African relevance
-
•
Sub-Saharan Africa is experiencing an increasing burden of stroke, with current prevalence as high as 1.46 per 1000 population.
-
•
Limited cohort studies exist to profile the epidemiological, clinical and paraclinical characteristics of stroke in this setting.
-
•
Identifying the most at-risk population for ischemic and haemorrhagic stroke can improve approaches for prevention in this setting.
Keywords: Stroke, Djibouti, Khat; Epidemiology, Cardiovascular risk factors
Abstract
Background
Stroke is a neurological emergency affecting both developed and developing countries. In Djibouti, stroke is the fourth leading cause of death. Our objective was to describe the demographic, clinical, paraclinical profile of stroke in Djibouti and identify the possible underlying risk factors.
Methods
We conducted a cross-sectional multicentre study carried out over a period of 6 months in the medical services of the Soudano-Djibouti military hospital, the General Peltier hospital and the emergency department of the National fund for social security health centre.
Results
A total of seventy patients were included. The mean age was 59.61 years with a male predominance (sex ratio: 2.5) and a statistically significant female-related difference beyond the age of 60 years (p <10−3). Cardiovascular risk factors were mainly hypertension (73%), khat chewing (64%) and tobacco use (50%). Khat chewing and tobacco use were associated with a younger age of occurrence of stroke (p=0.020 and p=0.004, respectively). Diabetes mellitus and hypercholesterolemia were found respectively in 30% and 19% of cases, and were more associated with ischemic stroke. Coronary disease (11%), heart failure (3%) and obesity (4%) (significantly associated with the female gender; p= 0,021) were less common. Motor deficits (94%) were the most common clinical manifestations, followed by sensory deficits (51%) and alteration of consciousness (37%). Stroke was ischemic in 61.5% of patients. The most affected territory in ischemic stroke was the territory of the middle cerebral artery, and capsulo-thalamic involvement in haemorrhagic stroke which was significantly associated with the alteration of consciousness(p=0,003).
Discussion
Stroke had primarily modifiable risk factors in Djiboutian patients dominated by high blood pressure, tobacco use and khat chewing especially in the male population under the age of 60 years. These findings could have implications on future preventive measures and a better approach to public health policy.
Introduction
Stroke is the second leading cause of death worldwide (5.781 million deaths in 2016) after ischemic heart disease, and the leading cause of acquired disability in adults [1]. In Djibouti, strokes were the third cause of death (464 deaths in 2017) after the lower respiratory infections and the Human Immunodeficiency Virus / Acquired Immunodeficiency Syndrome (HIV / AIDS) [2]. Strokes constitute a real public health problem by their annual cost, their frequency and their severity at the origin of serious disabling consequences. Nevertheless, they remain accessible to prevention. In Djibouti, the evolution of the current health situation characterised by a lack of human resources mainly trained health practitioners for the management of the disease, technical platforms and especially a specific program for the prevention of cardiovascular diseases, does not seem very favourable. Recent work has highlighted the importance and specificity of stroke in developing countries and particularly in Sub-Saharan Africa (SSA) [3]. With increasing burden of stroke, SSA has a high prevalence (up to 1.46 per 1000 population), and age-standardised stroke incidence (up to 316 per 100,000) rate [4]. The pooled estimates is currently 3.5 per 1000 population with an annual increase of 12.0% [5].The rare epidemiological data on strokes concerning Djibouti are those established by the World Health Organization (WHO) and the study by Benois and al., in 2009 [6] on 16 cases of haemorrhagic stroke. To our knowledge, there are currently no cohort studies conducted on the profile of stroke in Djibouti. In this context, the aim of this work was to describe the epidemiological, clinical and paraclinical characteristics of stroke in Djibouti, and to identify the underlying risk factors in these patients.
Methods
A cross-sectional multicentre study was carried out over a period of 6 months (July to December 2017) in the Departments of: Medicine at the Soudano-Djibouti Military Hospital, the General Peltier Hospital and Emergency of the National fund for social security health centre.
Were included all adult patients, regardless of age, hospitalised for the diagnosis of stroke of arterial origin (ischemic and haemorrhagic) during the study period. Our definition of stroke was established based on the latest update from the American Heart Association / American Stroke Association in 2013 [7]. We used the International Classification of Diseases Version 10 (ICD-10) for the classification of stroke [8]. We excluded from the study all adult patients with a stroke of venous origin (cerebral venous thrombosis), subarachnoid haemorrhage as well as patients without brain imaging. We collected the demographic, clinical and laboratory data. Vascular risk factors (hypertension, heart diseases, diabetes, and dyslipidaemia) were established upon obtaining personal history of patients in whom these factors were already diagnosed. In those who were newly diagnosed with such diseases during their hospitalisation, the diagnosis was established by the specialists of each disease (cardiologists and endocrinologists in each centre). Obesity was defined as a body mass index (BMI) over 30. Toxic habits were also noted including tobacco use, khat chewing and alcohol consumption and were classified as never, former, and current use. All the included patients had brain CT scan. For strokes of the middle cerebral artery territory, the Alberta Stroke Program Early CT score (ASPECTS score) was calculated.
The data were collected after oral consent of the patients or a family member in case of impossibility to communicate with the patient, and the agreement of the referring physician. Patients were informed of the aim of the work and of their anonymity.
