Abstract
A man in his late 70s presented to the emergency department endorsing a week of malaise. He was recently hospitalised for 2 days for new back pain and was discharged with non-opioid pain medications but continued to seek care as he felt unwell. On presentation, he was afebrile with a leukocytosis. Physical examination revealed a painful left knee with no evidence of trauma. Arthrocentesis revealed purulent fluid with elevated white blood cell consistent with septic arthritis. He was started on broad-spectrum antibiotics and underwent irrigation and synovectomy of the left knee. Aspirate and blood cultures grew Streptococcus agalactiae. Transthoracic echocardiogram showed no vegetations; however, an MRI of lumbar spine showed L2–L3 and L4–L5 osteomyelitis. He was treated with intravenous ceftriaxone for 3 weeks and then oral levofloxacin for 3 weeks, for a total 6 week course of antibiotics.
Keywords: Orthopaedics, Bone and joint infections
Background
Although Staphylococcus aureus has historically been the most common cause of bacterial infection in the USA, Streptococcus agalactiae (GBS; group B streptococcus), it has recently emerged as another serious and increasingly common cause of bacterial infection. Streptococcus agalactiae is a Gram-positive, catalase-negative organism that appears as cocci in pairs and chains on Gram stain.1 It is an opportunistic commensal bacterial that is part of the intestinal and vaginal physiologic microbiome and can live on the skin of colonised patients, putting them at risk of inoculation from skin breaks or procedures while in the hospital.2 3 Approximately, 25% of the population is colonised with GBS, and the prevalence of colonisation is higher among those who are sexually active and those who have multiple sex partners.3–5 Sexual transmission of GBS between partners has been cited with college students; however, whether frequent sexual intercourse or multiple sexual partners is an absolute risk factor for increased GBS colonisation is still controversial.6 GBS has multiple virulence factors that account for its morbidity, including a polysaccharide capsule containing sialic acid that mimics human cells to evade neonatal immune cells, pili that help attach the bacteria to host cells and beta-haemolysin, a pore-forming toxin that causes haemolysis by destroying host red blood cells.1 GBS can also induce apoptosis in host macrophages and monocytes.3
GBS has long been recognised as a common infectious agent in the peripartum period and in neonates, but recent studies have implicated it in a growing number of infections within the non-pregnant adult population.7–11 According to one population study, the incidence of adult GBS disease doubled in 10 years, representing a substantial disease burden.11 Primary bactereamia (24%) and skin and soft tissue infections (22%) are the most commonly seen GBS infections, with bone and joint involvement much less common (8%).8 11 The known risk factors for GBS disease in adults include diabetes, older age and malignancy; however, up to 24% of patients with GBS infections have no known risk factors.3 The clinical course of GBS infection in non-pregnant adults is still poorly understood, making it hard to diagnose, especially in the early stages of disease, and severe complications such as bacteraemia and septic arthritis can occur if left untreated.4 9 10 12 13 Moreover, a source of infection is never identified in a large number of cases, making counselling and avoidance of reinfection more difficult.8 9 11 This case highlights the need to better understand the risk factors for GBS infection and to better identify the signs and symptoms of early infection before bacteraemia and its sequelae develop.
Case presentation
A man in his late 70s with previous medical history of gastro-oesophageal reflux disease, treated hepatitis C, atrial fibrillation presented for fatigue, back pain and left knee pain. He first presented 8 days prior to another hospital for chills and weakness and was discharged from the emergency department (ED) after an unrevealing work-up. Four days later, he presented again to the same ED with the same complaints as well as new back pain and was admitted. MRI lumbar spine at that time showed central canal stenosis. Urinalysis was inconsistent with urinary tract infection, and his back pain was thought to be unrelated to a genitourinary aetiology. The patient’s back pain improved with tylenol, gabapentin and ketorolac, and he was discharged home. One day after discharge, he presented to our hospital endorsing progressive fatigue, continued back pain and new left knee pain. His vital signs on arrival were T 36.5 C, HR 122 bpm, BP 143/80 mm Hg, O2 sat 98% on rooom air. Laboratory data showed leukocytosis (22.2×109 /L with 89% neutrophils and an elevated absolute neutrophil count 19.8). Physical examination was notable for exquisite left knee tenderness with limited range of motion due to pain, a moderate suprapatellar effusion and mild erythema and warmth of the anterior knee, but no skin breaks. Sensation to light touch was intact and he demonstrated 5/5 strength in all four extremities. Paraspinal muscle tenderness was present at the level of L2–L5. He did not display saddle paraesthesia. The patient denied any trauma to that left knee, prior joint surgeries or indwelling prosthesis. He was not currently sexually active but had vaginal intercourse with multiple women without condoms in the past.
