Abstract
Olfactory dysfunction consistently occurs in patients with Alzheimer’s disease (AD), beyond the mild and gradual decline in olfactory ability found in normal aging. This dysfunction begins early in the disease course, typically before clinical diagnosis, and progresses with disease severity. While odor identification and detection deficits clearly differentiate AD from controls, there remains uncertainty as to whether these are determined by olfactory threshold. The purpose of the current preliminary fMRI study was to examine the neural correlates of olfactory processing in healthy young and old adults and compare them with AD patients. We also explored the interplay between age and disease-related psychophysical olfactory declines and odorant-induced brain activation. Results indicated AD patients had decreased odor detection task-related signal in all regions of the primary olfactory cortex, with activity in the entorhinal cortex best differentiating the groups. Moderated-mediation analyses on neuro-psychophysical relationships found that increased brain activation in the entorhinal cortex moderated the negative effect of disease-related threshold changes on olfactory detection. Therefore, even in the face of higher (worse) olfactory thresholds, older adults and AD patients compensated for this effect with increased brain activation in a primary olfactory brain region. This was the case for odor detection but not odor identification. fMRI activation induced by an olfactory detection task may eventually be useful in improving early discovery of AD and may, eventually, facilitate early treatment interventions in subjects at risk for AD.
Keywords: Alzheimer’s disease, Aging, Olfaction, Functional magnetic resonance imaging
1. Introduction
All studies examining AD-related olfactory function have demonstrated robust olfactory deficits relative to healthy controls, particularly involving higher-order olfactory functions such as quality discrimination, recognition memory, and odor identification [1–7]. These deficits represent an olfactory decline that cannot be explained by other cognitive problems, begins early in the disease course, and progresses with disease severity [8,9].
Olfactory functions are organized in a parallel and hierarchical fashion, such that all olfactory tasks involve sensory-level processing to enable perception of the odors, and increasingly diverse and distinct recruitment of brain regions specific to the cognitive demands of the odor task [10]. On a continuum from least to most cognitively demanding, olfactory tasks can be organized by odor threshold, detection, intensity discrimination, quality discrimination, recognition memory, cued identification, and non-cued identification [11,12]. In the present study, we consider the effects of AD and aging on detection, threshold, and cued identification (henceforth referred to as simply ‘odor identification’). Odor threshold is the lowest concentration at which subjects can perceive odors. Threshold tasks involve trials with varying odor concentration conditions interwoven with no-odor conditions, where the subject must make comparisons between multiple response options and identify which contained the odor. Optimal odor threshold performance occurs when subjects can perceive odors even at low concentrations, when they are hardest to notice. Odor detection is the ability to perceive odor onset (usually with a button press, or in a yes/no response format), irrespective of odorant concentration or in comparison to other presented conditions. Optimal odor detection performance occurs when subjects indicate they have perceived the odor immediately following odor onset, and not at times when an odor is not present. Odor identification involves the perception of an odor and the selection of the name associated with the odor (usually from words presented visually as cued response options). Optimal odor identification performance occurs when subjects select the correct odor name from foils.
Anatomical and brain-imaging studies have documented possible underlying neural correlates of age- and AD-related olfactory decline. Pathological changes in peripheral and central olfactory structures have been associated with increasing age, including diminished regenerative capacity of neurons in the olfactory epithelium and bulb [13,14], misrouted olfactory nerve fibers and displaced glomeruli [15], and the occurrence of intracellular neurofibrillary tangles, to a lesser extent amyloid plaques, and neuronal cell loss in primary olfactory cortex (POC) projection areas [13,15,16]. Structural MRI studies also show significant correlations between olfactory impairment and decreased brain volumes in olfactory bulbs [17] and hippocampus [18] of healthy individuals. Moreover, the few functional imaging studies (PET and fMRI) consistently report an age-related decline in the degree of odorant-induced activation in olfactory-related brain regions [17,19–24].
In AD patients relative to healthy old controls, anatomical studies have consistently reported neurofibrillary tangles and amyloid plaques in the olfactory epithelium, the olfactory bulb, and POC brain regions [15]. While few in number, functional brain imaging studies using olfactory stimulation paradigms corroborate these findings. Vasavada and colleagues (2017) reported that AD patients performing an odor detection task had lower POC activation than controls [25]. Lu and colleagues (2019) further demonstrated that AD patients had reduced odor identification-related activation in the olfactory network, which consists of the POC and secondary olfactory areas including the hippocampus, insula, and striatum [26]. Reduced activation in these areas was also reported by Wang and colleagues (2010), who additionally reported the magnitude of signal in the hippocampus positively related to odor identification performance [27]. Together, the psychophysical, anatomical and imaging studies suggests that AD-related olfactory deficits, particularly those related to higher order olfactory functions involving odor identification, likely result from AD-related pathology that damages the primary and secondary olfactory areas, and neocortical association areas [28].
While odor identification deficits clearly differentiate AD from controls, there remains uncertainty as to what extent these deficits are determined by olfactory threshold. The goal of the current fMRI study was to examine the neural correlates of olfactory processing as a function of age and AD by directly comparing odorant-induced brain activation in healthy young and old subjects and AD patients. Additionally, we sought to explore the influence of odor threshold on higher-order olfactory functions. Based on the extant literature, we hypothesized that, relative to group-matched healthy young individuals, the healthy old would show an age-related decline on psychophysical tests of olfaction and a concomitant decrease in odorant-induced brain activation in olfactory-related brain areas. Likewise, relative to group-matched healthy old controls, we hypothesized that AD patients would demonstrate a marked decline on psychophysical testing and a decrease in odorant-induced brain activation. Lastly, we hypothesized that group differences in odor detection and identification would be mediated by odor threshold.
2. Materials and methods
2.1. Subjects
Ten healthy young (mean age = 23.9, SD = 5.40), 10 healthy old (mean age = 71.6, SD = 7.8) volunteers, and 12 patients with probable AD (mean age = 69.4, SD = 10.3) participated in the current study. The healthy young and old subjects were group-matched on sex, education and self-reported ethnicity. The AD patients were group-matched to the old controls on sex, age, education, and self-reported ethnicity (Table 1). All subjects were right-handed except for one young control. Young subjects were recruited by word of mouth, and all healthy old and AD patients were consecutive participants in a larger longitudinal study of putative early diagnostic markers of AD conducted at the Memory Disorders Clinic (Devanand et al., 2008). The New York State Psychiatric Institute and Columbia Presbyterian Medical Center IRBs approved the study and all subjects provided informed consent prior to participation.
Table 1.
Demographic, clinical, and olfaction variables.
| Variable | Healthy Young (n = 10) | Healthy Old (n = 10) | p-value (Young vs. Old) | AD Patients (n = 12) | p-value (Old vs. AD) |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
|
| |||||
| Age, mean years | 23.9 (5.4) | 71.6 (7.8) | <0.001 | 69.4 (10.3) | 0.60 |
| Sex, % female | 50.0 | 40.0 | 0.50 | 58.0 | 0.30 |
| Education, years completed | 15.4 (2.4) | 16.8 (3.4) | 0.31 | 14.6 (3.2) | 0.10 |
| Handedness, % right | 90.0 | 100.0 | 0.50 | 100.0 | — |
| Self-Reported Ethnicity | |||||
| White/Non-Hispanic, % | 70.0 | 80.0 | 67.0 | ||
| Black/Non-Hispanic, % | 0.0 | 10.0 | 0.17 | 0.0 | 0.40 |
| Hispanics, % | 0.0 | 10.0 | 25.0 | ||
| Asian, % | 30.0 | 0.0 | 8.0 | ||
| Folstein MMSE (max = 30) | 29.8 (0.4) | 28.9 (1.0) | 0.02 | 22.9 (3.2) | <0.001 |
| Folstein MMSE Recall at 5 min (max = 3) | 3 (–) | 3 (–) | – | 1.1 (1.2) | <0.001 |
| Sniffin’ Sticks Threshold (max = 16) | 8.9 (2.3) | 7.8 (1.9) | 0.29 | 4.5 (3.0) | 0.005 |
| UPSIT score (max = 40) | 36.7 (1.9) | 34.7 (3.6) | 0.15 | 23.4 (8.1) | 0.001 |
| PIT (max = 40) | 40 (0.0) | 39.7 (0.5) | 0.07 | 38.1 (2.7) | 0.06 |
| fMRI Odor Detection Task (% accuracy) | 98 (3.5) | 97 (4.8) | 0.60 | 64 (29.4) | 0.003 |
For young and old subjects, inclusion criteria were age (20–39 and 50–95 years, respectively), no history of intellectual or cognitive impairment or learning disability, and a Folstein Mini Mental Status exam (MMSE) score ≥ 27/30 with recall of at least 2 out of 3 objects at five minutes (Table 1). All AD patients (Inclusion criteria age 50–95) met DSM-III-R criteria for dementia and NINCDS-ADRDA criteria for probable AD [29]. Diagnoses were determined by consensus between neurologists, psychiatrists, and neuropsychologists. In the AD group, only mildly affected patients with a Clinical Dementia Rating score (CDR) of 1 [30] and MMSE scores ≥ 18/30 were recruited to participate in this study (Table 1).
