Abstract
The new severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) is the etiological agent of Coronavirus disease 2019 (COVID-19), which becomes an eventual pandemic outbreak. Lack of proper therapeutic management has accelerated the researchers to repurpose existing drugs with known preclinical and toxicity profiles, which can easily enter Phase 3 or 4 or can be used directly in clinical settings. Vitamins are necessary nutrients for cell growth, function, and development. Furthermore, they play an important role in pathogen defence via cell-mediated responses and boost immunity. Using a computational approach, we intend to identify the probable inhibitory effect of all vitamins on the drug targets of COVID-19. The computational analysis demonstrated that vitamin B12 resulted in depicting suitable significant binding with furin, RNA dependent RNA polymerase (RdRp), Main proteases (Mpro), ORF3a and ORF7a and Vitamin D3 with spike protein and vitamin B9 with non structural protein 3 (NSP3). A detailed examination of vitamins suggests that vitamin B12 may be the component that reduces virulence by blocking furin which is responsible for entry of virus in the host cell. Details from the Molecular Dynamics (MD) simulation study aided in determining vitamin B12 as a possible furin inhibitor.
Keywords: Vitamins, Furin, Vitamin B12, SARS-CoV-2, Drug targets, Covid-19
Graphical abstract
1. Introduction
The outbreak of novel coronavirus disease (COVID-19) has been declared as the global pandemic by the world health organization. The world experienced a pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It represents an unprecedented challenge to the medical sciences, as the virus is highly contagious [1] and is mutating to different strains. The first case was reported in December 2019 and it spread across the globe through human-to-human transmission. During the past two decades, three outbreaks of coronavirus have ensued worldwide: (i) severe acute respiratory syndrome coronavirus (SARS-CoV), (ii) Middle East respiratory syndrome coronavirus (MERS-CoV) and (iii) SARS-CoV-2 [2]. Coronaviruses are single-stranded RNA viruses (+ssRNA) with 26–32 kb in size and appear with crown-like structure due to the presence of spike glycoproteins on their surface. They are categorized into four genera: Alpha (α), Beta (β), Gamma (γ), and Delta (δ) coronavirus. β-coronavirus include SARS-CoVs, MERS-CoVs, and SARS-CoV-2 and can infect both humans and animals [3]. SARS-CoV and MERS-CoV have developed mechanisms to reduce IFN production ensuing increased inflammatory host responses, which produce powerful inflammatory cytokines (cytokine storm) and severe lung injury [4]. Cytokine storms found in SARS-CoV and MERS-CoV infected patients correlate with disease severity and poor prognosis [5].
SARS-CoV-2, also known as novel coronavirus (nCOV-2), encodes for various structural as well as non-structural proteins, facilitating viral entry as well as replication inside the host cell. SARS-CoV-2 encodes the enveloped spike glycoprotein which is cleaved by furin proteases and binds to its cellular receptor, angiotensin-converting enzyme 2 (ACE2) and stimulates membrane fusion and endorsements of the virus into human cells [6]. The affinity of SARS-CoV-2 towards the ACE-2 is much higher compared to SARS-CoV. Furin also known as proprotein convertase 3 (PC3) is responsible for activation of SARS-CoV-2 which may be the reason for higher severity in terms of rapid transmission as compared to SARS- CoV. PCs are responsible for activation of many enveloped viruses. Hence, furin is considered as promising antiviral drug target for several viruses causing severe infections. SARS-CoV-2 encodes various nonstructural proteins (NSP), including mainly the protease (Mpro), papain-like protease (PL pro), RNA dependent RNA polymerase (RdRp) and Nsp3 as largest protein encoded by coronavirus. The structural and non-structural proteins of SARS-CoV-2 are promising antiviral therapeutic targets, and their availability enables for structure-based screening of current drugs against them [7]. Various studies have been already done on molecular docking-based screening of existing antiviral, antibacterial and other FDA approved drugs [8]. Nanotechnology may be used to treat covid infections as green nanotechnology has greater impact than conventional therapy nowadays and shown diversified application in several research areas such as medical sciences, pharmacy etc. [[9], [10], [11]], Gene editing technology such as Crispr/cas 9 technology can also play a role to combat covid pandemic [12]. Although to find a specific therapy for the recent pandemic of COVID-19 is still on the way, a clinically approved antiviral drug or vaccine is still being developed, the effect is yet to be confirmed against the SARS-CoV-2 medication both during and post-infection. Several drugs have been reported for in vitro activity against different coronaviruses. Drug repositioning may offer a strategy hence, many drugs have been repurposed, including Remdesivir, Hydroxychloroquine and Chloroquine, Umifenovir (Arbidol), Lopinavir-Ritonavir, Favipiravir (Avigan), Oseltamivir (Tamiflu), Sofosbuvir, Galidesivir and Tenofovir showed promising results for treatment of newly emerged strain of coronavirus [13,14]. Other antiviral drugs including Ribavirin, Zanamivir, Acyclovir, Peramivir and Ganciclovir which are commonly used in clinical practice, are currently not recommended for COVID-19. Molecular docking of selected phyto chemicals of nine medicinal plants and network analysis, sequence and structure dynamics of key proteins of coronavirus and human host has been reported by toluwase and group [15]. Our group working in this area has reported through immunoinformatics approach for immunogenic protein from the proteome of Leptospira and a codon-based analytical study for susceptible hosts of Nipah Virus [[43], [44]]. The “repurposed” drugs that have been approved by the FDA in the USA for other indications have been used profusely to treat COVID-19 patients. Until a perfect treatment guideline is available including treatment of co-morbidities, one must switch to suitable and balanced nutrition for appropriate body functioning and enhancing of the immune system especially with the help of various vitamins.
