Abstract
Purpose:
Questions remain about whether moderately hypofractionated whole breast irradiation is appropriate for patients with triple-negative breast cancer.
Methods:
Using a prospective database of a multicenter, collaborative quality improvement consortium, we identified patients with node-negative, triple-negative breast cancer who received whole breast irradiation with either moderate hypofractionation or conventional fractionation. Using inverse probability of treatment weighting (IPTW), we compared outcomes using the product-limit estimation method of Kaplan and Meier with Cox regression models estimating the hazard ratio for time-to-event endpoints between groups.
Results:
The sample included 538 patients treated at 18 centers in one state in the United States, of whom 307 received conventionally fractionated whole breast irradiation and 231 received moderately hypofractionated whole breast irradiation. The median follow-up time was 5.0 years (95% CI 4.77–5.15). The 5-year IPTW estimates for FFLR were 93.6% (95% CI 87.8%−96.7%) in the moderately hypofractionated group and 94.4% (95% CI 90.3%−96.8%) in the conventionally fractionated group. The hazard ratio was 1.05 (95% CI 0.51–2.17), p=0.89. The 5-year IPTW estimates for RFS were 87.8% (95% CI 81.0%−92.4%) in the moderately hypofractionated group and 88.4% (95% CI 83.2%−92.1%) in the conventionally fractionated group. The hazard ratio was 1.02 (95% CI 0.62–1.67), p=0.95. The 5-year IPTW estimates for OS were 96.6% (95% CI 92.0%−98.5%) in the moderately hypofractionated group and 93.4% (95% CI 88.7%−96.1%) in the conventionally fractionated group. The hazard ratio was 0.65 (95% CI 0.30–1.42), p=0.28.
Conclusion:
Analysis of outcomes in this large observational cohort of patients with triple-negative, node-negative breast cancer treated with whole breast irradiation reveals no differences by dose fractionation. This adds evidence to support the use of moderate hypofractionation in patients with triple-negative disease.
Introduction
Whole breast moderate hypofractionation is a less costly and less burdensome approach to adjuvant radiotherapy for women with breast cancer, requiring only 3 weeks (15–16 fractions to 40–42.5Gy), as compared to conventional fractionation, which requires 5 weeks or longer (50 to 50.4 Gy in 25–28 fractions). Large randomized trials from Canada1 and the United Kingdom2 established the overall safety and efficacy of whole breast moderate hypofractionation among patients with early-stage invasive breast cancer, leading clinical practice guidelines to embrace this as the preferred approach for whole breast irradiation.3
Questions have lingered, however,4 particularly regarding the appropriateness of moderate hypofractionation among patients with triple-negative disease. Breast and prostate cancers may generally have a lower alpha-beta ratio than the head and neck squamous cell carcinomas that were evaluated to derive our initial understanding of sensitivity to dose fractionation.5 However, questions remain about whether the lower alpha-beta ratio is specific to hormone-sensitive subtypes, which constitute the majority of these cancers, and whether using a higher dose per fraction to a lower total dose as prescribed by modern schedules of moderate hypofractionation is also equally effective in triple-negative cancers. Existing data to address these questions is limited in that the large British trials did not collect subtype information. Therefore, the only evidence describing subtype-specific outcomes from randomized comparison that existed to inform the most recent ASTRO guidelines used data from a subset of patients enrolled on the Canadian (OCOG) randomized trial.6 In that analysis, breast cancer subtype (luminal A, luminal B, HER2 enriched and basal) was measured using immunohistochemistry and fluorescence in situ hybridization (FISH). Risk of local recurrence did not differ significantly by treatment arm when stratified by molecular subtype, but the point estimates reported were in the direction of improved outcomes with hypofractionation for luminal A tumors (HR=0.56, 95% CI 0.24–1.33), whereas the point estimate was in the opposite direction for those with basal (triple-negative) disease (HR=1.27, 95% CI 0.21–7.58). Although this interaction was not statistically significant, the comprehensive ASTRO consensus guideline on whole breast fractionation emphasized the limitations of existing evidence and importance of additional research in this area because of the low power to detect an interaction between subtype and treatment arm based on the size of the subgroups in the OCOG analysis, which included only 125 patients with basal tumors.7
Since the publication of the aforementioned guideline, informative data have emerged from two additional trials. Rates of local recurrence were similar after conventional fractionation and moderate hypofractionation in the subgroup of 77 patients with triple-negative disease in a randomized trial from China8 and also in a subgroup of 188 patients with triple-negative disease in a randomized trial from Denmark, Germany, and Norway.9 In an editorial accompanying the publication of the trials, Abram Recht notes that these contributions help to advance understanding but continues to maintain that “there is not yet sufficient evidence to confidently reach a verdict on many of the important questions outlined above [including whether moderate hypofractionation is equally effective in patients with triple-negative disease]. Ongoing and future trials and retrospective analyses of existing studies will need to focus on those questions.”10 One such study was recently led using a large prospective observational cohort from Canada, which included 603 patients with triple-negative cancer, finding no difference in local recurrence-free survival in those patients.11 Using a similarly large observational cohort from the United States, we sought to collect additional evidence to address the gap in knowledge on this important question.
Methods
Sample Design and Data Collection
We queried a prospective database of a statewide collaborative quality improvement consortium that enrolls all patients receiving whole breast irradiation at participating facilities. We identified 672 patients with invasive, node-negative, triple-negative breast cancer treated between 1/1/12 and 12/31/18 (dates selected to allow a median follow-up of approximately five years). Because the consortium does not follow patients for disease control, we initiated a voluntary research study with physician leads from 18 of the 23 centers with eligible cases, in order to gather disease control information.