The data were entered and analysed using the Statistical Package for the Social Sciences (SPSS) version 23 software. We calculated simple frequencies and relative frequencies (percentages) for the qualitative variables. We calculated means (or medians in case of a non-Gaussian distribution), standard deviations (inter quartile difference in case of a non-Gaussian distribution) and the range (minimum and maximum) for the quantitative variables. The statistical significance (p) was set at 0.05 for all statistical tests. We used the Chi-squared test to compare the percentages and we used Fisher's exact test if the theoretical size was less than five. We used the Student's t-test (t) for the comparison of 2 means or the Mann-Whitney U test in case of a non-Gaussian distribution.
Results
A total of 70 patients were included. Three patients were excluded because they could not have a CT scan done for socioeconomic reasons. The average age of our study population was 59.61 years (ranging from 33 to 85 years) with a male predominance (sex ratio 2.5). A statistically significant difference related to the female gender was noted beyond the age of 60 years (p<10−3). Sixty-six percent of patients were of Djiboutian origin. The patients of foreign geographic origin were of Ethiopian origin (n=11), Somali (n=12), and Yemeni (n=1). Ethnically, 76% of patients were community Somali against 16% Afar and 7% Arab. Family history of stroke was found in 13% (n=9). Half of the patients (n=35) had no social security coverage.
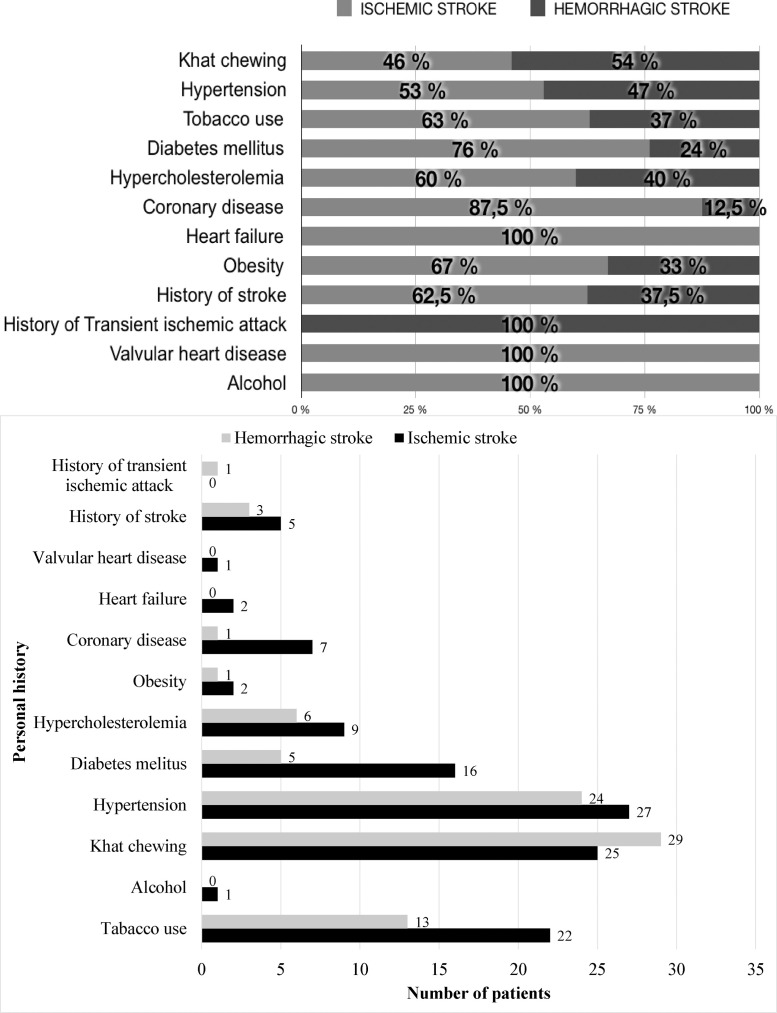
The most common cardiovascular risk factor was hypertension (73%) followed by the khat chewing (64%) and tobacco use (50%) (Table 1). These three factors were significantly associated with the male gender (p=0.009 for hypertension, p<10−3 for khat chewing and tobacco use). The average tobacco use rate measured for conventional tobacco was 29.36 Packets-year and it was associated with a younger age of stroke (p=0.004). Fifty-one percent of the patients were both hypertensive and khat-consumers. Among khat consuming patients, 47% had concomitant tobacco use with the consumption of khat. Consumption of khat was associated with a younger age of stroke less than 60 years (p = 0.020) (Table 2). Diabetes mellitus and hypercholesterolemia were present in 30% and 21% of cases, respectively. The frequencies of coronary artery disease (11%), heart failure (3%) and obesity (4%) were low. The obesity was significantly associated with the female gender (p=0.021). In patients with ischemic stroke, 76% had diabetes mellitus, 63% with tobacco use and 60% had hypercholesterolemia. Khat chewing and hypertension were at similar proportions in ischemic and haemorrhagic strokes (46% versus 54% and 53% versus 47% respectively) (Fig. 1).
Table 1.
