Key Points
Question
Is it possible to assess adverse events associated with drug-drug interactions (DDIs) by drug interaction checkers in patients with COVID-19?
Findings
The DDIs identified in this systematic review involved 46 different drugs, with 575 DDIs for 58 drug pairs (305 associated with at least 1 adverse drug reaction) reported. Drug interaction checkers could have identified such events, including severe and life-threatening ones.
Meaning
Notwithstanding the emergency context of the COVID-19 pandemic, DDI-related adverse events should never be overlooked to customize the most effective and safest therapy.
This systematic review explores drug-drug interactions associated with adverse clinical outcomes and/or adverse drug reactions in patients with COVID-19 and assesses whether drug interaction checkers are helpful in identifying such events.
Abstract
Importance
During the COVID-19 pandemic, urgent clinical management of patients has mainly included drugs currently administered for other diseases, referred to as repositioned drugs. As a result, some of these drugs have proved to be not only ineffective but also harmful because of adverse events associated with drug-drug interactions (DDIs).
Objective
To identify DDIs that led to adverse clinical outcomes and/or adverse drug reactions in patients with COVID-19 by systematically reviewing the literature and assessing the value of drug interaction checkers in identifying such events.
Evidence Review
After identification of the drugs used during the COVID-19 pandemic, the drug interaction checkers Drugs.com, COVID-19 Drug Interactions, LexiComp, Medscape, and WebMD were consulted to analyze theoretical DDI-associated adverse events in patients with COVID-19 from March 1, 2020, through February 28, 2022. A systematic literature review was performed by searching the databases PubMed, Scopus, and Cochrane for articles published from March 1, 2020, through February 28, 2022, to retrieve articles describing actual adverse events associated with DDIs. The drug interaction checkers were consulted again to evaluate their potential to assess such events.
Findings
The DDIs identified in the reviewed articles involved 46 different drugs. In total, 575 DDIs for 58 drug pairs (305 associated with at least 1 adverse drug reaction) were reported. The drugs most involved in DDIs were lopinavir and ritonavir. Of the 6917 identified studies, 20 met the inclusion criteria. These studies, which enrolled 1297 patients overall, reported 115 DDI-related adverse events: 15 (26%) were identifiable by all tools analyzed, 29 (50%) were identifiable by at least 1 of them, and 14 (24%) remained nonidentifiable.
Conclusions and Relevance
The main finding of this systematic review is that the use of drug interaction checkers could have identified several DDI-associated adverse drug reactions, including severe and life-threatening events. Both the interactions between the drugs used to treat COVID-19 and between the COVID-19 drugs and those already used by the patients should be evaluated.
Introduction
The COVID-19 pandemic has overwhelmed a completely unprepared world. Physicians have been faced with the challenge of caring for infected patients in the absence of consolidated scientific evidence and guidelines.1 As a consequence, they have used drugs already approved for other diseases, referred to as repositioned drugs.1,2 Especially at the beginning of the pandemic, the potential efficacy of these repositioned drugs against SARS-CoV-2 was often based on in vitro or in vivo evidence.3 Some of these drugs have been used without considering their potential to cause adverse outcomes associated with drug-drug interactions (DDIs).4,5
Drug-drug interactions, determined by pharmacokinetic and pharmacodynamic mechanisms, occur with high frequency in polytreated patients, such as patients with COVID-19.5 The increase in adverse outcomes associated with DDIs and/or adverse drug reactions (ADRs) leads to increased hospital admissions and health care costs. Therefore, it is essential to avoid potential DDIs when establishing therapy. Drug interaction checkers are tools used to identify potential DDIs, supporting safe prescribing. This study aimed to identify DDIs that led to adverse clinical outcomes and/or ADRs in patients with COVID-19 by systematically reviewing the literature and assessing the value of drug interaction checkers in identifying such events.
Methods
The study design for this systematic review involved 4 steps. Step 1 involved the identification of all drugs used during the pandemic by consulting the European Medicines Agency and the Italian Medicines Agency websites, ClinicalTrials.gov database, and literature data. Step 2 involved searching for potential DDIs that involved each drug identified in step 1 using the following drug interaction checkers: Drugs.com, COVID-19 Drug Interactions, LexiComp, Medscape, and WebMD. Step 3 involved a literature systematic review to identify articles that reported adverse clinical outcomes and/or ADRs related to DDIs among COVID-19 treatments and with coadministered drugs. Step 4 involved evaluating whether the DDIs identified in step 3 could have been identified by using the tools listed in step 2.
Systematic Review
To conduct a comprehensive systematic literature search, we used both controlled vocabulary and free-text terms. The following Medical Subject Heading terms were applied by using the Boolean operator AND: DDIs, COVID-19, patients with COVID-19, comedications, and ADRs. The PubMed, Scopus, and Cochrane databases were searched from the pandemic inception (March 1, 2020) up to February 28, 2022. Our research was limited to articles that involved patients with COVID-19 without sex and age restriction. Articles of any language that identified potential associations between DDIs and relevant clinical outcomes in patients with COVID-19 were included. A systematic review was performed, which identified 6917 studies, following the recommendations of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline6 and the PRISMA statement of reporting systematic review and meta-analysis.7 This study did not need approval from an ethics committee or written informed consent from patients because it is a systematic review without meta-analysis.
Inclusion criteria were as follows: articles involving patients with a diagnosis of COVID-19, case reports and case series, letters to the editor and communications, observational studies, and interventional clinical trials. Exclusion criteria were as follows: articles that did not report a direct association between DDIs and clinically relevant outcomes in patients with COVID-19, reviews and meta-analyses, conference papers and book chapters, and studies in silico or based on in vitro experiments.
Drug Interaction Checkers
The drug interaction checkers used in this study were Drugs.com, COVID-19 Drug Interactions, LexiComp, Medscape, and WebMD. Drugs.com8 generates a list of DDIs that are marked by a colored dot. Major DDIs (highly clinically significant; avoid combinations) are in red, moderate DDIs (moderately clinically significant; usually avoid combinations; use it only under special circumstances) are in orange, and minor DDIs (minimally clinically significant; minimize risk; assess risk and consider an alternative drug; take steps to circumvent the interaction risk and/or institute a monitoring plan) are in yellow. In COVID-19 Drug Interactions (made by Liverpool University),9 the drugs are divided according to the risk of clinically significant interaction as follows: do not coadminister (with a red circle), potential interaction (with an orange square), potential weak interaction (with a yellow triangle), and no interaction expected (with a green rhombus). The LexiComp interactions tool10 identifies DDIs, assigning the following risk rating: A, no known interaction; B, no action needed; C, monitor therapy; D, consider therapy modification; and X, avoid combination. LexiComp reports the drug class and the mechanism responsible for the interaction. The Medscape tool11 classifies the DDIs as follows: contraindicated (in red), serious–use alternative (in orange), monitor closely (in green), and minor (in blue). The degree of severity is indicated by different shades of red: contraindicated (in dark red), serious–use alternative (in red), monitor closely (in pink), and minor (in light pink). The WebMD tool12 classifies the DDI risk as follows: don’t use together (in red), serious (in orange), monitor closely (in yellow), and minor (in green).
