Abstract
Study Objective
This study investigated race and sex differences in tacrolimus pharmacokinetics and pharmacodynamics in stable kidney transplant recipients.
Design and Setting
A cross‐sectional, open‐label, single center, 12‐h pharmacokinetic‐pharmacodynamic study was conducted. Tacrolimus pharmacokinetic parameters included area under the concentration‐time curve (AUC0–12), AUC0–4, 12‐h troughs (C 12 h), maximum concentrations (C max), oral clearance (Cl), with dose‐normalized AUC0–12, troughs, and C max with standardized adverse effect scores. Statistical models were used to analyze end points with individual covariate‐adjustment including clinical factors, genotypic variants CYP3A5*3, CYP3A5*6, CYP3A5*7(CYP3A5*3*6*7) metabolic composite, and ATP binding cassette gene subfamily B member 1 (ABCB1) polymorphisms.
Patients
65 stable, female and male, Black and White kidney transplant recipients receiving tacrolimus and mycophenolic acid ≥6 months post‐transplant were evaluated.
Measurements and Main Results
Black recipients exhibited higher tacrolimus AUC0–12 (Race: p = 0.005), lower AUC* (Race: p < 0.001; Race × Sex: p = 0.068), and higher Cl (Race: p < 0.001; Sex: p = 0.066). Greater cumulative (Sex: p < 0.001; Race × Sex: p = 0.014), neurologic (Sex: p = 0.021; Race × Sex: p = 0.005), and aesthetic (Sex: p = 0.002) adverse effects were found in females, with highest scores in Black women. In 84.8% of Black and 68.8% of White patients, the target AUC0–12 was achieved (p = 0.027). In 31.3% of White and 9.1% of Black recipients, AUC0–12 was <100 ng‧h/ml despite tacrolimus troughs in the target range (p = 0.027). The novel CYP3A5*3*6*7 metabolic composite was the significant covariate accounting for 15%–19% of tacrolimus variability in dose (p = 0.002); AUC0–12 h* (p < 0.001), and Cl (p < 0.001).
Conclusions
Tacrolimus pharmacokinetics and adverse effects were different among stable kidney transplant recipient groups based upon race and sex with interpatient variability associated with the CYP3A5*3*6*7 metabolic composite. More cumulative, neurologic, and aesthetic adverse effects were noted among females. Tacrolimus regimens that consider race and sex may reduce adverse effects and enhance allograft outcomes by facilitating more patients to achieve the targeted AUC0–12 h.
Keywords: immunosuppression, race, renal transplantation, sex, tacrolimus pharmacokinetics
1. INTRODUCTION
Tacrolimus and mycophenolic acid (MPA) provide an efficacious immunosuppressive regimen to prevent kidney allograft rejection. 1 Tacrolimus exhibits inter‐ and intrapatient pharmacokinetic variability reflecting variation in cytochrome P‐450 3A5 (CYP3A5) isoenzymes and P‐glycoprotein (P‐gp). 2 , 3 , 4 , 5 Trough concentration monitoring is used to guide dosing of tacrolimus. 5 , 6 , 7 , 8 , 9 The trough tacrolimus concentration and effect relationships for pharmacotherapeutic responses and adverse effects have not been clearly defined in sex or racial sub‐populations. 5 , 10 , 11
Past calcineurin inhibitor protocols recommend lower target tacrolimus troughs to reduce adverse effects and maintain long‐term renal allograft function. 9 , 10 , 11 Limited pharmacokinetic data exist to confirm drug exposure over a dosing interval to achieve the target of 100–190 ng∙h/ml in sub‐populations ≥1‐year post‐transplant. 7 , 8 , 9 , 12 , 13 , 14 Recent studies indicate increased donor‐specific antibodies with allograft failure when tacrolimus troughs are below the target. 13 , 14
Chronic renal allograft survival in Black recipients is shorter compared with other races treated with comparable immunosuppression. 1 , 15 , 16 , 17 , 18 , 19 , 20 , 21 Factors contributing to this disparity include medication adherence, genomics, socioeconomics, pharmacokinetic and pharmacodynamic variability, donor‐recipient mismatches, and racial variation in immunodynamic responses. 15 , 16 , 17 , 18 , 19 , 20 , 21 To achieve similar allograft outcomes, Black patients require higher tacrolimus doses compared with Whites. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 Tacrolimus bioavailability is reduced in healthy Blacks compared with other races. 22 , 23 Limited data are available comparing tacrolimus pharmacokinetics and adverse effects between stable White and Black kidney transplant recipients receiving lower maintenance dosing. 23 , 24 , 25
Sex is another factor that influences CYP3A4/5 isoenzyme and P‐gp activity and may impact tacrolimus dosing. 26 , 27 , 28 , 29 The importance of investigating female‐focused diseases, biomarker responses, and pharmacologic knowledge gaps, including combined sex and racial differences, has been reported. 30 , 31 , 32 Studies that combine race with sex influences on tacrolimus pharmacokinetics and adverse effects are lacking. 24 , 25 , 30 , 31 , 32 , 33
Studies of calcineurin inhibitors have reported on CYP3A5 and P‐gp pharmacogenomics. 2 , 3 , 4 , 7 , 20 , 34 , 35 , 36 The CYP3A5*1 (wild‐type) variant is more common in Blacks than the major variant CYP3A5*3 (rs776746), which has been reported to result in loss of function. 2 , 4 , 34 The CYP3A5*3 variant contributes to interpatient variability in tacrolimus pharmacokinetics reported as area under the concentration versus time curve (AUC), dose‐normalized trough, and Cl. 20 , 37 , 38 , 39 , 40 , 41 Other CYP3A5 variants in Black subjects include CYP3A5*6 (rs10264272) and CYP3A5*7 (rs41303343), which are associated with loss of function and may contribute to pharmacokinetic differences among races. 39 , 42 Black kidney transplant recipients with CYP3A5*1 genotype need higher daily tacrolimus doses than White patients with the variant CYP3A5*3 or Blacks exhibiting variants CYP3A5*3, CYP3A5*6, and/or CYP3A5*7 in order to achieve similar concentrations. 39 , 42 To address this racial disparity, we previously reported the novel CYP3A5*3*6*7 metabolic composite that incorporates these loss‐of‐function variants with a tacrolimus population pharmacokinetic model. 43 Most pharmacokinetic‐pharmacogenomic studies between races have been limited to tacrolimus trough monitoring rather than intensive pharmacokinetic studies. 7 , 39 , 42 Although tacrolimus troughs are important monitoring tools, these parameters do not consistently predict AUC0–12 and contribute to interpatient pharmacokinetic variability. 5 , 6 The sex effect on tacrolimus pharmacokinetics is not well‐studied in spite of in vivo gender differences for CYP3A4/5 substrates and in vitro studies supporting greater expression in females. 26 , 44 , 45 The impact of common ABCB1 single nucleotide polymorphisms (SNPs) that encode for P‐glycoprotein: 1236C>T (rs1128503), 2677G>T/A (rs2032582), and 3435C>T (rs1045642) are associated with tacrolimus pharmacokinetics or pharmacodynamics (eg, acute rejection and nephrotoxicity). 2 , 7 , 34 , 35 Initial associations between extrarenal adverse effects, sex, and ABCB1 haplotypes have been reported. 46 , 47
We designed our study to address limitations and knowledge gaps by including an analysis of race and sex associations to intensive tacrolimus pharmacokinetics. The primary objective was to determine tacrolimus oral Cl among Black and White male and female kidney transplant recipients. Additional pharmacokinetic parameters included AUC 0–12 h (AUC0–12 h), AUC0–4 h, 12‐h troughs (C 12 h), maximum concentration (C max), and oral Cl, comparing stable Black and White kidney transplant recipients. Secondary objectives included (a) dose‐normalized AUC0–12 h, C 12 h, C max; (b) covariate analysis of common clinical factors and CYP3A5 and ABCB1 polymorphisms on tacrolimus pharmacokinetics; and (c) race and sex associations to extrarenal immunosuppressive adverse effects.
