Abstract
Witches were popularly imagined as older women (above middle age), with large warty noses, whose clothes were shabby and used pointy hats. They are usually associated with a cauldron and the presence of a black cat that accompany them in this imagery projection. The fact is that, historically, many women have suffered countless physical and emotional acts of violence, for which different analysis can be made from the perspective of the Human Sciences. Of the historical narratives that deal with this violence, the Salem witch trials stand out as the biggest witch hunt in history, where a series of hearings and trials of people accused of witchcraft took place in colonial Massachusetts, between February 1693 and May of 1694, episodes in which more than two hundred people were accused of practices of heresy. However, it is necessary to recognize that many of these women considered witches were, in fact, profound connoisseurs of plant species with biological properties, even though there was not precise information about the active compounds of these plants. With the development of characterization techniques for organic compounds, like spectrometric and spectroscopic analyses, most of the metabolites present in the “potions” had their structures elucidated, allowing a more appropriate knowledge of the possible metabolic pathways. In this article, we report a study of the structure–activity relationships for two of the most famous potions in history: the sleep potion and the love potion, with the aim of presenting new discussions within the scope of medicinal chemistry that can contribute to the process of science diffusion.
In this review we present the bioactive compounds of two classic potions: love potion and sleeping potion. This review also includes details on the presence of these bio-active molecules in current medicine and their effects.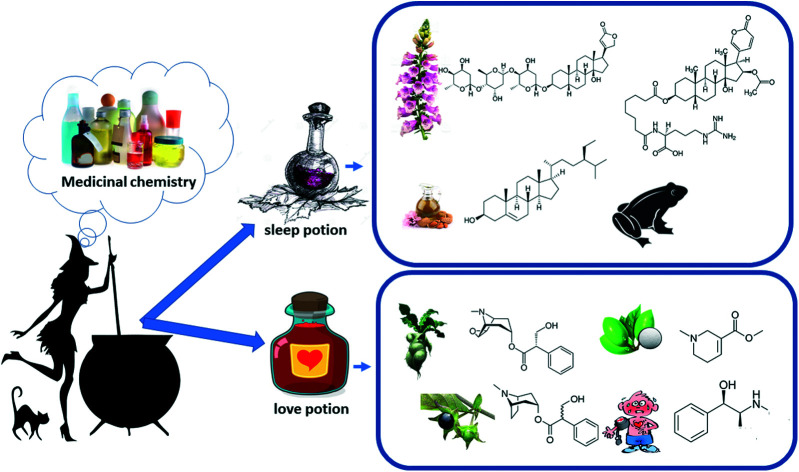
Introduction
“Thou shalt not suffer a witch to live” – Exodus 22.18. Persecution and assassination of people, mostly women, accused of witchcraft marked our history and influenced our recent culture. Despite many studies in the field, it is difficult to estimate the number of women (mainly elderly and poor) who were burned, hanged, and tortured accused of witchcraft in Europe. Estimates can range from 40 thousand to millions. These persecutions were attributed to a complexity of issues including gender, politics, and religion.1–4
Beliefs in witchcraft and magic were always part of the popular imagination, even before the episodes involving witch hunts from the late Middle Ages.5 Prior to 1350, witchcraft was seen as a way of controlling nature in favour of the person's own interest. However, witchcraft was judged as a crime only when it resulted in some form of harm.6
In the 15th century, Europe went through two outbreaks of persecution and witch hunts, with a peak between 1600 and 1650.7 The most striking feature of this period was the targeting of wise women, endowed with knowledge, especially those related to plants. Thus, from the 16th century onwards, the stereotype of witchcraft appears as a demonic practice and woman were seen as the main agent of the devil.8 Of innumerable and diverse episodes of violence, the Salem witch trials stand out. These trials took place in 1693 and became a landmark in terms of attempts to eliminate any practice considered heretical,9 as it will be discussed further in this review.
The mid-seventeenth century experienced a change. The tradition of magic still strongly influenced the popular class, but no longer the scholar's class.10 The Scientific Revolution was happening at that time, motivated by the work of Copernicus, the discoveries of Galileo and Kepler, and the triumph of mechanistic philosophy.11 A separation between spirit and matter happened at that period, which was a fundamental step in the development of science, as it left the strength and authority of faith intact while it allowed experimentation to be used in research.12 However, this transition period from the magical to the scientific universe was not a smooth process.
The history of the Salem witches and ergotism
February of 1962 was marked by the first accusation of witchcraft in the city of Salem. The city was experiencing some anomalies as citizens were showing change in behaviour, such as rolling on the floor and manifesting changes in their state of consciousness.13 Allegations initially targeted girls and teenagers who had some degree of relationship with the city's pastor, resulting in accusations of witchcraft and pact with the devil.14 Despite the behavioral changes being attributed to demonic actions at the time, in 1976, Linda Caporel published one of the best accepted hypotheses to explain the events, known as ergotism.15
Ergotism or ergot poisoning, is a condition caused by rye spurs (Claviceps purpurea), a type of fungus found in cereals, mainly rye, widely used at that time in Massachusetts.16 Nowadays is easy to understand how contamination of many villagers with ergotism occurred, considering the cold, humid climate of Massachusetts plus the long-term grain storage process which were common practices in the Middle Ages.17 Ingestion of contaminated flour, even in the presence of small amounts of Claviceps purpure, is enough to cause ergotism. The poor elderly women who lived on the surroundings of the community and survived on their herbal knowledge, did not consumed the village's flour since they couldn't afford it. Therefore, they were not contaminated by the fungus, making them vulnerable to accusations of witchcraft.
