ABSTRACT
Critically ill patients with coronavirus disease 2019 (COVID-19) may develop COVID-19-associated pulmonary aspergillosis (CAPA), which impacts their chances of survival. Whether positive bronchoalveolar lavage fluid (BALF) mycological tests can be used as a survival proxy remains unknown. We conducted a post hoc analysis of a previous multicenter, multinational observational study with the aim of assessing the differential prognostic impact of BALF mycological tests, namely, positive (optical density index of ≥1.0) BALF galactomannan (GM) and positive BALF Aspergillus culture alone or in combination for critically ill patients with COVID-19. Of the 592 critically ill patients with COVID-19 enrolled in the main study, 218 were included in this post hoc analysis, as they had both test results available. CAPA was diagnosed in 56/218 patients (26%). Most cases were probable CAPA (51/56 [91%]) and fewer were proven CAPA (5/56 [9%]). In the final multivariable model adjusted for between-center heterogeneity, an independent association with 90-day mortality was observed for the combination of positive BALF GM and positive BALF Aspergillus culture in comparison with both tests negative (hazard ratio, 2.53; 95% CI confidence interval [CI], 1.28 to 5.02; P = 0.008). The other independent predictors of 90-day mortality were increasing age and active malignant disease. In conclusion, the combination of positive BALF GM and positive BALF Aspergillus culture was associated with increased 90-day mortality in critically ill patients with COVID-19. Additional study is needed to explore the possible prognostic value of other BALF markers.
KEYWORDS: CAPA, GM, biomarker, galactomannan, Aspergillus, COVID-19, BALF
INTRODUCTION
Critically ill patients with coronavirus disease 2019 (COVID-19) may develop COVID-19-associated pulmonary aspergillosis (CAPA), and development of CAPA has been recently recognized as an independent predictor of 90-day mortality in this patient population (1–7).
According to the 2020 European Confederation of Medical Mycology/International Society for Human and Animal Mycology (ECMM/ISHAM) consensus criteria, the diagnosis of CAPA in critically ill patients with COVID-19 is categorized as proven, probable, and possible (8–10). Among patients with the highest probability of true disease (proven or probable), most patients are diagnosed with probable disease, owing to the frequent lack of histology or culture from sterile sites for defining proven disease (2, 8). Besides detection of tracheobronchial lesions or radiological pulmonary infiltrates/cavitary lesions, and the presence of clinical factors (e.g., persisting fever), the evidence of fungi or fungal antigens in blood/plasma/serum or in bronchoalveolar lavage fluid (BALF) is necessary for defining probable CAPA (9). In a recent multicenter, multinational, observational study that we conducted in 20 different centers worldwide, serum galactomannan (GM), BALF GM, and BALF Aspergillus culture were the most frequently performed of such tests in critically ill patients with COVID-19 and suspicion of CAPA (2).
While a positive serum GM in patients with CAPA has been recently associated with an unfavorable outcome (11), it is positive in only a minority of CAPA patients due the primary airway invasive character of the disease (2, 11). What remains unclear is whether, for CAPA patients with a negative serum GM, BALF GM and/or BALF Aspergillus culture results could also predict outcomes (12, 13). For this reason, we conducted a post hoc analysis of our previous multicenter observational study (2), with the aim of assessing the different combinations of BALF GM and BALF culture results for prediction of mortality.
MATERIALS AND METHODS
This was a post hoc analysis of a multicenter observational study conducted in 20 different hospitals worldwide (2). Briefly, in different periods between March 2020 and April 2021, 8 centers (Graz/Austria, Genoa/Italy, Cologne/Germany, Manchester/United Kingdom, Leuven, Bruges, Antwerp, and Roeselare/all Belgium) provided prospectively collected data on consecutive critically ill patients with COVID-19 (i.e., during the center-specific different enrollment periods), whereas the other 12 centers provided data from a limited numbers of patients with CAPA and/or without CAPA (2). The study population of the present post hoc analysis was composed by critically ill patients with COVID-19 enrolled in the main study that underwent at least once BALF GM testing and BALF culture during their ICU stay. In line with the purpose of the study, the following patients were excluded: (i) patients with a positive serum GM, (ii) patients with probable CAPA defined microbiologically only by positivity of BALF tests other than BALF GM and BALF Aspergillus (e.g., BALF Aspergillus PCR), and (iii) patients with possible CAPA. Included patients were categorized as patients with CAPA and patients without CAPA according to the 2020 ECMM/ISHAM consensus criteria (9). The primary outcome measure was 90-day mortality as a time-to-event endpoint. Details regarding the different local ethical approval procedures and numbers are available in the main study (2).
Data collection.
