Abstract
The remarkable success of the US government-backed COVID-19 vaccine development in 2020 offers several lessons on how to effectively foster rapid vaccine discovery and development. Conceptually, the formation of a public–private partnership that included innovative government and academic involvement at all levels of the program was instrumental in promulgating and overseeing the effort. Decades of NIH-sponsored research on vaccine backbones, immunogen design, and clinical trial operations enabled evaluation of vaccine candidates within months of pathogen discovery. Operation Warp Speed fostered industry participation, permitted accelerated movement from preclinical/early phase to efficacy trials, and developed structured clinical trial testing that allowed independent assessment of, yet reasonable comparison between, each vaccine platform by harmonizing protocols, endpoints, laboratories, and statistical analytical criteria for efficacy. This coordinated effort by the US government, pharmaceutical companies, regulators, and academic research institutions resulted in the streamlined, safe, and transparent development and deployment of multiple COVID-19 vaccines in under a year. Lessons learned from this collaborative endeavor should be used to advance additional vaccines of public health importance.
Current Opinion in Immunology 2022, 76:102206
This review comes from a themed issue on Vaccines
Edited by Mariagrazia Pizza and Rino Rappuoli
For complete overview of the section, please refer to the article collection, “Vaccines (August 2022)”
Available online 20th April 2022
https://doi.org/10.1016/j.coi.2022.102206
0952-7915/© 2022 Elsevier Ltd. All rights reserved.
Introduction
The rapidity and magnitude of the COVID-19 pandemic resulted in an unprecedented response from the United States government (USG) for developing COVID-19 vaccines that provides a historic case study for future vaccine development. Record speed from pathogen discovery to highly effective vaccines was achieved with several novel vaccine technologies. This was helped by an unparalleled influx of USG dollars to oversee the vaccine development process for all 5 of the companies involved in the initial effort and assist in moving vaccine products forward with oversight at all levels. Preclinical manufacturing, animal studies, supply-chain logistics, initial Phase-1 and -2 clinical trial funding, provision of laboratories for immunogenicity studies, preclinical use of nonhuman primate (NHP) challenge models, and funding of large-scale efficacy trials with a common design and overseen by a common Data and Safety Monitoring Board were all components of the program 1, 2••, 3. Importantly, government officials and government-supported contractors experienced in vaccine discovery, process development, and manufacturing served as constant liaisons with Operation Warp Speed (OWS) officials and the company executives involved in manufacturing and preclinical/clinical oversight of the program. Crucially, the USG agreed to buy at-risk from 50 to 100 million doses of vaccine from each company at ‘negotiated but market prices,’ essentially insuring both investment and development costs of their programs. In exchange, the USG assured that if the vaccine was useful, it would be distributed to all its citizens free of charge. OWS, a partnership between the Department of Health and Human Services and Department of Defense (DOD), was impressively successful and expensive (estimated $37 billion as of November 2021) [4]. It has also led to striking profits for the companies developing the mRNA vaccines. While the other industry partners sponsored by OWS have yet to ‘book such values’ in their bottom line, their subsidized costs from the OWS manufacturing and clinical trial programs make it likely that significant margins were or will be achieved by all pharmaceutical participants in the program. It is clear that the value investment was worth it. The health and economic benefits of the vaccines — population health, medical expenses averted, and economic recovery — are far greater than these costs 5, 6 and offer future models for vaccine and therapeutic drug development in high-priority areas. One avenue toward greater applicability to such models is making the vaccine-manufacturing program costs transparent and the effect sizes of the outcomes available publicly.
Public–private partnerships (PPP) for innovative vaccine design and development
The time period from pathogen discovery to vaccine approval, 11 months for mRNA and 13 months for adenovirus (Ad)-vectored vaccines, was in reality achieved from conscious planning and many years of prior hard work and scientific investment [7]. Derisking the entire process, both from the USG and the companies’ perspective, resulted from a large, established effort in basic, clinical, and vaccine science that, for most components of these vaccines, extends back at least 15 years. Preclinical NHP studies and human clinical trials defining Ad26 vector dose and safety were previously conducted by publicly supported HIV and Ebola programs 8, 9, 10, 11, 12. Development of mRNA vaccine platforms that include efficient delivery systems and overcoming issues with innate immune stimulation has been ongoing for decades 13, 14, 15. Extensive research on respiratory syncytial virus and human coronaviruses SARS-CoV-1 and Middle East respiratory syndrome (MERS) demonstrated that the spike protein could be an excellent immunogen 16, 17, 18 and its immunogenicity enhanced by stabilizing the prefusion conformation 19, 20, 21, 22, 23. This proline-stabilized prefusion protein sequence [24], developed at the National Institute of Allergy and Infectious Diseases (NIAID) Vaccine Research Center, was used in all but one vaccine in the USG portfolio; AstraZeneca/Oxford had shown that the full-length MERS spike protein elicited high neutralizing activity against MERS [25] and used full-length rather than stabilized SARS-CoV-2 spike. Thus, the years of publicly funded national and academic lab research on the vaccine insert and backbones provided a critically important ‘leg up’ going into the COVID-19 pandemic.