Prior to participation, all subjects completed a detailed medical history form and structural MRI scan (done as part of the larger longitudinal study) to rule out neurological, psychiatric, and other general medical conditions that could independently explain olfactory dysfunction. Specific exclusion criteria included current smoker, history of neurological and psychiatric disease (except for probable AD), pregnancy (for females of childbearing potential only), uncorrectable visual problems (e.g., amblyopia, cataracts, glaucoma, and macular degeneration), current nasal and/or sinus problems by history (e.g., congestion, asthma, emphysema, nasal polyps, chronic sinusitis, upper respiratory infection), deviated nasal septum, broken nose, history of rhinoplasty, radiation or chemotherapy, and head injury.
2.2. Psychophysical olfactory testing
Psychophysical testing occurred outside of the scanner prior to scanning sessions. Odor thresholds were determined with the “Sniffin’ Sticks” threshold test [31]. High scores on this test indicate subjects are able perceive odors even at low concentrations, i.e., low thresholds. Correspondingly, having a ‘lower threshold’ corresponds to better performance, and ‘higher threshold’ signifies worse performance. Odor identification was assessed using the University of Pennsylvania Smell Identification Test (UPSIT) [32]. Higher UPSIT scores indicate better performance. The Picture Identification Test (PIT) [33,34] was also administered. This test is identical in content and format to the UPSIT except that pictures serve as stimulus items.
2.3. Odorant delivery
Odor detection was measured in-scanner during an fMRI paradigm. A custom-built fMRI compatible olfactometer that allows for the presentation of up to 12 different odorants with a rise and odor-off time of < 250 ms was used [35,36]. A dual lumen nasal cannula (Nellcor Puritan Bennett) was used to deliver odorants bilaterally to the nose and to simultaneously monitor nasal breathing via a Sleepscan II pressure transducer (Bio-logic Systems Corporation). A laptop computer (Dell 5150) using a custom developed program (LabView 7.1, National Instruments Corp.) was used to control stimulus presentation.
2.4. fMRI Odorant presentation paradigm
The selection of odorants was based on their similarity to those in the UPSIT and has been previously described [36]. Two sets of five odorants were created and matched on perceived intensity and pleasantness. Set 1 comprised: Strawberry, Clove, Lemon, Lilac, and Menthol; and set 2 comprised: Orange, Root beer, Cherry, Pine, and Peppermint. The two sets were alternated across four scans to ensure that no odorant was repeated until all other odorants were presented once and were counterbalanced across subjects. The presentation order of the five odorants within each set was also counterbalanced across subjects. Scans were separated by a five min rest period. Two additional odorants (Grape and Soap) were used during practice sessions prior to fMRI scanning. As was previously shown [36], the counterbalanced presentation of odorants, along with the five minute rest periods between scans, prevented habituation of the odorant-induced brain response across trials and scans (Fig. 1).
Fig. 1. fMRI odorant presentation paradigm.
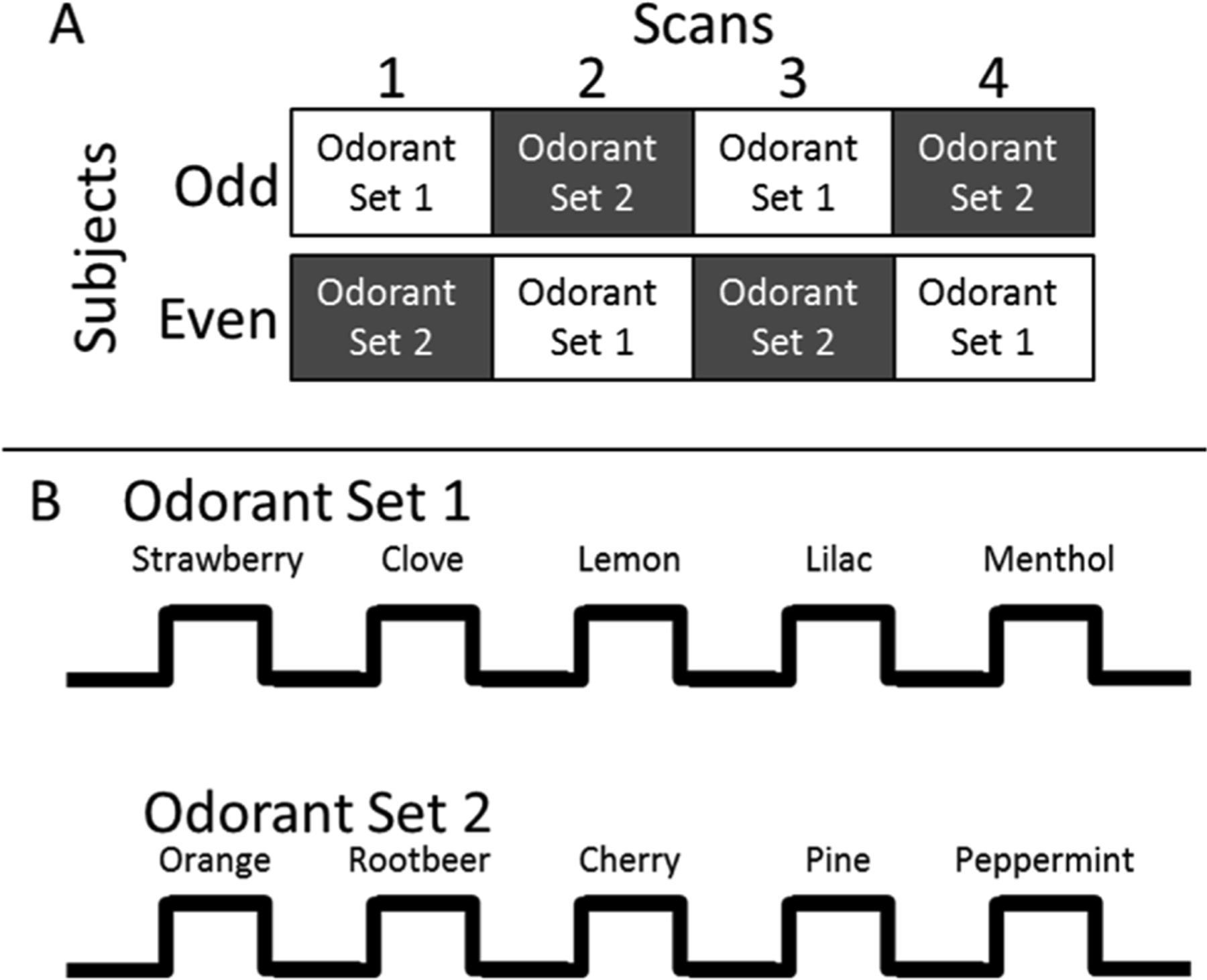
Notes: The top panel illustrates the counterbalanced order of odorant scans across subjects. The presentation order of the two odorant-sets across scans was counterbalanced, such that half of the subjects received odorant-set 1 first (i.e., odd numbered subjects) and the other half received odorant-set 2 first (even numbered subjects). The lower panel illustrates the odorants and timing parameters used during each scan. A simple Latin square design was used to counterbalance the presentation order of the five odorants within each odorant-set across subjects. Scans were separated by a five min rest period.
Each scan began with a 36-s baseline period of clear air and odorants were presented for 12 s (ON) alternating with 30 s of clear air (OFF; Fig. 1). The 12 s odorant ON period was chosen to allow subjects adequate time to sample each odorant (i.e., 2–3 times during each odorant-ON period) prior to making a behavioral response while breathing at a comfortable rate (see Paced breathing section below). The timing parameters also allowed for the presentation of 10 different odorants to prevent across trial and scan habituation.
Subjects were instructed to indicate the onset of odorants via a button press with the index finger of their right hand using a LUMItouch fiber optic response button box (Photon Control Inc.). Responses were monitored and recorded for accuracy with the same custom developed program used to control stimulus presentation (LabView 7.1, National Instruments Corp.). Higher detection scores indicate better performance.
2.5. Paced breathing
As has been previously described in detail [36], during scans breathing was paced with a visual cue to ensure that 2–3 inhalations of each presented odorant were taken during the 12-s odorant ON period. Breathing at this preset rate was practiced prior to scanning. Subjects were also instructed to maintain a “normal” breathing depth and not to sniff as sniffing can elicit task related signal change in POC in the absence of odorants [37].
