To the Editor:
Cerebral amyloid angiopathy (CAA) is the deposition of β-amyloid protein in the vessel walls (particularly the tunica media) of arteries and arterioles of the central nervous system. While CAA may be clinically silent, it is a frequent underlying cause of intracerebral hemorrhage (ICH) and contributor to cognitive impairment in elderly populations (1), although there is significant variation in the incidence and prevalence of CAA between cohorts (2). Although CAA (including CAA causing ICH) can be found in individuals lacking Alzheimer disease (AD) pathology, the vast majority of AD patients have some degree of CAA pathology. Studies have suggested that 83%–98% of AD patients have at least mild CAA (normal appearing vessels with immunohistochemical evidence of β-amyloid in smooth muscle cells) (2–5), and approximately 25%–60% of these subjects were reported to have moderate to severe levels of CAA (3, 4), where “moderate CAA” was defined as β-amyloid replacement of the tunica media in parenchymal vessels without hemorrhage, and “severe CAA” was defined as moderate CAA with additional evidence of vessel wall damage and erythrocyte leakage (4–6). Examination of our own cohorts revealed a 39.3% incidence of moderate to severe CAA in patients with histopathologically confirmed AD. This vascular pathology and resulting hemorrhage have been associated with the presence of the Apolipoprotein E (APOE) ε2 allele as well as the APOE ε4 allele (7–9).
Primary age-related tauopathy (PART) is a β-amyloid-independent tauopathy with some key similarities and differences to AD. PART is currently defined by the presence of phospho-tau (p-tau)-immunoreactive neurofibrillary tangles (NFTs) composed of both 3R- and 4R-tau isoforms primarily deposited in the temporal allocortex, corresponding to Braak stages I–IV, although unlike AD there is minimal to no NFT spread to neocortical structures (10) and a different pathoanatomical pattern of hippocampal NFT deposition (11, 12). Furthermore, PART is associated with an increased frequency of the APOE ε2 allele while sporadic AD is associated with increased APOE ε4 (13), and unlike AD, clinical symptoms in PART do not correlate well with Braak staging (12, 14). The diagnosis of PART is divided into 2 subcategories: “definite PART,” comprised of cases completely devoid of β-amyloid (Thal phase 0 and CERAD neuritic plaque score “none”), and “possible PART,” or cases with a small degree of β-amyloid deposition (Thal phase 1-2 and/or CERAD neuritic plaque score “sparse”). Given the relative lack of parenchymal amyloid deposition in PART, we investigated the incidence of moderate to severe CAA in 2 cohorts of PART, a multi-institutional cohort (n = 477) and a smaller institutional cohort (n = 74), using standard neuropathologic methods.
Our results indicate a significantly higher frequency of moderate to severe CAA in possible PART cases (those with early stages of parenchymal β-amyloid deposition; 17.8%–21.9%) compared to definite PART cases in both cohorts (3.7–4.8%; p < 0.0001; Table). These groups were significantly different in terms of the frequency of CAA using both Thal and CERAD criteria to distinguish between definite and possible PART cases. Previous work on the National Alzheimer’s Coordinating Center (NACC) dataset revealed a similar trend, but higher frequencies of CAA in both neuritic plaque-negative (19.3%; n = 166) and neuritic plaque-sparse cases (54.1%; n = 205; p < 0.0001), although it is worth noting that Thal phase was not available for the neuritic plaque-negative cases, so it is possible that this group includes cases that meet the criteria of both “definite PART” and “possible PART” (15). No significant relationship was identified between patient age or Braak stage and the frequency of CAA (p = 0.3145 and p = 0.4562, respectively), and no significant differences in the frequency of CAA were found with the presence of any particular APOE allele (ε2, ε3, ε4) or with any APOE allele combination in the cases for which APOE genotyping was available (Table).
TABLE.
