Abstract
Background
Lifestyle intervention is the mainstay therapy for Non-Alcoholic Fatty Liver Disease (NAFLD). We aimed to assess the efficacy of an intensive (9 contact points in 6 months) weight-loss intervention among patients with obesity (BMI 25–39.9 kg/m2) and NAFLD in north India.
Methods
A total of 140 patients (18–60 years) with obesity and NAFLD were randomized into intervention (n = 70) and control (n = 70) groups, at a tertiary-care hospital. Weight, anthropometric parameters, Controlled Attenuation Parameter (CAP), Liver Stiffness Measurement (LSM), liver enzymes, grade of fatty liver and HOMA-IR were measured at baseline (T0) and 6 months (T6). There was a high drop-out, exacerbated by the Covid-19 pandemic. Completers comprised of 59 participants (n = 30 intervention, n = 29 control). Intention to treat analysis was done.
Results
At T6, ALT normalized in significantly higher (p = 0.03) number of cases in the intervention arm (66.7%) versus control arm (18.2%). No significant improvement was seen in other metabolic, ultrasound or anthropometric outcomes. Weight (p < 0.001), AST (p = 0.01), ALT (p = 0.02), body fat% (p < 0.001), WC (p < 0.001) and CAP (p < 0.001) significantly improved within the intervention arm along with a trend of improvement in steatosis and HOMA-IR. Control group showed significant decrease in weight (p < 0.001), WC (p < 0.001) and CAP (p = 0.02). Twice the number of patients in intervention arm (46.7%) lost ≥5% weight, compared to control arm (24.1%) (p = 0.07).
Conclusion
The intensive weight-loss intervention was not effective in improving the treatment outcomes among patients with obesity and NAFLD. However, given the potential of our intervention, we recommend larger trials with more intensive weight-loss interventions.
Subject terms: Non-alcoholic fatty liver disease, Lifestyle modification
Introduction
Weight loss (5–10%) through lifestyle management is the first-line treatment for patients with obesity and Non Alcoholic Fatty Liver Disease (NAFLD) [1]. The amount of weight loss is proportional to the degree of liver histological improvement [2, 3]. Other benefits include improvement in enzymes, fat content and histology of the liver [4, 5].
However, adherence to lifestyle change is usually difficult to maintain as NAFLD is an asymptomatic disease [6]. The success of treatment depends on the intensity of the weight-loss interventions and frequency of visits to the healthcare professionals [7]. Prescriptions need to be reinforced time to time through structured programs to have positive patient outcomes [8].
Trials with different treatment intensities have shown success of intensive weight–loss interventions in NAFLD [9, 10]. Results from western trials cannot be generalized for patients across developing countries because of differences in socioeconomic, cultural, dietary and lifestyle factors [11]. To our knowledge, no trials from India have investigated the impact of intensive weight-loss interventions to treat NAFLD. Some prospective follow up studies have shown positive effects of diet and physical activity counseling in NAFLD, but the quality of evidence generated from these studies is arguable [12, 13].
Intensive weight-loss interventions have the potential to come out as sustainable and cost effective therapy for obese patients with NAFLD seeking treatment in hospital and community settings. Thus, this preliminary phase II trial was planned, based on the hypothesis that intensive weight-loss intervention along with standard care may be superior to standard care alone in improving metabolic, ultrasound and anthropometric parameters in obese patients with NAFLD.
Patients and methods
This six month long, open-label, parallel group, randomized–controlled preliminary phase II study was carried out in a tertiary-care center in India. The study was approved by the Institute Ethics Committee (IEC- 434/04.08.2017) and was registered on the CTRI website (CTRI/2018/04/013179), available as supplementary file 1. Informed consent was taken from all subjects. Study methods and reporting were conducted in accordance with the CONSORT 2010 guidelines. The primary objective was to assess the efficacy of an intensive weight-loss intervention on metabolic, anthropometric, and ultrasound parameters of NAFLD patients as compared to the standard care.
Study subjects
Among the patients attending the Gastroenterology and Medicine outpatient clinic between July 2018-November 2019, 313 patients with ultrasonography (USG) diagnosed NAFLD were screened. Of these, 140 patients, age 18–60 years, with ability to read and write in English/Hindi and willing to give informed consent were included. Males and females consuming >30 g/day or >20 g/day of alcohol respectively, pregnant/lactating women, diagnosed cases of endocrine disorders including Cushing’s syndrome, uncontrolled diabetes (HbA1c > 6.8%) and thyroid disease, patients with a history of long-term steroid intake, with any other secondary causes of fatty liver, psychiatric illness, current participation in a formal weight loss programme, or who had made significant changes in diet and exercise habits in previous 3 months resulting in weight loss >5% of body weight were excluded from the trial.
Sample size
In the absence of any prior Indian data on the efficacy of weight loss interventions in NAFLD, sample size calculation was not feasible. Therefore, a preliminary phase II RCT was planned. Seventy patients were recruited in each arm (140 patients in total) in this trial, keeping in mind the available cases of NAFLD at the tertiary care hospital, expected dropout of 20% and existing resources to carry out intensive weight-loss intervention.