Each of the 18 centers submitted applications to their IRBs and received approval to conduct this research study. Sites collected data using standardized forms with anonymous identifiers that allowed the newly collected information to be merged with the data already present in the consortium database, including radiation dose-fractionation, for centralized analysis.
The 18 centers included 573 of the 672 potential cases. We received data for 558 cases, with 13 of 18 centers returning data for all cases and with the lowest return rate being 81.82% from two institutions. Further we re-queried the received cases and excluded any cases that were missing receptor status for ER, PR, or HER2/Neu, resulting in an exclusion of 14 additional cases. Six cases had no follow-up information, leaving 538 cases in the final analytical sample.
Outcomes Measures
We considered three outcomes using time-to-event endpoints. First, we considered freedom from local recurrence (FFLR), with the time constructed from the date of lumpectomy until date of local recurrence or censored on date of mastectomy (for patients who elected later to have mastectomy unrelated to recurrence), death, or last known contact. Second, we considered recurrence-free survival (RFS), with the time constructed from the date of lumpectomy until the first of date of recurrence (any location) or date of death, or censored on date of last known contact. Finally, we determined overall survival (OS), with the time constructed from date of lumpectomy until date of death or censored on date of last known contact.
Analytic Approach
The effects of moderately hypofractionated and conventionally fractionated regimens were compared for the three time-to-event endpoints using the product-limit estimation method of Kaplan and Meier. Because fractionation treatment decisions were made based upon provider preference and the patient’s clinical characteristics, we expected some bias in treatment selection to be present in this observational sample. Comparisons in an unadjusted fashion for time-to-event endpoints would therefore be biased by any differences in predictive and prognostic characteristics between the treatment groups. We assessed the degree of difference in covariates between treatment groups using the standardized difference, finding that several covariates had absolute difference values of 10 or greater, suggesting significant imbalance between groups.12 Therefore, we proceeded to implement a balancing technique, using propensity score creation for the treatment assignment and weighting subsequent analyses by the inverse probability of treatment assignment in order to correct these imbalances.
Propensity scores were calculated using multiple variable logistic regression with the following covariates: age groups (<50, 50-<60, 60-<70, 70+), race (White vs Black/other), BMI categories (<25 under/normal weight, 25-<30 overweight, 30-<35 obesity I, 35+ obesity II/III), comorbidity group (zero, 1, 2, 3 or more comorbidities), smoking status (never, former, current), chemotherapy (yes/no), T-stage (0/1 vs. 2/3), tumor grade [1(well)/2(moderately) vs 3(poorly) differentiated], surgical margins (close/positive vs. negative), and breast volume modeled using a restricted cubic spline with 5 knots spaced using the observed percentiles. The covariates for the propensity model were chosen using subject matter knowledge about appropriate predictive and prognostic characteristics and categorization was modified so extremely small groups of patients were avoided [Black patients grouped with other race patients (a very small group), certain T-stages were grouped together (0 with 1 and 2 with 3), and tumor grades were grouped (1 with 2). Further, propensity score calculation requires complete information for the chosen characteristics; otherwise the propensity score is missing. Therefore, the amount of missingness needs to remain low (<3% of total sample) for chosen covariates. A decision was made not to include lymphovascular invasion (LVI), which was collected with a higher degree of missingness (16.2%). Using the propensity model as described above, propensity scores could be calculated for 520 of the 538 cases comprising the analytical sample. Because the total amount of missingness for propensity scores was low, methods to impute the limited missing data were not necessary to implement.
Product-limit 5-year estimates for the time to event endpoints were created after inverse probability of treatment weighting (IPTW). Finally, Cox regression models were created to estimate the hazard ratio for time-to-event endpoints between groups. A sensitivity analysis restricted to cases that received boost radiotherapy was also conducted. All statistical analyses were conducted using the SAS System version 9.4 [Cary, NC, USA].
Results
Of the 538 cases in the analytical sample, 307 received conventionally fractionated whole breast irradiation and 231 received moderately hypofractionated whole breast irradiation. The vast majority of these cases received a boost (93.3%: 502/538). Median patient age was 63 years (60 among conventionally fractionated patients and 67 among moderately hypofractionated cases). Table 1 shows the characteristics of the patients both before and after IPTW using the calculated propensity scores. The calculated propensity scores had sufficient overlap between the moderately hypofractionated and conventionally fractionated populations, and when converted into inverse probability weights for the treatment received, all patient weights were below 3, suggesting no unduly influential cases for weighted analyses. Further, one can observe from the weighted sample description that balance in the covariates has been obtained through propensity weighting.
Table 1.