From the beginning, the PPP strategy assumed that each vaccine platform would be different, thus likely derisking the USG investment. Subsequent clinical trial data have shown that vaccine efficacy (VE) and durability, as well as the flavor and magnitude of immune responses, are influenced by the regimen in entirety: platform, dose, and schedule 26, 27, 28•, 29, 30, 31. It is remarkable that several very different vaccine platforms did indeed work well 29, 30, 32, 33. When widespread vaccine rollout demonstrated some safety complications that varied by platform and age 34, 35, 36, this plurality approach was appreciated and instrumental to public health. The lesson learned is that government-backed approaches with a little of bit of scientific competition and transparent, uniform assessment of safety, immunogenicity, and preclinical VE provide for an even playing field; thus allowing clinical trial assessments and implementation science to define future development.
The seamless movement from Phase 1–2 to 3 relied on rapid and thorough review of safety and efficacy data with the prenegotiated commitment that if endpoints were met and regulatory review was achieved, advancement to efficacy trials would ensue. Public funding of efficacy trials meant that the cadence of development moved efficiently, quickly, and with momentum. It also erased the need for the significant pauses (months to years) that most companies insert before initiating a large and expensive Phase-3 investment. The initial corporate-designed trials ranged from 5000 to 8000 volunteers and did not stipulate those at greatest risk — such as Black and Latinx populations — would be enrolled with adequate power for subpopulation VE analyses. Public funding allowed the Phase-3 trials to be many times larger, with 30 000–40 000 participants, and analyses of subpopulation effects and evaluation of risk factors associated with efficacy made it possible to influence public policy decisions. Importantly, persons at highest risk and highest benefit were enrolled in the trials, ensuring knowledgeable population effects within each trial and collectively. All of this was altered through the PPP and by the power that public money and trial oversight by government/academic officials provided.
The ACTIV model
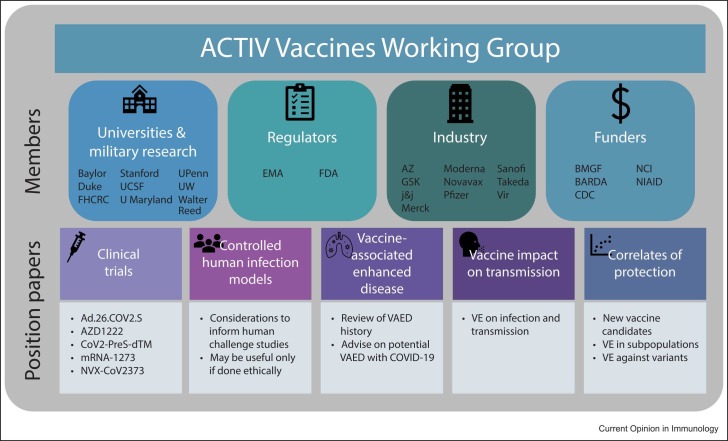
The plurality of vaccine platforms and manufacturers necessitated an open forum to discuss and deliberate problems/issues that arose, including how to define study endpoints, which laboratory assays would be most appropriate, and ensure use of robust statistical analysis plans. This forum was facilitated through the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) program coordinated by the NIH and Foundation for the NIH [37]. ACTIV is comprised of the Biomedical Advanced Research and Development Authority, Centers for Disease Control & Prevention, US Food and Drug Administration (FDA), DOD, Veterans Affairs, Countermeasures Acceleration Group (CAG, formerly OWS), representatives from academia and pharmaceutical companies, and, at times, European Medicines Agency regulators. Government officials and government- supported contractors experienced in vaccine discovery, process development, and manufacturing served as liaisons with CAG officials. The ACTIV Vaccine Working Group ( Figure 1), held weekly meetings April–August 2020 to fine-tune every component of each vaccine study. These deliberations led to consensus modifications that were acceptable to all parties involved.
Figure 1.