Results
Identification of Drugs Used During the COVID-19 Pandemic and Potential DDIs
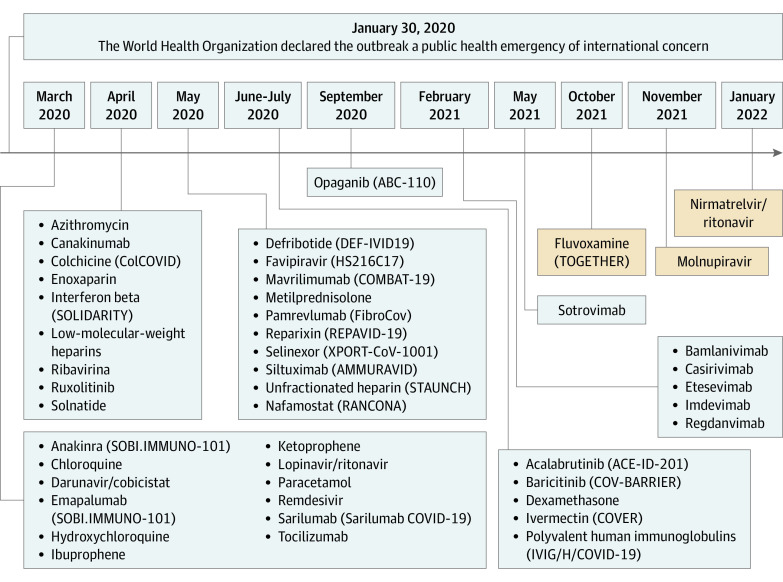
The drugs used during the COVID-19 pandemic were identified by consulting the website of the European Medicines Agency,13 Italian Medicines Agency,14 ClinicalTrials.gov,15 and literature data (Figure). The Figure shows all the 46 drugs listed in chronological order according to their period of use. Most of them were used under the concept of repurposing; some have been included in clinical trials or administered as off-label or compassionate use.
Figure. Timeline of the Drugs Used During the COVID-19 Pandemic.
ABC-110 indicates Study of Opaganib in Coronavirus Disease 2019 Pneumonia (COVID-19); ACE-ID-201, A Phase 2, Open Label, Randomized Study of the Efficacy and Safety of Acalabrutinib with Best Supportive Care Vs Best Supportive Care in Subjects Hospitalized with COVID-19; AMMURAVID, Factorial Randomized Trial of Remdesivir and Baricitinib Plus Dexamethasone for COVID-19; ColCOVID, Colchicine Counteracting Inflammation in COVID-19 Pneumonia; COMBAT-19, Mavrilimumab in Severe COVID-19 Pneumonia and Hyper-inflammation; COV-BARRIER, Study of Baricitinib (LY3009104) in Children With COVID-19; COVER, COVID Ivermectin - Randomized, Double-blind, Multi Centre Phase II, Proof of Concept, Dose Finding Clinical Trial on Ivermectin for the Early Treatment of COVID-19; DEF-IVID19, Defibrotide in COVID-19 Pneumonia - Use of Defibrotide to Reduce Progression of Acute Respiratory Failure Rate in Patients With COVID-19 Pneumonia; FibroCov, Open-label, Randomized, Parallel-arm Study Investigating the Efficacy and Safety of Intravenous Administration of Pamrevlumab Vs Standard of Care in Patients With COVID-19; HS216C17, Clinical Study to Evaluate the Performance and Safety of Favipiravir in COVID-19; IVIG/H/COVID-19, High Dose Intravenous Polyvalent Immunoglobulin (IVIG) in Patients With Early Inflammatory COVID-19; RANCONA, A Randomized Clinical Trial of Nafamostat: A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor for the Treatment of Covid-19; REPAVID-19, Reparixin in COVID-19 Pneumonia - Efficacy and Safety; SOBI.IMMUNO-101, Efficacy and Safety of Emapalumab and Anakinra in Reducing Hyperinflammation and Respiratory Distress in Patients With COVID-19 Infection; SOLIDARITY, Efficacy of Different Anti-viral Drugs in COVID 19 Infected Patients; STAUNCH, Steroids and Unfractionated Heparin in Critically Ill Patients With Pneumonia From COVID-19 Infection; TOGETHER, Trial to Evaluate the Effect of Peginterferon Lambda for the Treatment of COVID-19; XPORT-CoV-1001, Evaluation of Activity and Safety of Oral Selinexor in Participants With Severe COVID-19 Infection.
eTable 1 in the Supplement reports the number of potential DDIs for each drug administered against COVID-19 with the degree of severity of the associated adverse outcomes and/or ADRs found by using the drug interaction checkers. The tools differed from each other regarding the number of potential DDIs identified and the classification of the severity grade of the DDI-associated clinical outcomes. Drugs.com identified the largest numbmer of DDIs, followed by Medscape, WebMD, LexiComp, and COVID-19 Drug Interactions. Drugs.com identified the lirgest number of highly clinically significant DDI-associated adverse outcomes, followed by LexiComp, COVID-19 Drug Interactions, WebMD, and Medscape (eTable 1 in the Supplement).
The drugs most involved in DDIs were lopinavir and ritonavir, followed by nirmatrelvir and ritonavir, darunavir and cobicistat, chloroquine, acetazolamide, and hydroxychloroquine. The drug interaction checkers agree with each other (even if with different classification methods) in considering lopinavir and ritonavir as the drug involved in the most serious DDI-associated ADRs (eTable 1 in the Supplement).
Systematic Review Results
A systematic review was performed to identify adverse clinical outcomes and/or ADRs related to DDIs among treatments of COVID-19 and between COVID-19 treatments and drugs coadministered in patients with COVID-19. The PRISMA algorithm7 shows the research workflow (eFigure in the Supplement). Twenty articles16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 that involved 46 interacting drugs that led to DDI-associated adverse outcomes were evaluated (Table 1). The most frequent DDIs were hydroxychloroquine and lopinavir-ritonavir. Most DDIs resulted in QT-interval prolongation. Such a dangerous alteration was found 20 times, and in 12 of these 20 cases, it occurred together with other adverse outcomes, even leading to the death of 8 patients (Table 1).
Table 1. Study Design, DDIs, DDI-Associated Adverse Outcomes, and Mechanisms Reported by the Reviewed Studies.
