Key Points
Question
Is there a difference in incidence of dementia by race and ethnicity among enrollees in the US Veterans Health Administration (VHA)?
Findings
In this retrospective cohort study of 1 869 090 older adults receiving care at VHA medical centers, the age-adjusted incidence of dementia per 1000 person-years over a mean follow-up of 10.1 years was 14.2 for American Indian or Alaska Native participants, 12.4 for Asian participants, 19.4 for Black participants, 20.7 for Hispanic participants, and 11.5 for White participants. After adjustment, the hazard ratios compared with White participants were significantly higher in all subgroups except among American Indian or Alaska Native participants.
Meaning
Among adults who received care at VHA medical centers, significant differences in dementia incidence existed based on race and ethnicity.
Abstract
Importance
The racial and ethnic diversity of the US, including among patients receiving their care at the Veterans Health Administration (VHA), is increasing. Dementia is a significant public health challenge and may have greater incidence among older adults from underrepresented racial and ethnic minority groups.
Objective
To determine dementia incidence across 5 racial and ethnic groups and by US geographical region within a large, diverse, national cohort of older veterans who received care in the largest integrated health care system in the US.
Design, Setting, and Participants
Retrospective cohort study within the VHA of a random sample (5% sample selected for each fiscal year) of 1 869 090 participants aged 55 years or older evaluated from October 1, 1999, to September 30, 2019 (the date of final follow-up).
Exposures
Self-reported racial and ethnic data were obtained from the National Patient Care Database. US region was determined using Centers for Disease Control and Prevention (CDC) regions from residential zip codes.
Main Outcomes and Measures
Incident diagnosis of dementia (9th and 10th editions of the International Classification of Diseases). Fine-Gray proportional hazards models were used to examine time to diagnosis, with age as the time scale and accounting for competing risk of death.
Results
Among the 1 869 090 study participants (mean age, 69.4 [SD, 7.9] years; 42 870 women [2%]; 6865 American Indian or Alaska Native [0.4%], 9391 Asian [0.5%], 176 795 Black [9.5%], 20 663 Hispanic [1.0%], and 1 655 376 White [88.6%]), 13% received a diagnosis of dementia over a mean follow-up of 10.1 years. Age-adjusted incidence of dementia per 1000 person-years was 14.2 (95% CI, 13.3-15.1) for American Indian or Alaska Native participants, 12.4 (95% CI, 11.7-13.1) for Asian participants, 19.4 (95% CI, 19.2-19.6) for Black participants, 20.7 (95% CI, 20.1-21.3) for Hispanic participants, and 11.5 (95% CI, 11.4-11.6) for White participants. Compared with White participants, the fully adjusted hazard ratios were 1.05 (95% CI, 0.98-1.13) for American Indian or Alaska Native participants, 1.20 (95% CI, 1.13-1.28) for Asian participants, 1.54 (95% CI, 1.51-1.57) for Black participants, and 1.92 (95% CI, 1.82-2.02) for Hispanic participants. Across most US regions, age-adjusted dementia incidence rates were highest for Black and Hispanic participants, with rates similar among American Indian or Alaska Native, Asian, and White participants.
Conclusions and Relevance
Among older adults who received care at VHA medical centers, there were significant differences in dementia incidence based on race and ethnicity. Further research is needed to understand the mechanisms responsible for these differences.
This study evaluated racial and ethnic and regional differences in dementia risk within a large, diverse national cohort of older adults receiving care in the Veterans Health Administration.
Introduction
Studies examining racial and ethnic disparities in dementia incidence in the US have consistently reported higher rates of dementia for Black adults.1,2,3 Hispanic older adults are less well studied but also have greater dementia incidence than have White older adults.2,4,5 Much less is known about dementia incidence for American Indian or Alaska Native or for Asian individuals.6
Structural and systemic factors including unequal access to health care, the health effects of racism, and differences in quality of care, as well as discrepancies in the prevalence of dementia risk factors (such as cardiovascular disease) play important roles in these differences.2,4,5,7,8 However, most prior studies of racial and ethnic differences in dementia incidence compared only 1 or 2 racial or ethnic groups, were limited to a specific geographical area, and often did not account for competing mortality.1,2,3,6,7
US veterans are at high risk of dementia because of exposure to military-related risk factors (eg, traumatic brain injury [TBI] and posttraumatic stress disorder [PTSD]) and high prevalence of cardiovascular and other nonmilitary risk factors.9,10,11,12,13 Studying individuals enrolled in the Veterans Health Administration (VHA) integrated nationwide health care system provides a opportunity to study a large, diverse, and national sample in which availability of care is held relatively equal regardless of demographics. Moreover, VHA data allow for exploration of the association of US region with dementia incidence, a key question given regional differences in risk factor distribution.14,15
This study evaluated racial and ethnic and regional differences in dementia risk within a large, diverse national cohort of older adults receiving care in the VHA, the largest integrated health care system in the US. Differences in incidence rates by geographical region across the US were also examined.
Methods
Standard Protocol Approvals
All study procedures were approved by institutional review boards at the University of California, San Francisco; San Francisco Veterans Affairs Medical Center; and the US Army Medical Research and Material Command, Office of Research Protections, Human Research Protection Office. Informed consent was waived because data were deidentified administrative data.