For data collection and storage, we used FungiScope (NCT01731353), which allowed inclusion of data in an anonymized electronic case report form (14). Besides results of BALF mycological tests, the following variables included in the survival analysis of the main study were also included in the present post hoc analysis: age in years, sex, study center, obesity (defined as body mass index of ≥30), presence of active malignant disease, previous solid-organ transplantation, presence of cardiovascular disease, presence of pulmonary disease, presence of diabetes mellitus, number of coexisting comorbidities, history of smoking, extracorporeal membrane oxygenation (ECMO), invasive mechanical ventilation, and noninvasive mechanical ventilation.
Statistical analysis.
The main study analysis was the identification of factors associated with 90-day mortality, with particular attention to the impact of the results of BALF mycological tests. To this aim, the results of BALF mycological tests were categorized as a dummy variable {both negative BALF GM and negative BALF Aspergillus culture as the reference category; positive BALF GM (optical density index of ≥1.0, in line with the ECMM/ISHAM criteria [9], instead of recommended cutoff of ≥0.5 provided by the manufacturers) and negative BALF Aspergillus culture, negative BALF GM (optical density index of <1.0) and positive BALF Aspergillus culture, and both positive BALF GM and positive BALF Aspergillus culture}. Notably, this dummy variable was categorized according to the results of BALF tests (and not based on the diagnosis of probable/proven CAPA), in the attempt to evaluate the prognostic performance of BALF tests independently from the knowledge a priori of the unfavorable prognostic impact of CAPA diagnosis in the same cohort (2). The possible association of BALF mycological tests and other demographic and clinical variables with 90-day mortality was first tested in univariable Cox regression models with the time of origin set at the day of intensive care unit (ICU) admission and with BALF mycological tests considered a time-dependent covariate. Then, in addition to the variable “results of BALF mycological tests” (deemed as to be included in all multivariable models independently of P value in univariable comparisons, in line with the aim of the study), all the other factors potentially associated with 90-day mortality in univariable comparisons (P < 0.10) were initially included in a multivariable Cox regression model and further selected for inclusion in a final multivariable model (model A) by means of a stepwise backward procedure. In addition, variables included in model A were also included in a second multivariable Cox regression model (model B), which also included center as shared frailty (15). The following additional multivariable models were built as secondary analyses: (i) one also including patients with positive serum GM and with serum GM included as an independent variable in the model and (ii) one with 28-day mortality as the dependent variable.
Data availability.
After deidentification, data could be made available to researchers providing a methodologically sound research proposal in the 5 years after publication.
RESULTS
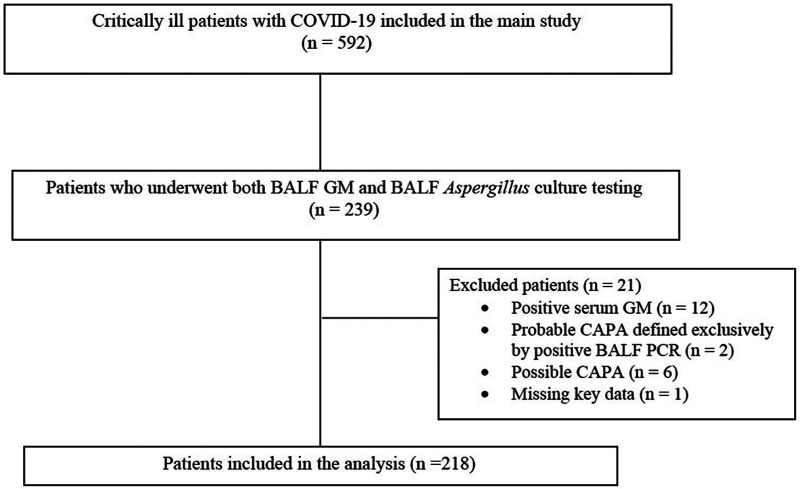
Of the 592 patients enrolled in the main study, 239 underwent both BALF GM and BALF Aspergillus culture testing. Overall, 218 patients were eventually included in the present post hoc analysis (Fig. 1). The clinical characteristics of the study population are summarized in Table 1. Median age was 65 years (interquartile range [IQR], 57 to 73) and 64/218 were females (29%). CAPA was diagnosed in 56/218 patients (26%), at a median time of 7 days after ICU admission (IQR, 3 to 11). Most cases were probable (51/56 [91%]) and few were proven (5/56 [9%]). Among patients with CAPA, 23/56 (41%) had positive BALF GM and negative BALF Aspergillus culture, 5/56 (9%) had negative BALF GM and positive BALF Aspergillus culture, and 28/56 (50%) had both positive BALF GM and positive BALF Aspergillus culture. The median BALF GM optical density index in patients with CAPA was 2.8 (IQR, 2.5 to 5.8; quantitative information available for 38/56 patients [68%]). BALF PCR was performed for 19/56 (34%) patients with CAPA (18/19 were positive) and in 43/162 (27%) patients without CAPA (all were negative). Among patients without CAPA, 4/162 (2%) had a positive BALF GM (no quantitative results available), and none had a positive BALF Aspergillus culture.
