Abstract
Photodynamic therapy (PDT) has historically been used as a means to treat cancerous tumors but has recently been used to kill bacterial cells through the use of targeted photosensitizers. PDT is a potential adjunct to scaling and root planing in the treatment of periodontal disease. However, the effectiveness of porphyrin derivatives against microorganisms has been limited because some gram-negative bacteria are refractory to photodynamic treatment with these agents. We have designed a porphyrin derivative conjugated to a pentalysine moeity that endows the molecule with activity against gram-positive and gram-negative bacteria. Whereas the porphyrin, chlorin e6, showed in vitro activity against a limited spectrum of bacteria, chlorin e6 conjugated to pentalysine showed in vitro activity against all oral microorganisms tested, including Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans, Bacteroides forsythus, Campylobacter rectus, Eikenella corrodens, Fusobacterium nucleatum subsp. polymorphum, Actinomyces viscosus, and the streptococci. Potent antimicrobial activity (≥5-log-unit reduction in the numbers of CFU per milliliter) was retained in the presence of up to 25% whole sheep blood. The use of potent, selective agents such as this chlorin e6–pentalysine conjugate to more effectively reduce the pathogenic bacteria in the periodontal pocket may be a significant tool for the treatment of periodontal disease.
Periodontal disease is caused by the overgrowth of oral pathogens such as the gram-negative anaerobe Porphyromonas gingivalis, which results in the formation of periodontal pockets surrounding the affected tooth. Successful periodontal therapy must restrain the growth of these infectious agents. Treatment of periodontal disease relies primarily on the use of scaling and root planing procedures but has increasingly been augmented with antibiotic therapy (7, 14). Despite these measures, infection recurs in a significant number of patients (22), necessitating additional treatment. Furthermore, the widespread use of antibiotics may lead to drug resistance in the bacterial pathogens responsible for infection (21). In view of this situation, an alternative treatment that is capable of killing periodontal pathogens in situ subsequent to scaling and root planing and that does not promote the selection of resistant organisms would be highly desirable.
Photodynamic therapy (PDT) is a process in which the activation of photoreactive compounds (photosensitizers) by light energy results in the production of singlet oxygen and free radicals that are cytotoxic (6). Due to the highly reactive nature of the radicals formed through this process, activity is confined to their immediate environment. Thus, activity is selective and dependent on the delivery of the photosensitizer to the target (3). In recent years, PDT has been used successfully in the treatment of certain tumors (6).
Research in a number of laboratories has demonstrated the potential of PDT as a treatment for localized microbial infections (8, 20, 24). Application of PDT to periodontal infection could prove to be a valuable adjunct to mechanical procedures, provided that the photosensitizer has broad-spectrum activity against bacterial pathogens and selectivity for procaryotic cells. The susceptibilities of both gram-positive and gram-negative oral bacteria to cyanine photosensitizers have been demonstrated (24). Cyanine photosensitizers have also been observed to have activity against oral streptococci present in plaque samples (15, 26) and in a biofilm (25).
In previous studies with porphyrin photosensitizers, gram-positive bacteria were found to be susceptible to the activated compounds (12). However, activity against gram-negative bacteria was limited to instances in which outer membrane permeability was achieved through the use of membrane-active agents (13, 19). Analysis of the antibacterial activities of a series of porphyrin photosensitizers revealed that net charge influences the spectrum of activity (9, 10). This suggested that it may be possible to broaden the spectrum of activity by combining a moiety with affinity for bacterial cell surfaces with a photoreactive agent. This hypothesis was initially examined by using a conjugated photosensitizer consisting of a polylysine mixture (average size, 20 residues) and chlorin e6, a porphyrin derivative photosensitizer (18). The positively charged polylysine could potentially facilitate binding to the negatively charged bacterial surface, thus allowing the photosensitizer to selectively target bacterial cells. This “targeted photosensitizer” was active against the periodontal pathogen Porphyromonas gingivalis, whereas the unconjugated photosensitizer was less active against P. gingivalis and was more cytotoxic to mammalian cells.
In the present study we report on the activity of a defined chlorin e6 photosensitizer, designated ce6-5K, that is composed of a lysine pentamer linked covalently through the N terminus to the C-20 carboxymethyl group of chlorin e6. We demonstrate that the addition of the lysine pentapeptide increases the spectrum of activity against periodontal pathogens and retains a strong killing effect.