Vitamins (Vita) are the essential elements required for cell proliferation, function and development. Moreover, they play a vital role against the pathogens via cell-mediated response and increase the immunity. All the vitamins except vitamin C are reported for the antibody production. Recent studies revealed that vitamins reduce the risk of pneumonia and other viral respiratory tract infections [16]. The vitamins also exhibit direct inhibition of viral replication or with immunomodulatory or anti-inflammatory means. Several vitamins, minerals and herbs have a treasured effect on mitochondrial function. Vitamins are essential nutrients as they serve a plethora of important metabolic functions in the body. It has been well documented that the protection provided by vitamins through the natural defence mechanism of the host not only functions against infectious diseases, but also against cancer and other degenerative diseases. The enhancement of host resistance to disease results due to biosynthetic, antioxidant and immunostimulatory activity. Direct antiviral action of some vitamins to protect against viral disease being inactivation of a wide spectrum of viruses as well as suppress viral replication and expression in infected cells. One mechanism of virus inactivation can be explained that depends on the presence of oxygen and production of reactive oxygen species (ROS) through the Nrf 2 pathway [17]. COVID-associated severe cases result in complex biological spectacles, such as apoptosis induction, macrophage activation, oxidative tissue damage and higher contents of pro-inflammatory cytokines [18]. The superoxide anion-mediated pathways denote severe tissue injury for that drug with antiviral and antioxidant activities may be the choice. Vitamins including B1, C (Ascorbic acid), D, E and Omega-3, minerals such as magnesium and manganese and herb like thyme play an important role on the innate system under the virus infection [19]. Studies have suggested a possible role of vitamin C is to combat COVID-19 [20]. Moreover, along with vitamin C, Vitamin D selenium also aids in COVID-19 management [21] Recent study reported efficacy of ascorbic acid against COVID-19 [22]. One more study suggests the need with the higher content of Vitamin C and Vitamin B-Complex micro-nutrients to manage this pandemic successfully [23,24]. The group of vitamin B has a prime role for the immune response to antigen as well; some of the water-soluble vitamins act as a coenzyme in the body for certain biochemical reactions to enhance the immune system. Thiamine, also known as Vitamin B1 has a role in B and T cell production. Riboflavin (B2) in combination with UV hinders DNA replication in MERS CoV. Hence it may have a role in SARS- CoV prevention. Nicotinamide (vitamin B3) is the building block of NAD+, which acts as a coenzyme in various metabolic pathways. Hence, it plays a significant role in the inflammatory response and prevents lung tissue damage. Pyridoxal 5′-phosphate is the active form of vitamin B6 and is involved in carbohydrate, protein and lipid metabolism. BAN (vitamin B6-derived bananin) inhibits the SARS-helicase enzyme, which hinders the viral replication process. Vitamins B6, B12, and B9 (folic acid) proliferate the activity of natural killer cells, which offers a significant antiviral effect. Vitamin B12 inhibits the RNA-dependent RNA polymerase, which leads to the SARS-CoV-2 viral replication. In a nutshell, vitamin B complexes have the potential to frontier the complications associated with COVID-19 infection [25].
2. Materials and methods
2.1. Retrieval of vitamin structures
In total, 15 molecular structures of vitamins were retrieved from the PubChem database (Table S1). The three-dimensional structure of the molecules was downloaded in SDF format and the molecules whose only two-dimensional structures were available, were converted into their three-dimensional structure using Marvin Sketch and the best confirmation obtained is minimised in semi-empirical PM3 method using Polak-Ribeire algorithm in Hperchem Student evaluation version. The minimised structure is then converted to pdbqt by AutoDock Tools [26].
2.2. Protein preparation
The high resolution three dimensional X-ray crystal structures of the target proteins of COVID-19 retrieved from protein data bank (PDB) ( http://www.rcsb.org/ ) using their accession IDs 6LU7 (main proteases), 6M71 (RdRp), 6VXX (spike protein), 6W6Y (NSP3), 6W37 (ORF7a),6XDC (ORF3a) and Human proprotein convertase furin 5JXG respectively. The selected target proteins were prepared for docking using AutoDockTools 1.5.6 [26]. The structures were saved in PDBQT format.
2.3. Molecular docking study
The binding affinity of each vitamin compound with the SARS-Cov-2 structural and non-structural targets was determined by molecular docking method. The molecular docking was performed using blind docking method in Autodock Vina 1.1.2 [27]. The grid box is outsized enough to cover the entire protein structure to encounter any probable protein-ligand interactions. The binding poses were clustered and ranked in the order of their binding affinities. The molecular interactions (hydrogen bonds and hydrophobic interactions between the target proteins and compounds were studied using LigPlot + version 1.4.5 [28].