ACTIV Vaccines Working Group membership and objectives. The working group consisted of 26 members from academia, regulatory organizations, industry partners, and funding bodies. FHCRC, Fred Hutchinson Cancer Research Center; UCSF, University of California at San Francisco; U Maryland, University of Maryland; UPenn, University of Pennsylvania; UW, University of Washington; EMA, European Medicines Agency; AZ, AstraZeneca; GSK, GlaxoSmithKline; J&J, Johnson & Johnson; BMGF, Bill & Melinda Gates Foundation; NCI, National Cancer Institute; VAED, vaccine-associated enhanced disease.
Transparency was fundamental to this system, and thus while individuality of manufacturing processes occurred, the clinical trial programs were placed into a common framework that allowed a level playing field in clinical efficacy and safety. While each company developed their own vaccine product for testing, the trials were designed to have the same timepoints, sampling, and lab procedures to allow reasonable cross-trial comparisons [2]. This approach has stood the test of time and even non-USG-sponsored companies have modeled their efficacy trials after the USG approach [38].
Manufacturing oversight
Another unique, and perhaps most innovative and impactful, OWS/CAG feature was that the USG actively monitored the areas of manufacturing and preclinical development. With the government footing the bill, it was deemed both necessary and appropriate for outside experts to oversee supply-chain logistic issues, the pace of preclinical studies, and manufacturing processes. Here the experience of the DOD was sought out. Outside logistic and manufacturing consultants with biological/vaccine expertize ensured that the contract-manufacturing organizations would meet specifications and deadlines with transparency. Corporate partners with the USG, outside consultants, and FDA regulators identified problems and defined appropriate solutions. There were many manufacturing delays, quality- control issues, and need to redefine process development; issues that rarely come to scrutiny outside corporate walls. Decisions were public and such transparency in this back-room part of vaccine production was unprecedented. This inside look at corporate performance was instrumental in achieving the deadlines required for rapid public vaccination campaigns. As manufacturing delays, often from underresourced management oversight, are common in vaccine development, the transparency of contractual expectations was an exceptionally important part of the OWS/CAG program and its inclusion in all future PPP should be considered.
Engaging the public
One advantage of the PPP model over a traditional company-led Phase-3 trial was the ability to engage and recruit individuals for enrollment from diverse populations. This factor was especially critical for COVID-19, which has affected Black and Brown Americans 3–5 times more frequently than Caucasians 39, 40, 41. The NIAID supported COVID-19 Prevention Network (CoVPN), which helped design the Phase-3 studies and served as the operations center for academic clinical trial sites, initiated a large-scale education campaign that included developing public service announcements, an online registry for volunteers interested in enrolling in the efficacy trials, and relationships with local community experts (e.g. faith leaders) to disseminate accurate information to their communities. The clinical trial protocols were posted online, so that experts in affected communities could review the studies. Educational materials distributed to clinics helped with recruitment, and a webinar series focused on Black, Indigenous and People of Color (BIPOC), and COVID-19 have been virtually held since the inception of the trials. These programs facilitated COVID-19 vaccine-trial participation of individuals seeking to positively affect their communities rather than to help company product licensure.
The decades-long community engagement infrastructure developed by the NIAID- supported HIV Vaccine and Prevention Network programs played a critical role in successfully enrolling BIPOC individuals into the vaccine trials. In fact, because the Moderna-supported contract research organizations were initially unsuccessful in recruiting BIPOC communities, the trial enrollment period was prolonged by reducing enrollment of Caucasian subgroups, so that effective evaluation of VE in Black and Latinx populations could be assessed [42].
Another facet of the CoVPN public engagement strategy was to mitigate any perceived lack of credibility in the scientific process resulting from rapid vaccine development and approval. A symposium in October 2020 cosponsored by Johns Hopkins University and the University of Washington targeting journalists, regulators, companies, and other political stakeholders focused on several key areas: 1) science behind the COVID-19 vaccine efficacy trials; 2) protecting scientific integrity; 3) frameworks for assessing vaccine safety and efficacy; 4) ethical aspects; 5) trial inclusivity and diversity allowing assessment of highly affected communities; and 6) vaccine access and allocation [43]. This well-attended symposium highlighted the importance of each trial maintaining its scientific integrity. The process of each company submitting trial data to the FDA for Emergency Use Authorization was not affected by the 2020 US presidential election and, instead, proceeded on a scientific rather than political timeline. The subsequent vaccine hesitancy and vaccine protest that has occurred over the course of 2021 and now into 2022 can, unfortunately, be partly attributed to political ideology.