MATERIALS AND METHODS
Strains and growth conditions.
All the strains and media used in this study are listed in Table 1. All media except FF medium were obtained from Binax/NEL (Waterville, Maine) (Table 1). Anaerobic organisms (P. gingivalis, Bacteroides forsythus, Eikenella corrodens, Campylobacter rectus, Actinobacillus actinomycetemcomitans, and Fusobacterium nucleatum) were cultured in an anaerobic chamber with an atmosphere composed of nitrogen-hydrogen-carbon dioxide (80:10:10) at 35°C. All the streptococcal strains and Actinomyces viscosus were grown at 35°C in a Brewer jar with a GasPak carbon dioxide generator (Becton Dickinson, Cockeysville, Md.), which created an aerobic atmosphere with approximately 10% carbon dioxide.
TABLE 1.
Oral bacteria tested
| Organism | Strain | Source | Mediuma |
|---|---|---|---|
| Porphyromonas gingivalis | ATCC 33277 | ATCCb | HK |
| Porphyromonas gingivalis | 7-1-4 (patient isolate) | A. Tanner | HK |
| Bacteroides forsythus | ATCC 43037 | ATCC | NAM |
| Fusobacterium nucleatum subsp. polymorphum | ATCC 10953 | ATCC | TSBY |
| Campylobacter rectus | ATCC 33238 | ATCC | FF |
| Eikenella corrodens | ATCC 23834 | ATCC | TSBY |
| Actinobacillus actinomycetemcomitans | ATCC 29523 | ATCC | Chocolate |
| Actinomyces viscosus | ATCC 15987 | ATCC | BHI |
| Streptococcus sobrinus | ATCC 6715 | ATCC | TSBY |
| Streptococcus mitis | ATCC 15914 | ATCC | TSBY |
| Streptococcus oralis | ATCC 35037 | ATCC | TSBY |
| Streptococcus mutans | ATCC 25175 | ATCC | TSBY |
HK, hemin-menadione; NAM, N-acetylmuramic acid; TSBY, tryptic soy brain heart infusion yeast extract; FF, Fumarate and formate medium (brain heart infusion agar, 52 g/liter; yeast extract, 10 g/liter; sodium formate, 2 g/liter; disodium fumarate, 4.1 g/liter; hemin, 5 g/liter; 50 ml of sheep red blood cells [pH 7.5]); Chocolate, chocolate agar; BHI, brain heart infusion agar.
ATCC, American Type Culture Collection.
Photosensitizers.
Chlorin e6 (ce6) was purchased from Porphyrin Products (Logan, Utah). The chlorin e6–(Lys)5–OH (ce6-5K) conjugate used in the study was custom synthesized by Multiple Peptide Systems (San Diego, Calif.). The final product (ce6-5K) was more than 99% pure, as assessed by analytical high-pressure liquid chromatography. The identity of the product was verified by mass spectrometry and amino acid analysis.
Laser apparatus.
Light for photoactivation was generated with a laser diode (250-670-9mm-SMA; Polaroid Inc., Norwood, Mass.) with a central wavelength of 662 nm. The laser light was coupled into an SMA connectorized 200-μm quartz fiber with an attached graded-index (GRIN) microlens (Rare Earth Medical, Inc., West Yarmouth, Mass.). The laser possessed a laser spectral stability of ±2 nm with an output power stability of ±10 mW. Power measurements were measured with a Lasermate/D power meter with a Lasermate 3 detector head (Coherent, Inc., Auburn, Calif.). Distance adjustments between the lens and the illuminated plates created fields of irradiation with appropriate dimensions and power densities.
PDT assay.
The assay used to demonstrate photosensitizer antimicrobial activity was adapted from the work of Wilson (23) with the following modifications. Each strain was cultured on plates anaerobically (approximately 7 days) or aerobically with CO2 (24 h). The cells were then suspended in one-quarter-strength phosphate-buffered saline buffer (2 g of NaCl per liter, 0.5 g of KCl per liter, 0.36 g of Na2HPO4 per liter, 0.06 g of KH2PO4 per liter) to an A560 of 0.01, or approximately 107 CFU/ml. The ce6 or ce6-5K photosensitizer containing EDTA was added to 1 ml of a cell suspension to give final concentrations of 5 μM photosensitizer and 2.5 mM EDTA (pH 7.0). The photosensitizer was incubated with the cell suspension for 2 min to allow binding and/or uptake. Two hundred microliters of the cell suspension was placed in a 48-well microtiter plate for light activation. The samples were exposed to 662-nm light from above with a fluence of 15 J/cm2 at a fluence rate of 100 mW/cm2 and a time of illumination of 150 s (unless noted otherwise). The irradiated cells were serially diluted in buffer and were plated on the appropriate medium. The numbers of CFU were enumerated after adequate incubation. Sheep erythrocytes were obtained from Binax/NEL (Waterville, Maine).