2.6. Acquisition of fMRI
Imaging was performed on a Philips Medical Systems Intera 1.5 T Research dedicated whole body MRI scanner equipped with echo planar capabilities (EPI) and a SENSE head coil. A standard EPI gradient echo sequence was used: repetition time (TR) =3000 ms, echo time (TE) =40 ms, and flip angle = 90°. Spatial resolution was set by a 64 × 64 voxel matrix covering a 200 × 200 mm2 field of view (FOV) resulting in an in-plane resolution of 3.13 × 3.13 mm2. Twenty-eight 5 mm thick slices with no gap covering the whole brain were acquired with a slice orientation of 30° clockwise to the anterior commissure to posterior commissure (AC-PC) plane. This slice orientation was chosen to minimize signal dropout in the orbitofrontal and medial temporal areas due to in-plane susceptibility gradients [38]. Stimulus presentation was automatically triggered by the scanner 6 s after scanning onset, allowing the first two frames to be discarded from the fMRI dataset. This eliminated transients arising before the achievement of dynamic equilibrium. Subjects completed four olfactory scans during a single scanning session. A T1 - weighted whole brain image was also acquired at the same plane (2 mm slices with no gap, TR =25 ms, TE =3 ms, flip angle = 45°, NEX = 1, 256 × 256, FOV = 230 × 230 mm2) to aid in anatomical localization. In addition, a high-resolution EPI gradient echo image (at the same locations as the standard EPI time series images with an in-plane resolution of 0.78 × 0.78 mm2) was acquired to aid in the co-registration of the EPI data and the T1-weighted anatomical images.
2.7. Preprocessing of fMRI
Functional data at the individual subject level were preprocessed with the FMRI Expert Analysis Tool (FEAT) from FMRIB’s Software Library (FSL), version 5.4 (www.fmrib.ox.ac.uk/fsl). Each functional scan (4 per subject) was composed of 82 whole brain images, each containing 28 oblique slices. The first two images from each time series were eliminated, leaving a total of 80 images for statistical analyses. Motion correction [39], brain extraction [40], spatial smoothing (FWHM = 8 mm), intensity normalization, temporal smoothing, correction for local autocorrelation [41], co-registration, and spatial normalization into Talairach space [42,43] were applied. Brain tissue volume, normalized for subject head size, was estimated with SIENAX [44].
2.8. Statistical analysis
2.8.1. Psychophysical and fMRI behavioral response accuracy
To evaluate age- and AD-related changes in odor thresholds, UPSIT scores, and response accuracy during fMRI scanning, two-sample independent t-tests were used to compare the performance of healthy young versus old subjects and old controls versus AD patients on these measures.
2.8.2. fMRI voxel-wise analyses
Whole brain GLM analyses were applied voxel-wise in FEAT to each of the olfactory scans for each subject (i.e., first-level analyses). To account for rapid habituation in olfactory-related brain areas [45–47], first-level GLM analyses modeled an early transient odorant-induced neuronal response by shortening the odorant-ON period of the experimental paradigm to be less than the actual 12-s stimulation period (i.e., 6 s ON and 36 s OFF). As has been previously reported [36], a shortened 6-s odorant-ON boxcar function, convolved with a double gamma hemodynamic response function (HRF), is highly sensitive and specific to odorant-induced activation in primary and higher-order olfactory-related areas.
Higher-level analyses (across scans, subjects and groups) used FLAME stage 1 (FMRIB’s Local Analysis of Mixed Effects) modeling and estimation. Resulting Z-statistic images at each level were corrected for multiple comparisons using both the default FSL cluster threshold determined by Z scores > 2.3 and a more conservative threshold of 4.0, and a corrected cluster significance of p = 0.05. Motion parameters were included in the models. Contrasts were specified to identify both odorant-induced activity increases (contrast: Odorant-ON minus Clear Air) and decreases (contrast: Clear Air minus Odorant-ON).
2.8.3. fMRI ROI analyses
ROIs were automatically created using the Pickatlas software [48] and based on the Automated Anatomical Labeled Atlas [49]. Three POC ROIs, which receive direct projections from the olfactory bulb (i.e., right and left amygdala, piriform cortex [Brodmann area 34] and entorhinal cortex [Brodmann area 28]), and three secondary olfactory ROIs, which receive direct projections from primary projection areas (i.e., right and left hippocampus, insula, and orbitofrontal cortex) where chosen a priori, and used to further test our hypotheses of an age- and AD-related decrease in odorant-induced brain activation in olfactory-related brain areas. For each primary and secondary ROI, the Z value from each voxel was averaged across all four scans from each subject, separately for the left and right hemispheres. This measure of brain activation was used as the dependent variable to directly compare odorant-induced activation in left- and right-sided primary and secondary ROIs across subject groups. Models were repeated with brain volume and sex additionally evaluated as covariates.
2.8.4. Neuro-psychophysical integration
A series of analyses explored the interactions between the psychophysical and neuroimaging results in the old adults (healthy and patients) from the entorhinal region of interest using mediation and moderated-mediation analyses. Fig. 2 diagrams this model. The mediation analyses used the Process toolbox for SPSS [50] to test whether the effects of disease on detection, identification and mean brain activity within the entorhinal cortex were mediated by threshold. Significance of the mediation effect size was assessed using 10,000 bootstrap resamples and bias-corrected accelerated confidence intervals. Significance of the moderated-mediation analyses was assessed using the Johnson-Neyman technique [51–53].
Fig. 2. Mediation and moderated-mediation models.
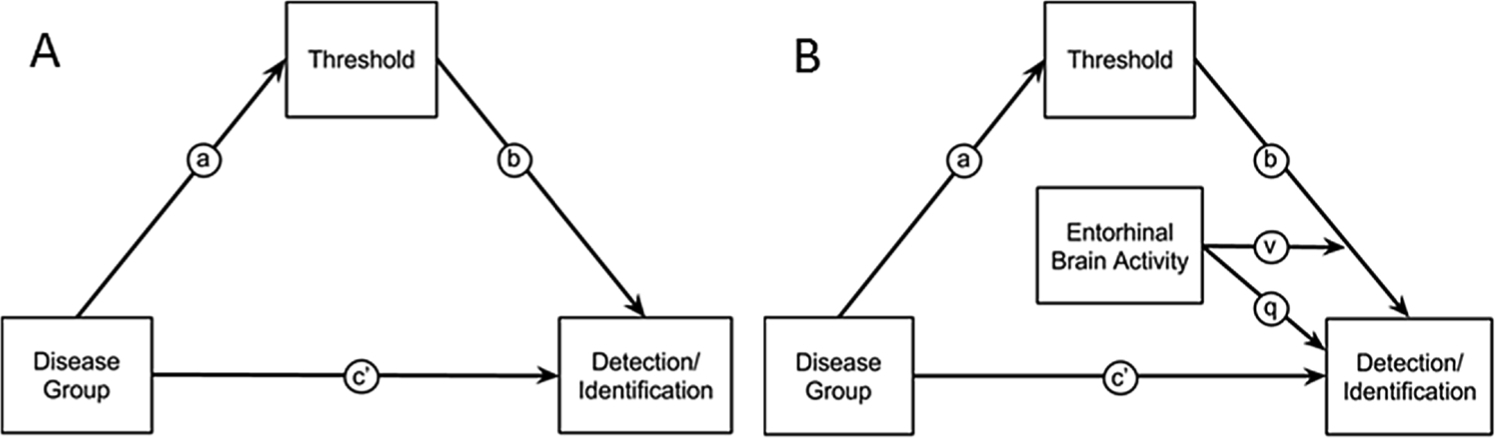
Notes: A) Model relating disease group (AD patients vs. healthy older adults) to olfactory detection/identification via a direct effect, path c’, or through an indirect effect of threshold, path through a and b. B) Moderated-mediation model that tests whether an indirect effect of group on detection/identification via threshold is moderated by the level of entorhinal brain activity. The moderating effect enters the regression equations as an interaction term, v and a main effect q.
3. Results
3.1. Psychophysical data
The healthy young and old subjects had normal olfactory function as assessed by widely used and standardized psychophysical tests (Table 1). Relative to the old controls, AD patients had significantly lower mean odor threshold scores (lower threshold test scores indicate that subjects required a higher minimum odor concentration in order to provide consistently correct responses, which corresponds to higher threshold and worse overall performance) and lower UPSIT scores (lower UPSIT scores correspond to worse odor identification performance). AD patients were not impaired on the PIT relative to the healthy old subjects.
Across all subjects, odor threshold and identification scores were significantly correlated (r = 0.72, p < 0.001). A significant correlation was also observed between PIT and UPSIT scores (r=0.37, p = 0.038), but not PIT and threshold (r=0.18, p = 0.347) scores. Both olfactory measures were correlated with performance on the Folstein MMSE (Threshold test r=0.57, p = 0.001; UPSIT r=0.72, p < 0.001), a measure of general cognitive function. Except for the threshold and UPSIT scores of AD patients (r=0.67, p = 0.018), significant correlations between any of the above-mentioned measures were not observed within subject groups (p > 0.1).
3.2. Behavioral response accuracy during fMRI scans
One-way ANOVA determined there was a significant effect of group on absolute motion (F(2,31) = 3.953, p = .030). A post hoc LSD test revealed this was driven by the AD group (0.43 ± 0.21 mm) having significantly greater (p = .042) absolute motion than the young group (0.25 ± 0.12 mm), whereas there was no significant difference (p = .087) between the AD and old group (0.27 ± 0.15 mm) or between the old and young groups (p = .941). There was no significant effect of group (AD, 0.14 ± 0.14 mm; Old, 0.13 ± 0.06 mm; Young, 0.10 ± 0.05 mm) on relative motion (F(2,31) = 1.595, p = .220).