Sample Description (covariates used in the propensity model are shaded):
| Variable/Level | Summary | Unweighted sample description | Propensity (IPTW‡) weighted sample description | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ALL | CWBI | AWBI | D† | ALL | CWBI | AWBI | D† | ||
| Age: Continuous | Mean (Median) [IQR2] | 62.49 (63.35) [53.80 – 70.70] | 59.13 (59.9) [51.60 – 67.40] | 66.95 (67.5) [57.90 – 76.20] | 67.906 | 61.77 (62.2) [53.40 – 70.20] | 61.45 (61.7) [53.10 – 70.10] | 62.19 (63.4) [53.50 – 70.80] | 6.041 |
| Age group: Age < 50 | N (%) | 82 (15.24) | 66 (21.50) | 16 (6.93) | −42.672 | 82 (15.96) | 46 (15.75) | 36 (16.23) | 1.330 |
| Age group: 50 <= Age < 60 | N (%) | 146 (27.14) | 90 (29.32) | 56 (24.24) | −11.476 | 148 (28.50) | 85 (28.86) | 63 (28.03) | −1.844 |
| Age group 60 <= Age < 70 | N (%) | 163 (30.30) | 101 (32.90) | 62 (26.84) | −13.268 | 153 (29.47) | 88 (30.04) | 64 (28.73) | −2.866 |
| Age group: 70 <= Age | N (%) | 147 (27.32) | 50 (16.29) | 97 (41.99) | 58.976 | 135 (26.07) | 74 (25.35) | 60 (27.00) | 3.754 |
| Race: 1 = White | N (%) | 333 (61.90) | 173 (56.35) | 160 (69.26) | 26.958 | 313 (60.20) | 178 (60.44) | 134 (59.88) | −1.137 |
| Race: 2 = Black | N (%) | 177 (32.90) | 113 (36.81) | 64 (27.71) | −19.565 | 176 (33.96) | 98 (33.41) | 78 (34.69) | 2.685 |
| Race: 3 = Other | N (%) | 28 (5.20) | 21 (6.84) | 7 (3.03) | −17.658 | 30 (5.84) | 18 (6.15) | 12 (5.43) | −3.063 |
| Comorbidity group: 0 | N (%) | 200 (37.17) | 119 (38.76) | 81 (35.06) | −7.667 | 198 (38.16) | 110 (37.59) | 87 (38.91) | 2.711 |
| Comorbidity group: 1 | N (%) | 185 (34.39) | 105 (34.20) | 80 (34.63) | 0.905 | 172 (33.08) | 97 (33.19) | 74 (32.95) | −0.511 |
| Comorbidity group: 2 | N (%) | 99 (18.40) | 52 (16.94) | 47 (20.35) | 8.760 | 97 (18.76) | 56 (19.09) | 41 (18.33) | −1.955 |
| Comorbidity group: 3+ | N (%) | 54 (10.04) | 31 (10.10) | 23 (9.96) | −0.469 | 51 (9.99) | 29 (10.13) | 22 (9.81) | −1.049 |
| Smoking Status: Missing | N (%) | 3 (0.56) | 2 (0.65) | 1 (0.43) | −2.977 | . | |||
| Smoking Status: Never | N (%) | 295 (54.83) | 172 (56.03) | 123 (53.25) | −5.585 | 290 (55.92) | 163 (55.42) | 127 (56.58) | 2.341 |
| Smoking Status: Former | N (%) | 175 (32.53) | 96 (31.27) | 79 (34.20) | 6.245 | 166 (32.10) | 95 (32.44) | 71 (31.64) | −1.727 |
| Smoking Status: Current | N (%) | 65 (12.08) | 37 (12.05) | 28 (12.12) | 0.212 | 62 (11.99) | 35 (12.14) | 26 (11.78) | −1.097 |
| Chemotherapy: Missing | N (%) | 1 (0.19) | 1 (0.43) | . | . | ||||
| Chemotherapy: No | N (%) | 132 (24.54) | 41 (13.36) | 91 (39.39) | 61.852 | 119 (23.05) | 65 (22.32) | 53 (23.99) | 3.956 |
| Chemotherapy: Yes | N (%) | 405 (75.28) | 266 (86.64) | 139 (60.17) | −62.800 | 400 (76.95) | 229 (77.68) | 171 (76.01) | −3.956 |
| Neoadjuvant: Missing | N (%) | 1 (0.19) | 1 (0.43) | . | . | ||||
| Neoadjuvant: No | N (%) | 451 (83.83) | 251 (81.76) | 200 (86.58) | 13.237 | 425 (81.87) | 246 (83.55) | 179 (79.66) | −10.044 |
| Neoadjuvant: Yes | N (%) | 86 (15.99) | 56 (18.24) | 30 (12.99) | −14.512 | 94 (18.13) | 48 (16.45) | 45 (20.34) | 10.044 |
| Adjuvant: Missing | N (%) | 1 (0.19) | 1 (0.43) | . | . | ||||
| Adjuvant: No | N (%) | 208 (38.66) | 92 (29.97) | 116 (50.22) | 42.228 | 202 (38.92) | 107 (36.59) | 94 (41.99) | 11.086 |
| Adjuvant: Yes | N (%) | 329 (61.15) | 215 (70.03) | 114 (49.35) | −43.133 | 317 (61.08) | 187 (63.41) | 130 (58.01) | −11.086 |
| Any anthracyclines: Missing | N (%) | 1 (0.19) | 1 (0.43) | . | . | ||||
| Any anthracyclines: No | N (%) | 310 (57.62) | 157 (51.14) | 153 (66.23) | 31.020 | 293 (56.45) | 170 (57.69) | 123 (54.82) | −5.777 |
| Any anthracyclines: Yes | N (%) | 227 (42.19) | 150 (48.86) | 77 (33.33) | −31.958 | 226 (43.55) | 124 (42.31) | 101 (45.18) | 5.777 |