FIG 1.
Flowchart of the patient inclusion process. BALF, bronchoalveolar lavage fluid; CAPA, COVID-19-associated pulmonary aspergillosis; COVID-19, coronavirus disease 2019; GM, galactomannan; PCR, polymerase chain reaction.
TABLE 1.
Demographic and clinical characteristics of critically ill patients with COVID-19 who underwent BALF culture and BALF GM testinga
| Variable | No. of patientsb | % |
|---|---|---|
| Demographic variables | ||
| Age in yrs, median (IQR) | 65 (57–73) | |
| Female sex | 64/218 | 29 |
| Medical history | ||
| No. of coexisting conditions, median (IQR) | 1 (0–2) | |
| Obesity | 44/218 | 20 |
| Active malignant disease | 19/218 | 9 |
| Solid-organ transplantation | 9/218 | 4 |
| Cardiovascular disease | 113/218 | 52 |
| Structural lung disease | 39/218 | 18 |
| Diabetes mellitus | 49/218 | 22 |
| History of smoking | 20/218 | 9 |
| ECMO | 13/218 | 6 |
| Invasive mechanical ventilation | 156/218 | 72 |
| Noninvasive ventilation | 86/218 | 39 |
| Results of BALF mycological tests | ||
| Negative BALF GM and negative BALF culture | 158/218 | 72 |
| Positive BALF GM and negative BALF culture | 27/218 | 12 |
| Negative BALF GM and positive BALF culture | 5/218 | 2 |
| Positive BALF GM and positive BALF culture | 28/218 | 13 |
BALF, bronchoalveolar lavage fluid; CAPA, COVD-19-associated pulmonary aspergillosis; ECMO, extracorporeal membrane oxygenation; GM, galactomannan; IQR, interquartile range.
Results are presented as no. of patients/total unless otherwise indicated.
Crude 90-day mortality rates were 54% (30/56) and 52% (85/162) in patients with CAPA and patients without CAPA, respectively. According to BALF results, crude 90-day mortality rates were 84/158 (53%) in patients with both tests negative, 11/27 (41%) in patients with positive GM and negative culture, 3/5 (60%) in patients with negative GM and positive culture, and 17/28 (61%) in patients with both tests positive. The results of the univariable and multivariable analyses of factors associated with 90-day mortality are presented in Tables 2 and 3, respectively. In univariable analysis, increasing age, presence of an active malignant disease, and presence of cardiovascular disease were associated with 90-day mortality. In addition, an association with 90-day mortality in univariable models was observed for both positive BALF GM and positive BALF Aspergillus culture compared with both tests negative as the reference category. In multivariable analysis (model A), increasing age (hazard ratio [HR], 1.23 per 5-year increase; 95% confidence interval [CI], 1.12 to 1.35; P < 0.001) and presence of an active malignant disease (HR, 1.98; 95% CI, 1.12 to 3.51; P = 0.019) retained an independent association with 90-day mortality. In addition, when center was included in the multivariable model as a random effect (model B), an independent association with 90-day mortality was also retained for both positive BALF GM and positive BALF Aspergillus culture in comparison with both tests negative (HR, 2.53; 95% CI, 1.28 to 5.02; P = 0.008). In a subgroup analysis in patients with positive BALF GM and available quantitative GM value (38/55 [69%]), no association was found between quantitative BALF GM and 90-day mortality (HR, 1.00 per one-point increase; 95% CI, 0.99 to 1.02; P = 0.382). Results of additional models including serum GM and for predictors of 28-day mortality are available in the supplemental material.
TABLE 2.
Univariable analysis of factors associated with 90-day mortality
| Variable | Hazard ratio | 95% CI | P a |
|---|---|---|---|
| Age (per 5 yrs) | 1.23 | 1.12–1.35 | <0.001* |
| Female sex | 1.32 | 0.90–1.95 | 0.16 |
| No. of coexisting conditions | 1.17 | 1.01–1.37 | 0.046* |
| Obesity | 0.84 | 0.52–1.35 | 0.47 |
| Active malignant disease | 1.76 | 1.01–3.09 | 0.048* |
| Solid-organ transplantation | 1.60 | 0.78–3.28 | 0.20 |
| Cardiovascular disease | 1.48 | 1.02–2.15 | 0.039* |
| Structural lung disease | 1.23 | 0.78–1.94 | 0.38 |
| Diabetes mellitus | 1.12 | 0.73–1.71 | 0.60 |
| History of smoking | 0.75 | 0.38–1.49 | 0.41 |
| ECMO | 1.04 | 0.51–2.14 | 0.91 |
| Invasive mechanical ventilation | 0.68 | 0.46–1.02 | 0.062 |
| Noninvasive ventilation | 0.75 | 0.51–1.10 | 0.14 |
| Results of BALF mycological tests | 0.28 | ||
| Negative BALF GM and negative BALF culture | Reference | ||
| Positive BALF GM and negative BALF culture | 1.05 | 0.54–2.04 | 0.87 |
| Negative BALF GM and positive BALF culture | 1.38 | 0.43–4.39 | 0.59 |
| Positive BALF GM and positive BALF culture | 1.72 | 1.02–2.92 | 0.043* |
*, P < 0.05.