2. METHODS
2.1. Study population
Sixty‐five stable male and female Black and White kidney transplant recipients receiving tacrolimus (Prograf ®) [Astellas Pharma US] and mycophenolic acid as enteric‐coated mycophenolate sodium (ECMPS; (Myfortic® ) [Novartis] for ≥6 months participated in a 12‐h pharmacokinetics‐pharmacodynamic study (Figure 1). Clinical stability was determined by physical examination, comprehensive metabolic panel, and complete blood count. Prior to study, tacrolimus troughs were adjusted using therapeutic drug monitoring target range: 4–10 ng/ml. ECMPS was dose adjusted based upon clinical response. Medication adherence and ethnicity for two previous generations were verified. Estimated glomerular filtration rate (eGFR) was calculated using the four‐factor Modification of Diet in Renal Disease (MDRD) equation. 48
FIGURE 1.
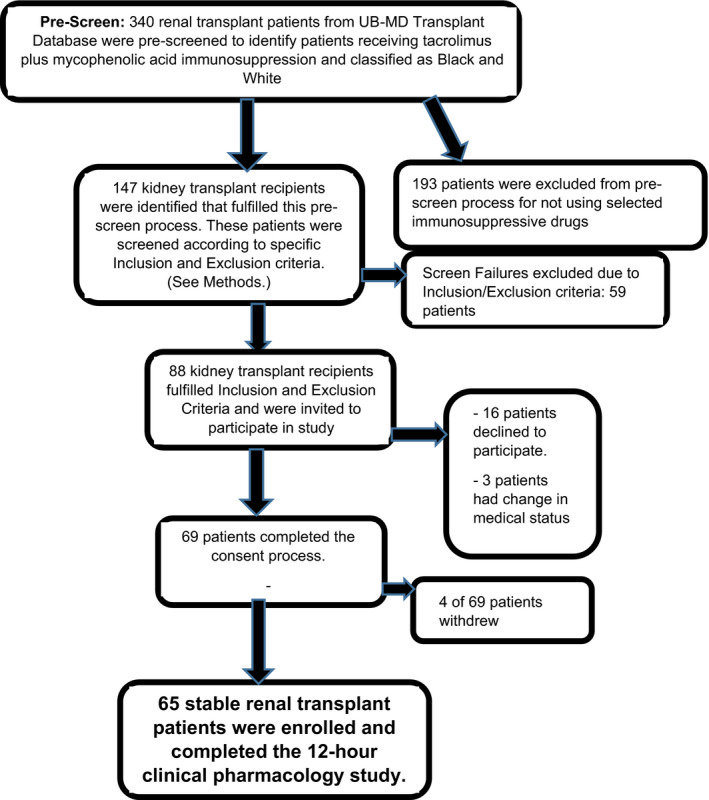
Study flow diagram for patient enrollment in cross‐sectional, open‐label single‐center clinical pharmacology study
Inclusion criteria were as follows: (1) ≥6 months post‐kidney transplant; (2) age 25–70 years; (3) first or second deceased‐donor or living allograft recipient; (4) receipt of the tacrolimus and MPA regimen for ≥3 months and on the same immunosuppressive doses for ≥7 days; (5) baseline eGFR >30 ml/min/1.73 m2 with no change greater than 20% from baseline eGFR during prior 2 clinic visits with confirmation by nephrologist for clinical stability; and (6) leukocyte count ≥3000/mm3 and hemoglobin ≥8.0 g/dl. Exclusion criteria were as follows: (1) infection or acute rejection within 2 weeks; (2) drugs interfering with tacrolimus or MPA absorption; (3) cytochrome P‐450 3A4/3A5 or P‐gp inhibitors or inducers within 4 weeks; and (5) significant medical or psychiatric diseases that would limit participation.
2.2. Study procedure
This was a cross‐sectional, single‐center, open‐label clinical pharmacology study in stable male and female Black and White kidney transplant recipients conducted at the University at Buffalo (UB) Renal Research Center at the Erie County Medical Center (ECMC). UB Health Sciences Institutional Review Board approved the study (IRB# PHP0599703‐4) which was conducted in accordance with the ethical standards for human subjects and the 1964 Helsinki Declaration. Upon enrollment, patients provided written consent.
Participants were at steady state for tacrolimus and ECMPS. Proton pump inhibiters, H2 antagonists, and antacids were discontinued for the prior 36 h. Immunosuppressives were taken at 5:30–6:30 PM prior to study, participants fasted and abstained from caffeine and alcohol for the prior 12 h. At 6:00 AM, patients were admitted, vital signs documented, and an intravenous angiocatheter inserted. A 0‐h sample (~15 ml) was collected prior to immunosuppressives for drug troughs and laboratory tests (ECMC Clinical Chemistry Laboratory). Oral study medications [(single lot of tacrolimus (Prograf ®) and ECMPS (Myfortic® )] were administered at 7:00 AM. Patients remained upright throughout the study. Standardized low‐fat meals were provided after 4 h. Antihypertensives were administered after 1.5 h and non‐immunosuppressives after 4 h. Blood samples (12 ml) were collected at 0 h and 1, 1.5, 2, 3, 4, 6, 8, 10, and 12 h after drug administration. Whole blood samples were aliquoted within 30 min and stored at −70°C.
2.3. Adverse effect assessment
Patients were evaluated using a validated immunosuppressive adverse effect (AE) rating system of 14 extrarenal AEs. 47 Nephrologists used physical examination, review of systems, laboratory results, and medication adherence assessment and assigned a ranked score of 0 (no AE), +1, +2, and +3 (severe AE). See Table S1. A ratio of the sum of AE scores to the maximum possible score was determined (AE ratio). 47 Individual AE was combined into four composite categories: gastrointestinal (GI) including vomiting, diarrhea, dyspepsia, and acid suppressive therapy; neurologic (headache, tremor, and insomnia); aesthetic (acne, skin changes, hirsutism, and gingival hyperplasia); and cumulative AE (sum of GI, neurologic, and aesthetic AEs).
2.4. Genetic analysis
Blood was collected pre‐dose in Cell Preparation Tubes (CPT® ‐BD Vacationer) for separation of peripheral blood mononuclear cells (PBMCs) at 25ºC. The PBMCs were harvested and transferred to cryovial aliquots, immediately frozen in liquid nitrogen, and stored at −70ºC.
Samples were all viable and analyzed in a genomics laboratory (University of New England's Genomics Analytical Core). The genotypes were determined using validated TaqMan allelic discrimination assays (Applied Biosystems) with a CFX96 Real‐Time Polymerase Chain Reaction Detection System (Bio‐Rad). Personnel were de‐identified to patient demographics and assayed in duplicate the ABCB1: 1236C>T (rs1128503), 2677G>T/A (rs2032582), 3435C>T (rs1045642) and CYP3A5*3 (rs776746), CYP3A5*6 (rs10264272), and CYP3A5*7 (rs41303343). All protocols and sample handling were in accordance with published guidelines. 49 Allele frequencies were confirmed in Hardy‐Weinberg equilibrium (HWE) when adjusted for race. A metabolic composite CYP3A5*3*6*7 was generated based upon the combined allelic status for each SNP and classified as extensive, intermediate, and poor metabolizers (Figure 2). 43
FIGURE 2.
