Abstract
Background
With large waves of infection driven by the B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), alongside evidence of waning immunity after the booster dose of coronavirus disease 2019 (Covid-19) vaccine, several countries have begun giving at-risk persons a fourth vaccine dose.
Methods
To evaluate the early effectiveness of a fourth dose of the BNT162b2 vaccine for the prevention of Covid-19–related outcomes, we analyzed data recorded by the largest health care organization in Israel from January 3 to February 18, 2022. We evaluated the relative effectiveness of a fourth vaccine dose as compared with that of a third dose given at least 4 months earlier among persons 60 years of age or older. We compared outcomes in persons who had received a fourth dose with those in persons who had not, individually matching persons from these two groups with respect to multiple sociodemographic and clinical variables. A sensitivity analysis was performed with the use of parametric Poisson regression.
Results
The primary analysis included 182,122 matched pairs. Relative vaccine effectiveness in days 7 to 30 after the fourth dose was estimated to be 45% (95% confidence interval [CI], 44 to 47) against polymerase-chain-reaction–confirmed SARS-CoV-2 infection, 55% (95% CI, 53 to 58) against symptomatic Covid-19, 68% (95% CI, 59 to 74) against Covid-19–related hospitalization, 62% (95% CI, 50 to 74) against severe Covid-19, and 74% (95% CI, 50 to 90) against Covid-19–related death. The corresponding estimates in days 14 to 30 after the fourth dose were 52% (95% CI, 49 to 54), 61% (95% CI, 58 to 64), 72% (95% CI, 63 to 79), 64% (95% CI, 48 to 77), and 76% (95% CI, 48 to 91). In days 7 to 30 after a fourth vaccine dose, the difference in the absolute risk (three doses vs. four doses) was 180.1 cases per 100,000 persons (95% CI, 142.8 to 211.9) for Covid-19–related hospitalization and 68.8 cases per 100,000 persons (95% CI, 48.5 to 91.9) for severe Covid-19. In sensitivity analyses, estimates of relative effectiveness against documented infection were similar to those in the primary analysis.
Conclusions
A fourth dose of the BNT162b2 vaccine was effective in reducing the short-term risk of Covid-19–related outcomes among persons who had received a third dose at least 4 months earlier. (Funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.)
The B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first identified in November 2021, has generated the largest waves of infection in the coronavirus disease 2019 (Covid-19) pandemic thus far, even in countries with successful mass-vaccination campaigns.1,2 Although early data from South Africa3 and subsequently from the United Kingdom4 suggested that the omicron variant was less virulent than the B.1.617.2 (delta) variant, with lower rates of hospitalization and severe disease, the large number of infections observed over a short period of time led to concerns that health care resources might be overwhelmed.
Initial evidence indicated that two doses of vaccine (BNT162b2 [Pfizer–BioNTech], mRNA-1273 [Moderna], or ChAdOx1 nCoV-19 [AstraZeneca]) offered limited protection against the omicron variant and that a recently administered third (booster) dose was effective in preventing symptomatic and severe disease.5,6 Laboratory and real-world studies have since shown evidence of waning immunity as early as 10 weeks after the third dose.5-7 In countries with early booster-dose campaigns such as Israel, the United Kingdom, and the United States, the onset of the omicron wave occurred at a time when many persons — especially those who were more vulnerable to severe Covid-19 — had received their booster dose several months earlier. Therefore, policymakers considered offering a fourth vaccine dose to the most vulnerable persons as possible protection against the omicron variant.
On January 3, 2022, the Israeli Ministry of Health launched a national fourth-dose vaccination campaign for high-risk persons (i.e., those who were ≥60 years of age or who had an immune deficiency) at least 4 months after their third vaccine dose. To date, more than 700,000 people in Israel have received a fourth BNT162b2 mRNA vaccine dose.8,9 In the United States, in response to the omicron wave, the Centers for Disease Control and Prevention (CDC) reduced the period between the third and fourth vaccine doses for immunocompromised persons from 6 months to 5 months.10 Other countries, including the United Kingdom, have also started rolling out targeted fourth-dose vaccination campaigns.11
Real-world evidence of the effectiveness of the fourth dose of BNT162b2 was published in a recent study,12 which showed that a fourth dose is more effective in preventing SARS-CoV-2 infection and severe Covid-19 than three doses. However, evidence regarding the effectiveness of a fourth dose in preventing additional outcomes, such as Covid-19–related hospitalization and Covid-19–related death, was not included in the study, and some potentially important confounders, such as coexisting conditions, were unable to be addressed.
We used the data repositories of the largest health care organization in Israel to estimate the relative effectiveness of a fourth dose of the BNT162b2 vaccine, as compared with three doses, in preventing a range of Covid-19–related outcomes among persons 60 years of age or older, while taking into account potential confounders.
Methods
Setting and Data
We used data collected between January 3 and February 18, 2022, when the omicron variant was predominant in Israel,13 to emulate a target trial evaluating the effectiveness of a fourth vaccine dose as compared with three vaccine doses. We analyzed data from Clalit Health Services (CHS), the largest integrated payer–provider health care organization in Israel. With more than 4.7 million members, CHS covers more than half of the population of Israel. The CHS population is largely representative of the general Israeli population.14,15 CHS health records have been fully digitized since 2000, and its data repositories include demographic, diagnostic, pharmacologic, laboratory, procedure, imaging, and hospitalization data. Data related to SARS-CoV-2 infections (polymerase-chain-reaction [PCR] and antigen tests) and Covid-19 outcomes (including hospitalization, severe illness, and death) are stored centrally by the Israeli Ministry of Health and delivered daily to the four national health organizations.