Randomization and blinding
The eligible 140 patients were randomized 1:1 to receive either intensive weight-loss intervention or standard care for 6 months. Randomization was done using a computer-generated permuted-block randomization sequence generated by a statistician who was not associated with the conduct of study. Allocation of participants was concealed through a sealed opaque envelope method. Radiographers and pathologists who analyzed the results were blinded to the treatment.
Treatment details
Control group
Patients in the control group received standard care that included evaluation of the patient by clinician, biochemical investigations (liver function test (LFT), fasting blood sugar, fasting insulin, USG and Fibroscan). Medications were prescribed as per co-morbidities (diabetes, blood pressure, dyslipidemia, hypothyroidism), if required. A 10–15 minutes diet-counseling session coupled with general exercise advice was given by the hospital dietitian. A follow up at three and six months was advised.
Intervention group
Patients in the intervention group participated in a scientifically designed, education-based, personalized intensive lifestyle intervention, along with standard care. A comprehensive table describing the intervention framework is given as supplementary Table 1. Full details describing the session in terms of length, type and content and tools used in each session are already published by the authors [14]. The intervention comprised of 9 individual-based sessions (5 face to face and 4 telephonic) within 6 months. During content validation by the experts, the intensity of the intervention was set in a manner that it does not overwhelm the participants (5 face to face and 4 telephonic within 6 months), nor does it create excessive burden on the health professional working in resource-constrained settings [14].
Evaluation and monitoring of patients
Weight, height and waist circumference (WC), hip circumference (HC) were measured using standard procedures. Body fat % was calculated using bioelectrical impedance analyser (Bodyvis BCA-2A). Liver enzymes (AST and ALT), fasting glucose, insulin resistance index HOMA-IR were acquired/calculated from medical records. Severity of NAFLD was assessed by USG and results were interpreted by a single experienced investigator. The degree of steatosis during USG was graded as absent (grade 0), mild (grade 1), moderate (grade 2) and severe (grade 3). LSM (Liver Stiffness Measurement) and CAP (Controlled Attenuation Parameter) measurement for liver stiffness and liver fat content (10 successful readings) were performed using a FibroScan touch 502 (Echosens, Paris, France) by a single operator. An M and XL probes were used for patients with a body mass index of <30 kg/m2 and ≥30 kg/m2 respectively [15].
Data collection
Data were collected for all outcomes such as weight, WC, body-fat%, grade of steatosis, CAP, LSM, liver enzymes and glycemic profile at baseline (T0) and at six months (T6). In addition, weight, WC and body-fat % were collected at three months as a part of standard care for both groups.
Drop out
In the intervention group, drop-out was determined if a patient missed two out of five face to face sessions and was unable to be contacted via phone/refused to come for further visits. In control group, drop-out was defined when the patient did not return for the final assessment at T6.
Study outcomes
The primary outcome of the intervention was weight-loss which was set at 5% for patients with normal liver enzymes and at 10% for patients with raised liver enzymes. Raised liver enzymes was defined as AST levels >40 IU/L and ALT levels greater than 45 IU/L, as per the cut offs used in the tertiary care hospital laboratory.
Other outcomes studied were: (a) Improvement in CAP (b) Improvement in liver enzymes (c) Reduction in grade of steatosis (d) Reduction in WC (e) Reduction in body-fat% (f) Improvement in HOMA-IR.
