Abstract
Coevolution occurs between viruses and their hosts. The hosts need to evolve means to eliminate pathogenic virus infections, and the viruses, for their own survival and multiplication, have to develop mechanisms to escape clearance by hosts. Hepatitis C virus (HCV) of Flaviviridae is a pathogen which infects human liver and causes hepatitis, a condition of liver inflammation. Unlike most of the other flaviviruses, HCV has an excellent ability to evade host immunity to establish chronic infection. The persistent liver infection leads to chronic hepatitis, liver cirrhosis, hepatocellular carcinoma (HCC), as well as extrahepatic HCV-related diseases. HCV genomic RNA only expresses 10 proteins, many of which bear functions, in addition to those involved in HCV life cycle, for assisting the virus to develop its persistency. HCV core protein is a structural protein which encapsulates HCV genomic RNA and assembles into nucleocapsids. The core protein is also found to exert functions to affect host inflammation and immune responses by altering a variety of host pathways. This paper reviews the studies regarding the HCV core protein-induced alterations of host immunity and inflammatory responses, as well as the involvements of the HCV core protein in pro- and anti-inflammatory cytokine stimulations, host cellular transcription, lipid metabolism, cell apoptosis, cell proliferations, immune cell differentiations, oxidative stress, and hepatocyte steatosis, which leads to liver fibrosis, cirrhosis, and HCC. Implications of roles played by the HCV core protein in therapeutic resistance are also discussed.
Keywords: Hepatitis C virus core protein, Host immunity, Inflammation modulation, Virus persistent infection
INTRODUCTION
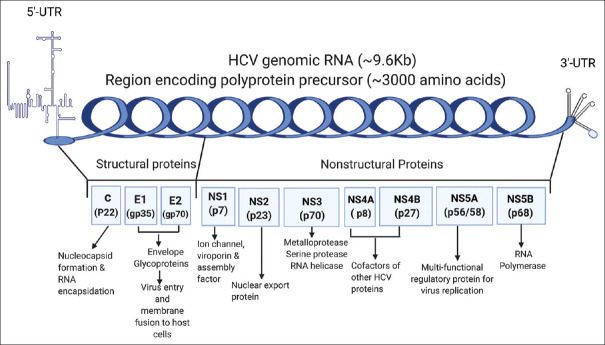
Despite the characteristic similarities of viruses within the genera Flaviviridae, hepatitis C virus (HCV) possess unique epidemiological and pathophysiological properties among the viruses in the family: HCV is most frequently transmitted via blood-to-blood contact between humans, while the majority of flaviviruses are vectored by mosquitos or ticks [1]; HCV has an exceptional ability to evade clearance by host immune response to develop its chronicity, with 60%–85% of infected patients develop into chronic infections [2,3] no known other flaviviruses have this high chronicity rate in humans [4]. The persistent liver infection can lead to chronic hepatitis, liver cirrhosis, and in some cases, hepatocellular carcinoma (HCC) [5]. HCV is an enveloped RNA virus containing a positive-sense, single-stranded genomic RNA of approximately 9600 nucleotides. The HCV genomic RNA and the translated proteins are schematically indicated in Figure 1. Sandwiched by 5'- and 3'-untranslated regions, the open reading frame of genomic RNA encodes a polyprotein with approximately 3000 amino acids [6]. The polyprotein is cleaved post-translationally by virus and host proteases into structural proteins and non-structural proteins. The primary functions of HCV proteins in virus infection, replication, and assembly are also indicated in Figure 1. The structural proteins, including core, E1, and E2, are the building blocks assembling into virus particles. The non-structural proteins, including ion channel protein p7 (NS1), NS2, NS3, NS4A, NS4B, NS5A, and NS5B, are responsible for forming virus replication systems and virus productions [6]. The best-known primary function of HCV core protein is to assemble into nucleocapsid and encapsulate the virus genomic RNA. However, additional properties of the HCV core protein for modulating host cell transcription, inhibition or stimulation of apoptosis, and suppression of host immunity have been documented [7,8,9,10,11]. These functions and properties will be discussed and summarized in the following sections.
Figure 1.
Hepatitis C virus genomic RNA, the translated proteins, and protein functions. Hepatitis C virus genomic RNA consists of a long open reading frame, sandwiched by 5'- and 3'-untranslated regions. The encoded polyprotein by the hepatitis C virus genome is cleaved by hepatitis C virus and host proteases into structural and nonstructural proteins. The putative primary functions of the hepatitis C virus proteins are indicated in the figure
HEPATITIS C VIRUS-INDUCED HOST INFLAMMATION
HCV infection triggers host inflammation, which can be acute or chronic. While acute inflammation is considered to play a protective role and one of the means for the host to fight against infections and speed up the healing process, chronic inflammation can cause serious problems in the host. Apart from the problems caused in liver, chronic liver inflammation is suggested to associate with heart disease, diabetes, bowel disease, arthritis, and cancer [12]. It has been indicated that broadly directed CD4+ and CD8+ T cell responses are associated with clearance of HCV infection [13]. However, when the host fails to eliminate HCV virion, the infection develops into chronic hepatitis. During inflammatory response in the liver, cells such as macrophages, dendritic cells, hepatic stellate cells, Kupffer cells, bile duct epithelial cells, and sinusoidal endothelial cells are recruited in the fight against invading pathogens [14]. However, the development of effective immunity to fight against HCV is complicated, because of the persistent changes in the virion genome [15]. In addition to liver inflammation, it is suggested that systemic inflammation can be developed in HCV-infected patients due to elevated pro-inflammatory cytokine levels and monocyte activations [16]. Thus, knowledge of the underlying mechanisms of HCV-induced inflammation and disease evolution can be a significant aid for disease prognosis and development of therapeutic strategies [17].
HEPATITIS C VIRUS CORE PROTEIN
HCV core protein, derived from N-terminal 191 amino acids of the polyprotein, is the only HCV protein to directly interact with and encapsulate HCV genomic RNA and form nucleocapsids. The HCV core protein consists of three domains: the highly cationic domain 1, with approximately 117 amino acids, is the major domain to associate with HCV genomic RNA and to form nucleocapsids; the hydrophobic domain 2 is responsible for association with lipid droplets crucial for infectious virus particle productions [18]; the domain 3, also hydrophobic, is the endoplasmic reticulum (ER) anchoring domain [19]. The majority of HCV core protein is found to locate at the ER, mitochondria, and lipid droplets in cytoplasm. The interaction between HCV core protein and mitochondria reduces electron transfer complex I activity and increases reactive oxygen species (ROS) productions [20]. On the other hand, nuclear localization of the core protein has been detected and was found to be mediated by nuclear localization signal-like sequences within protein domain 1 [21]. HCV core protein can be secreted from HCV-infected hepatocytes and detected in the circulating bloodstream [22,23]. The roles played by the core protein in the modulations of host transcription and lipid metabolism, stimulation or inhibition of apoptosis, alterations of immune cell differentiations, and pro- and anti-inflammatory cytokine releases have been investigated [2,9,10,11,24] and the ability of HCV core protein to trigger hepatic angiogenesis was also discovered [25]. The core protein-induced dysfunction of components of several important pathways, including p53, AP-1, MAPK extracellular signal-regulated kinase-extra-cellular signal-regulated kinase, transforming growth factor β, vascular endothelial growth factor, Wnt/β-catenin, cyclooxygenase 2 (COX-2) and peroxisome proliferator-activated receptor α (PPARα), are suggested to be involved in the development of HCC [26,27,28]. Immune suppression and liver damage induced by HCV core protein were also demonstrated [29]. The effects caused by the HCV core protein on host immune and inflammatory response are discussed in the following sections.