Investigations
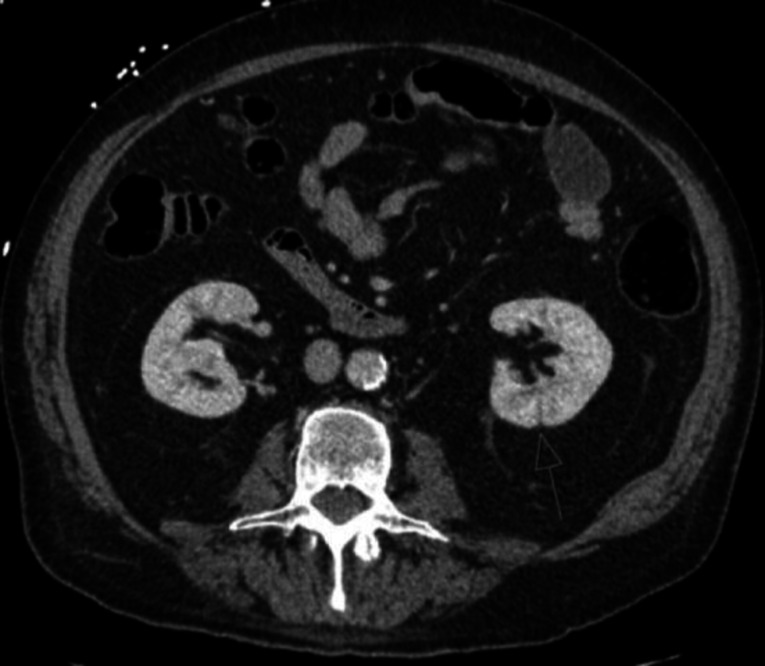
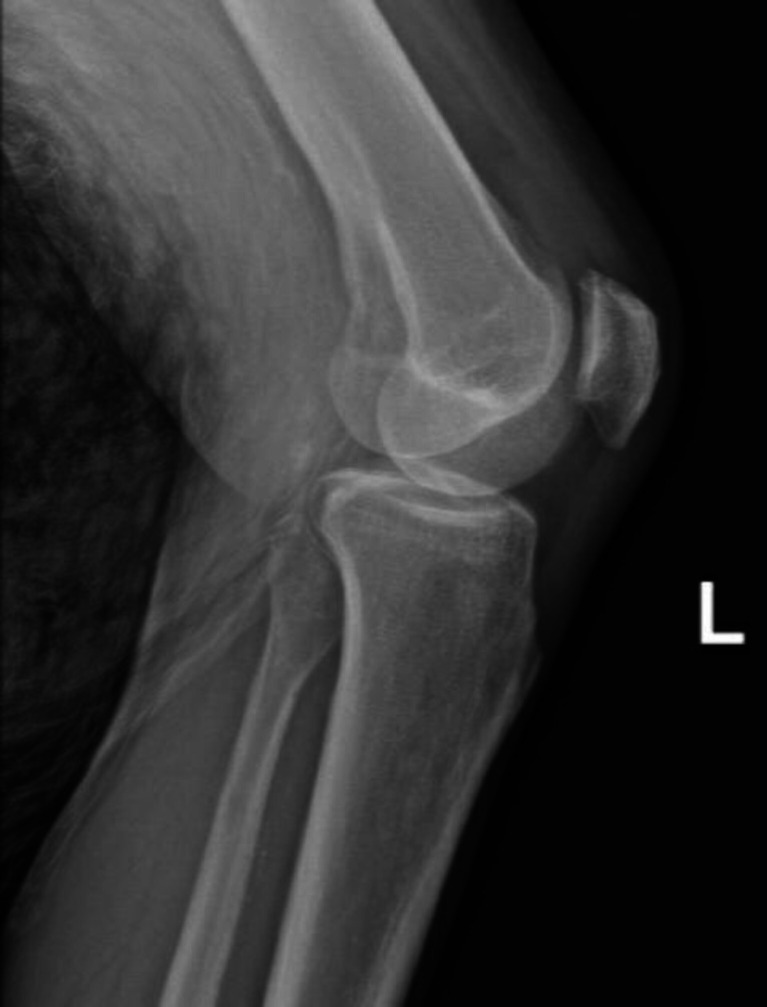
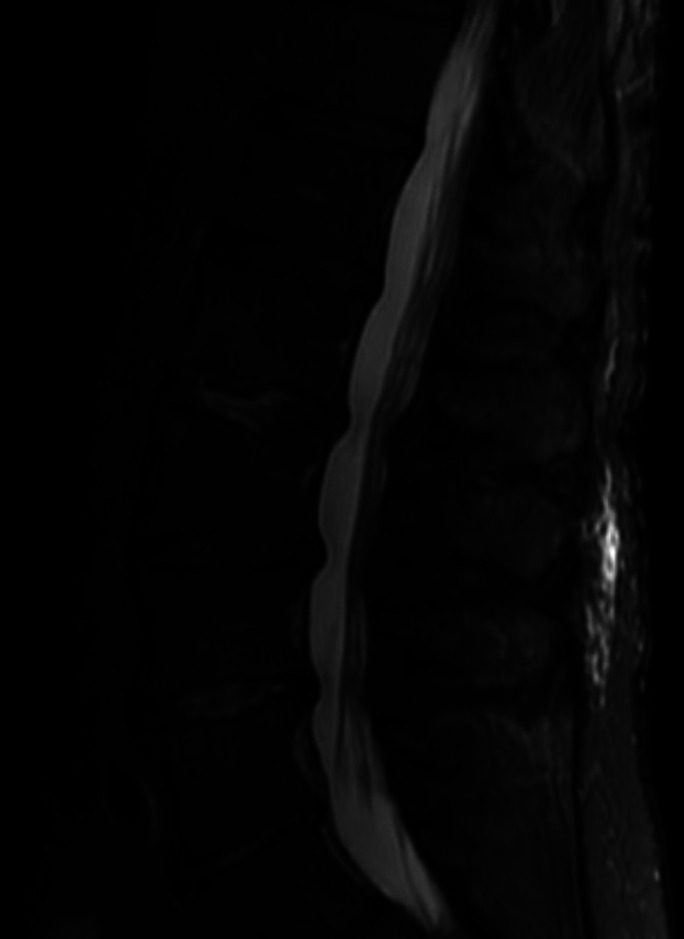
Complete blood count showed leukocytosis with neutrophilic predominance (22.2×109 /L with 89% neutrophils). Urinalysis showed 0–5 white blood count (WBCs) per high power field. CT abdomen and pelvis showed renal perfusion defects with areas of loss of corticomedullary differentiation, concerning for pyelonephritis (figure 1). X-ray of the left knee showed moderate suprapatellar effusion without evidence of trauma (figure 2). Synovial fluid was cloudy with 135000 WBCs and 0 crystals. Aspirate culture (figure 3) and blood cultures grew Streptococcus agalactiae with resistance to clindamycin and erythromycin. MRI of the lumbar spine showed osteomyelitis at L2–L3 and L4–L5.
Figure 1.
CT abdomen without contrasts with arrows showing cortical perfusion defect of the left kidney, concerning for pyelonephritis.