Alkaloids are nitrogenous heterocyclic compounds produced in the secondary metabolism of several species (including Claviceps purpurea).18 Ingestion can result in a variety of symptoms that change according to the concentration, including seizures, apoplectic attack, diarrhea, manic behavior, hallucinations, limb distortion, vomiting, spasms, tingling, numbness of hands and feet and a burning sensation which becomes extremely painful as it gangrenes.19
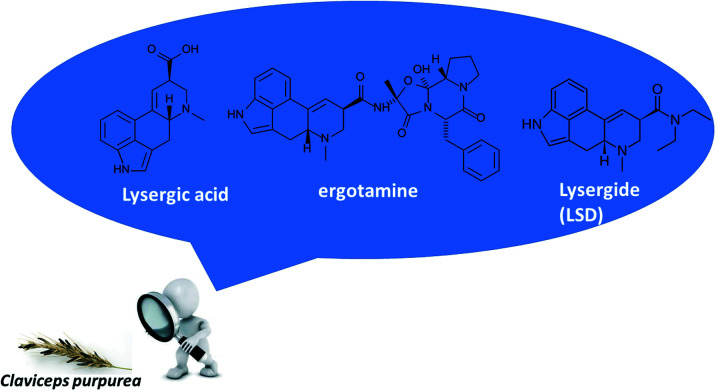
Claviceps purpurea produces a variety of alkaloids depending on external factors, such as the production region, harvest conditions, in addition to seasonal conditions.20 However, the most found metabolites and present in greater quantities are ergot alkaloids, such as ergotamine and lysergic acid diethylamide (LSD-25, currently known as LSD), both derivatives of lysergic acid whose hydroxyl group is replaced by another functional group (Fig. 1).21 Ergotamine has a peptide group derived from the amino acids l-alanine, l-phenylalanine, and l-proline, while in LSD the hydroxyl is replaced by a diethylamine.22
Fig. 1. Structures for lysergic acid, ergotamine, and LSD from Claviceps purpurea.
LSD was first synthesized at Sandoz's laboratories, and its hallucinogenic properties were unknown. Like the alkaloids found in cocaine, although toxic and dangerous, they have a long history of therapeutic use, in wich ergotines continue to play a role in medicine.23 This group of chemical substances had, and still has, a great impact on human history because alkaloids are physiologically active. Thus, a greater understanding of this class of compounds and some of its implications in history is significant.
This review does not aim to discuss new paradigms in the development of the area of medicinal chemistry, however, it discusses a topic of great popularity from a scientific perspective, which emphasizes the biological mechanisms linked to the components of two famous potions: the sleep potion and love potion.
Witches, potions and medicinal chemistry
Among the women accused of witchcraft, many were herbalists proficient in using local plants to cure illnesses and alleviate pain, as well as providing love potions, making incantations, and undoing witchcraft. The healing power that some herbs possess was believed to be magical at the time, as well as the incantations and rituals surrounding the other ceremonies they performed. If the successful cure of diseases is related to the use of these herbal remedies, some of which are still used nowadays in the form of herbal medicines, we can infer those herbalists who had the most mastery and knowledge of the effects of herbs on the organism were the most labeled as witches.24,25
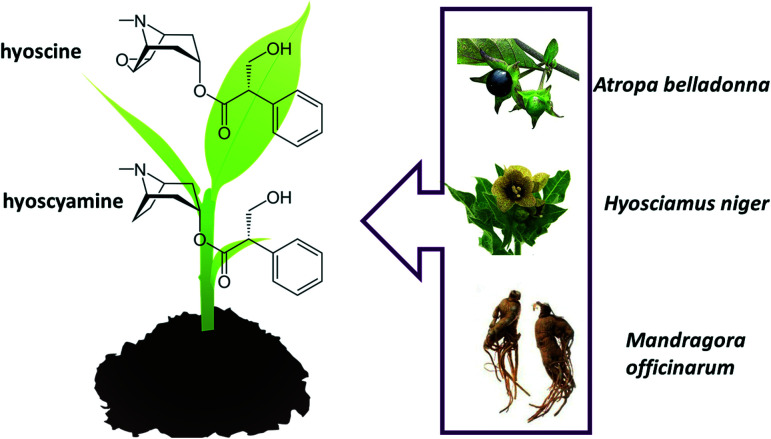
Alkaloids also play a role in witches and magician practices. Many chemical substances belonging to this class of compounds are associated with cults practiced by witches and magicians, linked to the consumption of belladonna (Atropa belladona), henbane (Hyosciamus niger) and mandrake (Mandragora officinarum). These three herbs contain several similar alkaloids, the main ones being hyoscyamine and hyoscine (or scopolamine) (Fig. 2). Examples of derivatives of these alkaloids are, respectively, atropine, used in pupil dilation in eye exams, and hyoscine, which have both anti-secretory and euphoric properties.26–28
Fig. 2. Structural formula of alkaloids hyoscyamine and hyoscine.
The knowledge on the biological effect of these alkaloids, even without a proper understanding of the chemical structures, boosted several actions on the social plane. This understanding influenced the witches' activities, for example, with the preparation of flight ointments.29 The “flight ointments” were applied to certain parts of the body and rubbed over the handle of a broom, which was placed between the legs by the “witches” as if it were a flight instrument.30 This technique for applying the ointments was recorded hundreds of years ago and is explained by a more efficient absorption of the compound where the skin is thinner and there are blood vessels located just below the surface. It is interesting to note that these women seemed to have the knowledge about the low solubility of alkaloids – in particular atropine and hyoscine – in water, as well as recognizing that the ingestion of these compounds would lead to death instead of causing the desired sensations, as they used oils and fats to mix the ointment extracts, and these greases were applied to the skin.31 Currently this technique – transdermal delivery – is a standard method for certain drugs, such as the nicotine patch, some drugs to combat nausea and hormone replacement therapies.32
It is noteworthy that, in addition to these, other plants were used in the preparation of “flight ointments” and in various witches' potions, each with its distinct biological effect.30 Next, the process of elaboration of two of the most common potions will be presented, with discussions involving the structure–activity relationship.
The sleep potion
What do the literary and film works “Sleeping Beauty”, “Snow White” and “Romeo and Juliet” have in common? All these stories portray a certain potion that triggers deep sleep, with a decrease in the state of consciousness, along with a reduction in skeletal muscle movements and a slowing of metabolism. Sleep performs a restorative function that is essential to the body and involves signaling various neurotransmitters that control this process. Did this “magic” potion only appear with the creation of this stories? What are the ingredients of this potion that lead to a dangerous and deep sleep?