TABLE 3.
Multivariable analysis of factors associated with 90-day mortality
| Model and factor | Hazard ratio (95% CI) | P a |
|---|---|---|
| Model A | ||
| Age (per 5 yrs) | 1.23 (1.12–1.35) | <0.001* |
| Active malignant disease | 1.98 (1.12–3.51) | 0.019* |
| Results of BALF mycological tests | 0.62 | |
| Negative BALF GM and negative BALF culture | Reference | |
| Positive BALF GM and negative BALF culture | 0.90 (0.46–1.76) | 0.77 |
| Negative BALF GM and positive BALF culture | 1.30 (0.41–4.14) | 0.66 |
| Positive BALF GM and positive BALF culture | 1.39 (0.82–2.37) | 0.22 |
| Model Bb | ||
| Age (per 5 yrs) | 1.27 (1.14–1.40) | <0.001* |
| Active malignant disease | 2.02 (1.11–3.68) | 0.021* |
| Results of BALF mycological tests | 0.11 | |
| Negative BALF GM and negative BALF culture | Reference | |
| Positive BALF GM and negative BALF culture | 1.30 (0.62–2.70) | 0.49 |
| Negative BALF GM and positive BALF culture | 1.53 (0.42–5.54) | 0.52 |
| Positive BALF GM and positive BALF culture | 2.53 (1.28–5.02) | 0.008* |
*, P < 0.05.
Model B included center as shared frailty.
DISCUSSION
In this post hoc analysis of a large multinational study, we observed that the prognosis of critically ill patients with COVID-19 may be different according to the results of BALF GM and BALF Aspergillus culture, being more unfavorable in patients with both tests positive.
Recently, Ergün and colleagues reported an increased mortality in CAPA patients with positive serum GM compared with patients without CAPA, which is consistent with angioinvasion as a marker of increased disease severity (11). On the other hand, the authors did not find an association between BALF GM levels and mortality (although they acknowledged the low power of the analysis), which is in contrast with the unfavorable association previously observed by Bartoletti and colleagues (4, 11). Trying to enrich our knowledge on this topic (which touches on the core diagnostic aspect of differentiating Aspergillus colonization versus infection), we selected a subset of patients without relevant confounding factors (i.e., concomitantly positive serum GM) to assess the independent prognostic impact of different combination of BALF GM and BALF Aspergillus culture results. Overall, we think our results provide novel information, at the same time raising some intriguing questions to be further explored. The first relevant point is that an independent association of the combination of both positive mycological BALF tests with mortality was observed only in the multivariable model adjusted for center heterogeneity and not in the multivariable model with only fixed effects. While we were ultimately unable to find a between-center critical difference in the therapeutic management that could explain these findings, we cannot exclude that some unexplored local factors (e.g., type of local BALF collection procedures and/or differences in strategies for diagnostic testing across centers) may have exerted a significant modifying effect on the prognostic ability of BALF tests. This hypothesis could have clinically significant implication on the real-life local diagnostic value of such tests and deserves further investigation. The second point is that while one-test-only positive (either BALF GM or BALF culture) results did not show a statistically significant association with mortality, the direction of the effect was still toward increased mortality in the center-adjusted multivariable model. In our opinion, this gradient in the size of the unfavorable effect raises the following nonmutually exclusive hypotheses: (i) the positivity of both BALF GM and BALF culture may reflect a greater disease burden, which is in line with a higher probability of unfavorable prognosis; (ii) at the same time, the positivity of both BALF GM and BALF culture may reflect a lower probability of false-positive results/colonization, again resulting in a more evident unfavorable prognostic effect. From a practical standpoint, the consistent direction of the effect toward increased mortality may pragmatically support the clinical usefulness of the 2020 ECMM/ISHAM consensus for the diagnosis of probable CAPA, since the risk of losing true cases by deeming one test-only positivity as colonization/false positivity could be nonnegligible and may theoretically lead to delays in antifungal treatment. In the future, a better definition of the additional prognostic value of other BALF tests (e.g., PCR) may help fine-tune our ability to distinguish colonization and false positivity from true infection. Unfortunately, BALF PCR was performed only for a minority of patients included in this post hoc analysis, thereby precluding a reliable assessment of its possible prognostic potential (and, indirectly, its contribution to diagnostic specificity).
