Abstract
Investigation of relationships between age-related changes in regional brain volumes and changes in domain-specific cognition could provide insights into the neural underpinnings of individual differences in cognitive aging. Domain-specific cognition (memory, verbal fluency, visuospatial ability) and tests of executive function and attention (Trail-Making Test Part A and B) and 47 brain volumes of interest (VOIs) were assessed in 836 Baltimore Longitudinal Study of Aging participants with mean follow-up of 4.1 years (maximum 23.1 years). To examine the correlation between changes in domain-specific cognition and changes in brain volumes, we used bivariate linear mixed effects models with unstructured variance-covariance structure to estimate longitudinal trajectories for each variable of interest and correlations among the random effects of these measures. Higher annual rates of memory decline were associated with greater volume loss in 14 VOIs primarily within the temporal and occipital lobes. Verbal fluency decline was associated with greater ventricular enlargement and volume loss in 24 VOIs within the frontal, temporal, and parietal lobes. Decline in visuospatial ability was associated with volume loss in 3 temporal and parietal VOIs. Declines on the attentional test were associated with volume loss in 4 VOIs located within temporal and parietal lobes. Greater declines on the executive function test were associated with greater ventricular enlargement and volume loss in 10 frontal, parietal, and temporal VOIs. Our findings highlight domain-specific patterns of regional brain atrophy that may contribute to individual differences in cognitive aging.
Keywords: Neurodegeneration, Cognitive decline, Brain aging, Structural MRI
1. Introduction
There are individual differences among cognitively normal older adults as they age, both with respect to longitudinal change in cognitive performance and regional brain atrophy. While there is ample evidence of age-related declines in cognition and brain volume, the relationships between the two are still not completely understood (Raz and Rodrigue, 2006). Further investigation of these links could provide insights into the neurobiology of individual differences in cognitive aging (Saur et al., 2008).
Other studies have examined associations between cognitive performance and brain structure at either one timepoint or over a relatively short follow-up. Correlations of domain-specific cognition, i.e., memory and executive function, with specific brain areas, e.g., hippocampus and prefrontal cortex, have been more difficult to establish, even though a moderate relationship between cognitive performance and brain volume has been reported (Fjell and Walhovd, 2010). For example, studies have shown that age-related atrophy in temporal lobe structures is correlated with changes in memory performance (van Petten et al., 2004; Yonelinas et al., 2007; Ystad et al., 2009; Chen et al., 2010). Other studies have found associations between memory performance and both hippocampal and entorhinal volumes (Cardenas et al., 2011) or only in entorhinal cortex (Raz and Rodrigue, 2006). Regarding the relationship between executive function and brain volumes of interest (VOIs), higher global gray matter (GM) and white matter (WM) volumes are associated with higher executive function, as represented by Switching of Attention (Brickman et al., 2006), category verbal fluency test, design fluency test, and the Trail-Making Test Part B (TMT-B) (Chee et al., 2009), and a selection of tests based on Miyake’s conceptual framework for executive function (Miyake et al., 2000) (measures of mental set shifting, working memory, and inhibition). Based on measures from Miyake’s conceptual framework (Miyake et al., 2000), executive function was associated with prefrontal GM volumes (Bettcher et al., 2016). Also, lower frontal lobe volumes have been associated with lower baseline executive function and greater longitudinal decline in executive function, as measured by Wisconsin Card Sorting Test, Phonemic Verbal Fluency, and Stroop Color Word Interference Test in a meta-analysis (Alvarez and Emory, 2006).
Given the great variability in overall levels of cognitive performance and brain structure among individuals, findings from cross-sectional studies are often inconsistent. Longitudinal study designs allow for the assessment of age-related changes in brain volume and cognition within the same individual. Raz, Rodrigue (Raz and Rodrigue, 2006) suggested that a longer follow-up time measuring cognitive trajectories may yield more consistent results than those observed cross-sectionally or with short follow-up intervals. Few longitudinal studies have examined correlated changes between brain aging and cognitive performance. In one such study, Leong et al. (2017) found that longitudinal decline in global cognition was associated with volume declines in total brain, parietal and temporal GM, and hippocampus, while ventricular expansion and hippocampal volume loss were associated with age-related declines in verbal memory, as measured by the Rey Auditory Verbal Learning Test, and executive function, as measured by the category fluency test, design fluency test, and TMT-B, over a maximum follow-up period of 8 years in healthy older adults. They also reported that greater frontal and parietal WM atrophy were associated with faster decline in verbal memory performance (Leong et al., 2017). In another study, relationships between cognitive trajectories and brain volume changes were examined over up to a 10-year interval in 460 individuals who were either cognitively impaired or unimpaired (Fletcher et al., 2018). These authors found that global cognitive decline was strongly influenced by temporal lobe GM volume change across both impaired and unimpaired older adults. However, these prior studies only examined global and lobar regions as well as several specific VOIs, i.e., hippocampus.
To investigate associations among a broader range of cognitive domains and brain regions, we leveraged data from the Baltimore Longitudinal Study of Aging (BLSA), a longitudinal study of physical and psychological aging among community-dwelling older adults (Shock et al., 1984). We examined associations between changes in domain- and test-specific cognitive performance and regional brain volumes over a long follow-up interval. We evaluated associations between changes in five cognitive domains, i.e., verbal memory, verbal fluency, visuospatial ability, attention, and executive function, and 47 brain VOIs. This approach allowed us to evaluate domain-specific patterns of associations between changes in cognition and brain structure, which we expected would vary by cognitive domains and measures.
2. Methods and materials
2.1. Sample characteristics
Participants were enrolled in the Baltimore Longitudinal Study of Aging (BLSA), a longitudinal study of physical and psychological aging among community-dwelling older adults (Shock et al., 1984). Our sample included two epochs of data. A subset of BLSA participants enrolled in the BLSA neuroimaging substudy in 1994 where participants underwent annual magnetic resonance imaging (MRI), PET and comprehensive neuropsychological testing and inclusion/exclusion criteria have been described previously (Resnick et al., 2003). Starting in 2009, all BLSA participants (without contraindications) were continuously enrolled in the MRI study. Individuals aged < 60 years were seen every four years, aged 60–79 years were seen biennially, and aged ≥ 80 years were seen annually, where they underwent MRI, neuropsychological testing, and a variety of physical assessments. Supplementary Fig. 1 shows participant selection. The final sample consisted of 836 participants aged ≥ 55 years with a total of 2853 concurrent MRI and neuropsychological evaluations conducted from 1994–2018. There are 664 (79.4%) active participants, 110 (13.2%) participants who died during the follow-up period, and 62 (7.4%) who refused contact, are lost to follow-up, or dropped out of the study. We excluded assessments after the onset of the cognitive impairment, and assessments after the development of certain health conditions, including brain injury, brain tumor, strokes, and Parkinson’s disease.
Determination of cognitive status was done through a two-step process. First, clinical and neuropsychological data from participants were reviewed at consensus diagnostic case conference if their Clinical Dementia Rating score (Morris, 1993) was ≥ 0.5 or if they had > 3 errors on the Blessed Information-Memory-Concentration Test (Fuld, 1978). Second, adjudicated diagnoses of dementia and AD, respectively, were based on criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, 3rd ed., Revised (Association, 1987) and the National Institute of Neurological and Communication Disorders and Stroke –Alzheimer’s Disease and Related Disorders (McKhann et al., 1984). MCI was based on the Petersen criteria (Petersen et al., 1999).
The BLSA protocol has been approved by the Intramural Research Program of the National Institute on Aging and the Institutional Review Board of the National Institute of Environmental Health Sciences, National Institutes of Health. All participants provided written informed consent before each assessment.