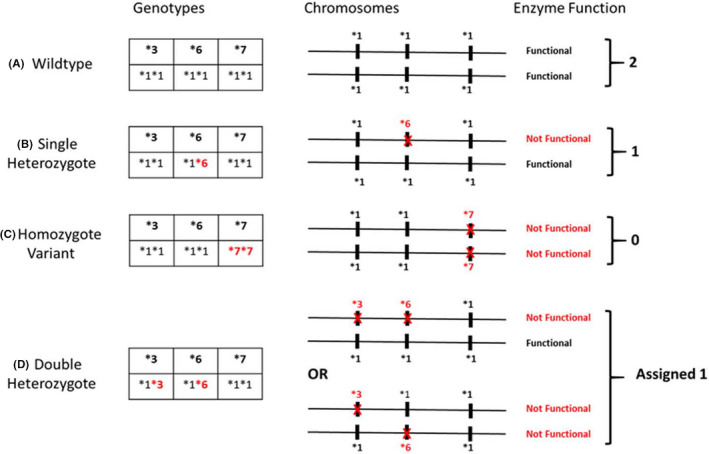
CYP3A5*3*6*7 metabolic composite scoring algorithm. Any of the three single nucleotide polymorphisms (SNPs), CYP3A5*3, CYP3A5*6, and CYP3A5*7 independently result in loss of protein gene expression from the carrying chromosome. Metabolic composite status for each patient based upon the combined allelic status from each chromosome is summarized in (A–D). (A) Depicts an extensive metabolizer with two completely functional genes. (C) Depicts one possible example of a poor metabolizer: individuals who carry a loss‐of‐function allele on both chromosomes. Similarly, (B and D) represent examples of possible intermediate metabolizers: individuals who are heterozygous at one or more loci. Note that for the double heterozygote case (D), the genotyping assay used could not differentiate between cis‐ and trans‐SNPs; therefore, the trans condition was conservatively assigned the intermediate metabolizer though such individuals would have no functional enzyme. 43 (Permission to reproduce this figure was granted by Journal of Clinical Pharmacology)
2.5. Assay methodology for tacrolimus
Tacrolimus concentrations were analyzed within 24 h using the ARCHITECT tacrolimus assay (Abbott), a chemiluminescent microparticle immunoassay. The lower limit of detection was 0.5 ng/ml, and intraday assay variability was <7%. The calibration curve ranged from 1 to 30 ng/ml and quality controls (QC) were 3.0, 12.0, and 25 ng/ml (Bio‐Rad). The interday coefficient of variation (CV) for each QC was <4%, and intraday CV was <5%. Random selected troughs and peaks (N = 40 samples) were analyzed using a validated liquid chromatography with tandem mass spectrometry (LCMSMS) assay that was conducted by a Clinical Laboratory Improvement Amendments (CLIA)‐certified external analytical laboratory and compared with the ARCHITECT tacrolimus assay with excellent agreement (R 2 = 0.98). For the LCMSMS assay, the interday and intraday CV were <5% at the low and high concentration QC.
2.6. Pharmacokinetic analysis
From intensive 12‐h serial sampling, tacrolimus pharmacokinetic parameters included AUC0–12 ng∙h/ml, AUC0–4 h, 12‐h trough (C 12 h; ng/ml), and peak concentration (C max; ng/ml). Oral clearance (Cl; L/h) was the ratio of dose to AUC0–12 h. AUC0–12 was determined by the linear trapezoidal rule using non‐compartmental methods (Phoenix WINNONLIN Version 6.3.; Pharsight Corp). Dose normalization of the C 12 h (ng/ml/mg), C max (ng/ml/mg), and AUC0–12 (AUC0–12; ng∙h/ml/mg) to 1‐mg dose equivalent was used to account for tacrolimus doses. The tacrolimus therapeutic exposure guide of 100–190 ng∙h/ml was used for comparisons. 7 , 8 , 12
2.7. Statistical analysis
Sample size was determined using a power of 80% to detect a difference of 30% in Cl for the main effect of race (ie, Black and White) or sex (ie, male vs female) with a coefficient of variation of 30% at the significance level of 0.05. This required at least 13 patients per race and sex group to address the primary objective.
Descriptive statistics were computed for all categorical and numeric variables and summarized using the mean and standard deviation. Main effects of race, sex, and the interaction are assessed by f tests, while pairwise comparisons are made using Tukey adjusted tests about the appropriate contrasts of model estimates. All model assumptions are verified graphically and transformation (ie, Box‐Cox) applied as appropriate. Categorical measures were summarized by race‐sex groups using frequencies and relative frequencies. These measures were modeled as a function of race, sex, and their interaction using logistic regression models. Main effects of race, sex, and the interaction are assessed by Wald tests with pairwise comparisons using Tukey adjusted tests for the appropriate contrasts of model estimates. 50 , 51
Multivariable linear regression models were considered for tacrolimus pharmacokinetics and adverse effects scores, and modeled as a function of race, sex, their interaction, and each demographic, clinical, or genotypic covariate in a one‐at‐a‐time manner. The race and sex associations (adjusting for each covariate) were assessed as described above, and the partial R 2 was reported for each covariate. A sub‐analysis was completed using Wilcoxon rank sums or Fisher's exact tests to compare tacrolimus pharmacokinetics in recipients with tacrolimus AUC0–12 <100 ng‧h/ml compared to ≥100 ng‧h/ml.
The race and sex groups and distributions of CYP3A5*3*6*7 metabolic composites were examined using an extension of Fisher's exact test. 51 All tests were two‐sided with nominal significance level of 0.05 (version 9.3; SAS Institute).
3. RESULTS
3.1. Patients
Sixty‐five recipients (13 Black females, 16 White females, 20 Black males, and 16 White males) completed the study with no statistical differences in age or time post‐transplant. Demographics and clinical characteristics are summarized in Table 1. Albumin, liver function tests, and hematologic parameters were within normal range with no group differences. Hemoglobin was within the acceptable range for renal function with males exhibiting a higher range (Sex: p < 0.001). There were no differences for glucose, prednisone or statin use, total cholesterol, low‐density lipoprotein, or triglycerides. Females had a modest increase in high‐density lipoproteins (Sex: p < 0.001). Black recipients demonstrated 60% higher serum creatinine which was adjusted using the eGFR. MPA doses were not different among groups. No data were missing for the primary end points.
TABLE 1.