All healthy young and old subjects accurately detected the onset of the suprathreshold odorants during the olfactory fMRI scans with at least 85 % accuracy (Table 1), demonstrating attendance to the stimuli for the duration of the scanning session. Consistent with their poor performance on psychophysical testing, patients performed significantly worse than the healthy old controls in detecting the onset of the odorants during scanning (Table 1). During debriefing after scans, AD patients reported being able to detect the different odorants during scanning, but often forgot to press the response button. Hence the response rate of 64 % may underestimate the detection of the presented odorants by the AD patients. Across all subjects, mean response accuracy was correlated with odor threshold (r = 0.66, p < 0.001) and UPSIT (r = 0.58, p = 0.001) scores, and with MMSE scores (r = 0.70, p < 0.001).
3.3. Whole brain activation
Fig. 3 shows the SPM maps and Table 2 lists the location, size and maximum Z values of the observed clusters of activation for each subject group using a conservative threshold of Z>4.0. For the healthy young, two separate clusters of voxels extended through POC areas (i.e., amygdala, piriform and entorhinal cortices) and into the hippocampus of both hemispheres.
Fig. 3. Statistical Parametric maps showing odorant-induced voxel-wise activation derived from GLM analyses averaged across the four olfactory scans for each subject group.
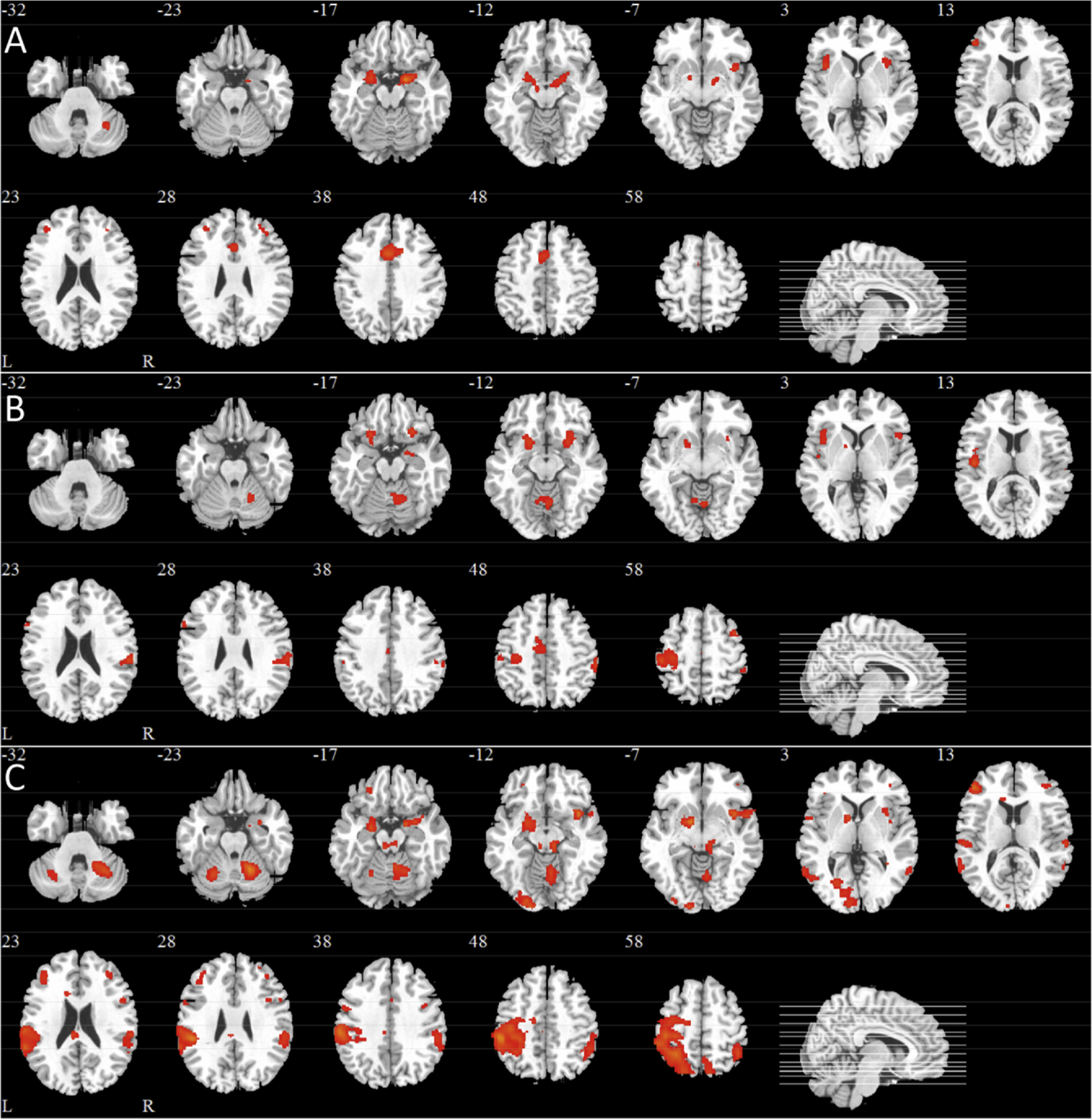
Notes: A) Healthy Young, B) Healthy Old, C) AD Patients. All images were thresholded using clusters determined by Z scores 4.0, respectively, and a corrected cluster significance of p = 0.05. Twelve slices were selected (i.e., the Z Talairach coordinate for each slice is indicated and corresponding slice lines are shown in the sagittal image on the bottom right of each figure). L, left hemisphere; R, right hemisphere.
Table 2.
Clusters of odorant-induced activation found with voxel-wise GLM analyses.
| Group | Brain Area | H | Talairach coordinates |
Z (max) | k | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
|
| |||||||
| Amygdala/Piriform/Entorhinal/Hippocampus/Globus Pallidus (BA 34/37/28) | L | −20 | 1 | −14 | 5.07 | 241 | |
| Piriform/Amygdala/Entorhinal/Hippocampus (BA 34/28) | R | 20 | −3 | −15 | 5.85 | 334 | |
| Insula (BA 1¾8) | L | −34 | 14 | 1 | 4.77 | 152 | |
| Healthy Young | Insula (BA 1¾8) | R | 42 | 9 | −7 | 4.66 | 177 |
| Cingulate, anterior/middle (BA 24/32) | BL | −2 | 13 | 36 | 5.55 | 761 | |
| Frontal, middle gyrus (BA 10/45/46) | L | −42 | 39 | 9 | 4.78 | 114 | |
| Frontal, middle gyrus (BA 10/46) | R | 34 | 42 | 22 | 4.44 | 55 | |
| Cerebellum | R | 36 | −56 | −26 | 4.81 | 65 | |
| Piriform/Amygdala/Entorhinal/Hippocampus/Globus Pallidus/Putamen (BA 34) | L | −20 | −4 | −12 | 4.52 | 227 | |
| Piriform/Amygdala/Entorhinal/Hippocampus/Insula (BA 34/28/13) | R | 20 | −5 | −17 | 4.60 | 262 | |
| Cingulate, middle (BA 24/32) | R | 4 | 12 | 38 | 4.31 | 42 | |
| Frontal, Middle gyrus (BA 10/45/46) | L | −42 | 41 | 11 | 5.46 | 359 | |
| Frontal, Middle gyrus (BA 9/44) | R | 44 | 13 | 31 | 4.64 | 84 | |
| Frontal, Middle gyrus/Inferior Orbitofrontal cortex (BA 10/46/47) | R | 38 | 40 | −7 | 4.53 | 222 | |
| Frontal, Middle Orbitofrontal (BA 11) | L | −22 | 36 | −17 | 4.63 | 39 | |
| Frontal, Inferior gyrus (BA 44/9) | R | 51 | 11 | 22 | 4.39 | 60 | |
| Healthy Old | Frontal, Precentral gyrus (BA 6) | L | −50 | 2 | 35 | 4.58 | 92 |
| Frontal, Precentral gyrus (BA 6) | R | 40 | −9 | 59 | 4.47 | 71 | |
| Temporal, Superior pole (BA 22/38) | L | −51 | 6 | −2 | 4.79 | 101 | |
| Parietal, Supramarginal/Inferior & Superior lobule (BA 2/7/40/48) | L | −46 | −32 | 27 | 5.95 | 7053 | |
| Parietal, Supramarginal/Inferior & Superior lobule (BA 2/7/40/48) | R | 57 | −33 | 31 | 5.27 | 1455 | |
| Parietal, Precuneus (BA 7) | R | 6 | −56 | 53 | 4.92 | 297 | |
| Occipital, Inferior gyrus/Precuneus (BA 18) | L | −22 | −92 | −6 | 5.31 | 655 | |
| Cerebellum | L | −20 | −59 | −16 | 5.1 | 295 | |
| Cerebellum | R | 20 | −53 | −19 | 5.84 | 1039 | |
| Cerebellum | R | 26 | −60 | −42 | 4.94 | 197 | |
| Inferior Orbitofrontal cortex /Putamen/Globus Pallidus (BA 47/48) | L | −22 | 17 | −14 | 5.13 | 256 | |
| Inferior Orbitofrontal cortex/Piriform/Amygdala/ Entorhina/Hippocampusl (BA 47/48/32) | R | 28 | 21 | −14 | 5.17 | 263 | |
| Insula (BA 13) | L | −38 | 19 | −1 | 5.1 | 323 | |
| Cingulate, Middle (BA 23/24) | BL | 0 | −14 | 32 | 4.44 | 229 | |
| Frontal, Inferior gyrus (BA 44) | L | −57 | 15 | 23 | 4.42 | 53 | |
| Frontal, Inferior gyrus (BA 47) | R | 50 | 18 | 1 | 4.27 | 53 | |
| Frontal, Middle gyrus (BA 6) | R | 40 | 8 | 53 | 4.82 | 92 | |
| Frontal, Pre-/ Postcentral gyrus (BA 4/6) | L | −28 | −16 | 67 | 5.4 | 1371 | |
| Parietal, Supramarginal/ (BA 40/48) | R | 59 | −31 | 44 | 5.22 | 528 | |
| Cerebellum | R | 20 | −53 | −16 | 4.87 | 380 | |
Notes: GLM analyses used a cluster threshold determined by Z scores>4.0 and a corrected cluster significance of p = 0.05.