This study was approved by the institutional review board of CHS. An exemption from the requirement for informed consent was granted. The authors vouch for the accuracy and completeness of the data in this report.
Eligibility Criteria
We included persons who, at baseline (defined below), were 60 years of age or older, had been members of CHS for at least 1 year, and were eligible to receive the fourth vaccine dose at any time during the study period (i.e., had been vaccinated with a third dose of BNT162b2 at least 4 months earlier16) and had no previous PCR-confirmed SARS-CoV-2 infection. As in previous studies,17-19 we also excluded health care workers, persons in long-term care facilities, persons confined to the home, and persons who had interacted with the health care system (e.g., saw a doctor or had blood tests performed) during the previous 3 days. This last exclusion criterion reduces the probability that persons who opted to delay receipt of a fourth vaccine dose because they were feeling unwell (possibly with symptoms of Covid-19) would be included in the control group. Given the rarity of missing data in the CHS data set (<1%), we also excluded persons with missing data on body-mass index (BMI), population sector, or residency area. A detailed description of all the study variables is provided in Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Outcomes
We examined five outcomes: PCR-confirmed SARS-CoV-2 infection, symptomatic Covid-19, Covid-19–related hospitalization, severe Covid-19 (defined according to National Institutes of Health criteria), and Covid-19–related death. All outcomes were assessed over two follow-up periods of interest: days 7 to 30 after the fourth dose and days 14 to 30 after the fourth dose. In addition, to estimate the gradual build-up of immunity and evaluate the similarity of the study groups during the initial days after vaccination (the negative control period20), PCR-confirmed infection was also assessed separately during each day of follow-up.
Study Design
The study design of the primary analysis was similar to that used in our previous vaccine-effectiveness studies,17,19 which examined the same population in a similar setting. On each day of the study period, eligible persons who received the fourth dose of the BNT162b2 mRNA vaccine on that day (four-dose group) were exactly matched to eligible persons who had not yet received a fourth dose as of that day (control group) according to a set of potential confounders: age (categorized into 1-year bins), sex, residency area, population sector (three categories: Arab, General Jewish, and Ultra-Orthodox Jewish), calendar month in which each person received the third vaccine dose, number of preexisting chronic conditions defined by the CDC (on December 20, 202021) as risk factors for severe Covid-19 (categorized into four bins: 0, 1, 2, and ≥3), and number of hospital admissions in the previous 3 years (categorized into 5 bins: 0, 1, 2, 3 or 4, and ≥5). The latter two variables, together, were designed to capture the load and stability of chronic conditions.
Each matched pair was followed from the matching date until the earliest of the following events: the outcome of interest; death; 30 days of follow-up; February 18, 2022 (the final day of data collection); or fourth-dose vaccination of the control member of the matched pair (at which point data for both members of the matched pair were censored). Controls who received a fourth vaccine dose after they had been matched as controls became eligible to be rerecruited to the four-dose group and matched to a new control.
Statistical Analysis
Cumulative incidence curves were constructed with the use of the Kaplan–Meier estimator. For each follow-up period, only matched pairs in which data for both members had not been censored as of the beginning of the follow-up period were included. Risk was defined as the probability of a given outcome developing during the follow-up period. The estimated risks in each group were compared both as risk ratios and as risk differences. Vaccine effectiveness was estimated as 1 minus the risk ratio. We calculated 95% confidence intervals using the nonparametric bootstrap method with 500 repetitions. The widths of the confidence intervals have not been adjusted for multiplicity and should not be used to infer statistical significance.
We performed two sensitivity analyses to explore the robustness of our estimates. First, our estimates of the observational analogue of the per-protocol effect, in which data from matched pairs were censored when the control received a fourth dose, would have been biased if the probability of vaccination changed around the time of infection (i.e., nonrandom censoring). We therefore performed an analysis identical to the primary analysis except that when the control received a fourth vaccine dose, the censoring of data from the matched pair was delayed by 7 days,17 a period during which the additional dose was not yet expected to have taken effect. In this sensitivity analysis, controls did not subsequently undergo rerecruitment to the four-dose group.
Second, as an alternative to our nonparametric Kaplan–Meier approach, we also fit three parametric Poisson regression models with a log-link function22 on all eligible persons, with each model incorporating a different definition of time-varying exposure: no fourth vaccine dose, days 1 to 4 after the fourth vaccine dose, days 5 and 6, and day 7 and onward; no fourth vaccine dose, days 1 to 4, days 5 and 6, days 7 to 13, and day 14 and onward; and no fourth vaccine dose and each day of follow-up treated as a separate category. Persons were able to contribute follow-up data to each of these four-dose groups (i.e., the groups based on time since receipt of the fourth dose) and to the control group dynamically and regardless of interactions with the health care system. The outcome of interest was PCR-confirmed documented SARS-CoV-2 infection. All models included, as covariates, the calendar date of each day of follow-up and the matching factors described above, with residency area (a covariate with hundreds of categories) replaced by a measure of local Covid-19 burden (the proportion of positive PCR tests in the residency area on the previous day) (Methods section S1). In this analysis, vaccine effectiveness was defined as 1 minus the incidence rate ratio estimated from the model.