2.4. Molecular dynamics simulation study
A molecular dynamic (MD) simulation of the Furin-Vitamin B12 complex was performed using Schrodinger's Desmond module. Molecular dynamics simulations of these compounds was run for 100 ns (ns). In the beginning, the furin-vitamin B12 complex was prepared using the Schrodinger suite's Protein Preparation Wizard with default settings that included hydrogen atom and bond reassignment, addition of missing side chain atoms of amino acid residues, optimization of loops residues, and water orientation sampling at pH 7.4. A periodic simulation box was developed using the system builder module, the system was solvated using the TIP3P (Transferable Intermolecular Potential with 3 Points) water model and neutralised by adding counter ions, and energy minimization was achieved using the OPLS with 1000 iterations of the steepest descent technique (Optimized Potentials for Liquid Simulations) all atoms force field. After achieving a state of equilibrium, an unrestrained production phase with NPT (constant atoms, pressure, and temperature) ensemble was developed for 100 ns under the supervision of a Nose–Hoover thermostat (300 K, relaxation time = 1 ps) and an isotropic Mar-tyna–Tobias–Klein barostat (1.01325 bar, relaxation time = 2 ps). The smooth particle mesh Ewald (PME) approach with the RESPA integrator was used to evaluate short-range interactions (cut-off = 9 A) and long-range Coulomb interactions. At a 5 ps period, the frames capturing the system's dynamic motions were exported. Plotting histograms for root mean square deviation (RMSD), root mean square fluctuations (RMSF), Hydrogen bond analysis was used to investigate the system's stability [29].
3. Results and discussion
3.1. Molecular docking of vitamins with COVID-19 drug targets
In this study, vitamins were docked against non-structural proteins (NSP3, NSP5, ORF7a, NSP12 and ORF3a), structural spike protein and one host protease furin. Since the presence of furin cleavage site (PRRA motif) in the spike has been confirmed [30], researchers are engaged in looking for a satisfactory mechanism to explain the importance of furin activity in spike maturation thus viral infectivity. Furin was shown to facilitate virus entry into the cell after receptor binding for other coronaviruses, e.g., MERS-CoV. Furin is generally membrane bound, but an active isoform has been described that can be secreted, potentially facilitating cleavage of the SARS-CoV-2 spike protein in the cellular neighbourhood. Realising the important role of this host protease in spike activation we have also targeted the protein for inhibitor binding that resulted with a very exciting outcome. The arrangement of the binding mode of the ligands is considered mainly based on the positions of hydrogen bond interaction with protein and minimum binding energy. Amongst all molecules vitamin B12, vitamin D3 and vitamin B9 exhibited good affinity, and vitamin B15 showed strong interaction in terms of H-bonds. Table 1 illustrates binding energies of all vitamins with target protein of SARS-CoV-2.
Table 1.
Binding energies of Covid-19 targets with Vitamins.
| Mpro (NSP5) | RDRP (NSP12) | NSP3 | ORF7a | ORF3a | Spike protein | Furin | |
|---|---|---|---|---|---|---|---|
| Vitamin B12 | −7.3 | −8.3 | −6.9 | −6.1 | −6.9 | 0 | −9.2 |
| Vitamin D3 | −6.8 | −7.4 | −7.6 | −7.4 | −6.3 | −7.4 | −8.2 |
| Vitamin B9 | −6.6 | −7.6 | −7.8 | −7.1 | −6.6 | −7.4 | −8.2 |
| Vitamin K1 | −6.3 | −4.9 | −6.9 | −7.2 | −4.5 | −5.3 | −6.1 |
| Vitamin B1 | −5.2 | −5.6 | −6.4 | −5.3 | −5.4 | 0 | −6.3 |
| Vitamin A | −6 | −6.5 | −6.5 | −6.5 | −6.1 | −7 | −7.1 |
| Vitamin B2 | −5.8 | −6.9 | −6.2 | −6.1 | −5.8 | 0 | −6.8 |
| Vitamin B3 | −4.2 | −4.3 | −4.6 | −4.2 | −4 | −4.4 | −4.8 |
| Vitamin B5 | −4.5 | −4.9 | −5.6 | −4.8 | −4.6 | −5.2 | −5.7 |
| Vitamin B6 | −4.4 | −4.8 | −5.3 | −4.9 | −4.6 | −4.9 | 0 |
| Vitamin B7 | −4.1 | −5.4 | −5.5 | −4.9 | −4.1 | −4.5 | −5.8 |
| Vitamin B15 | −5.3 | −4.8 | −4.8 | −4.2 | −4.8 | −4.6 | −5.3 |
| Vitamin C | −4.7 | −5.6 | −4.5 | −4.6 | −4.3 | −5.1 | −5.2 |
| Vitamin E | −5.4 | −6.3 | −6.6 | −6.5 | −5.7 | −5.5 | −7.5 |
| Vitamin K2 | −5.9 | −6.1 | −7.1 | −6.5 | −6.5 | −6.7 | −7.1 |
3.1.1. Vitamin B12 and B9 had the strongest inhibitory effect on furin and NSPs