2.2. Data and code availability
Data from the BLSA are available on request by proposal submission through the BLSA website (blsa.nih.gov). All requests are reviewed by the BLSA Data Sharing Proposal Review Committee. In terms of code availability, all code was written in terms of the procedure NLMIXED in SAS 9.4 (Institute, 2015).
2.3. Neuropsychological assessments
We initially calculated composite scores from five cognitive domains, i.e., memory, verbal fluency, visuospatial ability, attention, and executive function. The composite score for each cognitive domain was calculated using baseline means and standard deviations to compute the average z-scores of measures comprising that domain. The cognitive domains and labels were determined using a rational approach, based on the neuropsychological literature and informed by prior statistical approaches in a subset of BLSA participants (unpublished data). Memory consisted of the immediate and long-delayed free recall scores from the California Verbal Learning Test (Delis et al., 1987). The delay time on the CVLT was approximately 20 min. Verbal fluency consisted of the total numbers of correct words generated in 60 s for the letter (Benton, 1968) and category fluency (Newcombe, 1969) tests. Visuospatial ability consisted of a modified version of the Educational Testing Service Card Rotations test (Wilson et al., 1975), based on the total number correct minus total number incorrect, and the Clock Drawing Test (Rouleau et al., 1992), using times of 11:10 and 3:25. Attention was comprised of TMT Part A (TMT-A) (Reitan, 1958) and Digit Span Forward (Wechsler, 1981). Executive function consisted of completion time for TMT-B (Reitan, 1958) and the total score from Digit Span Backward (Wechsler, 1981). Completion times of TMT-A and TMT-B were truncated at 300 seconds. Due to the distributions of TMT-A and TMT-B, we log-transformed the completion times, standardized these tests, and reversed the signs so that higher scores reflected better performance.
We then performed a series of confirmatory factor analyses to evaluate our theoretical approach to definition of composite scores (see Supplement for the confirmatory factor analyses). While the factor analyses supported the use of composite scores for verbal memory, verbal fluency and visuospatial ability, the theoretically based composite scores representing attention and executive function were not empirically homogeneous. Thus, we present findings from single tests of attention (TMT-A) and executive function (TMT-B), given their widespread use in epidemiological studies and investigations of early markers of cognitive impairment. We removed Digit Span Forward and Backward from the analyses.
2.4. Structural magnetic resonance image acquisition
MRI scanning was performed on a General Electric (GE) Signa 1.5-T scanner (Milwaukee, WI) or a 3-T Philips Achieva scanner. The GE 1.5-T scans used a high-resolution volumetric spoiled gradient re-called acquisition in a steady state (SPGR) series (axial acquisition, repetition time = 35 ms, echo time = 5 ms, flip angle = 45°, field of view = 24 cm, matrix = 256 × 256, number of excitations = 1, voxel dimensions = 0.94 × 0.94 × 1.5 mm slice thickness). T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) scans were acquired on a 3-T Philips Achieva (repetition time [TR] = 6.8 ms, echo time [TE] = 3.2 ms, flip angle = 8°, image matrix = 256 × 256, 170 slices, pixel size = 1 × 1 mm, slice thickness = 1.2 mm). Of 2,853 MRI scans, 1073 (37.6%) scans were from the GE Signa 1.5-T scanner and 1780 (62.4%) scans were from the 3-T Philips Achieva scanner.
2.5. Harmonization of MUSE anatomical labels across 1.5-T SPGR and 3-T MPRAGE
A new automated labeling method specifically designed to achieve a consistent parcellation of brain anatomy in longitudinal MRI studies with scanner and imaging protocol differences was used to harmonize MR data in the BLSA from 1994 to 2018. This method combines the MUSE anatomical labeling approach (Doshi et al., 2016) with harmonized acquisition-specific atlases (Erus et al., 2018). The approach is detailed in Erus, Doshi, An, Verganelakis, Resnick, Davatzikos (Erus et al., 2018). Briefly, using 35 labeled 3-T MPRAGE brain MRIs from the OASIS data set (available for download at https://masi.vuse.vanderbilt.edu/workshop2012) as atlases, we first performed the MUSE labeling method on 3-T MPRAGE images for 32 BLSA participants with 1.5-T SPGR at an earlier time point. Among these participants, the average time interval between 1.5-T SPGR and 3-T MPRAGE images was 4.2 (SD = 0.4) years. Then, for each participant, we deformably registered their 1.5-T SPGR image to their 3-T MPRAGE image using a robust registration strategy that combines an ensemble of registrations obtained using two different algorithms and multiple smoothness parameters. From this approach, we obtained 32 pairs of 1.5-T SPGR and 3-T MPRAGE images in the same space with common anatomical labels. These sets were used as atlases in the MUSE approach to obtain labels on the entire collection of 1.5-T SPGR and 3-T MPRAGE images in the BLSA. This workflow for anatomical labeling has been extensively validated on the BLSA MRI data set (Erus et al., 2018). Stability measures for longitudinal VOIs over time were high, with intraclass correlations (ICC) ranging from 0.89 to 0.99.
2.6. Statistical analyses
Baseline characteristics of the sample were defined using means and standard deviations for continuous variables and numbers and frequencies for categorical variables.
To estimate the correlation between longitudinal changes in brain VOIs and longitudinal changes in cognition, we used bivariate linear mixed-effects models. This approach allows longitudinal trajectories of brain VOIs and cognitive function to be modeled jointly and correlations between changes to be estimated directly (Grimm et al., 2016). Since these models were comprised of two types of outcomes, there are two different sets of fixed effects and random effects for brain VOIs and cognitive measures. For both outcomes, the fixed effects included sex, race, baseline age, years of education, time since first concurrent neuroimaging and neuropsychological assessments, and two-way interactions between sex, race, baseline age, and years of education with time. For brain VOIs only, we added baseline intra-cranial volume and scanner type (1.5-T SPGR vs. 3-T MPRAGE), as fixed effects. We chose these covariates a priori because these covariates could confound the association of the outcomes. The random effects for each outcome included intercept and time, and we specified the 4 × 4 variance-covariance of the random effects to be unstructured. Each linear mixed-effects model assumes independent, normally distributed residuals conditional on the random effects, with the random effects and residuals being independent from one another. The models also assume multivariate normal distributions of random intercepts and slopes. We assume linear change during follow-up for each participant, given that the average follow-up is 4.1 years. Even in the case of nonlinearity in some measures, this linear change represents the average change over follow-up.
The correlations between longitudinal changes in brain VOIs and cognition were directly estimated from this variance-covariance matrix after accounting for fixed effects. By estimating the correlation of changes from the variance-covariance matrix of random effects, this approach fully accounts for changes considered as latent constructs. The linear mixed effects model accounts for the variability in visits, follow-up time, unequal measurements, and age, since participants enter the BLSA at different ages, have been in the BLSA for various lengths of time, have unequally spaced follow-up visits, and have unequal numbers of measurements (Morrell et al., 2009). Missing data are assumed to be missing at random, and all available data are used for each visit. There was no significant difference in proportion of lost to follow-up during our designed observation period between those who remained cognitively normal (n = 58, 7.9%) and those who developed subsequent cognitive impairment (n = 4, 3.9%) (p = 0.16 from Fisher’s exact test). The bivariate linear mixed effects models were fit using procedure NLMIXED in SAS 9.4 (Institute, 2015). We present results for correlation coefficients at p < 0.05, p < 0.01, and false discovery rate (FDR) correction by each cognitive domain or test in the case of attention and executive function.
To further investigate whether results varied by hemisphere, we estimated the correlation between cognitive changes and brain volume changes for left and right hemisphere, separately. We also performed sensitivity analyses to include hypertension and the two-way interaction between hypertension and time since baseline in the models, since hypertension may negatively affect both cognition and brain volumes over time.