Distribution of history and toxic habits according to gender.
| Gender |
||||
|---|---|---|---|---|
| Total | Male | Female | P | |
| N | 70 | 50 | 20 | |
| Personal history of stroke | 8(11%) | 6(12%) | 2(10%) | 0.588 |
| Personal history of transient ischemic attack | 1(1%) | 1(2%) | 0 | |
| Hypertension | 51(73%) | 41(82%) | 10(50%) | 0.009 |
| Diabetes mellitus | 21(30%) | 14(28%) | 7(35%) | 0.381 |
| History of hypercholesterolemia | 15(21%) | 8(16%) | 7(35%) | 0.079 |
| Coronary artery disease | 8(11%) | 7(14%) | 1(5%) | |
| Heart failure | 2(3%) | 2(4%) | 0 | |
| Valvular heart disease | 1(1%) | 1(2%) | 0 | |
| Obesity | 3(4%) | 0 | 3(15%) | 0.021 |
| Tobacco use | 35(50%) | 32(64%) | 3(9%) | <10-3 |
| Khat chewing | 44(64%) | 42(84%) | 2(10%) | <10-3 |
| Alcohol | 1(1%) | 1(2%) | 0 | |
Table 2.
Characteristics of khat consumption.
| Values | P | ||
|---|---|---|---|
| Prevalence of khat consumption, N (%) | 44 (63) | ||
| Gender | M, N (%)F, N (%) | 42 (95)2 (5) | <10−3 |
| Average consumption frequency, Day / week | 5,52 | ||
| Daily consumption, N (%) | 24 (34) | ||
| Consumption time before stroke, Year | 34,87 | ||
| Weaning, N (%) | 3 (7) | ||
Fig. 1.
Personal history according to the type of stroke.
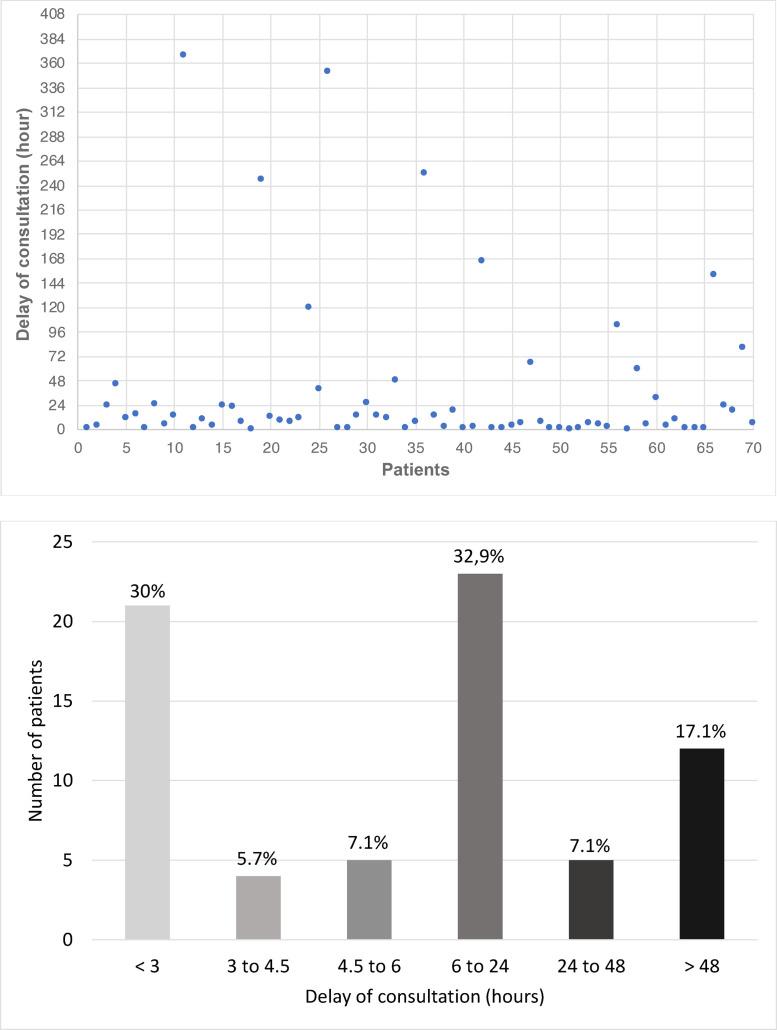
The average consultation time was 36 hours and 46 minutes (ranging from 20 minutes to 368 hours) (Fig. 2). On a clinical level, motor deficits (94%) were the most frequent clinical manifestations. Sensory deficits were found in 51%; and 37% of the patients had presented alertness disturbances. Impairment of higher functions was found in half of our patients with mainly language disorders such as Broca's motor aphasia (21%) and dysarthria (16%). Vision disorders (7%), cerebellar syndrome (4%) and the cranial nerves impairment (3%) were uncommon manifestations in our study population.
Fig. 2.
Distribution of patients according to the delay of consultation.
The brain CT scan was performed in all of our included patients. Sixty-one and a half percent had ischemic stroke and 38.5 % had haemorrhagic stroke. The most affected territory in ischemic stroke was the territory of the middle cerebral artery (14%). Haemorrhagic stroke was capsulo-thalamic in 51% of cases and significantly associated with alertness disturbances (p = 0.003). The median of the ASPECTS score, calculated in all patients with ischemic lesion in the territory of the middle cerebral artery was eight (interquartile range at 1).