EFFECTS OF HEPATITIS C VIRUS CORE PROTEIN ON APOPTOSIS
Apoptosis is programmed cell death naturally essential in the development and maintenance of homeostasis in multicellular organisms. As viruses must utilize host machinery for their multiplication, apoptosis of the infected cells is used as an effective weapon to eliminate virus infections by multicellular organisms. The core protein in both positive and negative regulation of cell death have been described.Cellular experiments indicated that HCV core protein induced apoptosis via casein kinase 1α-p53 signaling [9]. The Bcl-2 family proteins are famous for their roles in the regulation of apoptosis [30]. Interestingly, a Bcl-2 homology 3 (BH3) domain is identified in the core protein, and this domain induced apoptosis by specifically interacting with human myeloid cell factor 1 (Mcl-1) in virus-infected Huh-7 cells [31]. The core protein was found also to trigger apoptosis by causing ER stress and ER calcium depletion in replicon-expressing cells and in the liver of HCV core transgenic mice [32]. However, other studies suggested an apoptosis inhibitory role played by the core protein. The core protein inhibits apoptosis in HepG2 cells by inhibiting p53 signaling through sirt1-p53-bax pathway [10] or inhibiting TNF-α mediated pathway through NF-κB activation [33,34]. The core protein was also found to inhibit Huh-7 cell apoptosis despite mitochondrial ROS were generated [35].In addition, antiapoptotic effect of HCV core gene in Huh-7 cells was also found to associate with downregulation of expression of proapoptosis proteins caspases, cytochrome C, and p53 [36] and was found to promote cell proliferation by elevation of cellular p-Akt levels [36]. The core protein was found to be able to even immortalize primary human hepatocytes [37]. In liver, the proapoptotic properties of HCV core protein may cause liver damage and result in inflammation, while the role played by the core protein to prevent the host from apoptosis may aid the viral persistence and lead to the development of HCC. HCV also infects immune cells [38] and is suggested to promote apoptosis of immune cells through the Fas-signaling pathway [11]. HCV core protein was also found to induce apoptosis in mature DCs [39]. Apoptosis of the infected cells is a natural defense mechanism of the host to eliminate virus infection. Indeed, hepatocyte apoptosis can be triggered by HCV core protein. However, interestingly, the core protein also shows the ability to inhibit apoptosis and even promote proliferation of host cells favorable for virus survival and amplification. On the other hand, in the cases of immune cells, which are important for fighting the virus infection, the core protein induces their apoptosis. The proapoptotic properties of HCV core protein targeting immune cells might also contribute to impaired host immunity and lead to HCV infection persistency.
PRO- AND ANTI-INFLAMMATORY REGULATIONS BY HEPATITIS C VIRUS CORE PROTEIN
The inflammation regulations by HCV core protein are very complicated and still not fully understood. Both pro- and anti-inflammatory roles have been suggested by different studies. The induction of oxidative stress in hepatocyte by the HCV core protein was observed [40]. Microarray analysis on immortalized human hepatocytes suggested that the HCV core protein induces inflammatory cytokines through the STAT3 signaling pathway [41] and extracellular HCV core protein activations of STAT3 in human monocytes, macrophages, and dendritic cells (DCs) were observed to related to an interleukin (IL)-6 pathway [22]. HCV core protein in a primate model was found to induce hepatic inflammation through stimulated expression of IL-32 via phosphoinositide 3-kinases pathway [42]. On the other hand, the anti-inflammatory effects by the HCV core protein were also evidenced. Multiple studies have identified that the HCV core protein suppressed IL-12 and NO production in activated macrophages and human blood monocytes, thus inhibiting inflammatory responses [2,43,44]. IL-10 is considered as an anti-inflammatory cytokine, which played a central role in the production of other cytokines [45]. While a study claimed that the lipopolysaccharide-induced level of IL-10 was not upregulated by HCV core protein [44] other studies observed elevated level of IL-10 produced from HCV core protein-stimulated monocytes, cultured DCs [2,45] and HCV patients' DCs [2]. Cellular inflammatory response can also be inhibited by the core protein in cells via inhibition of COX-2 expressions [46]. Acute inflammation is a mean for the host to defend against virus infection. Host inflammation can indeed be triggered by HCV core protein. On the other hand, the core protein is found to be able to downregulate the host inflammation, contributing the development of persistent virus infection.
HEPATITIS C VIRUS CORE PROTEIN AND TOLL-LIKE RECEPTORS
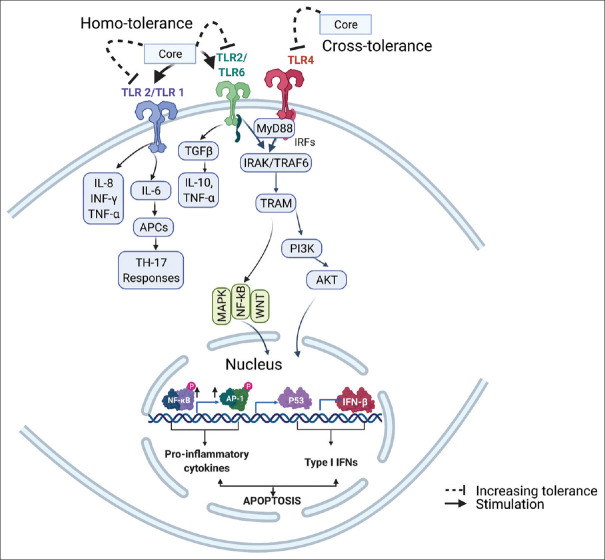
Toll-like receptors (TLRs), a family of pattern recognition receptors, play crucial roles in the innate immune system [47]. They are expressed in a variety of cells and are used by the host defense system to sense viral structural proteins [48]. As mentioned previously, HCV is one of the most efficient viruses in developing themselves to persistent infections by means of escaping host immune detections and/or avoiding the attack from host immune responses [49]. HCV core antigen induction of conjunctival inflammation via TLR-mediated signaling is indicated [50]. Among TLRs, TLR2 is identified to be the primary target of HCV core protein [51] and TLR2 was found to sense only the core protein but not the complete enveloped infectious virus particles, indicating that the monomeric core protein is the one sensed by TLR2 [48]. HCV core protein-related TLRs and their downstream molecular signaling are schematically summarized in Figure 2. The core protein monomer is suggested to bind to the TLR heterodimers [49]. The coreceptors to TLR2 for core protein interaction are identified to be TLR1 and TLR6 [49,52]. Th17 cells are considered important in the development and progression of autoimmune diseases, and the activation of TLR2 by agonist was found to increase IL-17 production and promote Th17 cell responses [53]. The interaction between HCV core protein and TLR2 of human monocytes was found to increase the production of pro-inflammatory IL-8 and TNF-α [51]. However, it was also found that stimulation of TLR2 by HCV core protein can cause homotolerance effects on TLR2 and cross-tolerance effects on TLR4 to their ligands [54] leading to downregulations of pro-inflammatory IL-6 and IL-8 productions induced by TLR ligands in monocytes of infected patients [54]. In host immunity, the core protein is sensed by TLR1/2 or TLR1/6 receptors for the detection of HCV infection, leading to the activation of host innate immune responses for virus clearance. However, the core protein has the ability to induce homo- and cross-tolerance of TLRs, thus desensitizing the TLR signaling.
