Abstract
Despite their excellent, useful, and stable properties, thermoplastics are constantly subject to environmental risks because of their low degradability under thermal, chemical, and mechanical stresses. To overcome the aforementioned issues, we hereby introduce an eco-friendly camphor (Ct) cyclic diester. The Ct diester is designed as a monomer, including a ketal group from the Ct, and shows high thermal stability via a rigid spiro-ring and a bridged bicyclic structure. A series of polyester was synthesized using the Ct diester, including various types of diols and dimethyl terephthalate. PETxCty copolyesters showed appropriate thermal stability up to 414 °C and a high glass transition temperature. This thermal behavior led to amorphous regions as the Ct diester content increased. Regarding the proportion of the Ct diester in the polyester, it was sensitive to hydrolysis and contributed to the degradation of the polyester in acid buffer conditions.
Graphical Abstract
Keywords: Polyesters, Biodegradability, Cyclic diester, Camphor, Tartaric acid
Introduction
COVID-19 caused an "explosion" in the use of disposable masks and the abuse of disposable plastics that eventually appeared on beaches and in rivers globally. Since the waste plastic does not degrade, it is an environmental problem with an urgent need for a solution [1–4]. Over the past few decades, a significant number of studies have been conducted on bio-based materials developed from partly or wholly from sustainable plant sources, and composting them at the end of their useful life [5, 6]. For polyesters, polyethylene terephthalate (PET) is one of the most commonly used thermoplastic polymers, which can be found in beverage bottles, coatings, elastomer, fiber, and food packaging. However, PETs present an environmental problem because of their high mechanical, thermal, and gas barrier properties. With a wide range of applications, aromatic diesters of polyesters are used because of their high performance with good thermal and mechanical properties. Therefore, it is advisable to enhance the biodegradability of aromatic polyester used for products with a short life span. Copolymerization controls the properties of the aromatic polyesters. Preferably, bio-based comonomers used to make these materials environmentally friendly and sustainable [7]. Yet, polyesters using bio-based aliphatic monomers generally result in reduced stiffness and a low glass transition temperature (Tg) [8–12]. By introducing polar groups into polyesters, it may be possible to devise a new drug delivery system that has bio-medical applications.
However, preparation of functional linear polyesters typically requires elaborate synthetic protocols, with multiple steps of protection and deprotection and then often induces the degradation or gelation of the main chain in the polymers. On the other hand, several studies have been made on bio-based terephthalate polyester containing sustainable bio-mass. Studies have focused on promising building blocks for natural bio-based monomers; isosorbide (ISB), vanillin, camphor, tartaric acid, citric acid, itaconic acid, and more. ISB, derived from starch and cellulose, has chiral, rigid, nontoxic, hydrophilic, and degradable characteristics. It has been widely used as a diol comonomer for the synthesis of thermoplastic polymers [13–15]. However, low reactivity of secondary hydroxy group and limited conversion to other forms are the major drawbacks of ISB for a wide application. To compensate for the low reactivity of isosorbide, bicyclic di-acetalized diols (e.g. Galx-OH, Manx-OH and Glux-OH) containing a high-active primary hydroxyl group have been studied by Muñoz-Guerra’s group [15, 16]. The reactivity is much higher than that of isosorbide, and it is similar to the reactivity of linear alkyl diols. Tartaric acid occurs naturally in many fruits including grapes, bananas, tamarinds, and citrus fruits. As for the molecular structure, it is characterized by accessibility and relative simplicity [17–25]. New bio-monocyclic dimethyl 2,3-O-methylene-L-threarate (Thx-diester) monomer are tartaric acid-derived monomers and are introduced to improve the chain’s stiffness in polyesters [10, 21, 26]. Although organic acids were used, they showed a relatively low thermal stability compared to polyester using dimethyl terephthalate. DL-Camphor had a rigid body due to natural terpenoids, and it is used for fragrances, preservative treatment fluids, medicinal purposes, and bio-based terephthalate polyesters. Recently, co-polyesters with bicyclic diacetalized CaG diol revealed a high Tg from 78 to 129 °C, and an acceptable molecular weight (Mw). However, in this case, the production cost increases as the monomer production needs two steps by converting camphor to camphorquinone.
Here, we introduce an eco-friendly camphor dimethyl DL-tartrate (referred to as Ct diester) to solve existing polymer degradation issues. Ct diester was synthesized using DL-camphor and DL-tartaric acid, to be used in advanced aliphatic polyesters. The resulted Ct diester is consisted of a bicyclic structure in the body, diester at the ends, and contained a spiro-ring linked with camphor. Judging from the above, Ct diester would help enhance structural rigidity and reactivity. The primary innovation is that the Mw was significantly enhanced and also a high Tg was observed using the homopolymerized Ct diester. We further show numerically that the copolymerization with dimethyl terephthalate (DMT) and Ct diester was successfully synthesized.
Experimental Section
Materials
1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one (( ±)-Camphor, > 95%), DL-2,3-dihydroxybutanedioic acid (DL-tartaric acid, ≥ 99%), trimethyl orthoformate (99%), sulfuric acid (95–98%), sodium bicarbonate (NaHCO3, 99.0%), ethylene glycol (EG, 1,2-ethanediol ≥ 99.8%), 1,4-butanediol (99%), 1,6-hexanediol (99%), dimethyl terephthalate (99%), titanium(iv) isopropoxide, (99.995%) and chloroform-d (99.8 atom % D), and deuterated trifluoroacetic acid were purchased from Sigma-Aldrich. ( ±)-Camphor was recrystallized from ethanol.