Analyses were performed with the use of R software, version 4.1.0, and the additional freely available R software packages “tidyverse,” version 1.3.1, and “survminer,” version 0.4.9.
Results
Four-Dose Recipients and Matched Controls
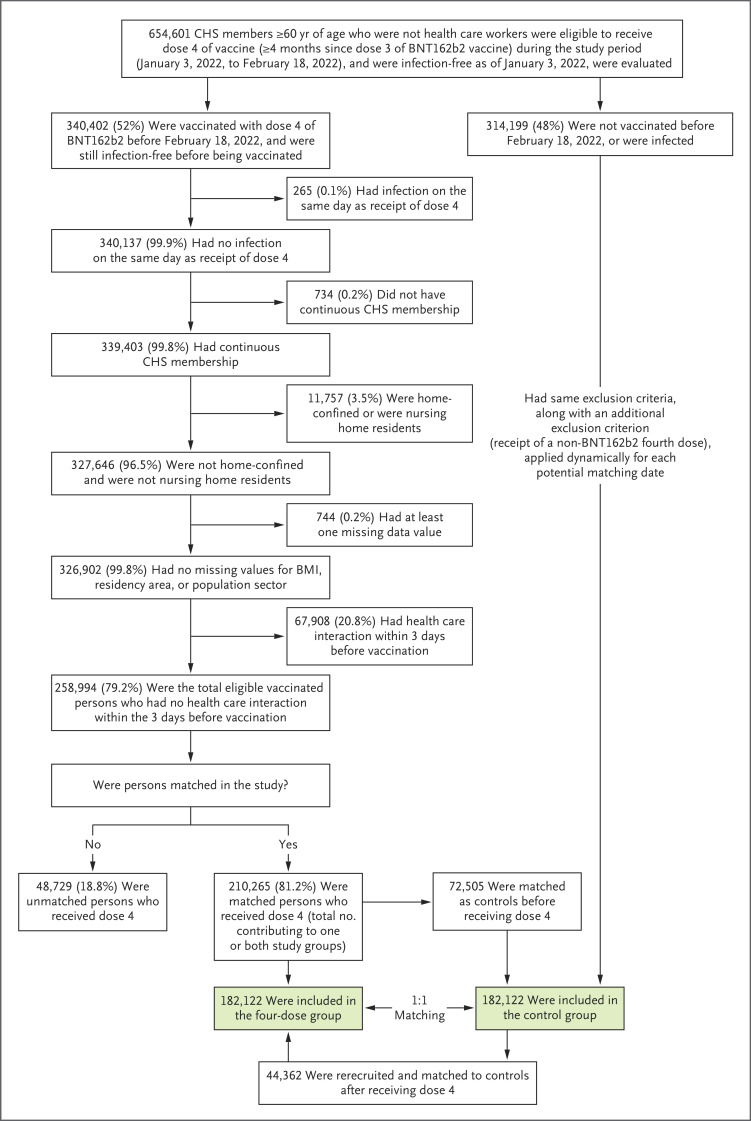
Of the 340,402 persons who received the fourth vaccine dose during the study period (Figure 1), 258,994 (76.1%) met the eligibility criteria, and 182,122 (70.3%) of those who were eligible were successfully matched to a control. A total of 44,362 persons who were initially matched as controls were rerecruited into the four-dose group after receiving a fourth vaccine dose and were matched to a new control.
Figure 1. Selection of Persons in the Four-Dose Group and the Matching Control Group.
Of 258,994 eligible vaccinated persons, 210,265 (81.2%) were successfully matched and included in at least one of the study groups as follows: 182,122 participated as members of the four-dose group and 72,505 participated as members of the control group, with an overlap of 44,362 persons who were initially matched as members of the control group and then were rerecruited as members of the four-dose group after receiving a fourth dose, along with a new matched control. CHS denotes Clalit Health Services.
The median age of the matched pairs was 72 years (interquartile range, 67 to 78), and 53% were women (Table 1). The two groups had the same distribution of matching factors and a similar distribution of conditions identified by the CDC as risk factors for severe Covid-19. Matched persons were generally similar to the total eligible population who had received a fourth dose with regard to the distribution of matching factors. Some differences with regard to age, sex, population sector and number of hospital admissions in the previous 3 years were noted when matched persons who had received a fourth dose were compared directly with unmatched persons who had received a fourth dose (Table S2). These differences related to the difficulty in finding persons who had not received a fourth dose and who could be exactly matched to persons who had received a fourth dose and were from smaller subgroups (e.g., men who were >80 years of age and had numerous hospital admissions). The maximum follow-up was 30 days after the fourth vaccine dose, with a median follow-up of 26 days (interquartile range, 7 to 30).