Figure 2.
Hepatitis C virus core protein related TLRs and downstream pathways involved in innate immunity. Hepatitis C virus core protein is found to interact with TLR2 and stimulate TLR2 signaling pathway. Hepatitis C virus core protein interact with TLR2 in TLR1/TLR2 and TLR2/TLR4 heterodimers. The core protein is also found to induce homo- and cross-tolerance effects on TLR2 and TLR4. NFκB: Nuclear factor κ B; IFN-γ: Interferon γ; APCs: Antigen-presenting cells; TGF-β: Transforming growth factor β; IRF: Interferon regulatory factor; IRAK: Interleukin-1 receptor-associated kinase; TRAF: TNF receptor-associated factor protein; TRAM: Translocating chain-associating membrane protein
HEPATITIS C VIRUS CORE PROTEIN ON IMMUNE CELL ACTIVITIES AND DIFFERENTIATIONS
HCV core protein is an effector to immune related cell differentiations. HCV core protein inhibits monocyte differentiation into DCs [24] and suppresses monocyte differentiation into both M1 and M2 macrophages through a TLR2/STATs signaling pathway [24]. While M1 macrophages produce pro-inflammatory cytokines, nitric oxide (NO) or ROS, phagocytize microbial pathogens, and initiate an immune response to eliminate virus infections, M2 macrophages, activated by molecules such as IL-10 or C1q, are associated with wound healing and tissue repair.CD4+ and CD8+ T cells play important roles in clearance of virus infections [55,56]. HCV core protein inhibits phagocytosis ability of both M1 and M2 macrophages and inhibits M1 macrophage-induced autologous and allogeneic CD4+ T cell proliferation [57]. The inhibition of M1 macrophage activities, CD4+ T cell differentiation, and CD8+ cell proliferation by the core protein might contribute to HCV persistence. On the other hand, the core protein was found to promote M2 macrophage-induced autologous and allogeneic CD4+ T cell proliferation [57]. Interaction of HCV core protein with gC1q receptor, a multifunctional pattern recognition protein, inhibits Th1 differentiation of CD4+ T cells via suppression of IL-12 production in DCs [58]. Prolonged exposure to HCV core protein results in the development of APCs with a limited ability to drive differentiation of the pro-inflammatory Th17, and this condition might be due to reduced TLR driven IL-6 production [49]. Reduction of CD8+ T-cell proliferation by HCV core protein has also been shown in a recent study [59]. In transgenic mouse models, immune suppression was identified to correlate with diminishing of INF-γ and IL-2 production in T cell responses [29]. In addition to T cells, HCV core protein also exerts inhibitory activity on B cells. The expression of HCV core protein downregulates MHC class II molecules [60].
It is suggested that there is a vicious cycle of inflammation and insulin resistance. Linkage of between adipose tissue insulin resistance and liver macrophages in patients has been suggested [61]. Insulin resistance in adipocytes was found to cause production of monocyte chemoattractant protein 1 (MCP1), which recruits monocytes and activates pro-inflammatory macrophages [62]. Transition in macrophage polarization from an alternative M2 activation state, maintained by STAT6 and PPARs, to a classical M1 activation state, induced by transcription factors including NF-κB, and AP1, promotes insulin resistance in hepatocyte [63]. HCV core protein impairs downstream signaling of insulin and regulate insulin growth factor binding protein-1 expression [64] whose levels are correlated with non-alcoholic fatty liver disease, and are suggested as a marker for advanced fibrosis [65]. The ability of HCV core protein to induce insulin resistance should also trigger the insulin resistance-inflammation cycle. In summary, HCV core protein plays roles in inhibiting the activation, differentiation, as well as functions of immune cells, thus reduces the virus-clearance ability of host immune system, contributing the persistence of HCV infection.