Study Population
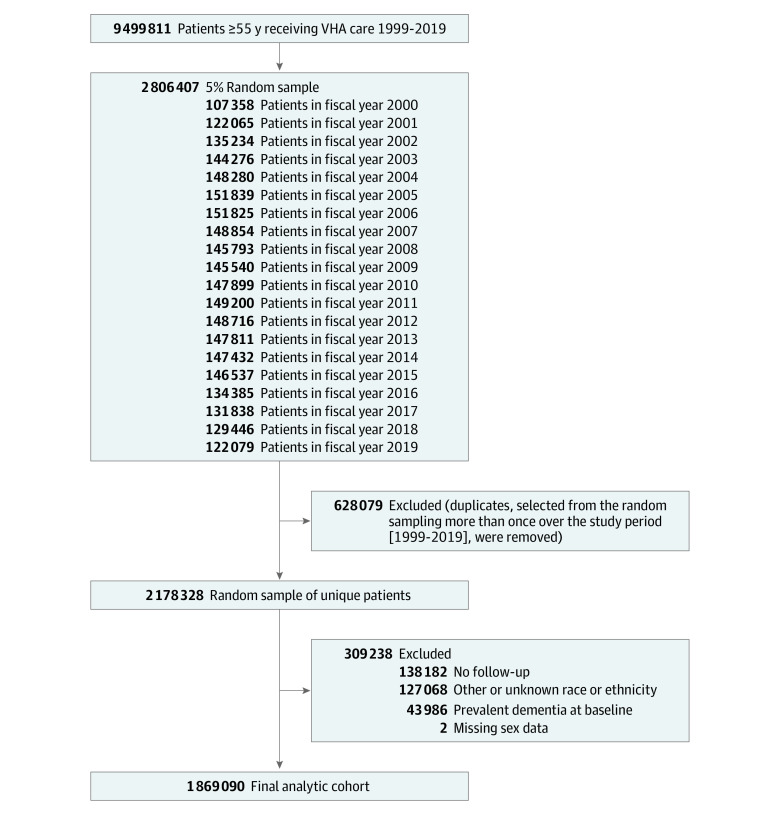
We identified a random sample of all adults aged 55 years or older who obtained care at VHA medical centers from October 1, 1999, to September 30, 2019. For each fiscal year from 2000 through 2019, we selected a 5% random sample from all patients, and then merged these samples for all years (Figure 1). Patients who were selected more than once from the random sampling procedures were flagged as duplicates and were not included in this study. This resulted in a random sample of all VHA patients who received care in that period.
Figure 1. Cohort Development in a Study of the Association of Race and Ethnicity With Incidence of Dementia.
Patients were required to have at least 1 visit during the 2-year period before their random selection date (baseline) and at least 1 follow-up visit.
For all participants, we obtained demographic information and dementia diagnoses from all inpatient and outpatient visits from the National Patient Care Databases and mortality from the Vital Status File database. Participants were required to have at least 1 visit during the 2-year period before their random selection date (baseline) and at least 1 follow-up visit. We excluded individuals with prevalent dementia over the 2-year baseline period and those with missing data for sex and those characterized as other or unknown race.
Exposures
Race and Ethnicity
Self-reported race and ethnicity were categorized into 5 groups: American Indian or Alaska Native, Asian, Black, Hispanic, and White. The study did not distinguish between Hispanic and non-Hispanic Black and White individuals. Participants identifying as “other” or whose race and ethnicity was unknown were excluded from analyses.
US Geographical Region
We used self-reported address zip codes to derive data on geocoded regions, based on the Centers for Disease Control and Prevention (CDC) National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP) regions. The NCCDPHP divides the US into 10 regions, each composed of 4 to 7 states.16
Other Demographic Characteristics
Demographic data included age, sex, and education. Zip codes and 2016 American Community Survey data were used to categorize participants’ residences into educational categories (≤25% of the adult population has earned a bachelor’s degree or higher vs >25%).
Comorbid Conditions
Medical and psychiatric comorbidities as identified by International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes were assessed during the 2-year baseline. Comorbidities included hypertension, obesity, stroke or transient ischemic attack, diabetes, TBI, PTSD, and alcohol use disorder.
Outcome
Dementia
We defined prevalent dementia during baseline (for exclusion) and incident dementia over follow-up using a comprehensive list of inpatient and outpatient ICD-9 and ICD-10 codes recommended by the VHA Dementia Steering Committee.17 The use of ICD codes in defining medical conditions among older adults has repeatedly been shown to be valid against external reference standards,18 including specifically for dementia, with positive predictive values ranging from 78.9% to 96.3%.19
Statistical Analyses
Baseline characteristics were compared across the 5 racial and ethnic groups using analysis of variance for continuous variables and χ2 analysis for categorical variables. Age-adjusted dementia incidence was calculated using direct standardization20 to compare rates among racial and ethnic groups. Fine-Gray proportional hazards regression was used to examine the association between race and ethnicity and time to dementia diagnosis with age as the time scale while accounting for the competing risk of death. We estimated an additional Fine-Gray proportional hazards model to test the interactive effect between the 5 racial and ethnic groups across US region by including race and ethnicity and region as the interaction terms in the model; a significant P value is consistent with a significant interaction effect (P < .05). Time to event was calculated from baseline until dementia diagnosis or death (whichever occurred first). Participants who did not die or develop dementia during follow-up were censored at the last medical encounter. Fine-Gray regression handles death as an alternate competing risk, which provides a more conservative estimate of the association between race and ethnicity and dementia than statistical approaches that treat patients who die as censored, which assumes they would still be at risk if additional follow-up data had been available. Fine-Gray models were (1) unadjusted (with age as the time scale and accounting for the competing risk of death) and (2) adjusted for sex, education, and medical and psychiatric comorbidities and are reported as hazard ratios (HRs) with 95% CIs. Values for education were missing for 40 602 participants (2.2%). Because the amount of missing education data was minimal, these data points were excluded from the multivariable models. We also estimated age-adjusted dementia incidence rates according to race and ethnicity and US region (categorized as CDC NCCDPHP regions.16) The proportional hazards model assumption was tested by examining cumulative residuals with respect to time (the ASSESS statement) and met for all final models. P values were 2-sided with significance defined as P < .05. Analyses were performed with SAS version 9.4 (SAS Institute Inc).