3. Results
3.1. Sample characteristics
Table 1 shows the overall sample characteristics (N = 836, 53% female). The mean age of the sample was 71.1 (Standard Deviation, [SD] = 8.8) years with an average follow-up time of 4.1 (SD = 4.7) years. Average number of assessments per participant was 3.3 (SD = 3.4). The mean follow-up time for participants with two or more assessments (n = 579) was 5.9 (SD = 4.6) years. There were 103 (12.3%) participants who developed subsequent incident cognitive impairment (MCI/dementia).
Table 1.
Sample characteristics.
| Characteristics | Overall sample (N=836) |
|---|---|
|
| |
| Females | 443 (53%) |
| Race, n (%) | |
| White | 588 (70.3%) |
| African American | 199 (23.8%) |
| Other | 49 (5.9%) |
| Years of Education, mean (SD) | 16.9 (2.5) |
| Range of Years of Education | 8.0–21.0 |
| Total Number of Assessments | 2853 |
| Baseline Age, in years, mean (SD) | 71.1 (8.8) |
| Range of Ages | 55.0–92.4 |
| Baseline Hypertension, n (%) | 410 (49.0%) |
| Baseline Systolic Blood Pressure, in mm Hg, mean (SD) | 119.6 (18.5) |
| Baseline Diastolic Blood Pressure, in mm Hg, mean (SD) | 68.5 (11.1) |
| Baseline Diabetes, n (%) | 131 (15.7%) |
| Baseline History of Myocardial Infarction, n (%) | 14 (1.7%) |
| Subsequent cognitive impairment (mild cognitive impairment or dementia), n (%) | 103 (12.3%) |
| Number of Assessments, mean (SD) | 3.4 (3.3) |
| Range of Number of Assessments | 1–20 |
| Number of Assessments among those with ≥2 | 4.5 (3.5) |
| assessments (n=579), mean (SD) | 2–20 |
| Range of Number of Assessments for those with ≥ 2 assessments | |
| Follow-up Time, in years, mean (SD) | 4.1 (4.7) |
| Range of Follow-up Time | 0–23.1 |
| Follow-up Time among those with ≥2 assessments | 5.9 (4.6) |
| (n=579), in years, mean (SD) | 0.8–23.1 |
| Range of Follow-up Time for those with ≥2 assessments | |
| Number of Total Concurrent Assessments, n (%) | |
| 1 | 257 (30.7) |
| 2 | 169 (20.2) |
| 3 | 158 (18.9) |
| 4 | 102 (12.2) |
| 5 or more and up to 20 | 150 (17.9) |
SD = standard deviation
3.2. Associations of changes in domain-specific cognitive performance with changes in brain volumes
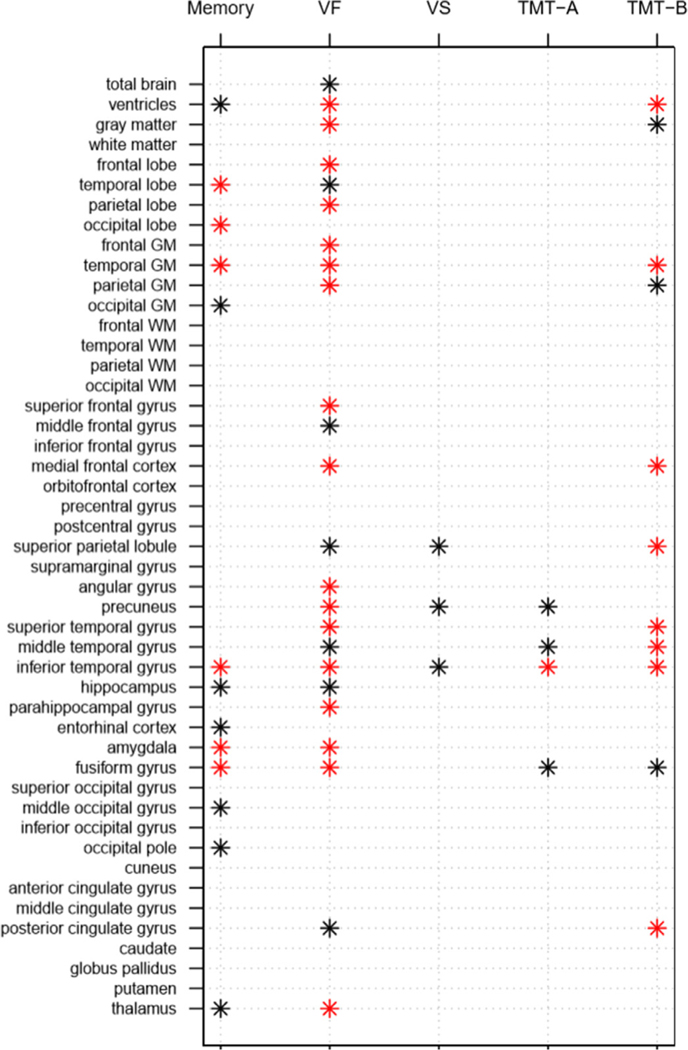
Fig. 1 summarizes the significant associations among the 3 domains and specific tests of attention and executive function, and the 47 brain VOIs.
Fig. 1.
Summary of findings for correlations of rates of change between each cognitive domain/test and each brain region of interest in cognitively normal older adults in the Baltimore Longitudinal Study of Aging. Note: red * indicates p < 0.01 and black * indicates p < 0.05. VF – verbal fluency; VS – visuospatial ability; GM – gray matter; WM – white matter; TMT – Trail-Making Test.
Table 2 shows the correlation estimates between changes in cognitive domains/tests and changes in brain VOIs. Supplementary Fig. 1 shows all results in dot plots. Results for the correlations between change in each cognitive domain and brain VOI are summarized in the following sections. All significant correlations (p < 0.05) in our analysis showed correlations between higher rates of cognitive decline and higher rates of volumetric decline or ventricular enlargement. In models including hypertension and the two-way interaction between time since baseline as covariates, the direction and magnitude of correlation coefficients remain the same (Supplementary Table 1).
Table 2.
Correlations between change in cognitive domains and change in brain regions of interest in the Baltimore Longitudinal Study of Aging (N = 836).