Table 1. Baseline Characteristics of Persons in the Study.*.
| Variable | Total Eligible for Inclusion in Four-Dose Group (N=258,994) |
Four-Dose Group (N=182,122) |
Control Group (N=182,122) |
|---|---|---|---|
| Median age (IQR) — yr | 73 (67–79) | 72 (67–78) | 72 (67–78) |
| Age group | |||
| 60–69 yr | 90,701 (35) | 67,778 (37) | 67,778 (37) |
| 70–79 yr | 107,620 (42) | 76,630 (42) | 76,630 (42) |
| ≥80 yr | 60,673 (23) | 37,714 (21) | 37,714 (21) |
| Sex | |||
| Female | 133,282 (51) | 97,113 (53) | 97,113 (53) |
| Male | 125,712 (49) | 85,009 (47) | 85,009 (47) |
| Population sector | |||
| General Jewish | 243,651 (94) | 173,689 (95) | 173,689 (95) |
| Arab | 9,178 (4) | 4,828 (3) | 4,828 (3) |
| Ultra-Orthodox Jewish | 6,165 (2) | 3,605 (2) | 3,605 (2) |
| No. of hospital admissions in previous 3 yr | |||
| 0 | 184,499 (71) | 140,226 (77) | 140,226 (77) |
| 1 | 44,502 (17) | 27,612 (15) | 27,612 (15) |
| 2 | 16,295 (6) | 8,310 (5) | 8,310 (5) |
| 3 or 4 | 10,116 (4) | 4,692 (3) | 4,692 (3) |
| ≥5 | 3,582 (1) | 1,282 (1) | 1,282 (1) |
| No. of CDC-defined risk factors for severe Covid-19 | |||
| 0 | 43,408 (17) | 31,533 (17) | 31,533 (17) |
| 1 | 59,304 (23) | 42,236 (23) | 42,236 (23) |
| 2 | 58,992 (23) | 40,275 (22) | 40,275 (22) |
| ≥3 | 97,290 (38) | 68,078 (37) | 68,078 (37) |
| CDC risk factors | |||
| Cancer | 14,123 (5) | 9,228 (5) | 8,665 (5) |
| Chronic kidney disease | 47,529 (18) | 32,343 (18) | 32,999 (18) |
| Chronic obstructive pulmonary disease | 15,882 (6) | 10,919 (6) | 11,675 (6) |
| Heart disease | 61,521 (24) | 41,018 (23) | 40,270 (22) |
| Solid-organ transplantation | 250 (<1) | 171 (<1) | 174 (<1) |
| Obesity† | 68,436 (26) | 48,949 (27) | 48,818 (27) |
| Severe obesity† | 4,911 (2) | 3,548 (2) | 3,877 (2) |
| Sickle cell disease | 14 (<1) | 4 (<1) | 8 (<1) |
| Smoking | 27,962 (11) | 20,224 (11) | 24,904 (14) |
| Type 2 diabetes mellitus | 84,137 (32) | 59,936 (33) | 60,138 (33) |
| Possible CDC risk factors | |||
| Asthma | 18,193 (7) | 12,806 (7) | 12,609 (7) |
| Cerebrovascular disease | 27,902 (11) | 18,319 (10) | 19,447 (11) |
| Other respiratory disease | 3,170 (1) | 2,232 (1) | 2,202 (1) |
| Hypertension | 148,096 (57) | 103,260 (57) | 101,536 (56) |
| Immunosuppression | 16,214 (6) | 11,304 (6) | 10,890 (6) |
| Neurologic disease | 31,546 (12) | 21,295 (12) | 22,793 (13) |
| Liver disease | 8,889 (3) | 6,221 (3) | 6,657 (4) |
| Overweight† | 109,322 (42) | 76,447 (42) | 75,086 (41) |
| Thalassemia | 1,061 (<1) | 732 (<1) | 821 (<1) |
| Type 1 diabetes mellitus | 2,393 (1) | 1,614 (1) | 1,432 (1) |
Persons 60 years of age or older with no previous confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection who had received a fourth vaccine dose (four-dose group) were matched with those who had received only a third dose given at least 4 months earlier (control group). CDC denotes Centers for Disease Control and Prevention, and IQR interquartile range.
Overweight was defined as a body-mass index (BMI; the weight in kilograms divided by the square of the height in meters) of 25 to <30, obesity as a BMI of 30 to <40, and severe obesity as a BMI of 40 or greater.
Effectiveness
During days 7 to 30, the estimated relative effectiveness of the fourth BNT162b2 dose as compared with three doses was 45% (95% confidence interval [CI], 44 to 47) against PCR-confirmed SARS-CoV-2 infection, 55% (95% CI, 53 to 58) against symptomatic Covid-19, 68% (95% CI, 59 to 74) against Covid-19–related hospitalization, 62% (95% CI, 50 to 74) against severe Covid-19, and 74% (95% CI, 50 to 90) against Covid-19–related death (Table 2). During this period, the risk of Covid-19–related hospitalization was 86.6 events per 100,000 persons in the four-dose group, as compared with 266.7 events per 100,000 persons in the control group — a difference in risk of 180.1 events per 100,000 persons (95% CI, 142.8 to 211.9). The risk of severe Covid-19 in this period was 42.1 events per 100,000 persons in the four-dose group, as compared with 110.8 events per 100,000 persons in the control group, corresponding to a difference in risk of 68.8 cases per 100,000 persons (95% CI, 48.5 to 91.9). During days 14 to 30, the estimated relative effectiveness of the fourth BNT162b2 dose was 52% (95% CI, 49 to 54) against PCR-confirmed SARS-CoV-2 infection, 61% (95% CI, 58 to 64) against symptomatic Covid-19, 72% (95% CI, 63 to 79) against Covid-19–related hospitalization, 64% (95% CI, 48 to 77) against severe Covid-19, and 76% (95% CI, 48 to 91) against Covid-19–related death.