Figure 2.
Plain film X-ray of the left knee, without factures or dislocation, but shows moderate suprapatellar effusion.
Figure 3.
MRI lumbar spine sagittal view demonstrating T2 hyperintensity at the L2 and L3 and L4 and L5 endplates, with associated hyperintensity in the intervertebral disc spaces at L2 and L3 and L4 and L5.
Differential diagnosis
On presentation, the patient showed signs and symptoms of systemic inflammation. His knee pain is most concerning for septic arthritis or inflammatory arthritis, which prompted synovial aspiration. Additionally, his blood cultures grew Streptococcus agalactiae, which made full infectious evaluation necessary. His back pain can either be due to epidural abscess, septic arthritis of the spine or osteomyelitis for which MRI spine would distinguish the aetiologies. Pyelonephritis was unlikely given negative urine culture. Finally, because patient has infection of different sites in the context of bacteraemia, endocarditis must be ruled out.
Treatment
He was empirically started on intravenous vancomycin and ceftriaxone. Orthopaedic surgery performed irrigation and synovectomy of the left knee. All the cultures (operative tissue culture, aspirate, blood) speciated to GBS sensitive to cephalosporins, levofloxacin, linezolid, penicillin G and vancomycin but resistant to clindamycin and erythromycin and so he was switched to ceftriaxone 2 g/day monotherapy.
Outcome and follow-up
He remained on 2 g/day of intravenous ceftriaxone for 3 weeks and then was transitioned to oral levofloxacin 750 mg/day for an additional 3 weeks to complete a total 6-week course of antibiotics for his osteomyelitis. The patient was discharged to a skilled nursing facility for ongoing treatment and physical rehabilitation. He followed with physical therapy to improve his overall strength and mobility and for rehabilitation of his left knee and lower back. Eight weeks after his surgery has not displayed any further infectious signs or symptoms, and his left knee has regained full range of motion without varus or valgus instability or joint line pain with flexion and extension. However, he remains grossly deconditioned and reports difficulty ambulating and has been mostly bed bound since discharge. He was referred to physical medicine and rehabilitation specialists to develop a comprehensive rehabilitation regimen and regain functional status.
Discussion
Four cases of GBS septic arthritis in non-pregnant adults had been reported as of January 2022.14–17 Two cases described patients without any known risk factors for GBS infection, similar to our patient. All of the cases describe significant disease burden, with associated endocarditis,14 multiple joint involvement15 16 or associated necrotising skin and soft tissue infection16 17 demonstrating the severity of invasive GBS infection. Isolated vertebral osteomyelitis due to streptococcus agalactiae is slightly more commonly reported, with over 30 cases reported on review of NCBI PubMed database and most often presents without concurrent bacteraemia or septic arthritis.18
Our case highlights the insidious nature of GBS infection and the need to better understand the risk factors and signs and symptoms of early GBS infection. It took three clinical encounters and 8 days for our patient to get the correct diagnosis and treatment of his serious, joint-threatening and life-threatening infection. Our case further demonstrates the need to retain a high clinical suspicion for vertebral infection in patients presenting with back pain and fevers, even in the setting of negative imaging, as radiographic changes may not be present early in the disease course. Fortunately, GBS remains susceptible to most standard antibiotic regimens and will be covered by most empiric antibiotic regimens until culture data are available.8 9 The main barrier to care, therefore, is diagnostic delay, demonstrating the need for better understanding of the risk factors for GBS infections in non-pregnant adults as well as the signs and symptoms of invasive GBS infections, in order to promptly treat and prevent morbidity and mortality in infected patients.