Statistical analysis
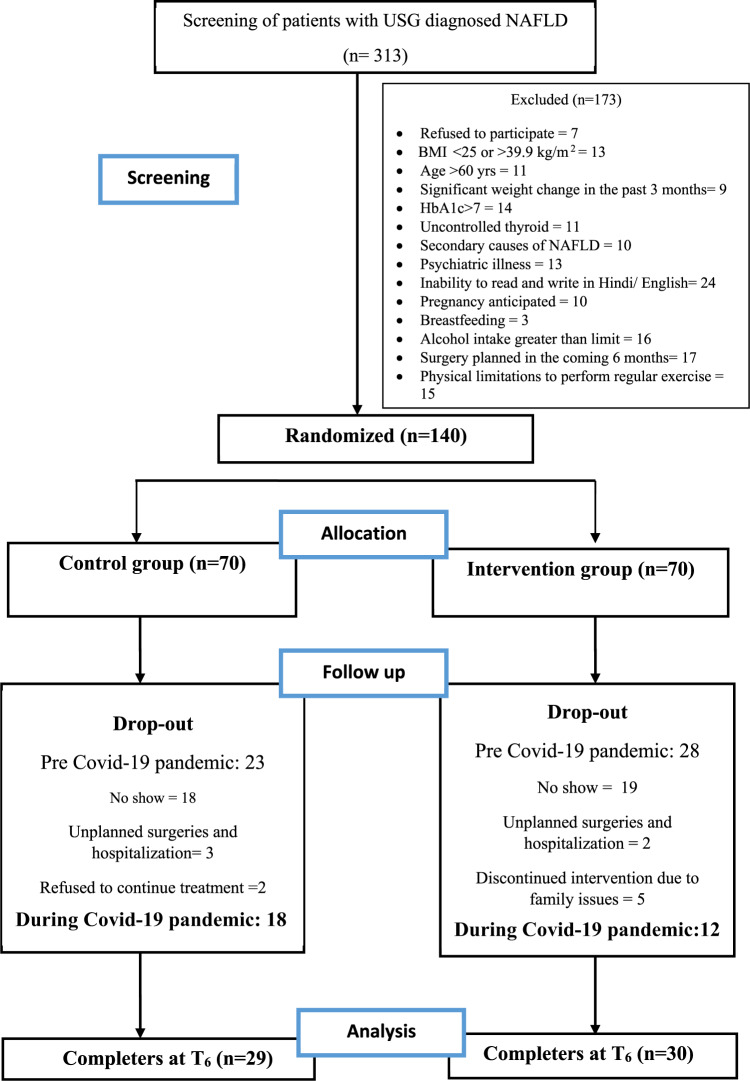
A total of 140 patients were recruited in the study. In the current analysis, we included only those who participated in both the baseline (T0) and end line measurement (T6) (Fig. 1). A total of 59 participants remained for the present analysis: 30 in intervention group and 29 in control group.
Fig. 1.
Flow chart of recruitment and participants.
Data analysis was done in a blinded manner by a statistician. Intention to treat analysis was done to compare the outcome measures between the intervention and control groups for all the participants whose end point measures were available. Normally distributed quantitative variables were expressed as mean ± standard deviation and those variables with skewed distribution as median with range. Categorical data were presented as frequency with percentages. Chi square test/Fisher exact test was used to find association between qualitative variables. Unpaired t test/Wilcoxon rank-sum test was used to compare quantitative characteristics between two groups. To compare quantitative variables within the group from pre to post, paired t test/Wilcoxon’s sign rank test was performed. Linear regression was also performed to find out the relationship of outcome variables with group variable with adjustment of baseline values. To compare categorical variables within the group, McNemar’s chi-squared test was performed. All p-values were two sided, and values less than 0.05 were considered statistically significant.
Baseline characteristics were also compared between those who dropped out from the study and those who did not (Supplementary Table 2). Apart from being younger and having a lower AST, there were no differences between the completers or drop outs. Exploratory correlation analysis was also performed correlating changes in weight with changes in liver markers.
Results
A total of 313 USG diagnosed NAFLD patients, were screened for inclusion in the study. Of these, 140 patients who fulfilled the eligibility criteria were randomized to either control (n = 70) or intervention (n = 70) group. Drop-out in control and intervention arm was 23 and 28 (pre-Covid-19) and 18 and 12 (during Covid-19) respectively (Fig. 1). 12 out of 30 (40%) participants in the intervention group attended all 9 sessions while rest attended 6 to 8 sessions.
Baseline characteristics of the participants
The mean age of participants was 42.79 ± 10.30 years (control) and 41.1 ± 10.75 years (intervention) and the proportion of females was lower [12 (41.38%) control; 11(36.67%) intervention, p = 0.13]. Overall, baseline socio-demographic, clinical characteristics and anthropometric parameters of participants were similar in both groups (Table 1).
Table 1.
Baseline characteristics of completers in control and intervention groups.