| Source | Study design | Study quality score | Drugs involved in potential DDIs | DDIs mechanism | DDI-associated adverse clinical outcomes and/or ADRs |
|---|---|---|---|---|---|
| Anmella et al,16 2020 | Case series | 4 | Acetazolamide, hydroxychloroquine, lopinavir-ritonavir, paroxetine, risperidone, and topiramate | PK | Behavioral disturbances |
| Case series | 4 | Acetazolamide, hydroxychloroquine, lopinavir and ritonavir, venlafaxine | PK | Mild QT-interval prolongation (443 ms) | |
| Bartiromo et al,17 2020 | Case report | 5 | Darunavir-cobicistat, hydroxychloroquine, and tacrolimus | PK | Tacrolimus trough levels found to be extremely high (90.5 ng/mL), intermittent abdominal pain, nausea and vomiting |
| Borba et al,18 2020 | Original investigation | 1 | Acetazolamide, ceftriaxone, and chloroquine | PK | Rhabdomyolysis, myocarditis, severe arrhythmias, QT-interval prolongation |
| Original investigation | 1 | Acetazolamide, ceftriaxone, chloroquine, and oseltamivir | PK | Rhabdomyolysis, myocarditis, severe arrhythmias, QT-interval prolongation | |
| Crescioli et al,19 2021 | Observational study | 3 | Amiodarone, acetazolamide, darunavir-cobicistat, and hydroxychloroquine | PK and PD | QT-interval prolongation |
| Observational study | 3 | Acetazolamide, citalopram, hydroxychloroquine, and lopinavir and ritonavir | PK and PD | QT-interval prolongation | |
| Observational study | 3 | Acetazolamide, darunavir-cobicistat, and hydroxychloroquine | PK and PD | Diarrhea, vomiting | |
| Observational study | 3 | Acetazolamide, haloperidol, hydroxychloroquine, levomepromazine, lopinavir-ritonavir, and zuclopenthixol | PK and PD | QT-interval prolongation | |
| Observational study | 3 | Acetazolamide, haloperidol, hydroxychloroquine, and lopinavir-ritonavir | PK and PD | QT-interval prolongation | |
| Observational study | 3 | Acetazolamide, hydroxychloroquine, and lopinavir-ritonavir | PK and PD | QT-interval prolongation | |
| Observational study | 3 | Acetazolamide, hydroxychloroquine, and lopinavir-ritonavir | PK and PD | QT-interval prolongation, vomiting | |
| Observational study | 3 | Acetazolamide, hydroxychloroquine, and sertraline | PK and PD | QT-interval prolongation | |
| Observational study | 3 | Citalopram and hydroxychloroquine | PK and PD | QT-interval prolongation | |
| Observational study | 3 | Darunavir-cobicistat, haloperidol, and hydroxychloroquine | PK and PD | QT-interval prolongation | |
| Observational study | 3 | Darunavir-cobicistat and hydroxychloroquine | PK and PD | QT-interval prolongation | |
| Observational study | 3 | Darunavir-cobicistat, hydroxychloroquine, lopinavir-ritonavir, and tocilizumab | PK and PD | Hypertransaminasemia | |
| Observational study | 3 | Darunavir-cobicistat, hydroxychloroquine, and tocilizumab | PK and PD | Psychosis, agitation, delirium, aggressiveness | |
| Observational study | 3 | Darunavir-cobicistat and tacrolimus | PK and PD | Nausea, vomiting, abdominal pain, drug level modification | |
| Observational study | 3 | Hydroxychloroquine and lopinavir-ritonavir | PK and PD | QT-interval prolongation, hypokalemia | |
| Observational study | 3 | Hydroxychloroquine and lopinavir-ritonavir | PK and PD | QT-interval prolongation | |
| Observational study | 3 | Hydroxychloroquine and magnesium sulfate | PK and PD | QT-interval prolongation, hypokalemia | |
| Observational study | 3 | Hydroxychloroquine and risperidone | PK and PD | QT-interval prolongation, atrial flutter, hemiplegia, hypokalemia, major depression | |
| Observational study | 3 | Hydroxychloroquine and trazodone | PK and PD | QT-interval prolongation | |
| Dajti et al,20 2020 | Case report | 5 | DRV/c, hydroxychloroquine, prednisone, and tacrolimus | PK | Increased tacrolimus levels |
| Gautret et al,21 2021 | Letter | 5 | Acetazolamide and hydroxychloroquine | PK | QT-interval prolongation (>60 ms), discontinuation of treatment |
| Ghani et al,22 2020 | Case series | 4 | Apixaban, enoxaparin, hydroxychloroquine, and corticosteroids | PK | A large intraparenchymal hemorrhage and cerebral edema |
| Case series | 4 | Apixaban, hydroxychloroquine, corticosteroids, and unfractionated heparin | PK | Scattered subarachnoid hemorrhages, a subdural hematoma | |
| Case series | 4 | Hydroxychloroquine, corticosteroids, and unfractionated heparin | PK | Acute subarachnoid and intraparenchymal hemorrhages within the posterior fossa | |
| Li et al,23 2020 | Observational study | 3 | Ganciclovir, lopinavir-ritonavir, oseltamivir, peramivir, penciclovir, rubavirin, and umifenovir | PK | Increase in D-dimer, hematologic abnormalities |
| Macías et al,24 2020 | Cross-sectional study | 4 | Amiodarone and lopinavir-ritonavir | PK | Orthostatic syncope |
| Martínez-López-de-Castro et al,25 2021 | Cohort, retrospective and single-center study | 3 | Alprazolam and lopinavir-ritonavir | PK | Psychiatric disorders |
| Cohort, retrospective and single-center study | 3 | Aripiprazole, digoxin, fentanyl, lithium, lopinavir-ritonavir, and tacrolimus | PK | Alteration of the concentration of blood levels | |
| Cohort, retrospective and single-center study | 3 | Acetazolamide and hydroxychloroquine | PK | Cutaneous reactions | |
| Cohort, retrospective and single-center study | 3 | Acetazolamide and lopinavir-ritonavir | PK | Gastrointestinal disorders | |
| Cohort, retrospective and single-center study | 3 | Hydroxychloroquine and lopinavir-ritonavir | PK | Hyperglycemia | |
| Cohort, retrospective and single-center study | 3 | Hydroxychloroquine and lopinavir-ritonavir | PK | Cutaneous reactions | |
| Cohort, retrospective and single-center study | 3 | Hydroxychloroquine and lopinavir-ritonavir | PK | Gastrointestinal disorders | |
| Cohort, retrospective and single-center study | 3 | Hydroxychloroquine and lopinavir-ritonavir | PK | Psychiatric disorders | |
| Cohort, retrospective and single-center study | 3 | Hydroxychloroquine and tacrolimus | PK | Alteration of the concentration of blood levels | |
| Cohort, retrospective and single-center study | 3 | Interferon beta and metamizole | PK | Hematologic toxicity | |
| Cohort, retrospective and single-center study | 3 | Lopinavir-ritonavir and methylprednisolone or prednisone | PK | Hyperglycemia | |
| Cohort, retrospective and single-center study | 3 | Lopinavir-ritonavir and midazolam or diazepam | PK | Increased sedative effect | |
| Cohort, retrospective and single-center study | 3 | Lopinavir-ritonavir and propofol | PK | Increased triglyceride level | |
| Cohort, retrospective and single-center study | 3 | Lopinavir-ritonavir and simvastatin | PK | Liver toxicity | |
| Cohort, retrospective and single-center study | 3 | Lopinavir-ritonavir and valproate | PK | Seizures | |
| Meriglier et al,26 2021 | Observational study | 2 | Darunavir-ritonavir and hydroxychloroquine | PK | Diarrhea grade I and II; ECG abnormalities; hepatic enzyme elevation |
| Observational study | 2 | Hydroxychloroquine and lopinavir-ritonavir | PK | Diarrhea grade I and II; ECG abnormalities; severe nausea | |
| Meziyerh et al,27 2020 | Case report | 5 | Everolimus, hydroxychloroquine, and lopinavir-ritonavir | PK | Dyspnea or tachypnea, everolimus plasma concentrations increased |
| Nham et al,28 2020 | Case report | 5 | Ceftriaxone, levofloxacin, and lopinavir-ritonavir | PK | Severe thrombocytopenia with epistaxis and petechiae |
| Ramireddy et al,29 2020 | Original research | 3 | Acetazolamide and hydroxychloroquine | PK | QT-interval prolongation |
| Skroza et al,30 2020 | Case report | 5 | Ceftriaxone, enoxaparin, hydroxychloroquine, and lopinavir-ritonavir | PK | Urticarial vasculitis attributable to adverse drug reaction |
| Szekely et al,31 2020 | Original research | 2 | Chloroquine, letrozole, and memantine | PK | Extreme QT-interval prolongation (720 ms), torsades de pointes |
| Teoli et al,32 2021 | Case report | 5 | Remdesivir and tramadol | PK | Severe pain localized in the legs |
| Thammathiwat et al,33 2021 | Case report | 5 | Darunavir-ritonavir and tacrolimus | PK | Tacrolimus levels turned significantly high, acute kidney injury, lymphopenia, Pio2/Fio2 was lowered, tacrolimus withdrawn for 10 d |
| Treon et al,34 2020 | Letter | 5 | Acetazolamide, hydroxychloroquine, and ibrutinib | PK | Wide QRS complex tachyarrhythmia |
| Yekedüz et al,35 2020 | Case report | 5 | Antidiabetics and hydroxychloroquine | PK | Hypoglycemia |
Abbreviations: ADRs, adverse drug reactions; DDIs, drug-drug interactions; DRV/c, darunavir-cobicistat; ECG, electrocardiography; Fio2, fraction of inspired oxygen; PD, pharmacodynamic; Pio2, inspired oxygen tension; PK, pharmacokinetic.