Demographics by race and sex groups (n = 65)
|
Parameter Mean (SD) |
Black females (n = 13) | Black males (n = 20) | White females (n = 16) | White males (n = 16) | p‐Values for the effect of | Significant pairwise comparisons (Tukey adjusted) | ||
|---|---|---|---|---|---|---|---|---|
| Race | Sex | Race × Sex interaction | ||||||
| Age (years) b | 47.9 (9.9) | 47.1 (12.5) | 50.4 (13.6) | 50.4 (10.3) | 0.350 | 0.902 | 0.780 | — |
| Time Post‐Transplant (years) a | 2.99 (2.20) | 2.59 (1.68) | 3.06 (2.89) | 3.59 (3.40) | 0.589 | 0.963 | 0.644 | — |
| Total body weight (kg) a | 87.9 (24.2) | 91.6 (18.8) | 73.1 (16.0) | 94.8 (17.6) | 0.244 | 0.007 | 0.064 |
BM vs. WF p = 0.021 WM vs. WF p = 0.008 |
| BMI (kg/m2) a | 32.5 (7.9) | 30.1 (6.1) | 28.1 (6.0) | 30.3 (4.2) | 0.250 | 0.861 | 0.124 | — |
| Albumin (g/dl) | 4.02 (0.33) | 4.24 (0.25) | 4.04 (0.28) | 4.17 (0.29) | 0.676 | 0.019 | 0.571 | — |
| Glucose (mg/dl) c | 121.7 (76.8) | 114.2 (62.3) | 92.8 (28.8) | 133.8 (95.2) | 0.380 | 0.058 | 0.278 | — |
| Total cholesterol (mg/dl) a | 161.6 (20.3) | 157.7 (59.3) | 163.8(34.3) | 144.4 (34.2) | 0.550 | 0.076 | 0.563 | — |
| Low‐density Lipoprotein (mg/dl) b | 82.1 (19.0) | 76.7 (30.9) | 86.9 (31.6) | 75.6 (30.9) | 0.887 | 0.183 | 0.757 | — |
| High‐density Lipoprotein (mg/dl) b | 56.9 (12.9) | 45.3 (12.8) | 54.6 (15.0) | 42.5 (13.4) | 0.403 | <0.001 | 0.903 |
WF vs. WM p = 0.055 BF vs. WM p = 0.023 |
| Triglycerides (mg/dl) b | 113.3 (43.03) | 188.8 (204.2) | 111.1 (43.8) | 132.1(51.4) | 0.779 | 0.142 | 0.830 | — |
| White blood cells (×103 cells/mm3) a | 5.64 (2.10) | 4.84 (1.40) | 4.93 (1.49) | 5.93 (2.62) | 0.752 | 0.895 | 0.126 | — |
| Neutrophils (×103 cells/mm3) a | 3.55 (1.66) | 3.16 (1.25) | 3.24 (1.23) | 4.19 (2.19) | 0.368 | 0.581 | 0.163 | — |
| Lymphocytes (×103cells/mm3) b | 1.39 (0.44) | 0.95 (0.48) | 1.02 (0.58) | 1.00 (0.39) | 0.215 | 0.061 | 0.065 | BF vs. BM p = 0.047 |
| Platelets (cells × 106) b | 207.7 (35.6) | 185.9 (39.3) | 216.3 (70.1) | 186.6 (50.8) | 0.934 | 0.044 | 0.833 | — |
| Hemoglobin (g/dl) b | 11.19 (0.88) | 12.73 (1.20) | 11.62 (0.84) | 13.43 (1.37) | <0.046 | <0.001 | 0.781 |
BF vs. BM p < 0.001 BF vs. WM p < 0.001 BM vs. WF p = 0.021 WF vs. WM p < 0.001 |
| Serum creatinine (mg/dl) b | 1.52 (0.54) | 1.65 (0.37) | 1.22 (0.28) | 1.26(0.29) | <0.001 | 0.213 | 0.459 |
BM vs. WF p = 0.003 BM vs. WM p = 0.008 |
| eGFR (ml/min/1.73 m2) | 49.9 (17.7) | 57.4 (13.4) | 49.8 (11.5) | 64.5 (16.2) | 0.343 | 0.003 | 0.379 |
BF vs. WM p = 0.044 WF vs. WM p = 0.034 |
| Mycophenolic acid study dose (mg) | 636.9 (157.9) | 639.0 (222.2) | 562.5 (159.3) | 663.8 (108.4) | 0.520 | 0.224 | 0.289 | — |
| Tacrolimus study dose a (mg) | 5.1 (1.7) | 3.7 (1.6) | 2.7 (1.2) | 2.2 (0.7) | <0.001 | 0.020 | 0.262 |
BF vs. WF p < 0.001 BF vs. WM p = 0.001 BM vs. WM p = 0.008 BF vs. BM p = 0.073 |
Data displayed as mean (standard deviation). Significant p values are denoted by bold type.
Abbreviations: B, Blacks; BMI, body mass index; eGFR, estimated glomerular filtration rate; F, females; M, males; SD, standard deviation; W, White.
Parameters that were log transformed during statistical analysis.
Square root transformation of parameter.
Inverse transformation of parameter.
The use of group comparisons was included to provide objective evaluations between sub‐populations and identify covariates that may impact pharmacologic parameters and address study objectives.
3.2. Tacrolimus pharmacokinetics
Comparison of tacrolimus pharmacokinetic parameters between groups is summarized in Table 2 with concentration versus time curves presented in Figure S1. Figure 3 Panels A–D present the target pharmacokinetic parameters stratified by race and sex. Black recipients exhibited higher tacrolimus AUC0–12 (Race: p = 0.005; Figure 3 Panel C) and AUC0–4 (Race: p = 0.007) with lower dose‐normalized AUC (Race: p < 0.001; Race × Sex: p = 0.068) (Figure 3 Panel D). Faster tacrolimus Cl (Race: p < 0.001; Sex: p = 0.066; Race × Sex: p = 0.085) and Cl/total body weight [TBW] (Race: p = 0.007; Sex: p = 0.006) were found in Black patients, which was ~twofold faster in Black females compared with White groups (Figure 3 Panel B). The CYP3A5*3*6*7 metabolic composite was the major significant covariate accounting for 15%–19% of tacrolimus variability in dose (p = 0.002); dose‐normalized AUC0–12 (p < 0.001) and Cl (p < 0.001).
TABLE 2.
Tacrolimus pharmacokinetic parameters by race and sex groups (n = 65)
|
Parameter Mean (SD) |
Black females (n = 13) | Black males (n = 20) | White females (n = 16) | White males (n = 16) | p‐Values for the effect of | Significant pairwise comparisons (Tukey adjusted) | ||
|---|---|---|---|---|---|---|---|---|
| Race | Sex | Race × Sex interaction | ||||||
| Primary measured pharmacokinetic parameters | ||||||||
| C12 h trough (ng/ml) | 7.5 (1.4) | 8.0 (2.0) | 6.8 (2.1) | 6.5 (1.4) | 0.021 | 0.800 | 0.439 | BM vs. WM p = 0.098 |
| Cmax (ng/ml) a | 22.9 (11.5) | 19.4 (6.7) | 18.8 (8.6) | 13.8 (3.7) | 0.012 | 0.067 | 0.452 |
BF vs. WM p = 0.021 BM vs. WM p = 0.071 |
| AUC0‐12 (ng h/ml) | 134.7 (31.7) | 138.5 (32.5) | 123(31.9) | 106.3(21.4) | 0.005 | 0.389 | 0.178 |
BM vs. WM p = 0.011 BF vs. WM p = 0.061 |
| AUC0‐4h (ng h/ml) b | 62.1 (20.3) | 60.2 (17.5) | 54.5 (17.5) | 44.1(10.9) | 0.007 | 0.157 | 0.293 |
BF vs. WM p=0.030 BM vs. WM p = 0.027 |
| Cl (L/h) a | 40.0(16.8) | 28.2 (13.2) | 22.2 (8.9) | 21.3 (6.8) | <0.001 | 0.066 | 0.085 |
BF vs. WF p = 0.002 BF vs. WM p = 0.001 BF vs. BM p = 0.062 |
| Cl/TBW a (L/h/kg) | 0.47 (0.23) | 0.34 (0.20) | 0.32 (0.15) | 0.23(0.07) | 0.007 | 0.006 | 0.515 |
BF vs. WM p = 0.002 BF vs. BM p = 0.075 |
| Dose normalized pharmacokinetic parameters: represents 1 mg tacrolimus exposure | ||||||||
| C12h trough dose a (ng/ml/mg) | 1.61 (0.60) | 2.68 (1.78) | 3.04 (1.60) | 3.20 (1.10) | <0.001 | 0.025 | 0.192 |
BF vs. WF p = 0.005 BF vs. WM p < 0.001 BF vs. BM p = 0.062 |
| C max/dose a (ng/ml/mg) | 5.12 (2.97) | 5.96 (2.67) | 8.15 (4.01) | 6.70 (2.45) | 0.013 | 0.825 | 0.149 | BF vs. WF p = 0.042 |
| AUC0‐12 dose a (ng∙h/ml/mg) | 29.6 (12.5) | 44.9 (25.5) | 54.6 (25.9) | 51.7 (16.8) | <0.001 | 0.084 | 0.068 |
BF vs. WF p = 0.002 BF vs. WM p = 0.002 |
Data displayed as mean (standard deviation). Significant p values are denoted by bold type.