| Parameter | Control group (Mean ± SD) (n = 29)T0 | Intervention group (Mean ± SD) (n = 30) T0 | p value |
|---|---|---|---|
| Socio-demographic profile n (%) | |||
| Age (years) | 42.8 ± 10.3 | 41.1 ± 10.8 | 0.53 |
| Females | 12 (41.4%) | 11 (36.7%) | 0.13 |
| Education | |||
| Graduate and above | 9 (31.0) | 18 (60.0) | 0.07 |
| 9th to 12th standard | 15 (51.7) | 8 (26.7) | |
| Upto 8th class | 5 (17.2) | 4 (13.3) | |
| Employed | 19 (65.5) | 20 (66.7) | 0.92 |
| Married | 26 (89.7) | 27 (90.0) | 0.96 |
| Socio-economic status | |||
| Upper class | 11 (37.9) | 18 (60.0) | 0.39 |
| Upper middle class | 15 (51.7) | 10 (33.3) | |
| Lower middle class | 1 (3.4) | 1 (3.3) | |
| Upper lower class | 2 (6.9) | 1 (3.3) | |
| Anthropometric and biochemical profile (Mean ± SD) | |||
| Weight (kg) | 81.8 ± 10.3 | 82.3 ± 14.8 | 0.88 |
| Height (cm) | 163.9 ± 10.0 | 163.4 ± 10.3 | 0.83 |
| BMI (kg/m2) | 30.5 ± 3.4 | 30.7 ± 3.8 | 0.82 |
| Waist circumference (cm) | 106.6 ± 7.6 | 105.2 ± 11.8 | 0.58 |
| Hip circumference (cm) | 104.8 ± 7.0 | 107.4 ± 8.9 | 0.23 |
| Body fat % | 33.5 ± 7.3 | 34.3 ±7.1 | 0.65 |
| Fat free mass (kg) | 54.8 ± 9.8 | 54.4 ± 11.9 | 0.89 |
| Total body water (kg) | 39.9 ± 7.4 | 39.4 ± 8.6 | 0.81 |
| Protein % | 13.7 ± 1.5 | 13.5 ± 1.5 | 0.67 |
| Skeletal muscle mass (kg) | 36.5 ± 6.8 | 36.2 ± 7.9 | 0.86 |
| BMR (kcal/day) | 1564 ± 268 | 1604 ± 358 | 0.62 |
| Trunk fat mass (kg) | 13.6 ± 3.0 | 13.9 ± 3.9 | 0.70 |
| Insulin resistance Median (min - max) | |||
| Fasting insulina (μU/mL) | 9.5 (1.3-27.4) | 10 (1.17-31) | 0.46 |
| HOMA IRa | 2.2 (0.5-7.2) | 2.3 (0.3-6.4) | 0.81 |
| Liver enzymes | |||
|
AST (IU/L) Median (Range) |
35.2 ± 16.0 32 (16-91) |
40.9 ± 33.1 26.5 (14-159) |
0.52 |
|
ALT (IU/L) Median (Range) |
47.4 ± 28.2 36 (11-121) |
51.7 ± 41.6 35.5 (14-158) |
0.81 |
| Raised ALT levels | |||
| n (%) | 11 (37.93%) | 12 (40%) | 0.87 |
|
ALP (IU/L) Median (Range) |
240.9 ± 84.9 260 (54-418) |
209.3 ± 95.0 217.5 (57-402) |
0.14 |
| Liver stiffness measurement | |||
| (LSM) (kPa) | 6.7 ± 2.7 | 8.7 ± 6.2 | 0.23 |
| Liver fat | |||
| CAP (Db/m) | 329.2 ± 30.3 | 329.5 ± 31.2 | 0.96 |
| Grade of fatty liver | |||
| Grade 1 n(%) | 14 (48.27) | 14 (46.67) | 0.89 |
| Grade 2 n(%) | 1 4 (48.27) | 16 (53.33) | |
| Grade 3 n(%) | 1 (3.44) | 0 | |
Chi-square, Fisher’s exact, Mann-Whitney or Student’s t test, as appropriate. Data are presented as mean ± SD, Median (Range) or as n(%). ALT alanine aminotransferase, AST aspartate aminotransferase, CAP controlled attenuation parameter, LSM liver stiffness measurement.
an = 16 in control arm and n = 23 in intervention arm for Fasting insulin and HOMA IR.
Effect of intervention on anthropometric and body composition parameters
Weight-loss was defined as 5% for patients with normal liver enzymes and 10% for patients with raised liver enzymes at 6 months. Significant weight loss was seen within both the control (p < 0.001) and in the intervention arm (p < 0.001). Though the mean weight loss at T6 was higher in the intervention group (3.7 kg) than the control group (2.22 kg), the difference between the two groups was not significant (Table 2). The proportion of patients who lost weight in the intervention arm (n = 8; 26.67%) was four times that (n = 2; 6.9%) in the control arm (p = 0.08).
Table 2.
Comparison of outcome measures within and between control and intervention group.