Eleven DDI-associated ADRs were diarrhea and vomiting as well as liver disorders. Six neurologic or psychiatric DDIs were reported. Three of 6 were serious neurovascular hemorrhages. One of them involved corticosteroids, hydroxychloroquine, and unfractionated heparin, another one implicated the aforementioned drugs coadministered with apixaban, and the last one included fractionated heparin. Surprisingly, none of the reviewed studies reported DDIs that involved ritonavir and anticoagulants. However, all drug interaction checkers agreed that the most severe DDIs occurred with ritonavir and direct factor Xa inhibitors.
Drugs coadministered with hydroxychloroquine were lopinavir-ritonavir (24 cases), acetazolamide (20 cases), and darunavir-cobicistat (15 cases). Of the 53 DDIs in which hydroxychloroquine was involved, 31 were associated with QT-interval prolongation. Four of these 31 DDIs led to patient death.
Only 3 of 56 DDIs involved coadministration of chloroquine with other drugs, including ceftriaxone, acetazolamide, and oseltamivir18 or memantine and letrozole.31 The major complication linked to chloroquine and hydroxychloroquine, in monotherapy or in combination and in short or low-dose regimens, was again QT-interval prolongation, which also caused fatal arrhythmias.18
DDI-Associated Clinical Outcomes and/or ADRs Identified by Drug Interaction Checkers
The DDIs identified in the reviewed articles involved 46 different drugs (Table 1). Many of them were administered for patients’ comorbidities. Table 2 lists all the drugs reported in the articles and identified as triggers of DDIs by at least 1 of the drug interaction checkers used. Drugs.com was the most complete tool. Conversely, COVID-19 Drug Interactions, WebMD, Medscape, and LexiComp did not include some medications, such as memantine, letrozole, and magnesium sulfate (Table 2).
Table 2. DDIs Reported in the Reviewed Studies and the Results of the 5 Consulted Drug Interaction Checkers.
| Drugs involved in DDIs reported in the reviewed studies | Drugs.com | COVID-19 Drug Interactions | LexiComp | Medscape | WebMD |
|---|---|---|---|---|---|
| Alprazolam and lopinavir-ritonavir | Moderate | Potential interaction | X: Avoid combination | Serious - use alternative | Serious |
| Amiodarone and DRV/c | Moderate | No interaction found | C: Monitor therapy | Monitor closely | Monitor closely |
| Amiodarone and hydroxychloroquine | Major | Do not coadminister | No interaction found | Serious - use alternative | Serious |
| Amiodarone and lopinavir-ritonavir | Major | Do not coadminister | X: Avoid combination | Contraindicated | Don’t use together |
| Antidiabetics and hydroxychloroquine | Moderate | Potential interaction | C: Monitor therapy | No interaction found | No interaction found |
| Apixaban and enoxaparin | Major | Potential interaction | X: Avoid combination | Serious - use alternative | Serious |
| Apixaban and hydroxychloroquine | No interaction found | Potential weak interaction | No interaction found | No interaction found | No interaction found |
| Apixaban and unfractionated heparin | No interaction found | No interaction found | X: Avoid combination | Serious - use alternative | Serious |
| Aripiprazole and digoxin | Moderate | No interaction found | No interaction found | No interaction found | No interaction found |
| Aripiprazole and fentanyl | No interaction found | No interaction found | D: Consider therapy modification | Monitor closely | Monitor closely |
| Aripiprazole and lithium | No interaction found | No interaction found | C: Monitor therapy | Monitor closely | No interaction found |
| Aripiprazole and lopinavir-ritonavir | Moderate | Potential interaction | D: Consider therapy modification | Serious - use alternative | Serious |
| Aripiprazole and tacrolimus | No interaction found | No interaction found | No interaction found | No interaction found | Serious |
| Acetazolamide and amiodarone | Major | Do not coadminister | D: Consider therapy modification | Serious - use alternative | Monitor closely |
| Acetazolamide and ceftriaxone | No interaction found | No interaction expected | No interaction found | No interaction found | No interaction found |
| Acetazolamide and citalopram | Major | Do not coadminister | C: Monitor therapy | Monitor closely | Monitor closely |
| Acetazolamide and chloroquine | Major | Potential interaction | C: Monitor therapy | Monitor closely | Monitor closely |
| Acetazolamide and DRV/c | Major | No interaction expected | No interaction found | Serious - use alternative | Serious |
| Acetazolamide and haloperidol | Major | Do not coadminister | C: Monitor therapy | Monitor closely | Monitor closely |
| Acetazolamide and hydroxychloroquine | Major | Potential interaction | B: No action needed | Serious - use alternative | Serious |
| Acetazolamide and levomepromazine | No interaction found | Potential interaction | B: No action needed | No interaction found | No interaction found |
| Acetazolamide and lopinavir | Moderate | No interaction found | B: No action needed | Monitor closely | No interaction found |
| Acetazolamide and lopinavir and ritonavir | Moderate | Potential interaction | B: No action needed | Monitor closely | Monitor closely |
| Acetazolamide and oseltamivir | No interaction found | No interaction expected | No interaction found | No interaction found | No interaction found |
| Acetazolamide and paroxetine | No interaction found | No interaction expected | No interaction found | Minor | Minor |
| Acetazolamide and risperidone | No interaction found | No interaction expected | C: Monitor therapy | Minor | No interaction found |
| Acetazolamide and ritonavir | Moderate | Potential interaction | B: No action needed | Monitor closely | Monitor closely |
| Acetazolamide and sertraline | Moderate | No interaction expected | C: Monitor therapy | Minor | Minor |
| Acetazolamide and zenlafaxine | No interaction found | Do not coadminister | No interaction found | Minor | Minor |
| Acetazolamide and zuclopenthixol | No interaction found | Do not coadminister | B: No action needed | No interaction found | No interaction found |
| Ceftriaxone and chloroquine | No interaction found | No interaction expected | No interaction found | No interaction found | No interaction found |
| Ceftriaxone and enoxaparin | Minor | No interaction found | No interaction found | Serious - use alternative | Serious |
| Ceftriaxone and lopinavir-ritonavir | No interaction found | No interaction expected | No interaction found | No interaction found | No interaction found |
| Citalopram and hydrochloroquine | Major | Do not coadminister | C: Monitor therapy | Serious - use alternative | Serious |
| Citalopram and lopinavir-ritonavir | Major | No interaction found | B: No action needed | Monitor closely | Monitor closely |
| Citalopram and ritonavir | Minor | No interaction found | No interaction found | No interaction found | No interaction found |
| Cobicistat and ritonavir | Moderate | No interaction found | No interaction found | No interaction found | No interaction found |
| Chloroquine and oseltamivir | No interaction found | No interaction expected | No interaction found | No interaction found | No interaction found |
| Darunavir and lopinavir | Moderate | No interaction found | No interaction found | Serious - use alternative | No interaction found |
| Darunavir and prednisone | Moderate | No interaction found | No interaction found | Monitor closely | Monitor closely |
| Darunavir and tacrolimus | Major | No interaction found | D: Consider therapy modification | Monitor closely | Monitor closely |
| Darunavir-ritonavir and hydroxychloroquine | Moderate | Potential weak interaction | No interaction found | No interaction found | No interaction found |
| Darunavir-ritonavir and tacrolimus | Major | No interaction found | D: Consider therapy modification | Monitor closely | Monitor closely |
| Darunavir and hydroxychloroquine | Moderate | No interaction found | No interaction found | No interaction found | No interaction found |
| Darunavir and ritonavir | No interaction found | No interaction found | No interaction found | Serious - use alternative | Serious |
| Diazepam and lopinavir | No interaction found | No interaction found | No interaction found | Monitor closely | No interaction found |
| Diazepam and lopinavir and ritonavir | Moderate | Potential interaction | C: Monitor therapy | Monitor closely | Monitor closely |
| Digoxin and hydroxychloroquine | No interaction found | No interaction found | No interaction found | Serious - use alternative | No interaction found |
| Digoxin and lopinavir and ritonavir | Moderate | No interaction found | No interaction found | No interaction found | No interaction found |
| Digoxin and tacrolimus | No interaction found | No interaction found | No interaction found | Monitor closely | Monitor closely |
| Darunavir-cobicistat and haloperidol | Moderate | No interaction found | No interaction found | Monitor closely | Monitor closely |
| Darunavir-cobicistat and hydroxychloroquine | Moderate | Potential weak interaction | No interaction found | No interaction found | No interaction found |