Synthesis of Camphor Dimethyl DL-Tartrate
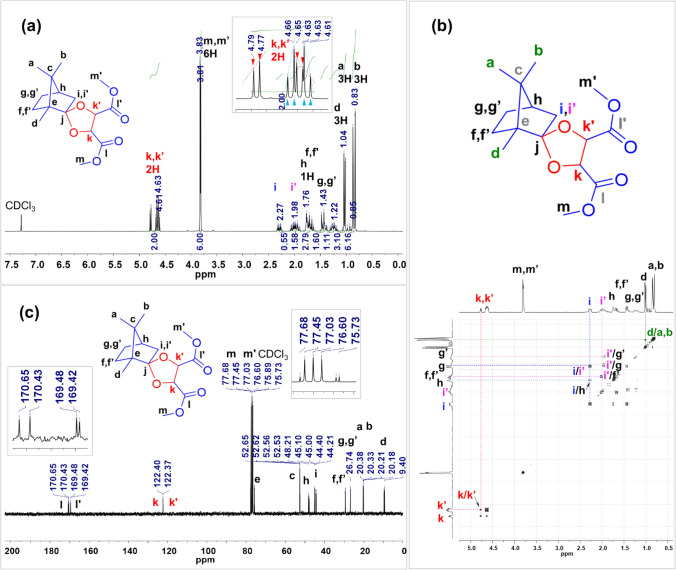
Ct diester was synthesized from bio-based monomers from camphor and tartaric acid according to the reported procedure [27, 28]. 60.89 g (0.400 mol, 152.24 g∙mol−1, 1 eq.) of DL-camphor, 100 mL (196 eq.) of dry methanol, and 160 mL of trimethyl orthoformate (1.46 mol, 60 eq.) were mixed in a two-necked round bottom flask (500 mL) under nitrogen condition. Thereafter, approximately 32 drops of sulfuric acid are added to the solution. The mixture was refluxed for 48 h under nitrogen. After the reaction, the solution is carefully neutralized with sodium hydrogen carbonate. The organic layer was dried on magnesium sulfate. Methanol, methyl formate, and trimethyl orthoformate were evaporated using a rotary evaporator and a straight path distilling unit. The residue is distilled under reduced pressure to obtain 59.5 g of dimethyl DL-tartrate, a colorless liquid. The raw material was purified using column chromatography (eluent n-Hexane: ethyl acetate = 9:1, Rf = 0.4, silica gel). The resulting molecular formula: C16H24O6; Mw: 312.36 g mol−1; yield: 70%. 1H-NMR (300 MHz, CDCl3): δ 4.68 (ddd, J = 20.4, 13.5, 6.0 Hz, 2H, acetal, –CH), 3.82 (dd, J = 3.2, 1.3 Hz, 6H, –COOCH3), 2.35–1.88 (m, 2H, –CH2–), 1.80–1.61 (m, 3H, –CH2–, –CH–), 1.50–1.35 (m, 2H, –CH2–), 1.32–1.16 (m, 1H, –CH2–), 1.03 (d, J = 6.9 Hz, 3H, –CH3), 0.84 (dd, J = 10.0, 2.6 Hz, 6H, –CH3). 13C-NMR (300 MHz, CDCl3): δ (ppm) = 170.54, 169.45, 122.38, 77.6, 76.2, 75.8, 52.6, 52.4, 47.9, 45.1, 44.3, 29.5, 26.8, 20.3, 20.2, 9.5. FT-IR: ν (cm−1) = 2954, 1740, 1437, 1208, 1122, 1031, 1002, 986.
Melt Polymerization
General Procedure
Poly(ethylene terephthalate) polyesters are abbreviated as PET100, PETxCty and PECt100. The samples were denoted by feeding ratio of DMT to Ct dister 100/0, 90/10, 70/30, 60/40, 50/50, 0/100, relatively. PETxCty copolyesters were synthesized using a reaction of dimethyl terephthalate with ethylene glycol and Ct diester, respectively. PETxCty copolyesters were synthesized from a mixture of ethylene glycol (1,4-buthanediol or 1,6-hexanediol), dimethyl terephthalate, and Ct diester in the reported compositions, and titanium(iv) isopropoxide (Ti {OCH (CH3) 2}4) was used as a catalyst. The polymerization was done in a three-neck round-bottomed flask with three outlets with a nitrogen inlet, a vacuum distillation outlet, and a mechanical stirrer. The transesterification was performed under a flow of nitrogen, and then the polycondensation progressed under a vacuum of approximately 2–5 Torr (2.67–6.67 mbar). The reagents were cooled to ambient temperature, and the low-pressure condition was restored using nitrogen. The reagents were dissolved in a mix of chloroform and trifluoroacetic acid (volume fraction, VCHCl3: VCF3COOH = 9:1), followed by precipitation into methanol. Lastly, the polymers were filtered, washed with methanol, and dried in a vacuum oven.
PET100 Homo-Polyester
A 22% molar excess of ethylene glycol was added to dimethyl terephthalate. Transesterification reactions were performed at 200 °C for 2 h and at 240 °C for 0.5 h. Polycondensation reactions were performed at 240 °C under a 2–5 Torr (2.67–6.67 mbar) vacuum for 2 h. 1H-NMR (300 MHz, CDCl3-TFA), δ (ppm): 8.12 (s, 4H, ArH), 4.75 (s, 4H, –CH2–). 13C-NMR (300 MHz, CDCl3 − TFA), δ (ppm): 166 (C=O), 133.5 (ArC), 130 (ArCH), 63.5(CH2). FT-IR: ν (cm−1) = 3462 (OH group), 2923 (symmetrical stretching of CH), 2955, 2915 (C–H, symmetrical stretching), 1713 (stretching of C=O of the R(C=O)OH group), 1578, 1505 (vibrations aromatic skeleton with stretching C=C), 1450, 1408, 1371 (stretching of the C–O group deformation of the O–H group and bending and wagging vibrational modes of the HOCH2CH2OH), 1250 (OOCC6H4–COO group), 1092, 1019 (–CH2– group and vibrations of the ester C–O bond), 939, 874, 840 (aromatic rings; tetra replaced), 1953, 794 (vibrations of adjacent two aromatic H in p-substituted compounds and aromatic bands), and 727 (interaction of polar ester groups and benzene rings).
PETxCty Co-Polyester
A 22% molar excess of diol monomer ethylene glycol was added to dimethyl terephthalate. Transesterification reactions were performed at 180 °C for 2 h and at 240 °C for 0.5 h. Polycondensation reactions were performed at 240 °C under a 2–5 Torr (2.67–6.67 mbar) vacuum for 2 h. 1H-NMR spectrum of PETxCty co-polyesters. 1H-NMR (300 MHz, CDCl3), δ (ppm): 8.11 (s, 4H, ArH), 4.84–4.39 (10H, m, ethylene group –(CH2)2–, ketal group, –O–CH–COO–), 2.25–1.1 (7H, m, –CH2CCH2–), 0.95 (3H, m, J = 29.8 Hz, –CCHCH3C–), 0.8–0.7 (6H, d, J = 9.9 Hz, –CCH3CH3C–). FT-IR: ν (cm−1) = 3462 (OH group, hydroxyl), 2955, 2875 (C–H, symmetrical stretching), 1720 (stretching of C=O of the carboxylic acid group), 1579, 1505 (vibrations aromatic skeleton with stretching C=C), 1476, 1451, 1409 (stretching of the C–O group deformation of the O–H group and bending and wagging vibrational modes of the ethylene glycol segment), 1247 (terephthalate Group (OOCC6H4–COO), 1097, 1017 (methylene group and vibrations of the ester C–O bond), 968, 876, 847 (aromatic rings; Tetra replaced), and 729 (interaction of polar ester groups and benzene rings).