| Outcome measure | Control group (Mean ± SD) (n = 29) | Intervention group (Mean ± SD) (n = 30) | Mean differenceb with 95% CI | ||||
|---|---|---|---|---|---|---|---|
| T0 | T6 | Mean difference with 95%CI | T0 | T6 | Mean difference with 95%CI | ||
| Weight status | |||||||
| Weight | 81.8 ± 10.3 | 79.6 ± 10.9* | -2.22 (−3.33, -1.11) | 82.3 ± 14.8 | 78.6 ± 13.6* | -3.69 (−4.99, -2.39) | -1.44 (−0.20 to 3.09) |
| Weight loss >5% n (%) | - | 7 (24.1%) | - | - | 14 (46.7%) | - | |
| Weight loss >10% n (%) | - | 1 (3.4%) | - | - | 2 (6.7%) | - | |
| Overall weight lossa n (%) | - | 2 (6.9%) | - | - | 8 (26.7%) | - | |
| Anthropometry and body composition | |||||||
| Waist circumference | 106.6 ± 7.6 | 104.1 ± 8.0* | -2.57 (−3.67, -1.47) | 105.2 ± 11.8 | 101.3±11.6* | -3.9 (−5.50, -2.30) | -1.42 (−3.32 to 0.47) |
| Body fat % | 33.5 ± 7.3 | 32.8 ± 7.6 | -0.64 (−1.66, 0.37) | 34.3 ±7.1 | 32.5 ± 7.12* | -1.77 (−2.46, -1.08) | -1.11 (−2.31 to 0.08) |
| Liver enzymes | |||||||
|
AST (IU/L) Median (Range) |
35.2 ± 16.0 32 (16-91) |
31.1 ± 10.3 31 (22-63) |
-4.09 (−10.05, 1.87) |
40.9 ± 33.1 26.5 (14-159) |
27.6 ± 10.8* 24 (12-38) |
-12.77 (−22.56, -2.98) | -4.29 (−0.09 to 8.68) |
|
ALT (IU/L) Median (Range) |
47.4 ± 28.2 36 (11-121) |
41.9 ± 30.8 32 (13-170) |
−5.48 (−13.44, 2.48) |
51.7 ± 41.6 35.5 (14-158) |
34.1 ± 14.8* 35.5 (10-73) |
-17.6 (−30.5, -4.70) | -9.55 (−0.58 to 19.69) |
| Fibroscan | |||||||
| LSM (kPa) | 6.7 ± 2.7 | 6.5 ± 2.5 | −0.18 (−0.96, 0.60) | 8.7 ± 6.2 | 7.5 ± 4.5 | -1.13 (−2.12, -0.15) | -0.28 (−1.22 to 0.66) |
| CAP (dB/m) | 329.2 ± 30.3 | 312.3 ±39.0* | −16.90 (−31.81, −1.98) | 329.5 ± 31.2 | 293.8 ± 41.8* | -35.7 (−52.27, -19.13) | -18.61 (−38.71 to 1.47) |
| Grade of steatosis | |||||||
|
Grade 0 Grade 1 Grade 2 Grade 3 |
0 14 (48.27) 14 (48.27) 1(3.44) |
1 (3.4) 15 (51.7) 13 (44.8) 0 |
- |
0 14 (46.67) 16(53.33) 0 |
3 (10) 17 (56.7) 9 (30) 1 (3.3) |
- | -0.13 (−0.13 to 0.40) |
| Insulin resistance | |||||||
|
HOMA IR Median (range) (n) |
2.2 (0.5-7.2) (16) |
2.6 (0.36-4.7) (13) |
-0.21 (0-0.98, 0.56) |
2.3 (0.3-6.4) (23) |
1.9 (0.28-5.94) (19) |
-0.4 (−0.78, -0.02) | -0.20 (−0.82 to 0.41) |
aWeight loss for the purpose of the trial was defined as 5% loss of weight in patients with normal liver enzymes and 10% weight loss in patients with raised liver enzymes.
bAverage change between the groups obtained through regression analysis after adjusting baseline value for each outcome measure.
*comparison within group (T6 vs T0), p < 0.05.
The number of patients with normal liver enzymes was 36 (18 in each arm). Of these, 2 (11.1%) in the control arm, and 7 (38.9%) in the intervention arm lost ≥5% weight. Among the patients who had raised liver enzymes (n = 23; 11 in control, 12 in intervention arm), none in control arm and only one in the intervention arm lost ≥ 10% weight. Irrespective of the level of liver enzymes, only three patients lost ≥10% body weight. We also computed the percent change in weight from T0 to T6 individually for each patient and calculated the mean, which was found to be higher in the intervention arm (4%) as compared to the control arm (2.9%).
The mean WC at T6 was 2.8 cm lower in the intervention arm (101.3 ± 11.6 cm) than the control group (104.1 ± 8.0 cm), though the difference was not significant (Table 2). The WC reduced significantly from T0 to T6 within both the arms - control (p < 0.001) and intervention (p < 0.001).
No significant change in body fat % was seen between the two groups at T6. Body-fat % significantly reduced from T0 to T6 within the intervention arm (p < 0.001) only.
Effect of intervention on metabolic parameters
Normalization of ALT was seen in a significantly higher (p = 0.03) number of cases in the intervention arm (n = 8; 66.7%) than the control arm (n = 2; 18.2%) at T6. An improvement was also observed in liver enzymes—AST (31.1 ± 10.3 IU/L versus 27.6 ± 10.8 IU/L) and ALT (41.9 ± 30.8 IU/L versus 34.1 ± 14.8 IU/L) at T6, but the change was not significant between the control versus intervention groups (Table 2). However, a significant decline was seen in AST (p = 0.01) and ALT (p = 0.02) within the intervention group (Table 2).
Average HOMA-IR decreased by 0.4 (2.3–1.9) units in the intervention arm and increased by 0.39 (2.6 from 2.2) units in the control arm, but the change was not significant either within or between the two arms at T6.
Effect of intervention on ultrasound parameters
The distribution of patients among the three grades of steatosis was similar in both the groups at T0 (Table 1). At T6, though improvement in steatosis was better in intervention arm, the change was not significant between the two groups.