Results
From a total random sample of 2 178 328 patients aged 55 years or older who received care at the VHA from October 1, 1999, to September 30, 2019, 138 182 (6.3%) were excluded due to no follow-up visit; 127 068 (5.8%) were excluded due to race and ethnicity classified as other or unknown; 43 986 (2.0%) were excluded due to prevalent dementia at baseline; and 2 were missing sex data. Overall, 1 869 090 VHA patients were included in this retrospective cohort study (Figure 1). At baseline, study participants had a mean (SD) age of 69.4 (7.9) years and 42 870 (2.3%) were women. Consistent with previous reports of the racial and ethnic distribution of older persons receiving care at the VHA, 6865 (0.4%) were American Indian or Alaska Native participants; 9391 (0.5%), Asian participants; 176 795 (9.5%), Black participants; 20 663 (1%), Hispanic participants; and 1 655 376 (88.6%), White participants.21 Participants were followed up for a mean of 10.1 years (median, 10.3; range, 0.1-17.9 years) until they developed dementia, died, or completed their last medical encounter, whichever occurred first.
Most demographics and comorbidities varied by race and ethnicity (Table 1) with greater educational attainment among White and Asian participants and lower among American Indian or Alaska Native participants. All groups had high prevalence of cardiovascular risk factors, with Asian participants having relatively lower prevalence of most conditions (except hypertension) than other groups. Prevalence of PTSD and alcohol use ranged from 3% to 11%, with higher prevalence among the non-White participant groups than among the White participant group. The proportion of participants who died during follow-up was significantly lower among all non-White groups (34.7% for American Indian or Alaska Native participants, 31.4% for Asian participants, 29.8% for Black participants, and 32.9% for Hispanic participants) than 36.7% among White participants (P < .001).
Table 1. Baseline Characteristics of Older Participants by Race and Ethnicitya.
| Characteristic | No. (%) of participants | ||||
|---|---|---|---|---|---|
| American Indian or Alaska Native (n = 6865) |
Asian (n = 9391) |
Black (n = 176 795) |
Hispanic (n = 20 663) |
White (n = 1 655 376) |
|
| Age, mean (SD), y | 67.7 (7.8) | 70.4 (9.0) | 67.1 (7.6) | 71.8 (8.2) | 69.6 (7.9) |
| Men | 6667 (97.1) | 8880 (94.6) | 171 900 (97.2) | 20 345 (98.5) | 1 618 428 (97.8) |
| Women | 198 (2.9) | 511 (5.4) | 4895 (2.8) | 318 (1.5) | 36 948 (2.2) |
| >25% college-educated zip codeb | 1787 (26.9) | 5109 (59.0) | 58 566 (34.1) | 6646 (34.0) | 708 201 (43.7) |
| Comorbidities | |||||
| Hypertension | 4139 (60.3) | 5859 (62.4) | 130 329 (73.7) | 14 089 (68.2) | 1 078 818 (65.2) |
| Diabetes | 2253 (32.8) | 2694 (28.7) | 56 019 (31.7) | 7451 (36.1) | 403 629 (24.4) |
| Obesity | 1123 (16.4) | 730 (7.8) | 28 318 (16.0) | 2419 (11.7) | 260 947 (16.0) |
| Posttraumatic stress disorder | 771 (11.2) | 701 (7.5) | 14 931 (8.4) | 1112 (5.4) | 82 252 (5.0) |
| Alcohol use disorder | 628 (9.1) | 254 (2.7) | 14 620 (8.3) | 1238 (6.0) | 76 378 (4.6) |
| Stroke or transient ischemic attack | 507 (7.4) | 614 (6.5) | 13 966 (7.9) | 1700 (8.2) | 123 610 (7.5) |
| Traumatic brain injury | 25 (0.4) | 43 (0.5) | 714 (0.4) | 102 (0.5) | 4647 (0.3) |
The baseline period reflects the 2 years prior to the random selection date for each participant.
Missing values amounted to 2.2%.