PBT Homo-Polyester
A 22% molar excess of diol monomer 1,4-buthanediol was added to dimethyl terephthalate. Transesterification reactions were performed at 180 °C for 2 h and at 230 °C for 0.5 h. Polycondensation reactions were performed at 220 °C under a 2–5 Torr (2.67–6.67 mbar) vacuum for 2 h. 1H-NMR (300 MHz, CDCl3-TFA), δ (ppm): 8.13 (s, 4H, ArH), 4.49 (s, 4H, –CH2–), 2.01 (s, 4H, –CH2–).
PBTxCty Co-Polyester
A 22% molar excess of diol monomer 1,4-buthanediol was added to dimethyl terephthalate. Transesterification reactions were performed at 180 °C for 2 h and at 230 °C for 0.5 h. Polycondensation reactions were performed at 220 °C under a 2–5 Torr (2.67–6.67 mbar) vacuum for 2 h. 1H-NMR spectrum of PBTxCty co-polyesters. 1H-NMR (300 MHz, CDCl3), δ (ppm): 8.12 (s, 4H, ArH), 4.76–4.24 (10H, m, butylene group –(CH2)4–, ketal group, –O–CH–COO–), 2.3–1.1 (7H, m, –CH2CCH2–), 1.02 (3H, d, J = 7.9 Hz, –CCHCH3C–), 0.8–0.7 (6H, d, J = 12.9 Hz, –CCH3CH3C–).
PHT100 Homo-Polyester
A 22% molar excess of diol monomer 1,6-hexanediol was added to dimethyl terephthalate. Transesterification reactions were performed at 180 °C for 2 h and at 220 °C for 0.5 h. Polycondensation reactions were performed at 200 °C under a 2–5 Torr (2.67–6.67 mbar) vacuum for 2 h. 1H-NMR spectrum of PHTxCty co-polyesters. 1H-NMR (300 MHz, CDCl3), δ (ppm): 8.11 (s, 4H, ArH), 4.38 (s, 4H, –CH2–), 1.85 (s, 4H, –CH2–), 1.55 (s, 4H, –CH2–).
PHTxCty Co-Polyester
A 22% molar excess of diol monomer 1,6-hexanediol was added to dimethyl terephthalate. Transesterification reactions were performed at 180 °C for 2 h and at 220 °C for 0.5 h. Polycondensation reactions were performed at 200 °C under a 2–5 Torr (2.67–6.67 mbar) vacuum for 2 h. 1H-NMR spectrum of PHTxCty co-polyesters. 1H-NMR (300 MHz, CDCl3), δ (ppm): 8.11 (4H, dd, m-ArH), 4.7–3.6 (9H, m, -(CH2)6-), 2.3–1.1 (14H, m, –CH2CHCH2–, –CCHCH3C–), 1.03 (d, 3H, –CCHCH3C–, J = 8.1 Hz), 0.91–0.77 (m, 6H, –CCH3CH3C–).
Hydrolytic Degradation of Film
Films with a thickness of about 200 μm were prepared from a solution (100 g∙L−1) of chloroform and hexafluoroisopropanol (4:1). The films were dried under atmospheric pressure at room temperature for 72 h and retained a thickness between 15 and 20 mg. The polymers were immersed in 10 mL of citric acid buffer (pH 2.0) at 80 °C. After the scheduled period, the films were rinsed with distilled water, then dried. The Mw of the hydrolyzed polymers was analyzed by GPC chromatography, and the average value after being repeated three times was used.
Measurements
The synthesized polyesters were determined through FT-NMR spectroscopy (Bruker, Avance III 300 MHz, Germany) and FT-IR spectrometer (PerkinElmer Instrument, spectrum 400 FT-IR with UATR accessory, diamond/ZnSe, 1 reflection, U.S.A.) at 25 °C. The NMR solvents were trifluoroacetic acid and deuterated chloroform (CDCl3, 1H: δ = 7.26 ppm) (1:9) with TMS as the internal standard. The average Mw and dispersity Đ of the synthesized polymers were measured by gel permeation chromatography (GPC) (high-temperature chromatography, PL-GPC 220, USA and HPLC Column Plgel, Olexis, 13 µm, 7.5 × 300 mm, particle size 13.0 µm, Agilent HPLC Guard Column PLgel 10 µm, 7.5 × 50 mm, particle size 10.0 µm). m-Cresol was used as the eluent (flow rate: 1 mL∙min−1) at 100 °C, and about 100 μL of each sample was injected into the GPC to achieve a 1.0% (wt/volume) solution in m-cresol. The Mw was calibrated by polystyrene standards. Tg was measured via differential scanning calorimetry (DSC, PerkinElmer Instrument, DSC8000, U.S.A.). The measurements were carried out at a heating/cooling rate of 10 °C∙min−1 from 60 to 200 °C. The thermal data acquisition was carried out using PerkinElmer’s Pyris thermal analysis software. The polyesters were analyzed regarding their thermal properties to verify their degradation temperatures. The TGA (PerkinElmer Instrument, TGA8000, U.S.A.) was used to analyze the thermal stabilities of the co-polyesters using nitrogen gas (99.99%) at a temperature range of from 50 to 600 °C and a heating rate of 20 °C ∙min−1.
Results and Discussion
Synthesis and Chemical Structure
Schemes 1 and 2 show a melt polymerization process from Ct diester monomer, ethylene glycol, and dimethyl terephthalate (DMT) using titanium (iv) isopropoxide as a catalyst. Melt polymerization is a two-step polymerization process. The first step is transesterification. This step was carried out at a relatively low temperature and atmospheric pressure, thereby reducing the volatilization rate of the diol and preventing the crystallization of the oligomer generated at the beginning of the process. The second step is polycondensation. This step was carried out at low pressure to efficiently remove by-products, methanol, and excess diol. Polymer purification was carried out by precipitating the polymerized polyester in chloroform or chloroform/trifluoroacetic acid in excess methanol.
Scheme 1.
Synthesis of camphor dimethyl DL-tartrate (Ct diester) monomer: dry trimethyl orthoformate, dry methanol, 98% sulfuric acid, reflex, 48 h
Scheme 2.