RESULTS
Determination of optimal photosensitizer concentration.
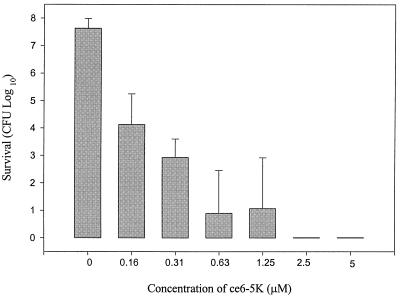
In order to determine the optimal concentration of photosensitizer required to effectively kill the oral pathogen P. gingivalis, various concentrations of the photosensitizer ce6-5K were tested. Total killing of P. gingivalis ATCC 33277 was observed with concentrations of ce6-5K that were equal to or greater than 2.5 μM (3.2 μg/ml) (Fig. 1). The killing activity of ce6-5K was diminished as the drug concentration was reduced over the range of 1.25 to 0.16 μM (1.6 to 0.2 μg/ml) (Fig. 1). When the P. gingivalis cells were incubated with the photosensitizer and in the absence of light, there was no loss of viability. Similarly, when the cells were irradiated in the absence of the photosensitizer, there was no loss of viability. Only when cells were incubated with the photosensitizer and irradiated was killing observed.
FIG. 1.
Effect of photosensitizer concentration on PDT killing of P. gingivalis ATCC 33277. The photosensitizer ce6-5K concentration was varied, as indicated. The values are the averages of three independent experiments, and the error bars represent standard deviations. Assays performed with 5 μM ce6, the highest photosensitizer concentration tested, demonstrated an average P. gingivalis survival 5.25 ± 1.53 log10 (standard deviation) greater than the survival demonstrated with the same concentration of ce6-5K (data not shown).
Determination of minimum effective irradiation.
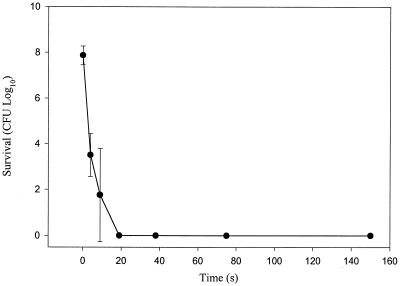
The minimum energy requirements for the killing of P. gingivalis were established by varying the irradiation time, which is directly proportional to the total energy delivered. Complete killing of P. gingivalis cells was observed when the cells were irradiated for just 19 s or 1.9 J in the presence of 5 μM ce6-5K. Therefore, the standard treatment time of 150 s provides an eightfold excess of energy for complete killing of P. gingivalis (Fig. 2).
FIG. 2.
Effect of total energy delivered on killing of P. gingivalis ATCC 33277 with the photosensitizer ce6-5K. Cell suspensions were treated with 5 μM ce6-5K and were irradiated for times ranging from 0 to 150 s. The values are the averages of three independent experiments, and the error bars represent standard deviations. Assays performed with the same concentration of ce6 at the longest irradiation time, 150 s, demonstrated an average P. gingivalis survival 5.25 ± 1.53 log10 (standard deviation) greater than the survival obtained after 150 s of irradiance with ce6-5K (data not shown).
Activity against oral pathogens.
The activities of the photosensitizers ce6-5K and ce6 were examined against a spectrum of aerobic and anaerobic oral pathogens (Table 2). The ce6-5K photosensitizer was extremely effective in killing all the oral bacteria tested, showing at least 6 logs of killing of all the organisms listed in Table 2. In contrast, the ce6 compound lacking the pentalysine moiety had a much narrower spectrum of activity. The photosensitizer ce6 was effective in killing the streptococci, A. viscosus, and the anaerobic bacterium P. gingivalis ATCC 33277, but the patient isolate, strain P. gingivalis 7-1-4, was more refractory to PDT treatment with ce6. PDT treatment with ce6 also had little or no effect on the gram-negative oral pathogens A. actinomycetemcomitans, C. rectus, E. corrodens, F. nucleatum subsp. polymorphum, and B. forsythus.