HEPATITIS C VIRUS CORE PROTEIN VARIABILITY IN RELATION WITH HOST INFLAMMATION AND THERAPY RESISTANCE
One of the important features of RNA viruses is their genetic variability resulting largely from the lack of proofreading mechanism during viral RNA replication [66]. HCV has currently been classified into 7 genotypes and 67 subtypes [67]. The HCV genotypes, subtypes, and their major geographical distributions are summarized in Table 1 [67,68,69,70,71]. In infected patients, HCV forms a mixture of genetically distinct but closely related variant, the so-called quasispecies, duo to high viral mutation rate [66]. Different from other HCV proteins, the core protein is highly conserved. However, the differences in its sequence are associated with different pathogenic consequences and therapeutic outcomes of the infection [72]. Before the applications of direct-acting antiviral (DAA) drugs, such as telaprevir, boceprevir, simeprevir, and sofosbuvir, interferon (IFN)-α combined with ribavirin was used as the standard treatment for chronic hepatitis C [73]. IFN-α is an innate immune response mediator, which induced expressions of IFN-stimulated genes to perform non-specific antiviral effects within the cells [74]. In the presence of HCV core protein, inhibition of IFN-α-induced signaling through the JAK-STAT pathway, essential for establishment of the cellular antiviral state, was observed in the liver of transgenic mice [75]. HCV core protein expression in HepG2 cells was found to downregulate IFN-α-induced downstream antiviral genes expression [76]. The core protein binds to STAT1 resulting in decreased phosphorylated STAT1 level, leading to blockage of STAT1 heterodimerization with STAT2, thus disrupting binding of IFN-stimulated gene factor-3-mediated gene transcription [77]. The efficacy of the IFN treatment is largely depending on the HCV genotype [78]. Amino acid variations in HCV core protein have been suggested to link to resistance to IFN therapy [78]. It was indicated that substitutions of amino acids 70 and/or 91 of core protein of genotype 1b patients can be acting as indicators of poorer sustained virologic response (SVR) to PEG-IFN-ribavirin treatment and can be considered risk factors for HCC development [79]. A study compared full-length HCV sequence from patients with or without HCC using a logistic regression model identified 7 polymorphisms, including the codon for amino acid 70 in HCV core protein sequence, significantly associated with increased HCC risk [72]. In the cases of DAA treatments, different SVR rates of the treatments are found to associate with different HCV genotypes [80,81,82,83]. HCV genotype 3 seems to be more resistant to DAA treatments [83]. The direct roles played by core protein viability in DAA resistance have not yet been identified. Lipid metabolism has also linked to inflammation pathways [84]. HCV genotypes are associated with different degrees of hepatic steatosis. Transcriptional alterations of host genes involved in lipid metabolism in HCV genotype 1 and genotype 3 are linked to inflammation in HCV-infected patients [85]. The relationships between HCV core protein and host lipid metabolism have been documented [86] and the HCV core protein–lipid droplet interaction is believed to play a role in virus-induced steatosis [87]. Liver steatosis is common in patients with chronic HCV infection. Studies expressed HCV core protein from patients with no liver steatosis (genotype 1b, 2a, 3h, 4h, and 5a) and with severe liver steatosis (genotype 3a, core protein Y164F) in hepatocytes found that the genotype 3a core protein causes the highest level of cellular lipid accumulation, as compared to those induced by other genotypes [87,88]. Although the new generation of DAAs has increased the SVR rate to more than 95%, between 2%–5% of the infected patients are not responding to the treatments [89,90]. The DAA therapies may also cause selection of drug-resistant virus strain. In addition, as the HCV infections are very often mild-symptomatic or asymptomatic, approximately 80% of the infected individuals are unaware of their infection [91]; it is impossible to eliminate the pathogenic virus just by applying therapeutic treatments. The best way to eliminate infectious virus in human population is vaccination. However, because of the fact that HCV is a high variant virus, it is difficult to develop vaccines against it. The core protein is highly conserved among different HCV strains; as a result, it was once considered to be a candidate for vaccine developments [92]. Nevertheless, until now, the development of core protein-based vaccine has not yet been successful. The development and application of HCV core protein-based vaccines still need to consider the ability of the core protein to interfere with innate and adaptive antiviral immunity, thus reducing the efficacy of the vaccination, and the unwanted effects induced by the core protein such as those leading to oncogenesis in HCC.
Table 1.
Hepatitis C virus genotypes, subtypes, and their geographical distributions
| Genotypes | Subtypes | Major distributions |
|---|---|---|
| 1 | a, b, c, e, g, h, i | The most prevalent worldwide and has a widespread geographic distribution, including Europe, North America, Central Africa, Oriental countries, such as Japan and Taiwan, representing approximately 46% of all HCV infections |
| 2 | a, b, c, d, e, i, j, k, m, q, r | Western Africa, Mediterranean countries, oriental countries |
| 3 | a, b, g, h, i, k | The second most prevalent genotype accounting for approximately 30% of global infections. Major distribution: South Asia, Southeast Asia, Australasia, and some countries in Europe |
| 4 | a, b, c, d, f, g, k, i, m, n, o, p, q, r, t, v, w | Central and Eastern Africa, North Africa, the Middle East |
| 5 | a | South Africa |
| 6 | a, b, c, d, e, f, g, h, i, j, k, l, m, n, o, p, q, r, s, t, u, v, w, xa | Southeast Asia (including Hong Kong, Vietnam), Australia |
| 7 | a | Central Africa |
HCV: Hepatitis C virus
The highly mutated nature of RNA viruses results in a fast evolution rate of HCV. Resistance to currently applied antivirals might soon be developed in HCV. Compared to other HCV proteins, HCV core protein is highly conserved [93] thus can be considered as a target for antiviral drug developments. Inhibitory effects of designed and optimized peptides, small molecules, and aptamers on HCV core protein dimerization, crucial for nucleocapsid assembly, and virus production have been investigated for their possible antiviral applications [94,95,96]. On the other hand, highly conserved virus proteins are normally considered ideal candidates for vaccine developments. However, as reviewed by this study, although HCV core protein is highly conserved, the core protein exerts effects on immune suppression and direct/indirect induction of HCC; therefore, it might not be a great choice for vaccine developments, unless modifications are made for elimination of its detrimental effects.
CONCLUSION
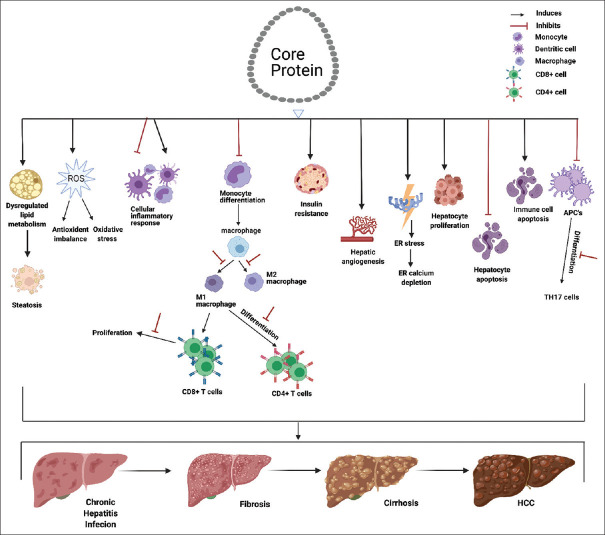
Alterations in host immunity and chronic inflammation are linked to the development of persistent virus infection and HCC. The related pathways in which the HCV core protein is involved are graphically summarized in Figure 3. As illustrated in Figure 3, the nucleocapsid forming HCV core protein exerts effects on host cell apoptosis, pro- and anti-inflammatory regulations, alterations of TLR signaling, and immune cell activations and differentiations. These effects play crucial roles in the progression from the persistent infection, chronic inflammation, fibrosis, and cirrhosis to the HCC. According to the literature review in this study, it seems that the core protein may exhibit opposite/contrary effects on the host immune and inflammatory responses (stimulative and suppressive effects). We suggest that these opposite effects might be resulted from strategies adopted by the host or the virus. The pro-inflammatory effects and immunity stimulated by the core protein might be due to the ability of the host to sense the structural proteins of invading virus and initiate the immune and inflammatory responses aiming for virus elimination and clearance. On the other hand, the anti-inflammatory effects and immunosuppression induced by the core protein are possibly coming from the results of virus evolved to resist and escape the host virus clearance mechanisms for virus survival and multiplication. The core protein is able to stimulate anti-inflammatory effects by altering expression of cellular proteins, suppressing pro-inflammatory cytokine productions, and increasing anti-inflammatory cytokine productions. To maintain efficient virus multiplication, it is important to keep the host cell alive. The core protein inhibits apoptosis, boots proliferation, and even promote immortalization of hepatocytes, which might link to HCC development. For the virus to evade the attack from host immunity, the core protein can also alter immune cell differentiations and promote the apoptosis of immune cells. HCV core protein is able to directly interact with cellular machinery involved in lipid metabolism and is suspected to play a role in liver steatosis [97,98] a contributor of HCC development. Owning to the high mutation nature of single-stranded RNA virus, HCV presents a high degree of genetic variability. The degree of effectiveness of therapeutic treatments for HCV infection is correlate to the genetic strains of HCV treated. However, the highly conserved nature of HCV core protein makes it a promising target for antiviral drug developments [94,95,96]. As the core protein is an effector of host inflammatory and immunological responses, the detailed knowledge to HCV core protein regarding these issues should provide crucial considerations for future design of novel therapeutic strategies and development of effective HCV vaccines.