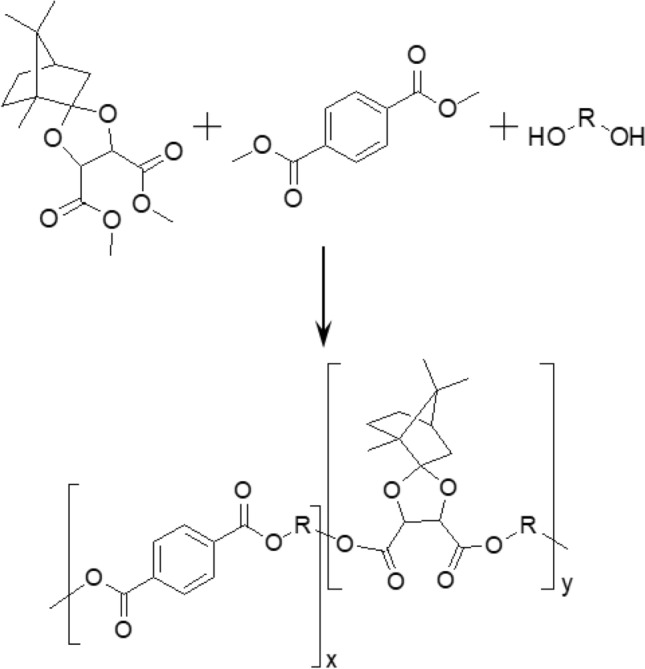
Melt polymerization of PET100 homo-polyester and PETxCty (PBTCt and PHTCt) co-polyester (R = –(CH2)2–, –(CH2)4–, –(CH2)6–, x = 90, 70, 60, 50 and y = 10, 30, 40, 50, x and y: feed ratio of DMT to Ct diester)
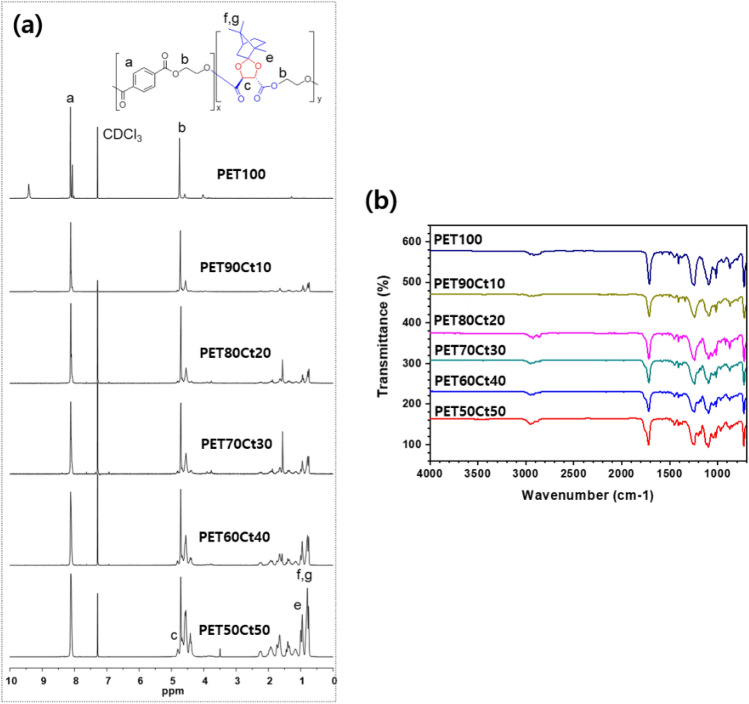
The chemical structure and composition of the polyester were analyzed by 1H-NMR, and GPC measured the Mw (Fig. 1 and Table 1). The molar ratio of the resulting polyester was calculated using the peak ratio of the 1H NMR spectrum (Fig. 2). The peaks of Ct diester and EG appeared at 4.84–4.39 ppm. The integration of proton signals indicated the content of each part. The following FT-IR spectra was in accordance with the results in 1H-NMR spectra (Fig. 2b).
Fig. 1.
a 1H-NMR, b 2D COSY(1H-1H correlation) NMR, and c 13C-NMR spectra of the Ct diester monomer
Table 1.
Molar composition, yield, and molecular weight of homo-polyester PET100 and co-polyesters PETxCty
| No | Copolyester | Yield (%) | Molar composition | Molecular weight | |||||
|---|---|---|---|---|---|---|---|---|---|
| % Feed | a% Incorporation | ||||||||
| DMT | Ct diester | DMT | Ct diester | bMn (g∙mol−1) | cMw (g∙mol−1) | dĐ | |||
| 1 | PET100 | 98 | 100 | 0 | 100 | 0 | 16,000 | 51,000 | 3.22 |
| 2 | PET90Ct10 | 60 | 90 | 10 | 81 | 19 | 14,000 | 48,000 | 3.42 |
| 3 | PET70Ct30 | 55 | 70 | 30 | 71 | 29 | 16,000 | 50,000 | 3.22 |
| 4 | PET60Ct40 | 60 | 60 | 40 | 63 | 38 | 14,000 | 51,000 | 3.62 |
| 5 | PET50Ct50 | 50 | 50 | 50 | 50 | 50 | 20,000 | 54,000 | 2.72 |
| 6 | PECt100 | 48 | 0 | 100 | 0 | 100 | 9,000 | 22,000 | 2.51 |
Measured conditions; high temperature GPC in m-cresol (flow rate: 1 mL min−1) at 100 °C against PS standards
a% Incorporation; 1H NMR by integrating the 1-methyl group of the Ct diester (peak f, g, e, 0.77 ppm) versus the aromatic protons (peak a, ~ 8 ppm)
bMn; Number-average molecular weights
cMw; Weight-average molecular weights
dĐ; dispersity index
Fig. 2.
Comparison of a 1H-NMR and b FT-IR spectra of PET100 and co-polyester; PETxCty: PET90Ct10, PET80Ct20, PET70Ct30, PET60Ct40, PET50Ct50. The peaks of Ct diester moiety appeared at 4.77–4.63 ppm and 1.04–0.83 ppm and ketal O-C-O 1200 cm−1 in FT-IR
The reactivity of ester group in Ct diester unit was lower than the reactivity of ester group adjacent to the aromatic ring in DMT unit. The Es values were defined from the following equation.
where k and k0 are the rate constants for acid hydrolysis of the substituted ester and the ester containing a -CH3 group in the acyl group. In the case of substituents conjugated with the ester carbonyl group, Es is thought to include resonance effects. ν is a van der Waals radii parameter. The rate of esterification of small molecules decreased with the increase in the steric hindrance from benzoic acid (aryl, Ph; ν = 0.57) to pivalic acid (t-Bu, 2°; ν = 1.24) [29, 30]. There is no significant difference with the raw material, and this difference in responsive can be overcome by controlling the feed rate. PETxCty copolyesters were obtained at yields above 48% and the Mw was 24–54 kDa with dispersity values that increased with the content of Ct diester unit (Table 1). The ketal group of the Ct diester unit is adjacent, which is formed by the reaction of tartaric acid with the ketone group of camphor. The increase in diversity value is also expected to be related to entanglement due to the structure of Ct moiety.
Thermal Properties
As shown in Table 2 and Fig. 3, the thermal behavior of PET homopolyester and PETxCty copolyester was measured by TGA and DSC, and the temperature corresponding to a 5% weight loss (T5%), the maximum decomposition rate (Td), and the residual weight in a nitrogen atmosphere were recorded.
Table 2.
