ABSTRACT
Detection of botulinum neurotoxin or isolation of the toxin-producing organism is required for the laboratory confirmation of botulism in clinical specimens. In an effort to reduce animal testing required by the gold standard method of botulinum neurotoxin detection, the mouse bioassay, many technologies have been developed to detect and characterize the causative agent of botulism. Recent advancements in these technologies have led to improvements in technical performance of diagnostic assays; however, many emerging assays have not been validated for the detection of all serotypes in complex clinical and environmental matrices. Improvements to culture protocols, endopeptidase-based assays, and a variety of immunological and molecular methods have provided laboratories with a variety of testing options to evaluate and incorporate into their testing algorithms. While significant advances have been made to improve these assays, additional work is necessary to evaluate these methods in various clinical matrices and to establish standardized criteria for data analysis and interpretation.
KEYWORDS: Clostridium botulinum, Endopep-MS, botulinum neurotoxin, botulism, diagnostic assay, toxins
INTRODUCTION
Botulism is a rare illness that occurs when botulinum neurotoxins (BoNT) enter nerve cells and block communication with muscle cells. This signal disruption at the neuromuscular junction leads to flaccid descending paralysis (1). Although antitoxin is available for treatment, it can only prevent the progression of the disease and cannot reverse damage (2). Without prompt administration of antitoxin and supportive care, this illness can be fatal. While most people who develop botulism will recover, complications may still lead to death (3, 4).
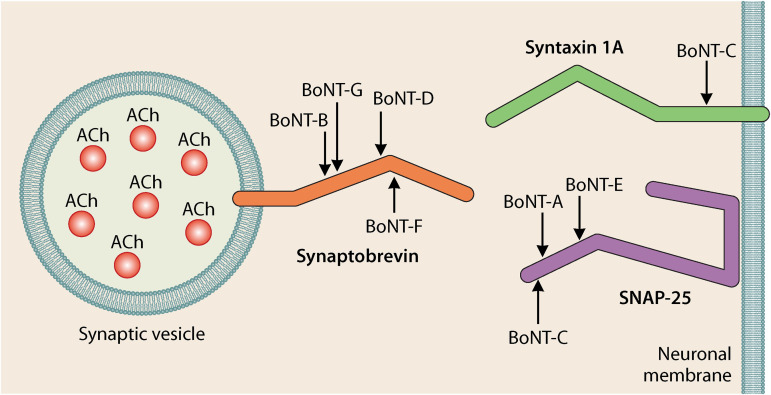
BoNTs are produced by members of the Gram-positive spore-forming anaerobic Clostridium species, including C. botulinum, C. baratii, C. butyricum, and C. argentinense. These metalloproteases are among the most lethal substances known (5). The BoNT protein structure consists of a 100-kDa heavy chain (HC) which aids in nerve cell receptor binding and translocation of the 50-kDa light chain (LC). The LC is then released into the cytosol to cleave SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex proteins necessary for signal transduction in highly specific locations (Fig. 1) (6). The HC and LC are linked by a disulfide bond and are surrounded by complexes which allow BoNT to persist in the environment. Traditionally, BoNTs have been divided into seven serotype groups (A to G) based on immunological cross-reaction with monovalent antitoxins in a standard neutralization assay in mice. They can be further divided into subtypes based on their amino acid sequence derived by sequencing bont genes found on chromosomes, plasmids, or prophage form (7, 8). Separately, the organisms that produce these BoNTs can also be classified into six phylogenetically distinct groups (I to VI) based on their 16S rRNA sequence, phenotypic characteristics, and phylogenetic distance (9). Significant genetic diversity across the six phylogenetic groups has led to a call from some for their reclassification (10).
FIG 1.
BoNT cleavage sites on SNARE complex components are highly specific in location and prevent the release of acetylcholine at the neuromuscular junction, leading to paralysis. (Reproduced from reference 83 with permission.)
Recently, additional serotypes have been proposed and are currently being studied. Hybrid or mosaic toxins such as BoNT/CD, BoNT/DC, or BoNT/H (also known as BoNT/FA or BoNT/HA) have been identified which exhibit properties from a combination of serotypes (11). Bivalent strains such as C. botulinum Ab, Af, Ba, or Bf may produce two active botulinum toxins in unequal amounts, while certain strains such as C. botulinum A(B) contain silent bont/B genes that are not expressed (8, 12). Recent studies have identified bont-like gene clusters in Weissella oryzae (BoNT/Wo), Chryseobacterium piperi (BoNT/Cp1), and Enterococcus faecium (eBoNT/J); however, these proteins have not been confirmed as BoNTs (13–16). Typically, human botulism is attributed to intoxication with BoNT/A, B, E, or F; however, all vertebrates are susceptible to various degrees to BoNTs depending on the level of exposure and the presence and structure of their receptors and targets (17). C. botulinum type G has been isolated from human specimens but has not been directly linked to the root cause of illness (18).
While the clinical presentation of patients with botulism may be sufficient for timely administration of antitoxin, it is important to confirm the clinical diagnosis. Laboratory investigations are needed to rule out other diseases that mimic botulism and determine the root cause of the illness. Laboratory confirmation of botulism can be achieved by detecting BoNT in clinical specimens from patients presenting with symptoms consistent with botulism or by isolating BoNT-producing species of Clostridium from these clinical or associated environmental specimens (19). Many analytical approaches to detect BoNTs in human specimens and environmental samples can be utilized; however, not all assays will provide the same information. For example, some molecular assays can screen for the presence of toxin genes, while other assays will detect enzymatic activity of BoNTs as they cleave SNARE complex components. Incorporation of advanced molecular detection methods into testing algorithms can provide supportive evidence to epidemiological investigations on relatedness and can link contamination sources to illnesses in the event of an outbreak.
TESTING CONSIDERATIONS
Botulism testing has traditionally been performed within public health laboratories due to the specialized testing methods that are currently only available in that setting. Most diagnostic testing for BoNTs can be performed in a biosafety level 2 laboratory following the appropriate biosafety practices outlined by an institution, using class II biological safety cabinets, and wearing the appropriate personal protective equipment (PPE). Testing that involves the use of large volumes of purified toxin, or has the potential to produce aerosols or droplets, requires a risk assessment to be completed and may require the assays to be performed in a biosafety level 3 laboratory with enhanced PPE (20). Laboratories in the United States may also need to register with the Federal Select Agent Program, which is jointly regulated by the Centers for Disease Control and Prevention (CDC) Division of Select Agents and Toxins and the Animal and Plant Health Inspection Service (APHIS) Division of Agricultural Select Agents and Toxins.