Figure 3.
Roles played by hepatitis C virus core protein in relation with host inflammation and the evolution from hepatitis C virus infection in liver to hepatocellular carcinoma. Repeated injury of liver tissue and chronic inflammation resulted from chronic hepatitis C, accompanied with liver tissue repair process leads to liver fibrosis that might further results in cirrhosis and hepatocellular carcinoma. The hepatitis C virus core protein plays multiple rules in the process. The core protein is suggested to trigger host inflammation by interaction with pro-inflammatory cell surface receptors, induction of release of pro- and anti-inflammatory cytokines, as well as induction of oxidative stress. The core protein is also identified to involve in promoting the persistent infection by inhibiting host apoptosis, promoting host cell proliferation, and suppression of immune cell differentiations and functions. The core protein is also able to alter the lipid metabolism of the host and induce steatosis which also contribute to the development of liver fibrosis
Financial support and sponsorship
This work is supported by Tzu Chi Foundation; Grant number: TCIRP106001-01.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Professor Ji-Hshiung Chen for valuable suggestions to this manuscript. The graphics in the manuscript was prepared with software from BioRender.com.
REFERENCES
- 1.Chevaliez S, Pawlotsky JM. HCV genome and life cycle. In: Tan SL, editor. Hepatitis C Viruses: Genomes and Molecular Biology. UK: Norfolk; 2006. [Google Scholar]
- 2.Dolganiuc A, Kodys K, Kopasz A, Marshall C, Do T, Romics L., Jr Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J Immunol. 2003;170:5615–24. doi: 10.4049/jimmunol.170.11.5615. [DOI] [PubMed] [Google Scholar]
- 3.Castello G, Scala S, Palmieri G, Curley SA, Izzo F. HCV-related hepatocellular carcinoma: From chronic inflammation to cancer. Clin Immunol. 2010;134:237–50. doi: 10.1016/j.clim.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Li J, Wang X, Sang M, Ho W. Hepatic stellate cells, liver innate immunity, and hepatitis C virus. J Gastroenterol Hepatol. 2013;28((Suppl 1)):112–5. doi: 10.1111/jgh.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goto K, Roca Suarez AA, Wrensch F, Baumert TF, Lupberger J. Hepatitis C virus and hepatocellular carcinoma: When the host loses its grip. Int J Mol Sci. 2020;21:3057. doi: 10.3390/ijms21093057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penin F. Structural biology of hepatitis C virus. Clin Liver Dis. 2003;7:1–21. doi: 10.1016/s1089-3261(02)00066-1. vii. [DOI] [PubMed] [Google Scholar]
- 7.Lai MM, Ware CF. Hepatitis C virus core protein: Possible roles in viral pathogenesis. Curr Top Microbiol Immunol. 2000;242:117–34. doi: 10.1007/978-3-642-59605-6_6. [DOI] [PubMed] [Google Scholar]
- 8.McLauchlan J. Properties of the hepatitis C virus core protein: A structural protein that modulates cellular processes. J Viral Hepat. 2000;7:2–14. doi: 10.1046/j.1365-2893.2000.00201.x. [DOI] [PubMed] [Google Scholar]
- 9.Shen S, Li C, Dai M, Yan X. Induction of Huh-7 cell apoptosis by HCV core proteins via CK1α-p53-Bid signaling pathway. Mol Med Rep. 2018;17:7559–66. doi: 10.3892/mmr.2018.8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng S, Li M, Zhang J, Liu S, Wang Q, Quan M, et al. Regulation of HepG2 cell apoptosis by hepatitis C virus (HCV) core protein via the sirt1-p53-bax pathway. Virus Genes. 2015;51:338–46. doi: 10.1007/s11262-015-1253-2. [DOI] [PubMed] [Google Scholar]
- 11.Hahn CS, Cho YG, Kang BS, Lester IM, Hahn YS. The HCV core protein acts as a positive regulator of fas-mediated apoptosis in a human lymphoblastoid T cell line. Virology. 2000;276:127–37. doi: 10.1006/viro.2000.0541. [DOI] [PubMed] [Google Scholar]
- 12.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park) 2002;16:217–26. 229. [PubMed] [Google Scholar]
- 13.Koziel MJ. Cellular immune responses against hepatitis C virus. Clin Infect Dis. 2005;41((Suppl 1)):S25–31. doi: 10.1086/429492. [DOI] [PubMed] [Google Scholar]
- 14.Weiskirchen R, Tacke F. Cellular and molecular functions of hepatic stellate cells in inflammatory responses and liver immunology. Hepatobiliary Surg Nutr. 2014;3:344–63. doi: 10.3978/j.issn.2304-3881.2014.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103–7. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 16.Zampino R, Marrone A, Restivo L, Guerrera B, Sellitto A, Rinaldi L, et al. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol. 2013;5:528–40. doi: 10.4254/wjh.v5.i10.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933–8. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa K, Hishiki T, Shimizu Y, Funami K, Sugiyama K, Miyanari Y, et al. Hepatitis C virus utilizes lipid droplet for production of infectious virus. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:217–28. doi: 10.2183/pjab.85.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hourioux C, Ait-Goughoulte M, Patient R, Fouquenet D, Arcanger-Doudet F, Brand D, et al. Core protein domains involved in hepatitis C virus-like particle assembly and budding at the endoplasmic reticulum membrane. Cell Microbiol. 2007;9:1014–27. doi: 10.1111/j.1462-5822.2006.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, et al. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem. 2005;280:37481–8. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki R, Sakamoto S, Tsutsumi T, Rikimaru A, Tanaka K, Shimoike T, et al. Molecular determinants for subcellular localization of hepatitis C virus core protein. J Virol. 2005;79:1271–81. doi: 10.1128/JVI.79.2.1271-1281.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tacke RS, Tosello-Trampont A, Nguyen V, Mullins DW, Hahn YS. Extracellular hepatitis C virus core protein activates STAT3 in human monocytes/macrophages/dendritic cells via an IL-6 autocrine pathway. J Biol Chem. 2011;286:10847–55. doi: 10.1074/jbc.M110.217653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabile A, Perlemuter G, Bono F, Kohara K, Demaugre F, Kohara M, et al. Hepatitis C virus core protein binds to apolipoprotein AII and its secretion is modulated by fibrates. Hepatology. 1999;30:1064–76. doi: 10.1002/hep.510300429. [DOI] [PubMed] [Google Scholar]