Thermal properties of PET homo-polyester and PETxCty co-polyesters
| No | Copolyester | Thermal properties | ||||
|---|---|---|---|---|---|---|
| TGA | DSC | |||||
| T5% (°C)a | Td (°C)b | Wc (%)c | Tg (°C) | Tm (°C) | ||
| 1 | PET100 | 463 | 474 | 17.58 | 74.52 | 228.25, 238.88 |
| 2 | PET90Ct10 | 430 | 453 | 16.31 | 74.52 | 217.96, 227.25 |
| 3 | PET70Ct30 | 429 | 452 | 14.89 | 72.52 | 212.26, 233.26 |
| 4 | PET60Ct40 | 398 | 423 | 11.08 | 71.63 | 200.42 |
| 5 | PET50Ct50 | 394 | 414 | 10.63 | 64.30 | – |
aT5%; Temperature at which 5% weight loss was observed
bTd; Temperature for maximum degradation rate
cWc; Remaining weight at 600 °C
Fig. 3.
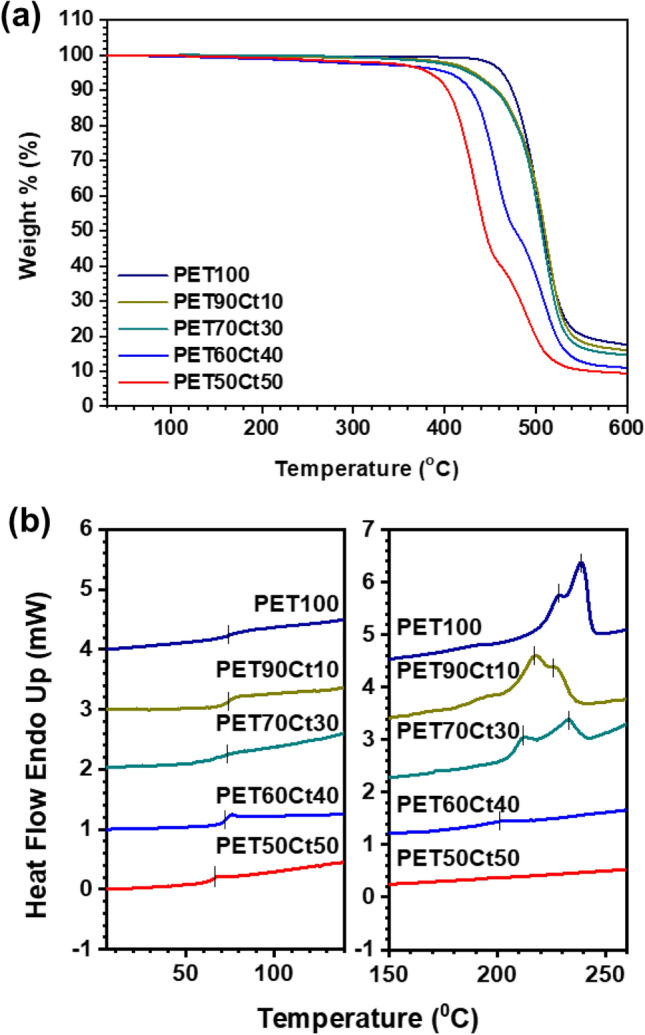
a TGA and b DSC curves of PET100 homo-polyester and PETxCty co-polyesters
PETxCty copolyester was relatively stable at a high temperature due to the structural stiffness of Ct diester. Weight loss for all polyesters started above 394 °C, and these copolyesters decomposed, leaving less than 17% residue at 600 °C. The results of this behavior indicated that the heat resistance of polyester was sufficient to allow conventional processing.
The Tg of the polymer was observed during the 2nd heating scan at a heating rate of 10 °C min−1 in the DSC trace (Fig. 3b). Except for PETCt100, with the lowest Mw, co-polyester PETxCty obtained Tg at 64–74 °C. Relatively, the Tg value of PET50Ct50, which has a large amount of Ct diester, and low Mn, less than 10,000, was observed at a low temperature. Low Mw polymers exhibit reduced Tg because the ends of the polymer chain produce a free volume and interfere with chain packing according to the Flory-Fox equation (Tg = Tg,∞−K/Mn). By observing the change in the DSC curve, the PET90Ct10 co-polyester exhibited a Tg of 74 °C, similar to that of the PET homo-polyester, due to the structural stiffness of Ct diester. This is mainly due to the Ct diester having a bridged 6-membered rings, 3 methyl branches, and a 5-membered spiro ring. This feature induces rigidity in the polymer chain and exhibits a high Tg, similar to the aromaticity of PET100. In particular, the six-membered rings were tightly bound and the methyl branches prevented the movement of rings. Due to their bulky and rigid structure, Ct diesters improved the Tg by limiting vibration. A portion of the low Mw polymer remaining in the polymerization acted as a plasticizer and showed Tg lowering behavior. PETs had a distinct melting point, which is one of the two primary phase transitions of crystalline polymers, but this gradually disappeared with increased Ct diester content. As previously reported, the heat resistance of polymers reinforced with heterocyclic ring structures is increased [31, 32]. The introduction of cyclic repeating units into the polymer backbone provides stiffness and improves the thermal stability of the resulting polymer. When the Ct moiety content increases, the shape of the melting peaks becomes broad and the size of the peak decreases in the DSC curves of PETxCty co-polymers. Wide endotherms were shown, composed of melting peaks indicative of crystallization defects in polyester. Multiple melting peaks of PET100, PET90Ct10 and PET80Ct20 were due to the presence of alternate crystal changes and molecular weight separation the accompanies crystallization [33]. If the content of Ct moiety is lower than 30%, melting peaks cannot be observed. This behavior is similar to that of reported for polyesters based on natural bicyclic monomers such as mannitol and glucose [34].
Degradability
As the crystallinity of the PET increases, the penetration of the solvent into the crystalline domain across the hard chain slows and the rate of decomposition rate decreases [35]. It is a heterogeneous reaction that the aqueous hydrolysis of PET between two incompatible phases such as PET suspended in an aqueous phase. One of the biggest problems for hydrolysis in aqueous medio is the incompatibility between the liquid phased and the polymer. The ketal group of co-ployesters can be a strategy to improve the reaction efficiency. PETxCty copolymers with new Ct diester monomer have the characteristics of acceleration of polymer degradation due to having ketal group, tunable functionality and primary alcohol group for high reactivity compared to ISB. Over the past few years, several studies have been made on polyesters and their polymers containing based on sugar monomers for hydrolysis at high-rated when incubated under acidic or basic solution [36–39]. Yet, polymers containing based on camphor and tartaric acid have not been widely studied. Especially, very few attempts have been made at hydrolytic surface observation using 3D profiler system. Figure 4 shows the hydrolytic degradation with time in buffer solutions (80 °C) of pH 2.0 and 10.0 in PET100 homo-polyester, PET90Ct10, and PET50Ct50 co-polyesters using a 3D optical surface profiler. The arithmetic mean height (Sa) of the surface was measured and observed. Sa is the absolute value of the difference in the height of each point compared to the arithmetic mean of the surface. This parameter is a numerical value generally used to evaluate surface roughness. Looking at the time-dependent degradation results, the higher the incorporation of Ct diester into the copolyester, the higher the Sa. Indeed, the hydrolysis of the ketal bonds in the PETxCty polymer chains is more rapid than the hydrolysis of the ester bonds at acidic buffer solutions [40].