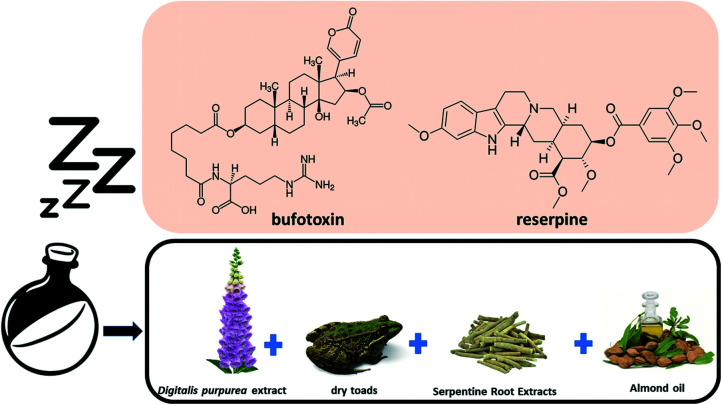
Records found through history indicate that the preparation of a sleeping potion required the following ingredients: foxglove flower extract (containing digitoxin and digoxin), dry frog extract (containing bufotoxin), root extract serpentine (containing reserpine) and almond oil (Fig. 3).33
Fig. 3. Components used in the making of the sleeping potion.
In the following section, we detail each of the components involved in the preparation of the sleep potion, to understand some structure–activity relationships and to promote an understanding of the process from the perspective of medicinal chemistry.
Foxglove flower extract
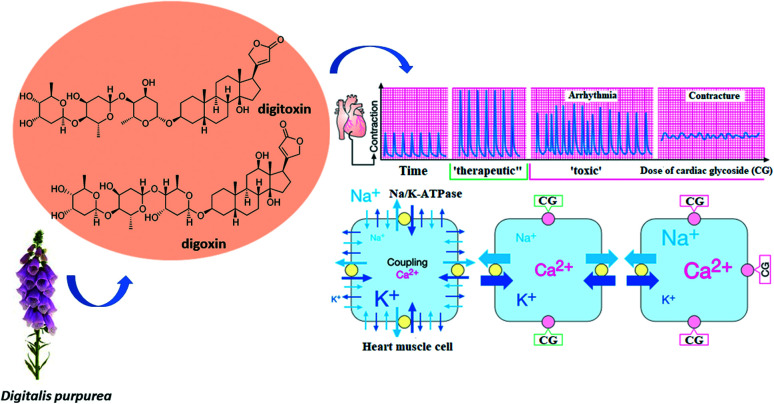
Foxglove extract, Digitalis purpurea, contains cardiac glycosides (or digitalis, or cardenolides), such as digitoxin and digoxin (Fig. 4), substances that cause a reduction on heartbeat. Cardiac glycosides slow down the repolarization of the atria and ventricles, decreasing the refractory period of the myocardium, thus increasing automaticity and the risk of arrhythmias. The effects of cardiac glycosides on vasoconstriction are variable. Digitalis can reduce plasma renin concentrations in patients with advanced decompensated heart failure, causing peripheral vasodilation. In patients without heart failure, digitalis may increase vasoconstriction. This difference is probably due to the greater responsiveness of baroreceptors in patients with chronic heart failure. As can be seen in Fig. 4, if the therapeutic dosage to achieve the effect of increasing the force of contraction is slightly exceeded, adverse effects arise, such as arrhythmia and contracture.
Fig. 4. Therapeutic and toxic effects of cardiac glycosides on protein Na+, K+–ATPase. Source: adapted from Lüllmann, Mohr and Hein (2019).34.
Analysis of the chemical structures show that digitoxin contains one less hydroxyl group than digoxin, making it more lipophilic and resulting in a longer metabolic half-life, smaller distribution volume and greater protein binding. Cardiac glycosides inhibit the Na+/K+/ATPase pump, increasing the intracellular sodium concentration, which reduces calcium expulsion by the Na+Ca+2 exchanger. This sequence increases the concentration of free calcium, also increasing muscle contraction, causing inotropic (contraction force) and chronotropic (contraction time) effects on the cardiac muscle. For this reason, digoxin is administered in safe dosages for patients with heart failure, as it slows down the ventricular response and helps control symptoms of the disease. Both digitoxin and digoxin interact with the same target, the transmembrane protein Na+/K+–ATPase, an ATP-powered ion pump that ensures cell maintenance through ion homeostasis. Na+/K+–ATPase main function is to maintain the electrochemical gradients of sodium and potassium in the animal cell membrane. Cardiac glycosides interact with this transmembrane protein and inhibit its action, causing a decrease in potassium ions and an increase in calcium ions in the intracellular environment (Fig. 4). Digoxin ingestion leads to a reduction in sympathetic flow and increases parasympathetic tone in the autonomic nervous system, while in the central nervous system some of the neurological effects caused by its ingestion include headache, malaise, drowsiness, dizziness, apathy, and blurred vision.