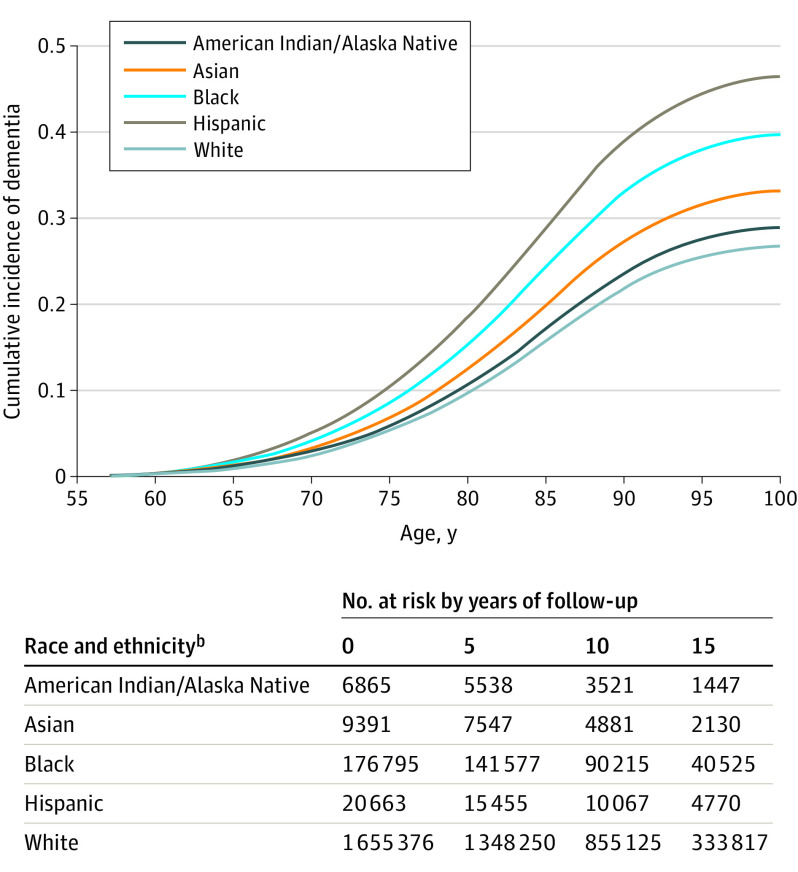
Overall, 243 272 participants (13%) developed dementia during follow-up. (The number of participants at risk of dementia in each racial and ethnic group at 5-year intervals during follow-up is shown in Figure 2.) Age-adjusted dementia incidence (Table 2) was highest for Hispanic participants (20.7 per 1000 person-years, 95% CI, 20.1-21.3) followed by and Black participants (19.4 per 1000 person-years, 95% CI, 19.2-19.6), lower among American Indian or Alaska Native (14.2 per 1000 person-years, 95% CI, 13.3-15.1) and Asian participants (12.4 per 1000 person-years, 95% CI, 11.7-13.1), and lowest among White participants (11.5 per 1000 person-years, 95% CI, 11.4-11.6). Compared with White participants, the HRs for developing dementia in unadjusted models with age as the timescale and accounting for the competing risk were 1.08 (95% CI, 1.01-1.16) for American Indian or Alaska Native participants, 1.24 (95% CI, 1.17-1.30) for Asian participants, 1.55 (95% CI, 1.53-1.57) for Black participants, and 1.99 (95% CI, 1.93-2.04) for Hispanic participants. In the fully adjusted model (including further adjustment for sex, education, and medical and psychiatric comorbidities), compared with White participants, the HRs were 1.05 (95% CI, 0.98-1.13) for American Indian or Alaska Native participants, 1.20 (95% CI, 1.13-1.28) for Asian participants, 1.54 (95% CI, 1.51-1.57) for Black participants, and 1.92 (95% CI, 1.82-2.02) for Hispanic participants.
Figure 2. Cumulative Incidence of Dementia by Racial and Ethnic Groupa.
aAge is used as the timescale to indicate age at dementia diagnosis, and death is treated as a competing risk. Participants contributed follow-up data either until the age they died or developed dementia (whichever came first); patients who did not develop dementia or die continued to contribute to follow-up data until the age of their last medical encounter date. Dementia is defined using a comprehensive list of International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes recommended by the Veterans Health Administration Dementia Steering Committee.17
bThe table shows participant numbers at 0, 5, 10, and 15 years. The curves as plotted with competing risk models do not permit the creation of a more typical table of participants at risk.
See eTable 1 in the Supplement for cumulative incidence of dementia with associated 95% CIs.
Table 2. Dementia Incidence by Race and Ethnicity Among 1 869 090 Older Participantsa.
| American Indian or Alaska Native (n = 6865) |
Asian (n = 9391) |
Black (n = 176 795) |
Hispanic (n = 20 663) |
White (n = 1 655 376) |
|
|---|---|---|---|---|---|
| New diagnoses of dementia, No. (%)a | 858 (12.5) | 1373 (14.6) | 29 615 (16.8) | 5189 (25.1) | 206 237 (12.5) |
| No. person-years | 73 061 | 101 024 | 1 890 444 | 212 801 | 17 614 826 |
| Incidence rate/1000 person-years (95% CI) | |||||
| Crude | 11.7 (11.0-12.5) | 13.6 (12.9-14.3) | 15.7 (15.5-15.8) | 24.4 (23.7-25.0) | 11.7 (11.6-11.8) |
| Age-adjustedb | 14.2 (13.3-15.1) | 12.4 (11.7-13.1) | 19.4 (19.2-19.6) | 20.7 (20.1-21.3) | 11.5 (11.4-11.6) |
| No. mortality over follow-upc | 2385 | 2946 | 52 602 | 6807 | 608 117 |
Dementia is defined using a comprehensive list of International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes recommended by the Veterans Health Administration Dementia Steering Committee..17
Age-adjusted incident rates per 1000 person-years were calculated using direct standardization20 for the comparison of rates among levels of race and ethnicity.