Our results reveal that vitamin B12 is an efficient nutraceutical for almost all the COVID-19 drug targets. Methylcobalamin unveiled high binding affinity with furin (−9.2 kcal/mol), RdRP (−8.0 kcal/mol), NSP5 (−7.3 kcal/mol) NSP3 (−6.9 kcal/mol), ORF7a (−6.1 kcal/mol), ORF3b (−6.9 kcal/mol) proteins. However, the spike protein of SARS-CoV-2 defended and demonstrated zero binding affinity with this vitamin. In this study, Vitamin B12 exemplifies fair interaction with furin in terms of seven H-bonds and nine hydrophobic interactions. The interacting residues of furin in context to H-bonds and hydrophobic contact are Glu 256 (3.11 Å), Gly 255 (2.90 Å), Ser 253 (3.13 Å), Ser 368(3.20 Å), Thr 365(3.0 Å), Arg 193 (3.21 Å), Asp 191(2.80 Å) and Trp 254, Leu 227, Gly 229, Asp 154, Asn 190, His 364, His 194, Asn 295, Asp 258 respectively (Fig. 1 ). Vitamin B12 also shows good hydrophobic interactions with 15 amino acids (leu 172, Phe 169, Asp 170, Arg 173, Arg 249, Thr 319, Tyr 265, Phe 321, Pro 323, Arg 349, Phe 396, Thr 394, Leu 460, Pro 667, Pro 461) and four H-bonds Tyr 465 (3.19 Å). Asn 459 (3.15 Å), Cys 395 (2.95 Å), Arg 415 (2.80 Å) with RNA dependent RNA polymerase enzyme. Fig. 2 shows that vitamin B12 has decent interactions with all viral targets. Vitamin B12, cobalamin, is a water-soluble vitamin produced in nature by microorganisms mainly from animal proteins and deficiency caused with different disease states. It is involved in different pathways as well in haematopoiesis. B12 is an important factor for activation of transcription factors like NF-ĸB, Myc and Fos and thought to potentiate anti-inflammatory effects. Though vitamin B12 has been used to improve immunity in HIV infected patients, there is no evidence of direct inactivation of any virus by this compound till the date. The insilico analysis explained that vitamin B9 also proved one of the superlative inhibitors for all viral targets. Amongst the viral targets vitamin B9 illustrates best binding with Furin (−8.2 kcal/mol), spike (−7.4 kcal/mol), RdRp (−7.6 kcal/mol), NSP3 proteins (−7.6 kcal/mol) (Fig. 3 .). Our frequent observation denotes (Fig. 3.) that amino acid residues of furin interrelates with vitamin B9 and vitamin B12 at distinguished domains. LigPlot exposed two strong H-bonds Asn310 (3.15 Å, 3.02 Å), Asn529 (3.22 Å, 2.79 Å), Gln488 (3.07 Å, 2.92 Å) and single H-bond Val263 (3.03 Å), Glu271 (2.78 Å), Tyr571 (3.05 Å) between furin and this vitamin.
Fig. 1.
Interaction profile for vitamin B12 in docked complex with furin (PDB Id: 5JXG).
Fig. 2.
Interaction plot of vitamin B12 with (a) RDRP (b) MPRO (c) NSP3 (d) Orf7a (e) Orf3a. (f.)Interaction description.
Fig. 3.
Interaction plot of vitamin B12 with (a) furin (b) NSP3 (c) ORF7a (d) Mpro (e) RdRp (f) ORF3a.
Vitamin B9, Folic acid, is a type of vitamin B normally found in foods rich in iron and dried beans and mainly helps the body to produce and maintain new cells as well as helps in DNA repair mechanisms.
It is well known that folate intake is essential for human health because it prevents megaloblastic anemia and neural tube birth defects as well as cardiovascular disease, dementia, cognitive function alterations, osteoporosis and several types of cancer. Recent computer simulation studies on antiviral activity of vitamin B9 have been reported [31] where the authors have suggested that B9 can inactivate the furin endoprotease that is crucial for the SARS-CoV-2 virus to enter its host cell [30]. While another work [16] explained that vitamin B9 inactivates protease 3CLpro, which is vital in the replication of all coronaviruses. Amongst vitamin B1 to B6, vitamin B2, B5, and B6 manifest good H-bond networks with all viral targets (Fig. S1&S2). Our results unveil zero affinity of vitamin B1 and B2 and no interaction with spike protein. In contrast to that, vitamin B3, B5, and B6 have connections with spike protein. Vitamin B5 shows a maximum of 8 H-bonds with S protein and 7 H-bonds with furin.
The structure of the vitamin B15 also known as pangamic acid complexed with ORF7a, NSP5 and NSP12 highlighted hydrogen bond interaction to be the main contributor in protein–ligand interaction whereas binding energy contributes moderately (Fig. 4 .) in this study. This vitamin shows maximum 10 H-bond interaction with ORF7a protein and 7 with NSP5. It is reported that assorted strong-weak H-bond unions decrease ligand binding affinity and sometimes connect poorly with experimental binding affinity [32]. The robust hydrogen bonding and hydrophobic interaction between vitamins with the enzyme infer it may be a potent ORF7a and NSP5 inhibitor. Laboratory experiments have proven the protective effect of B15 on coronary artery occlusion and myocarditis in animals. Though controversy exists in categorising B15 under vitamin group it has received the most attention for its effect in stress and on athletic and physical effort. Apart from its protective role in hypoxia, another important function of cellular detoxification associated with cytochrome p450, B15 has a protective role in various health conditions like fatty infiltration of liver, atherosclerosis, alcoholism etc [33]. Anticipating its role in covid-19 induced hypoxia the present investigation also focused on its antiviral activity.
Fig. 4.
Interaction plot of vitamin B15 with (a) ORF7a (b) RdRp (c) Mpro (d) ORF3a (e) NSP3 (f) furin.