| Brain regions of interest | Memory | Verbal Fluency | Visuospatial Ability | Trail-Making Test Part A | Trail-Making Test Part B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||||
| ρ | SE | p-value | ρ | SE | p-value | ρ | SE | p-value | ρ | SE | p-value | ρ | SE | p-value | |
|
| |||||||||||||||
| Total brain | 0.077 | 0.135 | 0.571 | 0.400 | 0.183 | 0.029 | −0.102 | 0.244 | 0.677 | 0.042 | 0.176 | 0.813 | 0.093 | 0.228 | 0.682 |
| vCSF | −0.190 | 0.096 | 0.049 | −0.718 | 0.084 | <0.0001 * § | −0.258 | 0.231 | 0.264 | −0.105 | 0.150 | 0.482 | −0.503 | 0.143 | 0.0005 * § |
| Frontal Lobe | 0.098 | 0.134 | 0.462 | 0.635 | 0.160 | <0.0001 * § | −0.060 | 0.283 | 0.832 | 0.070 | 0.205 | 0.733 | 0.365 | 0.228 | 0.110 |
| Temporal Lobe | 0.384 | 0.132 | 0.0037 * § | 0.427 | 0.191 | 0.026 | 0.275 | 0.270 | 0.308 | 0.232 | 0.177 | 0.189 | 0.296 | 0.251 | 0.238 |
| Parietal Lobe | 0.096 | 0.135 | 0.480 | 0.523 | 0.194 | 0.007 * § | 0.028 | 0.330 | 0.933 | 0.085 | 0.214 | 0.693 | 0.329 | 0.240 | 0.170 |
| Occipital Lobe | 0.389 | 0.147 | 0.0083 * | 0.344 | 0.208 | 0.098 | −0.146 | 0.321 | 0.650 | 0.340 | 0.179 | 0.058 | 0.712 | 0.465 | 0.126 |
| GM | 0.240 | 0.125 | 0.055 | 0.559 | 0.138 | <0.0001 * § | 0.025 | 0.240 | 0.919 | 0.117 | 0.170 | 0.490 | 0.406 | 0.177 | 0.022 |
| Frontal | 0.156 | 0.127 | 0.221 | 0.566 | 0.134 | <0.0001 * § | 0.061 | 0.241 | 0.802 | 0.027 | 0.171 | 0.873 | 0.298 | 0.202 | 0.140 |
| Temporal | 0.381 | 0.113 | 0.0008 * § | 0.586 | 0.147 | <0.0001 * § | 0.191 | 0.227 | 0.400 | 0.309 | 0.162 | 0.056 | 0.510 | 0.175 | 0.0036 * § |
| Parietal | 0.132 | 0.132 | 0.318 | 0.586 | 0.168 | 0.0005 * § | −0.005 | 0.271 | 0.985 | −0.033 | 0.164 | 0.843 | 0.490 | 0.235 | 0.038 |
| Occipital | 0.348 | 0.140 | 0.013 | 0.362 | 0.197 | 0.067 | 0.135 | 0.329 | 0.682 | 0.219 | 0.178 | 0.219 | 0.512 | 0.279 | 0.067 |
| WM | 0.130 | 0.151 | 0.390 | 0.300 | 0.214 | 0.161 | −0.194 | 0.304 | 0.523 | 0.105 | 0.185 | 0.570 | −0.027 | 0.267 | 0.919 |
| Frontal | 0.033 | 0.149 | 0.826 | 0.384 | 0.197 | 0.052 | −0.401 | 0.311 | 0.198 | −0.069 | 0.191 | 0.717 | −0.103 | 0.273 | 0.707 |
| Temporal | 0.155 | 0.150 | 0.301 | 0.031 | 0.221 | 0.887 | 0.080 | 0.301 | 0.791 | 0.133 | 0.183 | 0.465 | −0.082 | 0.276 | 0.766 |
| Parietal | 0.046 | 0.142 | 0.744 | 0.144 | 0.203 | 0.479 | −0.260 | 0.313 | 0.406 | 0.194 | 0.176 | 0.273 | −0.075 | 0.261 | 0.773 |
| Occipital | 0.140 | 0.155 | 0.370 | 0.081 | 0.225 | 0.718 | −0.395 | 0.333 | 0.237 | 0.295 | 0.183 | 0.107 | 0.117 | 0.301 | 0.698 |
| Superior frontal gyrus | 0.141 | 0.131 | 0.282 | 0.467 | 0.149 | 0.0018 * § | −0.102 | 0.237 | 0.668 | −0.028 | 0.173 | 0.871 | 0.056 | 0.221 | 0.798 |
| Middle frontal gyrus | 0.0099 | 0.126 | 0.938 | 0.393 | 0.153 | 0.010 | −0.254 | 0.197 | 0.198 | −0.038 | 0.155 | 0.808 | 0.282 | 0.188 | 0.135 |
| Inferior frontal gyrus | 0.182 | 0.129 | 0.160 | 0.216 | 0.211 | 0.307 | 0.025 | 0.311 | 0.935 | −0.027 | 0.205 | 0.896 | 0.086 | 0.231 | 0.710 |
| Medial frontal cortex | 0.049 | 0.143 | 0.733 | 0.468 | 0.178 | 0.0085 * § | 0.146 | 0.287 | 0.612 | 0.164 | 0.183 | 0.371 | 0.644 | 0.162 | <0.0001 * § |
| Orbitofrontal cortex | 0.066 | 0.152 | 0.666 | 0.392 | 0.202 | 0.053 | 0.116 | 0.298 | 0.697 | −0.060 | 0.192 | 0.756 | 0.034 | 0.264 | 0.897 |
| Precentral gyrus | −0.040 | 0.133 | 0.763 | 0.168 | 0.178 | 0.344 | 0.151 | 0.208 | 0.468 | −0.091 | 0.157 | 0.563 | 0.179 | 0.193 | 0.353 |
| Postcentral gyrus | 0.042 | 0.137 | 0.762 | 0.255 | 0.188 | 0.174 | 0.022 | 0.229 | 0.923 | −0.039 | 0.170 | 0.818 | 0.037 | 0.213 | 0.862 |
| Superior parietal lobule | 0.147 | 0.120 | 0.221 | 0.391 | 0.153 | 0.011 § | 0.480 | 0.195 | 0.014 | 0.166 | 0.159 | 0.296 | 0.513 | 0.158 | 0.0012 * § |
| Supramarginal gyrus | 0.195 | 0.127 | 0.126 | 0.055 | 0.198 | 0.780 | −0.058 | 0.224 | 0.796 | 0.143 | 0.162 | 0.380 | 0.012 | 0.212 | 0.953 |
| Angular gyrus | 0.204 | 0.145 | 0.160 | 0.524 | 0.177 | 0.0031 * § | 0.089 | 0.282 | 0.751 | 0.016 | 0.171 | 0.924 | 0.320 | 0.242 | 0.187 |
| Precuneus | 0.241 | 0.127 | 0.058 | 0.537 | 0.148 | 0.0003 * § | 0.545 | 0.237 | 0.022 | 0.371 | 0.178 | 0.038 | 0.359 | 0.196 | 0.067 |
| Superior temporal gyrus | 0.227 | 0.135 | 0.093 | 0.501 | 0.161 | 0.002 * § | 0.233 | 0.234 | 0.320 | 0.275 | 0.180 | 0.127 | 0.535 | 0.201 | 0.0079 * § |
| Middle temporal gyrus | 0.206 | 0.118 | 0.081 | 0.350 | 0.178 | 0.050 | −0.066 | 0.215 | 0.758 | 0.296 | 0.145 | 0.042 | 0.468 | 0.167 | 0.0052 * § |
| Inferior temporal gyrus | 0.401 | 0.127 | 0.0016 * § | 0.711 | 0.216 | 0.001 * § | 0.708 | 0.304 | 0.020 | 0.627 | 0.217 | 0.004 * | 0.748 | 0.230 | 0.0012 * § |
| Hippocampus | 0.264 | 0.107 | 0.014 | 0.463 | 0.190 | 0.015 § | −0.142 | 0.258 | 0.583 | −0.257 | 0.176 | 0.144 | 0.084 | 0.190 | 0.658 |
| Parahippocampal gyrus | 0.242 | 0.142 | 0.089 | 0.635 | 0.192 | 0.001 * § | −0.133 | 0.325 | 0.682 | −0.148 | 0.220 | 0.500 | 0.014 | 0.240 | 0.953 |
| Entorhinal cortex | 0.289 | 0.119 | 0.016 | 0.316 | 0.190 | 0.097 | 0.002 | 0.239 | 0.994 | −0.066 | 0.166 | 0.692 | 0.008 | 0.219 | 0.969 |
| Amygdala | 0.406 | 0.098 | <0.0001 * § | 0.455 | 0.141 | 0.0013 * § | 0.208 | 0.208 | 0.318 | 0.008 | 0.140 | 0.955 | 0.203 | 0.177 | 0.251 |
| Fusiform gyrus | 0.387 | 0.114 | 0.0007 * § | 0.509 | 0.149 | 0.0007 * § | 0.205 | 0.221 | 0.353 | 0.309 | 0.153 | 0.044 | 0.426 | 0.179 | 0.017 |
| Superior occipital gyrus | 0.209 | 0.115 | 0.070 | 0.201 | 0.168 | 0.232 | 0.320 | 0.183 | 0.080 | 0.150 | 0.146 | 0.306 | −0.033 | 0.184 | 0.858 |
| Middle occipital gyrus | 0.312 | 0.133 | 0.019 | 0.115 | 0.190 | 0.544 | 0.148 | 0.255 | 0.563 | 0.159 | 0.160 | 0.320 | 0.308 | 0.249 | 0.216 |
| Inferior occipital gyrus | 0.235 | 0.159 | 0.141 | 0.255 | 0.219 | 0.244 | 0.059 | 0.310 | 0.850 | −0.002 | 0.193 | 0.994 | 0.474 | 0.284 | 0.096 |
| Occipital pole | 0.323 | 0.148 | 0.029 | −0.061 | 0.217 | 0.779 | −0.058 | 0.292 | 0.843 | −0.189 | 0.181 | 0.296 | 0.405 | 0.268 | 0.132 |
| Cuneus | −0.049 | 0.134 | 0.716 | 0.299 | 0.196 | 0.127 | −0.156 | 0.242 | 0.518 | 0.245 | 0.170 | 0.149 | 0.004 | 0.234 | 0.986 |
| Anterior cingulate gyrus | 0.056 | 0.139 | 0.685 | 0.235 | 0.193 | 0.222 | −0.259 | 0.232 | 0.264 | −0.065 | 0.174 | 0.707 | 0.120 | 0.219 | 0.582 |