| Any taxanes: Missing | N (%) | 1 (0.19) | 1 (0.43) | . | . | ||||
| Any taxanes: No | N (%) | 158 (29.37) | 56 (18.24) | 102 (44.16) | 58.260 | 150 (28.87) | 78 (26.74) | 71 (31.67) | 10.846 |
| Any taxanes: Yes | N (%) | 379 (70.45) | 251 (81.76) | 128 (55.41) | −59.196 | 369 (71.13) | 216 (73.26) | 153 (68.33) | −10.846 |
| T-stage: 0/1 | N (%) | 362 (67.29) | 196 (63.84) | 166 (71.86) | 17.231 | 340 (65.53) | 195 (66.26) | 145 (64.57) | −3.542 |
| T-stage: 2/3 | N (%) | 176 (32.71) | 111 (36.16) | 65 (28.14) | −17.231 | 179 (34.47) | 99 (33.74) | 79 (35.43) | 3.542 |
| BMI: Continuous | Mean (Median) [IQR2] | 30.15 (29.29) [24.87 – 34.01] | 31 (30.43) [25.69 – 34.57] | 29.03 (27.98) [24.09 – 32.92] | −27.970 | 30.15 (29.34) [25.00 – 33.66] | 30.22 (29.35) [25.00 – 33.46] | 30.04 (29.13) [24.94 – 33.71] | −2.609 |
| BMI category: Underweight/Normal <25 | N (%) | 141 (26.21) | 64 (20.85) | 77 (33.33) | 28.377 | 130 (25.11) | 73 (24.82) | 57 (25.50) | 1.574 |
| BMI category: Overweight 25-<30 | N (%) | 152 (28.25) | 81 (26.38) | 71 (30.74) | 9.645 | 150 (28.95) | 85 (29.11) | 64 (28.74) | −0.811 |
| BMI category: Obesity I 30-<35 | N (%) | 130 (24.16) | 89 (28.99) | 41 (17.75) | −26.801 | 132 (25.57) | 74 (25.32) | 58 (25.89) | 1.292 |
| BMI category: Obesity II 35-<40 | N (%) | 73 (13.57) | 45 (14.66) | 28 (12.12) | −7.454 | 65 (12.64) | 36 (12.48) | 28 (12.85) | 1.093 |
| BMI category: Obesity III >40 | N (%) | 42 (7.81) | 28 (9.12) | 14 (6.06) | −11.573 | 40 (7.73) | 24 (8.27) | 15 (7.03) | −4.677 |
| Margin Status: Missing | N (%) | 7 (1.30) | 6 (1.95) | 1 (0.43) | −14.045 | . | |||
| Margin Status: Close | N (%) | 71 (13.20) | 37 (12.05) | 34 (14.72) | 7.837 | 65 (12.65) | 37 (12.63) | 28 (12.69) | 0.182 |
| Margin Status: Negative | N (%) | 451 (83.83) | 261 (85.02) | 190 (82.25) | −7.479 | 445 (85.76) | 254 (86.31) | 191 (85.03) | −3.643 |
| Margin Status: Positive | N (%) | 9 (1.67) | 3 (0.98) | 6 (2.60) | 12.252 | 8 (1.59) | 3 (1.06) | 5 (2.28) | 9.496 |
| Tumor grade: Missing | N (%) | 6 (1.12) | 3 (0.98) | 3 (1.30) | 3.032 | . | |||
| Tumor grade: 1 | N (%) | 16 (2.97) | 5 (1.63) | 11 (4.76) | 17.886 | 15 (3.03) | 8 (2.77) | 7 (3.37) | 3.440 |
| Tumor grade: 2 | N (%) | 103 (19.14) | 51 (16.61) | 52 (22.51) | 14.911 | 97 (18.74) | 54 (18.45) | 43 (19.12) | 1.715 |
| Tumor grade: 3 | N (%) | 413 (76.77) | 248 (80.78) | 165 (71.43) | −22.066 | 406 (78.23) | 232 (78.78) | 174 (77.51) | −3.057 |
| Breast volume: Continuous | Mean (Median) [IQR2] | 1130.72 (997.4) [684.40 – 1483.10] | 1205.51 (1067.65) [702.10 – 1575.90] | 1031.66 (906) [627.00 – 1359.30] | −27.570 | 1125.84 (1008.5) [657.20 – 1453.70] | 1134 (1008.5) [674.50 – 1470.60] | 1115.15 (1006.9) [627.00 – 1401.60] | −2.973 |
| D50 breast: Continuous | Mean (Median) [IQR2] | 48.4 (47.79) [44.64 – 51.89] | 50.95 (51.22) [47.96 – 52.81] | 45.09 (44.41) [43.62 – 45.46] | −164.271 | 48.31 (47.68) [44.61 – 51.88] | 50.93 (51.28) [47.93 – 52.80] | 44.93 (44.36) [43.55 – 45.46] | −181.040 |
D is the Standardized difference. Values of 10 or above suggest significant imbalance between fractionation groups.
IPTW is the Inverse Probability of Treatment Weighting.
IQR is the Interquartile range, value for the 25th and 75th percentiles are reported.
The overall study median follow-up time is 5.0 years, 95% CI 4.77–5.15, as calculated using the reverse censoring method of the product-limit overall survival estimate. The median follow-up time for conventional cases is 5.4 years [95% CI: 5.13 – 5.75] and for hypofractionated cases is 4.3 years [95% CI: 3.91 – 4.57].
The 5-year IPTW estimates for FFLR were 93.6% (95% CI 87.8%−96.7%) in the moderately hypofractionated group and 94.4% (95% CI 90.3%−96.8%) in the conventionally fractionated group. The hazard ratio was 1.05 (95% CI 0.51–2.17), p=0.89 (Figure 1).