Table 2. Vaccine Effectiveness of a Fourth Dose of BNT162b2 as Compared with Three Doses.*.
| Period and Outcome | No. at Risk at Start of Follow-up in Each Group | Four-Dose Group | Control Group | Relative Vaccine Effectiveness of Fourth Dose (95% CI)† | Risk Difference (95% CI)‡ | ||||
|---|---|---|---|---|---|---|---|---|---|
| Events | Tests | Risk§ | Events | Tests | Risk§ | ||||
| number | events/ 100,000 persons | number | events/ 100,000 persons | percent | events/100,000 persons | ||||
| Days 7–30 | |||||||||
| PCR-confirmed SARS-CoV-2 infection | 135,921 | 5040 | 40,480 | 4436.9 | 9021 | 38,534 | 8105.5 | 45 (44–47) | 3668.5 (3473.3–3856.3) |
| Symptomatic Covid-19 | 138,850 | 1664 | 43,577 | 1430.9 | 3661 | 42,350 | 3204.8 | 55 (53–58) | 1773.9 (1645.1–1888.2) |
| Covid-19–related hospitalization | 140,936 | 101 | 45,480 | 86.6 | 303 | 44,927 | 266.7 | 68 (59–74) | 180.1 (142.8–211.9) |
| Severe Covid-19 | 140,992 | 48 | 45,535 | 42.1 | 125 | 45,026 | 110.8 | 62 (50–74) | 68.8 (48.5–91.9) |
| Covid-19–related death | 141,007 | 9 | 45,561 | 8.3 | 35 | 45,085 | 31.7 | 74 (50–90) | 23.4 (11.8–34.6) |
| Days 14–30 | |||||||||
| PCR-confirmed SARS-CoV-2 infection | 109,929 | 2586 | 24,510 | 2619.8 | 5343 | 21,911 | 5406.1 | 52 (49–54) | 2786.3 (2613.6–2961.5) |
| Symptomatic Covid-19 | 116,126 | 842 | 29,213 | 811.2 | 2194 | 27,971 | 2094.3 | 61 (58–64) | 1283.2 (1173.2–1376.4) |
| Covid-19–related hospitalization | 120,134 | 59 | 32,031 | 54.4 | 205 | 31,967 | 191.1 | 72 (63–79) | 136.7 (106.3–165.0) |
| Severe Covid-19 | 120,278 | 33 | 32,112 | 30.4 | 92 | 32,156 | 85.2 | 64 (48–77) | 54.8 (34.7–75.9) |
| Covid-19–related death | 120,327 | 7 | 32,163 | 6.7 | 30 | 32,238 | 27.8 | 76 (48–91) | 21.1 (10.4–32.8) |
Events were included either from day 7 after the index date or from day 14 after the index date. Covid-19 denotes coronavirus disease 2019, PCR polymerase chain reaction, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Vaccine effectiveness was calculated as 1 minus the risk ratio.
The risk difference was calculated as the risk in the control group minus that in the four-dose group.
Risk was calculated with the use of the Kaplan–Meier estimator.
PCR testing for Covid-19 was transiently less frequent in the four-dose group than in the control group at the beginning of the study period. However, this difference was not seen during the follow-up period of interest (i.e., from day 7 onward) (Fig. S1 and Table 2).