| Middle cingulate gyrus | −0.031 | 0.131 | 0.814 | 0.020 | 0.181 | 0.911 | −0.255 | 0.198 | 0.198 | −0.157 | 0.150 | 0.293 | 0.063 | 0.189 | 0.738 |
| Posterior cingulate gyrus | 0.214 | 0.131 | 0.104 | 0.362 | 0.178 | 0.042 | 0.319 | 0.224 | 0.153 | 0.262 | 0.165 | 0.113 | 0.472 | 0.161 | 0.0035 * § |
| Caudate nucleus | −0.220 | 0.115 | 0.056 | −0.131 | 0.202 | 0.517 | 0.185 | 0.283 | 0.514 | −0.188 | 0.192 | 0.330 | −0.208 | 0.204 | 0.309 |
| Globus pallidus | −0.072 | 0.156 | 0.646 | −0.095 | 0.216 | 0.659 | −0.365 | 0.316 | 0.248 | −0.060 | 0.190 | 0.752 | −0.558 | 0.323 | 0.084 |
| Putamen | 0.0033 | 0.112 | 0.977 | 0.010 | 0.197 | 0.961 | 0.320 | 0.263 | 0.225 | −0.182 | 0.181 | 0.313 | −0.186 | 0.213 | 0.383 |
| Thalamus | 0.251 | 0.119 | 0.035 | 0.459 | 0.147 | 0.0018 * § | 0.026 | 0.223 | 0.906 | 0.106 | 0.157 | 0.502 | 0.187 | 0.198 | 0.347 |
SE – standard error, vCSF-ventricles, GM – gray matter, WM – white matter. All P values shown are unadjusted. Bolded values indicate p < 0.05, and
indicates p < 0.01.
indicates significance at FDR corrected p < 0.05.
3.2.1. Memory
Longitudinal verbal memory declines were correlated with volumetric declines in 6 VOIs at p < 0.01: temporal and occipital lobe, temporal gray matter (GM), inferior temporal gyrus, amygdala, and fusiform gyrus. All these correlations survived FDR correction. Additionally, at p < 0.05, we observed correlations of memory decline with greater ventricular enlargement and volumetric declines in occipital GM, hippocampus, entorhinal cortex, middle occipital gyrus, occipital pole, and thalamus. None of these survived FDR correction.
3.2.2. Verbal fluency
Longitudinal declines in verbal fluency were correlated with greater ventricular enlargement and volumetric declines in 16 VOIs at p < 0.01: GM, frontal lobe, parietal lobe, frontal GM, temporal GM, parietal GM, superior frontal gyrus, medial frontal cortex, angular gyrus, precuneus, superior temporal gyrus, inferior temporal gyrus, parahippocampal gyrus, amygdala, fusiform gyrus, and thalamus. Longitudinal declines in verbal fluency were correlated additionally with 7 VOIs at p < 0.05: total brain, temporal lobe, middle frontal gyrus, superior parietal lobule, middle temporal gyrus, hippocampus, and posterior cingulate gyrus. The VOIs that survived FDR correction were frontal lobe, parietal lobe, GM, frontal GM, temporal GM, parietal GM, superior frontal gyrus, medial frontal cortex, superior parietal lobule, angular gyrus, precuneus, inferior temporal gyrus, hippocampus, parahippocampal gyrus, amygdala, fusiform gyrus, and thalamus.
3.2.3. Visuospatial ability
Longitudinal declines in visuospatial ability were correlated with volumetric declines in superior parietal lobule, precuneus, and inferior temporal gyrus at p < 0.05. None of these correlations were significant at p < 0.01, and none of them survived FDR correction.
3.2.4. Trail-making test part A
Longitudinal declines in TMT-A were associated with volumetric declines in inferior temporal gyrus at a p < 0.01, which did not survive FDR correction. At p < 0.05, declines in TMT-A were correlated with volumetric declines in precuneus, middle temporal gyrus, and fusiform gyrus. None of these estimates survived FDR correction.
3.2.5. Trail-making test part B
Longitudinal declines in TMT-B were correlated with greater ventricular enlargement and steeper volumetric decline in temporal GM, medial frontal cortex, superior parietal lobule, superior, middle, and inferior temporal gyri, and posterior cingulate gyrus at p < 0.01, with all surviving FDR correction. Additionally, at p < 0.05, we observed correlations with volumetric declines in GM, parietal GM, and fusiform gyrus. These estimates did not survive FDR correction.
3.3. Associations of changes in domain-specific cognitive performance with changes in brain volumes by hemisphere
The patterns of correlations for the right and left hemispheres are similar to those of the combined hemispheres, except for visuospatial ability where associations were more pronounced for right than left hemisphere structures. Supplementary Fig. 2 shows the results from the secondary analyses examining the correlations between rates of change in visuospatial ability and rates of change in brain regional VOIs by hemisphere.
3.4. Additional results
The primary aim of the current study was to investigate associations between cognitive change and brain volume changes. While not the primary aim of this study, we also include results of the models examining baseline cognition with change in brain volumes, as well as baseline brain volumes with change in cognition (Supplementary Tables 2 and 3). The lack of consistent patterns across these analyses, with both positive and negative correlations, illustrates the challenge in investigating associations between individual differences in cognition and brain structure without considering intra-individual change, where each person serves as their own control.
4. Discussion
We found that annual rates of cognitive change were associated with domain-specific patterns of regional volume changes among cognitively normal older adults. All findings indicated that faster rates of cognitive decline were associated with faster rates of brain volume loss or ventricular expansion. Overall, longitudinal declines in verbal fluency were associated with the most widespread regions of volume loss, followed by associations with memory. Longitudinal decline in visuospatial ability was associated with the fewest and most specific regions of volume loss. Among brain regions, inferior temporal gyrus volume loss was most commonly associated with declines in the cognitive domains. Together, these results demonstrate specific patterns of brain volume loss in association with domain-specific cognitive declines over time in cognitively normal older individuals. Since the preclinical AD stage occurs prior to symptom onset and there are subtle differences between normal and pathological cognitive and brain aging, we will consider the patterns observed in light of the possibility that some (but not all) changes may be associated with emerging pathology.