Conclusions
The success of the US COVID-19 vaccine program raises the question of why ever go back to the old days when industry chose the immunogen and conducted the trial at its pace and value evaluation? This question is not to demonize the past, but to state that more effective models of success do exist. Involving academia and the government makes the entire process more transparent, of higher quality, quicker, and more applicable to public health. In addition, corporations achieve success for a product already approved by key stakeholders. Companies are needed to manufacture and distribute vaccines, as well as democratize therapy and vaccination availability. Conversely, government and academia can provide intellectual and practical resources to the entire discovery landscape. The COVID-19 pandemic has revealed a roadmap for successful vaccine development, and we now have a path to follow. It is imperative to continue this strategy going forward.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) grant UM1 AI068614-14.
Conflict of interest statement
The authors report no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest.
References
- 1.Cable J., Rappuoli R., Klemm E.J., et al. Innovative vaccine approaches-a Keystone Symposia report. Ann N Y Acad Sci. 2022 doi: 10.1111/nyas.14739. [DOI] [PubMed] [Google Scholar]
- 2••.Corey L., Mascola J.R., Fauci A.S., Collins F.S. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368:948–950. doi: 10.1126/science.abc5312. [DOI] [PubMed] [Google Scholar]; This paper outlined a framework for harmonized COVID-19 vaccine clinical trial design, implementation and analysis.
- 3.Joffe S., Babiker A., Ellenberg S.S., et al. Data and safety monitoring of COVID-19 vaccine clinical trials. J Infect Dis. 2021;224:1995–2000. doi: 10.1093/infdis/jiab263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slaoui M., Hepburn M. Developing safe and effective covid vaccines - operation warp speed’s strategy and approach. N Engl J Med. 2020;383:1701–1703. doi: 10.1056/NEJMp2027405. [DOI] [PubMed] [Google Scholar]
- 5.Bartsch S.M., Wedlock P.T., O’Shea K.J., et al. Lives and costs saved by expanding and expediting coronavirus disease 2019 vaccination. J Infect Dis. 2021;224:938–948. doi: 10.1093/infdis/jiab233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moghadas S.M., Vilches T.N., Zhang K., et al. The impact of vaccination on coronavirus disease 2019 (COVID-19) outbreaks in the United States. Clin Infect Dis. 2021;73:2257–2264. doi: 10.1093/cid/ciab079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fauci A.S. The story behind COVID-19 vaccines. Science. 2021;372:109. doi: 10.1126/science.abi8397. [DOI] [PubMed] [Google Scholar]
- 8.Baden L.R., Stieh D.J., Sarnecki M., et al. Safety and immunogenicity of two heterologous HIV vaccine regimens in healthy, HIV-uninfected adults (TRAVERSE): a randomised, parallel-group, placebo-controlled, double-blind, phase 1/2a study. Lancet HIV. 2020;7:e688–e698. doi: 10.1016/S2352-3018(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephenson K.E., Wegmann F., Tomaka F., et al. Comparison of shortened mosaic HIV-1 vaccine schedules: a randomised, double-blind, placebo-controlled phase 1 trial (IPCAVD010/HPX1002) and a preclinical study in rhesus monkeys (NHP 17-22) Lancet HIV. 2020;7:e410–e421. doi: 10.1016/S2352-3018(20)30001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baden L.R., Walsh S.R., Seaman M.S., et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001) J Infect Dis. 2013;207:240–247. doi: 10.1093/infdis/jis670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barry H., Mutua G., Kibuuka H., et al. Safety and immunogenicity of 2-dose heterologous Ad26.ZEBOV, MVA-BN-Filo Ebola vaccination in healthy and HIV-infected adults: a randomised, placebo-controlled Phase II clinical trial in Africa. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milligan I.D., Gibani M.M., Sewell R., et al. Safety and immunogenicity of novel adenovirus Type 26- and modified vaccinia Ankara-vectored ebola vaccines: a randomized clinical trial. JAMA. 2016;315:1610–1623. doi: 10.1001/jama.2016.4218. [DOI] [PubMed] [Google Scholar]
- 13.Kariko K., Buckstein M., Ni H., Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Sahin U., Kariko K., Tureci O. mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 15.Pardi N., Hogan M.J., Pelc R.S., et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543:248–251. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L., Shi W., Joyce M.G., et al. Evaluation of candidate vaccine approaches for MERS-CoV. Nat Commun. 2015;6 doi: 10.1038/ncomms8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchholz U.J., Bukreyev A., Yang L., et al. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci U S A. 2004;101:9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L., Shi W., Chappell J.D., et al. Importance of neutralizing monoclonal antibodies targeting multiple antigenic sites on the middle east respiratory syndrome coronavirus spike glycoprotein to avoid neutralization escape. J Virol. 