Isolation and culture of BoNT-producing organisms from these samples is essential—not only to confirm the diagnosis, but also to support epidemiological investigations. In cases where levels of organism are present below assay limits of detection (LOD), sample volumes are insufficient for testing, or matrix interferences prevent detection, culture of BoNT-producing organisms is critical. Axenic culture can then be utilized for molecular methods, including whole-genome sequencing (WGS). Due to the severity of this illness, it is preferable that diagnostic tests produce results rapidly and with high levels of sensitivity and specificity. While there are many diagnostic tests available to detect BoNTs, many assays are lacking in one aspect or another, so a combination of the following methods may be appropriate.
Regardless of the test selected for the laboratory confirmation of botulism, there are several factors that should be considered that may affect test results. Sample collection timing should be considered, as collection after the administration of antitoxin may inhibit some assays that detect toxin activity. Additionally, samples collected further from the time of exposure may not produce positive results due to absorption, degradation, excretion, or irreversible receptor binding (21). Sample storage and transport conditions may also affect results. If samples are shipped or stored frozen, it may reduce the chances that viable organism will be recovered from or detected in clinical specimens (22). With specimens containing low levels of BoNT, repeated freeze-thaws may hinder detection. Certain sample types may contain substances that can inhibit molecular reactions, and appropriate steps should be taken to understand and reduce these effects (23). For many sequencing-based assays, isolation of BoNT-producing organisms is necessary to generate results. Epidemiological investigations performed upon laboratory confirmation of botulism are essential to identify sources of contamination and to prevent further illness. Without the sampling of environmental or food sources, it may not be possible to gather the isolates needed to establish criteria to definitively link cases.
SAMPLE PROCESSING AND CULTURE
BoNTs and the organisms that produce them have been detected in a variety of clinical matrices, including serum, stool, digestive contents, wound samples, and culture supernatants. They have also been detected in food or environmental matrices such as improperly preserved or minimally processed vegetables, meats, dairy products, beverages, honey, soil, dust, and sediment (24–27). Isolation and culture of BoNT-producing organisms from these samples are essential for the laboratory confirmation of botulism and epidemiological investigations. Although BoNT and organisms that produce them may be present in a sample, assay limitations may prevent their detection. In such cases, enrichment and isolation of BoNT producers is critical.
Due to their potential for misuse for bioterrorism, BoNT-producing species of Clostridium have been classified as tier 1 select agents (28). As such, culture of these bacteria must be performed in secure facilities with limited access. Prior to testing, many samples will need to be homogenized, which can be performed by stomaching with an equal volume of gelatin diluent in filtered bags. The resulting filtrate can then be used in downstream testing. Complex matrices will require additional centrifugation and filtration. Nonselective culture media typically used to grow BoNT producers from clinical and environmental samples include egg yolk agar (EYA), sheep blood agar, Trypticase-peptone-glucose-yeast extract broth, and cooked meat medium (29). BoNT-producing Clostridium will produce a lipase reaction on EYA, except for C. butyricum and C. argentinense, which produce lipase-negative colonies, and C. baratii, which produces a lecithinase reaction on EYA (30). Clinical specimens and environmental and food samples submitted for testing may contain high numbers of other organisms, making isolation of BoNT producers very difficult. In this case, spore selection using heat or ethanol treatment may aid in isolation (22). Botulinum selective media may also be used for isolation of BoNT producers from stool specimens (31). Recently, a new method for isolation of group III organisms involved with avian botulism outbreaks has been developed to combat poor growth in mixed cultures on traditional medium and loss of bont genes located on phages. As the protocol relies on the ability of C. botulinum to survive an InstaGene extraction (32), it is possible that this method could be used to isolate C. botulinum from other matrices as well.
MOUSE BIOASSAY
Historically, the gold standard for the laboratory confirmation of botulism has been the mouse bioassay (MBA). This method is preferred by many because it can confirm the ability of BoNTs to bind to and enter nerve cells and cause symptoms by means of proteolytic activity in vivo. While this assay is sensitive, it may take up to 4 days to confirm test results, can be costly, can be arduous, and requires skilled personnel. Additionally, this assay is not ideal in a situation that requires high-throughput testing. Limited diagnostic age and size restrictions on mice used for this assay can be problematic, as larger mice may have a higher tolerance for BoNT before becoming symptomatic (33). While the MBA is a robust assay, the presence of pathogenic organisms or other interfering substances may lead to nonspecific systemic effects, confounding results (34, 35). Fortunately, false-positive results can be ruled out via BoNT neutralization with specific mono- or polyvalent antitoxin. Finally, the ethical concerns involved with the use of live animals for testing should be considered, as other assays are now available which can provide comparable information. One improvement to the traditional MBA based on previous work in rats uses low-volume nonlethal injections. With this assay, BoNT is administered through intramuscular injection, and mice are then observed for paralysis at the site of administration, using software to analyze changes in toe-spread reflex (36). This assay can produce results in 24 h and reduces the possibility of a false positive due to nonspecific systemic effects, although live animals must still be used for testing.
CELL-BASED ASSAYS
Cell-based assays have been suggested as a replacement for the MBA, as they can provide evidence of BoNT intoxication. Currently, these assays are the only alternative to the MBA that can demonstrate this process. Briefly, neuronal cells are cultured, samples containing BoNT are added to these cells, and evidence of BoNT activity via receptor binding, internalization, and proteolysis can be measured by a variety of methods (37). Although these assays can be more sensitive than the MBA, performance may be affected by the selected cell lines, growth conditions, or other factors that can affect cell health (33). Additionally, these tests may take up to 3 days to complete (38). Sterile samples are required for testing, which could be problematic for the testing of clinical, food, and environmental samples. Recently, cell-based assays to detect BoNT/A, B, and E have been developed (39, 40). While some cell-based assays have received FDA, Health Canada, or European Union approval for pharmaceutical potency testing of BoNT/A-based drug products (41, 42), they have not been implemented for diagnostic testing.