4.1. Memory
Greater longitudinal declines in verbal episodic memory, measured by immediate and delayed free recall on the CVLT, were associated with greater rates of volume loss in 14 VOIs: temporal and occipital lobes, temporal GM, parietal GM, precuneus, inferior temporal gyrus, hippocampus, entorhinal cortex, amygdala, fusiform gyrus, middle occipital gyrus, occipital pole, caudate nucleus, and thalamus. This pattern of brain volume loss involves regions within the medial temporal lobe memory system, which is crucial for memory processes (Eichenbaum et al., 2012). These findings are also consistent with other studies that showed associations between annual rates of decline in memory and hippocampal volume (Cardenas et al., 2011; Leong et al., 2017, Mungas et al., 2005; Gorbach et al., 2017). Additionally, the CVLT includes an auditory component of listening to a list of words and reciting them either immediately or after approximately 20 min, and it is plausible that we are observing temporal regions that are associated with the ventral language pathway, such as the middle and inferior temporal cortices. The ventral language pathway involves regions that are necessary for mapping sounds to higher-level language comprehension (Saur et al., 2008).
The regions which show a relationship between memory function and brain volume change are also affected by early AD pathology. In AD, the hippocampus, entorhinal cortex, and fusiform gyrus show accelerated volume loss prior to appearance of clinical symptoms (Armstrong et al., 2019). Additionally, recent in vivo PET imaging studies have shown that tau deposition, a hallmark of AD, accumulates in the inferior temporal (Johnson et al., 2016) and fusiform gyri (Schöll et al., 2016) in individuals with incident cognitive impairment. Tau deposition has also been observed in regions of the medial temporal lobe in cognitively normal older adults (Schöll et al., 2016).
4.2. Verbal fluency
Verbal fluency was measured by the average performance on tests of semantic and phonemic fluency. We observed that declines in verbal fluency were associated with widespread declines in regional brain volumes, including total brain and GM volumes, and total lobar and GM volumes of the frontal, temporal, and parietal lobes. Greater longitudinal declines in verbal fluency also were associated with greater ventricular enlargement and volumetric declines in 14 other ROIs: superior and middle frontal gyri, orbitofrontal cortex, superior parietal lobule, angular gyrus, precuneus, superior and inferior temporal gyri, hippocampus, parahippocampal gyrus, amygdala, fusiform gyrus, posterior cingulate, and thalamus.
Since we combined both semantic (category) and phonemic (letter) verbal fluency tasks in our definition of verbal fluency, we may be observing relationships associated with either of these tasks as well as relationships reflecting executive function, as verbal fluency tasks also tap executive function. In addition to the regional associations with executive function described below, Leong et al. (2017) and Mungas et al. (2005) reported relationships among letter and category fluency task performance, greater ventricular enlargement, and hippocampal volumetric decline. Letter fluency tasks have also been associated with increased activation in frontal, parietal, and temporal lobes (Parks et al., 1988) as well as the thalamus (Paulesu et al., 1997; Friston et al., 1993), suggesting a link between phonemic fluency and these VOIs cross-sectionally. Moreover, studies in AD patients found that the neural pathways involved in semantic or category-specific information processing included temporal regions associated with knowledge of animals, and frontal and parietal regions associated with the knowledge of inanimate objects (Damasio et al., 1996; Martin et al., 1995, 1996; Mummery et al., 1996; Silveri et al., 1991; Perani et al., 1995). Together, these studies provide support for widespread relationships between verbal fluency and brain tissue loss in aging and possibly preclinical AD.
4.3. Visuospatial ability
The tests used to define visuospatial ability involve spatial working memory, spatial orientation, and visuo-constructional skills. For the Clock Drawing Test, individuals must remember the location of the short and long hands of the clock for 3:25 and 11:15 before drawing these times (Jäncke and Jordan, 2007). Similarly, for the Card Rotations Test, an individual must be able to rotate shapes in their mind before deciding whether the test shape matches the designated target shape. We observed associations between declines in visuospatial ability and volume loss in the superior parietal lobule, precuneus, and inferior temporal gyrus. These results highlight some of the key circuitry patterns related to the dorsal stream of visual processing. The parieto-prefrontal and parieto-medial temporal pathways are responsible for spatial working memory and spatial navigation, respectively, as reported from various lesion and neuroimaging studies (Kravitz et al., 2011). Mental rotations performance has been associated with frontal, temporal, and occipital areas as well as the superior parietal lobe and intraparietal sulcus (Jäncke and Jordan, 2007). Change in some VOIs related to change in visuospatial ability were also associated with change in attention, consistent with the fact that visual attention is a key component of visuospatial ability. Deficits in visuospatial ability are common in early AD, indicating parietal lobe involvement, especially of the precuneus (Blair et al., 2006; Heinik et al., 2000). Besides correlations between change in visuospatial ability and both superior parietal lobule and precuneus, change in attention could be related to change in inferior temporal gyrus volume, given that neurons within the inferior temporal gyrus selectively respond to shape, color, and texture or combination of these features (Desimone et al., 1984; Tanaka et al., 1991).
Declines in visuospatial ability were associated with regional volume declines in the right hemisphere, consistent with greater right compared to left hemisphere processing of visuospatial stimuli. In a prior study, we observed right lateralized activation in the temporal lobe during processing of a figural memory task in which participants encode and recognize a series of pictures. During encoding, there was greater activation of the medial temporal lobe and inferior temporal association areas in the right hemisphere, which aligns with one of the three VOIs showing correlations with change in visuospatial ability. During recognition, we observed greater activation in the right inferior temporal gyrus (Beason-Held et al., 2005).
4.4. Attention
Attention was measured by performance on TMT-A. We found correlations between declines in TMT-A and volumetric declines in four VOIs: precuneus, middle and inferior temporal gyri, and fusiform gyrus. TMT-A has a visual scanning component (Sánchez-Cubillo et al., 2009), which requires sensorimotor and visuo-perceptual integration and integrity of the occipitotemporal visual pathway, which includes the precuneus, temporal association areas and the fusiform gyrus (Mishkin et al., 1983). The precuneus is also involved in focal attention processes (Petersen and Posner, 2012). While these associations did not survive FDR correction, the specific regional relationships can guide future studies as larger samples with longer longitudinal follow-ups become available.
4.5. Executive function
We assessed executive function by performance on the TMT-B, which measures higher order cognitive processes involved in attentional control, cognitive inhibition, working memory, and cognitive flexibility. Greater longitudinal decline in TMT-B was associated with greater ventricular enlargement and steeper volumetric declines in global GM, temporal and parietal GM, medial frontal cortex, superior parietal lobule, superior, middle, and inferior temporal gyri, fusiform gyrus, and posterior cingulate. Both lesion and neuroimaging studies have shown that intact frontal lobe function is essential for multiple aspects of executive function. Patients with frontal lobe lesions have impairments in planning and problem-solving, and greater activation in frontal regions has been observed in cognitively normal individuals during functional MRI (fMRI) tasks tapping executive function (Jurado and Rosselli, 2007). Within the frontal lobe, the medial prefrontal cortex has been associated with executive function, especially working memory, hierarchical organization of information processing (Rushworth et al., 2004; Alexander et al., 2007), and error awareness (Klein et al., 2007).
We also observed associations between declines in TMT-B and inferior temporal volume loss over time. Some studies suggest a role of the temporal lobe in executive function (Jurado and Rosselli, 2007). For instance, increased activation of the inferior temporal gyrus is observed with executive function performance (Bush et al., 1998), and task performance during sustained attention is associated with increased glucose metabolic rate in the lateral inferior temporal gyrus (Siegel Jr et al., 1995). Previous studies have also reported associations of abnormal posterior cingulate connectivity with impaired performance on tasks involving executive function, albeit cross-sectionally, in cognitively normal older adults (Andrews-Hanna et al., 2007; Damoiseaux et al., 2007; Sambataro et al., 2010; Prakash et al., 2012). Our finding involving ventricular enlargement is also consistent with a previous study using data from the Rotterdam Study (Breteler et al., 1994), which showed associations of poorer executive function with ventricular enlargement.