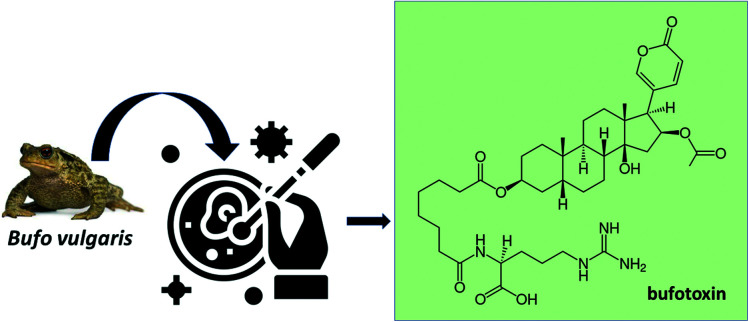
Dry toads: bufotoxin
All amphibian species can produce toxins and many of them are pharmacologically active towards invertebrates or vertebrates. The wide range of activities observed in these toxins has attracted interest in research, which culminated in the discovery of several classes of compounds.35 In view of the great biodiversity of amphibians on the planet, especially in Brazil, animals not yet cataloged and chemically unexplored have great potential in generating novelties that can increase the collection of biologically active compounds. Furthermore, research can be directed towards discovering new mechanisms of physiological action for biological receptors, increasing knowledge of the innate immune system of amphibians, and leading to rational drug development.35
The systematic study of animal-derived bufadienolides materialized after the discovery of numerous of these compounds in Ch'an Su (Venenum Bufonis), a galenic preparation used in traditional Chinese medicine. Ch'an Su originates from the venom of the frog glands B. gargarizans and B. melanostictus and its main pharmacological effect is positive inotropism, which leads to an increase in the force of contraction in the cardiac muscle due to the interaction of cardiotonic steroids with Na+/K+–ATPase.36 Since then, numerous bufadienolides have been discovered in amphibians of the Bufonidae family – which includes the genera Rhinella, Bufo, Atelopus, Ansonia, Lephtophryne and Pedostipes.37 In addition to amphibians, bufadienolides are accumulated in nurchaal glands of snakes (Rhabdophis tigrinus, Colubridae) because of the acquisition of this compounds through their frog-based diet.38 In arthropods, compounds of this class have been isolated from fireflies (Photinus pyralis, P. ignites and P. marginellus, Lampiridae).39
Plants containing bufadienolides are important in African and Asian traditional medicine and, in contrast to their therapeutic effects, some of them are related to serious poisoning. The structural diversity of bufadienolides in bufonid toads appears to be extensive.40 Several chromatographic techniques are used to isolate the components of the venom in sufficient quantity to chemically characterize the compounds present in each species. After partitioning or treatment with organic solvents, bufadienolides and bufotoxins have been isolated from natural sources by classical chromatographic methods or fast chromatographic columns (dry flash). Column chromatography is usually carried out on silica gel and Sephadex©-LH 20.41
Bufadienolides have a carbon skeleton like cardiotonic glycosides, and both inhibit Na+, K+–ATPase. However, unlike cardiac glycosides, bufadienolides are obtained from amphibians and have a six-membered lactone ring at the end of the steroid. Fig. 5 shows the structure of bufotoxin, a buphanolide present in dry toads that results in decreased body temperature and heart rate.
Fig. 5. Molecular structure of bufotoxin found in Bufo vulgaris species.
To understand how the biological activity of bufadienolides is affected by the molecules conformation or by the substituent groups, several studies of the structure–activity relationship (SAR) have been proposed. Bufadienolides have a low therapeutic index, therefore numerous derivatives have been synthesized to reduce the compounds toxic effect.42,43
Through QSAR (quantitative structure–activity relationship) studies, Kamano and colleagues analyzed the three-dimensional structural features common to bufadienolides and cardenolides relative to activity against tumor cells. By this technique, important information about the functional substituents that can improve the pharmacological profile of bufadienolides is obtained.44 In this context, the presence of more potent or less toxic compounds for eukaryotes may assist in designing new drugs.
It is important to point out that there are several biologically active compounds in amphibian venom, such as peptides, biogenic amines, steroids, and alkaloids, which can cause neurotoxic, cardiotoxic, hemotoxic and myotoxic effects.45
Extracts of serpentine roots
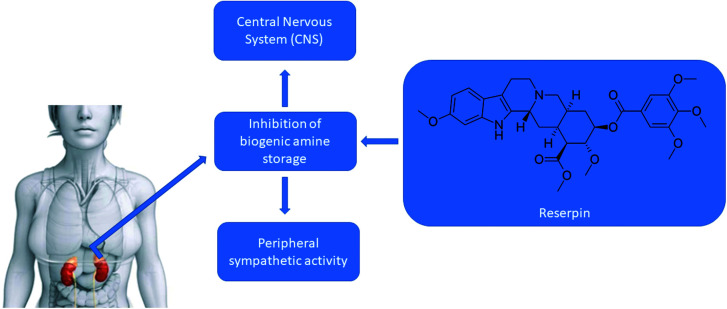
Serpentine root, Rauwolfia serpentina, Indian snake root or even devil's pepper is defined as a flower native species of the Indian subcontinent and East Asia that belongs to the Apocynaceae family.46 Species belonging to this family, in general, produce bioactive substances, such as cardiac glycosides and alkaloids, especially indole alkaloids, which are nitrogenous compounds derived from tryptophan, whose heterocyclic ring contains five or six carbons and one nitrogen as a heteroatom.47Rauwolfia serpentina produces several alkaloids, including reserpine, which has been widely used for the treatment of hypertensive, nervous and mental disorders48 (Fig. 6). Reserpine blocks the possibility of storage of biogenic amines (NA, dopamine = DA, serotonin = 5-HT), inhibiting the necessary mechanism of part of the storage vesicles. The amount of NA releasable per stimulus decreases. The release of adrenaline from the adrenal is also affected, to a lesser extent. Reserpine crosses the central nervous system, where the storage capacity of biogenic amines by vesicles is also influenced.49
Fig. 6. Actions of reserpine on the central nervous system.