| Darunavir-cobicistat and lopinavir-ritonavir | No interaction found | Do not coadminister | No interaction found | No interaction found | Serious |
| Darunavir-cobicistat and tacrolimus | Major | No interaction found | D: Consider therapy modification | Monitor closely | Monitor closely |
| Darunavir-cobicistat and tocilizumab | No interaction found | No interaction expected | No interaction found | No interaction found | No interaction found |
| Enoxaparin and hydroxychloroquine | No interaction found | No interaction expected | No interaction found | No interaction found | No interaction found |
| Enoxaparin and lopinavir-ritonavir | No interaction found | No interaction expected | No interaction found | No interaction found | No interaction found |
| Enoxaparin and corticosteroids | No interaction found | No interaction found | No interaction found | Monitor closely | Monitor closely |
| Everolimus and hydroxychloroquine | No interaction found | No interaction found | No interaction found | Serious - use alternative | No interaction found |
| Everolimus and lopinavir-ritonavir | No interaction found | No interaction found | X: Avoid combination | Serious - use alternative | No interaction found |
| Fentanyl and lopinavir | No interaction found | No interaction found | No interaction found | Serious - use alternative | No interaction found |
| Fentanyl and lopinavir-ritonavir | Major | Potential interaction | D: Consider therapy modification | Serious - use alternative | No interaction found |
| Ganciclovir and peramivir | No interaction found | No interaction found | No interaction found | Monitor closely | Monitor closely |
| Haloperidol and hydroxychloroquine | Major | Do not coadminister | C: Monitor therapy | Serious - use alternative | Serious |
| Haloperidol and lopinavir | No interaction found | No interaction found | C: Monitor therapy | Serious - use alternative | No interaction found |
| Haloperidol and lopinavir-ritonavir | Major | Do not coadminister | C: Monitor therapy | Serious - use alternative | Serious |
| Haloperidol and ritonavir | Moderate | No interaction found | No interaction found | No interaction found | No interaction found |
| Haloperidol and zuclopenthixol | No interaction found | No interaction found | C: Monitor therapy | No interaction found | No interaction found |
| Hydroxychloroquine and levomepromazine | No interaction found | Potential interaction | No interaction found | No interaction found | No interaction found |
| Hydroxychloroquine and lopinavir | Major | Potential interaction | No interaction found | Serious - use alternative | No interaction found |
| Hydroxychloroquine and lopinavir and ritonavir | Major | Potential interaction | No interaction found | Serious - use alternative | Serious |
| Hydroxychloroquine and magnesium sulfate | Moderate | No interaction found | No interaction found | No interaction found | No interaction found |
| Hydroxychloroquine and paroxetine | No interaction found | Potential interaction | C: Monitor therapy | No interaction found | No interaction found |
| Hydroxychloroquine and prednisone | No interaction found | No interaction expected | No interaction found | No interaction found | No interaction found |
| Hydroxychloroquine and risperidone | Major | Potential interaction | B: No action needed | Serious - use alternative | Serious |
| Hydroxychloroquine and ritonavir | Moderate | No interaction found | No interaction found | Serious - use alternative | Serious |
| Hydroxychloroquine and sertraline | Major | No interaction expected | No interaction found | Serious - use alternative | Serious |
| Hydroxychloroquine and corticosteroids | No interaction found | No interaction expected | No interaction found | No interaction found | No interaction found |
| Hydroxychloroquine and tacrolimus | Major | Potential interaction | No interaction found | Serious - use alternative | Serious |
| Hydroxychloroquine and tocilizumab | Moderate | Potential interaction | No interaction found | Serious - use alternative | Serious |
| Hydroxychloroquine and topiramate | Moderate | No interaction expected | No interaction found | No interaction found | No interaction found |
| Hydroxychloroquine and trazodone | Major | Potential interaction | No interaction found | No interaction found | No interaction found |
| Hydroxychloroquine and unfractionated heparin | No interaction found | No interaction expected | No interaction found | Monitor closely | No interaction found |
| Hydroxychloroquine and venlafaxine | Major | Do not coadminister | No interaction found | No interaction found | No interaction found |
| Hydroxychloroquine and zuclopenthixol | No interaction found | Do not coadminister | No interaction found | No interaction found | No interaction found |
| Interferon beta and metamizole | No interaction found | Do not coadminister | No interaction found | No interaction found | No interaction found |
| Levofloxacin and lopinavir-ritonavir | No interaction found | Potential interaction | No interaction found | No interaction found | No interaction found |
| Levomepromazine and lopinavir-ritonavir | No interaction found | Potential interaction | No interaction found | No interaction found | No interaction found |
| Lithium and lopinavir-ritonavir | Moderate | Potential interaction | No interaction found | No interaction found | No interaction found |
| Lopinavir and methylprednisolone | No interaction found | No interaction found | No interaction found | Monitor closely | No interaction found |
| Lopinavir and midazolam | No interaction found | No interaction found | No interaction found | Serious - use alternative | No interaction found |
| Lopinavir and prednisone | No interaction found | No interaction found | No interaction found | Monitor closely | No interaction found |
| Lopinavir and tacrolimus | No interaction found | No interaction found | No interaction found | Serious - use alternative | No interaction found |
| Lopinavir and venlafaxine | No interaction found | No interaction found | No interaction found | Monitor closely | No interaction found |
| Lopinavir-ritonavir and methylprednisolone | Major | No interaction expected | C: Monitor therapy | Serious - use alternative | Serious |
| Lopinavir-ritonavir and midazolam | Major | Do not coadminister | X: Avoid combination | Serious - use alternative | Serious |
| Lopinavir-ritonavir and paroxetine | Moderate | Potential interaction | No interaction found | No interaction found | No interaction found |
| Lopinavir-ritonavir and prednisone | Moderate | Potential interaction | C: Monitor therapy | Monitor closely | Monitor closely |
| Lopinavir-ritonavir and propofol | Moderate | Potential interaction | No interaction found | No interaction found | No interaction found |
| Lopinavir-ritonavir and risperidone | Moderate | Potential interaction | C: Monitor therapy | No interaction found | No interaction found |
| Lopinavir-ritonavir and simvastatin | Major | Potential interaction | No interaction found | Contraindicated | Don’t use together |
| Lopinavir-ritonavir and tacrolimus | Major | Potential interaction | D: Consider therapy modification | Serious - use alternative | Serious |
| Lopinavir-ritonavir and topiramate | No interaction found | No interaction found | No interaction found | Monitor closely | Monitor closely |
| Lopinavir-ritonavir and valproate | Moderate | Potential interaction | C: Monitor therapy | No interaction found | No interaction found |
| Lopinavir-ritonavir and venlafaxine | Moderate | Potential interaction | B: No action needed | No interaction found | Monitor closely |
| Lopinavir-ritonavir and zuclopenthixol | No interaction found | Potential interaction | No interaction found | No interaction found | No interaction found |
| Methylprednisolone and ritonavir | Major | No interaction found | No interaction found | No interaction found | No interaction found |
| Paroxetine and risperidone | No interaction found | No interaction found | D: Consider therapy modification | Monitor closely | Monitor closely |
| Paroxetine and topiramate | No interaction found | No interaction found | B: No action needed | No interaction found | No interaction found |
| Prednisone and ritonavir | Moderate | No interaction found | No interaction found | Monitor closely | Monitor closely |
| Prednisone and tacrolimus | Moderate | No interaction found | C: Monitor therapy | Minor | Minor |
| Remdesivir and tramadol | No interaction found | No interaction expected | C: Monitor therapy | No interaction found | No interaction found |
| Risperidone and topiramate | No interaction found | No interaction found | C: Monitor therapy | Monitor closely | Monitor closely |
| Ritonavir and tacrolimus | Major | No interaction found | D: Consider therapy modification | Monitor closely | Monitor closely |
| Corticosteroids and unfractionated heparin | No interaction found | No interaction found | No interaction found | Monitor closely | Monitor closely |
Abbreviation: DDIs, drug-drug interactions; DRV/c, darunavir-cobicistat.