The present study has some other important drawbacks. For example, an important limitation of the present study is that it was a post hoc analysis of a secondary survival analysis of a multinational study primarily aimed to assess predictors of CAPA development and not prognostic predictors. For this reason, the study was not designed to adequately assess the possible prognostic impact of either the treatment of CAPA or the immunosuppression connected to COVID-19 and its treatment, which were eventually not included in the prognostic models. Although possibly less likely connected to the prognostic impact of CAPA than immunosuppression and antifungal therapy, it is worth noting that other potential prognostic predictors, such as biomarkers of inflammation, lymphopenia, thromboembolic complications, and concomitant documented bacterial infection, were unavailable for the present post hoc analysis. Another important limitation is that our selected subgroup may be not representative of the initial population of 592 critically ill patients with COVID-19. This selection was necessary since the independent prognostic effect of BALF GM or BALF Aspergillus culture could not be assessed without knowing the results of both tests. However, it should be noted that our study population may reflect those patients in which physicians requested both tests due to clinical suspicion of CAPA, rather than for other reasons (e.g., surveillance cultures); thus, this selection may not necessarily represent a disadvantage and may more properly reflect the clinical population of interest, although a more standardized prospective collection of samples remains necessary to ultimately confirm this hypothesis. Regarding other possible limitations, it should be acknowledged that we had limited information to reliably explore the possible prognostic impact of quantitative BALF GM in patients with test positivity (information available only for 38 cases); therefore, the lack of association found in our study should be extrapolated with due caution. Pending further study, caution should also be adopted with respect to the results of the additional prognostic model for 28-day mortality, due to the reduced number of events precluding adequate adjustment and generalization. Finally, we did not collect information on serum beta-d-glucan values, which have also been suggested to be associated with increased mortality in patients with CAPA (11, 16).
In conclusion, when between-center heterogeneity was considered, the presence of both positive BALF GM and positive BALF Aspergillus culture was associated with increased 90-day mortality in critically ill patients with COVID-19. Most patients with at least one of the two tests positive had probable CAPA according to ECMM/ISHAM consensus criteria. Additional study is needed to explore the possible prognostic value of other BALF markers, such as Aspergillus PCR, Aspergillus lateral flow device, or Aspergillus galactomannan lateral flow assay.
ACKNOWLEDGMENTS
R.R.-R. was supported by the NIHR Manchester Biomedical Research Centre. P.K. is supported by the German Federal Ministry of Research and Education and the State of North Rhine-Westphalia, Germany, and has received nonfinancial scientific grants from the Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases, University of Cologne, Cologne, Germany. M.H. is supported by the NIH (UL1TR001442) and investigator-initiated grants from Astellas, Gilead, and Pfizer. S.F. is funded by a Research Foundation Flanders (FWO) Ph.D. fellowship (11M6922N).
Outside the submitted work, D.R.G. reports an unconditional grant from Correvio Italia, and investigator-initiated grants from Pfizer Inc. and Gilead Italia. J.P. has received personal fees from Gilead Sciences and Pfizer and research funding from MSD outside the submitted work and is a stakeholder of AbbVie and Novo Nordisk. J.W. reports grants and personal fees from Gilead and Pfizer: investigator-initiated grants, personal fees and also non-financial support from MSD, outside the submitted work. J.M. reports grants, personal fees and nonfinancial support from MSD, grants, personal fees, and nonfinancial support from Pfizer Inc., grants, personal fees and nonfinancial support from Gilead Sciences, personal fees and nonfinancial support from Astellas Pharma, personal fees and nonfinancial support from Cidara, personal fees and nonfinancial support from F2G, personal fees and nonfinancial support from Mundipharma, and personal fees and nonfinancial support from