Figure 1:
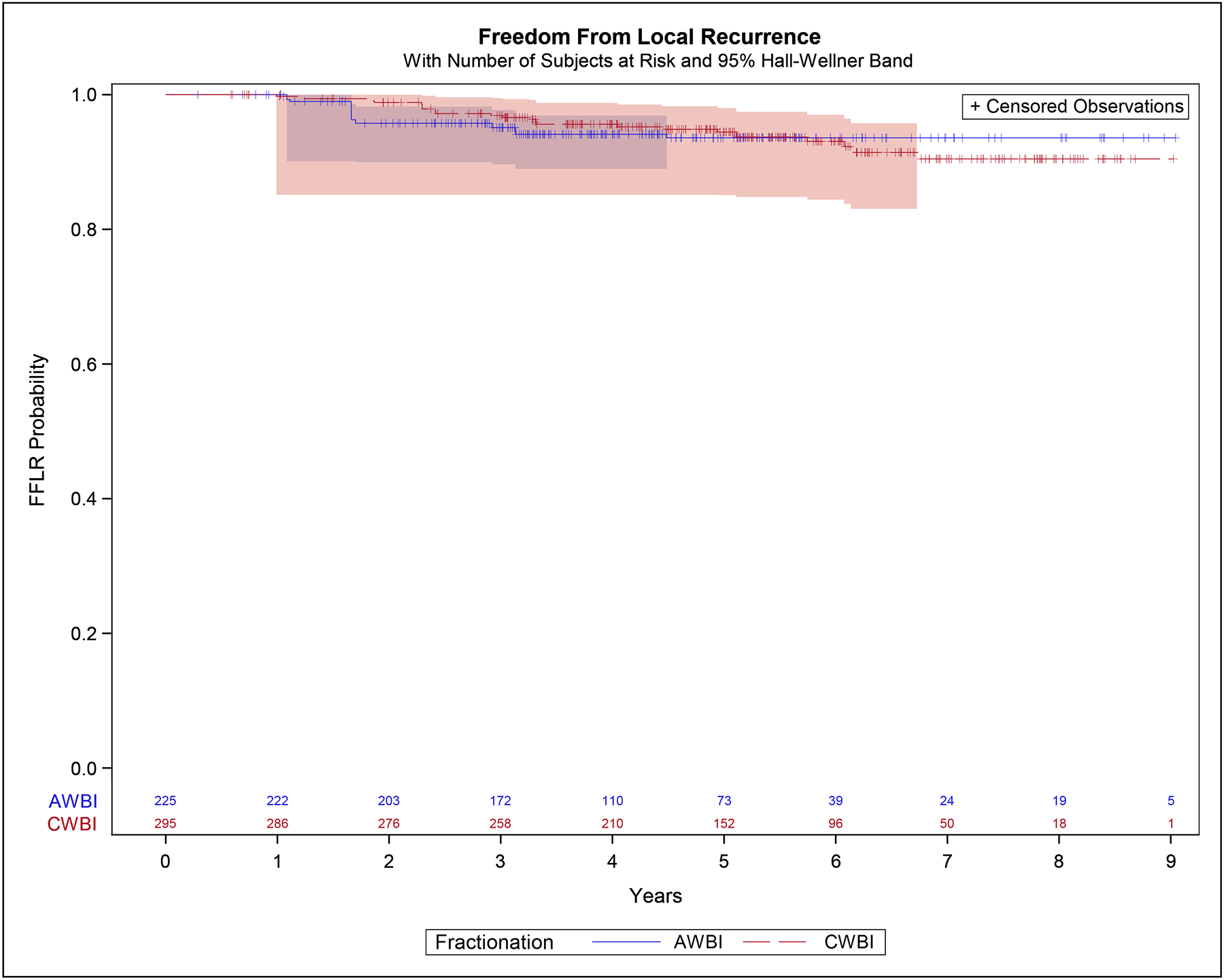
Freedom From Local Recurrence – inverse-probability of treatment weight adjusted
The 5-year IPTW estimates for RFS were 87.8% (95% CI 81.0%−92.4%) in the moderately hypofractionated group and 88.4% (95% CI 83.2%−92.1%) in the conventionally fractionated group. The hazard ratio was 1.02 (95% CI 0.62–1.67), p=0.95 (Figure 2).