In total, 575 DDIs for 58 drug pairs (305 associated with at least 1 ADR) were reported. Such DDIs were identified as follows: 70 by Medscape, 68 by COVID-19 Drug Interactions, 64 by Drugs.com, 55 by WebMD, and 48 by LexiComp. In 271 of 580 cases, no interactions were found. LexiComp reported the fewest DDIs, classified into B (no action needed) (10 [20%]), C (monitor therapy) (22 [45%]), D (consider modifying therapy) (10 [23%]), and X (avoid combinations) (6 [12%]).
The number of the identified severe-moderate DDI-associated adverse events was comparable among Drugs.com, Medscape, and WebMD. An equivalent classification was found using the latter 2 tools. Most DDIs were classified as major (30 [48%]) and moderate (32 [49%]) by Drugs.com, as serious (32 [46%]) and monitor closely (31 [44%]) by Medscape, and as serious (23 [43%]) and monitor closely (26 [46%]) by WebMD (Table 2). In addition, DDI-associated adverse events were classified as minor by Drugs.com in 2 cases (3%), by Medscape in 5 cases (7%), and by WebMD in 4 cases (7%).
COVID-19 Drug Interactions identified DDI-associated adverse outcomes as follows: 15 (22%) as do not coadminister, 32 (46%) as potential interaction, and 3 (4%) as potential weak interaction (Table 2). According to Medscape and WebMD, the most severe DDIs were caused by the association of amiodarone with lopinavir and ritonavir and lopinavir and ritonavir with simvastatin, classified as contraindicated (2 [3%]) by Medscape and as don’t use together (2 [4%]) by WebMD.
Globally, the reviewed studies described 15 patients taking lopinavir and ritonavir plus simvastatin25 and only 1 taking lopinavir and ritonavir plus amiodarone.24 The studies24,25 reported liver toxicity (related to lopinavir and ritonavir plus simvastatin) and orthostatic syncope (related to lopinavir and ritonavir plus amiodarone). For all the tools, besides these serious DDI-associated adverse outcomes already described, the remaining 301 can be divided into 117 (39%) classified as severe, 132 (43%) as moderate, and 52 (17%) as minor.
eTable 2 in the Supplement details the last step of the study. Of the 6917 studies identified, 20 studies, which enrolled 1297 patients, reported 115 DDI-related adverse events: 15 (26%) were identifiable by all tools analyzed, 29 (50%) were identifiable by at least 1 of them, and 14 (24%) remained nonidentifiable. Most of these involved psychotic disorders or cutaneous reactions.
Discussion
Therapeutic strategy to treat COVID-19 has rapidly changed during the pandemic, above all based on experimental and real-world data and following the concept of repurposing. Some drugs have fallen out of use, whereas others represent a cornerstone of treatment.2,36,37,38,39 Both real-world data and results of clinical trials have highlighted the need to review all steps of the care process from the beginning of the pandemic to today.37 In particular, what seems clear is the large variability in the therapeutic response of patients with COVID-19 and therefore the urgent need to use a personalized approach.38,39,40 One important issue is that patients with comorbidities (thus polytreated), who represent most patients with COVID-19, are likely to experience ADRs, including those related to DDIs. Therefore, regardless of the drugs used for SARS-CoV-2 clearance and to treat COVID-19, it is crucial to take into account the risk of DDIs.41
The current study was planned to analyze DDI-associated clinical outcomes that occurred in clinical practice during the pandemic and to investigate whether and how drug interaction checkers might be useful to assess them. Our main finding is that the use of these tools could have identified several DDI-associated ADRs, including severe and life-threatening events. However, the interactions between the drugs used to treat COVID-19 and between the COVID-19 drugs and those already used by the patients should be evaluated.
At the beginning of the pandemic, chloroquine and hydroxychloroquine were largely used because of their ability (assessed in vitro) to modify cellular pH, thus interfering with SARS-CoV-2 replication and its fusion with the host cells.2 Then, as shown in the current study, hydroxychloroquine was recognized to interfere with the antiviral agents lopinavir-ritonavir, darunavir-cobicistat, and acetazolamide, causing QT-interval prolongation, ventricular arrhythmias, and torsade de pointes.19,31 In the study by Borba et al,18 several patients treated with chloroquine died after drug administration. Most patients (89.6%) with increased QT-interval prolongation were taking oseltamivir as well as acetazolamide and ceftriaxone. Crescioli et al19 reported 5 deaths among 23 patients. These patients had developed QT-interval prolongation after the coadministration of hydroxychloroquine with at least 1 of the following drugs: darunavir-cobicistat, acetazolamide, amiodarone, lopinavir-ritonavir, haloperidol, citalopram, and trazodone. Martínez-López-de-Castro et al25 reported that 3 of 44 deceased patients also had alteration of the QT interval associated with DDIs.
Lopinavir-ritonavir and darunavir-cobicistat were involved in most of the DDI-associated ADRs. Of importance, all the drug interaction checkers used in our study could have identified such events. This finding is not surprising, because these antivirals are inhibitors of cytochrome CYP3A4, which is the most involved isoenzyme of drug metabolism.
The interaction among hydroxychloroquine, darunavir-cobicistat, and tocilizumab can also lead to psychiatric disorders, such as behavioral disturbances, psychosis, agitation, delirium, and aggression. However, psychiatric ADRs were difficult to identify by the DDI tools. Martínez-López-de-Castro et al25 evaluated 2 patients taking hydroxychloroquine and lopinavir-ritonavir who experienced psychiatric disorders, whereas Anmella et al16 described 1 patient treated with acetazolamide, hydroxychloroquine, lopinavir-ritonavir, paroxetine, risperidone, and topiramate who had disturbing behavior.
None of the interaction tools identified the cutaneous ADRs that emerged from the systematic review. Martínez-López-de-Castro et al25 identified 8 patients with COVID-19 who reported cutaneous reactions following administration of acetazolamide plus hydroxychloroquine and hydroxychloroquine plus lopinavir-ritonavir. Skroza et al30 described erythematous rash, urticaria, and varicella-like blisters in 18 patients and 1 patient with a history of COVID-19 and late-onset urticarial vasculitis after healing.
Therapy must be chosen wisely, especially when dealing with drugs known to favor DDIs, such as anticoagulants.42 In this regard, Ghani et al22 described 3 patients treated with hydroxychloroquine and unfractionated or fractionated heparins or apixaban who had subarachnoid, severe cerebral edema, and intraparenchymal hemorrhages. A recent review43 also highlighted the risk of QT-interval prolongation and cardiomyopathy attributable to the possibility of interaction between apixaban and hydroxychloroquine because of a mechanism of inhibition of CYP2C8 and P-glycoprotein.