ENDOPEPTIDASE MASS SPECTROMETRY ASSAYS
The endopeptidase-based assay is the major contender to replace the MBA for the laboratory confirmation of botulism in clinical and associated environmental samples. Comparatively, these assays can be less expensive to perform, can produce results within hours, and most importantly, do not require the use of live animals for testing. Additionally, these assays are highly specific, and their analytical sensitivity allows for detection of active BoNT at levels at or below the MBA LOD.
Endopeptidase-based assays developed by the CDC using high-resolution mass spectrometers have been successfully transitioned for use in laboratories utilizing mass spectrometers commonly used for the identification of microbiological isolates (43). Detection of BoNT via matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF/MS) has been thoroughly assessed. Using this method, confirmation of active BoNT can be achieved for serotypes A through G in various matrices and specimen types at levels near or below the MBA LOD (43–46). With this Endopep-MS assay, antibody-coated beads specific to each BoNT serotype are added to a sample and subsequently incubated. If BoNT is present in the sample, it will bind to the serotype-specific beads prior to washing away any nonspecific proteases. Peptide substrates, mimicking SNARE complex targets for each BoNT serotype, are incubated with the corresponding antibody-coated beads. Due to the highly specific enzymatic activity of each BoNT serotype, if toxin is present, these peptides will be cleaved in exact locations, producing fragments of a defined mass. The fragmented reaction solutions are spotted onto a MALDI target plate and analyzed using the MALDI-TOF/MS instrument to determine if these cleavage products are present in the sample (Fig. 2).
FIG 2.
Endopep-MS method summary. BoNT is captured by serotype-specific antibody-coated magnetic beads, and nonspecific proteases are removed. Peptide substrates mimicking SNARE complex components will be cleaved if toxin is present. (Reproduced from reference 84 with permission from Elsevier.)
This Endopep-MS assay has been successfully utilized to detect active BoNT in clinical and environmental samples directly related to human illness. Studies have demonstrated that peptide substrate modifications can lead to significant improvements in analytical sensitivity specifications, producing positive results approximately one to two log values below the MBA LOD (47). Conversely, studies have been performed which show that modifications to peptide substrates may also prevent the detection of certain BoNT subtypes (48), stressing the importance of thorough evaluation of assay component alterations. Interestingly, the Endopep-MS assay has been used with veterinary specimens to detect outbreaks of botulism in livestock (49). Researchers have modified the Endopep-MS protocol to include salt washes and protease inhibitors that decrease inhibition due to nonspecific cleavage of peptide substrates and increase assay sensitivity in liver specimens for some serotypes associated with animal outbreaks (50). While this Endopep-MS assay has been successfully incorporated into clinical laboratory testing algorithms, further evaluation of assay performance specifications when challenged with food, environmental, and veterinary matrices would be beneficial. Furthermore, it is important to consider the possibility that the serotype-specific antibodies used may not capture novel serotypes or subtypes and that currently used peptide substrates may not encompass novel BoNT target regions.
In addition to providing information on toxin serotype, it is possible to obtain BoNT subtype information from the products of the Endopep-MS assay. Briefly, endopeptidase digests of toxin are prepared and queried against a database containing BoNT protein sequences to determine the subtype (51). This method has been used to identify novel subtypes (52, 53) and could be beneficial in the event of an outbreak, as serotype and subtype information can be provided to epidemiologists within 24 h even if DNA is not present in a sample.
IMMUNOASSAYS
Immunological assays have been studied for the detection of BoNT in various clinical and food matrices (54, 55). Many of these assays, such as enzyme-linked immunosorbent assay (ELISA) and lateral flow assays, are simple to perform and can produce results rapidly for many samples (56, 57). Although immunoassays can be powerful screening tools to identify samples that contain BoNT, they are not typically used for confirmatory testing. Previously, toxin endopeptidase activity could not be observed using ELISA or lateral flow assays; however, using a combination of technologies and enhanced reagents, this is now possible (58, 59). Certain immunoassay platforms, including lateral flow, microsphere, and multiarray technologies using optical or electrochemical reporting, allow for multiplexing, saving on labor and reagent costs, which can otherwise be prohibitive (60). Immunoassays may be beneficial in instances where samples contain only toxin, which may occur with a bioterrorism event or foodborne botulism.
Immuno-PCR (iPCR) has been used successfully to detect some BoNT serotypes with higher sensitivity than the MBA and with higher specificity (61); however, on-site detection capabilities and performance in complex matrices have not been thoroughly evaluated (60). With iPCR, nucleic acid reporters are coupled to detector antibodies and amplified to produce a signal when bound to the BoNT target. This amplification in signal can produce up to a 105-fold increase in sensitivity compared to traditional ELISA performance (62).
Centrifugal microfluidic immunoassays, such as SpinDx, have also been assessed and can detect BoNT at levels below the MBA LOD within 30 min using a fraction of the sample size required by most available assays (63). With this platform, BoNTs are captured on an antibody-coated microsphere, tagged with quantum dot labeled antibodies, and then passed through a density medium to filter out matrix and inhibiting particles. Fluorescence is then quantified after excitation with a laser. Since no sample preparation is required, this can be used with a variety of food and clinical matrices.
Although immunoassay platforms can be used to detect BoNTs at very low levels, it is possible that they may produce false-positive results due to the presence of inactivated toxin or interfering matrix components. Whichever method of immunological detection is used, the challenge of matrix interference persists, and the assay should be validated for its fitness and intended purpose.
NUCLEIC ACID TESTING
Due to the genetic diversity among BoNT-producing species, molecular assays are useful to screen clinical and associated environmental samples for the presence of genes related to BoNT toxin production or to detect and differentiate organisms that produce the toxin. While these assays do not provide information on the viability of organisms or their ability to produce BoNTs, PCR-based assays can be extremely useful to screen primary clinical and environmental samples for the presence of bont and associated genes quickly and with high sensitivity and specificity (64). Additionally, the ability to multiplex reactions targeting these genes reduces turnaround time and costs associated with labor and reagents. As new subtypes or serotypes are identified, it is essential that laboratories confirm that currently employed assays will detect them.