Incidence of CAA in Subjects with PART
| PART Cohort 1 |
PART Cohort 2 |
||||
|---|---|---|---|---|---|
| Definite | Possible | Definite | Possible | p Value | |
| Age (years) | 84.4 ± 1.0 | 85.9 ± 1.1 | 77.6 ± 1.5 | 75.3 ± 1.7 | 0.31 |
| CAA frequency | 3.7% (12/325) | 17.8% (27/152) | 4.8% (2/42) | 21.9% (7/32) | <0.0001 |
| Braak stage | |||||
| I | 0% (0/50) | 28.6% (4/14) | 0% (0/10) | 25.0% (1/4) | |
| II | 6.5% (3/46) | 22.2% (4/18) | 0% (0/6) | 0% (0/6) | |
| III | 4.6% (4/87) | 15.6% (7/45) | 0% (0/12) | 22.2% (2/9) | |
| IV | 3.5% (5/142) | 16.0% (12/75) | 14.3% (2/14) | 30.8% (4/13) | 0.46 |
| Thal phase | |||||
| 0 | 3.7% (12/325) | – | 4.8% (2/42) | – | |
| 1 | – | 19.6% (20/102) | – | 20.0% (4/20) | |
| 2 | – | 14.0% (7/50) | – | 25.0% (3/12) | <0.0001 |
| CERAD score | |||||
| 0 | 3.7% (12/325) | 28.6% (2/7) | 4.8% (2/42) | 9.5% (2/21) | |
| 1 | – | 17.2% (25/145) | – | 45.5% (5/11) | <0.0001 |
| APOE genotype | |||||
| ε2,ε2 | 0% (0/1) | – | – | – | |
| ε2,ε3 | 15.0% (3/20) | 16.7% (2/12) | – | – | |
| ε2,ε4 | – | 0% (0/2) | – | – | |
| ε3,ε3 | 6.6% (4/61) | 16.7% (6/36) | – | – | |
| ε3,ε4 | 0% (0/5) | 30.0% (3/10) | – | – | |
| ε4,ε4 | – | – | – | – | 0.33 |
| APOE classification | |||||
| ≥1 ε2 Allele | 14.3% (5/35) | – | – | ||
| Lacking ε2 Allele | 16.1% (8/112) | – | – | 0.81 | |
| ≥1 ε3 Allele | 12.5% (18/144) | – | – | ||
| Lacking ε3 Allele | 0% (0/3) | – | – | 0.66 | |
| ≥1 ε4 Allele | 20.0% (3/15) | – | – | ||
| Lacking ε4 Allele | 11.4% (15/132) | – | – | 0.40 | |
Bold values indicate significance <0.05. PART, primary age-related tauopathy; CAA, cerebral amyloid angiopathy, all cases included as positive for CAA had either moderate or severe CAA (4–6); CERAD, Consortium to Establish a Registry for Alzheimer's Disease; APOE, Apolipoprotein E.
These cohorts of definite and possible PART, in combination with previous observations regarding the high frequency of CAA in cohorts of neuropathologically confirmed AD cases, reinforce the correlation between parenchymal and vascular β-amyloid deposition. The presence of any amyloid (by either Thal or CERAD criteria) appears to increase the likelihood of CAA. Unlike previous studies (7–9), we found no association between APOE allele frequency and the presence of CAA, although APOE status was only available in 142 of our 551 total cases. It is worth noting, however, that the presence of CAA in cases completely devoid of any cerebral β-amyloid positivity is consistent with the idea that these 2 disease processes are not inextricably linked and although rare, it is possible to have moderate or severe CAA in “definite PART” cases (Thal phase 0, CERAD neuritic plaque score “none”; Fig. 1). Additionally, previous studies have identified moderate or severe CAA in 9.2% of chronic traumatic encephalopathy cases, another 3R/4R-tauopathy (4). Given the significant risk of morbidity and mortality associated with CAA and ICH, as well as the finding that CAA is an independent risk factor for dementia (4), these data demonstrate that presence of CAA should not be ruled out clinically, even in patients with amyloid-negative (A-), tau-positive (T+) biomarkers.
FIGURE 1.