4.6. Strengths and limitations
Strengths of this study include the large and well-characterized sample with repeated neuropsychological and neuroimaging assessments with up to 23.1 years of follow-up. The harmonization of MRI volumes across different scanners and field strengths is an innovative approach that is being utilized in BLSA neuroimaging studies (Armstrong et al., 2019), and there is high (ICC ≥ 0.89) intra-individual stability over time. The use of bivariate linear mixed effects models to estimate the correlations between changes directly from the variance-covariance matrix of random effects is also a strength. This approach allows us to utilize all available longitudinal data, estimating the correlations while considering that the changes are latent constructs. Despite the numerous strengths, there are limitations to the study. The definitions of our cognitive domain scores were initially based on theoretical constructs. Confirmatory factor analysis validated our cognitive composites for the memory, verbal fluency, and visuospatial domains. Model fit was lower when the executive function (TMT-B and Digit Span Backward) and attention (TMT-A and Digit Span Forward) domains were defined theoretically from cognitive constructs, as compared to models where the TMT-A and TMT-B define one factor and the Digit Span Forward and Backward tests define another. This result highlights the challenge in developing and validating meaningful composite measures of cognitive constructs. Theoretically defined constructs yielded some domain scores that were not empirically homogeneous, while empirical approaches can yield factors that are test-specific and reflect multiple cognitive processes. For this reason, we restricted our final analysis of attention and executive function to single measures of TMT-A and TMT-B, respectively, which are widely used but do not capture the complexity of these cognitive constructs. In addition, our results are correlational. We cannot make causal inferences because we did not investigate the relative temporal sequence of cognitive changes relative to brain changes. We performed numerous correlational analyses and present these exploratory results at both the p < 0.05 and p < 0.01 levels and indicate those that survived FDR correction. Notably, all significant associations were in the hypothesized direction, supporting the fact that these findings do not reflect spurious correlations. Also, generalizability of the findings may be limited, as BLSA participants have higher levels of education and socioeconomic status than the general population. Lastly, to some extent these findings may reflect emerging pathologies in our older sample. Longer follow-up times will be necessary to evaluate this possibility and may yield additional associations between domains of cognitive function and regional brain volume loss.
4.7. Conclusions
Taken together, our findings show that changes in domain-specific cognitive performance are related to regionally specific changes in brain volumes, with declining performance associated with declining regional tissue volumes. These associations vary by cognitive domain and highlight specific brain-behavior relationships in aging among cognitively normal older individuals. Our findings caution against considering global cognition as a unitary construct, because patterns of underlying brain changes differ by cognitive construct and may have different mechanistic implications.
Supplementary Material
Acknowledgments
We would like to thank the participants and staff of the Baltimore Longitudinal Study of Aging, the cognitive and neuroimaging staff of the Laboratory of Behavioral Neuroscience, and the staff of the Johns Hopkins and National Institute on Aging MRI facilities. Also, we would like to thank Dr. Andrea T. Shafer for her additional expertise.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Role of Sponsor
The authors of this manuscript include employees of the Intramural Research Program of the National Institute on Aging, who participated in all aspects of the project.
Footnotes
CRediT authorship contribution statement
Nicole M. Armstrong: Conceptualization, Methodology, Writing - original draft, Writing - review & editing. Yang An: Conceptualization, Methodology, Writing - original draft, Writing - review & editing, Validation, Formal analysis, Data curation. John J. Shin: Conceptualization, Methodology, Writing - review & editing. Owen A. Williams: Conceptualization, Methodology, Writing - review & editing. Jimit Doshi: Methodology, Validation, Writing - review & editing, Data curation. Guray Erus: Methodology, Validation, Writing - review & editing, Data curation. Christos Davatzikos: Methodology, Validation, Writing - review & editing, Data curation. Luigi Ferrucci: Resources, Writing - review & editing, Funding acquisition. Lori L. Beason-Held: Conceptualization, Methodology, Writing - review & editing. Susan M. Resnick: Conceptualization, Methodology, Resources, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
The authors report no conflicts of interest.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.neuroimage.2020.117289.
References
- Alexander MP, Stuss DT, Picton T, Shallice T, Gillingham S, 2007. Regional frontal injuries cause distinct impairments in cognitive control. Neurology 68 (18), 1515–1523. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E, 2006. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol. Rev. 16 (1), 17–42. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, et al. , 2007. Disruption of large-scale brain systems in advanced aging. Neuron 56 (5), 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong NM, An Y, Beason-Held L, et al. , 2019. Predictors of neurodegeneration differ between cognitively normal and subsequently impaired older adults. Neurobiol. Aging 75, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association AP., 1987. Diagnostic and Statistical Manual of Mental Health Disorders (DSM-III-R). American Psychiatric Association. [Google Scholar]
- Beason-Held L, Golski S, Kraut MA, Esposito G, Resnick S, 2005. Brain activation during encoding and recognition of verbal and figural information in older adults. Neurobiol. Aging 26 (2), 237–250. [DOI] [PubMed] [Google Scholar]
- Benton AL., 1968. Differential behavioral effects in frontal lobe disease. Neuropsychologia 6 (1), 53–60. [Google Scholar]
- Bettcher BM, Mungas D, Patel N, et al. , 2016. Neuroanatomical substrates of executive functions: beyond prefrontal structures. Neuropsychologia 85, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair M, Kertesz A, Mcmonagle P, Davidson W, Bodi N, 2006. Quantitative and qualitative analyses of clock drawing in frontotemporal dementia and Alzheimer’s disease. J. Int. Neuropsychol. Soc. 12 (2), 159–165. [DOI] [PubMed] [Google Scholar]
- Breteler M, van Amerongen N, van Swieten J, et al. , 1994. Cognitive correlates of ventricular enlargement and cerebral white matter lesions on magnetic resonance imaging. The Rotterdam Study. Stroke 25 (6), 1109–1115. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, et al. , 2006. Regional white matter and neuropsychological functioning across the adult lifespan. Biol. Psychiatry 60 (5), 444–453. [DOI] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL, 1998. The counting Stroop: an interference task specialized for functional neuroimaging–validation study with functional MRI. Hum Brain Mapp. 6 (4), 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Chao LL, Studholme C, et al. , 2011. Brain atrophy associated with baseline and longitudinal measures of cognition. Neurobiol. Aging 32 (4), 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Chen KHM, Zheng H, et al. , 2009. Cognitive function and brain structure correlations in healthy elderly East Asians. Neuroimage 46 (1), 257–269. [DOI] [PubMed] [Google Scholar]
- Chen Y, Rex CS, Rice CJ, et al. , 2010. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. PNAS 107 (29), 13123–13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR, 1996. A neural basis for lexical retrieval. Nature 380 (6574), 499–505. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Scheltens P, Beckmann CF, et al. , 2007. Reduced resting-state brain activity in the “default network ” in normal aging. Cereb. Cortex 18 (8), 1856–1864. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B, 1987. California Verbal Learning Test, Research edition Psychological Corporation, New York, NY. [Google Scholar]
- Desimone R, Albright TD, Gross CG, Bruce C, 1984. Stimulus-selective properties of inferior temporal neurons in the macaque. J. Neurosci. 4 (8), 2051–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi J, Erus G, Ou Y, et al. , 2016. MUSE: multi-atlas region segmentation utilizing ensembles of registration algorithms and parameters, and locally optimal atlas selection. Neuroimage 127, 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P, 2012. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci. Biobehav. Rev. 36 (7), 1597–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erus G, Doshi J, An Y, Verganelakis D, Resnick SM, Davatzikos C, 2018. Longitudinally and inter-site consistent multi-atlas based parcellation of brain anatomy using harmonized atlases. Neuroimage 166, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB., 2010. Structural brain changes in aging: courses, causes and cognitive consequences. Revneuro 21 (3), 187–222. [DOI] [PubMed] [Google Scholar]