TABLE 2.
Susceptibilities of oral bacteria to photosensitizers ce6 and ce6-5Ka
| Organism | Log10 survival of
|
||
|---|---|---|---|
| Untreated control | With ce6 treatment | With 5K-ce6 treatment | |
| P. gingivalis ATCC 33277 | 7.7 | 4.0 | <0.3b |
| P. gingivalis 7-1-4 | 7.8 | 6.5 | <0.3 |
| B. forsythus ATCC 43037 | 7.4 | 7.1 | <0.3 |
| F. nucleatum subsp. polymorphum ATCC 10953 | 7.0 | 6.6 | <0.3 |
| C. rectus ATCC 33238 | 7.5 | 6.7 | <0.3 |
| E. corrodens ATCC 23834 | 7.1 | 6.6 | <0.3 |
| A. viscosus ATCC 15987 | 7.5 | <0.3c | <0.3 |
| S. sobrinus ATCC 6715 | 6.9 | 1.1 | 0.3 |
| S. mitis ATCC 15914 | 7.3 | <0.3 | <0.3 |
| S. oralis ATCC 35037 | 6.5 | <0.3 | <0.3 |
| S. mutans ATCC 25175 | 7.2 | 1.2 | 0.6 |
| A. actinomycetemcomitans ATCC 29523 | 7.1 | 6.7 | <0.3 |
All strains except P. gingivalis 7-1-4 were obtained from the American Type Culture Collection. Strain 7-1-4 was provided by A. Tanner. The values are the averages of at least two independent experiments except when noted otherwise.
Value is the average of four independent experiments.
The lower limit of detectable bacterial survival.
Activity of ce6-5K was retained in presence of whole blood.
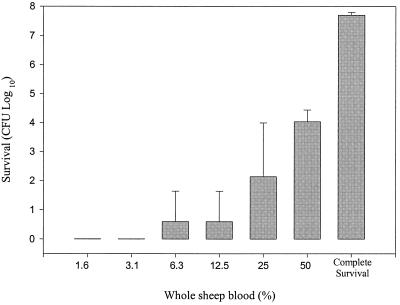
PDT is planned as an adjunct therapy to scaling and root planing, which is a procedure expected to result in some bleeding. It was therefore important to determine the interference of whole blood with the activity of the ce6-5K compound. P. gingivalis ATCC 33277 cells were suspended in 1× phosphate-buffered saline buffer, and then whole sheep blood and ce6-5K (5 μM) were added to the suspension. Figure 3 shows that the PDT procedure resulted in substantial killing activity even in 50% whole blood. In contrast, 1% whole blood was sufficient to completely inhibit the killing effects of ce6 (lacking the pentalysine tail) (data not shown). Thus, the ce6-5K photosensitizer, in contrast to the ce6 compound, showed the capacity to kill oral bacteria in the presence of whole blood.
FIG. 3.
Effect of whole sheep blood on killing activity of ce6-5K (5 μM). Blood was first added to the cell suspension, followed by the addition of the photosensitizer. The values are the averages of three independent experiments, and the error bars represent standard deviations. Complete survival is the maximum survival possible by cells not treated with photosensitizer. In comparison, the photosensitizer ce6 was completely inactive in the presence of 1% sheep blood.
DISCUSSION
Two photosensitizers, ce6 and ce6-5K, were tested for their killing activities against oral bacteria exposed to laser light. The addition of the pentalysine moiety, which adds four net charges to the ce6 porphyrin, endows the ce6-5K molecule with strong killing activity against a broad spectrum of oral bacterial cells (Table 2). Whereas ce6 showed some activity as a photosensitizer against gram-positive organisms, only the ce6-5K molecule, in combination with light, consistently killed both the gram-negative and gram-positive organisms. This difference in spectra of activity is particularly important because the gram-negative anaerobic microorganisms, such as P. gingivalis and B. forsythus, are closely associated with periodontal disease (5). In addition, A. actinomycetemcomitans, a gram-negative facultative bacterium, plays a crucial role in juvenile periodontitis (1, 11).