The electrocardiogram was performed in all patients. Abnormalities were dominated by signs of left ventricular hypertrophy (17%) and those of myocardial ischemia (11%). Forty-two patients (60%) had a transthoracic cardiac ultrasound with eight cases (11%) of hypertrophic cardiomyopathy. Ultrasound of the supra-aortic trunks was performed in 16 (23%) patients, a quarter of whom had atheromatous infiltration. Plasma cholesterol, measured in 68 patients (97%) was high in 19% of them, while plasma triglyceride, measured in 65 patients (93%) was higher than normal in 11% of the cases.
Discussion
Our study, having assessed prospectively a multicentre cohort of 70 patients from different ethnic groups with ischemic or haemorrhagic stroke, depicted preliminary demographic and clinical features of stroke in Djiboutian population. The average age of our population (59.61 years) was lower than that of Western countries (73 years) [9]. However, we found comparable values in the African series [10], [11], [12]. Age is in fact the first non-modifiable risk factor for stroke in the literature, and stroke incidence increases with age [13]. This younger age found in our cohort seems to be a common feature in the black race as has been shown in American studies [14]. In our series, male predominance was found regardless the age group (sex-ratio of 2.5) in accordance with most African series [15], [16], [17], [18]. However, a statistically significant difference in favour of the female gender was noted in our cohort from the age of 60 years. In fact, the male gender exposed more to the risk of stroke except in the advanced ages where the female gender dominated [19]. Hormonal factors may be incriminated. Series like that of N'goran and al., in 2015, showed a female predominance(56% women) but it was a single centre study despite a study duration of two years [11].
In our study, 73% of the patients were hypertensive; which was slightly lower than data from some sub-Saharan countries such as the Ivory Coast (86.4%) [11], the Congo (84.3%) [10]and Ghana (85%) [15]. But our rate was higher than that of Asian countries such as South Korea (64%) [20] and India (60%) [21]. Hypertension is the most important risk factor of all types of stroke combined. The relationship between the risk of stroke and the degree of hypertension is almost linear [22]. A third of our patients (30%) had diabetes mellitus. This rate is higher compared to what was found in Western countries (15.5%) as well as studies done in Ivory Coast in 2015 (11.4%). Larger proportions have been reported in African countries such as the Congo (36.1%), Nigeria (23.8%) and especially in other countries such as Iran (55.7%) [10,11,[23], [24], [25]]. This high rate of diabetes mellitus could be explained by sedentary lifestyle and overweight. A fifth of our patients (19 %) had a high level of cholesterol. This rate was higher than that of studies conducted in Nigeria (3%) in Mozambique (14.3%) but also in Yemen (8.7%) [10,26,27]. Yet, larger proportions have been reported in recent studies in Ethiopia (38.5%), Congo (45.2%) and Brazil (47%) [10,28,29].The relationship between cholesterol and stroke is not as obvious as that with coronary artery disease. The elevation of the cholesterol level was associated with an increased risk of ischemic stroke while an elevation of the HDL cholesterol level was associated with a reduction in this risk [30]. It was observed that the risk was different according to the type of stroke with an association between cholesterol levels and the obstruction of the large arteries in the ischemic stroke (Table 3) [10,21,26,[28], [29],[31], [32], [33]].
Table 3.
Comparison of stroke risk factors in our study and other previous studies.
| Author, country, year | N | Hypertension% | Diabetes mellitus% | Hypercholesterolemia% | History of stroke% | Heredity% |
|---|---|---|---|---|---|---|
| Bamekhlahetal., Yémen, 2014 [26] | 774 | 57,2 | 44,8 | 8,7 | 10,6 | 13,4 |
| Limboleet al., Congo, 2017 [10] | 166 | 84,3 | 36,1 | 45,2 | - | - |
| Deresse and Shaweno, Ethiopia, 2015 [28] | 163 | 50,9 | 7,4 | 38,5 | 2,5 | 1,2 |
| Sylajaetal., India, 2018 [21] | 2066 | 60.8 | 35.7 | 14.4 | 19.8 | 15,2 |
| Marroneet al., Brazil, 2011 [29] | 688 | 75,1 | 22,6 | 47,1 | - | 16,4 |
| Omori et al., Japan, 2012 [33] | 1087 | 70 | 39,8 | 50 | 21,3 | 30 |
| Ben Mahjoub et al., Tunisia, 2017 [35] | 107 | 62 | 35 | - | - | - |
| Our study, Djibouti, 2017 | 70 | 73 | 30 | 21 | 11 | 13 |
Half of our patients were tobacco consumers.Tobacco use is a factor that doubles the risk of stroke as demonstrated by the Framingham study [34]. There is also a dose-dependent relationship between smoking and the occurrence of stroke [35]. Tobacco consumption in our patients was higher than that observed in sub-Saharan countries such as Ethiopia (4.9%), Senegal (5.8%) and Nigeria (22.8%) [16,24,28]. But our values were close to those of studies conducted in South Korea (48.7%) [36] and in Tunisi a (42.1 %) [37]. Tobacco use was associated with the occurrence of stroke at younger age in our patients (p=0.040). Another explanation would be the tobacco / khat chewing association. Indeed, during the sessions of khat chewing, a large amount of tobacco use can be associated. As observed by Bawazeer and al., in 1999 in Yemen, khat consumers were great tobacco consumers (> 20 cigarettes / day) [38]. Indeed, in our series, the majority of patients were consumers of khat (63%) with a male predominance (95%). This consumption was daily among 34% of consumers. Khat was associated with a younger age of stroke (p=0.020). Indeed, there is a long history of Khat chewing in the Horn of Africa and the Arabian Peninsula. Khat chewing for social and psychological reasons has been practiced for many centuries in these countries including Djibouti. The role of khat consumption in cardiovascular risk has already been proven [39]. In fact, the leaves of the Khat plant contain amphetamine like compounds such as cathinone and cathine increasing both blood pressure and heart rate. Cathinone, which is the