3.1.2. Vitamin D3 exhibited a good inhibitory effect on spike protein and furin
The current study was able to discover a mechanism through binding vitamin D3 to several proteins of SARS-CoV-2. The spike glycoprotein is necessary for viral attachment, fusion, and entrance into the host cell. The S glycoprotein's surface position makes it a direct therapeutic target. Mature S glycoprotein on the viral surface is a highly glycosylated trimer with 1260 amino acids (residues 14–1273) in each protomer. S1 is made up of 672 amino acids (residues 14–685) and is divided into four domains: an N-terminal domain (NTD), a C-terminal domain (CTD, also known as the receptor-binding domain, RBD), and two subdomains (SD1 and SD2). Vitamin D3 demonstrated good binding affinity (−7.4 kcal/mol) with S glycoprotein. On the S1 subunit, the interacting residues of spike with vitamin D3 are Ile 128, Ile 119, Phe 168, Tyr 170, Val 227, Leu 229, Leu 226, Phe 194, Val 126, Ile 203, and Phe 192 (Fig. 5 a). The vitamin D3 also displays finest dock score with proprotein convertase 3 furin (−8.2 kcal/mol). At the S1/S2 cleavage site, cellular furin or furin-like proteases cut the SARS-CoV-2 S glycoprotein, which contains numerous arginine residues not present in the closely related SARS-CoV. Cleavage at the S1/S2 site produces a surface component S1, which connects the virus to the host cell surface receptor, and a transmembrane subunit S2, which facilitates viral-host cell membrane fusion. Furin-like cleavage is necessary for effective SARS-CoV-2 infection of human lung cells and is required for S-protein mediated cell-cell fusion and viral infectivity. According to recent findings, vitamin D3 has an inhibitory effect on viral entrance in the host cell because it inhibits both viral targets. In this study, we also observed that all viral targets have decent hydrophobic interactions with vitamins. Klebe in 2015 reported that binding affinity of several ligands is very high due to hydrophobic interactions. In hydrophobic interactions, ligand suppresses the lipophilic surface of target protein and dislocates the water. Vitamin D absorbed from diet and dietary supplements is converted to its biologically active form inside the human body. Photo-mediated conversion of 7- dehydrocholesterol in the skin is the primary source of Vitamin D inside our body. Thus exposure to sunlight is important to promote vitamin D production in the skin. Major sources of Vitamin D include egg yolks, saltwater oily fish and liver as food. Some other foods, like milk and cereal, mushrooms often have added vitamin D. To become biologically active D3, 25-hydroxylation of vitamin D is required in the liver and subsequent 1-hydroxylation in the kidney [34]. The fat soluble Vitamin D plays a central role in calcium and phosphate homeostasis that is essential for the proper development and maintenance of bone. It is also involved in cell proliferation, differentiation, and immunomodulation. Vitamin D deficiency is associated with increased risk of viral infections such as influenza, respiratory tract infections, and human immunodeficiency virus (HIV) along with many pathological conditions, including cancer, autoimmune diseases, cardiovascular disease, and diabetes [35] reported the reason for antiviral activity of vitamin D3 in HCV to be the increased expression of interferon [36]. In the context of increasing respiratory tract infection cases of COVID-19 patients a host of authors are suggesting vitamin D to be the wonder drug for cure. However, a proper mechanism to confirm the role of vitamin D is lacking.
Fig. 5.
Interaction profile for (a) vitamin D3 in docked complex with spike glycoprotein of SARS-CoV-2 (b) furin.
3.1.3. Vitamin C interacts well with RdRp and furin
The best docking energy is as predicted by AutoDock Vina of vitamin C with furin and RdRp proteins are −5.6 and −5.2 kcal/mol subsequently. The residues interacting with furin in context to hydrophobic interactions are Trp 390, Thr 389, Arg391, Gln 447and in six H-bonds interactions are Leu 285 (3.07 Å), Gly284 (2.88 Å, 3.19 Å), Val 278(3.05 Å), Ser279 (2.92 Å, 3.16 Å). The interacting residues of RdRp with vitamin C in hydrophobic interactions and H-bond interactions are Ser 814, Asp 618, Gly 616, Phe 812, Glu 811 and Asp 761 (2.86 Å),Trp 800 (3.07 Å), Cys 813 (2.98 Å), Trp 617 (2.81 Å, 2.98 Å). Vitamin C (ascorbic acid) is essentially required as a natural antioxidant in the body for the correct functioning of the immune system, it's a great immunity booster compared to all vitamins found in nature due to its antioxidative property. Thus, it shows potentiality against various infections, including coronavirus infections [37]. Its role in stress response after administration in critically ill COVID-19 patients is well documented. To understand the mechanism of vitamin C, structural and non-structural SARS-CoV-2 proteins were analysed. The ligand interaction maps of the compound with best dock conformation reported in Supplementary Fig. S3. This computational study ascertains the inhibitory effect of ascorbic acid on all viral proteins. Viral infections generate oxidative stress and certain redox-active substances such as antiviral drugs are expected to suppress oxidative stress and improve inflammatory symptoms. Influential scavenging and antioxidative properties of ascorbic acid have shown protective effects. Both in vitro and in vivo clinical trials with vitamin C treatments showed significant reduction of viral replication without any insight to proper inhibitory mechanisms. The present investigation may be for the first time able to find a plausible mechanism from the observed binding properties to the viral proteins.