- Fletcher E, Gavett B, Harvey D, et al. , 2018. Brain volume change and cognitive trajectories in aging. Neuropsychology 32 (4), 436–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RSJ, 1993. Functional connectivity: the principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow Metab. 13 (1), 5–14. [DOI] [PubMed] [Google Scholar]
- Fuld PA. Psychological Testing in the Differential Diagnosis of the Dementias. Alzheimer’s Disease: Senile Dementia and Related Disorders. 1978;7:185–193. [Google Scholar]
- Gorbach T, Pudas S, Lundquist A, et al. , 2017. Longitudinal association between hippocampus atrophy and episodic-memory decline. Neurobiol. Aging 51, 167–176. [DOI] [PubMed] [Google Scholar]
- Grimm KJ, Ram N, Estabrook R, 2016. Growth Modeling: Structural Equation and Multilevel Modeling Approaches. Guilford Publications, New York, NY. [Google Scholar]
- Heinik J, Lahav D, Drummer D, Vainer-Benaiah Z, Lin R, 2000. Comparison of a clock drawing test in elderly schizophrenia and Alzheimer’s disease patients: a preliminary study. Int. J. Geriatr. Psychiatry 15 (7), 638–643. [DOI] [PubMed] [Google Scholar]
- SAS Institute, 2015. Base SAS 9.4 Procedures Guide. [Google Scholar]
- SAS Institute, Cary NC. Jäncke L, Jordan K, 2007. Functional neuroanatomy of mental rotation performance. In: Mast FW, Jäncke L (Eds.), Spatial Processing in Navigation, Imagery and Perception. Springer, New York, NY, pp. 183–207. [Google Scholar]
- Johnson KA, Schultz A, Betensky RA, et al. , 2016. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol. 79 (1), 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M, 2007. The elusive nature of executive functions: a review of our current understanding. Neuropsychol. Rev. 17 (3), 213–233. [DOI] [PubMed] [Google Scholar]
- Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M, 2007. Neural correlates of error awareness. Neuroimage 34 (4), 1774–1781. [DOI] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Mishkin M, 2011. A new neural framework for visuospatial processing. Nat. Rev. Neurosci. 12 (4), 217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong RLF, Lo JC, Sim SKY, et al. , 2017. Longitudinal brain structure and cognitive changes over 8 years in an East Asian cohort. Neuroimage 147, 852–860. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG, 1995. Discrete cortical regions associated with knowledge of color and knowledge of action. Science 270 (5233), 102–105. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV, 1996. Neural correlates of category-specific knowledge. Nature 379 (6566), 649–652. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E, 1984. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34 (7), 939. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA, 1983. Object vision and spatial vision: two cortical pathways. Trends Neurosci. 6, 414–417. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD, 2000. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognit. Psychol. 41 (1), 49–100. [DOI] [PubMed] [Google Scholar]
- Morrell CH, Brant LJ, Ferrucci L, 2009. Model choice can obscure results in longitudinal studies. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 64A (2), 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC., 1993. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43 (11), 2412–2414. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Wise RJS, 1996. Generating ‘tiger’ as an animal name or a word beginning with T: differences in brain activation. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 263 (1373), 989–995. [DOI] [PubMed] [Google Scholar]
- Mungas D, Harvey D, Reed B, et al. , 2005. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology 65 (4), 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe F, 1969. Missile Wounds of the Brain: A Study of Psychological Deficits. Oxford University Press, Oxford, England. [Google Scholar]
- Parks RW, Loewenstein DA, Dodrill KL, et al. , 1988. Cerebral metabolic effects of a verbal fluency test: a PET scan study. J. Clin. Exp. Neuropsychol. 10 (5), 565–575. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Goldacre B, Scifo P, et al. , 1997. Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. Neuroreport 8 (8), 2011–2016. [DOI] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Bettinardi V, et al. , 1995. Different neural systems for the recognition of animals and man-made tools. Neuroreport 6 (12), 1637–1641. [DOI] [PubMed] [Google Scholar]
- Petersen R, Smith G, Waring S, Ivnik R, Tangalos E, Kokmen E, 1999. Mild cognitive impairment: clinical characterization and outcome. JAMA Neurol. 56 (3), 303–308. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI., 2012. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 35, 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash RS, Heo S, Voss MW, Patterson B, Kramer AF, 2012. Age-related differences in cortical recruitment and suppression: Implications for cognitive performance. Behav. Brain Res. 230 (1), 192–200. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM., 2006. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci. Biobehav. Rev. 30 (6), 730–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM., 1958. Validity of the Trail Making Test as an indicator of organic brain damage. Percept. Mot. Skills 8, 271–276. [Google Scholar]
- Resnick S, Pham D, Kraut M, Zonderman A, Davatzikos C, 2003. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J. Neurosci. 23 (8), 3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau I, Salmon DP, Butters N, Kennedy C, McGuire K, 1992. Quantitative and qualitative analyses of clock drawings in Alzheimer’s and Huntington’s Disease. Brain Cogn. 18, 70–87. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM, 2004. Action sets and decisions in the medial frontal cortex. Trends Cogn. Sci. 8 (9), 410–417. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, et al. , 2010. Age-related alterations in default mode network: impact on working memory performance. Neurobiol. Aging 31 (5), 839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Cubillo I, Periáñez JA, Adrover-Roig D, et al. , 2009. Construct validity of the trail making test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 15 (3), 438–450. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, et al. , 2008. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci. 105 (46), 18035–18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöll M, Lockhart Samuel N, Schonhaut Daniel R, et al. , 2016. PET imaging of tau deposition in the aging human brain. Neuron 89 (5), 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shock N, Greulich R, Andres R, et al. , 1984. Normal Human Aging: The Baltimore Longitudinal Study of Aging. US Government Printing Office, Washington, DC. [Google Scholar]
- Siegel BV Jr, Nuechterlein KH, Abel L, Wu JC, Buchsbaum MS, 1995. Glucose metabolic correlates of continuous performance test performance in adults with a history of infantile autism, schizophrenics, and controls. Schizophr. Res. 17 (1), 85–94. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Daniele A, Giustolisi L, Gainotti G, 1991. Dissociation between knowledge of living and nonliving things in dementia of the Alzheimer type. Neurology 41 (4), 545. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Saito H-a, Fukada Y, Moriya M, 1991. Coding visual images of objects in the inferotemporal cortex of the macaque monkey. J. Neurophysiol. 66 (1), 170–189. [DOI] [PubMed] [Google Scholar]
- van Petten C, Plante E, Davidson PSR, Kuo TY, Bajuscak L, Glisky EL, 2004. Memory and executive function in older adults: relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia 42 (10), 1313–1335. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 1981. Wechsler Adult Intelligence Scale-Revised, 1. Psychological Corporation, New York, NY. [Google Scholar]
- Wilson JR, De Fries JC, Mc Clearn GE, Vandenberg SG, Johnson RC, Rashad MN, 1975. Cognitive abilities: use of family data as a control to assess sex and age differences in two ethnic groups. Int. J. Aging Hum. Dev. 6 (3), 261–276. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Widaman K, Mungas D, Reed B, Weiner MW, Chui HC, 2007. Memory in the aging brain: doubly dissociating the contribution of the hippocampus and entorhinal cortex. Hippocampus 17 (11), 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad MA, Lundervold AJ, Wehling E, et al. , 2009. Hippocampal volumes are important predictors for memory function in elderly women. BMC Med. Imaging 9 (1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the BLSA are available on request by proposal submission through the BLSA website (blsa.nih.gov). All requests are reviewed by the BLSA Data Sharing Proposal Review Committee. In terms of code availability, all code was written in terms of the procedure NLMIXED in SAS 9.4 (Institute, 2015).