Previous studies with this class of porphyrin photosensitizers containing a peptide tail composed of a mixture of lysine multimers covalently bonded to ce6 showed little activity against cultured mammalian cells (17). Similar results were obtained with the ce6-5K compound (data not shown). Thus, the combination of broad-spectrum activity against dental pathogens and little or no activity against mammalian cells suggests that ce6-5K is a good potential lead compound to be carried forward for PDT as part of dental therapy.
It is likely that the addition of the pentalysine moiety of ce6-5K results in a photosensitizer that has a stronger affinity for binding to bacterial surfaces than to mammalian cells. Singlet oxygen and free radicals that are generated by irradiation with laser light are very short-lived. Consequently, the photosensitizer must be in close proximity to the target bacterium (2). The ce6 compound (lacking the pentalysine tail) exhibited activity against a limited spectrum of microorganisms. Our current hypothesis is that ce6 is active as a photosensitizer against gram-negative bacteria, such as P. gingivalis, that have an active porphyrin uptake system (4). In keeping with this idea, ce6 lost its activity in the presence of 1% whole blood, which probably contains ample porphyrins (e.g., heme) that could compete with ce6 for uptake (data not shown). In contrast, ce6-5K retained strong activity even in the presence of 50% whole blood (Fig. 3), suggesting that ce6-5K has a distinct binding mechanism.
In vivo treatment of periodontal disease will require administration of ce6-5K into the periodontal pocket by means of a cannula, with subsequent insertion of a diffuser-tipped fiber-optic laser probe for light delivery. The relatively short period of illumination time required for killing (Fig. 2) and the strong activity of ce6-5K in the presence of high concentrations of whole blood (Fig. 3) suggest that the in vivo requirements for irradiance and photosensitizer concentration may be similar to those required in vitro. However, the low level of toxicity exhibited to date by chlorin photosensitizers and the low power output of the diode laser would permit increases in either parameter, if necessary, to obtain optimal efficacy in vivo.
The in vitro activity of ce6-5K against the key dental pathogens shows that it may be a potentially valuable new tool for the treatment of periodontitis as an adjunct to scaling and root planing. It is encouraging that photofrin (a porphyrin derivative) has been approved by the U.S. Food and Drug Administration for cancer treatment (2) and that subsequent ce6 derivatives have been tested in clinical trials without significant side effects (16). In addition, PDT with potent and selective agents such as ce6-5K could be more broadly useful in endodontal work and implants, in which bactericidal action and sterilization are important considerations.
ACKNOWLEDGMENTS
We thank Mark Maiden and Anne Tanner for use of their facilities, strains, and helpful discussions. We also thank Tayyaba Hasan for insight and assistance.
REFERENCES
- 1.Baehni P, Tsai C C, McArthur W P, Hammond B F, Taichman N S. Interaction of inflammatory cells and oral microorganisms. VIII. Detection of leukotoxic activity of a plaque-derived gram-negative microorganism. Infect Immun. 1979;24:233–243. doi: 10.1128/iai.24.1.233-243.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dougherty T J, Gomer C J, Henderson B W, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gal D. Hunt for singlet oxygen under in vivo conditions. Biochem Biophys Res Commun. 1994;202:10–16. doi: 10.1006/bbrc.1994.1886. [DOI] [PubMed] [Google Scholar]
- 4.Genco C A, Simpson W, Forng R Y, Egal M, Odusanya B M. Characterization of a Tn4351-generated hemin uptake mutant of Porphyromonas gingivalis: evidence for the coordinate regulation of virulence factors by hemin. Infect Immun. 1995;63:2459–2466. doi: 10.1128/iai.63.7.2459-2466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haffajee A D, Socransky S S. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 6.Hassan T, Parrish J A. Photodynamic therapy of cancer. In: Holland J F, Frei E I, Bast R J C, Kufe D W, Morton D L, Weisehselbaum R R, editors. Cancer medicine. 4th ed. Baltimore, Md: The William & Wilkins Co.; 1996. pp. 739–751. [Google Scholar]