3.1.4. Vitamin K, E and vitamin A exhibited moderate inhibitory effect on drug targets
Fat-soluble vitamin K primarily affects blood clotting along with wound healing as well as modulates the calcium-binding in bones. Vitamin K deficiency reported a poor outcome of COVID-19 [38]. The ligand interaction plot (Supplementary Fig. S4.) displays more hydrophobic interactions with both vitamin K1 and K2. Vitamin K1 shows a good docking score with one target ORF7a (−7.2 kcal/mol) and the residues in context to hydrophobic interaction are Leu 16, Tyr 60, Gln 6, Tyr 5, His 4, Tyr 3, Glu 18, Leu 2, Pro 19, Lys 17. Vitamin K2 displays −7.1 kcal/mol affinity with NSP3 and furin. The interacting residues of NSP3 with vitamin K2 are Lys 55, Val 24, Ala 154, Ala 38, Val 49, Gly 130, Ala 129, Gly 48, Leu 126, Asp157, Phe156, Asp 22, Ala 52. The amino acid interacting with furin in context to hydrophobic interactions are Gln488, Arg490, Trp531, Asp530, Asn310, Asn529, Pro 266, Arg268, Glu272, Phe275, Glu271, Gly307, Ala532, Ser311. Vitamin E deficiency has shown increased levels of the viral (coxsackievirus B3) infection end up with a myocardial injury in mice, due to oxidative stress. Likewise, vitamin E deficiency in calves was associated with a high risk of bovine coronavirus infection. Alpha-tocopherol shows fair scores −7.5 and −6.6 kcal/mol with furin and NSP3 one-to-one. Vitamin A plays a key role in normal eye vision, functional immune system, and reproduction. Furthermore, it regulates the antigen-presenting cells and maintains the balance between Th1 and Th2 lymphocytes, and produces an antibody response against an antigen [39]. Hence, it is the most promising vitamin against lung damage due to COVID-19. Table S2 lists the interaction amino acids of SARS-CoV-2 drug targets with all vitamins.
The ingenious element of our study is that we are disseminating the full interaction analysis of all vitamins with all SARS-CoV-2 drug targets for the first time. This investigation provided a meticulous examination of vitamins with one-to-one drug targets. As all of the vitamins were docked one by one with the SARS-CoV-2 drug targets, it was determined that vitamin B12 maintained fair interactions with the majority of them, including Furin, RdRp, Mpro, and ORF3a. Vitamin D3 interacted well enough with structural protein spike. We propose that vitamin B12 has a considerable inhibitory effect on furin. The pathogenicity of SARS-CoV-2 is directly linked to furin-mediated cleavage of the viral spike glycoprotein. The inhibitory impact of vitamin B12 on furin prevents SARS-CoV-2 spike protein cleavage and viral entrance into host cells.
3.2. Molecular dynamics simulation of furin with Vitamin B12
We propose that vitamin B12 has a strong inhibitory potential against furin. The molecular dynamics simulation on the best docking score complex (Furin-Vitamin B12) for 100 ns measures up to examine the dynamic performance of the protein-ligand complex. The structure experienced initial fluctuation due to the kinetic shock applied during the MD simulation. Fig. 6 shows the graphical representation of the root mean square deviation (RMSD) and root mean square fluctuation (RMSF) of the complex in each amino acid. The amino acid in complex with vitamin B12 maintains equilibrium throughout the simulation. Around 1.75 ± 0.25 Å RMSD was reported for equilibration of the protein backbone. The complex re-equilibrated a few times during the 100 ns simulation. Fig. 6 illustrates the retreating of the ligand orientation near∼15 ns and ∼30 ns. The magnitude of fluctuation and the difference between the average RMSD values recommended that simulation produced stable trajectories. In the graphs of RMSD right Y-axis represents the ligand RMSD value. ‘Lig fit Prot’ signifies the RMSD values of ligand with reference to protein backbone. After the 50 ns of simulation, ‘Lig fit Prot’ value of the furin-vitamin B12 complex is in the range of 19.5 ± 2.5 Å.
Fig. 6.
A 100 ns simulation profile of Protein–ligand interaction, root-mean-square deviation (RMSD) for a furin-vitamin B12.
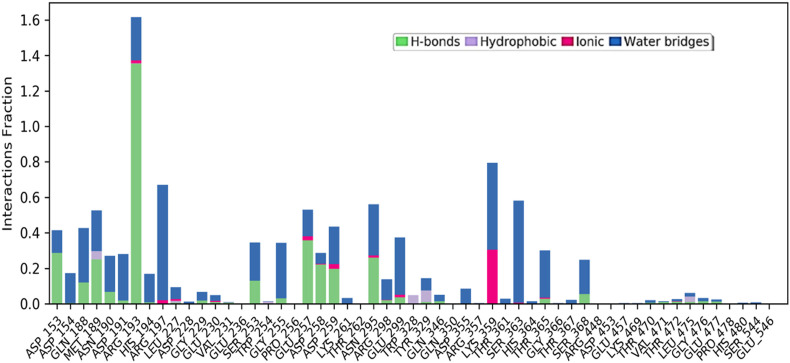
The root mean square fluctuation analysis (Fig. 7 ) correlates with the functional analysis and the highest fluctuation in this region. This reported decrease in scores of particular residues proposes their direct participation in enhancing the overall stability of the bound protein complex [40]. The RMSF value provides information on the dynamic behaviour of proteins in an aqueous simulated environment and is useful for displaying localised minute changes along protein chains. The peaks in the diagram represent the protein districts that change the greatest throughout the simulation. Protein tails (both N- and C-terminal) are more likely to alter than other parts of the protein. Auxiliary locations of proteins, such as alpha helices and beta strands, are often more rigid and inflexible than unstructured sections, and hence do not oscillate like circular framing parts of protein. The red and blue colours represent the alpha-helical and beta-strand sections, respectively. The amino acid residues 250, 300, and 350–375 showed high fluctuations. This type of illustration in an experimental setup will be time-consuming, thus creating a method for computational microscope MD to analyze the structural effects [41,42]. In a bar chart arrangement, the interaction analysis illustrates four types of connections established between protein and ligand in normalised form during the simulation duration. The interaction fraction is displayed on the Y-axis, showing the proportion of that specific contact, whereas various interactions between the residue and the ligand are displayed in two or three distinct colours in a bar column. Hydrogen bonds, hydrophobic interactions such as Pi cation, pi-pi stacking, water bridges and ionic interactions made by vitamin B12 with amino acids of furin during 100-ns MD simulation are denoted in this Fig. 8 . The molecular dynamics study of the best ligand-receptor complex also exhibits the stability of the compound during the simulation process, which validates the virtual screening and docking results.