active molecule, exerts both a positive inotropic and chronotropic effect on the heart leading to high blood pressure but also a vasoconstrictor and aggregating platelet effect through catecholamines [40,41]. The study of Mujili et al., noted, by studying ischemic stroke among 358 consumers of khat and 335 non-consumers, that the occurrence of ischemic stroke was significantly more frequent among consumers [42]. In the study of Ali et al. including 8176 consecutive patients presenting with acute coronary syndrome, khat chewing was associated with increased risk of both ischemic and haemorrhagic stroke and death [43]. These findings were in line with the conclusions of the systematic review and meta-analysis of Mega et al. assuming that khat was found to have either a causative or worsening effect on stroke, myocardial infarction and heart failure [39]. In Djibouti, khat consumption has been previously incriminated in cardiovascular complications including heart failure [44]. In the prospective study by Bénois et al. in 2009 assessing morbidity and mortality in haemorrhagic stroke in Djibouti, approximately 62% were daily consumers of khat [6].
The time between the onset of signs and the consultation of a medical centre was assessed in our study. There was an average of 36 hours and 46 minutes, so more than 24 hours. The time taken for care is decisive in the stroke as " Time is brain ". This significant delay found in our cohort could be justified by the distance from hospitals, but also by the lack of information about the disease by the patients and their entourage [45]. Shorter delays have been reported in regions of European countries such as Spain or in randomised controlled trials in Italy (respectively 3 hours 21 minutes and 2 hours 40 minutes) [46,47]. But a systematic review of the literature that described stroke care in 14 African country included, noted a median time interval of 31 hours [48].
In our study, all patients had a brain CT scan following the latest recommendations of the American Heart Association and the American Stroke Association of 2018 [49].
Ischemic stroke was found in 61.5 % of our cases (including 58.5 % of ischemic strokes formed) and haemorrhagic stroke in 38.5 %. This prevalence of ischemic stroke was the same as that observed across the world, in Europe as well as in Africa [9,12]. However, the proportions of haemorrhagic stroke were higher than in European countries (6.7-29.9%). This was known as a particularity of sub-Saharan countries with reports of more than 50% of haemorrhagic stroke [50,51]. Nonetheless, studies conducted the last ten years have shown a decline in haemorrhagic stroke which currently range from 30 - 40% of strokes [12], [24], [27]
In our study, the most frequent territory for ischemic stroke (56%) was that of the middle cerebral artery; and deep capsulo-thalamic hematoma was the most common location for haemorrhagic stroke (85%). These proportions were similar to those of the French studies (83.4%) for ischemic stroke and Senegalese (67.8%) for haemorrhagic stroke [52,53].
More than three quarters of the patients having an ischemic stroke in the territory of the middle cerebral artery in our series, had ASPECTS score higher than 7. Therefore, they could have had a better prognosis in case of intravenous thrombolysis within 3 hours of the cerebral infarction [54]. The main hindrance for thrombolysis therapy implementation in African studies was reported to be cost [55]. Additionally, as aforementioned, the average presenting time of our patients was 36 hours and 46 minutes which is way above the three to four and a half hours window of intervention. Also, the insufficiency of equipment and expert for thrombolysis therapy in stroke in Djibouti was the major obstacle.
Some limitations should be kept in mind though regarding the findings of this study and warrant analysis. The limited cohort size could reduce statistical study power and hamper association studies. Being a pioneer study on stroke in Djibouti, the lack of previously published comparative data hampered us from performing any a priori power calculations for sample size. Besides, study duration carried out over six months could ignore potential seasonal differences in stroke features and would require further prospective studies over longer duration. Additionally, the study was conducted in urban settings in the three main centres of Djibouti. All stroke patients in the regional hospitals were transferred to those centres but some rural undiagnosed cases or transient ischemic attacks could be missed. By excluding patients for not having imaging done because of socio-economic reasons, our study may have skewed towards a higher socioeconomic profile of patients who may not represent all stroke patients in Djibouti. However, only three subjects were excluded for such reasons. The limited resources deployed for exhaustive etiological assessment of stroke impede formulating valid conclusions on the spectrum of causes of stroke in Djiboutian patients. Taken all together, these limitations could hamper the generalisability of our results. Finally, our study lacks data on prognosis and mortality which could be later assessed in future prospective follow-up cohorts.
In conclusion, our prospective multi-centric study may help to provide healthcare interveners in Djibouti with preliminary data on stroke. Indeed, we identified in this cohort main risk factors of stroke in Djiboutian patients dominated by hypertension, smoking and khat consumption, especially in the male population under 60 years of age. Our work depicted a first profile of strokes to improve the preventive approach based mainly on education and the fight against modifiable risk factors, focusing on the identified mostly at-risk population of young male smokers and khat consumers. In the future, larger studies with longer duration, and prospective monitoring of prognosis would be useful to better understand the underlying risk factors specific to Djiboutian population and the possible effective interventions in this context.