A second pair of clusters occurred in the left and right insula and a third pair in the left and right middle frontal gyrus. A final cluster was observed in the right cerebellum.
The healthy old subjects also showed similar robust activation in all POC areas, which also extended to the hippocampus and right insula (Fig. 2b). Relative to the healthy young subjects, activation was observed in the orbitofrontal cortex, cingulate activation appeared somewhat reduced, insular activation was restricted to the right, occipital and cerebellar activation (right > left) was observed. Notably, in the healthy old subjects, activation outside of the core olfactory areas (i.e., primary and secondary olfactory projection areas) was much more widespread than that observed in the healthy young. In particular, more diffuse areas of activation were observed in parietal areas, particularly on the left side, extending from the pre- and postcentral gyrus into the supramarginal gyrus and inferior and superior parietal lobules.
AD patients also exhibited activation at the frontotemporal junction (Fig. 2c). Activation was centered in the orbitofrontal cortex and striatum extending into POC areas, particularly on the right side. Robust left side insular activation extending to frontal and temporal regions and bilateral cingulate activation was also observed. While AD patients showed activation in areas outside of the core olfactory regions, there was a marked reduction in posterior activation (e.g., supramarginal gyrus) relative to the healthy old subjects. Cerebellar activation was prominent on the right side.
3.4. ROI analyses
Table 3 lists the mean Z values of each primary and secondary ROI in the left and right hemispheres across subject groups.
Table 3.
Mean Z values for primary (POC) and secondary (SOC) olfactory projection areas across subject groups.
| POC | H | Young (n = 10) | Old (n = 10) | AD (n = 12) |
|---|---|---|---|---|
|
| ||||
| Amygdaloid | L | 3.32 (1.52) | 3.44 (1.84) | 1.96 (1.57) |
| Complex | R | 2.93 (1.54) | 2.86 (1.31) | 2.19 (1.35) |
| Piriform | L | 2.99 (1.20) | 2.99 (1.67) | 1.96 (1.51) |
| Cortex | R | 2.75 (1.27) | 2.51 (1.41) | 1.88 (1.13) |
| Entorhinal | L | 1.92 (0.87) | 2.26 (0.87) | 1.53 (0.72) |
| Cortex | R | 1.94 (0.64) | 2.48 (1.01) | 1.57 (0.73) |
| SOC | L | 1.50 (0.50) | 2.15 (0.84) | 1.56 (0.62) |
| Hippocampus | R | 1.60 (0.63) | 1.90 (0.67) | 1.61 (0.95 |
| Insular | L | 2.45 (1.16) | 2.24 (1.12) | 2.01 (0.90) |
| Cortex | R | 2.35 (0.89) | 2.41 (1.18) | 1.98 (0.99) |
| Orbital Frontal | L | 1.19 (0.38) | 1.70 (0.65) | 1.42 (0.46) |
| Cortex | R | 1.27 (0.56) | 1.97 (0.73) | 1.28 (0.40) |
For POC areas, a mixed, 3 (Group: young, old, and AD) by 3 (ROI: amygdala, piriform, and entorhinal) by 2 (Hemisphere: right and left) ANOVA revealed significant main effects of Group (F [2,29] = 4.33, P = 0.023) and ROI (Huynh-Feldt adjusted F [1.4, 55.7] = 4.84, P = 0.023). No main effect of hemisphere (p = 0.20) or higher-order interaction effects (p > 0.10) were observed. LSD pairwise comparisons for the main effect of Group revealed that AD patients (Mean Z (SE), 1.85 [0.23]) had less overall POC activation than the healthy old (Mean Z (SE), 2.75 [0.25], p=0.013) and young (Mean Z (SE), 2.64 [0.25], p = 0.027) subjects; activation did not significantly differ between the healthy young and old subjects (p = 0.75). LSD pairwise comparisons for the main effect of ROI revealed that in AD patients, the entorhinal cortex (Mean Z (SE), 1.95 [0.13]) showed overall less activation than the amygdala (Mean Z (SE), 2.78 [0.25], p < 0.001) and piriform cortex (Mean Z (SE), 2.51 [0.23], p = 0.05); activation did not significantly differ between the amygdala and piriform cortex (p = 0.4). After additionally controlling for brain volume and sex, there remained a significant effect of group (F [1,25] = 3.60, P = 0.042), but not ROI (Huynh-Feldt adjusted F [1.57, 39.34] = 0.592, P = 0.519). As in the prior model, LSD pairwise comparisons for the main effect of Group revealed that AD patients (Mean Z (SE), 1.87 [0.23]) had less overall POC activation than the healthy old (Mean Z (SE), 2.63 [0.29], p=0.047) and young (Mean Z (SE), 2.68 [0.29], p=0.039) subjects; activation did not significantly differ between the healthy young and old subjects (p=0.91). Further, no main effect of brain volume (F [1,25] = 0.09, P = 0.767) or sex was found (F [1,25] = 3.19, P = 0.090). Consistent with the previous analysis, no main effect of hemisphere (p=0.138) or higher-order interaction effects (p>0.20) were observed.
To further characterize the odorant-induced response in each primary ROI across groups, simple effects analyses averaging across hemisphere were conducted. For the entorhinal cortex there was a significant effect of group (F [2,31] = 3.29, p = 0.045). Pairwise comparisons of entorhinal activation between the three subject groups revealed a significant AD-related decrease relative to the healthy old (Mean (SE), 2.37 [0.86] vs. 1.55 [0.70], respectively, p = 0.016). The main effect of group for the amygdala, p = 0.15, and piriform cortex, p = 0.19, were not significant.
For secondary olfactory projection areas, a mixed, 3 (Group: young, old, and AD) by 3 (ROI: hippocampus, insula and orbitofrontal cortex) by 2 (Hemisphere: right and left) ANOVA revealed a significant main effect of ROI (F [2,56] = 18.31, p < 0.001). No significant main effects of Group (=0.31), hemisphere (p=0.70), or higher-order interaction effects (p > 0.05) were observed. All LSD pairwise comparisons for the main effect of ROI were significant at the p<0.05 level. The insular cortex (Mean Z (SE), 2.25 [0.19]) showed overall greater activation than the hippocampus (Mean Z (SE), 1.72 [0.21], p<0.001) and orbitofrontal cortex (Mean Z (SE), 1.47 [0.09], p=0.001). Hippocampal activation was significantly greater than that observed in the orbitofrontal cortex (p=0.026). However, after brain volume and sex were added to the model, there was no longer a significant effect of ROI (F [2,50] = 0.318, P = 0.729).
3.5. Neuro-psychophysical integration analyses
Significant mediation results demonstrated that differences in threshold between AD and healthy old subjects partially explained group differences in odor identification and detection (Table 4). Although olfactory threshold was a significant mediator of disease on identification and detection, these performance measures had much greater variability in the AD group as compared to their healthy counterparts. To explain this excessive variability, moderated-mediation models tested whether the disease group differences in identification and detection explained by disease-group differences in threshold were dependent on the level of brain activity. For odorant detection there was a significant effect. Once the mean brain activity within the entorhinal cortex exceeded a Z-value of 2.4, the negative effects of disease on detection performance via threshold was no longer significant (Fig. 4A). This Z-value was calculated using the Johnson-Neyman technique. Level of brain activity had no effect on moderating the relationship between disease related differences in threshold and disease related differences in odor identification (Fig. 4B).
Table 4.