Figure 2:
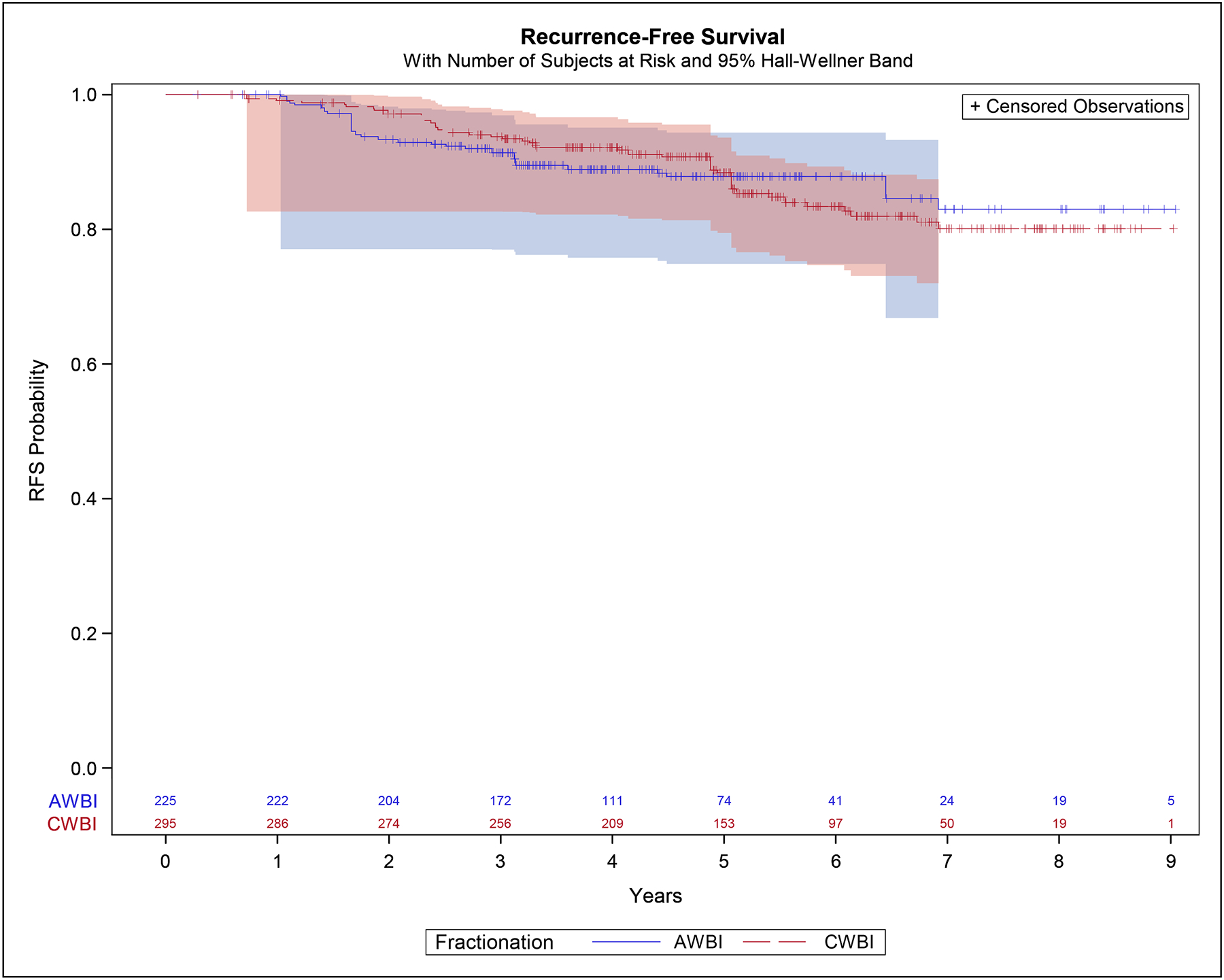
Recurrence-Free Survival – inverse-probability of treatment weight adjusted
The 5-year IPTW estimates for OS were 96.6% (95% CI 92.0%−98.5%) in the moderately hypofractionated group and 93.4% (95% CI 88.7%−96.1%) in the conventionally fractionated group. The hazard ratio was 0.65 (95% CI 0.30–1.42), p=0.28 (Figure 3).
Figure 3:
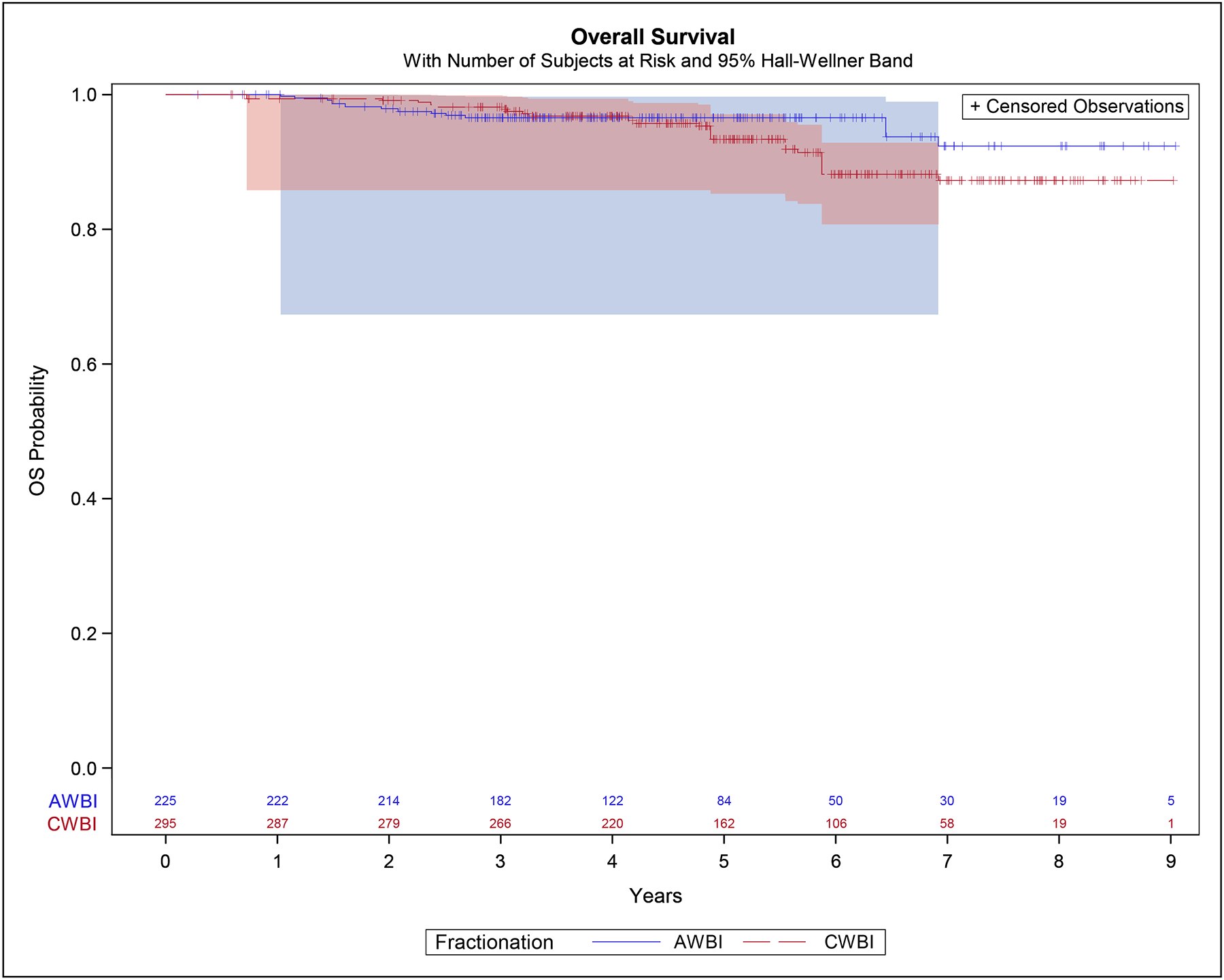
Overall Survival – inverse-probability of treatment weight adjusted
Of the 538 cases, 502 received a boost (301/307 in the conventionally fractionated group and 201/231 in the moderately hypofractionated group). Only one of the patients who did not receive boost experienced a recurrence and only two died. The estimates of FFLR, RFS, and OS were almost identical to those in the entire sample when we conducted a sensitivity analysis restricted to the cases that received boost.
Discussion
Analysis of disease control outcomes in this large observational cohort of patients with triple-negative, node-negative breast cancer treated with whole breast irradiation reveals no differences by dose fractionation. This adds meaningfully to the body of evidence supporting the use of moderate hypofractionation in patients with triple-negative disease.
Data now exist from several sources of information that taken together are reassuring on the important question of whether patients with triple-negative breast cancer are appropriate candidates for moderate hypofractionation. This includes 125 patients randomized to treatment with either conventional fractionation or moderate hypofractionation in a Canadian trial,6 77 patients randomized in a Chinese trial,8 and 188 patients randomized in a trial from Denmark, Germany, and Norway (DBCG HYPO).9 Specifically, in the OCOG trial, the hazard ratio comparing local recurrence outcomes of moderately hypofractionated to conventionally fractionated treatment in the 125 patients with triple-negative disease was slightly in favor of conventional fractionation, at 1.27, but with a very wide 95% CI from 0.21–7.58.6 In the Chinese trial, among 77 triple-negative patients, results were nearly identical in the two arms: 1/37 treated with moderately hypofractionated radiotherapy and 1/40 treated with conventionally fractionated radiotherapy had local recurrence (and 2/38 and 3/40 had locoregional recurrence).8 In the DBCG HYPO Trial, among 188 patients who were ER and HER2 negative, 7/98 patients treated with conventional fractionation and 2/90 patients treated with hypofractionation had locoregional recurrences,9 again not significantly different, and this time with the point estimate in the opposite direction from that in the OCOG analysis. Although none of these subgroup analyses within the randomized trials revealed a significant difference, given the small size of these subgroup analyses, concerns remained.