- 24.Tu Z, Hamalainen-Laanaya HK, Nishitani C, Kuroki Y, Crispe IN, Orloff MS. HCV core and NS3 proteins manipulate human blood-derived dendritic cell development and promote Th 17 differentiation. Int Immunol. 2012;24:97–106. doi: 10.1093/intimm/dxr104. [DOI] [PubMed] [Google Scholar]
- 25.Hassan M, Selimovic D, Ghozlan H, Abdel-kader O. Hepatitis C virus core protein triggers hepatic angiogenesis by a mechanism including multiple pathways. Hepatology. 2009;49:1469–82. doi: 10.1002/hep.22849. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda K, Tsuchihara K, Hijikata M, Nishiguchi S, Kuroki T, Shimotohno K. Hepatitis C virus core protein enhances the activation of the transcription factor, Elk1, in response to mitogenic stimuli. Hepatology. 2001;33:159–65. doi: 10.1053/jhep.2001.20794. [DOI] [PubMed] [Google Scholar]
- 27.Mahmoudvand S, Shokri S, Taherkhani R, Farshadpour F. Hepatitis C virus core protein modulates several signaling pathways involved in hepatocellular carcinoma. World J Gastroenterol. 2019;25:42–58. doi: 10.3748/wjg.v25.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zahra M, Azzazy H, Moustafa A. Transcriptional regulatory networks in hepatitis C virus-induced hepatocellular carcinoma. Sci Rep. 2018;8:14234. doi: 10.1038/s41598-018-32464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soguero C, Joo M, Chianese-Bullock KA, Nguyen DT, Tung K, Hahn YS. Hepatitis C virus core protein leads to immune suppression and liver damage in a transgenic murine model. J Virol. 2002;76:9345–54. doi: 10.1128/JVI.76.18.9345-9354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lutz RJ. Role of the BH3 (Bcl-2 homology 3) domain in the regulation of apoptosis and Bcl-2-related proteins. Biochem Soc Trans. 2000;28:51–6. doi: 10.1042/bst0280051. [DOI] [PubMed] [Google Scholar]
- 31.Mohd-Ismail NK, Deng L, Sukumaran SK, Yu VC, Hotta H, Tan YJ. The hepatitis C virus core protein contains a BH3 domain that regulates apoptosis through specific interaction with human Mcl-1. J Virol. 2009;83:9993–10006. doi: 10.1128/JVI.00509-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benali-Furet NL, Chami M, Houel L, De Giorgi F, Vernejoul F, Lagorce D, et al. Hepatitis C virus core triggers apoptosis in liver cells by inducing ER stress and ER calcium depletion. Oncogene. 2005;24:4921–33. doi: 10.1038/sj.onc.1208673. [DOI] [PubMed] [Google Scholar]
- 33.Marusawa H, Hijikata M, Chiba T, Shimotohno K. Hepatitis C virus core protein inhibits Fas- and tumor necrosis factor alpha-mediated apoptosis via NF-kappaB activation. J Virol. 1999;73:4713–20. doi: 10.1128/jvi.73.6.4713-4720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito K, Meyer K, Warner R, Basu A, Ray RB, Ray R. Hepatitis C virus core protein inhibits tumor necrosis factor alpha-mediated apoptosis by a protective effect involving cellular FLICE inhibitory protein. J Virol. 2006;80:4372–9. doi: 10.1128/JVI.80.9.4372-4379.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hara Y, Hino K, Okuda M, Furutani T, Hidaka I, Yamaguchi Y, et al. Hepatitis C virus core protein inhibits deoxycholic acid-mediated apoptosis despite generating mitochondrial reactive oxygen species. J Gastroenterol. 2006;41:257–68. doi: 10.1007/s00535-005-1738-1. [DOI] [PubMed] [Google Scholar]
- 36.Jahan S, Khaliq S, Siddiqi MH, Ijaz B, Ahmad W, Ashfaq UA, et al. Anti-apoptotic effect of HCV core gene of genotype 3a in Huh-7 cell line. Virol J. 2011;8:522. doi: 10.1186/1743-422X-8-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray RB, Meyer K, Ray R. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology. 2000;271:197–204. doi: 10.1006/viro.2000.0295. [DOI] [PubMed] [Google Scholar]
- 38.Joo M, Hahn YS, Kwon M, Sadikot RT, Blackwell TS, Christman JW. Hepatitis C virus core protein suppresses NF-kappaB activation and cyclooxygenase-2 expression by direct interaction with IkappaB kinase beta. J Virol. 2005;79:7648–57. doi: 10.1128/JVI.79.12.7648-7657.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siavoshian S, Abraham JD, Thumann C, Kieny MP, Schuster C. Hepatitis C virus core, NS3, NS5A, NS5B proteins induce apoptosis in mature dendritic cells. J Med Virol. 2005;75:402–11. doi: 10.1002/jmv.20283. [DOI] [PubMed] [Google Scholar]
- 40.Dionisio N, Garcia-Mediavilla MV, Sanchez-Campos S, Majano PL, Benedicto I, Rosado JA, et al. Hepatitis C virus NS5A and core proteins induce oxidative stress-mediated calcium signalling alterations in hepatocytes. J Hepatol. 2009;50:872–82. doi: 10.1016/j.jhep.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 41.Basu A, Meyer K, Lai KK, Saito K, Di Bisceglie AM, Grosso LE, et al. Microarray analyses and molecular profiling of Stat3 signaling pathway induced by hepatitis C virus core protein in human hepatocytes. Virology. 2006;349:347–58. doi: 10.1016/j.virol.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 42.Liu B, Ma X, Wang Q, Luo S, Zhang L, Wang W, et al. Marmoset viral hepatic inflammation induced by hepatitis C virus core protein via IL-32. Front Cell Infect Microbiol. 2020;10:135. doi: 10.3389/fcimb.2020.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee CH, Choi YH, Yang SH, Lee CW, Ha SJ, Sung YC. Hepatitis C virus core protein inhibits interleukin 12 and nitric oxide production from activated macrophages. Virology. 2001;279:271–9. doi: 10.1006/viro.2000.0694. [DOI] [PubMed] [Google Scholar]
- 44.Eisen-Vandervelde AL, Waggoner SN, Yao ZQ, Cale EM, Hahn CS, Hahn YS. Hepatitis C virus core selectively suppresses interleukin-12 synthesis in human macrophages by interfering with AP-1 activation. J Biol Chem. 2004;279:43479–86. doi: 10.1074/jbc.M407640200. [DOI] [PubMed] [Google Scholar]
- 45.Pang X, Wang Z, Zhai N, Zhang Q, Song H, Zhang Y, et al. IL-10 plays a central regulatory role in the cytokines induced by hepatitis C virus core protein and polyinosinic acid: Polycytodylic acid. Int Immunopharmacol. 2016;38:284–90. doi: 10.1016/j.intimp.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Jhaveri R, Kundu P, Shapiro AM, Venkatesan A, Dasgupta A. Effect of heptitis C virus core protein on cellular gene expression: Specific inhibition of cyclooxygenase 2. J Infect Dis. 2005;191:1498–506. doi: 10.1086/429301. [DOI] [PubMed] [Google Scholar]