Abbreviations: AUC, area under the concentration vs. time curve; B, Blacks; C12hr, 12‐hour trough concentration; CLss, steady state oral clearance; C max, maximum concentration; F, females; M, males; SD, standard deviation; TBW, total body weight; T max, time to maximum concentration; W, White.
Parameters that were log transformed during statistical analysis.
Square root transformation of parameter.
FIGURE 3.
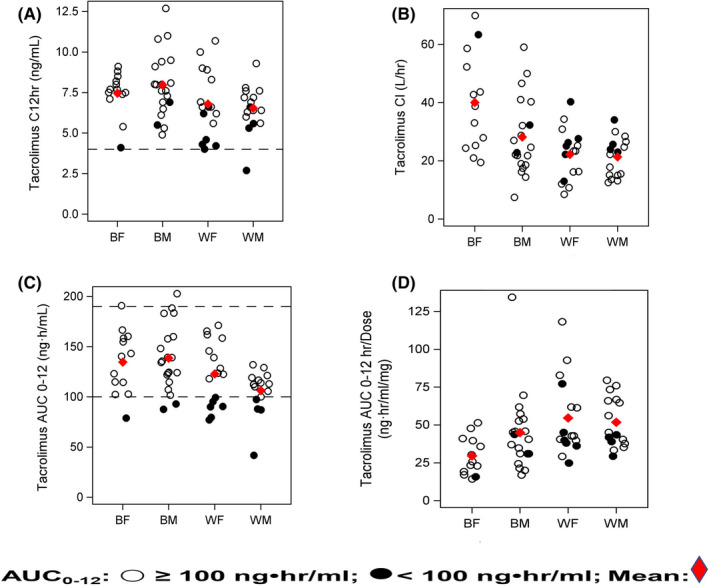
Panels A–D: Select tacrolimus pharmacokinetic parameters stratified by race‐sex groups and target AUC0–12 [Open dot represents AUC0–12 ≥100 ng‧h/ml; closed dot represents AUC0–12 <100 ng‧h/ml]: Panel A represents 12‐h concentration (Race: p = 0.021) achieved in each patient using the range of 4 (dotted line) to 12 ng/ml. Panel B depicts tacrolimus Cl for each group. Rapid Cl (Race: <0.001; Sex: p = 0.066; Race × Sex: p = 0.085) are noted in Black females and males compared to White recipients. Panel C depicts tacrolimus AUC0–12 relative to race and sex groups (Race: p = 0.005) using the tacrolimus therapeutic AUC0–12 guide of ≥100 ng‧h/ml, represented by the dotted line and less than or equal to 190 ng‧h/ml. 7 , 8 There were 68.8% (22/32) of White and 84.5% (28/33) of Black patients within the target AUC0–12 guide of 100–190 ng‧h/ml for recipients >1‐year post‐transplant. Note that the tacrolimus AUC0–12 was <100 ng‧h/ml in 31.3% of Whites and 9.1% of Blacks. Panel D presents the distribution of dose‐normalized AUC0–12 between race and sex groups (Race: <0.001; Sex: p = 0.084; Race × Sex: p = 0.068). See Results section and Table S2 for comparisons between tacrolimus AUC0–12 exposures < and ≥100 ng‧h/ml (p = 0.027). AUC0–12, area under the concentration time curve 0–12 h; BF, Black females; BM, Black males; C 12 h, 12‐h trough concentration; Cl, clearance; WF, White females; WM: White males
The target AUC0–12 sub‐analysis compared combined race groups with tacrolimus AUC0–12 <100 ng∙h/ml and ≥100 ng∙h/ml and is summarized in Table S2. In 84.8% of Black and 68.8% of White recipients, therapeutic AUC0–12 of 100–190 ng‧h/ml was achieved; (p = 0.027; Figure 3 Panel C). In 31.3% of White and 9.1% of Black patients, AUC0–12 was <100 ng‧h/ml despite therapeutic troughs (p = 0.027).
3.3. Adverse effects
Table 3 summarizes the cumulative, neurologic, aesthetic, and GI adverse effect AE ratios. A sex (p < 0.001) and race‐sex interaction (p = 0.014) was found with the cumulative AE ratio, with 1.5‐fold higher scores in Black females compared with Black males. Figure 4 represents cumulative AE ratios and tacrolimus AUC0–12 with 68% of females demonstrating an AE score >0.14. 9 The neurologic AE ratio had a sex effect (p = 0.021) and a race‐sex interaction (p = 0.005), with the highest score found in Black females. A sex effect was found with a twofold higher aesthetic AE ratio (p = 0.002) in White females followed by Black females when compared to males. Interactions between race and sex for the cumulative AE ratio (p = 0.014) and neurologic AE ratio (p = 0.005) were found.
TABLE 3.
Adverse effects by race and sex groups (n = 65)
| Parameter a | Black females (n = 13) | Black males (n = 20) | White females (n = 16) | White males (n = 16) | p‐Values for the effect of | Significant pairwise comparisons (Tukey adjusted) | ||
|---|---|---|---|---|---|---|---|---|
| Race | Sex | Interaction of race and sex | ||||||
| Cumulative AE ratio c | 0.21 (0.06) | 0.09 (0.06) | 0.16 (0.09) | 0.13(0.06) | 0.736 | <0.001 | 0.014 |
BF vs. BM p < 0.001 BF vs. WM p = 0.012 BM vs. WF p = 0.018 |
| GI AE ratio c | 0.35 (0.17) | 0.20 (0.18) | 0.28(0.28) | 0.26 (0.16) | 0.866 | 0.139 | 0.140 | — |
| Neurologic AE ratio b | 0.33 (0.17) | 0.13 (0.11) | 0.200 (0.13) | 0.22 (0.18) | 0.646 | 0.021 | 0.005 | BF vs. BM p = 0.002 |
| Aesthetic AE ratio d | 0.07 (0.08) | 0.02 (0.04) | 0.11 (0.13) | 0.031 (0.05) | 0.271 | 0.002 | 0.617 |
BM vs. WF p = 0.008 WF vs. WM p = 0.043 |
Significant p values are denoted by bold type.
Abbreviations: AE, adverse effect; B, Blacks; F, females; GI, gastrointestinal; M, males; W, White.
Data displayed as mean (standard deviation).
Parameters that were log transformed during statistical analysis.
Square root transformation of parameter.
Inverse transformation of parameter
FIGURE 4.
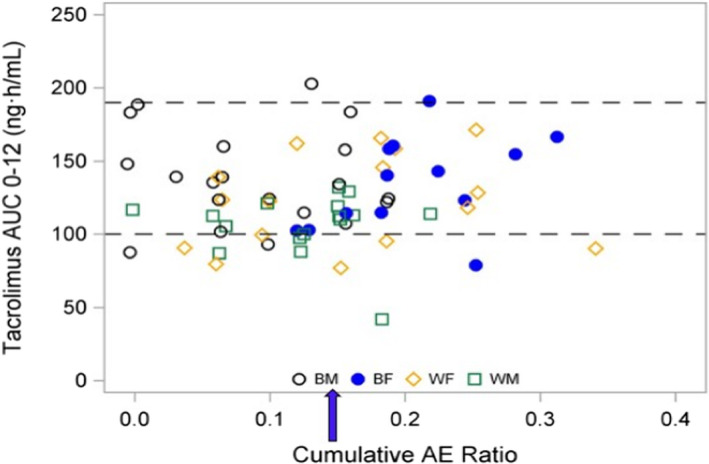
Tacrolimus AUC0–12 and relationship to cumulative AE ratio. This graph depicts the target tacrolimus AUC0–12 guide ranging from ≥100 to ≤190 ng‧h/ml represented by dotted lines from consensus recommendations 7 , 8 and the relationship to the cumulative adverse effect (AE) ratio for each patient stratified by race and sex. The ratio of 0.14 (blue arrow) was selected since it represents consistent and significant manifestation of cumulative adverse effects. 46 Note that 69% of the White and Black female recipients exhibited a cumulative adverse effect ratio ≥0.14 with a mean (SD) of 0.222 (0.049) indicating more severe adverse effects for women who were within or below the therapeutic tacrolimus AUC0–12 h. In contrast, 39% of males had a cumulative adverse effect ratio of 0.167 (0.019) at the time of study. AE, adverse effects; AUC0–12, area under the concentration time curve 0–12 h; BF, Black females [blue closed circle]; BM, Black males [black open circle]; WF, White females [yellow diamond]; WM, White males [green square]
3.4. CYP3A5*3*6*7 and ABCB1 genotypes—associations to tacrolimus pharmacokinetics
The distribution of CYP3A5*3*6*7 genotypes (n = 65) is summarized in Table 4 and explained in Figure 2A–D. Significant differences between race‐sex groups in allele frequencies were found (p < 0.001). Among White recipients expressing CYP3A5*3(rs776746), there were only three heterozygotes, and none were homozygous for the wild‐type allele, CYP3A5*1. See Table S3 for a complete summary. Table S4 summarizes the ABCB1 genotype distribution for 1236C>T (rs1128503), 2677G>T/A (rs2032582), 3435C>T (rs1045642) stratified by race and sex. For two of the three alleles: ABCB1 2677 & 3435, there were significant differences in allele frequencies between race as determined by the Goodness‐of‐fit test (G test).