Several potential DDIs that involved anticancer drugs used for the treatment of COVID-19 were also found (eTable 1 in the Supplement). This finding is important considering that anticancer agents have a narrow therapeutic index and the ADRs are responsible for approximately 12% of hospitalizations in oncology units, almost 3 times more than in other medical areas.44,45 Anticancer drugs belonging to the targeted therapy are mainly associated with QT liability and interact with concomitant medications, increasing the likelihood of life-threatening ventricular arrhythmia.43,46 Nevertheless, our systematic review retrieved only 2 studies that reported potential DDIs that involved anticancer drugs. Szekely et al31 indicated a potential DDI that involved letrozole coadministered with chloroquine and memantine, leading to torsade de pointes. However, none of the 5 drug interaction checkers detected such a DDI. Treon et al34 documented a tachyarrhythmia potentially associated with acetazolamide, hydroxychloroquine, and ibrutinib administration. However, 4 of 5 drug interaction checkers recognized acetazolamide and hydroxychloroquine but not ibrutinib as responsible drugs for this DDI. No other DDI-associated adverse outcomes that involved ibrutinib were found despite this drug being a P-glycoprotein inhibitor and CYP3A4 substrate.47
The experience of the pandemic offers the opportunity to improve therapy for patients with other diseases, such as rheumatological diseases, who have variable responses to the disease-modifying antirheumatic drugs. Identifying pretherapeutic and on-treatment factors associated with drug effectiveness is essential in this field.48 The same goes for all drugs, including antivirals, anticoagulants, hypoglycemic agents, and antibiotics, whose use is not avoidable, especially in hospitalized patients. Recently, 2 oral antivirals were approved. One of them is molnupiravir, originally developed against influenza viruses.49,50 The other one is an association of 2 protease inhibitors, nirmatrelvir and ritonavir.51
Drug interaction checkers identified potential DDIs that involved nirmatrelvir-ritonavir and several drugs, such as colchicine, statins, antithrombotic, immunosuppressant, and antineoplastic agents, and DDIs that involved fluvoxamine combined with antidepressants, antiplatelet agents, benzodiazepines, and fentanyl. Conversely, only LexiComp identified a DDI between molnupiravir and cladribine. The reviewed studies16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 did not report DDI-associated clinical outcomes, conceivably because of the recent use of these COVID-19 drugs. However, potential DDIs should never be underestimated. In particular, even if nirmatrelvir-ritonavir has been specifically developed for the treatment of COVID-19, the presence of ritonavir should be emphasized.
Limitations
This study has some limitations. Only 5 (although widely used and consolidated) available drug interaction checkers were accessed, with the risk of overlooking some DDI-associated ADRs that occurred in clinical practice. However, the concomitant use of tools with different classification methods can complicate the assessment of the DDI-associated outcomes. Similarly, we may have neglected studies included in gray literature (eg, congress proceedings) and emerging sources (eg, preprint websites). Moreover, except for the study by Crescioli et al,19 which used the Naranjo algorithm, the other reviewed studies did not implement a causality assessment to ascertain the relationship between DDIs and the ADRs described. However, the aim of drug interaction checkers is to highlight the risk of DDI-associated ADRs to help physicians and patients to follow the most appropriate therapy and set up monitoring actions.
Conclusions
The findings of this systematic review of drug interactions among patients with COVID-19 reported in databases and the literature suggest that extreme caution should be used in choosing COVID-19 therapy, especially in polytreated patients. Although a critical emergency, such as the COVID-19 pandemic, might justify an urgent clinical approach, possible DDIs should never be ignored when choosing the most effective and safest therapy. In this context, support could and can still derive from drug interaction checkers, which help to perform a therapeutic reconciliation by stopping use of or withholding drugs and by intensifying clinical monitoring. Attention must be paid to concomitantly examine different sources of information to manage old and new drugs. The COVID-19 pandemic offers learning and opportunity to draw on new ideas and stimuli to optimize the care of all patients with complex conditions.
eFigure. Flowchart of the Systematic Review
eTable 1. Potential DDIs for Each Drug Administered Against COVID-19 With the Degree of Severity of the Associated Adverse Outcomes and/or ADRs Found by Using the Drug Interaction Checkers
eTable 2. Reviewed Studies With Reported DDIs and Their Predictability (YES or NO) by Using the DDI Checkers
References
- 1.Pagliano P, Sellitto C, Conti V, Ascione T, Esposito S. Characteristics of viral pneumonia in the COVID-19 era: an update. Infection. 2021;49(4):607-616. doi: 10.1007/s15010-021-01603-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pagliano P, Scarpati G, Sellitto C, et al. Experimental pharmacotherapy for COVID-19: the latest advances. J Exp Pharmacol. 2021;13:1-13. doi: 10.2147/JEP.S255209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esposito S, Noviello S, Pagliano P. Update on treatment of COVID-19: ongoing studies between promising and disappointing results. Infez Med. 2020;28(2):198-211. [PubMed] [Google Scholar]
- 4.Perazzolo S, Zhu L, Lin W, Nguyen A, Ho RJY. Systems and clinical pharmacology of COVID-19 therapeutic candidates: a clinical and translational medicine perspective. J Pharm Sci. 2021;110(3):1002-1017. doi: 10.1016/j.xphs.2020.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantudo-Cuenca MD, Gutiérrez-Pizarraya A, Pinilla-Fernández A, et al. Drug-drug interactions between treatment specific pharmacotherapy and concomitant medication in patients with COVID-19 in the first wave in Spain. Sci Rep. 2021;11(1):12414. doi: 10.1038/s41598-021-91953-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drugs.com . Drug Interaction checker. Accessed February 13, 2022. https://www.drugs.com/
- 9.Covid 19 Drug Interaction. Drug Interaction checker. Accessed February 13, 2022. https://www.covid19-druginteractions.org/checker
- 10.Wolters Kluwer. Lexicomp User Academy. Accessed February 13, 2022. https://www.wolterskluwer.com/en/solutions/lexicomp/resources/lexicomp-user-academy/drug-interactions-analysis
- 11.Medscape Reference. Drug Interactions Checker. Accessed February 13, 2022. https://reference.medscape.com/
- 12.WebMD . Better information. Better health. Accessed February 13, 2022. https://www.webmd.com/
- 13.European Medicines Agency . Accessed February 13, 2022. https://www.ema.europa.eu/en
- 14.Agenzia Italiana del Farmaco . Accessed February 13, 2022. https://www.aifa.gov.it/
- 15.Clinicaltrials.gov . Accessed February 13, 2022. https://www.clinicaltrials.gov/
- 16.Anmella G, Arbelo N, Fico G, et al. COVID-19 inpatients with psychiatric disorders: real-world clinical recommendations from an expert team in consultation-liaison psychiatry. J Affect Disord. 2020;274:1062-1067. doi: 10.1016/j.jad.2020.05.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartiromo M, Borchi B, Botta A, et al. Threatening drug-drug interaction in a kidney transplant patient with coronavirus disease 2019 (COVID-19). Transpl Infect Dis. 2020;22(4):e13286. doi: 10.1111/tid.13286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borba MGS, Val FFA, Sampaio VS, et al. ; CloroCovid-19 Team . Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3(4):e208857. doi: 10.1001/jamanetworkopen.2020.8857 [DOI] [PubMed] [Google Scholar]