At T6, steatosis reversed completely among three patients in the intervention arm and one in the control arm. Though steatosis improved from grade 2 to 1 in six patients, it worsened from grade 2 to grade 3 in one patient in the intervention group. In the control group, steatosis improved from grade 3 to grade 2, and from grade 2 to grade 1 in one patient each.
In terms of liver fat content, CAP was lower in intervention group (293.8 ± 41.8 dB/m) as compared to control (312.3 ± 39.0 dB/m) at T6, but the difference between the two groups was not significant (Table 2). Reduction in liver fat was significant within both the arms—control (p = 0.02); intervention arm (p < 0.001) at T6. Liver stiffness measurement (LSM) at T6 improved by 0.09 kPa and 1.14kPa in the control and intervention arm respectively, though no significant change was seen within or between two groups.
An additional correlation analysis was performed correlating changes in weight with changes in liver markers. No significant correlation between change in weight and change in levels of AST, ALP and ALT was found in the intervention group. However, in the control group, a significant correlation was seen between change in weight and change in ALT (ρ = 0.55, p = 0.001). A significant positive correlation was also seen in change in weight and change in CAP score in both the intervention (ρ = 0.45, p = 0.01), as well as control arm (ρ = 0.50, p = 0.005).
Linear regression was performed to find out the effect of the intervention on the outcome at 6 months after adjustment for baseline values. The coefficient for AST at 6 months is −4.29 (−0.09 to 8.68) and ALT is −9.55 (−0.58 to 19.69) in the intervention arm, compared to the control arm. The coefficient for weight and CAP at 6 months was found to be −1.44 (−0.20 to 3.09) and −18.61 (−38.71 to 1.47) respectively in the intervention arm versus the control arm (Table 2).
Discussion
The efficacy of intensive weight-loss interventions in managing NAFLD remains largely unexplored in India. We evaluated the effect of an intensive weight-loss intervention on metabolic, ultrasound and anthropometric parameters among adult Indian patients with obesity and NAFLD.
At end line, a significantly higher number of patients in the intervention arm improved in ALT levels compared to the control arm. No significant difference was found between the two groups in any of the other metabolic, ultrasound or anthropometric outcomes. However, AST, ALT and body-fat% significantly improved within the intervention group. Probably a higher sample size would have shown a significant improvement in these outcomes. The trend of positive outcomes within our intervention arm highlights the potential of our intensive lifestyle intervention to improve NAFLD-related outcomes among Indian patients with obesity. Our results are in alignment with a recent Chinese trial comparing standard care to intensive lifestyle intervention in a 2-year intervention period [16].
Weight loss is the predictor of all NAFLD-related histologic improvements [17]. In our study, a considerably higher number of patients in the intervention group lost weight ≥5% (46.7%) and ≥10% (6.7%) as compared to the control group. However, this weight loss was less than that reported in a study, where a structured intensive lifestyle intervention delivered to patients with NAFLD for 6-months led to weight loss of ≥5% and ≥10% in 55.8% and 15.6% participants respectively. The weekly face-to-face contacts along with physiologist-supervised exercise sessions might have resulted in better weight loss outcome in this study [18].
A modest reduction in body-weight can result in improvement in insulin sensitivity, changes in body and liver fat depots and improvements in LFT [19, 20]. Our trial resulted in a modest 4% weight-loss in the intervention arm, along with marked improvement in whole-body adiposity and liver enzymes. A trend of improvement was also seen in WC, liver-fat content and severity of hepatic steatosis. Though a modest improvement in HOMA-IR is seen in our trial, a considerable improvement in the liver enzymes within the intervention arm reveals a positive trend. ALT may be the best indicator of hepatic injury due to NAFLD [21]. After 6 months of weight-loss intervention, ALT levels in our study improved significantly in the intervention arm, suggesting that weight loss improves ALT. In the LOOK AHEAD fatty liver ancillary study, diabetic participants lost 8% body-weight along with significant improvement in hepatic steatosis, in twelve months through a combination of moderate-caloric restriction and increased physical-activity. This trial included a more intensive intervention including thirty contact points (24 in first 6 months;6 sessions in subsequent 6 months) [10]. CAP and LSM are accurate non-invasive methods for assessing liver steatosis and fibrosis respectively [22]. Our trial shows significant improvement in the CAP within both control and intervention group, but no significant improvement in LSM within or between groups.
In clinical settings, with less intensive weight-loss interventions, the effect of the intervention is clearly lower [23]. Our findings are in sync with many international studies which report improvement in liver enzymes, metabolic parameters and steatosis after 6–12 months long intensive weight-loss interventions [20, 24]. Despite evidence, the use of intensive weight-loss interventions in clinical settings is lacking, especially in India and patients with NAFLD are given only a quick weight-loss advice by the clinicians. Often these patients discontinue treatment due to unsuccessful weight loss attempts [25].