Among reserpine adverse effects are hypotension, depression of the central nervous system, drowsiness, and hypothermia.50 Reserpine absorption occurs within 1 to 2 hours after its oral administration, in blood its half-life is approximately 5 hours, in plasma its elimination varies between 45 to 168 hours, and it has the ability to cross the blood–brain barrier.51 Reserpine has as its mechanism of action the inhibition of the vesicular monoamine transporter (VMAT), which is an antiproton carrier, as it irreversibly binds to these transporters in the membranes of secretory vesicles in presynaptic neurons, preventing the binding of intracellular neurotransmitters to the carrier.52 Two types of this transporter were identified, VMAT1, in endocrine tissues and paracrine cells, and VMAT2 found in monoaminergic neurons of the central nervous system, as it has a high affinity for monoamines. Reserpine competes with monoamines to bind to VMAT, binding three times more to VMAT2.53 Once this binding occurs, the function of these vesicles to store monoamines is lost, causing these neurotransmitters to remain in the cytoplasm, where it undergoes degradation by monoamine oxidase (MAO).54 With the metabolization of reserpine and/or the end of its use and with the passage of time, the cell begins to produce new vesicles that will store the transmitters, restoring cell function.55 Among its side effects, depression has been described for a long time. This effect comes from the depletion of monoamines, mainly related to the transmission of serotonin and norepinephrine.56
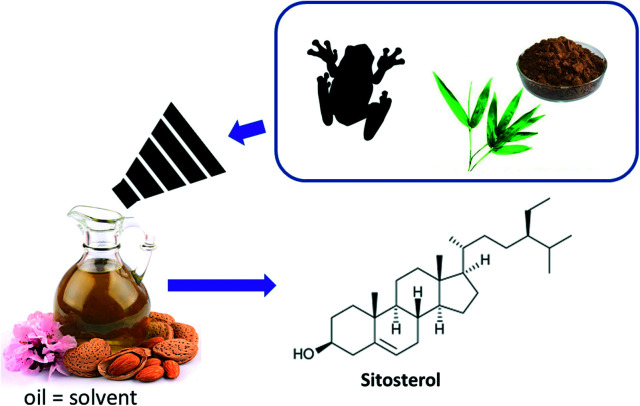
Almond oils
There are two groups of almonds, sweet and bitter. Sweet almonds contain about 50% oil, which is why the oils are mostly extracted from the sweet almond group. This unsaturated oil consists mainly of oleic acid, β-sitosterol and α-tocopherol (Fig. 7).57 Almond oil acted as a solvent in the sleeping potion, solubilizing the other components.
Fig. 7. Molecular structure of the sitosterol present in the oil, used as a solvent for the preparation of the sleeping potion.
The love potion
The concept of love is widely studied, mainly in the field of psychology. There are several definitions and theories that seek to explain this theme, one of which is the “Colors of love” theory.58 Based on this theory, it is possible to draw an analogy of love with colors, because in the same way that colors are not limited to black and white, love should not be abbreviated, as it is plural of meanings.58 However, in addition to the psychosocial implications, love can be perceived through some biological symptoms, such as shortness of breath, excessive sweating on the hands and difficulty in reasoning.59 Love is a complex neurobiological phenomenon – based on brain activities of trust, belief, pleasure, and reward. These activities involve many biochemical mechanisms and, therefore, the human body presents specific sensations and behaviors, which we associate with the feeling of love.60
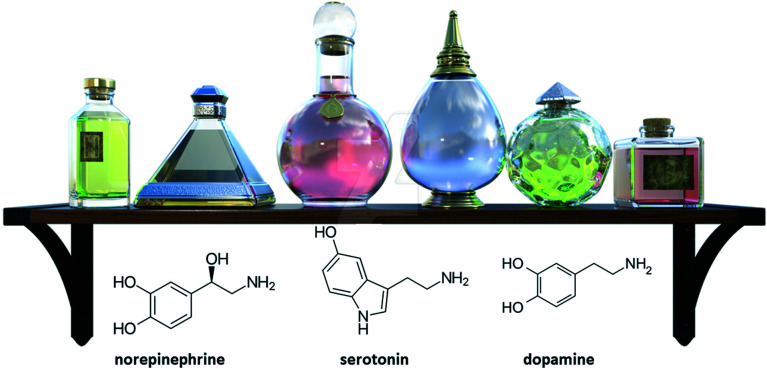
All these implications are the result of the behavior of our brain in the presence of certain chemical compounds, such as: norepinephrine (a compound associated with an increase in heart rate), serotonin (a compound associated with cognitive functions) and dopamine (a compound associated with the regulation of some emotions such as happiness).61 The structure of these compounds can be seen in Fig. 8.
Fig. 8. Structures of the compounds norepinephrine, serotonin and dopamine.
Like the “sleeping potions” the elixir of love is also very common in ancient tales. It contains ingredients such as mandrake root extract, henbane leaves, areca nut and yellow hemp.62
Mandrake roots are very common in witchcraft tales. The roots are extremely narcotic and aphrodisiac and contain tropane alkaloids. Mandrake (Mandragora Officinarum) is a plant native to the Mediterranean that has been widely used in witchcraft because it is considered magical.63 There are references to the use of this plant that predate the sacred scriptures and ancient oriental manuscripts. The narcotic effects caused to the human body, as well as the strange shape of its root – branched and contorted aspect that resembles the human body – made the Mandrake quite famous in magic and witchcraft.63 The Mandrake was also the result of a great theatrical success by Nicolau Machiavelli in 1524. The myth surrounding the collection of the plant is presented by J. K. Rowling, in 1998, in Harry Potter,64 gaining ground and spreading knowledge about this species among teenagers. In the book of Genesis, the mandrake is already mentioned associated with increased fertility, and its name, which has its origins in Hebrew, means “plant of love”. According to legend, this plant was born under the gallows, originating from the semen that the defendants let escape and that it screams in pain when it is pulled from the ground. Such a legend is quoted in an excerpt from Willian Shakespeare's Romeo and Juliet: “Screams like those of a mandrake plucked from the earth, which mortals go mad to hear.”
Mandrake belongs to the family of Solanaceae which main characteristics are the fact that it is herbaceous, stems, endowed with bell-shaped flowers and bears bacaceous fruits.65 Some plant species of the Solanaceae family, such as mandrake, are sources of hyoscyamine, hyoscine and atropine, which are toxic by blocking the parasympathetic nervous system, and may lead to symptoms of poisoning, which will be addressed later in this review.65 The use of these alkaloids, depending on the dosage, can lead to an increased heart rate, palpitation, severe dryness of the mouth, dilation of the pupils and blurring of near vision.