Real-time PCR (rtPCR) has been developed for the qualitative or quantitative detection of BoNT producers or toxin genes (64, 65). These assays can provide critical information within 4 h using automated extraction platforms and can be used with a variety of clinical and food matrices (64). With quick turnaround time to results and the potential for rapid serotype determination, rtPCR would be a useful screening tool in foodborne outbreaks or a bioterrorism event, as results could possibly differentiate contamination sources. rtPCR assays can also be useful to rapidly screen for toxin genes in cases where patients may experience atypical symptoms, including asymmetry of deficits, unreactive pupils, fever, symptomology limited to a particular location such as the eye, demyelination of cranial nerves, or unusual presentations in patients such as no typical prodrome in infants (4, 66–68). Recently, researchers developed a multiplex rtPCR assay that differentiates Clostridium groups I and II, which are mainly associated with human botulism, as well as genes associated with toxin production (69).
GENOMIC COMPARATIVE METHODS
Once botulism has been confirmed, it is essential to investigate these cases to determine the source of contamination and prevent further illness. Various assays have been used to compare the genomes of BoNT producers. While some gel-based techniques are still being used, there has been an increase in the use of sequencing methods to investigate outbreaks.
To strengthen epidemiological investigations, additional information on the relatedness of C. botulinum clinical and environmental or food isolates can be obtained by performing pulse field gel electrophoresis (PFGE) analysis. Nucleic acid digested by specific restriction enzymes is separated on a gel, and the resulting fingerprints can be compared. National databases may be queried to identify potentially related cases. For most epidemiologically related isolates, patterns may be indistinguishable; however, in rare instances confounding results may need to be supplemented with epidemiological data (70). Although PFGE has been used extensively for bacterial foodborne outbreak investigations, variations in testing protocols and run conditions, PFGE enzymes used, and database availability and completeness may make data interpretation difficult.
Another method that has been used to supplement initial testing is traditional multilocus sequence typing (MLST) analysis (71). Sequence data from fragments of seven genes from each isolate are assigned as unique alleles, and a sequence type is generated. This information can be compared to large databases to identify similar isolates. Whole-genome MLST can be performed, which relies on the same principles but allows for examination of many more genes, providing increased resolution (72). Recent publications have shown that a combination of bioinformatic techniques can be used to characterize and differentiate closely related C. botulinum isolates using WGS data (73, 74).
With increased availability and affordability of next-generation sequencing (NGS) protocols and platforms, many laboratories have gained the ability to sequence clinical and environmental isolates. Depending on assay design and output, it is possible to detect and characterize BoNT-producing species simultaneously; however, analysis of NGS data typically requires the use of high-performance computers and interpretation by trained personnel. WGS of Clostridium species can provide critical information to epidemiological investigations, potentially linking patient specimens to outbreak sources. In contrast, more targeted amplicon-based sequencing assays could potentially provide detailed information on bont genes present in a sample rapidly and directly within a secure laboratory space. With the ability to sequence isolates, it is possible that novel serotypes or subtypes with similar activities may also be discovered.
Two platforms that have recently been used to sequence C. botulinum are Illumina MiSeq and Oxford Nanopore MinION sequencers, which generate short reads and long reads, respectively (75). Both platforms are available at many institutions, and MinION devices have recently been incorporated into many workflows due to their long read length, small size, low cost, and rapid time to results and the ability to sequence directly within a secure laboratory space. Although MiSeq data can provide high-quality reads, it may be difficult to determine the location of bont gene clusters due to the short read lengths generated with this technology. On the other hand, base-calling in homopolymeric regions may be inaccurate with nanopore sequencing platforms. To address issues with each of the sequencing platforms, Illumina MiSeq and Oxford Nanopore MinION data have been combined to produce high-quality closed C. botulinum genomes (75). Using this approach for a given isolate, information including BoNT serotype and subtype, bont gene cluster locations, genome size, chromosome and plasmid synteny, and phylogeny can be generated simultaneously. While single nucleotide polymorphism (SNP)-based analysis has been used to compare and differentiate closely related group I isolates (76), currently published data suggest that group I epidemiologically linked isolates have shown little to no variation in genetic sequences using a SNP-based approach (27, 77). It is possible that as more C. botulinum genomes are available for analysis and comparison, small variations in genetic sequences may become increasingly significant, and a combination of analytical approaches may be needed to provide additional resolution. Without an established SNP threshold to definitively link related isolates, NGS findings must be considered in conjunction with other laboratory testing results and epidemiological data.
ELECTROCHEMICAL DETECTION AND BIOSENSORS
Recently, electrochemical assays to detect BoNT enzymatic activity have been developed. With these methods, biosensors can be coated with SNARE complex proteins. Once cleaved by BoNT, the change in the SNARE protein coating leads to measurable changes in electrochemical properties which are used to establish the presence of enzymatically active BoNT (78). Researchers have developed nanopore-based detection of BoNT enzymatic activity by measuring changes in ion current in nanopores as cleavage products of substrates mimicking SNARE complex regions are passed through aerolysin nanopores and digested. These assays are sensitive, some with detection levels in subnanomolar ranges, and can generate results within minutes once prepared samples are placed on the instrument (79). While promising results have been observed, additional studies incorporating all SNARE complex proteins for the detection of all serotypes should be performed.
Detection of active BoNT has been performed using nano-biosensors. Quantum dots, which fluoresce when excited, are linked to serotype-specific substrates with an attached quencher, allowing Förster resonance energy transfer (FRET) to occur. This method allows for rapid, specific, and quantitative detection of active BoNT at levels at or near the LOD of the MBA and, in some cases, requires no instrumentation to interpret results (80). This technology has been shown to work for serotypes A, B, and E and may be useful as a screening assay (81); however, further testing with the remaining serotypes and challenging the assay using various food and clinical matrices would be beneficial. Synthetic peptides mimicking SNARE complex proteins contain fluorophores and quenchers which may be incorporated into future FRET-based assays. Interestingly, differences in endopeptidase activity have been observed, depending on the form of toxin present in the sample and the length and structure of the peptide substrate being examined (82), which could have potential implications for future assay development. While electrochemical and biosensor assays show promise to produce rapid results with high sensitivity, these platforms are not currently being used in clinical settings. Additional work would need to be done to validate assay performance for all BoNT serotypes in various clinical and environmental matrices.
CONCLUSIONS
Botulism is diagnosed by observing clinical signs and symptoms that are consistent with the illness. Laboratory confirmation of the clinical case is important for public health response, and timely administration of antitoxin is critical for successful treatment of the disease. In cases where botulinum antitoxin has not yet been released, rapid detection of BoNT or identification of BoNT-producing organisms is essential. Therefore, turnaround time of testing should be considered when developing laboratory testing algorithms.