The high-dropout and poor-compliance to lifestyle change in our study may be attributed to multiple barriers specific to India, such as complex family dynamics, over-engagement of women in household work, feasting and fasting, social and environmental barriers, published earlier by the authors [11]. It is difficult to convince patients with NAFLD to change their lifestyle [26]. Moreover, our study was carried out at a tertiary-care center with limited resources and many outstation patients, which could have been an additional barrier. To better understand the efficacy of weight-loss interventions in NAFLD, future studies need to be performed in settings with more easily available resources that facilitate frequent intensive weight-loss counseling sessions and follow-ups with patients. Also, use of validated questionnaires to assess the motivation to change [8], use of technology (apps, pedometers, online sessions) [27], use of low calorie diets in NAFLD [28] are promising options that can be tested in future trials to reduce drop outs and increase adherence to the interventions
A robust methodology using objective outcomes is the strength of this trial. However, while interpreting our findings, several limitations, such as the use of USG to diagnose fatty liver, high drop out of patients, unexpected lockdown during Covid-19 pandemic and resource-constrained settings of work need to be considered. Further trials with larger sample size and more frequent contact points are warranted to confirm these findings.
Conclusion
The six-month long intensive weight-loss intervention was not effective in improving the treatment outcomes among NAFLD patients. However, given the potential of our intervention shown in this study, we recommend future trials to design more intensive interventions with frequent contact points, rigorous follow up and regular assessment of adherence to the weight loss intervention.
Supplementary information
Acknowledgements
We acknowledge University Grants Commission for providing Junior Research fellowship to Mrs. Charu Arora. [(UGC Reference number- 1332 (NET-DEC 2014))].
Author contributions
CA: Data collection, data analysis, writing of the manuscript; AM: concept and design of the paper, data analysis and interpretation, critically reviewing and finalizing the manuscript; PR: Corresponding author, concept and design of the paper, finalization of the paper; VS: Statistical analysis and data interpretation, critically revising the manuscript; NS: Data collection, analysis and interpretation of dietary data; Shalimar: Data collection, critical review of paper; SND: Critical review of paper; NKV: Revising and reviewing the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Institute Ethics Committee (IEC- 434/04.08.2017).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41430-022-01111-8.
References
- 1.Rinella ME, Sanyal AJ Management of NAFLD: a stage-based approach. Nature reviews Gastroenterology & hepatology. 2016;196–205. Available from: www.nature.com/nrgastro [DOI] [PubMed]
- 2.Koutoukidis DA, Koshiaris C, Henry JA, Noreik M, Morris E, Manoharan I, et al. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism. 2021;115:154455. Available from https://pubmed.ncbi.nlm.nih.gov/33259835/ [DOI] [PubMed]
- 3.Koutoukidis DA, Jebb SA, Tomlinson JW, Cobbold JF, Aveyard P. Association of weight changes with changes in histological features and blood markers in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2021;S1542-3565:00382–7. Available from https://pubmed.ncbi.nlm.nih.gov/33813074/ [DOI] [PubMed]
- 4.Ghaemi A, Taleban FA, Hekmatdoost A, Rafiei A, Hosseini V, Amiri Z, et al. How much weight loss is effective on nonalcoholic fatty liver disease? Hepat Mon.;13:15227. Available from:/pmc/articles/PMC3867211/?report=abstract [DOI] [PMC free article] [PubMed]
- 5.Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin Mol Hepatol Korean Assoc Study Liver. 2013;19:325–48. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3894432/ [DOI] [PMC free article] [PubMed]
- 6.Yasutake K, Kohjima M, Kotoh K, Nakashima M, Nakamuta M, Enjoji M. Dietary habits and behaviors associated with nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:1756–67. doi: 10.3748/wjg.v20.i7.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim SL, Johal J, Ong KW, Han CY, Chan YH, Lee YM, et al. Lifestyle intervention enabled by mobile technology on weight loss in patients with nonalcoholic fatty liver disease: Randomized controlled trial. JMIR mHealth uHealth. 2020;8:e14802. Available from https://mhealth.jmir.org/2020/4/e14802 [DOI] [PMC free article] [PubMed]
- 8.Centis E, Moscatiello S, Bugianesi E, Bellentani S, Fracanzani AL, Calugi S, et al. Stage of change and motivation to healthier lifestyle in non-alcoholic fatty liver disease. J Hepatol. 2013;58:771–7. doi: 10.1016/j.jhep.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 9.Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, et al. One-year intense nutritional counseling results in histological improvement in patients with nonalcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–81. Available from https://pubmed.ncbi.nlm.nih.gov/15842581/ [DOI] [PubMed]