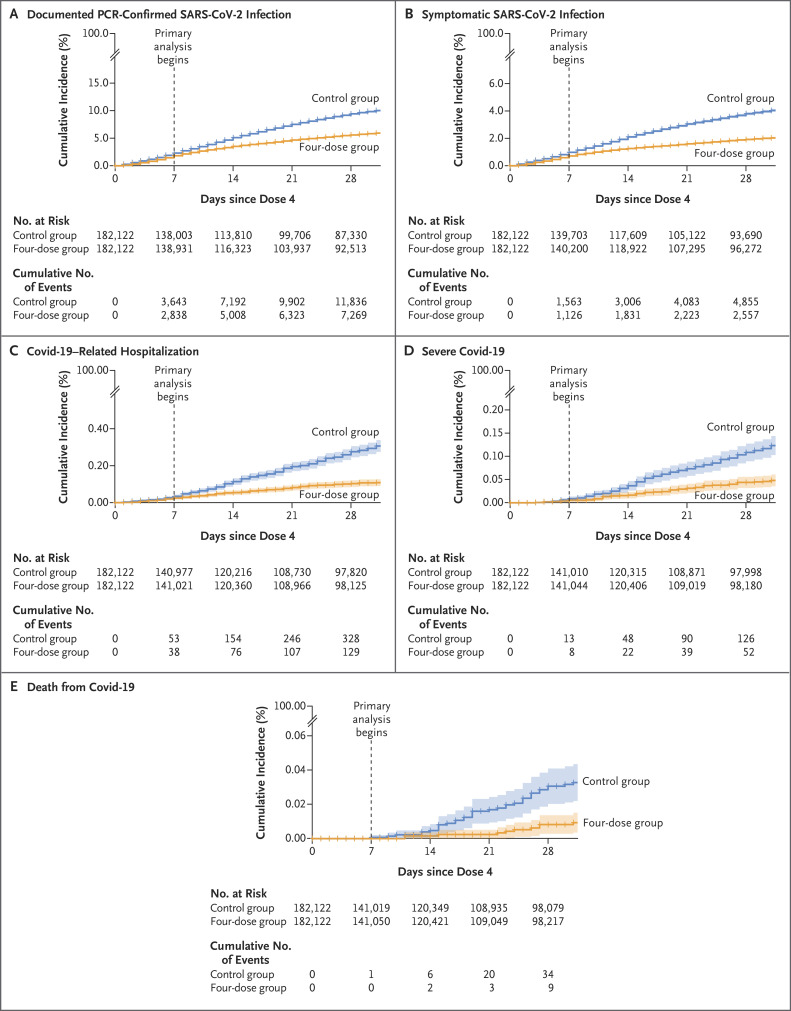
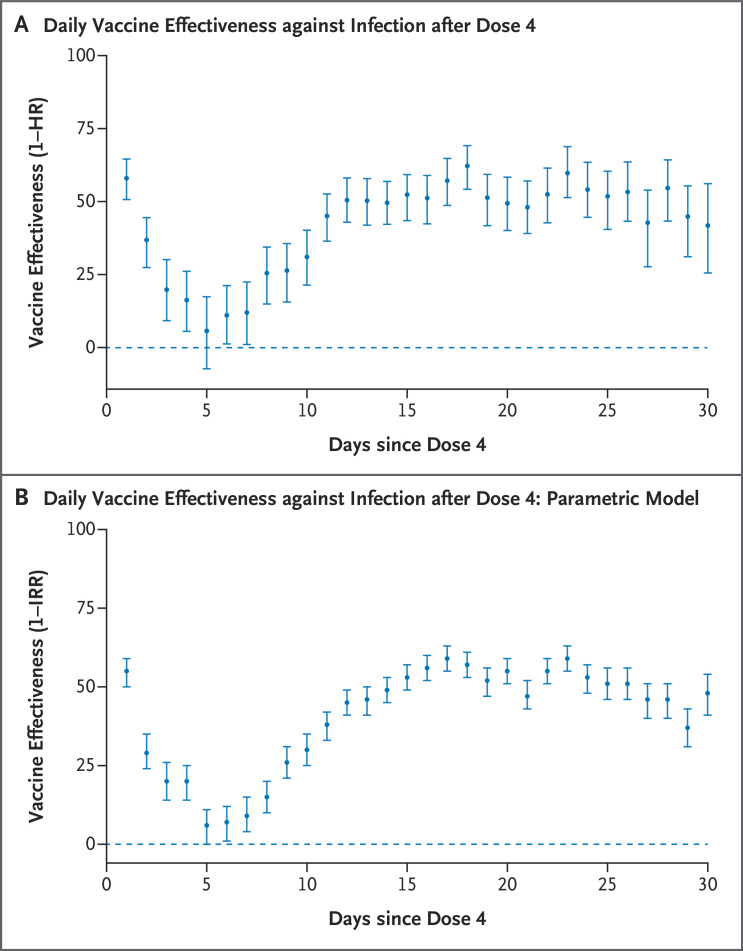
Cumulative incidence curves for all five primary outcomes are presented in Figure 2. These curves diverge mainly at approximately day 7 after the fourth vaccine dose. After an initial transient period of lower infection risk in the four-dose group, very small differences between the groups are seen by days 5 and 6 (Figure 3A). After day 7, the relative effectiveness gradually increases until it reaches a stable estimate of approximately 50% by day 14.
Figure 2. Cumulative Incidence of Covid-19 Outcomes in the Four-Dose Group and the Matching Control Group.
Shown are cumulative incidence curves (1 minus the Kaplan–Meier estimate) for the five outcomes in persons in the four-dose group (four doses of BNT162b2 vaccine) as compared with those in the control group (three doses of BNT162b2 at least 4 months earlier). Shaded areas indicate the 95% confidence intervals, and tick marks indicate censored data (with a mark appearing at each day of follow-up because of the large sample size). Covid-19 denotes coronavirus disease 2019, PCR polymerase chain reaction, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Figure 3. Daily Vaccine Effectiveness in the Primary Analysis and the Poisson Regression Analysis.
Panel A shows daily estimated vaccine effectiveness (1 minus the daily hazard ratio [HR]) against PCR-confirmed SARS-CoV-2 infection after a fourth vaccine dose as compared with a third vaccine dose (≥4 months earlier). 𝙸 bars indicate the 95% confidence interval. The analysis was performed with the bootstrap method (500 repetitions) on the Kaplan–Meier estimate. Panel B shows the daily estimated vaccine effectiveness (1 minus the incidence rate ratio [IRR]) against PCR-confirmed SARS-CoV-2 infection after a fourth vaccine dose as compared with a third vaccine dose (≥4 months earlier). 𝙸 bars indicate the 95% confidence interval. The analysis was performed with the use of Poisson regression as part of a sensitivity analysis. In each panel, values above the dashed line indicate that four doses are more effective than three doses received at least 4 months earlier.