- 47.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffmann M, Zeisel MB, Jilg N, Paranhos-Baccalà G, Stoll-Keller F, Wakita T, et al. Toll-like receptor 2 senses hepatitis C virus core protein but not infectious viral particles. J Innate Immun. 2009;1:446–54. doi: 10.1159/000226136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imran M, Waheed Y, Manzoor S, Bilal M, Ashraf W, Ali M, et al. Interaction of hepatitis C virus proteins with pattern recognition receptors. Virol J. 2012;9:126. doi: 10.1186/1743-422X-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajalakshmy AR, Malathi J, Madhavan HN, Srinivasan B, Iyer GK. Hepatitis C virus core and NS3 antigens induced conjunctival inflammation via toll-like receptor-mediated signaling. Mol Vis. 2014;20:1388–97. [PMC free article] [PubMed] [Google Scholar]
- 51.Dolganiuc A, Oak S, Kodys K, Golenbock DT, Finberg RW, Kurt-Jones E, et al. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513–24. doi: 10.1053/j.gastro.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 52.Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J Leukoc Biol. 2007;82:479–87. doi: 10.1189/jlb.0207128. [DOI] [PubMed] [Google Scholar]
- 53.Zhao RR, Yang XF, Dong J, Zhao YY, Wei X, Huang CX, et al. Toll-like receptor 2 promotes T helper 17 cells response in hepatitis B virus infection. Int J Clin Exp Med. 2015;8:7315–23. [PMC free article] [PubMed] [Google Scholar]
- 54.Chung H, Watanabe T, Kudo M, Chiba T. Hepatitis C virus core protein induces homotolerance and cross-tolerance to Toll-like receptor ligands by activation of Toll-like receptor 2. J Infect Dis. 2010;202:853–61. doi: 10.1086/655812. [DOI] [PubMed] [Google Scholar]
- 55.Sant AJ, McMichael A. Revealing the role of CD4(+) T cells in viral immunity. J Exp Med. 2012;209:1391–5. doi: 10.1084/jem.20121517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moskophidis D, Kioussis D. Contribution of virus-specific CD8+cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J Exp Med. 1998;188:223–32. doi: 10.1084/jem.188.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Q, Wang Y, Zhai N, Song H, Li H, Yang Y, et al. HCV core protein inhibits polarization and activity of both M1 and M2 macrophages through the TLR2 signaling pathway. Sci Rep. 2016;6:36160. doi: 10.1038/srep36160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waggoner SN, Hall CH, Hahn YS. HCV core protein interaction with gC1q receptor inhibits Th1 differentiation of CD4+T cells via suppression of dendritic cell IL-12 production. J Leukoc Biol. 2007;82:1407–19. doi: 10.1189/jlb.0507268. [DOI] [PubMed] [Google Scholar]
- 59.Khan ST, Karges W, Cooper CL, Crawley AM. Hepatitis C virus core protein reduces CD8+T-cell proliferation, perforin production and degranulation but increases STAT5 activation. Immunology. 2018;154:156–65. doi: 10.1111/imm.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu CG, Budhu A, Chen S, Zhou X, Popescu NC, Valerie K, et al. Effect of hepatitis C virus core protein on the molecular profiling of human B lymphocytes. Mol Med. 2006;12:47–53. doi: 10.2119/2006-00020.Wu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosso C, Kazankov K, Younes R, Esmaili S, Marietti M, Sacco M, et al. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J Hepatol. 2019;71:1012–21. doi: 10.1016/j.jhep.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 62.Shimobayashi M, Albert V, Woelnerhanssen B, Frei IC, Weissenberger D, Meyer-Gerspach AC, et al. Insulin resistance causes inflammation in adipose tissue. J Clin Invest. 2018;128:1538–50. doi: 10.1172/JCI96139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 64.Alberstein M, Zornitzki T, Zick Y, Knobler H. Hepatitis C core protein impairs insulin downstream signalling and regulatory role of IGFBP-1 expression. J Viral Hepat. 2012;19:65–71. doi: 10.1111/j.1365-2893.2011.01447.x. [DOI] [PubMed] [Google Scholar]
- 65.Hagström H, Stål P, Hultcrantz R, Brismar K, Ansurudeen I. IGFBP-1 and IGF-I as markers for advanced fibrosis in NAFLD-A pilot study. Scand J Gastroenterol. 2017;52:1427–34. doi: 10.1080/00365521.2017.1379556. [DOI] [PubMed] [Google Scholar]
- 66.Pawlotsky JM. Hepatitis C virus genetic variability: Pathogenic and clinical implications. Clin Liver Dis. 2003;7:45–66. doi: 10.1016/s1089-3261(02)00065-x. [DOI] [PubMed] [Google Scholar]
- 67.Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: Updated criteria and genotype assignment web resource. Hepatology. 2014;59:318–27. doi: 10.1002/hep.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murphy DG, Sablon E, Chamberland J, Fournier E, Dandavino R, Tremblay CL. Hepatitis C virus genotype 7, a new genotype originating from central Africa. J Clin Microbiol. 2015;53:967–72. doi: 10.1128/JCM.02831-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu CI, Chiang BL. A new insight into hepatitis C vaccine development. J Biomed Biotechnol. 2010;2010:548280. doi: 10.1155/2010/548280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsieh P, Kuo H, Cho W, Liao Y, Lin C. The genotype of hepatitis C virus has important clinical and therapeutic implications. J Intern Med Taiwan. 2009;20:309–19. [Google Scholar]
- 71.Schnell G, Krishnan P, Tripathi R, Beyer J, Reisch T, Irvin M, et al. Hepatitis C virus genetic diversity by geographic region within genotype 1-6 subtypes among patients treated with glecaprevir and pibrentasvir. PLoS One. 2018;13:e0205186. doi: 10.1371/journal.pone.0205186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fishman SL, Factor SH, Balestrieri C, Fan X, Dibisceglie AM, Desai SM, et al. Mutations in the hepatitis C virus core gene are associated with advanced liver disease and hepatocellular carcinoma. Clin Cancer Res. 2009;15:3205–13. doi: 10.1158/1078-0432.CCR-08-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Basyte-Bacevice V, Kupcinskas J. Evolution and revolution of hepatitis C management: From non-A, non-B hepatitis toward global elimination. Dig Dis. 2020;38:137–142. doi: 10.1159/000505434. [DOI] [PubMed] [Google Scholar]