The next ingredient, henbane leaves, is toxic, and can cause hallucinations in moderate doses. In ancient Greece, henbane was used by the oracles when talking to Apollo, which gave it the name Herba apollinaris.66
Henbane contains tropane alkaloids, hyoscine, and atropine, which makes it possible for its tea to be anesthetic and soporific, however, concentrated becomes a poison. The physiological action of atropine is very wide, as it is an antagonist of the acetylcholine receptor, which is the main neurotransmitter of the parasympathetic system.67
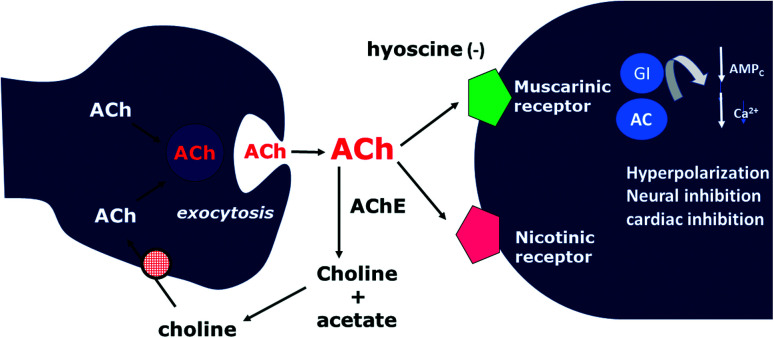
Hyoscine is used in the manufacture of the so-called “truth serum” used in police cases and to combat nausea in NASA astronauts. The only difference between atropine is the presence of an oxygen attached to the tropane ring. Hyoscine is classified as a competitive antagonist drug at the muscarinic receptor (M2), which is why it can also be called an anticholinergic or antimuscarinic drug (Fig. 9). Its tachycardia effect is pronounced by blocking the M2 receptor in the heart (M2 coupled to inhibitory G protein (Gi) that causes bradycardia – chronotropic and dromotropic effect, resulting from the action of the sinoatrial node (NSA) and atrioventricular (AV) septum, not presenting an inotropic effect, as the cardiac tissue does not have cholinergic receptors, only catecholamines).68
Fig. 9. Hyoscine and its relationship with muscarinic receptors.
Areca nut was used by “witches” as an alcoholic extract rich in an alkaloid known as Arecollis, which has an action like nicotine, making this extract a strong stimulant and euphoric.
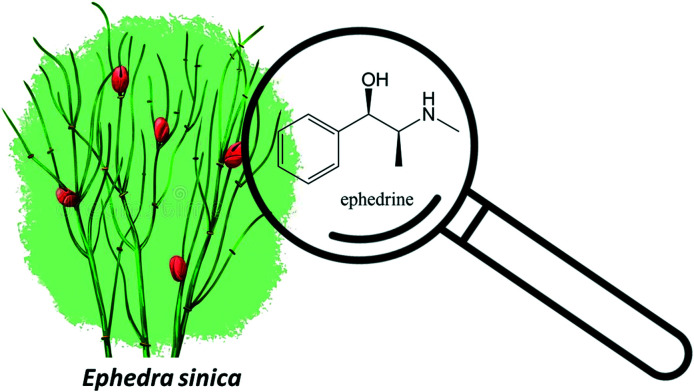
Finally, one of the most used ingredients is yellow hemp, which contains several alkaloids, including ephedrine (Fig. 10), secondary metabolite common in species of the genus Ephedra such as Ephedra Sinicus which has a stimulating and bronchodilator effect.69 This has direct action on adrenaline and noradrenaline adrenergic receptors.
Fig. 10. Molecular structure of ephedrine from Ephedra sinica.
Ephedrine is a sympathomimetic amine, non-specific agonist, it acts on alpha- and beta-adrenergic receptors, with effects on the cardiovascular system and the central nervous system, in addition to increasing serum levels of noradrenaline through its release from peripheral nerve endings to the extracellular fluid.69 Ephedrine is the main alkaloid found in plants of the genus Ephedra (Ephedraceae), also known as Ma Huang, which can be found in subtropical areas of Asia, Europe, and the Americas.70 They are present in several pharmaceutical specialties used in the treatment of respiratory diseases such as asthma, cold and nasal congestion, due to their decongestant and bronchodilator and hypertensive action.71
Ephedrine toxicity is manifested by upregulation of the adrenergic system and effects on the cardiovascular system, including headache, anxiety, insomnia, agitation, dizziness, nausea, vomiting, sweating, thirst, palpitations, muscle weakness and tremors, psychosis, and seizures.72
Adverse effects of ephedrine include hypertension, as it stimulates cardiac rate and output and variably increases peripheral resistance, resulting in an increase in blood pressure. Insomnia is a common adverse effect of the CNS, and tachyphylaxis may also occur.73
Ephedrine also has an ophthalmic decongestant action, which, when applied topically, will promote vasoconstriction, enlarge pupils (mydriasis) and decrease intraocular pressure. It is used in the treatment of glaucoma and some eye diseases, and in eye surgery and ophthalmoscopy.74
Regarding the mechanism of action, ephedrine is classified as a direct-acting sympathomimetic, acting on specific alpha and beta receptors. When administered orally, it can cause excitability and insomnia, due to action on the central nervous system. In overdose, effects include high blood pressure, tachyarrhythmias and myocardial infarction. In addition to the oral and topical route, it can be administered intravenously and intramuscularly.74
Currently, its use for weight loss and by bodybuilders (doping) has been reported, in association with other drugs to increase the caloric burning rate.75 However, studies show that this reduction is modest, in addition to the effect being established for a short period of time.76
Conclusions
This review carries out an analysis on two of the most famous witch potions, the so-called sleep and love potion, intersecting between chemistry and culture realms. The bio-active compounds of the potion's ingredients, from historical accounts, are detailed and physiological effects are explained throw a structure–activity relationship. This review also includes details the presence of this bio-active molecules are in current medicine and its effects.
Conflicts of interest
“There are no conflicts to declare”.
Supplementary Material
Acknowledgments
The authors would like to thank the Universidade Federal de Santa Catarina, Campus Blumenau for their support in carrying out the studies involving this review article.