The number of participants in each racial and ethnic group who died over follow-up. The median follow-up for the entire cohort was 10.3 years (IQR, 6.1-14.3 years).
Figure 2 shows the cumulative incidence of dementia according to race and ethnicity with age as the timescale, accounting for the competing risk of death. This figure demonstrates a similar pattern of results (higher cumulative incidence by age for Black and Hispanic participants, moderately higher for Asian and American Indian or Alaska Native participants, and lowest for White participants), and indicates that the curves for the racial and ethnic groups begin to diverge around age 65 years. CIs for the cumulative incidences are presented in eTable 1 in the Supplement.
Age-adjusted dementia incidence among the 5 racial and ethnic groups was variable across US CDC NCCDPHP geographical regions (P for interaction of race and ethnicity and region, <.001, eTable 2 in the Supplement). Compared with American Indian or Alaska Native, Asian, and White participants, incidence was higher among Black participants (range per 1000 person-years, 15.5 [95% CI, 15.0-16.0] to 20.7 [95% CI, 20.1-21.3]), and Hispanic participants (range per 1000 person-years, 14.0 [95% CI, 12.8-15.2] to 21.7 [95% CI, 15.3-28.1]) across most regions (Figure 3). (See eTable 2 in the Supplement for within-region statistical comparisons in age-adjusted incidence rate per 1000 person-years by race and ethnicity.)
Figure 3. Dementia Incidence Rate by Race and Ethnicity and Region.
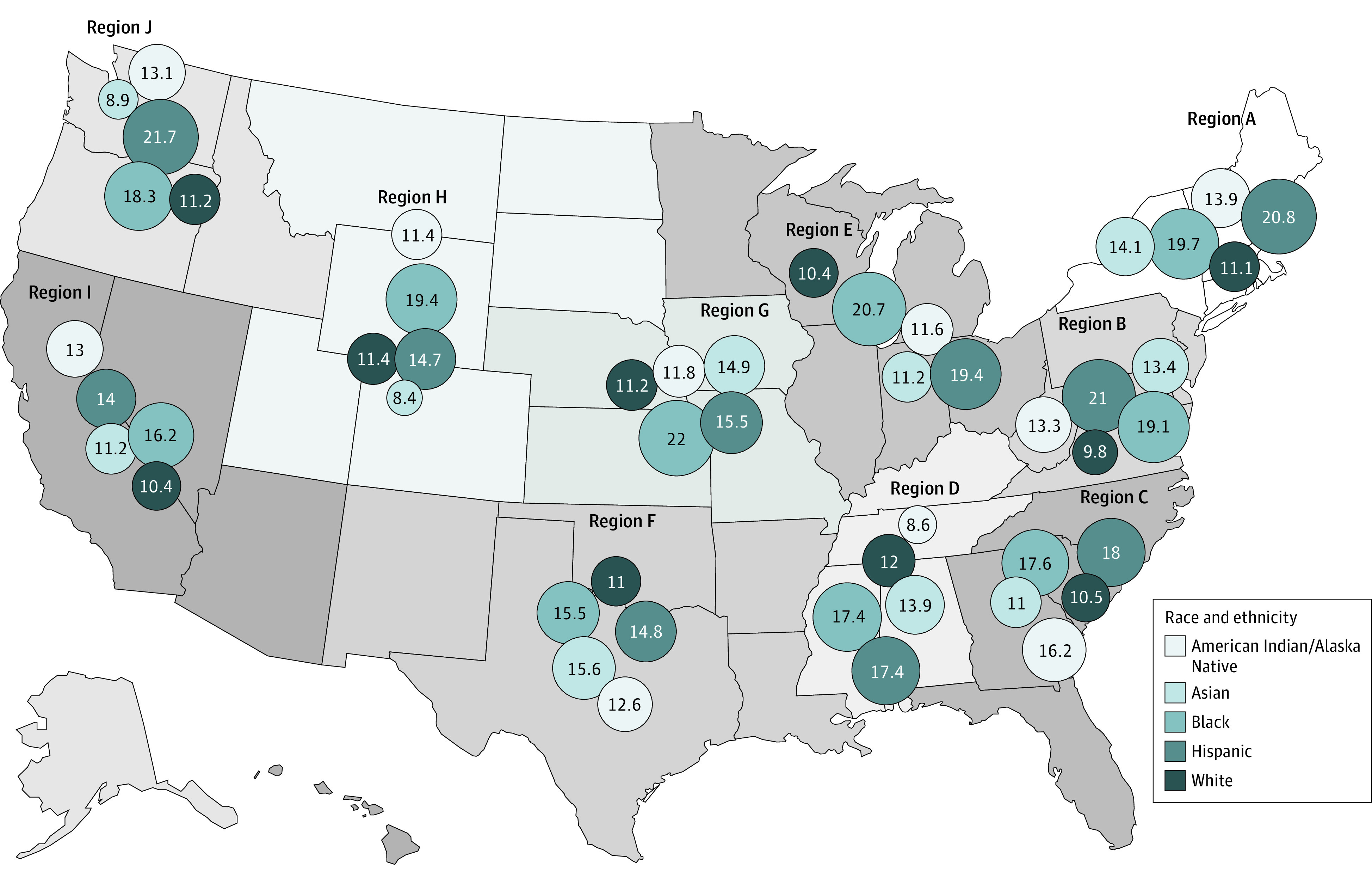
The area of the circles corresponds to the actual value of the rate presented in the circle for each racial and ethnic group. Dementia is defined using a comprehensive list of International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes recommended by the Veterans Health Administration Dementia Steering Committee.17 Of the more than 1.8 million Veterans Health Administration patients, who were a mean (SD) age of 69.4 (7.9) years and were followed up for a mean of 10.1 years (median, 10.3; range 0.7-17.9 years), 13% developed dementia.