Fig. 7.
A 100 ns simulation profile of Protein–ligand interaction, root-mean-square fluctuation (RMSF) for furin-vitamin B12.
Fig. 8.
Protein–ligand interaction diagram showing interaction fraction of crucial interacting amino acids of furin.
4. Conclusion
With the progression of SARS-CoV-2 infection resulting in various worldwide waves and the lack of any effective antiviral therapy against its treatment, vitamins are being investigated as a potential source for anti-viral therapeutic development. The present study revealed that the role of vitamins as immunonutrition supplements could be deployed as potential supplements to attenuate the severity of the COVID-19 infection. Our results clearly state that vitamin B12 has fair interaction with all COVID-19 drug targets. It demonstrated strong inhibitory effect on furin by docking analysis followed by md simulation. These upshots imply that Vitamin B12 could be the wonder molecule to shrink the virulence by hindering the furin mediated entry of spike to host cell. These identified vitamins may effectively assist in SARS-CoV-2 therapeutic management to boost the immunity by inhibiting the virus imparting relief in lung inflammation.
Ethical statement
The study does not involve the animals.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contribution of authors
The conception and design of study by JD & MP. Acquisition of data, analysis and interpretation DM, TJ, AP, AG & MP. Drafting and revising articles by SS, SD, & KJ.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to thank COVID-19 Omics Research Consortium (CORC) for providing this platform.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.imu.2022.100951.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wu C., Zhang Y. Furin : a potential therapeutic target for COVID-19 furin : a potential therapeutic target for COVID-19. iScience. 2020;23 doi: 10.1016/j.isci.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y., Liu Q., Guo D. 2020. Emerging coronaviruses : Genome structure , replication , and pathogenesis; pp. 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fung S., Yuen K. vol. 9. 2020. (A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence : lessons from other pathogenic viruses). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wit E De, Doremalen N Van, Falzarano D, Munster VJ. REVIEWS SARS and MERS : recent insights into emerging coronaviruses. Nat Publ Gr n.d. 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed]
- 5.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2019 doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alsaadi E., Jones I.M. Membrane binding proteins of coronaviruses. Future Virol. 2019;14:275. doi: 10.2217/fvl-2018-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.M Dhanalakshmi; Kajari Das; Medha Pandya; Sejal Shah ;Ayushman Gadnayak ;Sushma Dave;Jayashankar Das ; Artificial neural network-based study predicts GS441524 as a potential inhibitor of SARS COV-2 activator protein furin: a polypharmacology approach.Appl Biochem Biotechnol. 10.21203/rs.3.rs-513443/v1. [DOI] [PMC free article] [PubMed]
- 8.Roujian L., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turakhia B., D M.B., S M.S., shah Green synthesis of copper oxide nanoparticles: a promising approach in the development of antibacterial textiles. J Coating Technol Res. 2020;1034:531–540. [Google Scholar]
- 10.Tanzil Juneja, Medha Pandya, Shah Sejal. Molecular landscape and computational screening of the natural inhibitors against HPV16 E6 oncoprotein. Asian Pac J Cancer Prev APJCP. 2021;22:2461. doi: 10.31557/APJCP.2021.22.8.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dave Vishakha, Pandya Medha, Rawal Rakesh, Bhatnagar S.P., Mehta Rasbindu. Elsevier; 2022. Smart and intelligent vehicles for drug delivery: Theranostic nanorobots; pp. 541–564. Adv. Nanomater. Point Care Diagnosis Ther. [DOI] [Google Scholar]
- 12.Nalla Yashika, Shah Sejal. A new era in molecular biology clustered regularly interspaced short palindromic repeats/cas9 technology: a brief understanding. Adv Hum Biol. 2021:11. [Google Scholar]
- 13.Kumar V., Kancharla S., Kumar M. In silico virtual screening-based study of nutraceuticals predicts the therapeutic potentials of folic acid and its derivatives against. Virus Dis. 2021;32:29–37. doi: 10.1007/s13337-020-00643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020:1–10. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toluwase Hezekiah FatokiORCID Icon. Omodele Ibraheem, Ibukun Oladejo Ogunyemi. Clement Akinmoladun Afolabi, Harriet U. Ugboko, Catherine Joke Adeseko, Oladoja A. Awofisayo, Sunday Joseph Olusegunf Departamento de Quimica, Universidade Federal de Minas Ger B &Jesupemi ME. Network analysis, sequence and structure dynamics of key proteins of coronavirus and human host, and molecular docking of selected phytochemicals of nine medicinal plants. J Biomol Struct Dyn. 2021:6195–6217. doi: 10.1080/07391102.2020.1794971. [DOI] [PubMed] [Google Scholar]
- 16.Jovic TH, Ali SR, Ibrahim N, Jessop ZM, Tarassoli SP, Dobbs TD, et al. Could vitamins help in the Fight against COVID-19 ? n.d.. [DOI] [PMC free article] [PubMed]