Neuro-psychophysical mediation and moderated-mediation models.
| Mediation Models |
Model Parameters |
|
||||||||
| Independent Variable | Mediating Variable | Moderator Variable | Dependent Variable | a | b | c’ | R [2] | Indirect Effect | 95 % BcB CI | |
|
| ||||||||||
| Group | Threshold | None | Identification | −3.2* | 1.5* | −6.3* | 64 | −4.8* | −10.07 – −0.71 | |
| Group | Threshold | None | Detection | −3.2* | 5.3* | −19 | 55 | −16.9* | −33.45 – −2.58 | |
| Group | Threshold | None | Brain | −4.6* | −0.05 | −.93 | 35 | .23 | −0.40 – 1.19 | |
| Group | Brain | None | Identification | −0.70 | −2.3 | −14* | 50 | 1.6 | −2.62 – 5.14 | |
| Group | Brain | None | Detection | −0.70 | 11.7 | −27 | 44 | −8.2 | −29.47 – 1.12 | |
|
| ||||||||||
| Moderated-Mediation Models | p | v | ||||||||
|
| ||||||||||
| Group | Threshold | Brain | Identification | −4.6* | 1.6 | −6.2 | 66 | −1.5 | −0.0 | −7.13 – 10.02 |
| Group | Threshold | Brain | Detection | −4.6* | 13.2 | −1.2 | 79 | 33* | −3.6* | −1.44–45.75 |
Notes: The models refer to either the simple mediation model, Fig. 2A, or the moderated-mediation model, Fig. 2B. BcB CI: bias-corrected bootstrap confidence interval for the indirect effect in the mediation models, and for the conditional indirect effect in the moderated-mediation models. Brain: refers to mean functional brain activity in the entorhinal cortex. All models covary for sex. Models which include brain activity additionally covary for mean paced respiration adherence.
p < 0.05.
Fig. 4. Relationship between mean brain activity in the entorhinal cortex and the effect of disease group differences in threshold on odorant detection (A) and identification (B).
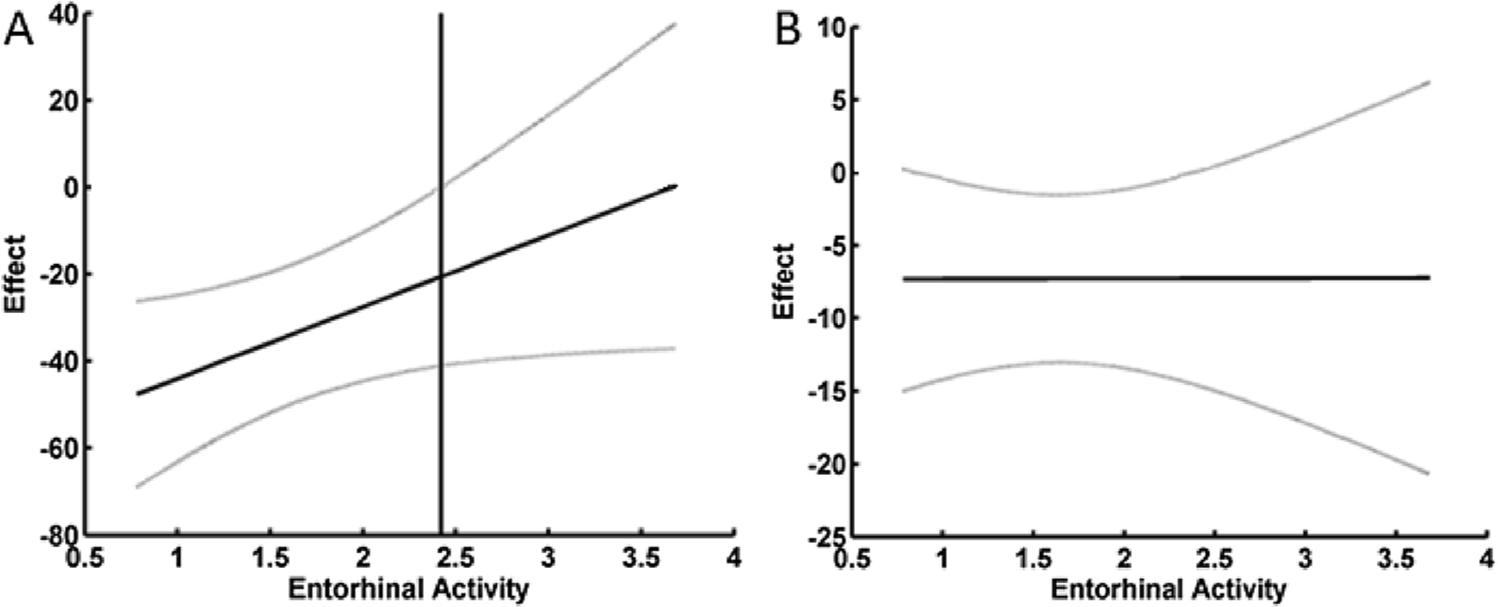
Notes: A) For odor detection there was a significant interaction between brain activity and threshold in predicting odorant detection. The vertical line is the point above which increasing brain activity renders the effect that disease-related differences in threshold have on detection non-significant at p < 0.05. This Z-value of 2.42 was calculated using the Johnson-Neyman technique for probing the interaction. B) For odorant identification there was no significant interaction between brain activity and threshold in predicting odorant identification. The grey lines are the 95 % confidence intervals around the moderated effect.
4. Discussion
The AD group performed significantly worse than the healthy old and younger adult controls on odorant threshold (corresponding to higher threshold), odorant identification, and odorant detection performance. This is in line with the known AD-related effects on olfactory functioning [7,54]. The groups did not differ on a picture identification analog to the odorant identification test, suggesting that the odorant identification deficits in mild AD patients do not have a cognitive basis (unlike odor identification deficits in more advanced AD, which are expected to be driven in part by cognitive impairments in memory, language, or attention). Contrary to expectations, the healthy old and younger adults did not differ on psychophysical measures of olfactory functioning, nor on ROI-level group analyses of task-related brain activity. As psychophysical performance amongst the healthy old adults non-significantly tended to be worse than in the young adults, and the magnitude of decline in olfactory functioning of healthy adults carefully screened for concomitant medical conditions is relatively small [55], this result likely reflects insufficient statistical power to detect significance in this small sample rather than equivalency in olfactory functioning. Interestingly, in the whole brain analyses, healthy older adults exhibited select clusters of greater activation outside of olfactory networks compared to healthy young adults, in the precentral gyrus, superior temporal pole, and supramarginal gyrus. These three regions are functionally connected with the piriform cortex [56]. The superior temporal pole is involved with the semantic memory system [57] and the supramarginal gyrus is involved with such functions as phonological storage and multisensory integration [58], thus it is tempting to speculate that such age-related increases in activation of these regions during odor detection represents a functional compensatory strategy to facilitate semantically labeling odors following age-related decline in odor identification abilities.
The three groups differed in their level of brain activity during the odorant detection task. The AD patients had decreased task-related signal change in all regions of the POC, with activity in the entorhinal cortex best differentiating the groups to a greater extent than the amygdala and piriform cortex, though not after adjusting for covariates. These results support previous findings in the literature that AD leads to decreases in both structural integrity and functional activity within the POC [25,59] and secondary olfactory regions [26,27,60].
Mediation results support the hypothesis that disease-related decrement in olfactory detection and identification scores are partially due to worse (higher) odorant threshold, which reflects differential disease progression and peripheral defects [61]. Moderated-mediation results support the hypothesis that there remains residual olfactory functioning (albeit greatly reduced) within more centrally located olfactory areas in AD patients. Increasing brain activation in the entorhinal cortex moderated the negative effect of disease-related threshold changes on olfactory detection. Therefore, even in the face of higher olfactory thresholds, older adults and AD patients may have the ability to compensate for this effect with increased brain activation in a POC brain region. This was the case only for odor detection and not odor identification. Support for the residual olfactory functioning hypothesis has been reported in a meta-analysis of olfaction in AD and Parkinson’s disease, where threshold scores were found to be the least reliably impaired olfactory psychophysical measure, such that threshold scores were not consistently impaired in AD and Parkinson’s patients to the same degree as identification and recognition. Further, AD patients were found to have residual odor detection function despite deficient odor thresholds [6]. Notably, this sample contained only patients with mild AD (CDR = 1, MMSE ≥ 18/30), therefore these compensatory and residual functioning findings may not extend across moderate and severe AD stages. It may be hypothesized that the progressive worsening of odor threshold seen during later stages of AD (even compared to mild AD) is influenced by continued atrophy of the entorhinal cortex, and in turn, an inability to rely on functionally recruiting it for offsetting loss of activation in peripheral areas and other POC regions.
Given the moderating effect that entorhinal cortex had on the relationship between odor threshold and odor detection, an open question from this result is whether there are subgroups of patients who have the ability to engage the brain activity in the entorhinal cortex and those who do not. The entorhinal cortex, along with the hippocampus, is thought to be the starting point of AD pathology [28,62] suggesting that the low performing AD patients may be at a more advanced stage of disease progression than their higher performing counterparts. This may be the result of an accumulation of neurofibrillary pathology in the entorhinal cortex [63]. This finding is supported by the hypothesis that lesions in the entorhinal and transentorhinal areas are disconnecting the primary and secondary olfactory areas, thus disrupting the flow of olfactory information necessary for accurate olfactory processing [28]. Furthermore, during the debriefing session after scanning, AD patients reported being able to detect odorants during scanning, but that they often forgot to press the response button. The performance may therefore underestimate the detection of odorants by the AD patients; however, it may also be an indication of disease progression.