Dissemination of results
Results from this study were shared with staff members at the data collection sites through informal presentations, This study was also the subject of thesis research presented on March, 22nd 2018 in Djibouti Faculty of Medicine /University of Djibouti.
Author contribution
Authors contributed as follow to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content: MAN contributed 30%; AN and RG contributed 20% each; SM, MAM, AGh, YA, AS, AGa, IK contributed 5% each. All authors approved the version to be published and agreed to be accountable for all aspects of the work.
Declaration of competing interest
The authors declare no conflicts of interest.
Contributor Information
Amina NASRI, Email: dr.nasri.amina@gmail.com.
Riadh GOUIDER, Email: riadh.gouider@gnet.tn.
References
- 1.2020. WHO | Top 10 causes of death. WHO World Health Organ n.d. [Google Scholar]; http://www.who.int/gho/mortality_burden_disease/causes_death/top_10/en/ accessed.
- 2.HEALTH PROFILE DJIBOUTI . April 20, 2020. World Life Expect n.d. [Google Scholar]; https://www.worldlifeexpectancy.com/country-health-profile/djibouti accessed.
- 3.Owolabi MO, Arulogun O, Melikam S, Adeoye AM, Akarolo-Anthony S, Akinyemi R, et al. The burden of stroke in Africa : a glance at the present and a glimpse into the future : review article. Cardiovasc J Afr. 2015;26:27–38. doi: 10.5830/CVJA-2015-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ezejimofor MC, Uthman OA, Maduka O, Ezeabasili AC, Onwuchekwa AC, Ezejimofor BC, et al. Stroke survivors in Nigeria: a door-to-door prevalence survey from the Niger Delta region. J Neurol Sci. 2017;372:262–269. doi: 10.1016/j.jns.2016.11.059. [DOI] [PubMed] [Google Scholar]
- 5.Ezejimofor MC, Chen Y-F, Kandala N-B, Ezejimofor BC, Ezeabasili AC, Stranges S, et al. Stroke survivors in low- and middle-income countries: a meta-analysis of prevalence and secular trends. J Neurol Sci. 2016;364:68–76. doi: 10.1016/j.jns.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Benois A, Raynaud L, Coton T, Petitjeans F, Hassan A, Ilah A, et al. [Morbidity and mortality after intensive care management of hemorrhagic stroke in Djibouti] Med Trop Rev Corps Sante Colon. 2009;69:41–44. [PubMed] [Google Scholar]
- 7.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st Century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. Buddy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buck CJ. Edition 1e. Philadelphia: Saunders; 2016. 2017 ICD-10-CM Physician Professional Edition. [Google Scholar]
- 9.Heuschmann PU, Wiedmann S, Wellwood I, Rudd A, Di Carlo A, Bejot Y, et al. Three-month stroke outcome: the European Registers of Stroke (EROS) investigators. Neurology. 2011;76:159–165. doi: 10.1212/WNL.0b013e318206ca1e. [DOI] [PubMed] [Google Scholar]
- 10.Limbole EB, Magne J, Lacroix P. Stroke characterization in Sun Saharan Africa: congolese population. Int J Cardiol. 2017;240:392–397. doi: 10.1016/j.ijcard.2017.04.063. [DOI] [PubMed] [Google Scholar]
- 11.N'goran YNK, Traore F, Tano M, Kramoh KE, Kakou J-BA, Konin C, et al. Aspects épidémiologiques des accidents vasculaires cérébraux (AVC) aux urgences de l'institut de cardiologie d'Abidjan (ICA) Pan Afr Med J. 2015;21:1–5. doi: 10.11604/pamj.2015.21.160.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagui E. Stroke in sub-Saharan Africa. Med Trop Rev Corps Sante Colon. 2007;67:596–600. [PubMed] [Google Scholar]
- 13.Hollander M, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Breteler MMB. Incidence, risk, and case fatality of first ever stroke in the elderly population. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2003;74:317–321. doi: 10.1136/jnnp.74.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard G, Kissela BM, Kleindorfer DO, McClure LA, Soliman EZ, Judd SE, et al. Differences in the role of black race and stroke risk factors for first vs recurrent stroke. Neurology. 2016;86:637–642. doi: 10.1212/WNL.0000000000002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarfo FS, Acheampong JW, Appiah LT, Oparebea E, Akpalu A, Bedu-Addo G. The profile of risk factors and in-patient outcomes of stroke in Kumasi, Ghana. Ghana Med J. 2014;48:127–134. doi: 10.4314/gmj.v48i3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sène Diouf F, Basse AM, Ndao AK, Ndiaye M, Touré K, Thiam A, et al. Pronostic fonctionnel des accidents vasculaires cérébraux dans les pays en voie de développement : Sénégal. Ann Réadapt Médecine Phys. 2006;49:100–104. doi: 10.1016/j.annrmp.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Keita AD, Toure M, Diawara A, Coulibaly Y, Doumbia S, Kane M, et al. Epidemiological aspects of stroke in CT-scan department of the Point-G Hospital in Bamako. Mali. Med Trop Rev Corps Sante Colon. 2005;65:453–457. [PubMed] [Google Scholar]