- 17.Bender D., Hildt E. vols. 1–37. 2019. (E ff ect of hepatitis viruses on the Nrf2/Keap1-signaling pathway and its impact on viral replication and pathogenesis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scavone C., Brusco S., Bertini M., Sportiello L., Rafaniello C., Zoccoli A., et al. 2020. Current pharmacological treatments for COVID-19 : what ’ s next ? pp. 4813–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abobaker Anis, Alzwi Aboubaker, Ahaa Overview of the possible role of vitamin C in management of COVID-19. Pharmacol Rep. 2020;72:1517–1528. doi: 10.1007/s43440-020-00176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bae Minkyung, K H. The role of vitamin C, vitamin D, and selenium in immune system against COVID-19. Molecules. 2020;25:5346. doi: 10.3390/molecules25225346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jariwalla Raxit J., S H. Antiviral and immunomodulatory activities of ascorbic acid. Subcell Biochem. 1996:215–231. [PubMed] [Google Scholar]
- 23.Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil Med Res. 2019;7:1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos D., Jeane L.M. Can vitamin B12 be an adjuvant to COVID-19 treatment? GSC Biol Pharm Sci. 2020;11 001–5. [Google Scholar]
- 25.Junaid K., Ejaz H., Abdalla A.E., Abosalif K.O.A., Ullah M.I., Yasmeen H., et al. E ff ective immune functions of micronutrients against SARS-CoV-2. Nutrients. 2020;2 doi: 10.3390/nu12102992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256.AutoDock4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2011;31:455–461. doi: 10.1002/jcc.21334.AutoDock. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laskowski R.A., Swindells M.B. LigPlot + : multiple ligand À protein interaction diagrams for. Drug Discov. 2011:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- 29.Krieger Elmar, Darden Tom, Nabuurs Sander B., Alexei Finkelstein G.V. Making optimal use of empirical energy functions: force-field parameterization in crystal space. Proteins. 2004;57:678–683. doi: 10.1002/prot.20251. [DOI] [PubMed] [Google Scholar]
- 30.Coutard B., Valle C., Lamballerie X De, Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin- like cleavage site absent in CoV of the same clade. Antivir Res. 2020 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Covid- C, Sheybani Z., Dokoohaki M.H., Negahdaripour M., Dehdashti M., Zolghadr H. 2020. The Role of Folic Acid in the Management of Respiratory Disease the role of folic acid in the management of respiratory disease caused by COVID-19. [DOI] [Google Scholar]
- 32.Chen D., Oezguen N., Urvil P., Ferguson C., Dann S.M., Savidge T.C. 2016. Regulation of protein-ligand binding affinity by hydrogen bond pairing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolton S., Null P.D.G. Vitamin B15 A review and update. Orthomol Psychiatr. 1982;11:260–266. [Google Scholar]
- 34.Barchetta I., Carotti S., Labbadia G., Gentilucci U.V., Muda A.O., Angelico F., et al. 2012. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. 2180–7. [DOI] [PubMed] [Google Scholar]
- 35.Munshi R., Hussein M.H., Toraih E.A., Elshazli R.M., Jardak C., Sultana N., et al. 2021. Vitamin D insufficiency as a potential culprit in critical COVID ‐ 19 patients; pp. 733–740. [DOI] [PubMed] [Google Scholar]
- 36.Gal-tanamy M., Bachmetov L., Ravid A., Koren R., Erman A., Tur-kaspa R., et al. 2011. Vitamin D: an innate antiviral agent suppressing hepatitis C virus in human hepatocytes; pp. 1570–1579. [DOI] [PubMed] [Google Scholar]
- 37.Baladia E., Pizarro A.B., Rada G. Vitamin C for the treatment of COVID-19: a living systematic review. medRxiv. 2020 doi: 10.5867/medwave.2020.06.7978. [DOI] [PubMed] [Google Scholar]
- 38.Dofferhoff ASM, Piscaer I, Schurgers LJ, Visser MPJ, Jong PA De, Gosens R, et al. Reduced vitamin K status as a potentially modifiable risk factor of severe COVID-19 Anton n.d. [DOI] [PMC free article] [PubMed]
- 39.Ross A.C. Vitamin A deficiency and retinoid repletion regulate the antibody response to bacterial antigens and the maintenance of natural killer cells 1. Clin Immunol Immunopathol. 1996;80:63–72. doi: 10.1006/clin.1996.0143. [DOI] [PubMed] [Google Scholar]
- 40.Pandya M.D., Dabhi S.D., Jha P.K., Rawal R. vol. 1141. 2016. pp. 115–120. (Targeting MLL-CXXC domain with synthetic CpG Dinucleotides : docking and molecular dynamics simulation based approach). [DOI] [Google Scholar]
- 41.Dave M. 2016. Structural and functional analysis of AF9-MLL Oncogenic Fusion Protein using homology modeling and simulation based approach. [Google Scholar]
- 42.Pandya M., Jani S., Dave V., Rawal R. Nanoinformatics: an emerging trend in cancer therapeutics. Nanobiotechnol Nanobiotechnol Concepts Appl Heal Agric Environ. 2020:135. [Google Scholar]
- 43.Khandia Rekha, Shailja Singhal, Utsang Kumar, Afzal Ansari, Ruchi Tiwari, Kuldeep Dhama. Analysis of Nipah virus codon usage and adaptation to hosts. Front Microbiol. 2019;10:886. doi: 10.3389/fmicb.2019.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhoyar, Rahul C., Abhinav Jain, Paras Sehgal, Mohit Kumar Divakar, Disha Sharma High throughput detection and genetic epidemiology of SARS-CoV-2 using COVIDSeq next-generation sequencing. PloS one. 2021;16(2) doi: 10.1371/journal.pone.0247115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.