TABLE 4.
Summary of CYP3A5*3*6*7 metabolic composite stratified by race and sex
| Race–sex groups | CYP3A5*3*6*7 metabolic composite [% (N)] a | |||
|---|---|---|---|---|
| Extensive | Intermediate | Poor | N | |
| White males | 0% | 6% (1) | 94% (15) | 16 |
| White females | 0% | 13% (2) | 88% (14) | 16 |
| Black males | 15% (3) | 60% (12) | 25% (5) | 20 |
| Black females | 31% (4) | 62% (8) | 8% (1) | 13 |
| N per CYP3A5*3*6*7 Metabolic Composite | 7 | 23 | 35 | |
There was a significant difference among CYP3A5*3*6*7 metabolic composite group frequencies between races (Fisher exact test, p = 0.0012). There was no significant difference among sexes.
CYP3A5*3*6*7 metabolic composites were the only significant covariate accounting for approximately 15%–of the tacrolimus pharmacokinetic variability after determination of race‐sex associations. 52 Significant covariate associations attributed to CYP3A5*3*6*7 metabolic composites were identified with tacrolimus dose (p = 0.007), dose/TBW (p = 0.025), 12‐h trough/dose (p < 0.001), AUC0–12/dose (p < 0.001), Cl (p = 0.002), and Cl/LBW (p = 0.003). No association of CYP3A5*3*6*7 metabolic composites with adverse effects was found.
No associations with individual ABCB1 genotypes were found with tacrolimus pharmacokinetic parameters and individual or composite adverse effects.
4. DISCUSSION
This is the first intensive pharmacokinetic study design used to examine race and sex differences in tacrolimus clearance, pharmacokinetics parameters, and AEs during maintenance tacrolimus and mycophenolic acid immunosuppression in stable kidney transplant recipients.
4.1. Tacrolimus clearance and AUC0–12
Our study design investigated tacrolimus pharmacokinetics in high‐risk Black recipients, points not addressed in the Elite‐Symphony trial, that clinically compared low‐dose tacrolimus to either cyclosporine or sirolimus within the first year in primarily white patients, that remains an important clinical issue. 10 , 11 , 15 , 18 , 19 Black patients, irrespective of sex, exhibited more rapid tacrolimus Cl with higher troughs and AUC0–12 that may reflect higher tacrolimus doses when compared to White recipients (Table 2). This Cl difference is consistent with a previous study in healthy subjects. 23 Factors contributing to elevated tacrolimus troughs and AUC in Black renal transplant recipients will require further investigation. A sex influence was determined in Black females, who exhibited the most rapid tacrolimus Cl adjusted for weight and reduced dose‐normalized AUC0–12. Sex differences have been suggested using a limited tacrolimus sampling approach after the first dose. 33 The tacrolimus AUC0–12 in our study was below the suggested target of 100 ng‧h/ml for ≥1‐year post‐transplant in 20% of recipients, irrespective of race or sex despite therapeutic troughs (Figure 3 Panels A and C). 7 This finding is important as sub‐therapeutic exposure may contribute to development of donor‐specific antibodies. 12 , 13 , 14 , 53 Therefore, in‐depth pharmacokinetic comparisons between sub‐populations are needed to develop individualized tacrolimus regimens. 7 , 8 , 24 , 25
4.2. Pharmacokinetics and pharmacodynamics
A sex effect was identified for females who had higher cumulative, neurologic, and aesthetic AE scores. The cumulative adverse effects score >0.14 was exhibited in 69% of females who were below the target tacrolimus AUC0–12 (Figure 4) for time post‐transplant. 7 Associations between extrarenal adverse effects, sex, and ABCB1 haplotypes have been reported, 46 but no AE association to ABCB1 genotypes was detected in this study. Several studies reported that tacrolimus‐related adverse effects were more frequent or severe at higher tacrolimus exposures. 7 , 8 , 49 With reduced targeted trough ranges, 10 , 11 the incidence of tacrolimus‐mediated AE has improved. 7 , 8 , 11 A tacrolimus pharmacokinetic‐pharmacodynamic population model that identified increased dose‐normalized AUC* and maximum concentration with decreased Cl exhibited significant associations to greater GI and neurologic AEs with sex as a covariate. 52 Since these extrarenal AEs have been linked to poor medication adherence, standardized AE assessment can be useful as a longitudinal monitoring guide.
4.3. CYP3A5*3*6*7 metabolic composite
The Clinical Pharmacogenetics Implementation Consortium (CPIC) provides tacrolimus dosing recommendations with CYP3A5*3*6*7 variants to account for pharmacokinetic variability. 7 , 39 , 42 Recently, a pharmacokinetic model incorporating CYP3A5*3*6*7 metabolic composite was reported to describe loss‐of‐function variants and tacrolimus disposition. 43 Approximately 88% of Black recipients were extensive and intermediate metabolizers attributed to CYP3A5*1 genotype (Table 4 and Figure 2). Higher daily doses were required to maintain comparable troughs due to rapid Cl. 42 CYP3A5*3*6*7 metabolic composite was the only significant covariate that accounted for 15%–19% of tacrolimus pharmacokinetic variability after adjustment for race‐sex influence. Investigations of troughs as a surrogate marker in Black recipients demonstrated associations with CYP3A5*1 alleles, with higher dose requirements needed to achieve therapeutic troughs comparable to CYP3A5*3*6*7. 39 Earlier studies lacked sex‐specific analysis or intensive pharmacokinetic sampling to verify tacrolimus AUC0–12. This parameter reflects drug exposure in high‐risk Black recipients expressing CYP3A5*1 genotype. The utility of genotype‐based dosing models to achieve target troughs has limitations and requires tacrolimus pharmacokinetic studies. 39
This report of race and sex differences in tacrolimus pharmacokinetics (ie, weight‐normalized Cl, dose‐normalized AUC0–12 and C 12 h) and adverse effects provides AUC0–12 ranges for dosing between Black and White recipients, and fills an important pharmacotherapeutic knowledge gap. The comparison of sex with race incorporates well‐established differences in gender biology. 33 , 54 The influence of sex is supported by in vivo gender differences for CYP3A4/5 substrates and in vitro reports of greater GI tract and liver expression in females. 26 , 27 , 29 The significant contribution of the covariate CYP3A5*3*6*7 metabolic composite on interpatient tacrolimus variability further supports these in vivo and in vitro findings. 26 , 27 , 29 , 44 , 45 Therefore, our study provides new data describing combined race and sex associations with tacrolimus pharmacokinetics.