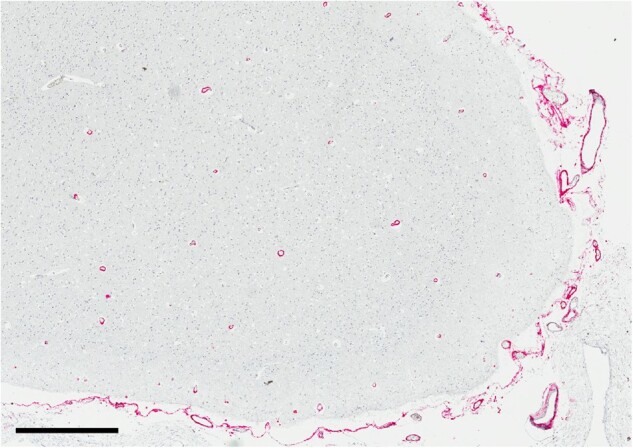
β-amyloid immunohistochemical stain (6E10) on a representative neocortical section from a patient with definite primary age-related tauopathy (PART) and moderate cerebral amyloid angiopathy (CAA), scale bar = 700 µm.
FUNDING
Portions of this work were supported by National Institute on Aging (NIA) grants P30 AG066514, P50 AG005138, R01 AG054008, and R01 NS095252.
COMPETING INTERESTS
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke 1987;18:311–24 [DOI] [PubMed] [Google Scholar]
- 2. Jellinger KA. Alzheimer disease and cerebrovascular pathology: An update. J Neural Transm (Vienna) 2002;109:813–36 [DOI] [PubMed] [Google Scholar]
- 3. Ellis RJ, Olichney JM, Thal LJ, et al. Cerebral amyloid angiopathy in the brains of patients with Alzheimer's disease: The CERAD experience, Part XV. Neurology 1996;46:1592–6 [DOI] [PubMed] [Google Scholar]
- 4. Standring OJ, Friedberg J, Tripodis Y, et al. Contact sport participation and chronic traumatic encephalopathy are associated with altered severity and distribution of cerebral amyloid angiopathy. Acta Neuropathol 2019;138:401–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vonsattel JPG, Myers RH, Hedley-Whyte ET, et al. Cerebral amyloid angiopathy without and with cerebral hemorrhages: A comparative histological study. Ann Neurol 1991;30:637–49 [DOI] [PubMed] [Google Scholar]
- 6. Greenberg SM, Vonsattel JP.. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke 1997;28:1418–22 [DOI] [PubMed] [Google Scholar]
- 7. Nicoll JA, Burnett C, Love S, et al. High frequency of apolipoprotein E epsilon 2 allele in hemorrhage due to cerebral amyloid angiopathy. Ann Neurol 1997;41:716–21 [DOI] [PubMed] [Google Scholar]
- 8. Chalmers K, Wilcock GK, Love S.. APOE epsilon 4 influences the pathological phenotype of Alzheimer's disease by favouring cerebrovascular over parenchymal accumulation of A beta protein. Neuropathol Appl Neurobiol 2003;29:231–8 [DOI] [PubMed] [Google Scholar]
- 9. Esiri M, Chance S, Joachim C, et al. Cerebral amyloid angiopathy, subcortical white matter disease and dementia: Literature review and study in OPTIMA. Brain Pathol 2015;25:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol 2014;128:755–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jellinger KA. Different patterns of hippocampal tau pathology in Alzheimer's disease and PART. Acta Neuropathol 2018;136:811–3 [DOI] [PubMed] [Google Scholar]
- 12. Walker JM, Richardson TE, Farrell K, et al. Early selective vulnerability of the CA2 hippocampal subfield in primary age-related tauopathy. J Neuropathol Exp Neurol 2021;80:102–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robinson AC, Davidson YS, Roncaroli F, et al. Influence of APOE genotype in primary age-related tauopathy. Acta Neuropathol Commun 2020;8:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iida MA, Farrell K, Walker JM, et al. Predictors of cognitive impairment in primary age-related tauopathy: An autopsy study. Acta Neuropathol Commun 2021;9:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Besser LM, Crary JF, Mock C, et al. Comparison of symptomatic and asymptomatic persons with primary age-related tauopathy. Neurology 2017;89:1707–15 [DOI] [PMC free article] [PubMed] [Google Scholar]