See eTable 2 in the Supplement for within-region statistical comparisons in age-adjusted dementia incidence rate per 1000 person-years by race and ethnicity, and interaction of race and ethnicity and region.
Region A: Connecticut, Maine, Massachusetts, New Hampshire, New York, Rhode Island, Vermont
Region B: Delaware, District of Columbia, Maryland, New Jersey, Pennsylvania, Virginia, West Virginia
Region C: Georgia, Florida, North Carolina, South Carolina
Region D: Alabama, Kentucky, Mississippi, Tennessee
Region E: Illinois, Indiana, Michigan, Minnesota, Ohio, Wisconsin
Region F: Arkansas, Louisiana, New Mexico, Oklahoma, Texas
Region G: Iowa, Kansas, Missouri, Nebraska
Region H: Colorado, Montana, North Dakota, South Dakota, Utah, Wyoming
Region I: Arizona, California, Hawaii, Nevada
Region J: Alaska, Idaho, Oregon, Washington
Discussion
Among older adults receiving care at VHA medical centers, there were significant differences in dementia incidence based on race and ethnicity. There was some variation by US geographical region, but the incidence rates were consistently highest for Black participants and Hispanic participants. After accounting for competing risk of death and key demographics and comorbidities, the comparative risk for dementia was significantly higher among older Hispanic participants, Black participants, and Asian participants than among White participants.
These results are consistent with the findings of several prior studies among populations not receiving VHA care. With a few exceptions, however, most previous studies have focused comparisons on Black participants vs White participants, frequently demonstrating greater dementia incidence among older Black adults.1,3,4,5,7 One systematic review of dementia prevalence and incidence studies reported that incidence was higher among Black and Hispanic Caribbean populations and lower among White and Asian populations.2 A study of older participants enrolled in Kaiser Permanente of Northern California reported higher age-adjusted dementia incidence rates among Black and American Indian or Alaska Native patients, intermediate incidence in Hispanic and White patients, and lower incidence in Asian patients, but results were not adjusted for education.6 Unlike most prior studies,1,3,5,7 the current study accounted for the competing risk of death, a key strength given the differences in mortality across racial and ethnic groups.
To our knowledge, this is the first study to report dementia incidence for American Indian or Alaska Native older adults using a nationwide sample. Although American Indian or Alaska Native individuals make up only 1.7% of the United States population,22,23 they serve in the armed forces at higher rates than other racial and ethnic groups.24 Previous studies of dementia among American Indian or Alaska Native individuals have been small and geographically limited and have produced conflicting evidence, with some studies showing higher and some showing lower rates of dementia compared with White individuals.6,25,26,27,28 In this study, after accounting for demographics (including education) and the high rates of medical and psychiatric risk factors in the American Indian or Alaska Native group, there was no significant difference in dementia incidence compared with White participants.
One explanation for the differences observed in dementia incidence across racial and ethnic groups is bias, including medical decision-making bias, test bias, or survival bias. It is possible that clinicians are more likely to diagnose dementia among Black participants or Hispanic participants due to conscious or unconscious bias.29,30 Moreover, non-White participants may be more likely to perform less well on cognitive tests that are often used in dementia evaluations for reasons other than actual cognitive impairment (ie, test bias, English as a second language, lower educational attainment),31 which may lead to inflated rates of dementia diagnosis. There is also a concern for a survival bias; however, this was accounted for statistically by using age as a timescale in models and by using Fine-Gray models to account for competing risk of death. Another possibility is that the racial and ethnic–based differences in dementia diagnosis reflect differences in susceptibility to dementia, influenced by socioeconomic and other structural factors,7 the health effects of racism,32 decreased cognitive reserve because of unequal access to educational opportunities or quality,31 and other social determinants of health.
Differences in dementia incidence could also be driven by discrepancies in risk factors or comorbid disease, such as cardiovascular risk factors and disease, which vary by race and ethnicity and are associated with socioeconomic and structural factors,33,34 and other risk factors that are more common among individuals receiving care at VHA facilities than patients receiving care at non-VHA facilities, such as PTSD.9,35 Important racial and ethnic differences in education and many key medical and psychiatric comorbidities were observed in this cohort. When accounting statistically for these differences, the findings were only slightly attenuated, suggesting that there may be other factors underlying the observed differences.
Additionally, access to health care, which varies by race and ethnicity,36 is associated with all health outcomes. Most veterans serve as young adults and remain eligible for VHA care for the rest of their lives, implying that access to health care in adulthood may be more equitable for individuals enrolled in the VHA than for the general population. This study showed differences in dementia incidence, despite this relatively equal access to care, which suggests that other mechanisms including early life circumstances may play a stronger role, or despite having access to care, there may be differences in quality of care.