Our study is novel in that the AUC0–12 was reported in high‐risk Blacks compared with White male and female recipients. Intensive pharmacokinetic sampling and a validated extrarenal adverse effect scoring evaluation (Table S1) combined with medication adherence assessment and tacrolimus assays from a CLIA‐certified laboratory enhance the rigor and reproducibility of our study. Another novel component is the inclusion of CYP3A5*3*6*7 metabolic composite as a covariate, its use to categorize variable metabolism, and its contribution to tacrolimus pharmacokinetic variability between race and sex groups. Incorporation of clinical covariate analysis further identifies patients at risk for adverse effects or interpatient pharmacokinetic variability. Our statistical model incorporated use of pairwise group comparisons between patient groups to substantiate pharmacokinetic differences and adverse effects that may improve our understanding of race and sex responses post‐transplant.
In conclusion, this is the first report of the combined influence of race with sex contributing to tacrolimus pharmacokinetic variability in Black and White stable kidney transplant recipients. Despite therapeutic tacrolimus troughs achieved in most recipients, 20% of patients had sub‐therapeutic AUC0–12. Extrarenal adverse effects were more evident in women and most prominent in Black females. The CYP3A5*3*6*7 metabolic composite was the major significant covariate contributing to additional interpatient pharmacokinetic variability between White and Black recipients. These findings may guide differential tacrolimus dosing using individualization approaches.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
Authors, using their initials, have contributed to this study and manuscript as follows: KMT[PI], GW, and RCV involved in research conception and study design. KMT[PI], VG, RCV, AG, and SC involved in data acquisition. KMT[PI], CJM, DB, GW, JC, LMC, and RCV involved in analysis and data interpretation. KMT[PI], CJM, DB, GW, JC, KA, and RCV involved in drafting and revising the final article. KMT[PI], CJM, DB, GW, JC, KA, SC, LMC, and RCV involved in providing intellectual content. KMT[PI], GW, RCV, KA, VG, and LMC involved in supervision and mentorship. KMT[PI], CJM, DB, GW, JC, KA, SC, LMC, VG, and RCV involved in final approval of manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
Dr. Rocco Venuto is deceased. He was involved in all aspects of this clinical pharmacology study. His clinical experience and efforts are greatly appreciated. Dr. Meaney was an Immunosuppressive Pharmacology Fellow in the Immunosuppressive Pharmacology Research Program at the School of Pharmacy and Pharmaceutical Sciences and New York State Center of Excellence for Bioinformatics and Life Sciences. Dr. Chang was an ECRIP Transplant Fellow during this research study in the UB Department of Medicine, Nephrology Division. Dr. Consiglio was a PhD student during this research project. The assistance of the following individuals is greatly appreciated: Lisa Venuto, Brenda Pawl, Ethel Kendricks, Kris Reed, Jean Meyers, and Joseph Kabacinski, from Erie County Medical Center and Renal Division.
Tornatore KM, Meaney CJ, Attwood K, et al. Race and sex associations with tacrolimus pharmacokinetics in stable kidney transplant recipients. Pharmacotherapy. 2022;42:94–105. doi: 10.1002/phar.2656
Funding information
This study was supported by grants from NIDDK ARRA R21: DK077325‐01A1 (KMT‐PI) and Investigator‐Initiated Research Grants (KMT‐PI) from Astellas Pharma Global Development, Inc. Funding sources had no role in conducting, analyzing, or interpretation of data and writing the manuscript.
REFERENCES
- 1. Hart A, Lentine KL, Smith JM, et al. OPTN/SRTR 2019 annual data report: kidney. Am J Transplant. 2021;21(Suppl 2):21‐137. [DOI] [PubMed] [Google Scholar]
- 2. Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Parts I and II. Clin Pharmacokinet. 2010;49(4):207‐221; 49(3):141‐175. [DOI] [PubMed] [Google Scholar]
- 3. Knops N, Levtchenko E, van den Heuvel B, Kuypers D. From gut to kidney: transporting and metabolizing calcineurin‐inhibitors in solid organ transplantation. Int J Pharm. 2013;452(1‐2):14‐35. [DOI] [PubMed] [Google Scholar]
- 4. Vanhove T, Annaert P, Kuypers DR. Clinical determinants of calcineurin inhibitor disposition: a mechanistic review. Drug Metab Rev. 2016;48(1):88‐112. [DOI] [PubMed] [Google Scholar]
- 5. Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol. 2007;2(2):374‐384. [DOI] [PubMed] [Google Scholar]
- 6. Shuker N, van Gelder T, Hesselink DA. Intra‐patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant Rev. 2015;29(2):78‐84. [DOI] [PubMed] [Google Scholar]
- 7. Brunet M, van Gelder T, Åsberg A, et al. Therapeutic drug monitoring of tacrolimus‐personalized therapy: second consensus report. Ther Drug Monit. 2019;41(3):261‐307. [DOI] [PubMed] [Google Scholar]
- 8. Wallemacq P, Armstrong VW, Brunet M, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit. 2009;31(2):139‐152. [DOI] [PubMed] [Google Scholar]
- 9. Bouamar R, Shuker N, Hesselink DA, et al. Tacrolimus predose concentrations do not predict the risk of acute rejection after renal transplantation: a pooled analysis from three randomized‐controlled clinical trials(dagger). Am J Transplant. 2013;13(5):1253‐1261. [DOI] [PubMed] [Google Scholar]
- 10. Ekberg H, Tedesco‐Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357(25):2562‐2575. [DOI] [PubMed] [Google Scholar]
- 11. Ekberg H, Bernasconi C, Tedesco‐Silva H, et al. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant. 2009;9(8):1876‐1885. [DOI] [PubMed] [Google Scholar]
- 12. Scholten EM, Cremers SCLM, Schoemaker RC, et al. AUC‐guided dosing of tacrolimus prevents progressive systemic overexposure in renal transplant recipients. Kidney Int. 2005;67(6):2440‐2447. [DOI] [PubMed] [Google Scholar]
- 13. Davis S, Gralla J, Klem P, et al. Lower tacrolimus exposure and time in therapeutic range increase the risk of de novo donor‐specific antibodies in the first year of kidney transplantation. Am J Transplant. 2018;18(4):907‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaynor JJ, Ciancio G, Guerra G, et al. Lower tacrolimus trough levels are associated with subsequently higher acute rejection risk during the first 12 months after kidney transplantation. Transpl Int. 2016;29(2):216‐226. [DOI] [PubMed] [Google Scholar]
- 15. Young CJ, Gaston RS. Understanding the influence of ethnicity on renal allograft survival. Am J Transplant. 2005;5(11):2603‐2604. [DOI] [PubMed] [Google Scholar]
- 16. Young CJ, Gaston RS. Renal transplantation in black Americans. N Engl J Med. 2000;343(21):1545‐1552. [DOI] [PubMed] [Google Scholar]
- 17. Young CJ, Kew C. Health disparities in transplantation: focus on the complexity and challenge of renal transplantation in African Americans. Med Clin North Am. 2005;89(5):1003‐1031, ix. [DOI] [PubMed] [Google Scholar]
- 18. Eckhoff DE, Young CJ, Gaston RS, et al. Racial disparities in renal allograft survival: a public health issue? J Am Coll Surg. 2007;204(5):894‐902. [DOI] [PubMed] [Google Scholar]
- 19. Fan P‐Y, Ashby VB, Fuller DS, , et al. Access and outcomes among minority transplant patients, 1999–2008, with a focus on determinants of kidney graft survival. Am J Transplant. 2010;10(4 Pt 2):1090‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andrews LM, De Winter BC, Van Gelder T, Hesselink DA. Consideration of the ethnic prevalence of genotypes in the clinical use of tacrolimus. Pharmacogenomics. 2016;17(16):1737‐1740. [DOI] [PubMed] [Google Scholar]