Our patient initially presented with a high leukocytosis and new knee pain, prompting immediate joint aspiration. The fluid analysis of the aspirate confirmed our diagnosis of septic arthritis. Septic arthritis is an orthopaedic emergency, requiring prompt diagnosis and intervention to prevent permanent joint destruction or death.19 Septic arthritis occurs when an infectious organism invades the synovium and joint space, causing a release of inflammatory cytokines and proteases that mediate joint destruction. Joint synovium is highly vascularised and lacks a basement membrane, putting it at risk of infection via haematogenous seeding from bacteraemia. Any clinical or radiographical suspicion should require immediate arthrocentesis with fluid analysis. The mainstay of treatment is removing the infected tissue from the joint and 4 weeks of intravenous antibiotics for a native joint.19 20 Our patient’s left knee septic arthritis was managed by surgical intervention within 24 hours and empiric then later targeted intravenous antibiotics.
The patient’s positive blood cultures and continued back pain prompted concern for osteomyelitis, which was confirmed by MRI lumbar spine. Osteomyelitis is most often caused by spread of bacteria from haematogenous spread, open fractures or surgery. MRI is the gold standard for diagnosing osteomyelitis and can detect early disease, within 3–5 days of disease onset.21 However, very early in the disease process, there may be no radiographic changes, which can lead to diagnostic delays. Thus, it is important to maintain a high threshold of suspicion in any patient with back pain and infectious signs, and consider reimaging if symptoms are worsening or not improving. The primary management of any infection is source control and antibiotics, which holds true for osteomyelitis. Surgical debridement of infected bone is recommended when possible, especially when associated with a surrounding abscess.21 However, surgical debridement of vertebrae is not without risk, so for, patients without neurologic deficits, a 6-week course of targeted intravenous antibiotic therapy or highly bioavailable oral antibiotic therapy is the current guideline-recommended treatment for preventing recurrent osteomyelitis.22 Our patient’s osteomyelitis was treated with a total 6-week course of targeted antibiotics based on sensitivity, as he had no neurological deficits requiring surgical debridement. Repeat blood cultures showed no additional growth and echocardiogram did not reveal any vegetations, ruling out endocarditis as a diagnosis. Based on the progression of the patient’s symptoms, it would be reasonable to suspect a haematogenous spread from GBS bacteraemia to his lumbar spine and later to his left knee, resulting in osteomyelitis and septic arthritis. However, the source of inoculation is unclear to date. Bacterial translocation from the Gastrointestinal/genitourinary tract is possible as the patient has radiographical findings concerning for pyelonephritis; however, his urinalysis did not suggest an urinary tract infection. A direct inoculation to his joints leading to bacteraemia is also possible, but this is inconsistent with his history and the natural progression of his symptoms.
Learning points.
Streptococcus agalactiae is emerging as a common and serious aetiology of bacteraemia and its associated sequelae (ie, septic arthritis, osteomyelitis).
Streptococcus agalactiae bacteraemia can occur in adults without classic risk factors for infection
Disseminated, life-threatening infection can occur insidiously and without severe symptoms, making prompt diagnosis difficult, particularly early on in the disease course.
Septic arthritis is a surgical emergency and arthrocentesis with fluid analysis should be immediately performed, if there is any clinical suspicion.
Back pain in a patient with chills and weakness is always suspicious for an underlying infection, and negative imaging findings do not exclude infection, as it may be too early in the clinical course for radiographic changes to appear back pain in a patient with a systemic infection should always be investigated to rule out osteomyelitis, as it alters the duration and choice of antibiotic therapy.