References
- Horsley R. A. J. Interdiscip. Hist. 1979;9(4):689–715. doi: 10.2307/203380. [DOI] [PubMed] [Google Scholar]
- Apps L. and Gow A., in Male witches in early modern Europe, 2018 [Google Scholar]
- Eboiyehi F. A. J. Int. Womens. Stud. 2017;18:247–265. [Google Scholar]
- Toivo R. M., Witch. Gend. Early Mod. Soc., 2016, pp. 1–21 [Google Scholar]
- Rowlands A. Past Present. 2001;173(1):50–89. doi: 10.1093/past/173.1.50. [DOI] [PubMed] [Google Scholar]
- Monter E. W. J. Interdiscip. Hist. 1972;2(4):435–451. doi: 10.2307/202315. [DOI] [Google Scholar]
- Ben-Yehuda N. Am. J. Sociol. 1980;86(1):1–31. doi: 10.1086/227200. [DOI] [Google Scholar]
- Anderson A. Gordon R. Br. J. Sociol. 1978;29(2):171–184. doi: 10.2307/589887. [DOI] [Google Scholar]
- Rosenthal B., in Records of the Salem Witch-Hunt, 2017 [Google Scholar]
- Ben-Yehuda N. Am. J. Sociol. 1983;88(6):1275–1279. doi: 10.1086/227807. [DOI] [PubMed] [Google Scholar]
- Hedesan J. and Tendler J., The structure of scientific revolutions, 2017 [Google Scholar]
- Suran M. EMBO Rep. 2010:11. doi: 10.1038/embor.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haaske L. A. Genes. 2021;2:34–46. [Google Scholar]
- Newman L., Teach. Hist. A J. Methods, Book Review of Vexed With Devils: Manhood and Witchcraft in Old and New England, ed. E. Gasser, 2019, vol. 43(2), pp. 51–53 [Google Scholar]
- Bennett J. W. Bentleyf R. Perspect. Biol. Med. 1999;42(3):333–355. doi: 10.1353/pbm.1999.0026. [DOI] [Google Scholar]
- Agriopoulou S. Agronomy. 2021;11:931. doi: 10.3390/agronomy11050931. [DOI] [Google Scholar]
- Caporael L. R. Science. 1976;192(4234):21–26. doi: 10.1126/science.769159. [DOI] [PubMed] [Google Scholar]
- Xu K. Yuan X. L. Li C. Li X. D. Mar. Drugs. 2020;18(317):1–32. doi: 10.3390/md18010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krska R. Crews C. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008;25(6):722–731. doi: 10.1080/02652030701765756. [DOI] [PubMed] [Google Scholar]
- Coufal-Majewski S. Stanford K. McAllister T. Blakley B. McKinnon J. Chaves A. V. Wang Y. Front. Vet. Sci. 2016;3(15):1–13. doi: 10.3389/fvets.2016.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemckmeier D. Galindo C. M. Melchioretto E. Gava A. Casa R. T. Pesq. Vet. Bras. 2018;38(5):875–882. doi: 10.1590/1678-5150-pvb-5130. [DOI] [Google Scholar]
- Wallwey C. Li S. M. Nat. Prod. Rep. 2011;28:496–510. doi: 10.1039/C0NP00060D. [DOI] [PubMed] [Google Scholar]
- Zamberlan F. Sanz C. Martínez Vivot R. Pallavicini C. Erowid F. Erowid E. Tagliazucchi E. Front. Integr. Neurosci. 2018;12(54):1–22. doi: 10.3389/fnint.2018.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashanipour R. A., A World of Cures: Magic and Medicine in Colonial Yucatán, dissertation, 2012, pp. 1–312 [Google Scholar]
- Lans C., Creole Remedies: Case Studies of Ethnoveterinary Medicine in Trinidad and Tobago, 2001, pp. 1–318 [Google Scholar]
- Mann J. Educ. Chem. 2008;45(1):14–16. [Google Scholar]
- Passos I. D. and Mironidou-Tzouveleki M., in Neuropathology of Drug Addictions and Substance Misuse, 2016, vol. 2 [Google Scholar]
- Kraft K. Zeitschrift fur Phyther. 1996;17(6):343–355. [Google Scholar]
- Ostling M. Magic Ritual Witch. 2016;11(1):30–72. doi: 10.1353/mrw.2016.0008. [DOI] [Google Scholar]
- Carruthers D. M. J. Mosaic: An Interdisciplinary Critical Journal. 2015;48:119–132. doi: 10.1353/mos.2015.0025. [DOI] [Google Scholar]
- Dey P., Kundu A., Kumar A., Gupta M., Lee B. M., Bhakta T., Dash S. and Kim H. S., in Recent Advances in Natural Products Analysis, 2020 [Google Scholar]
- Alkilani A. Z. McCrudden M. T. C. Donnelly R. F. Pharmaceutics. 2015;7(4):438–470. doi: 10.3390/pharmaceutics7040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couteur P. L. and Burreson J., Napoleon's Buttons: How 17 Molecules Changed History, Penguin Publishing Group, 2004 [Google Scholar]
- Lüllmann H., Mohr K. and Hein L., Color Atlas of Pharmacology, 2019 [Google Scholar]
- Filho G. S. A. C., Bufadienolídeos Da Secreção Cutânea de Rhinella Schneideri (Anura: Bufonidae) - Isolamento, Caracterização, Modificações Químicas e Avaliação Das Atividades Biológicas, 2010, pp. 1–164 [Google Scholar]
- dos Santos Cruz J. Matsuda H. Eur. J. Pharmacol. 1993;239(1–3):223–226. doi: 10.1016/0014-2999(93)90999-X. [DOI] [PubMed] [Google Scholar]
- Daly J. W. Noimai N. Kongkathip B. Kongkathip N. Wilham J. M. Garraffo H. M. Kaneko T. Spande T. F. Nimit Y. Nabhitabhata J. Chan-Ard T. Toxicon. 2004;44(8):805–815. doi: 10.1016/j.toxicon.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Hanwell M. D. Curtis D. E. Lonie D. C. Vandermeerschd T. Zurek E. Hutchison G. R. Aust. J. Chem. 2012;4:1–17. doi: 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner T. Wiemer D. F. Haynes L. W. Meinwald J. Proc. Natl. Acad. Sci. U. S. A. 1978;75(2):905–908. doi: 10.1073/pnas.75.2.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. L. Hsu Y. L. Wu T. S. Bastow K. F. Lee K. H. Org. Lett. 2006;8(23):5207–5210. doi: 10.1021/ol061873m. [DOI] [PubMed] [Google Scholar]