- 10.Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156–63. https://diabetesjournals.org/care/article/33/10/2156/28393/Effect-of-a-12-Month-Intensive-Lifestyle [DOI] [PMC free article] [PubMed]
- 11.Arora C, Malhotra A, Ranjan P, Vikram NK, Dwivedi SN, Singh N, et al. Perceived barriers and facilitators for adherence to lifestyle prescription: Perspective of obese patients with non alcoholic fatty liver disease from north India. Diabetes Metab Syndr Clin Res Rev. 2021;15:102138. doi: 10.1016/j.dsx.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Bhat G, Baba CS, Pandey A, Kumari N, Choudhuri G. Life style modification improves insulin resistance and liver histology in patients with non-alcoholic fatty liver disease. World J Hepatol. 2012;4:209–17. doi: 10.4254/wjh.v4.i7.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul J, Venugopal RV, Peter L, Hussain S, Naresh Kumar Shetty K, Shetti MP. Effects of lifestyle modification on liver enzyme and Fibroscan in Indian patients with non-alcoholic fatty liver disease. Gastroenterol Rep. 2018;6:49–53. Available from https://academic.oup.com/gastro/article/6/1/49/3829870 [DOI] [PMC free article] [PubMed]
- 14.Arora C, Malhotra A, Ranjan P, Vikram NK, Shalimar, Singh N, et al. Lifestyle intervention framework for obese patients with non-alcoholic fatty liver disease—a tool for health professionals in resource constraint settings. Cureus. 2019;11:e5999. doi: 10.7759/cureus.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shalimar, Kumar R, Rout G, Kumar R, Yadav R, Das P, et al. Body mass index–based controlled attenuation parameter cutoffs for assessment of hepatic steatosis in non-alcoholic fatty liver disease. Indian J Gastroenterol. 2020;39:32–41. Available from https://link.springer.com/article/10.1007/s12664-019-00991-2 [DOI] [PubMed]
- 16.Dong F, Zhang Y, Huang Y, Wang Y, Zhang G, Hu X, et al. Long-term lifestyle interventions in middle-aged and elderly men with nonalcoholic fatty liver disease: a randomized controlled trial. Sci Rep. 2016;6:1–8. Available from www.nature.com/scientificreports/ [DOI] [PMC free article] [PubMed]
- 17.Mundi MS, Velapati S, Patel J, Kellogg TA, Abu Dayyeh BK, Hurt RT. Evolution of NAFLD and Its Management [Internet]. Nutr Clin Pract. 2020;35:72–84. Available from https://aspenjournals.onlinelibrary.wiley.com/doi/full/10.1002/ncp.10449 [DOI] [PubMed]
- 18.Konerman MA, Walden P, Joseph M, Jackson EA, Lok AS, Rubenfire M. Impact of a structured lifestyle programme on patients with metabolic syndrome complicated by non-alcoholic fatty liver disease. Aliment Pharm Ther. 2019;49:296–307. Available from https://onlinelibrary.wiley.com/doi/full/10.1111/apt.15063 [DOI] [PubMed]
- 19.Thomas EL, Brynes AE, Hamilton G, Patel N, Spong A, Goldin RD, et al. Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:5813–9. Available from: /pmc/articles/PMC4100662/ [DOI] [PMC free article] [PubMed]
- 20.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–9. Available from https://aasldpubs.onlinelibrary.wiley.com/doi/full/10.1002/hep.23276 [DOI] [PMC free article] [PubMed]
- 21.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–70. Available from www.interscience.wiley.com [DOI] [PubMed]
- 22.Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of fibroscan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease.Gastroenterology. 2019;156:1717–30. Available from 10.1053/j.gastro.2019.01.042. [DOI] [PubMed]
- 23.Armandi A, Schattenberg JM. Beyond the paradigm of weight loss in non-alcoholic fatty liver disease: from pathophysiology to novel dietary approaches. Nutrients. 2021;13:1977. Available from https://www.mdpi.com/2072-6643/13/6/1977 [DOI] [PMC free article] [PubMed]
- 24.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Clinical-liver weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. 2015; Available from: 10.1053/j.gastro.2015.04.005 [DOI] [PubMed]
- 25.Hallsworth K, Avery L, Trenell MI. Targeting lifestyle behavior change in adults with nafld during a 20-min consultation: summary of the dietary and exercise literature. Curr Gastroenterol Rep. 2016;18:11. doi: 10.1007/s11894-016-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellentani S, Dalle Grave R, Suppini A, Marchesini G, Bedogni G, Bugianesi E, et al. Behavior therapy for nonalcoholic fatty liver disease: The need for a multidisciplinary approach. Hepatology. 47;746–54. Available from: https://aasldpubs.onlinelibrary.wiley.com/doi/full/10.1002/hep.22009 [DOI] [PubMed]
- 27.Mazzotti A, Caletti MT, Brodosi L, Di Domizio S, Forchielli ML, Petta S, et al. An internet-based approach for lifestyle changes in patients with NAFLD: two-year effects on weight loss and surrogate markers. J Hepatol. 2018;69:1155–63. doi: 10.1016/j.jhep.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Scragg J, Avery L, Cassidy S, Taylor G, Haigh L, Boyle M, et al. Feasibility of a very low calorie diet to achieve a sustainable 10% weight loss in patients with nonalcoholic fatty liver disease. Clin Transl Gastroenterol. 2020;11:e00231. Available from https://pubmed.ncbi.nlm.nih.gov/33094956/ [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.