A key limitation of this preliminary is small sample size, and in turn, low statistical power. Another limitation to the study is unequal motion across subject groups, potentially obscuring detection of group differences. Increased head motion during scanning is a common issue in samples with dementia. We attempted to mitigate this by including motion parameters in our group-level analyses. The present study contained patients within a single stage of AD (mild), thus findings cannot be generalized to earlier or more advanced stages. Future studies should aim to include MCI patients in order to grade development of olfactory deficits in earlier stages of AD. Because we were only able to determine if an accurate button press took place in the data, and not if an omitted button press was due to odor non-detection versus forgetting task instructions, the influence of AD-related attentional and memory impairments cannot be ruled out as contributing factors towards detection accuracy. Consideration should be given to the influence of trigeminally-involved odorants on BOLD response during fMRI studies. As is the case for detection threshold of olfactory stimuli, detection threshold for trigeminal stimuli changes across the lifespan [64], though the neural processing of trigeminal stimuli as distinct from pure odorants across AD stages is presently unclear.
5. Conclusions
Patients with AD exhibit worse (higher) odor threshold, odor identification, and odor detection-related brain activity in the POC and secondary olfactory regions. Odor threshold mediates the disease-related decrement in odor identification and odor detection. Entorhinal cortex activity best differentiates AD from healthy older and younger adults, with heightened brain activity in this region moderating the negative effect of disease-related threshold changes on olfactory detection. Wider recruitment of the entorhinal cortex during odor detection tasks may therefore represent a compensatory response [65–67] to disease progression as captured by olfactory threshold scores [6,61,68,69]. fMRI activation induced by an olfactory detection task may eventually be useful in improving early discovery of AD and may, eventually, facilitate early treatment interventions in subjects at risk for AD.
Acknowledgments
This research was supported by the National Institute on Aging, grant numbers 1K01AG21548 to M.H. Tabert, PhD, and R01AG17761 and R01AG041795 to D.P. Devanand, MD, National Institute of Mental Health fellowship 2T32MH020004-21 to J.N. Motter, PhD, and the Alzheimer’s Association, grant number IIRG-02-4126 to D.P. Devanand, MD. We wish to thank Felix Buccellato of Custom Essence Inc., Somerset NJ, for preparing the odorants used in this study; Bill Thomas for technical expertise and assistance in constructing the fMRI compatible olfactometer; Tyler Lorig, PhD, for helpful discussion and olfactometry expertise; Eric Zarahn, PhD., for help with image analysis of pilot data; and Richard L. Doty, PhD, and Scott A. Small, MD, for continued advice and support.
Declaration of Competing Interest
DPD: Consultant to Eisai, Genentech, Axovant, Astellas, Acadia. Research support: NIA, DOD, Avanir.
Footnotes
CRediT authorship contribution statement
Jason Steffener: Conceptualization, Methodology, Investigation, Formal analysis, Writing - original draft. Jeffrey N. Motter: Formal analysis, Data curation, Visualization, Writing - original draft. Matthias H. Tabert: Conceptualization, Methodology, Investigation, Writing - review & editing. D.P. Devanand: Conceptualization, Methodology, Supervision, Writing - review & editing.
References
- [1].Doty RL, Shaman P, Applebaum SL, et al. , Smell identification ability: changes with age, Science 226 (1984) 1441–1443. [DOI] [PubMed] [Google Scholar]
- [2].Cain WS, Stevens JC, Uniformity of olfactory loss in aging, Ann. N. Y. Acad. Sci 561 (1989) 29–38. [DOI] [PubMed] [Google Scholar]
- [3].Murphy C, Schubert CR, Cruickshanks KJ, et al. , Prevalence of olfactory impairment in older adults, JAMA J. Am. Med. Assoc 288 (2002) 2307–2312. [DOI] [PubMed] [Google Scholar]
- [4].Choudhury ES, Moberg P, Doty RL, Influences of age and sex on a microencapsulated odor memory test, Chem. Senses 28 (2003) 799–805. [DOI] [PubMed] [Google Scholar]
- [5].Mackay-Sim A, Grant L, Owen C, et al. , Australian norms for a quantitative olfactory function test, J. Clin. Neurosci 11 (2004) 874–879. [DOI] [PubMed] [Google Scholar]
- [6].Mesholam RI, Moberg PJ, Mahr RN, et al. , Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases, Arch. Neurol 55 (1998) 84–90. [DOI] [PubMed] [Google Scholar]
- [7].Murphy C, Olfactory and other sensory impairments in Alzheimer disease, Nat. Rev. Neurol 15 (2019) 11–24. [DOI] [PubMed] [Google Scholar]
- [8].Devanand DP, Michaels-Marston KS, Liu X, et al. , Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up, Am. J. Psychiatry 157 (2000) 1399–1405. [DOI] [PubMed] [Google Scholar]
- [9].Tabert MH, Liu X, Doty RL, et al. , A 10-item smell identification scale related to risk for Alzheimer’s disease, Ann. Neurol 58 (2005) 155–160. [DOI] [PubMed] [Google Scholar]
- [10].Savic I, Gulyas B, Larsson M, et al. , Olfactory functions are mediated by parallel and hierarchical processing, Neuron 26 (2000) 735–745. [DOI] [PubMed] [Google Scholar]
- [11].Zucco GM, Olfactory performance assessed via a new odour recognition test: Reliability and normative data, J. Cogn. Psychol 23 (2011) 1–7. [Google Scholar]
- [12].Zucco GM, Ingegneri G, Olfactory deficits in HIV-infected patients with and without AIDS dementia complex, Physiol. Behav 80 (2004) 669–674. [DOI] [PubMed] [Google Scholar]
- [13].Kovacs I, Torok I, Zombori J, et al. , Cholinergic structures and neuropathologic alterations in the olfactory bulb of Alzheimer’s disease brain samples, Brain Res. 789 (1998) 167–170. [DOI] [PubMed] [Google Scholar]
- [14].Meisami E, Mikhail L, Baim D, et al. , Human olfactory bulb: aging of glomeruli and mitral cells and a search for the accessory olfactory bulb, Ann. N. Y. Acad. Sci 855 (1998) 708–715. [DOI] [PubMed] [Google Scholar]
- [15].Kovacs T, Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders, Ageing Res. Rev 3 (2004) 215–232. [DOI] [PubMed] [Google Scholar]
- [16].Kovacs T, Cairns NJ, Lantos PL, Olfactory centres in Alzheimer’s disease: olfactory bulb is involved in early Braak’s stages, Neuroreport 12 (2001) 285–288. [DOI] [PubMed] [Google Scholar]
- [17].Yousem DM, Maldjian JA, Hummel T, et al. , The effect of age on odor-stimulated functional MR imaging, AJNR Am. J. Neuroradiol 20 (1999) 600–608. [PMC free article] [PubMed] [Google Scholar]
- [18].Murphy C, Jernigan TL, Fennema-Notestine C, Left hippocampal volume loss in Alzheimer’s disease is reflected in performance on odor identification: a structural MRI study, J. Int. Neuropsychol. Soc. JINS 9 (2003) 459–471. [DOI] [PubMed] [Google Scholar]
- [19].Suzuki Y, Critchley HD, Suckling J, et al. , Functional magnetic resonance imaging of odor identification: the effect of aging, J. Gerontol. A Biol. Sci. Med. Sci 56 (2001) M756–760. [DOI] [PubMed] [Google Scholar]
- [20].Cerf-Ducastel B, Murphy C, FMRI brain activation in response to odors is reduced in primary olfactory areas of elderly subjects, Brain Res. 986 (2003) 39–53. [DOI] [PubMed] [Google Scholar]
- [21].Ferdon S, Murphy C, The cerebellum and olfaction in the aging brain: a functional magnetic resonance imaging study, Neuroimage 20 (2003) 12–21. [DOI] [PubMed] [Google Scholar]
- [22].Kareken DA, Mosnik DM, Doty RL, et al. , Functional anatomy of human odor sensation, discrimination, and identification in health and aging, Neuropsychology 17 (2003) 482–495. [DOI] [PubMed] [Google Scholar]
- [23].Murphy C, Cerf-Ducastel B, Calhoun-Haney R, et al. , ERP, fMRI and functional connectivity studies of brain response to odor in normal aging and Alzheimer’s disease, Chem. Senses 30 (Suppl 1) (2005) i170–171. [DOI] [PubMed] [Google Scholar]
- [24].Wang J, Eslinger PJ, Smith MB, et al. , Functional magnetic resonance imaging study of human olfaction and normal aging, J. Gerontol. A Biol. Sci. Med. Sci 60 (2005) 510–514. [DOI] [PubMed] [Google Scholar]
- [25].Vasavada MM, Martinez B, Wang JL, et al. , Central olfactory dysfunction in Alzheimer’s disease and mild cognitive impairment: a functional MRI study, J. Alzheimer Dis 59 (2017) 359–368. [DOI] [PubMed] [Google Scholar]