Differences in dementia incidence were also observed by US region, but age-adjusted dementia incidence was highest for Black participants and Hispanic participants across regions. Drivers of these regional differences are unclear. They may reflect different baseline overall dementia incidence by region. Alternatively, these findings may reflect the association of regional differences with prevalence of medical comorbidities, dementia diagnostic practices, or social determinants of health. For American Indian or Alaska Native participants and Asian participants, rates of dementia were lower than rates among Black participants and Hispanic participants, and in many regions, similar to those observed for White participants. More research is needed to understand the source of regional differences in dementia risk based on race and ethnicity. Future studies should also seek to incorporate participants’ regions of birth, which influences risk for dementia and other medical conditions.37
Strengths
A strength of this study is the use of a large, representative national sample of older adults, allowing for testing inferences about racial and ethnic differences in dementia incidence across the US. It used national data from the VHA, the largest integrated health care system in the US, in which access to care is held relatively equal regardless of race and ethnicity. The analyses adjusted for important health and demographic variables and accounted for the competing risk of mortality, which is essential when assessing differential risk of dementia.
Limitations
This study has several limitations. First, the sample had a relatively small proportion of women, American Indian or Alaska Native, Asian, and Hispanic participants although consistent with previously reported sex and racial and ethnic distribution of the older US VHA population.21 Second, education in this study was defined by the rate of educational attainment in the participant’s zip code. Education is an important risk factor for dementia,2,38 and although this approach has been used in previous studies of health disparities,39,40 it may not have been an accurate measure of individual ascertainment. Third, this study used ICD-9 and ICD-10 codes from medical records for dementia diagnoses, which may have resulted in less accurate categorization of participants than a comprehensive dementia examination. However, ICD codes show very good criterion validity compared with medical record text review for many conditions, including dementia.18,19
Conclusions
Among older adults who received care at VHA medical centers, there were significant differences in dementia incidence based on race and ethnicity. Further research is needed to understand the mechanisms responsible for these differences.
eTable 1. Cumulative incidence of dementia adjusted for competing risk of death
eTable 2. Age-adjusted dementia incidence among older adults by race and ethnicity and United States region
References
- 1.Katz MJ, Lipton RB, Hall CB, et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26(4):335-343. doi: 10.1097/WAD.0b013e31823dbcfc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. 2017;13(1):72-83. doi: 10.1016/j.jalz.2016.06.2360 [DOI] [PubMed] [Google Scholar]
- 3.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52(2):195-204. doi: 10.1111/j.1532-5415.2004.52058.x [DOI] [PubMed] [Google Scholar]
- 4.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1-2):125-132. doi: 10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon H, Badana ANS, Hwang S-Y, Sears JS, Haley WE. Dementia prevalence in older adults: variation by race/ethnicity and immigrant status. Am J Geriatr Psychiatry. 2019;27(3):241-250. doi: 10.1016/j.jagp.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 6.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216-224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaffe K, Falvey C, Harris TB, et al. ; Health ABC Study . Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051. doi: 10.1136/bmj.f7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25(3):187-195. doi: 10.1097/WAD.0b013e318211c6c9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613. doi: 10.1001/archgenpsychiatry.2010.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, Yaffe K. Traumatic brain injury and risk of dementia in older veterans. Neurology. 2014;83(4):312-319. doi: 10.1212/WNL.0000000000000616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veitch DP, Friedl KE, Weiner MW. Military risk factors for cognitive decline, dementia and Alzheimer’s disease. Curr Alzheimer Res. 2013;10(9):907-930. doi: 10.2174/15672050113109990142 [DOI] [PubMed] [Google Scholar]
- 12.Hinojosa R. Cardiovascular disease among United States military veterans: evidence of a waning healthy soldier effect using the National Health Interview Survey. Chronic Illn. 2020;16(1):55-68. doi: 10.1177/1742395318785237 [DOI] [PubMed] [Google Scholar]
- 13.Walker LE, Poltavskiy E, Janak JC, Beyer CA, Stewart IJ, Howard JT. US military service and racial/ethnic differences in cardiovascular disease: an analysis of the 2011-2016 Behavioral Risk Factor Surveillance System. Ethn Dis. 2019;29(3):451-462. doi: 10.18865/ed.29.3.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baicker K, Chandra A, Skinner JS. Geographic variation in health care and the problem of measuring racial disparities. Perspect Biol Med. 2005;48(1)(suppl):S42-S53. doi: 10.1353/pbm.2005.0020 [DOI] [PubMed] [Google Scholar]
- 15.Hasnain-Wynia R, Baker DW, Nerenz D, et al. Disparities in health care are driven by where minority patients seek care: examination of the hospital quality alliance measures. Arch Intern Med. 2007;167(12):1233-1239. doi: 10.1001/archinte.167.12.1233 [DOI] [PubMed] [Google Scholar]
- 16.National Center for Chronic Disease Prevention and Health Promotion Regions . Centers for Disease Control and Prevention. Accessed March 18, 2022. https://www.cdc.gov/chronicdisease/index.htm