- 19.Crescioli G, Brilli V, Lanzi C, et al. Adverse drug reactions in SARS-CoV-2 hospitalised patients: a case-series with a focus on drug-drug interactions. Intern Emerg Med. 2021;16(3):697-710. doi: 10.1007/s11739-020-02586-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dajti E, Cristini F, Tamanini G, Cescon M, Bazzoli F, Tamè M. COVID-19 in a young liver transplant recipient: caution for drug-drug interactions. J Gastrointestin Liver Dis. 2020;29(3):470. doi: 10.15403/jgld-2672 [DOI] [PubMed] [Google Scholar]
- 21.Gautret P, Lagier JC, Honoré S, Hoang VT, Raoult D. Clinical efficacy and safety profile of hydroxychloroquine and azithromycin against COVID-19. Int J Antimicrob Agents. 2021;57(1):106242. doi: 10.1016/j.ijantimicag.2020.106242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghani MU, Kumar M, Ghani U, Sonia F, Abbas SA. Intracranial hemorrhage complicating anticoagulant prophylactic therapy in three hospitalized COVID-19 patients. J Neurovirol. 2020;26(4):602-604. doi: 10.1007/s13365-020-00869-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Yang Y, Liu L, et al. Effect of combination antiviral therapy on hematological profiles in 151 adults hospitalized with severe coronavirus disease 2019. Pharmacol Res. 2020;160:105036. doi: 10.1016/j.phrs.2020.105036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macías J, Pinilla A, Lao-Dominguez FA, et al. High rate of major drug-drug interactions of lopinavir-ritonavir for COVID-19 treatment. Sci Rep. 2020;10(1):20958. doi: 10.1038/s41598-020-78029-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martínez-López-de-Castro N, Samartín-Ucha M, Paradela-Carreiro A, et al. Real-world prevalence and consequences of potential drug-drug interactions in the first-wave COVID-19 treatments. J Clin Pharm Ther. 2021;46(3):724-730. doi: 10.1111/jcpt.13337 [DOI] [PubMed] [Google Scholar]
- 26.Meriglier E, Rivoisy C, Hessamfar M, et al. Safety of hydroxychloroquine and darunavir or lopinavir in COVID-19 infection. J Antimicrob Chemother. 2021;76(2):482-486. doi: 10.1093/jac/dkaa441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meziyerh S, Zwart TC, van Etten RW, et al. Severe COVID-19 in a renal transplant recipient: a focus on pharmacokinetics. Am J Transplant. 2020;20(7):1896-1901. doi: 10.1111/ajt.15943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nham E, Ko JH, Jeong BH, et al. Severe thrombocytopenia in a patient with COVID-19. Infect Chemother. 2020;52(3):410-414. doi: 10.3947/ic.2020.52.3.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramireddy A, Chugh H, Reinier K, et al. Experience with hydroxychloroquine and azithromycin in the coronavirus disease 2019 pandemic: implications for QT interval monitoring. J Am Heart Assoc. 2020;9(12):e017144. doi: 10.1161/JAHA.120.017144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skroza N, Bernardini N, Balduzzi V, et al. A late-onset widespread skin rash in a previous COVID-19-infected patient: viral or multidrug effect? J Eur Acad Dermatol Venereol. 2020;34(9):e438-e439. doi: 10.1111/jdv.16633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szekely Y, Lichter Y, Taieb P, et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142(4):342-353. doi: 10.1161/CIRCULATIONAHA.120.047971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teoli D, Thompson V, Wright J, et al. Acute pain crisis caused by tramadol remdesivir drug-drug interaction. J Palliat Med. 2021;24(10):1582-1584. doi: 10.1089/jpm.2021.0123 [DOI] [PubMed] [Google Scholar]
- 33.Thammathiwat T, Tungsanga S, Tiankanon K, et al. A case of successful treatment of severe COVID-19 pneumonia with favipiravir and tocilizumab in post-kidney transplant recipient. Transpl Infect Dis. 2021;23(1):e13388. doi: 10.1111/tid.13388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treon SP, Castillo JJ, Skarbnik AP, et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135(21):1912-1915. doi: 10.1182/blood.2020006288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yekedüz E, Dursun B, Aydın GÇ, et al. Clinical course of COVID-19 infection in elderly patient with melanoma on nivolumab. J Oncol Pharm Pract. 2020;26(5):1289-1294. doi: 10.1177/1078155220924084 [DOI] [PubMed] [Google Scholar]
- 36.Pagliano P, Sellitto C, Scarpati G, et al. An overview of the preclinical discovery and development of remdesivir for the treatment of coronavirus disease 2019 (COVID-19). Expert Opin Drug Discov. 2022;17(1):9-18. doi: 10.1080/17460441.2021.1970743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693-704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stasi C, Fallani S, Voller F, Silvestri C. Treatment for COVID-19: an overview. Eur J Pharmacol. 2020;889:173644. doi: 10.1016/j.ejphar.2020.173644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong CKH, Wong JYH, Tang EHM, Au CH, Wai AKC. Clinical presentations, laboratory and radiological findings, and treatments for 11,028 COVID-19 patients: a systematic review and meta-analysis. Sci Rep. 2020;10(1):19765. doi: 10.1038/s41598-020-74988-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conti V, Corbi G, Sellitto C, et al. Effect of tocilizumab in reducing the mortality rate in COVID-19 patients: a systematic review with meta-analysis. J Pers Med. 2021;11(7):628. doi: 10.3390/jpm11070628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preskorn SH, Quadri S. Why are patients with COVID-19 at risk for drug-drug interactions? J Psychiatr Pract. 2020;26(6):485-492. doi: 10.1097/PRA.0000000000000502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agenzia Italiana del Farmaco . Uso delle eparine nei pazienti adulti con COVID-19. Accessed February 13, 2022. https://www.aifa.gov.it/documents/20142/0/Eparine_Basso_Peso_Molecolare_13.05.2021.pdf
- 43.Gatti M, Raschi E, Poluzzi E, et al. The complex management of atrial fibrillation and cancer in the COVID-19 era: drug interactions, thromboembolic risk, and proarrhythmia. Curr Heart Fail Rep. 2020;17(6):365-383. doi: 10.1007/s11897-020-00485-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miranda V, Fede A, Nobuo M, et al. Adverse drug reactions and drug interactions as causes of hospital admission in oncology. J Pain Symptom Manage. 2011;42(3):342-353. doi: 10.1016/j.jpainsymman.2010.11.014 [DOI] [PubMed] [Google Scholar]
- 45.Chan A, Soh D, Ko Y, Huang YC, Chiang J. Characteristics of unplanned hospital admissions due to drug-related problems in cancer patients. Support Care Cancer. 2014;22(7):1875-1881. doi: 10.1007/s00520-014-2160-0 [DOI] [PubMed] [Google Scholar]
- 46.CredibleMeds . Accessed February 13, 2022. https://crediblemeds.org/
- 47.Chai KL, Rowan G, Seymour JF, Burbury K, Carney D, Tam CS. Practical recommendations for the choice of anticoagulants in the management of patients with atrial fibrillation on ibrutinib. Leuk Lymphoma. 2017;58(12):2811-2814. doi: 10.1080/10428194.2017.1315115 [DOI] [PubMed] [Google Scholar]
- 48.Conti V, Corbi G, Costantino M, et al. Biomarkers to personalize the treatment of rheumatoid arthritis: focus on autoantibodies and pharmacogenetics. Biomolecules. 2020;10(12):14. doi: 10.3390/biom10121672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon CJ, Tchesnokov EP, Schinazi RF, Götte M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J Biol Chem. 2021;297(1):100770. doi: 10.1016/j.jbc.2021.100770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Painter WP, Holman W, Bush JA, et al. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob Agents Chemother. 2021;65(5):e02428-e20. doi: 10.1128/AAC.02428-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374(6575):1586-1593. doi: 10.1126/science.abl4784 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flowchart of the Systematic Review
eTable 1. Potential DDIs for Each Drug Administered Against COVID-19 With the Degree of Severity of the Associated Adverse Outcomes and/or ADRs Found by Using the Drug Interaction Checkers
eTable 2. Reviewed Studies With Reported DDIs and Their Predictability (YES or NO) by Using the DDI Checkers