The results of the sensitivity analyses in which censoring was delayed by 7 days (Fig. S2 and Table S3) and the relative effectiveness against PCR-confirmed infection as estimated with a parametric model (Tables S4 and S5) were generally similar to those in the primary analysis, in terms of both the size of the estimates and their trajectories (Table 2). The estimates of relative effectiveness against Covid-19–related death in the sensitivity analysis had broad confidence intervals, which limits the ability to compare the results with those from the primary analysis. Estimated relative daily vaccine effectiveness against PCR-confirmed infection based on the parametric model followed a trajectory very similar to that in the primary analysis (Figure 3B and Table S6).
Discussion
The results of this study strongly suggest that, as compared with only a third dose of BNT162b2 received at least 4 months earlier, a fourth BNT162b2 dose provided early protection against PCR-confirmed SARS-CoV-2 infection (95% CI, 44 to 47% at days 7 to 30 and 49 to 54% at days 14 to 30), symptomatic Covid-19 (53 to 58% and 58 to 64%, respectively), Covid-19–related hospitalization (59 to 74% and 63 to 79%, respectively), severe Covid-19 (50 to 74% and 48 to 77%, respectively), and Covid-19–related death (50 to 90% and 48 to 91%, respectively).
In the initial days of follow-up, immediately after their fourth vaccination, persons in the four-dose group appeared to have a reduced risk of confirmed SARS-CoV-2 infection (Figure 3). This early lower risk has been observed in previous studies and may be the result of including some persons as controls who were already infected at baseline and, because of their symptoms, were less likely to opt to receive the vaccine on that specific day (“healthy vaccinee bias”).17,22,23 Vaccinated persons also underwent testing for Covid-19 relatively less frequently in the first few days after vaccination, possibly because they attributed any symptoms to vaccine side effects. However, this bias was a transient phenomenon, as indicated by the almost equal risks by days 5 and 6 in the two study groups (Figure 3A and 3B). After this, at approximately day 7, the vaccine begins to take effect, with effectiveness gradually increasing to a stable level at around day 14. Given the very small size of the difference between the two groups during days 5 and 6, most of this subsequent difference could be attributable to the effectiveness of a fourth vaccine dose.
The reference baseline for comparison used in this study was a third dose of BNT162b2 received at least 4 months before the index date, rather than other potential baselines, such as two doses of BNT162b2 or no vaccination, for two reasons. First, because of the widespread nature and success of multiple vaccination campaigns in Israel, few persons 60 years of age or older who have had two vaccine doses or fewer and who have not already had Covid-19 remain.8 Second, despite evidence supporting the effectiveness of a third vaccine dose, including effectiveness against the omicron variant,5,6 a growing body of evidence documents subsequent waning over time of the immune protection from the third dose.5-7 Therefore, and in the face of the large pandemic waves driven by the omicron variant, there is a need for an accurate assessment of the benefits of a fourth vaccine dose among persons who are candidates to receive it — namely, those who have already received the third dose.
This study is subject to several limitations. First, the follow-up time available was short, and therefore we were not yet able to assess longer-term effects, including possible waning of the effect. Second, as with any observational study, the potential for confounding exists; however, given the rigorous matching we performed and the very small difference in risk after the transient period of healthy vaccinee bias, we believe that little residual confounding remains. Third, it is important to acknowledge the trade-off between minimization of bias (through rigorous matching) and the generalizability of results (through maximization of sample size and sample diversity). Interpretation of the results should be made with respect to the population analyzed. Finally, outcomes may be differentially misclassified as a function of whether persons opt to undergo a PCR test. However, the numbers of tests in the four-dose group were similar to those in the control group during the follow-up period of interest. The more severe outcomes, such as Covid-19–related hospitalization, severe Covid-19, and Covid-19–related death, are less prone to such potential misclassification.
Potential concerns have been raised about the use of a fourth vaccine dose. The European Medicines Agency has asked whether vaccinating too frequently could result in a weaker immune response,24,25 whereas others have claimed that a fourth vaccine could “hone” the immune system “too effectively” against wild-type SARS-CoV-2, potentially reducing broad protection against future, increasingly diverging variants.26 Although the BNT162b2 vaccine was designed to target the spike protein of the original wild-type SARS-CoV-2,27 the omicron variant differs substantially from this original strain, with a large number of spike protein mutations.2 The results of our real-world study suggest that a fourth vaccine dose is, at least initially, effective against the omicron variant. Although these results may partially alleviate the concern raised, further studies will be needed to determine whether vaccinating less frequently or offering a combination of different Covid-19 vaccines may be a superior long-term strategy.
Our results indicate that a fourth dose of BNT162b2 vaccine increases protection against PCR-confirmed SARS-CoV-2 infection, symptomatic Covid-19, Covid-19–related hospitalization, severe Covid-19, and Covid-19–related death as compared with a third (booster) dose given at least 4 months earlier among persons 60 years of age or older. Additional follow-up will allow further assessment of the protection provided by the fourth dose over time.