Many of the assays available to confirm a clinical diagnosis of botulism rely on the function of specialized reagents, such as serotype-specific antibodies, peptide substrates, or fluorescent reporters. Specialized laboratory equipment or the use of animals for laboratory testing are also needed at the moment to confirm the presence of BoNT, as alternative methods have not been thoroughly validated in complex matrices for all serotypes. Stability and accessibility of reagents, consumables, and equipment to perform such testing are important considerations for laboratories performing botulism testing. Finally, emerging serotypes or subtypes may have genetic or physical properties that do not allow for their detection using current reagents or technologies, which should be considered when assays are being evaluated for incorporation into testing algorithms.
ACKNOWLEDGMENT
We have declared that no competing interest exists.
Contributor Information
Dominick A. Centurioni, Email: dominick.centurioni@health.ny.gov.
Romney M. Humphries, Vanderbilt University Medical Center
REFERENCES
- 1.Humeau Y, Doussau F, Grant NJ, Poulain B. 2000. How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie 82:427–446. 10.1016/S0300-9084(00)00216-9. [DOI] [PubMed] [Google Scholar]
- 2.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K, Working Group on Civilian Biodefense. 2001. Botulinum toxin as a biological weapon: medical and public health management. JAMA 285:1059–1070. 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 3.Dembek ZF, Smith LA, Rusnak JM. 2007. Botulism: cause, effects, diagnosis, clinical and laboratory identification, and treatment modalities. Disaster Med Public Health Prep 1:122–134. 10.1097/DMP.0b013e318158c5fd. [DOI] [PubMed] [Google Scholar]
- 4.Rao AK, Sobel J, Chatham-Stephens K, Luquez C. 2021. Clinical guidelines for diagnosis and treatment of botulism, 2021. MMWR Recomm Rep 70:1–30. 10.15585/mmwr.rr7002a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamanna C. 1959. The most poisonous poison. Science 130:763–772. 10.1126/science.130.3378.763. [DOI] [PubMed] [Google Scholar]
- 6.Rossetto O, Pirazzini M, Montecucco C. 2014. Botulinum neurotoxins: genetic, structural and mechanistic insights. Nat Rev Microbiol 12:535–549. 10.1038/nrmicro3295. [DOI] [PubMed] [Google Scholar]
- 7.Dong M, Stenmark P. 2021. The structure and classification of botulinum toxins, p 11–33. In Whitcup SM, Hallett M (ed), Botulinum toxin therapy. Springer International Publishing, Cham, Switzerland. [DOI] [PubMed] [Google Scholar]
- 8.Peck MW, Smith TJ, Anniballi F, Austin JW, Bano L, Bradshaw M, Cuervo P, Cheng LW, Derman Y, Dorner BG, Fisher A, Hill KK, Kalb SR, Korkeala H, Lindstrom M, Lista F, Luquez C, Mazuet C, Pirazzini M, Popoff MR, Rossetto O, Rummel A, Sesardic D, Singh BR, Stringer SC. 2017. Historical perspectives and guidelines for botulinum neurotoxin subtype nomenclature. Toxins (Basel 9) :38. 10.3390/toxins9010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill KK, Xie G, Foley BT, Smith TJ. 2015. Genetic diversity within the botulinum neurotoxin-producing bacteria and their neurotoxins. Toxicon 107:2–8. 10.1016/j.toxicon.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Smith T, Williamson CHD, Hill K, Sahl J, Keim P. 2018. Botulinum neurotoxin-producing bacteria. Isn’t it time that we called a species a species? mBio 9:e01469-18. 10.1128/mBio.01469-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maslanka SE, Lúquez C, Dykes JK, Tepp WH, Pier CL, Pellett S, Raphael BH, Kalb SR, Barr JR, Rao A, Johnson EA. 2016. A novel botulinum neurotoxin, previously reported as serotype H, has a hybrid-like structure with regions of similarity to the structures of serotypes A and F and is neutralized with serotype A antitoxin. J Infect Dis 213:379–385. 10.1093/infdis/jiv327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutson RA, Zhou Y, Collins MD, Johnson EA, Hatheway CL, Sugiyama H. 1996. Genetic characterization of Clostridium botulinum type A containing silent type B neurotoxin gene sequences. J Biol Chem 271:10786–10792. 10.1074/jbc.271.18.10786. [DOI] [PubMed] [Google Scholar]
- 13.Mansfield MJ, Adams JB, Doxey AC. 2015. Botulinum neurotoxin homologs in non-Clostridium species. FEBS Lett 589:342–348. 10.1016/j.febslet.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Wentz TG, Muruvanda T, Lomonaco S, Thirunavukkarasu N, Hoffmann M, Allard MW, Hodge DR, Pillai SP, Hammack TS, Brown EW, Sharma SK. 2017. Closed genome sequence of Chryseobacterium piperi strain CTM(T)/ATCC BAA-1782, a Gram-negative bacterium with clostridial neurotoxin-like coding sequences. Genome Announc 5:e01296-17. 10.1128/genomeA.01296-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunt J, Carter AT, Stringer SC, Peck MW. 2018. Identification of a novel botulinum neurotoxin gene cluster in Enterococcus. FEBS Lett 592:310–317. 10.1002/1873-3468.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zornetta I, Azarnia Tehran D, Arrigoni G, Anniballi F, Bano L, Leka O, Zanotti G, Binz T, Montecucco C. 2016. The first non clostridial botulinum-like toxin cleaves VAMP within the juxtamembrane domain. Sci Rep 6:30257. 10.1038/srep30257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasetti-Escargueil C, Lemichez E, Popoff MR. 2019. Public health risk associated with botulism as foodborne zoonoses. Toxins 12:17. 10.3390/toxins12010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonnabend O, Sonnabend W, Heinzle R, Sigrist T, Dirnhofer R, Krech U. 1981. Isolation of Clostridium botulinum type G and identification of type G botulinal toxin in humans: report of five sudden unexpected deaths. J Infect Dis 143:22–27. 10.1093/infdis/143.1.22. [DOI] [PubMed] [Google Scholar]