Footnotes
Contributors: All authors provided direct patient care. LG and MM-P wrote the manuscript and obtained the images. H-MY consented the patient and revised the manuscript. All authors approved the final version of the manuscript submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Hanna M, Noor A. Streptococcus group B. 12. StatPearls Publishing, 2021. [Google Scholar]
- 2.Shabayek S, Spellerberg B. Group B streptococcal colonization, molecular characteristics, and epidemiology. Front Microbiol 2018;9:437. 10.3389/fmicb.2018.00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sendi P, Johansson L, Norrby-Teglund A. Invasive group B Streptococcal disease in non-pregnant adults : a review with emphasis on skin and soft-tissue infections. Infection 2008;36:100–11. 10.1007/s15010-007-7251-0 [DOI] [PubMed] [Google Scholar]
- 4.Colford JM, Mohle-Boetani J, Vosti KL. Group B streptococcal bacteremia in adults. five years' experience and a review of the literature. Medicine 1995;74:176–90. 10.1097/00005792-199507000-00002 [DOI] [PubMed] [Google Scholar]
- 5.Farley MM. Group B streptococcal disease in nonpregnant adults. Clin Infect Dis 2001;33:556–61. 10.1086/322696 [DOI] [PubMed] [Google Scholar]
- 6.Meyn LA, Moore DM, Hillier SL, et al. Association of sexual activity with colonization and vaginal acquisition of group B Streptococcus in nonpregnant women. Am J Epidemiol 2002;155:949–57. 10.1093/aje/155.10.949 [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg DL, Reed JI. Bacterial arthritis. N Engl J Med 1985;312:764–71. 10.1056/NEJM198503213121206 [DOI] [PubMed] [Google Scholar]
- 8.Dubost JJ, Soubrier M, De Champs C, et al. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis 2002;61:267–9. 10.1136/ard.61.3.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muñoz P, Llancaqueo A, Rodríguez-Créixems M, et al. Group B Streptococcus bacteremia in nonpregnant adults. Arch Intern Med 1997;157:213–6. 10.1001/archinte.1997.00440230087011 [DOI] [PubMed] [Google Scholar]
- 10.Ruksasakul R, Narongroeknawin P, Assavatanabodee P, et al. Group B streptococcus is the most common pathogen for septic arthritis with unique clinical characteristics: data from 12 years retrospective cohort study. BMC Rheumatol 2019;3:1–10. 10.1186/s41927-019-0084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skoff TH, Farley MM, Petit S, et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990-2007. Clin Infect Dis 2009;49:85–92. 10.1086/599369 [DOI] [PubMed] [Google Scholar]
- 12.Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology 2001;40:24–30. 10.1093/rheumatology/40.1.24 [DOI] [PubMed] [Google Scholar]
- 13.Ho C-M, Chi C-Y, Ho M-W, C-M H, M-W H, et al. Clinical characteristics of group B Streptococcus bacteremia in non-pregnant adults. J Microbiol Immunol Infect 2006;39:396–401. [PubMed] [Google Scholar]
- 14.Takeda S, Tanaka Y, Takeichi Y, et al. A rare case of right-sided infective endocarditis caused by group B Streptococcus complicated with septic knee arthritis and subcutaneous abscess in the lower extremity. Acute Med Surg 2020;7:e456. 10.1002/ams2.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umemura H, Hiragushi K, Sasaki S, et al. A male with group B streptococcal necrotizing fasciitis at multiple sites secondary to multifocal septic arthritis. Acta Derm Venereol 2015;95:614–5. 10.2340/00015555-2015 [DOI] [PubMed] [Google Scholar]
- 16.Galloway A, Deighton CM, Deady J, et al. Type V group B streptococcal septicaemia with bilateral endophthalmitis and septic arthritis. Lancet 1993;341:960–1. 10.1016/0140-6736(93)91251-G [DOI] [PubMed] [Google Scholar]
- 17.Hammel JM, Kwon N. Septic arthritis of the acromioclavicular joint. J Emerg Med 2005;29:425–7. 10.1016/j.jemermed.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 18.Ganapathy ME, Rissing JP. Group B streptococcal vertebral osteomyelitis with bacteremia. South Med J 1995;88:350–1. 10.1097/00007611-199503000-00020 [DOI] [PubMed] [Google Scholar]
- 19.Momodu II, Savaliya V. Septic Arthritis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK538176/ [PubMed] [Google Scholar]
- 20.Ross JJ. Septic arthritis of native joints. Infect Dis Clin North Am 2017;31:203–18. 10.1016/j.idc.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 21.Momodu II, Savaliya V. Osteomyelitis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK532250/ [Google Scholar]
- 22.Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 infectious diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 2015;61:e26–46. 10.1093/cid/civ482 [DOI] [PubMed] [Google Scholar]