Fig. 4.
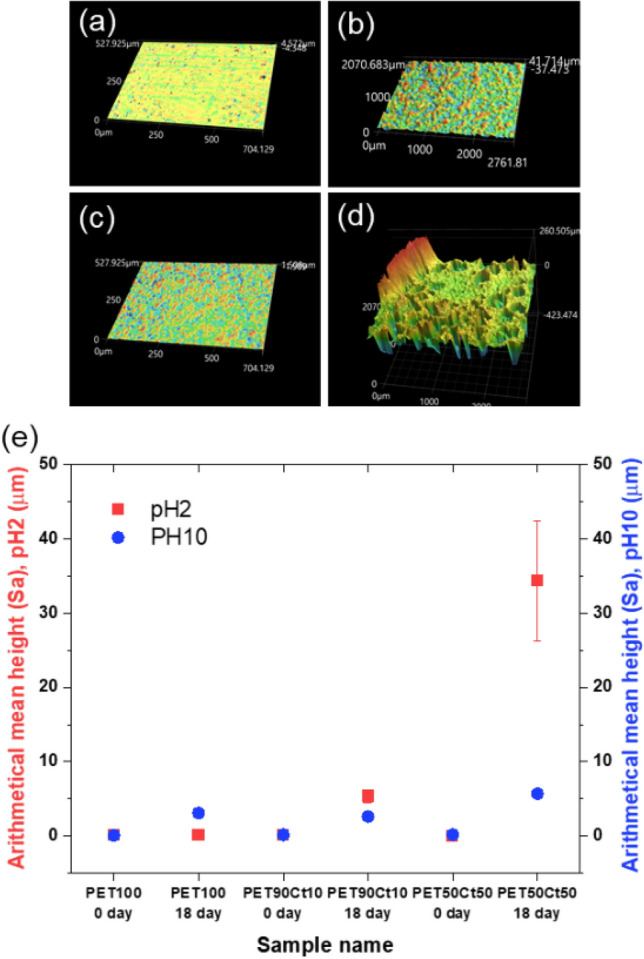
Appearance of the surface before and after 18 days degradation for a, b PET90Ct10, c, d PET50Ct50, e surface roughness of PET100, PET90Ct10, and PET50Ct50. The arithmetical mean height to a surface (Sa, surface parameter, ISO 25178) versus degradation time (0, 18 days) at 80 °C under pH 2.0 and pH 10.0 buffer solutions
As shown in Fig. 5a, PET100 polyester contained in pH 2.0 buffer solution at 80 °C showed a very low mass loss, and the residual amount of PET50Ct50 polyester film showed a tendency to decrease over time. After 18 days, the mass loss of PET100 homopolyester and PET50Ct50 copolyester was about 1.73% and 17.8%, respectively, and the weight average Mw reduction was 9.4% from 23.7%, respectively, of the initial value. In addition, the degradation rate of the polyester increased as the proportion of Ct diester increased. Figure 5b shows that hydrolysis of PET50Ct50 was confirmed at 80 °C for 18 h and a pH 2.0 acid buffer solution conditions using proton NMR analysis. It was assumed that the change of the bio-based polyester film resulted from the presence or absence of Ct diester attached to the surface of the polymer chain with ketal groups. The ketal group and carbonate group of the polymer are hydrolyzed in an acid buffer solution. Comparing the integrals of the protons corresponding to Ct diester units, it can be seen that this ratio is significantly reduced. A ketal group has two single-bonded oxygen atoms attached to the same carbon atom. When the oxygen is protonated, the adjacent carbon is activated, facilitating a reaction in the water, eventually resulting in the breakdown of the ketal group to an alcohol. Hydrolysis occurs in the ketal group on the polymer side to increase the hydrophilicity of the polymer, so water can easily penetrate the main chain of polymers. Based on these results, it was found that the degradation of PE50Ct50 occurs preferentially in the Ct diester unit due to the ketal bond of camphor. The conversion of polyester to a water-soluble material is expected to be applicable to drug delivery systems.
Fig. 5.
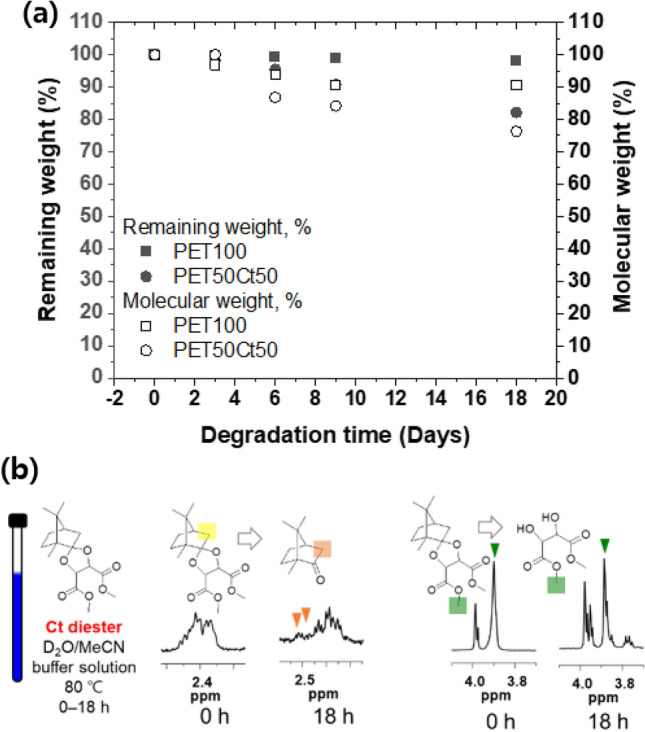
a Degradation of PET100 (square) and PET50Ct50 (circle). Remaining weight (%) and Mw (%) versus hydrolytic degradation time in pH 2.0 buffer solution at 80 °C. b 1H-NMR peaks before and after 18 days in pH 2.0 buffer solution at 80 °C (left). A peak of change occurs for the large ketal bond breaking and the small ester bond breaking in the PET50Ct50 co-polyester after 18 days (right)
Conclusion
Ct diester is derived from the abundantly available and inexpensive camphor and tartaric acid byproducts of the wine-making process. In this work, Ct diesters were successfully synthesized by a melt polymerization step. The combination of biomass tartaric acid and camphor creates a bio-based monomer that can improve the thermal condensation of polyester. The Mw of homo-polyester PET100 was 51 kDa. The copolymer significantly affected the Tg of PETxCty co-polyester compared to PET100 homo-polyester. The Tg of the polyester can be controlled by adjusting the content of the Ct diester. The thermal stability of PETxCty co-polyesters with Ct diesters showed a Td of 414 °C. Although copolymerization reduced the Mw and the Tg of PETxCty, copolymerized polyesters contain up to 50% bio-based monomers. The Ct diester monomer improved the Tg of the polyester and the thermal stability of the polymer due to the unique ring structure.