- 18.Diagana M, Traore H, Bassima A, Druet-Cabanac M, Preux PM, Dumas M. Contribution of computerized tomography in the diagnosis of cerebrovascular accidents in Nouakchott, Mauritania. Med Trop Rev Corps Sante Colon. 2002;62:145–149. [PubMed] [Google Scholar]
- 19.Kapral MK, Fang J, Hill MD, Silver F, Richards J, Jaigobin C, et al. Sex Differences in Stroke Care and Outcomes: Results From the Registry of the Canadian Stroke Network. Stroke. 2005;36:809–814. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]
- 20.Choi JC, Lee JS, Kang S-Y, Kang J-H, Bae J-M. Family history and risk for ischemic stroke: Sibling history is more strongly correlated with the disease than parental history. J Neurol Sci. 2009;284:29–32. doi: 10.1016/j.jns.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Sylaja PN, Pandian JD, Kaul S, Srivastava MVP, Khurana D, Schwamm LH, et al. Ischemic stroke profile, risk factors, and outcomes in India: the Indo-US collaborative stroke project. Stroke. 2018;49:219–222. doi: 10.1161/STROKEAHA.117.018700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacMahon S, Peto R, Collins R, Godwin J, Cutler J, Sorlie P, et al. Blood pressure, stroke, and coronary heart disease: Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. The Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 23.O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. The Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 24.Desalu OO, Wahab KW, Fawale B, Olarenwaju TO, Busari OA, Adekoya AO, et al. A review of stroke admissions at a tertiary hospital in rural Southwestern Nigeria. Ann Afr Med. 2011;10:80. doi: 10.4103/1596-3519.82061. [DOI] [PubMed] [Google Scholar]
- 25.Delbari A, Roghani RS, Tabatabaei SS, Rahgozar M, Lokk J. Stroke epidemiology and one-month fatality among an urban population in Iran. Int J Stroke. 2011;6:195–200. doi: 10.1111/j.1747-4949.2010.00562.x. [DOI] [PubMed] [Google Scholar]
- 26.Bamekhlah RM, Bin-Nabhan AS, Musaian NS. Risk factors and clinical presentation of Stroke in Mukalla, Hadhramout, Republic of Yemen. Alandalus J Appl Sci. 2014;391:1–19. doi: 10.12816/0028801. [DOI] [Google Scholar]
- 27.Damasceno A, Gomes J, Azevedo A, Carrilho C, Lobo V, Lopes H, et al. An epidemiological study of stroke hospitalizations in Maputo, Mozambique: a high burden of disease in a resource-poor country. Stroke. 2010;41:2463–2469. doi: 10.1161/STROKEAHA.110.594275. [DOI] [PubMed] [Google Scholar]
- 28.Deresse B, Shaweno D. Epidemiology and in-hospital outcome of stroke in South Ethiopia. J Neurol Sci. 2015;355:138–142. doi: 10.1016/j.jns.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Marrone LCP, Diogo LP, Oliveira FM de, Trentin S, Scalco RS, Almeida AG de, et al. Risk factors among stroke subtypes in Brazil. J Stroke Cerebrovasc Dis. 2013;22:32–35. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Kurth T, Everett BM, Buring JE, Kase CS, Ridker PM, Gaziano JM. Lipid levels and the risk of ischemic stroke in women. Neurology. 2007;68:556–562. doi: 10.1212/01.wnl.0000254472.41810.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tirschwell DL, Smith NL, Heckbert SR, Lemaitre RN, Longstreth WT, Psaty BM. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 2004;63:1868–1875. doi: 10.1212/01.WNL.0000144282.42222.DA. [DOI] [PubMed] [Google Scholar]
- 32.Visscher PM, Medland SE, Ferreira MAR, Morley KI, Zhu G, Cornes BK, et al. Assumption-free estimation of heritability from genome-wide identity-by-descent sharing between full siblings. PLOS Genet. 2006;2:e41. doi: 10.1371/journal.pgen.0020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omori T, Kawagoe M, Moriyama M, Yasuda T, Ito Y, Hyakuta T, et al. Multifactorial analysis of factors affecting recurrence of stroke in Japan. Asia Pac J Public Health. 2015;27:NP333–NP340. doi: 10.1177/1010539512441821. [DOI] [PubMed] [Google Scholar]
- 34.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham study. Stroke. 1991;22:312–318. doi: 10.1161/01.STR.22.3.312. [DOI] [PubMed] [Google Scholar]
- 35.Bhat VM, Cole JW, Sorkin JD, Wozniak MA, Malarcher AM, Giles WH, et al. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke. 2008;39:2439–2443. doi: 10.1161/STROKEAHA.107.510073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong K-S, Bang OY, Kang D-W, Yu K-H, Bae H-J, Lee JS, et al. Stroke Statistics in Korea: Part I. Epidemiology and Risk Factors: a Report from the Korean stroke society and clinical research center for stroke. J Stroke. 2013;15:2–20. doi: 10.5853/jos.2013.15.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.BEN MAHJOUB N. Facteurs prédictifs du pronostic fonctionnel et vital des hémiplégiques vasculaires. Thèse. Faculté de Médecine Ibn El Jazzar, 2017.