- 19.Lindström M, Korkeala H. 2006. Laboratory diagnostics of botulism. Clin Microbiol Rev 19:298–314. 10.1128/CMR.19.2.298-314.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meechan PJ, Potts J. 2020. Biosafety in microbiological and biomedical laboratories. U.S. Department of Health and Human Services, Washington, DC. [Google Scholar]
- 21.Woodruff BA, Griffin PM, McCroskey LM, Smart JF, Wainwright RB, Bryant RG, Hutwagner LC, Hatheway CL. 1992. Clinical and laboratory comparison of botulism from toxin types A, B, and E in the United States, 1975–1988. J Infect Dis 166:1281–1286. 10.1093/infdis/166.6.1281. [DOI] [PubMed] [Google Scholar]
- 22.National Center for Infectious Diseases (U.S.). Division of Bacterial and Mycotic Diseases. 1998. Botulism in the United States, 1899–1996. U.S. Dept. of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 23.Oikarinen S, Tauriainen S, Viskari H, Simell O, Knip M, Virtanen S, Hyöty H. 2009. PCR inhibition in stool samples in relation to age of infants. J Clin Virol 44:211–214. 10.1016/j.jcv.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Lúquez C, Edwards L, Griffin C, Sobel J. 2021. Foodborne botulism outbreaks in the United States, 2001–2017. Front Microbiol 10.3389/fmicb.2021.713101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nevas M, Lindström M, Virtanen A, Hielm S, Kuusi M, Arnon SS, Vuori E, Korkeala H. 2005. Infant botulism acquired from household dust presenting as sudden infant death syndrome. J Clin Microbiol 43:511–513. 10.1128/JCM.43.1.511-513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koepke R, Sobel J, Arnon SS. 2008. Global occurrence of infant botulism, 1976–2006. Pediatrics 122:e73–e82. 10.1542/peds.2007-1827. [DOI] [PubMed] [Google Scholar]
- 27.Bergeron G, Latash J, Costa-Carter C-AD, Egan C, Stavinsky F, Kileci JA, Winstead A, Zhao B, Perry MJ, Chatham-Stephens K, Sarpel D, Hughes S, Conlon MA, Edmunds S, Mohanraj M, Rakeman JL, Centurioni DA, Lúquez C, Chiefari AK, Harper S. 2019. Botulism outbreak associated with home-canned peas: New York City, 2018. Morbidity and Mortality Wkly Report 68:251–252. 10.15585/mmwr.mm6810a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Division of Select Agents and Toxins. 2021. Select agents and toxins list. https://www.selectagents.gov/sat/list.htm. Accessed 1 July 2021.
- 29.Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW. 2019. Manual of clinical microbiology, 12th ed. ASM Press, Washington, DC. [Google Scholar]
- 30.Versalovic J. 2011. Manual of clinical microbiology, 10th ed. ASM Press, Washington, DC. [Google Scholar]
- 31.Mills DC, Midura TF, Arnon SS. 1985. Improved selective medium for the isolation of lipase-positive Clostridium botulinum from feces of human infants. J Clin Microbiol 21:947–950. 10.1128/jcm.21.6.947-950.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Gratiet T, Poezevara T, Rouxel S, Houard E, Mazuet C, Chemaly M, Marechal CL. 2020. Development of an innovative and quick method for the isolation of Clostridium botulinum strains involved in avian botulism outbreaks. Toxins 12:42. 10.3390/toxins12010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pellett S, Tepp WH, Johnson EA. 2019. Critical analysis of neuronal cell and the mouse bioassay for detection of botulinum neurotoxins. Toxins 11:713. 10.3390/toxins11120713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dezfulian M, Bartlett JG. 1985. Detection of Clostridium botulinum type B toxin in the presence of a lethal substance interfering with toxin neutralization. Diagn Microbiol Infect Dis 3:105–112. 10.1016/0732-8893(85)90018-5. [DOI] [PubMed] [Google Scholar]
- 35.Horwitz MA, Hatheway CL, Dowell VR. 1976. Laboratory diagnosis of botulism complicated by pyridostigmine treatment of the patient. A method for selectively removing interfering substances from clinical specimens. Am J Clin Pathol 66:737–742. 10.1093/ajcp/66.4.737. [DOI] [PubMed] [Google Scholar]
- 36.Wilder-Kofie TD, Lúquez C, Adler M, Dykes JK, Coleman JD, Maslanka SE. 2011. An alternative in vivo method to refine the mouse bioassay for botulinum toxin detection. Comp Med 61:235–242. [PMC free article] [PubMed] [Google Scholar]
- 37.Stern D, von Berg L, Skiba M, Dorner MB, Dorner BG. 2018. Replacing the mouse bioassay for diagnostics and potency testing of botulinum neurotoxins: progress and challenges. Berl Munch Tierarztl Wochenschr 10.2376/0005-9366-17110. [DOI] [Google Scholar]
- 38.Pellett S, Tepp WH, Toth SI, Johnson EA. 2010. Comparison of the primary rat spinal cord cell (RSC) assay and the mouse bioassay for botulinum neurotoxin type A potency determination. J Pharmacol Toxicol Methods 61:304–310. 10.1016/j.vascn.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Rust A, Doran C, Hart R, Binz T, Stickings P, Sesardic D, Peden AA, Davletov B. 2017. A cell line for detection of botulinum neurotoxin type B. Front Pharmacol 8:796. 10.3389/fphar.2017.00796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bak N, Rajagopal S, Stickings P, Sesardic D. 2017. SiMa cells for a serotype specific and sensitive cell-based neutralization test for botulinum toxin A and E. Toxins 9:230. 10.3390/toxins9070230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernández-Salas E, Wang J, Molina Y, Nelson JB, Jacky BP, Aoki KR. 2012. Botulinum neurotoxin serotype A specific cell-based potency assay to replace the mouse bioassay. PLoS One 7:e49516. 10.1371/journal.pone.0049516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor K, Gericke C, Alvarez LR. 2019. Botulinum toxin testing on animals is still a Europe-wide issue. ALTEX 36:81–90. 10.14573/altex.1807101. [DOI] [PubMed] [Google Scholar]