- [26].Lu JM, Yang Q, Zhang H, et al. , Disruptions of the olfactory and default mode networks in Alzheimer’s disease, Brain Behav. 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang JL, Eslinger PJ, Doty RL, et al. , Olfactory deficit detected by fMRI in early Alzheimer’s disease, Brain Res. 1357 (2010) 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Murphy C, Olfactory functional testing: sensitivity and specificity for Alzheimer’s disease, Drug Dev. Res 56 (2002) 123–131. [Google Scholar]
- [29].McKhann G, Drachman D, Folstein M, et al. , Clinical diagnosis of alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on alzheimer’s disease, Neurology 34 (1984) 939–944. [DOI] [PubMed] [Google Scholar]
- [30].Hughes CP, Berg L, Danziger WL, et al. , A new clinical scale for the staging of dementia, Br. J. Psychiatry 140 (1982) 566–572. [DOI] [PubMed] [Google Scholar]
- [31].Kobal G, Klimek L, Wolfensberger M, et al. , Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds, Eur. Arch. Otorhinolaryngol 257 (2000) 205–211. [DOI] [PubMed] [Google Scholar]
- [32].Doty RL, Shaman P, Kimmelman CP, et al. , University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic, Laryngoscope 94 (1984) 176–178. [DOI] [PubMed] [Google Scholar]
- [33].Vollmecke T, Doty R, Development of the picture identification test (PIT)-a research companion to the University of Pennsylvania Smell Identification Test (UPSIT), Chem. Senses 10 (1985) 413–414. [Google Scholar]
- [34].Vollmecke T, Doty R, Development of the Picture Identification Test (PIT)-A research companion to the University-of-Pennsylvania Smell Identification Test (UPSIT), Oxford Univ Press Walton St Journals Dept, Oxford, ENGLAND OX2 6DP, 1985. [Google Scholar]
- [35].Lorig TS, Elmes DG, Zald DH, et al. , A computer-controlled olfactometer for fMRI and electrophysiological studies of olfaction, Behav. Res. Methods Instrum. Comput 31 (1999) 370–375. [DOI] [PubMed] [Google Scholar]
- [36].Tabert MH, Steffener J, Albers MW, et al. , Validation and optimization of statistical approaches for modeling odorant-induced fMRI signal changes in olfactory-related brain areas, Neuroimage 34 (2007) 1375–1390. [DOI] [PubMed] [Google Scholar]
- [37].Sobel N, Prabhakaran V, Desmond JE, et al. , Sniffing and smelling: separate subsystems in the human olfactory cortex, Nature 392 (1998) 282–286. [DOI] [PubMed] [Google Scholar]
- [38].Deichmann R, Gottfried JA, Hutton C, et al. , Optimized EPI for fMRI studies of the orbitofrontal cortex, Neuroimage 19 (2003) 430–441. [DOI] [PubMed] [Google Scholar]
- [39].Jenkinson M, Smith S, A global optimisation method for robust affine registration of brain images, Med. Image Anal 5 (2001) 143–156. [DOI] [PubMed] [Google Scholar]
- [40].Smith SM, Fast robust automated brain extraction, Hum. Brain Mapp 17 (2002) 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Woolrich MW, Ripley BD, Brady M, et al. , Temporal autocorrelation in univariate linear modeling of FMRI data, Neuroimage 14 (2001) 1370–1386. [DOI] [PubMed] [Google Scholar]
- [42].Talairach J, Tournoux P, Co-planar Stereotaxic Atlas of the Human Brain: 3-dimensional Proportional System: an Approach to Cerebral Imaging, G. Thieme, 1988. [Google Scholar]
- [43].Jenkinson M, Bannister P, Brady M, et al. , Improved optimization for the robust and accurate linear registration and motion correction of brain images, Neuroimage 17 (2002) 825–841. [DOI] [PubMed] [Google Scholar]
- [44].Smith SM, Zhang Y, Jenkinson M, et al. , Accurate, robust, and automated longitudinal and cross-sectional brain change analysis, Neuroimage 17 (2002) 479–489. [DOI] [PubMed] [Google Scholar]
- [45].Wilson DA, Habituation of odor responses in the rat anterior piriform cortex, J. Neurophysiol 79 (1998) 1425–1440. [DOI] [PubMed] [Google Scholar]
- [46].Sobel N, Prabhakaran V, Zhao Z, et al. , Time course of odorant-induced activation in the human primary olfactory cortex, J. Neurophysiol 83 (2000) 537–551. [DOI] [PubMed] [Google Scholar]
- [47].Poellinger A, Thomas R, Lio P, et al. , Activation and habituation in olfaction–an fMRI study, Neuroimage 13 (2001) 547–560. [DOI] [PubMed] [Google Scholar]
- [48].Maldjian JA, Laurienti PJ, Kraft RA, et al. , An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets, Neuroimage 19 (2003) 1233–1239. [DOI] [PubMed] [Google Scholar]
- [49].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. , Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain, Neuroimage 15 (2002) 273–289. [DOI] [PubMed] [Google Scholar]
- [50].Hayes AF, Introduction to Mediation, Moderation, and Conditional Process Analysis: a Regression-based Approach, The Guilford Press, New York, 2013. [Google Scholar]
- [51].Johnson PO, Neyman J, Tests of certain linear hypotheses and their application to some educational problems, Stat. Res. Mem 1 (1936) 57–93. [Google Scholar]
- [52].Johnson PO, Fay LC, The Johnson-Neyman technique, its theory and application, Psychometrika 15 (1950) 349–367. [DOI] [PubMed] [Google Scholar]
- [53].Hayes AF, Matthes J, Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations, Behav. Res. Methods 41 (2009) 924–936. [DOI] [PubMed] [Google Scholar]
- [54].Md M.E. Silva, Mercer PBS, Witt MCZ, et al. , Olfactory dysfunction in Alzheimer’s disease Systematic review and meta-analysis, Dement. Neuropsychol 12 (2018) 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mackay-Sim A, Johnston AN, Owen C, et al. , Olfactory ability in the healthy population: reassessing presbyosmia, Chem. Senses 31 (2006) 763–771. [DOI] [PubMed] [Google Scholar]
- [56].Zhou G, Lane G, Cooper SL, et al. , Characterizing functional pathways of the human olfactory system, eLife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mesulam M-M, Thompson CK, Weintraub S, et al. , The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia, Brain 138 (2015) 2423–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ripp I, Zur Nieden AN, Blankenagel S, et al. , Multisensory integration processing during olfactory-visual stimulation-An fMRI graph theoretical network analysis, Hum. Brain Mapp 39 (2018) 3713–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kareken DA, Doty RL, Moberg PJ, et al. , Olfactory-evoked regional cerebral blood flow in Alzheimer’s disease, Neuropsychology 15 (2001) 18–29. [DOI] [PubMed] [Google Scholar]
- [60].Buchsbaum MS, Kesslak JP, Lynch G, et al. , Temporal and hippocampal metabolic rate during an olfactory memory task assessed by positron emission tomography in patients with dementia of the Alzheimer type and controls. Preliminary studies, Arch. Gen. Psychiatry 48 (1991) 840–847. [DOI] [PubMed] [Google Scholar]
- [61].Murphy C, Gilmore MM, Seery CS, et al. , Olfactory thresholds are associated with degree of dementia in Alzheimer’s disease, Neurobiol. Aging 11 (1990) 465–469. [DOI] [PubMed] [Google Scholar]
- [62].Braak H, Braak E, Staging of Alzheimer-related cortical destruction, Int. Psychogeriatrics / IPA 9 (Suppl 1) (1997) 257–261, discussion 269–272. [PubMed] [Google Scholar]
- [63].Wilson RS, Arnold SE, Schneider JA, et al. , The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age, J. Neurol Neurosurg. Psychiatry 78 (2007) 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Murphy C, Age-related effects on the threshold, psychophysical function, and pleasantness of menthol, J. Gerontol 38 (1983) 217–222. [DOI] [PubMed] [Google Scholar]
- [65].Persson J, Nyberg L, Altered brain activity in healthy seniors: what does it mean? Prog. Brain Res 157 (2006) 45–56. [DOI] [PubMed] [Google Scholar]
- [66].Stern Y, Cognitive reserve and Alzheimer disease, Alzheimer Dis. Assoc. Disord 20 (2006) 112–117. [DOI] [PubMed] [Google Scholar]
- [67].Steffener J, Stern Y, Exploring the neural basis of cognitive reserve in aging, Biochim. Biophys. Acta 1822 (2012) 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Larsson M, Nilsson LG, Olofsson JK, et al. , Demographic and cognitive predictors of cued odor identification: evidence from a population-based study, Chem. Senses 29 (2004) 547–554. [DOI] [PubMed] [Google Scholar]
- [69].Rahayel S, Frasnelli J, Joubert S, The effect of Alzheimer’s disease and Parkinson’s disease on olfaction: a meta-analysis, Behav. Brain Res 231 (2012) 60–74. [DOI] [PubMed] [Google Scholar]