An Italian cohort study that included 48 triple-negative patients showed similar rates of relapse (21%) in patients treated with hypofractionation and those receiving conventionally fractionated radiation.13 A larger Canadian cohort study of 603 patients also revealed no differences in outcomes with 10-year LRFS of 93.9% vs 92.2% for hypofractionation versus conventional fractionation, p=0.47. Our findings are consistent with these results and add substantially to the number of patients with triple-negative disease whose outcomes have now been compared, as advocated by leaders in the field—including both those who developed the most recent consensus guidelines in this area encouraging use of hypofractionation3 and those who raised concerns about embracing hypofractionation too quickly.10
The primary limitation of this study is its observational design. Patients who received hypofractionation had more favorable disease characteristics and were less likely to receive chemotherapy. These imbalances would be expected to influence outcomes in opposite directions, with more favorable disease characteristics biasing estimates of disease control upwards in the hypofractionated group and lack of chemotherapy biasing estimates downwards in that same group. Importantly, efforts were made to address confounding by these and other known prognostic covariates using appropriate statistical techniques. Patients treated with hypofractionation on the whole were treated slightly more recently than those treated with conventional fractionation, and the overall follow-up time was limited. Because triple-negative disease has lower rates of late recurrence than hormone receptor positive disease, we believe that the five-year results presented here are informative. We are also reassured by the consistency of the findings of this and the other two observational studies on this point with the findings of subgroup analyses from the randomized trials.
Hypofractionated radiotherapy in the adjuvant setting after breast-conserving surgery for breast cancer is clearly more convenient for patients, less costly for both patients and society, and appears to have less acute14 and late toxicity15 as compared to conventionally fractionated regimens. Nevertheless, caution has been warranted when considering whether its application is equally effective for disease control in patients with the less common and more aggressive subtype of triple-negative disease,10 which might conceivably have different fractionation sensitivity as compared to more common hormone-sensitive subtypes and which has been shown to have a higher risk of local recurrence as compared to other subtypes.16 Taken together with other sources of information, this study provides evidence that supports the use of hypofractionated whole breast irradiation in triple-negative patients, as in other subtypes.