- 43.Perry MJ, Centurioni DA, Davis SW, Hannett GE, Musser KA, Egan CT. 2017. Implementing the Bruker MALDI biotyper in the public health laboratory for C. botulinum neurotoxin detection. Toxins (Basel) 9:94. 10.3390/toxins9030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalb SR, Krilich JC, Dykes JK, Luquez C, Maslanka SE, Barr JR. 2015. Detection of botulinum toxins A, B, E, and F in foods by Endopep-MS. J Agric Food Chem 63:1133–1141. 10.1021/jf505482b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalb SR, Smith TJ, Moura H, Hill K, Lou J, Geren IN, Garcia-Rodriguez C, Marks JD, Smith LA, Pirkle JL, Barr JR. 2008. The use of Endopep-MS to detect multiple subtypes of botulinum neurotoxins A, B, E, and F. Int J Mass Spectrom 278:101–108. 10.1016/j.ijms.2008.04.004. [DOI] [Google Scholar]
- 46.Boyer AE, Moura H, Woolfitt AR, Kalb SR, McWilliams LG, Pavlopoulos A, Schmidt JG, Ashley DL, Barr JR. 2005. From the mouse to the mass spectrometer: detection and differentiation of the endoproteinase activities of botulinum neurotoxins A−G by mass spectrometry. Anal Chem 77:3916–3924. 10.1021/ac050485f. [DOI] [PubMed] [Google Scholar]
- 47.Wang D, Baudys J, Hoyt KM, Barr JR, Kalb SR. 2017. Further optimization of peptide substrate enhanced assay performance for BoNT/A detection by MALDI-TOF mass spectrometry. Anal Bioanal Chem 409:4779–4786. 10.1007/s00216-017-0421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalb SR, Baudys J, Kiernan K, Wang D, Becher F, Barr JR. 2020. Proposed BoNT/A and /B peptide substrates cannot detect multiple subtypes in the Endopep-MS assay. J Anal Toxicol 44:173–179. 10.1093/jat/bkz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frye EA, Egan C, Perry MJ, Crouch EE, Burbank KE, Kelly KM. 2020. Outbreak of botulism type A in dairy cows detected by MALDI-TOF mass spectrometry. J Vet Diagn Invest 32:722–726. 10.1177/1040638720943127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tevell Aberg A, Karlsson I, Hedeland M. 2021. Modification and validation of the Endopep-mass spectrometry method for botulinum neurotoxin detection in liver samples with application to samples collected during animal botulism outbreaks. Anal Bioanal Chem 413:345–354. 10.1007/s00216-020-03001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalb SR, Goodnough MC, Malizio CJ, Pirkle JL, Barr JR. 2005. Detection of botulinum neurotoxin A in a spiked milk sample with subtype identification through toxin proteomics. Anal Chem 77:6140–6146. 10.1021/ac0511748. [DOI] [PubMed] [Google Scholar]
- 52.Kull S, Schulz KM, Weisemann J, Kirchner S, Schreiber T, Bollenbach A, Dabrowski PW, Nitsche A, Kalb SR, Dorner MB, Barr JR, Rummel A, Dorner BG. 2015. Isolation and functional characterization of the novel Clostridium botulinum neurotoxin A8 subtype. PLoS One 10:e0116381. 10.1371/journal.pone.0116381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalb SR, Baudys J, Rees JC, Smith TJ, Smith LA, Helma CH, Hill K, Kull S, Kirchner S, Dorner MB, Dorner BG, Pirkle JL, Barr JR. 2012. De novo subtype and strain identification of botulinum neurotoxin type B through toxin proteomics. Anal Bioanal Chem 403:215–226. 10.1007/s00216-012-5767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma SK, Ferreira JL, Eblen BS, Whiting RC. 2006. Detection of type A, B, E, and F Clostridium botulinum neurotoxins in foods by using an amplified enzyme-linked immunosorbent assay with digoxigenin-labeled antibodies. Appl Environ Microbiol 72:1231–1238. 10.1128/AEM.72.2.1231-1238.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Worbs S, Fiebig U, Zeleny R, Schimmel H, Rummel A, Luginbühl W, Dorner BG. 2015. Qualitative and quantitative detection of botulinum neurotoxins from complex matrices: results of the first international proficiency test. Toxins (Basel) 7:4935–4966. 10.3390/toxins7124857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hobbs RJ, Thomas CA, Halliwell J, Gwenin CD. 2019. Rapid detection of botulinum neurotoxins: a review. Toxins 11:418. 10.3390/toxins11070418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thirunavukkarasu N, Johnson E, Pillai S, Hodge D, Stanker L, Wentz T, Singh B, Venkateswaran K, McNutt P, Adler M, Brown E, Hammack T, Burr D, Sharma S. 2018. Botulinum neurotoxin detection methods for public health response and surveillance. Front Bioeng Biotechnol 6:80. 10.3389/fbioe.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J, Gao S, Kang L, Ji B, Xin W, Kang J, Li P, Gao J, Wang H, Wang J, Yang H. 2017. An ultrasensitive gold nanoparticle-based lateral flow test for the detection of active botulinum neurotoxin type A. Nanoscale Res Lett 12:227. 10.1186/s11671-017-1944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pellett S, Tepp WH, Johnson EA, Sesardic D. 2017. Assessment of ELISA as endpoint in neuronal cell-based assay for BoNT detection using hiPSC derived neurons. J Pharmacol Toxicol Methods 88:1–6. 10.1016/j.vascn.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pöhlmann C, Elßner T. 2020. Multiplex immunoassay techniques for on-site detection of security sensitive toxins. Toxins (Basel) 12:727. 10.3390/toxins12110727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajkovic A, El Moualij B, Fikri Y, Dierick K, Zorzi W, Heinen E, Uner A, Uyttendaele M. 2012. Detection of Clostridium botulinum neurotoxins A and B in milk by ELISA and immuno-PCR at higher sensitivity than mouse bio-assay. Food Anal Methods 5:319–326. 10.1007/s12161-011-9300-7. [DOI] [Google Scholar]
- 62.Chao HY, Wang YC, Tang SS, Liu HW. 2004. A highly sensitive immuno-polymerase chain reaction assay for Clostridium botulinum neurotoxin type A. Toxicon 43:27–34. 10.1016/j.toxicon.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 63.Koh CY, Schaff UY, Piccini ME, Stanker LH, Cheng LW, Ravichandran E, Singh BR, Sommer GJ, Singh AK. 2015. Centrifugal microfluidic platform for ultrasensitive detection of botulinum toxin. Anal Chem 87:922–928. 10.1021/ac504054u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis SW, Kelly-Cirino C, Cirino N, Hannett GE, Musser KA, Egan C. 2016. A 10 year analysis of the use of multiplex real-time PCR screening for botulinum neurotoxin-producing Clostridium species. J Bacteriol Mycol 3:1030. [Google Scholar]