- Shimada K. Sato Y. Nambara T. Chem. Pharm. Bull. 1987;35(6):2300–2304. doi: 10.1248/cpb.35.2300. [DOI] [PubMed] [Google Scholar]
- Mukherjee P. K., in Quality Control and Evaluation of Herbal Drugs, 2019 [Google Scholar]
- García-Pérez P., Barreal M. E., Rojo-De Dios L., Cameselle-Teijeiro J. F. and Gallego P. P., in Studies in Natural Products Chemistry, 2018, vol. 61 [Google Scholar]
- Gao H. Popescu R. Kopp B. Wang Z. Nat. Prod. Rep. 2011;28:953–969. doi: 10.1039/C0NP00032A. [DOI] [PubMed] [Google Scholar]
- Anjolette F. A. P. Leite F. P. Bordon K. C. F. Azzolini A. E. C. S. Pereira J. C. Pereira-Crott L. S. Arantes E. C. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015;21(25):1–12. doi: 10.1186/s40409-015-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. S. Lucky R. A. Univers. J. Pharm. Res. 2019;4(1):40–44. [Google Scholar]
- Agustiar A. B. Masyitoh D. Fibriana I. D. Khumairoh A. S. Rianti K. A. Fitriani N. Harissuddin M. Akmalia H. A. Bioeduscience. 2020;4(2):113–119. doi: 10.22236/j.bes/424945. [DOI] [Google Scholar]
- Lobay D. Integr. Med. 2015;14:40–46. [PMC free article] [PubMed] [Google Scholar]
- Elisabetsky E., in Advances in Phytomedicine, 2002, vol. 1 [Google Scholar]
- Nickell J. R. Siripurapu K. B. Vartak A. Crooks P. A. Dwoskin L. P. Adv. Pharmacol. 2014;69:71–106. doi: 10.1016/B978-0-12-420118-7.00002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M. and Parmar M., Reserpine, StatPearls Publishing, Nova Southeastern University, Treasure Island (FL), 2021 [Google Scholar]
- Mandela P. Chandley M. Xu Y. Y. Zhu M. Y. Ordway G. A. Neurochem. Int. 2010;56(6–7):760–767. doi: 10.1016/j.neuint.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khvotchev M. and Kavalali E. T., Pharmacology of neurotransmitter release: Measuring exocytosis, 2008, vol. 184 [DOI] [PubMed] [Google Scholar]
- Prah A. Purg M. Stare J. Vianello R. Mavri J. J. Phys. Chem. B. 2020;124(38):8259–8265. doi: 10.1021/acs.jpcb.0c06502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwer-Foner G. J. Ogle W. Can. Med. Assoc. J. 1956;74:526–532. [PMC free article] [PubMed] [Google Scholar]
- Delgado P. L. J. Clin. Psychiatry. 2000;61(Suppl 6):7–11. [PubMed] [Google Scholar]
- Fernandes G. D. Gómez-Coca R. B. Pérez-Camino M. D. C. Moreda W. Barrera-Arellano D. J. Chem. 2017:1–11. [Google Scholar]
- Lee J. A., The Colors of Love: An Exploration of the Ways of Loving, New Press, 1976 [Google Scholar]
- Chapman H., Sr. Honor. Proj., 2011, 254, pp. 6–7 [Google Scholar]
- Carter C. S. Porges S. W. EMBO Rep. 2013;14:12–16. doi: 10.1038/embor.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbar-Slamloo Y. Fazlali Z. Front. Mol. Neurosci. 2020;12(334):1–8. doi: 10.3389/fnmol.2019.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultes R. E., Hofmann A. and Rätsch C., Plants of the gods: their sacred, healing, and hallucinogenic powers, Healing Arts Press, Rochester, Vt., 2001 [Google Scholar]
- Dafni A. Blanché C. Khatib S. A. Petanidou T. Aytaç B. Pacini E. Kohazurova E. Geva-Kleinberger A. Shahvar S. Dajic Z. Klug H. W. Benítez G. J. Ethnobiol. Ethnomed. 2021;17:68. doi: 10.1186/s13002-021-00494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowling J. K., Harry Potter and the chamber of secrets, Scholastic, Inc., New York, 2000, ©1999 [Google Scholar]
- Gebhardt C. Theor. Appl. Genet. 2016:129. doi: 10.1007/s00122-016-2804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro G. and Thomas B., Drugs of the dreaming: Oneirogens: Salvia divinorum and other dream-enhancing plants, Simon and Schuster, 2007 [Google Scholar]
- Kohnen-Johannsen K. L. Kayser O. Molecules. 2019;24:796. doi: 10.3390/molecules24040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D. Belevych A. E. Br. J. Pharmacol. 2003:139. doi: 10.1038/sj.bjp.0705338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman R. B. Vu N. Partilla J. S. Roth B. L. Hufeisen S. J. Compton-Toth B. A. Birkes J. Young R. Glennon R. A. J. Pharmacol. Exp. Ther. 2003;307:138–145. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- Soni M. G. Carabin I. G. Griffiths J. C. Burdock G. A. Toxicol. Lett. 2004;150:97–110. doi: 10.1016/j.toxlet.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Gründlingh J. J. Forensic Leg. Med. 2014;28:54. doi: 10.1016/j.jflm.2014.10.001. [DOI] [Google Scholar]
- Llanes L. C. Sa N. B. Cenci A. R. Teixeira K. F. de França I. V. Meier L. de Oliveira A. S. MMWR. Morb. Mortal. Wkly. Rep. 1996;45:689–693. [Google Scholar]
- Brunton L. L., Chabner B. A. and Knollmann B. C., As bases farmacológicas da terapêutica Goodman & Gilman, 2012 [Google Scholar]
- Statler A. K., Maani C. V. and Kohli A., Ephedrine, 2022 [PubMed] [Google Scholar]
- Lee M. Bloor M. Dobash R. P. Dobash R. E. Sociol. Res. Online. 2000;5(2):45–56. doi: 10.5153/sro.489. [DOI] [Google Scholar]
- Wooltorton E. Sibbald B. Can. Med. Assoc. J. 2002;166:633. [PMC free article] [PubMed] [Google Scholar]