- 38.Bawazeer AA, Hattab AS, Morales E. First cigarette smoking experience among secondary-school students in Aden, Republic of Yemen. East Mediterr Health J Rev Sante Mediterr Orient Al-Majallah Al-Sihhiyah Li-Sharq Al-Mutawassit. 1999;5:440–449. [PubMed] [Google Scholar]
- 39.Mega TA, Dabe NE. Khat (Catha Edulis) as a risk factor for cardiovascular disorders: systematic review and meta-analysis. Open Cardiovasc Med J. 2017;11:146–155. doi: 10.2174/1874192401711010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Motarreb A, Al-Habori M, Broadley KJ. Khat chewing, cardiovascular diseases and other internal medical problems: the current situation and directions for future research. J Ethnopharmacol. 2010;132:540–548. doi: 10.1016/j.jep.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Haft JI, Kranz PD, Albert FJ, Fani K. Intravascular platelet aggregation in the heart induced by norepinephrine: microscopic studies. Circulation. 1972;46:698–708. doi: 10.1161/01.CIR.46.4.698. [DOI] [PubMed] [Google Scholar]
- 42.Mujlli HM, Bo X, L Z. The effect of Khat (Catha edulis) on acute cerebral infarction. Neurosci Riyadh Saudi Arab. 2005;10:219–222. [PubMed] [Google Scholar]
- 43.Ali WM, Zubaid M, Al-Motarreb A, Singh R, Al-Shereiqi SZ, Shehab A, et al. Association of khat chewing with increased risk of stroke and death in patients presenting with acute coronary syndrome. Mayo Clin Proc. 2010;85:974–980. doi: 10.4065/mcp.2010.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massoure PL, Roche NC, Lamblin G, Topin F, Dehan C, Kaiser E, et al. [Heart failure patterns in Djibouti: epidemiologic transition] Med Sante Trop. 2013;23:211–216. doi: 10.1684/mst.2013.0188. [DOI] [PubMed] [Google Scholar]
- 45.Ragoschke-Schumm A, Walter S, Haass A, Balucani C, Lesmeister M, Nasreldein A, et al. Translation of the ‘time is brain’ concept into clinical practice: focus on prehospital stroke management. Int J Stroke. 2014;9:333–340. doi: 10.1111/ijs.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.R García Ruiz, J Silva Fernández, RM García Ruiz, M Recio Bermejo, Á Arias Arias, del Saz Saucedo P, et al. Response to symptoms and prehospital delay in stroke patients. is it time to reconsider stroke awareness campaigns? J Stroke Cerebrovasc Dis. 2018;27:625–632. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 47.Denti L, Caminiti C, Scoditti U, Zini A, Malferrari G, Zedde ML, et al. Impact on prehospital delay of a stroke preparedness campaign: A SW-RCT (Stepped-Wedge Cluster Randomized Controlled Trial) Stroke. 2017;48:3316–3322. doi: 10.1161/STROKEAHA.117.018135. [DOI] [PubMed] [Google Scholar]
- 48.Urimubenshi G, Cadilhac DA, Kagwiza JN, Wu O, Langhorne P. Stroke care in Africa: a systematic review of the literature. Int J Stroke. 2018;13:797–805. doi: 10.1177/1747493018772747. [DOI] [PubMed] [Google Scholar]
- 49.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/American stroke association. Stroke. 2018;49:e46–110. doi: 10.1161/STR.0000000000000158. 2018. [DOI] [PubMed] [Google Scholar]
- 50.Longo-Mbenza B, Mbuilu Pukuta J, Tshinkwela ML. Rates and predictors of stroke-associated case fatality in black Central African patients. Cardiovasc J Afr. 2008;19:72–76. [PMC free article] [PubMed] [Google Scholar]
- 51.Matuja W, Janabi M, Kazema R, Mashuke D. Stroke subtypes in black tanzanians: a retrospective study of computerized tomography scan diagnoses at Muhimbili National Hospital, Dar es. Trop Doct. 2004;34:144–146. doi: 10.1177/004947550403400305. [DOI] [PubMed] [Google Scholar]
- 52.Soize S, Kadziolka K, Estrade L, Serre I, Bakchine S, Pierot L. Mechanical Thrombectomy in acute stroke: prospective pilot trial of the Solitaire FR Device while Under Conscious Sedation. Am J Neuroradiol. 2013;34:360–365. doi: 10.3174/ajnr.A3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sounga Bandzouzi PE, Sow Adjaratou D, Dadah SLM, Moustapha N, Diop Amadou G, Ndiaye Mouhamadou M. Aspects épidémiocliniques, évolutifs et paracliniques de l'accident vasculaire cérébral hémorragique du sujet âgé à Dakar. Rev Neurol. 2016;172:A72. doi: 10.1016/j.neurol.2016.01.171. [DOI] [Google Scholar]
- 54.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. The Lancet. 2000;355:1670–1674. doi: 10.1016/S0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 55.Napon C, Dabilgou A, Kyelem J, Bonkoungou P, Kaboré J. Therapeutic route of patients at the acute phase of their stroke in Burkina Faso. J Neurol Sci. 2017;372:75–77. doi: 10.1016/j.jns.2016.11.017. [DOI] [PubMed] [Google Scholar]