Acknowledgements:
The authors gratefully acknowledge the extensive support that made this work possible, including the assistance of Lauren Szczygiel, Chris Krenz, Rhonda Hubbard, and Melissa Mietzel at the University of Michigan and the contributions of the staff and patients at the centers that contributed data.
Funding statement
This study was supported by a Michigan Medicine Rogel Cancer Center Discovery Award and the Komen Foundation. Blue Cross Blue Shield of Michigan supports the Michigan Radiation Oncology Quality Consortium through payments to the institutions of all authors. None of these played any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Conflicts of Interest
RJ has stock options as compensation for her advisory board role in Equity Quotient, a company that evaluates culture in health care companies; she has received personal fees from the Greenwall Foundation, Doris Duke Foundation, and the National Institutes of Health and grants or contracts for unrelated work from the National Institutes of Health, the Doris Duke Foundation, the Greenwall Foundation, the Komen Foundation, Genentech, and Blue Cross Blue Shield of Michigan for the Michigan Radiation Oncology Quality Consortium. She has served as an expert witness for Sherinian and Hasso, Dressman Benzinger LaVelle, and Kleinbard, LLC.
EMW reports grants or contracts for unrelated work from the Pfizer and Genentech.
PGK reports honorarium for the Congdon lecture series from Ascension Genesys Hospital.
LP reports patents associated with PFS Genomics, a subsidiary of Exact Sciences. She has leadership or fiduciary roles with the American Society of Clinical Oncology and the BCRF Advisory Board.
Data Availability Statement for this Work:
Data are the property of the individual sites and cannot be shared.
References
- 1.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–520. doi: 10.1056/NEJMoa0906260 [DOI] [PubMed] [Google Scholar]
- 2.Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3 [DOI] [PubMed] [Google Scholar]
- 3.Smith BD, Bellon JR, Blitzblau R, Freedman G, Haffty B, Hahn C, Halberg F, Hoffman K, Horst K, Moran J, Patton C, Perlmutter J, Warren L, Whelan T, Wright JL, Jagsi R. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol. 2018;8(3):145–152. doi: 10.1016/j.prro.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 4.Recht A, McArthur H, Solin LJ, Tendulkar R, Whitley A, Giuliano A. Contemporary Guidelines in Whole-Breast Irradiation: An Alternative Perspective. Int J Radiat Oncol Biol Phys. 2019;104(3):567–573. doi: 10.1016/j.ijrobp.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 5.Thames HD Jr, Withers HR, Peters LJ, Fletcher GH. Changes in early and late radiation responses with altered dose fractionation: implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982;8(2):219–226. doi: 10.1016/0360-3016(82)90517-x [DOI] [PubMed] [Google Scholar]
- 6.Bane AL, Whelan TJ, Pond GR, et al. Tumor factors predictive of response to hypofractionated radiotherapy in a randomized trial following breast conserving therapy. Ann Oncol. 2014;25(5):992–998. doi: 10.1093/annonc/mdu090 [DOI] [PubMed] [Google Scholar]
- 7.Smith BD, Bentzen SM, Correa CR, et al. Fractionation for whole breast irradiation: an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;81(1):59–68. doi: 10.1016/j.ijrobp.2010.04.042 [DOI] [PubMed] [Google Scholar]
- 8.Wang SL, Fang H, Hu C, et al. Hypofractionated Versus Conventional Fractionated Radiotherapy After Breast-Conserving Surgery in the Modern Treatment Era: A Multicenter, Randomized Controlled Trial from China. J Clin Oncol. 2020;38(31):3604–3614. doi: 10.1200/JCO.20.01024 [DOI] [PubMed] [Google Scholar]
- 9.Offersen BV, Alsner J, Nielsen HM, et al. Hypofractionated Versus Standard Fractionated Radiotherapy in Patients with Early Breast Cancer or Ductal Carcinoma in Situ in a Randomized Phase III Trial: The DBCG HYPO Trial. J Clin Oncol. 2020;38(31):3615–3625. doi: 10.1200/JCO.20.01363 [DOI] [PubMed] [Google Scholar]
- 10.Recht A Hypofractionated Whole-Breast Irradiation: Case Closed?. J Clin Oncol. 2020;38(31):3584–3586. doi: 10.1200/JCO.20.02389 [DOI] [PubMed] [Google Scholar]
- 11.Lalani N, Voduc KD, Jimenez RB, et al. Breast Cancer Molecular Subtype as a Predictor of Radiation Therapy Fractionation Sensitivity. Int J Radiat Oncol Biol Phys. 2021;109(1):281–287. doi: 10.1016/j.ijrobp.2020.08.038 [DOI] [PubMed] [Google Scholar]
- 12.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazzari G, Terlizzi A, Leo MG, Silvano G. Tumor grade and molecular subtypes on local control in breast cancer radiotherapy: Does fractionation really matter? A retrospective control study group. Clin Transl Radiat Oncol. 2018;15:7–12. doi: 10.1016/j.ctro.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaitelman SF, Schlembach PJ, Arzu I, et al. Acute and Short-term Toxic Effects of Conventionally Fractionated vs Hypofractionated Whole-Breast Irradiation: A Randomized Clinical Trial. JAMA Oncol. 2015;1(7):931–941. doi: 10.1001/jamaoncol.2015.2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3 [DOI] [PubMed] [Google Scholar]
- 16.Arvold ND, Taghian AG, Niemierko A, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011;29(29):3885–3891. doi: 10.1200/JCO.2011.36.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are the property of the individual sites and cannot be shared.