- 21. Page TF, Woodward RS, Brennan DC. The Impact of Medicare's lifetime immunosuppression coverage on racial disparities in kidney graft survival. Am J Transplant. 2012;12(6):1519‐1527. [DOI] [PubMed] [Google Scholar]
- 22. Vadivel N, Garg A, Holt DW, Chang RW, MacPhee IA. Tacrolimus dose in black renal transplant recipients. Transplantation. 2007;83(7):997‐999. [DOI] [PubMed] [Google Scholar]
- 23. Mancinelli LM, Frassetto L, Floren LC, et al. The pharmacokinetics and metabolic disposition of tacrolimus: a comparison across ethnic groups. Clin Pharmacol Ther. 2001;69(1):24‐31. [DOI] [PubMed] [Google Scholar]
- 24. Huang SM, Temple R. Is this the drug or dose for you? Impact and consideration of ethnic factors in global drug development, regulatory review, and clinical practice. Clin Pharmacol Ther. 2008;84(3):287‐294. [DOI] [PubMed] [Google Scholar]
- 25. Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84(3):417‐423. [DOI] [PubMed] [Google Scholar]
- 26. Scandlyn MJ, Stuart EC, Rosengren RJ. Sex‐specific differences in CYP450 isoforms in humans. Expert Opin Drug Metab Toxicol. 2008;4(4):413‐424. [DOI] [PubMed] [Google Scholar]
- 27. Hu ZY, Zhao YS. Sex‐dependent differences in cytochrome P450 3A activity as assessed by midazolam disposition in humans: a meta‐analysis. Drug Metab Dispos. 2010;38(5):817‐823. [DOI] [PubMed] [Google Scholar]
- 28. Momper JD, Misel ML, McKay DB. Sex differences in transplantation. Transplant Rev. 2017;31(3):145‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cummins CL, Wu CY, Benet LZ. Sex‐related differences in the clearance of cytochrome P450 3A4 substrates may be caused by P‐glycoprotein. Clin Pharmacol Ther. 2002;72(5):474‐489. [DOI] [PubMed] [Google Scholar]
- 30. Hickson LJ, Balls‐Berry JE, Jaffe AS, Rule AD. Biomarkers associated with progression of diabetic kidney disease: do they hold the same meaning for blacks and women? J Am Soc Nephrol. 2018;29(6):1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coakley M, Fadiran EO, Parrish LJ, Griffith RA, Weiss E, Carter C. Dialogues on diversifying clinical trials: successful strategies for engaging women and minorities in clinical trials. J Women's Health. 2012;21(7):713‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anonymous Women's Health Research Roadmap – A Strategy for Science and Innovation to Improve the Health of Women. FDA. https://www.fda.gov/science‐research/womens‐health‐research/womens‐health‐research‐roadmap; published 2/16/2018; Accessed 9/5/21.
- 33. Velickovic‐Radovanovic R, Mikov M, Catic‐Djordjevic A, et al. Gender‐dependent predictable pharmacokinetic method for tacrolimus exposure monitoring in kidney transplant patients. Eur J Drug Metab Pharmacokinet. 2015;40(1):95‐102. [DOI] [PubMed] [Google Scholar]
- 34. Tang JT, Andrews LM, van Gelder T, et al. Pharmacogenetic aspects of the use of tacrolimus in renal transplantation: recent developments and ethnic considerations. Expert Opin Drug Metab Toxicol. 2016;12(5):555‐565. [DOI] [PubMed] [Google Scholar]
- 35. Hesselink DA, Bouamar R, Elens L, van Schaik RH, van Gelder T. The role of pharmacogenetics in the disposition of and response to tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2014;53(2):123‐139. [DOI] [PubMed] [Google Scholar]
- 36. Barbarino JM, Staatz CE, Venkataramanan R, Klein TE, Altman RB. PharmGKB summary: cyclosporine and tacrolimus pathways. Pharmacogenet Genomics. 2013;23(10):563‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Jonge H, de Loor H, Verbeke K, Vanrenterghem Y, Kuypers DR. In vivo CYP3A4 activity, CYP3A5 genotype, and hematocrit predict tacrolimus dose requirements and clearance in renal transplant patients. Clin Pharmacol Ther. 2012;92(3):366‐375. [DOI] [PubMed] [Google Scholar]
- 38. Shuker N, Bouamar R, van Schaik RH, et al. A randomized controlled trial comparing the efficacy of Cyp3a5 genotype‐based with body‐weight‐based tacrolimus dosing after living donor kidney transplantation. Am J Transplant. 2016;16(7):2085‐2096. [DOI] [PubMed] [Google Scholar]
- 39. Oetting WS, Schladt DP, Guan W, et al. Genomewide association study of tacrolimus concentrations in African American kidney transplant recipients identifies multiple CYP3A5 alleles. Am J Transplant. 2016;16(2):574‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. MacPhee IAM, Fredericks S, Tai T, et al. The influence of pharmacogenetics on the time to achieve target tacrolimus concentrations after kidney transplantation. Am J Transplant. 2004;4(6):914‐919. [DOI] [PubMed] [Google Scholar]
- 41. Thervet E, Loriot MA, Barbier S, et al. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin Pharmacol Ther. 2010;87(6):721‐726. [DOI] [PubMed] [Google Scholar]
- 42. Birdwell KA, Decker B, Barbarino JM, et al. Clinical pharmacogenetics implementation consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin Pharmacol Ther. 2015;98(1):19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Campagne O, Mager DE, Brazeau D, Venuto RC, Tornatore KM. Tacrolimus population pharmacokinetics and multiple CYP3A5 genotypes in black and white renal transplant recipients. J Clin Pharmacol. 2018;58(9):1184‐1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wolbold R, Klein K, Burk O, et al. Sex is a major determinant of CYP3A4 expression in human liver. Hepatology. 2003;38(4):978‐988. [DOI] [PubMed] [Google Scholar]
- 45. Meibohm B, Beierle I, Derendorf H. How important are gender differences in pharmacokinetics? Clin Pharmacokinet. 2002;41(5):329‐342. [DOI] [PubMed] [Google Scholar]
- 46. Venuto RC, Meaney CJ, Chang S, et al. Association of extrarenal adverse effects of posttransplant immunosuppression with sex and ABCB1 haplotypes. Medicine. 2015;94(37):e1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meaney CJ, Arabi Z, Venuto RC, Consiglio JD, Wilding GE, Tornatore KM. Validity and reliability of a novel immunosuppressive adverse effects scoring system in renal transplant recipients. BMC Nephrol. 2014;15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461‐470. [DOI] [PubMed] [Google Scholar]
- 49. Little J, Higgins JPT, Ioannidis JPA, et al. STrenthening the REporting of Genetic Associations Studies (STREGA) – an extension of STROBE statement. PLoS Medicine. 2009;6(2):e1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O, eds. SAS for Mixed Model. 2nd edn. SAS Institute, Inc.; 2006. [Google Scholar]
- 51. Freeman GH, Halton JH. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika. 1951;38(1‐2):141‐149. [PubMed] [Google Scholar]
- 52. Campagne O, Mager DE, Brazeau D, Venuto RC, Tornatore KM. The impact of tacrolimus exposure on extrarenal adverse effects in adult renal transplant recipients. Br J Clin Pharmacol. 2019;85(3):516‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shuker N, Shuker L, van Rosmalen J, et al. A high intrapatient variability in tacrolimus exposure is associated with poor long‐term outcome of kidney transplantation. Transplant Int. 2016;29(11):1158‐1167. [DOI] [PubMed] [Google Scholar]
- 54. Federman DD. The biology of human sex differences. N Engl J Med. 2006;354(14):1507‐1514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


