T, Okamura H, Kurimoto M, Saito A. IL-18 protects mice against pulmonary and disseminated infection with Cryptococcus neoformans by inducing IFN-γ production. J Immunol. 1997;159:5528–5534. [PubMed] [Google Scholar]
- 22.Kurita N, Brummer E, Yoshida S, Nishimura K, Miyaji M. Antifungal activity of murine polymorphonuclear neutrophils against Histoplasma capsulatum. J Med Vet Mycol. 1991;29:133–143. [PubMed] [Google Scholar]
- 23.Lieschke G J, Burgess A W. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. N Engl J Med. 1992;327:28–35. doi: 10.1056/NEJM199207023270106. [DOI] [PubMed] [Google Scholar]
- 24.Mandujano J F, D'Souza N B, Nelson S, Summer W R, Beckerman R C, Shellito J E. Granulocyte-macrophage colony-stimulating factor and Pneumocystis carinii pneumonia in mice. Am J Respir Crit Care Med. 1995;151:1233–1238. doi: 10.1164/ajrccm/151.4.1233. [DOI] [PubMed] [Google Scholar]
- 25.Metcalf D. Control of granulocytes and macrophages: molecular, cellular, and clinical aspects. Science. 1991;254:529–533. doi: 10.1126/science.1948028. [DOI] [PubMed] [Google Scholar]
- 26.Munker R, Gasson J, Ogawa M, Koeffler H P. Recombinant human TNF induces production of granulocyte-monocyte colony-stimulating factor. Nature. 1986;323:79–81. doi: 10.1038/323079a0. [DOI] [PubMed] [Google Scholar]
- 27.Muranaka H, Suga M, Nakawaga K, Sato K, Gushima Y, Ando M. Effects of granulocyte and granulocyte-macrophage colony-stimulating factors in a neutropenic model of trichosporonosis. Infect Immun. 1997;65:3422–3429. doi: 10.1128/iai.65.8.3422-3429.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray H W, Cervia J S, Hariprashad J, Taylor A P, Stoeckle M Y, Hockman H. Effect of granulocyte-macrophage colony-stimulating factor in experimental leishmaniasis. J Clin Investig. 1995;95:1183–1192. doi: 10.1172/JCI117767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman S L, Gootee L. Colony-stimulating factors activate human macrophages to inhibit intracellular growth of Histoplasma capsulatum yeasts. Infect Immun. 1992;60:4593–4597. doi: 10.1128/iai.60.11.4593-4597.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman S L, Gootee L, Gabay J E. Human neutrophil-mediated fungistasis against Histoplasma capsulatum. Localization of fungistatic activity to the azurophil granules. J Clin Investig. 1993;92:1422–1429. doi: 10.1172/JCI116630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed S G, Nathan C F, Pihl D L, Rodricks P, Shanebeck K, Conlon P J, Grabstein K H. Recombinant granulocyte/macrophage colony-stimulating factor activates macrophages to inhibit Trypansoma cruzi and release hydrogen peroxide. Comparison with interferon-γ. J Exp Med. 1987;166:1734–1746. doi: 10.1084/jem.166.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarmento A, Appelberg R. Involvement of reactive oxygen intermediates in tumor necrosis factor alpha-dependent bacteriostasis of Mycobacterium avium. Infect Immun. 1996;64:3224–3230. doi: 10.1128/iai.64.8.3224-3230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Storozynsky E, Woodward J G, Frelinger J G, Lord E M. Interleukin-3 and granulocyte-macrophage colony-stimulating factor enhance the generation and function of dendritic cells. Immunology. 1999;97:138–149. doi: 10.1046/j.1365-2567.1999.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou P, Miller G, Seder R A. Factors involved in regulating primary and secondary immunity to infection with Histoplasma capsulatum: TNF-α plays a critical role in maintaining immunity in the absence of IFN-γ. J Immunol. 1998;160:1359–1368. [PubMed] [Google Scholar]
- 35.Zhou P, Seder R A. CD40 ligand is not essential for induction of type I cytokine responses or protective immunity after primary or secondary infection with Histoplasma capsulatum. J Exp Med. 1998;187:1315–1324. doi: 10.1084/jem.187.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou P, Sieve M C, Bennett J, Kwon-Chung K J, Tewari R P, Gazzinelli R T, Sher A, Seder R A. IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-γ. J Immunol. 1995;155:785–795. [PubMed] [Google Scholar]