2018;92 doi: 10.1128/JVI.02002-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pallesen J., Wang N., Corbett K.S., et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci U S A. 2017;114:E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirchdoerfer R.N., Cottrell C.A., Wang N., et al. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham B.S., Gilman M.S.A., McLellan J.S. Structure-based vaccine antigen design. Annu Rev Med. 2019;70:91–104. doi: 10.1146/annurev-med-121217-094234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walls A.C., Tortorici M.A., Bosch B.J., et al. Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature. 2016;531:114–117. doi: 10.1038/nature16988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crank M.C., Ruckwardt T.J., Chen M., et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science. 2019;365:505–509. doi: 10.1126/science.aav9033. [DOI] [PubMed] [Google Scholar]
- 24.Wrapp D., Wang N., Corbett K.S., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Doremalen N., Haddock E., Feldmann F., et al. A single dose of ChAdOx1 MERS provides protective immunity in rhesus macaques. Sci Adv. 2020;6 doi: 10.1126/sciadv.aba8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldblatt D., Fiore-Gartland A., Johnson M., et al. Towards a population-based threshold of protection for COVID-19 vaccines. Vaccine. 2022;40:306–315. doi: 10.1016/j.vaccine.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atmar R.L., Lyke K.E., Deming M.E., et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046–1057. doi: 10.1056/NEJMoa2116414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first OWS vaccine efficacy trial published, outlining the safety and 94% efficacy of Moderna’s mRNA-1273 vaccine in preventing symptomatic COVID-19.
- 29.Dunkle L.M., Kotloff K.L., Gay C.L., et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022;386:531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falsey A.R., Sobieszczyk M.E., Hirsch I., et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N Engl J Med. 2021;385:2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadoff J., Gray G., Vandebosch A., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Sahly H.M., Baden L.R., Essink B., et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;385:1774–1785. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadoff J., Gray G., Vandebosch A., et al. Final analysis of efficacy and safety of single-dose Ad26.COV2.S. N Engl J Med. 2022;386:847–860. doi: 10.1056/NEJMoa2117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenblum H.G., Hadler S.C., Moulia D., et al. Use of COVID-19 vaccines after reports of adverse events among adult recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 vaccines (Pfizer-BioNTech and Moderna): update from the Advisory Committee on Immunization Practices - United States, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1094–1099. doi: 10.15585/mmwr.mm7032e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shay D.K., Gee J., Su J.R., et al. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 vaccine - United States, March-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:680–684. doi: 10.15585/mmwr.mm7018e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cines D.B., Bussel J.B. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384:2254–2256. doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Collins F.S., Stoffels P. Accelerating COVID-19 therapeutic interventions and vaccines (ACTIV): an unprecedented partnership for unprecedented times. JAMA. 2020;323:2455–2457. doi: 10.1001/jama.2020.8920. [DOI] [PubMed] [Google Scholar]; This viewpoint explains the origin, structure and strategic objectives of ACTIV.
- 38.Hager KJ, Marc GP, Gobeil P., et al. Efficacy and safety of a plant-based virus-like particle vaccine for COVID-19 adjuvanted with AS03. 2022:2022.01.17.22269242. N Engl J Med. 2022 May 4. doi: 10.1056/NEJMoa2201300 PMID: 35507508.
- 39.Kullar R., Marcelin J.R., Swartz T.H., et al. Racial disparity of coronavirus disease 2019 in African American Communities. J Infect Dis. 2020;222:890–893. doi: 10.1093/infdis/jiaa372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garg S., Kim L., Whitaker M., et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gross C.P., Essien U.R., Pasha S., Gross J.R., Wang S.Y., Nunez-Smith M. Racial and ethnic disparities in population-level Covid-19 mortality. J Gen Intern Med. 2020;35:3097–3099. doi: 10.1007/s11606-020-06081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrasik M.P., Broder G.B., Wallace S.E., et al. Increasing Black, Indigenous and People of Color participation in clinical trials through community engagement and recruitment goal establishment. PLoS One. 2021;16 doi: 10.1371/journal.pone.0258858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johns Hopkins University of Medicine UoW. Preserving the scientific integrity of getting to COVID-19 vaccines: from clinical trials to public allocation. Available at: 〈https://coronavirus.jhu.edu/live/events/covid-19-vaccine-symposium/jhu-uw-vaccine-symposium〉. (Accessed March 8, 2022).