- 7.Killoy W J. Chemical treatment of periodontitis: local delivery of antimicrobials. Int Dent J. 1998;48(3 Suppl. 1):305–315. doi: 10.1111/j.1875-595x.1998.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 8.Malik Z, Hanania J, Nitzan Y. Bactericidal effects of photoactivated porphyrins—an alternative approach to antimicrobial drugs. J Photochem Photobiol B. 1990;5:281–293. doi: 10.1016/1011-1344(90)85044-w. [DOI] [PubMed] [Google Scholar]
- 9.Merchat M, Bertolini G, Giacomini P, Villanueva A, Jori G. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J Photochem Photobiol B. 1996;32:153–157. doi: 10.1016/1011-1344(95)07147-4. [DOI] [PubMed] [Google Scholar]
- 10.Merchat M, Spikes J D, Bertoloni G, Jori G. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. J Photochem Photobiol B. 1996;35:149–157. doi: 10.1016/s1011-1344(96)07321-6. [DOI] [PubMed] [Google Scholar]
- 11.Meyer D H, Fives-Taylor P M. The role of Actinobacillus actinomycetemcomitans in the pathogenesis of periodontal disease. Trends Microbiol. 1997;5:224–228. doi: 10.1016/S0966-842X(97)01055-X. [DOI] [PubMed] [Google Scholar]
- 12.Nitzan Y, Wexler H M, Finegold S M. Inactivation of anaerobic bacteria by various photosensitized porphyrins or by hemin. Curr Microbiol. 1994;29:125–131. doi: 10.1007/BF01570752. [DOI] [PubMed] [Google Scholar]
- 13.Nitzan Y, Malik Z, Eherenberg B. Photosensitization of microbial cells. In: Riklis E, editor. Photobiology: the science and its applications. New York, N.Y: Plenum Press; 1991. pp. 815–820. [Google Scholar]
- 14.Rams T E, Slots J. Local delivery of antimicrobial agents in the periodontal pocket. Periodontol 2000. 1996;10:139–159. doi: 10.1111/j.1600-0757.1996.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar S, Wilson M. Lethal photosensitization of bacteria in subgingival plaque from patients with chronic periodontitis. J Periodontal Res. 1993;28:204–210. doi: 10.1111/j.1600-0765.1993.tb01070.x. [DOI] [PubMed] [Google Scholar]
- 16.Sickenberg M, Schmidt-Erfurth U, Miller J M. Preliminary results of photodynamic therapy for choroidal neovascularization in pathologic myopia, ocular histoplasmosis syndrome and idiopathic causes within a phase I/II study. Investig Ophthalmol Vis Sci. 1997;38:92. doi: 10.1001/archopht.118.3.327. [DOI] [PubMed] [Google Scholar]
- 17.Soukos N S, Hamblin M R, Hasan T. The effect of charge on cellular uptake and phototoxicity of polylysine chlorin (e6) conjugates. Photochem Photobiol. 1997;65:723–729. doi: 10.1111/j.1751-1097.1997.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 18.Soukos N S, Ximenez-Fyvie L A, Hamblin M R, Socransky S S, Hasan T. Targeted antimicrobial photochemotherapy. Antimicrob Agents Chemother. 1998;42:2595–2601. doi: 10.1128/aac.42.10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) J Antimicrob Chemother. 1998;42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 21.Walker C B. The acquisition of antibiotic resistance in the periodontal microflora. Periodontol 2000. 1996;10:79–88. doi: 10.1111/j.1600-0757.1996.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 22.Wasserman B, Hirschfeld L. The relationship of initial clinical parameters to the long-term response in 112 cases of periodontal disease. J Clin Periodontol. 1988;15:38–42. doi: 10.1111/j.1600-051x.1988.tb01552.x. [DOI] [PubMed] [Google Scholar]
- 23.Wilson M. Bactericidal effect of laser light and its potential use in the treatment of plaque-related diseases. Int Dent J. 1994;44:181–189. [PubMed] [Google Scholar]
- 24.Wilson M. Photolysis of oral bacteria and its potential use in the treatment of caries and periodontal disease. J Appl Bacteriol. 1993;75:299–306. doi: 10.1111/j.1365-2672.1993.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 25.Wilson M, Burns T, Pratten J. Killing of Streptococcus sanguis in biofilms using a light-activated antimicrobial agent. J Antimicrob Chemother. 1996;37:377–381. doi: 10.1093/jac/37.2.377. [DOI] [PubMed] [Google Scholar]
- 26.Wilson M, Burns T, Pratten J, Pearson G J. Bacteria in supragingival plaque samples can be killed by low-power laser light in the presence of a photosensitizer. J Appl Bacteriol. 1995;78:569–574. doi: 10.1111/j.1365-2672.1995.tb03101.x. [DOI] [PubMed] [Google Scholar]