Takeda/Shire, outside the submitted work. O.A.C. reports grants or contracts from Amplyx, Basilea, BMBF, Cidara, DZIF, EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer, and Scynexis, Consulting fees from Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, PSI, Scynexis, and Seres, honoraria for lectures from Abbott, Al-Jazeera Pharmaceuticals, Astellas, Grupo Biotoscana/United Medical/Knight, Hikma, MedScape, MedUpdate, Merck/MSD, Mylan, and Pfizer; payment for expert testimony from Cidara, participation on a data safety monitoring board or advisory board from Actelion, Allecra, Cidara, Entasis, IQVIA, Jannsen, MedPace, Paratek, PSI, and Shionogi; a pending patent currently reviewed at the German Patent and Trade Mark Office, and other interests from DGHO, DGI, ECMM, ISHAM, MSG-ERC, and Wiley, outside the submitted work. J.S.G. has received lecture honoraria from Gilead and Pfizer, outside the submitted work. M. Bassetti has received funding for scientific advisory boards and travel and speaker honoraria from Angelini, Astellas, Bayer, bioMérieux, Cidara, Cipla, Gilead, Menarini, MSD, Pfizer, and Shionogi. R.R.-R. has received speaker honoraria from Astellas Pharma, Gilead Sciences, and Pfizer and research funding from Associates of Cape Cod. P.K. reports grants or contracts from the German Federal Ministry of Research and Education and the State of North Rhine-Westphalia, consulting fees from Ambu GmbH, Gilead Sciences, Noxxon N.V., and Pfizer Pharma, honoraria for lectures from Akademie für Infektionsmedizin e.V., Ambu GmbH, Astellas Pharma, Bio-Rad Laboratories Inc., European Confederation of Medical Mycology, Gilead Sciences, GPR Academy Ruesselsheim, medupdate GmbH, MedMedia, Merck Sharp & Dohme GmbH, Pfizer Pharma GmbH, Scilink Comunicación Científica SC, and University Hospital and LMU Munich, participation on an advisory board from Ambu GmbH, Gilead Sciences, and Pfizer Pharma, a pending patent currently reviewed at the German Patent and Trade Mark Office, and other nonfinancial interests from Elsevier, Wiley, and Taylor & Francis online, outside the submitted work. K.L. received consultancy fees from SMB Laboratoires Brussels, MSD, and Gilead, travel support from Pfizer, speaker fees from FUJIFILM WAKO, Pfizer, and Gilead, and a service fee from Thermo Fisher Scientific. M.H. received research funding from Gilead Sciences, Astellas, Scynexis, F2G, MSD, and Pfizer, all outside the submitted work. All other authors declare no conflict of interest for this study.
D.R.G., J.P., J.W., M. Bassetti, J.M., R.R.-R., P.K., O.A.C., K.L., and M.H. made substantial contribution to study concept and design. J.P., J.W., D.R.G., J.S.-G., Marc Bourgeois, M.R., L.R., N.V.R., P.L., S.F., S.D., and M.H. made substantial contribution to the acquisition of data for the work. A.S., D.R.G., J.P., J.W., S.H., K.L., N.K., O.S., R.R.-R., and M.H. made substantial contribution to the statistical analysis or interpretation of data. D.R.G., J.P., M. Bassetti, and M.H. drafted the manuscript. All authors critically reviewed the manuscript and gave final approval for publication.
ECMM-CAPA Study Group contributors include Yves Debaveye (Surgical Intensive Care Unit, University Hospital Leuven, Belgium), Marisa H. Miceli (University of Michigan Hospitals, Ann Arbor, MI), Jean-Jacques Tudesq (Medical Intensive Care Unit, Saint-Louis Teaching Hospital, AP-HP, Université de Paris, Paris, France), Gregor Paul (Klinik für Gastroenterologie, Pneumologie und Infektiologie, Katharinenhospital Stuttgart, Zentrum Innere Medizin, Klinikum Stuttgart, Stuttgart, Germany), Robert Krause (Medical University of Graz, Graz, Austria), Marina Linhofer (Medical University of Graz, Graz, Austria), Jonas Frost (Medical University of Graz, Graz, Austria), Peter Zechner (LKH Graz II Standort West, Graz, Austria), Matthias Kochanek (University of Cologne, Medical Faculty and University Hospital Cologne, Department I of Internal Medicine, Excellence Centre for Medical Mycology [ECMM], Cologne, Germany), Philipp Eller (Medical University of Graz, Graz, Austria), Jeffrey D. Jenks (University of California San Diego, San Diego, CA), Sara Volpi (Infectious Disease Department of the University of Modena, Modena, Italy), Anne-Pauline Bellanger (Laboratoire de Parasitologie-Mycologie Pole Biologie Anatomie Pathologique CHRU Jean Minjoz, Besançon, France), P. Lewis White (Public Health Wales Microbiology Cardiff, University Hospital of Wales, Cardiff, UK), Gustavo H. Goldman (Faculdade de Ciencias Farmaceuticas de