- 65.Kirchner S, Krämer KM, Schulze M, Pauly D, Jacob D, Gessler F, Nitsche A, Dorner BG, Dorner MB. 2010. Pentaplexed quantitative real-time PCR assay for the simultaneous detection and quantification of botulinum neurotoxin-producing clostridia in food and clinical samples. Appl Environ Microbiol 76:4387–4395. 10.1128/AEM.02490-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.CDC. 2013. Botulism associated with home-fermented tofu in two Chinese immigrants: New York City, March-April 2012. MMWR Morb Mortal Wkly Rep 62:529–532. [PMC free article] [PubMed] [Google Scholar]
- 67.Mitchell WG, Tseng-Ong L. 2005. Catastrophic presentation of infant botulism may obscure or delay diagnosis. Pediatrics 116:e436-8. 10.1542/peds.2005-0297. [DOI] [PubMed] [Google Scholar]
- 68.Filozov A, Kattan JA, Jitendranath L, Smith CG, Luquez C, Phan QN, Fagan RP. 2012. Asymmetric type F botulism with cranial nerve demyelination. Emerg Infect Dis 18:102–104. 10.3201/eid1801.110471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williamson CHD, Vazquez AJ, Hill K, Smith TJ, Nottingham R, Stone NE, Sobek CJ, Cocking JH, Fernandez RA, Caballero PA, Leiser OP, Keim P, Sahl JW. 2017. Differentiating botulinum neurotoxin-producing clostridia with a simple, multiplex PCR assay. Appl Environ Microbiol 83:e00806-17. 10.1128/AEM.00806-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Halpin JL, Joseph L, Dykes JK, McCroskey L, Smith E, Toney D, Stroika S, Hise K, Maslanka S, Luquez C. 2017. Pulsotype diversity of Clostridium botulinum strains containing serotypes A and/or B genes. Foodborne Pathog Dis 14:494–501. 10.1089/fpd.2017.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacobson MJ, Lin G, Whittam TS, Johnson EA. 2008. Phylogenetic analysis of Clostridium botulinum type A by multi-locus sequence typing. Microbiology (Reading) 154:2408–2415. 10.1099/mic.0.2008/016915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maiden MC, Jansen van Rensburg MJ, Bray JE, Earle SG, Ford SA, Jolley KA, McCarthy ND. 2013. MLST revisited: the gene-by-gene approach to bacterial genomics. Nat Rev Microbiol 11:728–736. 10.1038/nrmicro3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosen HE, Kimura AC, Crandall J, Poe A, Nash J, Boetzer J, Tecle S, Mukhopadhyay R, McAuley K, Kasirye O, Garza A, Shahkarami M, Chaturvedi V, Kiang D, Vidanes J, McCoy K, Barcellos M, Derby T, Jain S, Vugia DJ. 2020. Foodborne botulism outbreak associated with commercial nacho cheese sauce from a gas station market. Clin Infect Dis 70:1695–1700. 10.1093/cid/ciz479. [DOI] [PubMed] [Google Scholar]
- 74.Halpin JL, Dykes JK, Katz L, Centurioni DA, Perry MJ, Egan CT, Luquez C. 2019. Molecular characterization of Clostridium botulinum harboring the bont/B7 gene. Foodborne Pathog Dis 16:428–433. 10.1089/fpd.2018.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonzalez-Escalona N, Sharma SK. 2020. Closing Clostridium botulinum group I genomes using a combination of short- and long-reads. Front Microbiol 11:239. 10.3389/fmicb.2020.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonzalez-Escalona N, Timme R, Raphael BH, Zink D, Sharma SK. 2014. Whole-genome single-nucleotide-polymorphism analysis for discrimination of Clostridium botulinum group I strains. Appl Environ Microbiol 80:2125–2132. 10.1128/AEM.03934-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raphael BH, Shirey TB, Lúquez C, Maslanka SE. 2014. Distinguishing highly-related outbreak-associated Clostridium botulinum type A(B) strains. BMC Microbiol 14:192. 10.1186/1471-2180-14-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Halliwell J, Savage AC, Buckley N, Gwenin C. 2014. Electrochemical impedance spectroscopy biosensor for detection of active botulinum neurotoxin. Sens Biosensing Res 2:12–15. 10.1016/j.sbsr.2014.08.002. [DOI] [Google Scholar]
- 79.Wang Y, Montana V, Grubisic V, Stout RF Jr, Parpura V, Gu LQ. 2015. Nanopore sensing of botulinum toxin type B by discriminating an enzymatically cleaved peptide from a synaptic protein synaptobrevin 2 derivative. ACS Appl Mater Interfaces 7:184–192. 10.1021/am5056596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Fry HC, Skinner GE, Schill KM, Duncan TV. 2017. Detection and quantification of biologically active botulinum neurotoxin serotypes A and B using a Forster resonance energy transfer-based quantum dot nanobiosensor. ACS Appl Mater Interfaces 9:31446–31457. 10.1021/acsami.7b08736. [DOI] [PubMed] [Google Scholar]
- 81.Wang Y, Schill KM, Fry HC, Duncan TV. 2020. A quantum dot nanobiosensor for rapid detection of botulinum neurotoxin serotype E. ACS Sens 5:2118–2127. 10.1021/acssensors.0c00738. [DOI] [PubMed] [Google Scholar]
- 82.Ambrin G, Kumar R, Singh BR. 2018. Differential endopeptidase activity of different forms of type A botulinum neurotoxin: a unique relationship between the size of the substrate and activity of the enzyme. Toxicon 144:34–41. 10.1016/j.toxicon.2017.12.055. [DOI] [PubMed] [Google Scholar]
- 83.Barr JR, Moura H, Boyer AE, Woolfitt AR, Kalb SR, Pavlopoulos A, McWilliams LG, Schmidt JG, Martinez RA, Ashley DL. 2005. Botulinum neurotoxin detection and differentiation by mass spectrometry. Emerg Infect Dis 11:1578–1583. 10.3201/eid1110.041279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang D, Baudys J, Ye Y, Rees JC, Barr JR, Pirkle JL, Kalb SR. 2013. Improved detection of botulinum neurotoxin serotype A by Endopep-MS through peptide substrate modification. Anal Biochem 432:115–123. 10.1016/j.ab.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]