- 17.US Department of Veterans Affairs . VHA dementia steering committee recommendations for dementia care in the VHA healthcare system 2016. Published September 2016. Accessed September 3, 2019. https://www.va.gov/GERIATRICS/docs/VHA_DSC_RECOMMENDATIONS_SEPT_2016_9-12-16.pdf
- 18.Wei MY, Luster JE, Chan C-L, Min L. Comprehensive review of ICD-9 code accuracies to measure multimorbidity in administrative data. BMC Health Serv Res. 2020;20(1):489. doi: 10.1186/s12913-020-05207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujiyoshi A, Jacobs DR Jr, Alonso A, Luchsinger JA, Rapp SR, Duprez DA. Validity of death certificate and hospital discharge ICD codes for dementia diagnosis: the Multi Ethnic Study of Atherosclerosis. Alzheimer Dis Assoc Disord. 2017;31(2):168-172. doi: 10.1097/WAD.0000000000000164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beiser A, D’Agostino RB Sr, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med. 2000;19(11-12):1495-1522. doi: [DOI] [PubMed] [Google Scholar]
- 21.VA Office of Health Equity . VA national veteran health equity report: distribution of race/ethnicity among veteran VHA patients, FY13. Published 2016. Accessed June 10, 2021. https://vha-healthequity.shinyapps.io/NVHER_Shiny/
- 22.Weiner MF, Hynan LS, Beekly D, Koepsell TD, Kukull WA. Comparison of Alzheimer’s disease in American Indians, whites, and African Americans. Alzheimers Dement. 2007;3(3):211-216. doi: 10.1016/j.jalz.2007.04.376 [DOI] [PubMed] [Google Scholar]
- 23.National Congress of American Indians. Demographics. Accessed August 24, 2021. https://www.ncai.org/about-tribes/demographics
- 24.National Indian Council on Aging, Inc . American Indian Veterans have highest record of military service. Published November 8, 2019. Accessed August 12, 2021. https://www.nicoa.org/american-indian-veterans-have-highest-record-of-military-service/
- 25.Hendrie HC, Hall KS, Pillay N, et al. Alzheimer’s disease is rare in Cree. Int Psychogeriatr. 1993;5(1):5-14. doi: 10.1017/S1041610293001358 [DOI] [PubMed] [Google Scholar]
- 26.Jervis LL, Manson SM. American Indians/Alaska natives and dementia. Alzheimer Dis Assoc Disord. 2002;16(suppl 2):S89-S95. doi: 10.1097/00002093-200200002-00011 [DOI] [PubMed] [Google Scholar]
- 27.Warren LA, Shi Q, Young K, Borenstein A, Martiniuk A. Prevalence and incidence of dementia among indigenous populations: a systematic review. Int Psychogeriatr. 2015;27(12):1959-1970. doi: 10.1017/S1041610215000861 [DOI] [PubMed] [Google Scholar]
- 28.Carty CL, Noonan C, Muller C, et al. Risk factors for Alzheimer’s disease and related dementia diagnoses in American Indians. Ethn Dis. 2020;30(4):671-680. doi: 10.18865/ed.30.4.671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spencer KL, Grace M. Social foundations of health care inequality and treatment bias. Annu Rev Sociol. 2016;42:101-120. doi: 10.1146/annurev-soc-081715-074226 [DOI] [Google Scholar]
- 30.Eliacin J, Cunningham B, Partin MR, et al. Veterans affairs providers’ beliefs about the contributors to and responsibility for reducing racial and ethnic health care disparities. Health Equity. 2019;3(1):436-448. doi: 10.1089/heq.2019.0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fyffe DC, Mukherjee S, Barnes LL, Manly JJ, Bennett DA, Crane PK. Explaining differences in episodic memory performance among older African Americans and Whites: the roles of factors related to cognitive reserve and test bias. J Int Neuropsychol Soc. 2011;17(4):625-638. doi: 10.1017/S1355617711000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyatt SB, Williams DR, Calvin R, Henderson FC, Walker ER, Winters K. Racism and cardiovascular disease in African Americans. Am J Med Sci. 2003;325(6):315-331. doi: 10.1097/00000441-200306000-00003 [DOI] [PubMed] [Google Scholar]
- 33.Tang M-X, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49-56. doi: 10.1212/WNL.56.1.49 [DOI] [PubMed] [Google Scholar]
- 34.Barnes LL, Leurgans S, Aggarwal NT, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85(6):528-534. doi: 10.1212/WNL.0000000000001834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yaffe K, Lwi SJ, Hoang TD, et al. Military-related risk factors in female veterans and risk of dementia. Neurology. 2019;92(3):e205-e211. doi: 10.1212/WNL.0000000000006778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rooks RN, Simonsick EM, Klesges LM, Newman AB, Ayonayon HN, Harris TB. Racial disparities in health care access and cardiovascular disease indicators in Black and White older adults in the Health ABC Study. J Aging Health. 2008;20(6):599-614. doi: 10.1177/0898264308321023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilsanz P, Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Association between birth in a high stroke mortality state, race, and risk of dementia. JAMA Neurol. 2017;74(9):1056-1062. doi: 10.1001/jamaneurol.2017.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guland B, Wilder D, Lantigua R, et al. Differences in rates of dementia between ethno-racial groups. In: Racial and Ethnic Differences in the Health of Older Americans. National Academies Press; 1997. [PubMed] [Google Scholar]
- 39.Javier SJ, Troszak LK, Shimada SL, et al. Racial and ethnic disparities in use of a personal health record by veterans living with HIV. J Am Med Inform Assoc. 2019;26(8-9):696-702. doi: 10.1093/jamia/ocz024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen AY, Schrag NM, Halpern M, Stewart A, Ward EM. Health insurance and stage at diagnosis of laryngeal cancer: does insurance type predict stage at diagnosis? Arch Otolaryngol Head Neck Surg. 2007;133(8):784-790. doi: 10.1001/archotol.133.8.784 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Cumulative incidence of dementia adjusted for competing risk of death
eTable 2. Age-adjusted dementia incidence among older adults by race and ethnicity and United States region