Ribeirao Preto, Universidade de Sao Paulo, Brazil), Paul Bowyer (The University of Manchester, Manchester, UK), Antonis Rokas (Department of Biological Sciences, Vanderbilt University, Nashville, TN), Sara Gago (The University of Manchester, Manchester, UK), Paolo Pelosi (Department of Surgical Sciences and Integrated Diagnostics, University of Genoa, Genoa, Italy), Chiara Robba (Department of Surgical Sciences and Integrated Diagnostics, University of Genoa, Genoa, Italy), Jean-Pierre Gangneux (Mycology-Parasitology Laboratory, Rennes Teaching Hospital, Rennes, France), Cornelia Lass-Flörl (Innsbruck Medical University, Austria), Marina Machado and Patricia Munoz (Clinical Microbiology and Infectious Diseases Department, Hospital General Universitario Gregorio Maranon, Madrid, Spain), Alexander Christian Reisinger (Medical University of Graz, Department of Internal Medicine, Intensive Care Unit, Graz, Austria), Stefan Hatzl (Medical University of Graz, Department of Internal Medicine, Intensive Care Unit, Graz, Austria), Tobias Lahmer (Klinik und Poliklinik für Innere Medizin II, Klinikum rechts der Isar der Technischen Universitat München, Munich, Germany), Maricela Valerio (Clinical Microbiology and Infectious Diseases Department, Hospital General Universitario Gregorio Maranon, Instituto de Investigación Sanitaria Gregorio Maranon, Madrid, Spain), Laurence Delhaes (Centre Hospitalier Universitaire de Bordeaux, ISERM U1045, Bordeaux, France), Kauser Jabeen (Aga Khan University, Karachi, Pakistan), Joerg Steinmann (Institute of Clinical Hygiene, Medical Microbiology and Infectiology, Paracelsus Medical University, Klinikum Nürnberg, Nuremberg, Germany), Mathilde Chamula (Manchester University NHS Foundation Trust, Wythenshawe Hospital and Division of Infection, Immunity and Respiratory Medicine, Faculty of Biology, Medicine and Health, University of Manchester, UK).
Footnotes
Supplemental material is available online only.
Contributor Information
Daniele Roberto Giacobbe, Email: danieleroberto.giacobbe@unige.it.
Kimberly E. Hanson, University of Utah
REFERENCES
- 1.Permpalung N, Chiang TP, Massie AB, Zhang SX, Avery RK, Nematollahi S, Ostrander D, Segev DL, Marr KA. 9 March 2021. COVID-19 associated pulmonary aspergillosis in mechanically ventilated patients. Clin Infect Dis 74:83–91. doi: 10.1093/cid/ciab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prattes J, Wauters J, Giacobbe DR, Salmanton-Garcia J, Maertens J, Bourgeois M, Reynders M, Rutsaert L, Van Regenmortel N, Lormans P, Feys S, Reisinger AC, Cornely OA, Lahmer T, Valerio M, Delhaes L, Jabeen K, Steinmann J, Chamula M, Bassetti M, Hatzl S, Rautemaa-Richardson R, Koehler P, Lagrou K, Hoenigl M, the ECMM-CAPA Study Group. 25 August 2021. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients—a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect. doi: 10.1016/j.cmi.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salmanton-Garcia J, Sprute R, Stemler J, Bartoletti M, Dupont D, Valerio M, Garcia-Vidal C, Falces-Romero I, Machado M, de la Villa S, Schroeder M, Hoyo I, Hanses F, Ferreira-Paim K, Giacobbe DR, Meis JF, Gangneux JP, Rodriguez-Guardado A, Antinori S, Sal E, Malaj X, Seidel D, Cornely OA, Koehler P, FungiScope European Confederation of Medical Mycology/The International Society for Human and Animal Mycology Working Group. 2021. COVID-19-associated pulmonary aspergillosis, March–August 2020. Emerg Infect Dis 27:1077–1086. doi: 10.3201/eid2704.204895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, Fornaro G, Tonetti T, Pizzilli G, Francalanci E, Giuntoli L, Rubin A, Moroni A, Ambretti S, Trapani F, Vatamanu O, Ranieri VM, Castelli A, Baiocchi M, Lewis R, Giannella M, Viale P, PREDICO Study Group. 2021. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis 73:e3606–e3614. doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borman AM, Palmer MD, Fraser M, Patterson Z, Mann C, Oliver D, Linton CJ, Gough M, Brown P, Dzietczyk A, Hedley M, McLachlan S, King J, Johnson EM. 2020. COVID-19-associated invasive aspergillosis: data from the UK National Mycology Reference Laboratory. J Clin Microbiol 59:e02136-20. doi: 10.1128/JCM.02136-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahmer T, Kriescher S, Herner A, Rothe K, Spinner CD, Schneider J, Mayer U, Neuenhahn M, Hoffmann D, Geisler F, Heim M, Schneider G, Schmid RM, Huber W, Rasch S. 2021. Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: results from the prospective AspCOVID-19 study. PLoS One 16:e0238825. doi: 10.1371/journal.pone.0238825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prattes J, Koehler P, Hoenigl M, Wauters J, Giacobbe DR, Lagrou K, Salmanton-García J, Rautemaa-Richardson R, Hatzl S, Maertens J, Debaveye Y, Bourgeois M, Reynders M, Rutsaert L, Van Regenmortel N, Lormans P, Feys S, Reisinger AC, Cornely OA, Lahmer T, Valerio M, Delhaes L, Jabeen K, Steinmann J, Chamula M, Bassetti M, the ECMM-CAPA Study Group. 2021. COVID-19 associated pulmonary aspergillosis: regional variation in incidence and diagnostic challenges. Intensive Care Med 47:1339–1340. doi: 10.1007/s00134-021-06510-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenks JD, Nam HH, Hoenigl M. 2021. Invasive aspergillosis in critically ill patients: review of definitions and diagnostic approaches. Mycoses 64:1002–1014. doi: 10.1111/myc.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, Klimko N, Lass-Florl C, Oladele RO, Vinh DC, Zhu LP, Boll B, Bruggemann R, Gangneux JP, Perfect JR, Patterson TF, Persigehl T, Meis JF, Ostrosky-Zeichner L, White PL, Verweij PE, Cornely OA, European Confederation of Medical Mycology, the International Society for Human and Animal Mycology, the Asia Fungal Working Group, the INFOCUS LATAM/ISHAM Working Group, the ISHAM Pan Africa Mycology Working Group, the European Society for Clinical Microbiology and Infectious Diseases Fungal Infection Study Group, the ESCMID Study Group for Infections in Critically Ill Patients, the Interregional Association of Clinical Microbiology and Antimicrobial Chemotherapy, the Medical Mycology Society of Nigeria, the Medical Mycology Society of China Medicine Education Association, Infectious Diseases Working Party of the German Society for Haematology and Medical Oncology, Association of Medical Microbiology and Infectious Disease Canada. 2021. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis 21:e149–e162. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prattes J, Wauters J, Giacobbe DR, Lagrou K, Hoenigl M, ECMM-CAPA Study Group. 2021. Diagnosis and treatment of COVID-19 associated pulmonary apergillosis in critically ill patients: results from a European confederation of medical mycology registry. Intensive Care Med 47:1158–1160. doi: 10.1007/s00134-021-06471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ergün M, Bruggemann RJM, Alanio A, Delliere S, van Arkel A, Bentvelsen RG, Rijpstra T, van der Sar-van der Brugge S, Lagrou K, Janssen NAF, Buil JB, van Dijk K, Melchers WJG, Reijers MHE, Schouten JA, Wauters J, Cordey A, Soni S, White PL, van de Veerdonk FL, Verweij PE. 2021. Aspergillus test profiles and mortality in critically ill COVID-19 patients. J Clin Microbiol 59:e01229-21. doi: 10.1128/JCM.01229-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassetti M, Giacobbe DR, Grecchi C, Rebuffi C, Zuccaro V, Scudeller L, FUNDICU investigators. 2020. Performance of existing definitions and tests for the diagnosis of invasive aspergillosis in critically ill, adult patients: a systematic review with qualitative evidence synthesis. J Infect 81:131–146. doi: 10.1016/j.jinf.2020.03.065. [DOI] [PubMed] [Google Scholar]
- 13.Rouze A, Lemaitre E, Nseir S. 2021. COVID-19-associated invasive pulmonary aspergillosis: high incidence or difficult diagnosis? Intensive Care Med 47:1337–1338. doi: 10.1007/s00134-021-06499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seidel D, Duran Graeff LA, Vehreschild M, Wisplinghoff H, Ziegler M, Vehreschild JJ, Liss B, Hamprecht A, Kohler P, Racil Z, Klimko N, Sheppard DC, Herbrecht R, Chowdhary A, Cornely OA, FungiScope G. 2017. FungiScope—global emerging fungal infection registry. Mycoses 60:508–516. doi: 10.1111/myc.12631. [DOI] [PubMed] [Google Scholar]
- 15.Balan TA, Putter H. 2020. A tutorial on frailty models. Stat Methods Med Res 29:3424–3454. doi: 10.1177/0962280220921889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Haese J, Theunissen K, Vermeulen E, Schoemans H, De Vlieger G, Lammertijn L, Meersseman P, Meersseman W, Lagrou K, Maertens J. 2012. Detection of galactomannan in bronchoalveolar lavage fluid samples of patients at risk for invasive pulmonary aspergillosis: analytical and clinical validity. J Clin Microbiol 50:1258–1263. doi: 10.1128/JCM.06423-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2. Download jcm.02298-21-s0001.pdf, PDF file, 0.05 MB (49.4KB, pdf)
Data Availability Statement
After deidentification, data could be made available to researchers providing a methodologically sound research proposal in the 5 years after publication.