- 74.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–72. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 75.Blindenbacher A, Duong FH, Hunziker L, Stutvoet ST, Wang X, Terracciano L, et al. Expression of hepatitis c virus proteins inhibits interferon alpha signaling in the liver of transgenic mice. Gastroenterology. 2003;124:1465–75. doi: 10.1016/s0016-5085(03)00290-7. [DOI] [PubMed] [Google Scholar]
- 76.Chang YZ, Lei YC, Hao YH, Chen SS, Wu W, Yang DL, et al. Study of the effect of hepatitis C virus core protein on interferon-induced antiviral genes expression and its mechanisms. Sheng Wu Gong Cheng Xue Bao. 2007;23:1000–4. [PubMed] [Google Scholar]
- 77.Lin W, Kim SS, Yeung E, Kamegaya Y, Blackard JT, Kim KA, et al. Hepatitis C virus core protein blocks interferon signaling by interaction with the STAT1 SH2 domain. J Virol. 2006;80:9226–35. doi: 10.1128/JVI.00459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khaliq S, Jahan S, Pervaiz A. Sequence variability of HCV Core region: Important predictors of HCV induced pathogenesis and viral production. Infect Genet Evol. 2011;11:543–56. doi: 10.1016/j.meegid.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 79.Akuta N, Suzuki F, Sezaki H, Suzuki Y, Hosaka T, Someya T, et al. Predictive factors of virological non-response to interferon-ribavirin combination therapy for patients infected with hepatitis C virus of genotype 1b and high viral load. J Med Virol. 2006;78:83–90. doi: 10.1002/jmv.20507. [DOI] [PubMed] [Google Scholar]
- 80.Calleja JL, Crespo J, Rincón D, Ruiz-Antorán B, Fernandez I, Perelló C, et al. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: Results from a Spanish real-world cohort. J Hepatol. 2017;66:1138–48. doi: 10.1016/j.jhep.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 81.Grebely J, Feld JJ, Wyles D, Sulkowski M, Ni L, Llewellyn J, et al. Sofosbuvir-based direct-acting antiviral therapies for HCV in people receiving opioid substitution therapy: An analysis of phase 3 studies. Open Forum Infect Dis. 2018;5:ofy001. doi: 10.1093/ofid/ofy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu CH, Su TH, Liu CJ, Hong CM, Yang HC, Tseng TC, et al. Sofosbuvir-based direct acting antiviral therapies for patients with hepatitis C virus genotype 2 infection. J Gastroenterol Hepatol. 2019;34:1620–5. doi: 10.1111/jgh.14615. [DOI] [PubMed] [Google Scholar]
- 83.Zhuang L, Li J, Zhang Y, Ji S, Li Y, Zhao Y, et al. Real-world effectiveness of direct-acting antiviral regimens against hepatitis C virus (HCV) genotype 3 infection: A systematic review and meta-analysis. Ann Hepatol. 2020;23:100268. doi: 10.1016/j.aohep.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 84.Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62:720–33. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 85.d'Avigdor WMH, Budzinska MA, Lee M, Lam R, Kench J, Stapelberg M, et al. Virus genotype-dependent transcriptional alterations in lipid metabolism and inflammation pathways in the hepatitis C virus-infected liver. Sci Rep. 2019;9:10596. doi: 10.1038/s41598-019-46664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Syed GH, Amako Y, Siddiqui A. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol Metab. 2010;21:33–40. doi: 10.1016/j.tem.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Piodi A, Chouteau P, Lerat H, Hézode C, Pawlotsky JM. Morphological changes in intracellular lipid droplets induced by different hepatitis C virus genotype core sequences and relationship with steatosis. Hepatology. 2008;48:16–27. doi: 10.1002/hep.22288. [DOI] [PubMed] [Google Scholar]
- 88.Abid K, Pazienza V, de Gottardi A, Rubbia-Brandt L, Conne B, Pugnale P, et al. An in vitro model of hepatitis C virus genotype 3a-associated triglycerides accumulation. J Hepatol. 2005;42:744–51. doi: 10.1016/j.jhep.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 89.Sepulveda-Crespo D, Resino S, Martinez I. Hepatitis C virus vaccine design: Focus on the humoral immune response. J Biomed Sci. 2020;27:78. doi: 10.1186/s12929-020-00669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cox AL. MEDICINE.Global control of hepatitis C virus. Science. 2015;349:790–1. doi: 10.1126/science.aad1302. [DOI] [PubMed] [Google Scholar]
- 91.Spearman CW, Dusheiko GM, Hellard M, Sonderup M. Hepatitis C. Lancet. 2019;394:1451–66. doi: 10.1016/S0140-6736(19)32320-7. [DOI] [PubMed] [Google Scholar]
- 92.Naderi M, Gholipour N, Zolfaghari MR, Moradi Binabaj M, Yegane Moghadam A, Motalleb G. Hepatitis C virus and vaccine development. Int J Mol Cell Med. 2014;3:207–15. [PMC free article] [PubMed] [Google Scholar]
- 93.Bukh J, Purcell RH, Miller RH. Sequence analysis of the core gene of 14 hepatitis C virus genotypes. Proc Natl Acad Sci U S A. 1994;91:8239–43. doi: 10.1073/pnas.91.17.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Strosberg AD, Kota S, Takahashi V, Snyder JK, Mousseau G. Core as a novel viral target for hepatitis C drugs. Viruses. 2010;2:1734–51. doi: 10.3390/v2081734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ni F, Kota S, Takahashi V, Strosberg AD, Snyder JK. Potent inhibitors of hepatitis C core dimerization as new leads for anti-hepatitis C agents. Bioorg Med Chem Lett. 2011;21:2198–202. doi: 10.1016/j.bmcl.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shi S, Yu X, Gao Y, Xue B, Wu X, Wang X, et al. Inhibition of hepatitis C virus production by aptamers against the core protein. J Virol. 2014;88:1990–9. doi: 10.1128/JVI.03312-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Asselah T, Rubbia-Brandt L, Marcellin P, Negro F. Steatosis in chronic hepatitis C: Why does it really matter? Gut. 2006;55:123–30. doi: 10.1136/gut.2005.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Modaresi Esfeh J, Ansari-Gilani K. Steatosis and hepatitis C. Gastroenterol Rep (Oxf) 2016;4:24–9. doi: 10.1093/gastro/gov040. [DOI] [PMC free article] [PubMed] [Google Scholar]