Acknowledgments
We thank Joseph Levy and Yatir Ben-Shlomo for their assistance.
Supplementary Appendix
Disclosure Forms
This article was published on April 13, 2022, at NEJM.org.
Footnotes
Supported by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute. Dr. Lipsitch’s work is supported by the Morris–Singer Foundation.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.SARS-CoV-2 sequences by variant. Our World in Data (https://ourworldindata.org/grapher/covid-variants-bar?country=AUS~GBR~USA~BEL~ITA~FRA~ESP~DEU~BWA~ZAF~CAN).
- 2.Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. Geneva: World Health Organization, November 26, 2021. (https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern).
- 3.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa. December 21, 2021. (https://www.medrxiv.org/content/10.1101/2021.12.21.21268116v1). preprint. [DOI] [PMC free article] [PubMed]
- 4.Sheikh A, Kerr S, Woolhouse M, McMenamin J, Robertson C. Severity of omicron variant of concern and vaccine effectiveness against symptomatic disease: national cohort with nested test negative design study in Scotland. University of Edinburgh Research Explorer, December 22, 2021. (https://www.research.ed.ac.uk/en/publications/severity-of-omicron-variant-of-concern-and-vaccine-effectiveness-). [DOI] [PMC free article] [PubMed]
- 5.Effectiveness of 3 doses of COVID-19 vaccines against symptomatic COVID-19 and hospitalisation in adults aged 65 years and older. London: UK Health Security Agency, 2022. (https://khub.net/documents/135939561/338928724/Effectiveness+of+3+doses+of+COVID-19+vaccines+against+symptomatic+COVID-19+and+hospitalisation+in+adults+aged+65+years+and+older.pdf/ab8f3558-1e16-465c-4b92-56334b6a832a).
- 6.Gardner BJ, Kilpatrick AM. Estimates of reduced vaccine effectiveness against hospitalization, infection, transmission and symptomatic disease of a new SARS-CoV-2 variant, Omicron (B.1.1.529), using neutralizing antibody titers. December 12, 2021. (https://www.medrxiv.org/content/10.1101/2021.12.10.21267594v2). preprint.
- 7.Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance — VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Israel Ministry of Health. Israel Covid-19 data dashboard (https://datadashboard.health.gov.il/COVID-19/general). (In Hebrew.)
- 9.Prime Minister’s Office. PM Bennett announces Israelis 60+ and medical workers to receive 4th vaccine. January 2, 2022. (https://www.gov.il/en/departments/news/event_statement020122).
- 10.CDC recommends Pfizer booster at 5 months, additional primary dose for certain immunocompromised children. Press release, January 4, 2022. (https://www.cdc.gov/media/releases/2022/s0104-Pfizer-Booster.html).
- 11.All adults to be offered COVID-19 boosters by end of January. Press release, November 30, 2021. (https://www.gov.uk/government/news/all-adults-to-be-offered-covid-19-boosters-by-end-of-january).
- 12.Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against omicron in Israel. N Engl J Med. DOI: 10.1056/NEJMoa2201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SARS-CoV-2 variants in analyzed sequences, Israel. Our World in Data (https://ourworldindata.org/grapher/covid-variants-area?country=~ISR).
- 14.Cohen R, Rabin G. National Insurance Institute R and PA. Membership in sick funds 2016. Period Surv 2017;289:104-104. (In Hebrew.) [Google Scholar]
- 15.Israel Ministry of Health. Collection of statistical data — 20 years of the national health insurance law. 2015. (https://www.health.gov.il/English/News_and_Events/Spokespersons_Messages/Pages/03062015_2.aspx#).
- 16.Clalit Health Services. COVID-19 vaccine doses 3 & 4 (booster): the complete guide. 2022. (https://www.clalit.co.il/he/your_health/family/Pages/3rd_corona_vaccine.aspx). (In Hebrew.)
- 17.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med 2021;385:1078-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021;398:2093-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010;21:383-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.COVID-19: people with certain medical conditions. Centers for Disease Control and Prevention (https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html). [PubMed]
- 22.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021;385:1393-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahmud SM, Righolt CH. Pitfalls of the healthy vaccinee effect. Lancet 2018;391:123-123. [DOI] [PubMed] [Google Scholar]
- 24.EMA regular press briefing on COVID-19. European Medicines Agency, January 11, 2022. (https://www.ema.europa.eu/en/events/ema-regular-press-briefing-covid-19-11#event-summary-section).
- 25.Pratt E. Why a 4th COVID-19 shot likely won’t provide more protection. Healthline, January 17, 2022. (https://www.healthline.com/health-news/why-a-4th-covid-19-shot-likely-wont-provide-more-protection).
- 26.Not so fast! Top immunologist says Israel shouldn’t rush fourth Corona shot. Israel Today, January 3, 2022. (https://www.israeltoday.co.il/read/not-so-fast-top-immunologist-says-israel-shouldnt-rush-fourth-corona-shot/).
- 27.Martínez-Flores D, Zepeda-Cervantes J, Cruz-Reséndiz A, Aguirre-Sampieri S, Sampieri A, Vaca L. SARS-CoV-2 vaccines based on the spike glycoprotein and implications of new viral variants. Front Immunol 2021;12:701501-701501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.