Further, we applied a 3D optical surface profiler to observe the hydrolytic surface of a polymer film for the first time. When these polyesters were incubated under pH 2.0, hydrolytic degradation was rapidly proceeded as the Ct diester in the polyesters increased. Here, a rapid degradation within the main chain of the polymer was achieved with the aid of the ketal group. The PET50Ct50 film acted as an acid buffer solution and revealed a higher hydrolysis efficiency than PET100. The weight loss and Mw loss of PET50 Ct50 were about 22.8% and 42.6%, respectively, of the initial values after 18 days. With these features in hand, the bio-based Ct diester has the potential to expand the applications by replacing conventional petrochemicals via the use of eco-friendly chemicals from renewable sources.
Author Contributions
JHK and DHS conceptualized and directed the research. JHK, SJS, and JHL performed the syntheses and collected the analytical data. SL performed the data curation and formal analysis. JHK and DHS carried out the visualization. JHK and DHS contributed to the manuscript writing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors.
Data Availability
Not applicable.
Declarations
Conflict of interest
The authors declare no competing financial interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Millican JM, Agarwal S. Plastic pollution: a material problem? Macromolecules. 2021;54:4455–4469. doi: 10.1021/acs.macromol.0c02814. [DOI] [Google Scholar]
- 2.Chamas A, Moon H, Zheng J, et al. Degradation rates of plastics in the environment. ACS Sustain Chem Eng. 2020;8:3494–3511. doi: 10.1021/acssuschemeng.9b06635. [DOI] [Google Scholar]
- 3.Wang Z, Ganewatta MS, Tang C. Sustainable polymers from biomass: bridging chemistry with materials and processing. Prog Polym Sci. 2020;101:101197. doi: 10.1016/j.progpolymsci.2019.101197. [DOI] [Google Scholar]
- 4.Nguyen HTH, Qi P, Rostagno M, et al. The quest for high glass transition temperature bioplastics. J Mater Chem A. 2018;6:9298–9331. doi: 10.1039/c8ta00377g. [DOI] [Google Scholar]
- 5.Zhang Q, Song M, Xu Y, et al. Bio-based polyesters: recent progress and future prospects. Prog Polym Sci. 2021;120:101430. doi: 10.1016/j.progpolymsci.2021.101430. [DOI] [Google Scholar]
- 6.Zia KM, Noreen A, Zuber M, et al. Recent developments and future prospects on bio-based polyesters derived from renewable resources: a review. Int J Biol Macromol. 2016;82:1028–1040. doi: 10.1016/j.ijbiomac.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Li WD, Zeng JB, Lou XJ, et al. Aromatic-aliphatic random and block copolyesters: synthesis, sequence distribution and thermal properties. Polym Chem. 2012;3:1344–1353. doi: 10.1039/c2py20068f. [DOI] [Google Scholar]
- 8.Pawar M, Kadam A, Yemul O, et al. Biodegradable bioepoxy resins based on epoxidized natural oil (cottonseed & algae) cured with citric and tartaric acids through solution polymerization: a renewable approach. Ind Crops Prod. 2016;89:434–447. doi: 10.1016/j.indcrop.2016.05.025. [DOI] [Google Scholar]
- 9.Oliva R, Ortenzi MA, Salvini A, et al. One-pot oligoamides syntheses from L-lysine and L-tartaric acid. RSC Adv. 2017;7:12054–12062. doi: 10.1039/c7ra00676d. [DOI] [Google Scholar]
- 10.Lavilla C, Gubbels E, Alla A, et al. Carbohydrate-based PBT copolyesters from a cyclic diol derived from naturally occurring tartaric acid: a comparative study regarding melt polycondensation and solid-state modification. Green Chem. 2014;16:1789–1798. doi: 10.1039/c3gc41759j. [DOI] [Google Scholar]
- 11.Zamora F, Hakkou K, Alla A, et al. Aromatic homo-and copolyesters from naturally occurring monosaccharides: PET and PEI analogs derived from l-arabinitol and xylitol. J Polym Sci Part A Polym Chem. 2005;43:6394–6410. doi: 10.1002/pola.21045. [DOI] [Google Scholar]
- 12.Zakharova E, Lavilla C, Alla A, et al. Modification of properties of poly (butylene succinate) by copolymerization with tartaric acid-based monomers. Eur Polym J. 2014;61:263–273. doi: 10.1016/j.eurpolymj.2014.09.024. [DOI] [Google Scholar]
- 13.Saxon DJ, Nasiri M, Mandal M, et al. Architectural control of isosorbide-based polyethers via ring-opening polymerization. J Am Chem Soc. 2019;141:5107–5111. doi: 10.1021/jacs.9b00083. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Wu J, Qi J, et al. Systematic study of thermal and (bio) degradable properties of semiaromatic copolyesters based on naturally occurring isosorbide. ACS Sustain Chem Eng. 2019;7:1061–1071. doi: 10.1021/acssuschemeng.8b04717. [DOI] [Google Scholar]
- 15.Monnery BD, Karanastasis A, Adriaensens P, et al. Mechanically versatile isosorbide-based thermoplastic copolyether-esters with a poly (ethylene glycol) soft segment. J Polym Sci. 2021;59:2809–2818. doi: 10.1002/pol.20210548. [DOI] [Google Scholar]
- 16.Lavilla C, Martínez De Ilarduya A, Alla A, Munoz-Guerra S, et al. PET copolyesters made from ad-mannitol-derived bicyclic diol. Polym Chem. 2013;4:282–289. doi: 10.1039/c2py20531a. [DOI] [Google Scholar]
- 17.Pang C, Jiang X, Yu Y, et al. Copolymerization of natural camphor-derived rigid diol with various dicarboxylic acids: access to biobased polyesters with various properties. ACS Macro Lett. 2019;8:1442–1448. doi: 10.1021/acsmacrolett.9b00570. [DOI] [PubMed] [Google Scholar]
- 18.Nsengiyumva O, Miller SA. Synthesis, characterization, and water-degradation of biorenewable polyesters derived from natural camphoric acid. Green Chem. 2019;21:973–978. doi: 10.1039/c8gc03990a. [DOI] [Google Scholar]
- 19.Li J, Zhang H, Liu F, et al. A new series of fluorinated alicyclic-functionalized polyimides derivated from natural-(D)-camphor: synthesis, structure–properties relationships and dynamic dielectric analyses. Polymer. 2013;54:5673–5683. doi: 10.1016/j.polymer.2013.08.014. [DOI] [Google Scholar]
- 20.Park JE, Hwang DY, Choi GH, et al. Fast hydrolysis polyesters with a rigid cyclic diol from camphor. Biomacromol. 2017;18:2633–2639. doi: 10.1021/acs.biomac.7b00761. [DOI] [PubMed] [Google Scholar]
- 21.Japu C, De Ilarduya AM, Alla A, et al. Bio-based poly (hexamethylene terephthalate) copolyesters containing cyclic acetalized tartrate units. Polymer. 2013;54:1573–1582. doi: 10.1016/j.polymer.2013.01.013. [DOI] [Google Scholar]
- 22.Kint DPR, Wigström E, de Ilarduya AM, et al. Poly (ethylene terephthalate) copolyesters derived from (2S, 3S)-2, 3-dimethoxy-1, 4-butanediol. J Polym Sci Part A Polym Chem. 2001;39:3250–3262. doi: 10.1002/pola.1308. [DOI] [Google Scholar]
- 23.Marín R, Muñoz-Guerra S. Linear polyurethanes made from threitol: acetalized and hydroxylated polymers. J Polym Sci Part A Polym Chem. 2008;46:7996–8012. doi: 10.1002/pola.23099. [DOI] [Google Scholar]
- 24.Alla A, Oxelbark J, Rodríguez-Galán A, et al. Acylated and hydroxylated polyamides derived from L-tartaric acid. Polymer. 2005;46:2854–2861. doi: 10.1016/j.polymer.2005.02.046. [DOI] [Google Scholar]
- 25.Alla A, Rodríguez-Galán A, Muñoz-Guerra S. Hydrolytic and enzymatic degradation of copoly (ester amide) s based on l-tartaric and succinic acids. Polymer. 2000;41(19):6995–7002. doi: 10.1016/S0032-3861(00)00070-7. [DOI] [Google Scholar]
- 26.Japu C, Martínez De Ilarduya A, Alla A, et al. Bio-based poly (ethylene terephthalate) copolyesters made from cyclic monomers derived from tartaric acid. Polymer. 2014;55:2294–2304. doi: 10.1016/j.polymer.2014.03.018. [DOI] [Google Scholar]
- 27.Mikołajczyk M, Mikina M, Wieczorek MW, et al. The first synthesis of enantiopure (–)- and (+)-isoterrein from optically inactive meso-tartaric acid. Angew Chemie Int Ed English. 1996;35:1560–1562. doi: 10.1002/anie.199615601. [DOI] [Google Scholar]
- 28.Ley SV, Osborn HM, Priepke HW, et al. (1’s,2’s)-methyl-3o,4o-(1’,2’-dimethoxycyclohexane-1’,2’-diyl)-a-d-mannopyranoside. Org Synth. 1998;75:170. doi: 10.15227/orgsyn.075.0170. [DOI] [Google Scholar]
- 29.Charton M. Nature of the ortho effect. II. Composition of the Taft steric parameters. J Am Chem Soc. 1969;91:615–618. doi: 10.1021/ja01031a016. [DOI] [Google Scholar]
- 30.Charton M. Steric effects. I. Esterification and acid-catalyzed hydrolysis of esters. J Am Chem Soc. 1975;97:1552–1556. doi: 10.1021/ja00839a047. [DOI] [Google Scholar]
- 31.Hashimoto T, Takagi H, Hasegawa Y, et al. Living/controlled cationic cyclopolymerization of divinyl ether with a cyclic acetal moiety: Synthesis of poly (vinyl ether) s with high glass transition temperature based on incorporation of cyclized main chain and cyclic side chains. J Polym Sci Part A Polym Chem. 2010;48:952–958. doi: 10.1002/pola.23851. [DOI] [Google Scholar]
- 32.Park JE, Kim WK, Hwang DY, et al. Thermally stable bio-based aliphatic polycarbonates with quadra-cyclic diol from renewable sources. Macromol Res. 2018;26:246–253. doi: 10.1007/s13233-018-6038-8. [DOI] [Google Scholar]
- 33.Gunaratne LMWK, Shanks RA. Multiple melting behaviour of poly (3-hydroxybutyrate-co-hydroxyvalerate) using step-scan DSC. Eur Polym J. 2005;41:2980–2988. doi: 10.1016/j.polymertesting.2009.11.011. [DOI] [Google Scholar]
- 34.Lavilla C, de Ilarduya AM, Alla A, et al. Bio-based aromatic polyesters from a novel bicyclic diol derived from D-mannitol. Macromolecules. 2012;45:8257–8266. doi: 10.1021/ma3013288. [DOI] [Google Scholar]
- 35.Ügdüler S, Van Geem KM, Denolf R, et al. Towards closed-loop recycling of multilayer and coloured PET plastic waste by alkaline hydrolysis. Green Chem. 2020;22:5376–5394. doi: 10.1039/D0GC00894J. [DOI] [Google Scholar]
- 36.Fenouillot F, Rousseau A, Colomines G, et al. Polymers from renewable 1, 4: 3, 6-dianhydrohexitols (isosorbide, isomannide and isoidide): a review. Prog Polym Sci. 2010;35:578–622. doi: 10.1016/j.progpolymsci.2009.10.001. [DOI] [Google Scholar]
- 37.Muñoz-Guerra S, Lavilla C, Japu C, et al. Renewable terephthalate polyesters from carbohydrate-based bicyclic monomers. Green Chem. 2014;16:1716–1739. doi: 10.1039/C3GC42394H. [DOI] [Google Scholar]
- 38.Sureshan KM, Shashidhar MS, Praveen T. Regioselective protection and deprotection of inositol hydroxyl groups. Chem Rev. 2003;103:4477–4504. doi: 10.1021/cr0200724. [DOI] [PubMed] [Google Scholar]
- 39.Lavilla C, Alla A, Martínez de Ilarduya A, et al. Carbohydrate-based polyesters made from bicyclic acetalized galactaric acid. Biomacromol. 2011;12:2642–2652. doi: 10.1021/bm200445w. [DOI] [PubMed] [Google Scholar]
- 40.Johnson AL, Parish JD. Recent developments in molecular precursors for atomic layer deposition. J Organomet Chem. 2018;42:1–53. doi: 10.1039/9781788010672-00001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.