Abstract
Background: While Molnupiravir and Paxlovid have recently been approved for use in some countries, there are no widely available treatments for COVID-19, the disease caused by SARS-CoV-2 infection. Herbal extracts have been used to treat respiratory clinical indications by Ayurvedic medicine practitioners with minimal adverse reactions and intense research efforts are currently under way to develop some of these formulations for COVID-19 treatment.
Methods: Literature search for in silico, in vitro, in vivo, and clinical studies on the topic of Ayurvedic formulations for potential COVID-19 treatment, in order to present the current state of current knowledge by integrating information across all systems.
Results: The search yielded 20 peer reviewed articles on in silico studies examining the interaction of phytoconstituents of popular Ayurvedic formulations with SARS-CoV-2 components and its receptors; five articles on preclinical investigations of the ability of selected Ayurvedic formulations to inhibit functions of SARS-CoV-2 proteins; and 51 completed clinical trials on the efficacy of using Ayurvedic formulations for treatment of mild to moderate COVID-19. Clinical data was available from 17 of the 51 trials. There was a considerable overlap between formulations used in the in silico studies and the clinical trials. This finding was unexpected as there is no clearly stated alignment between studies and the traditional pathway to drug discovery– basic discovery leading to in vitro and in vivo proof of concept, followed by validation in clinical trials. This was further demonstrated in the majority of the in silico studies where focus was on potential antiviral mechanisms, while the clinical trials were focused on patient recovery using oral treatments. In all 17 clinical trials where data was available, Ayurvedic treatments lead to a shorter period to recovery in participants with COVID-19.
Conclusion: The most commonly used Ayurvedic treatments for management of respiratory symptoms associated with SARS-CoV-2 infection appear to have prophylactic and/or therapeutic properties. It would be of particular interest to assess synergistic and concomitant systemic effects and antiviral activities of individual phytoconstituents and their combinations in the Ayurvedic treatments.
Keywords: COVID-19, Ayurvedic formulations, Antivirals, In silico molecular docking, Clinical trials
Graphical abstract
Abbreviations
- 3CLpro
3C like protease
- Mpro
Main protease
- ACE2
angiotensin converting enzyme 2
- COVID-19
coronavirus infectious disease 2019
- ERGIC
endoplasmic reticulum Golgi intermediate compartment
- KSK
Kabasura Kudineer
- (MERS)-CoV
Middle Eastern Respiratory Syndrome
- NSP
nonstructural protein
- NVK
Nilavembu Kudineer
- RBD
receptor binding domain
- SARS-CoV
Severe Acute Respiratory Syndrome coronavirus
- TMPRSS2
transmembrane serine protease 2
- uHPLCPDA
an ultra-high-performance liquid chromatographic-photodiode array
- vRNP
viral ribonucleoprotein
Introduction
Human coronaviruses usually cause common cold symptoms, except for the recently emerged betacoronaviruses, including Severe Acute Respiratory Syndrome coronavirus (SARS-CoV), Middle Eastern Respiratory Syndrome (MERS)-CoV (Fung and Liu, 2019) and SARS-CoV-2 that can cause severe disease and in extreme cases, death (WHO, 2021a). The SARS-CoV-2, identified as the causative agent for COVID-19 and the current pandemic, emerged in 2019 and has rapidly spread around the world, evolving into highly transmissible strains, the delta and omicron variants and recent subvariants (WHO, 2021a). Individuals infected with COVID-19 present with a range of clinical manifestations, from asymptomatic to mild (cough, chest pain, loss of taste, back pain) to fatal (death). There have been over 464 million confirmed cases and more than 6 million deaths worldwide (WHO, 2021b) (information current as of 20 March 2022), with a concerted effort around the world to develop and deploy vaccines for protection from emerging SARS-CoV-2 strains. Although several vaccines have been approved for use in adult, adolescent and older children populations (March 2022), no vaccine has been approved for infants and young children below the age of 5 years. It is envisaged that once the majority of the global eligible population has been vaccinated, the younger, currently ineligible, population will be at a high risk of infections. Access to safe and effective therapeutics would greatly enhance our ability to manage SARS-CoV-2 infections into the future.
Currently, there are no widely available therapeutics for the management of COVID-19 (Siemieniuk et al., 2020), however, recent clinical trial data from several drug treatment options are showing promising results. Fluvoxamine (Abbott Laboratories) has shown good results in treating COVID-19 by preventing patients from developing serious illness and reducing the risk of hospitalization (Reis et al., 2022), while Novartis has declared topline data from their Phase 2 study for Ensovibep showing viral load reduction over eight days. Pfizer has announced promising outcomes from clinical trials of Paxlovid™ that targets the SARS-CoV-2 3C-like protease, while Merck has announced similar results from trials of Molnupiravir, a nucleoside analog (Drozdzal et al., 2021; Jayk Bernal et al., 2021). The latter two experimental drugs have been authorized by the FDA and are approved for use in several countries.
The use of nutraceuticals and/or dietary supplements, including herbal medicines, is well established for the management of several respiratory indications (Chan et al., 2021; Chen et al., 2014) and the interest in their antiviral properties has also been investigated in the context of the current COVID-19 pandemic (Parisi et al., 2021; Singh et al., 2021; Steel et al., 2020). Many research groups have examined the potential use of natural products and substances already available for use for other indications, such as Ayurvedic formulations that are widely used in India and within the Indian diaspora (Ayatollahi et al., 2021). Ayurveda is an ancient Indian holistic system of medicine that utilizes plant extracts as one of the main ingredients for their formulations (National Center for Complementary and Integrative Health, 2022). Given the widespread use of Ayurvedic formulations as complementary medicine, they provide an attractive avenue for development of anti-COVID-19 treatments that would be safe and well tolerated (Singh et al., 2018; Steel et al., 2020).
In this review we aim to summarize the current literature on the efficacy of natural compounds most commonly used in Ayurvedic formulations with potential anti-SARS-CoV-2 properties, in order to gage the current stage of knowledge in this topic and provide the stepping-stone for development of successful anti-viral treatments.
SARS-CoV-2
The main structural components of coronaviruses are the spike glycoprotein S, the transmembrane proteins M (membrane) and E (envelope), and the nucleoprotein N. The S, M and E proteins are embedded in the virus envelope while N protein forms a viral ribonucleoprotein (vRNP) complex with the viral RNA (V'Kovski et al., 2021). The SARS-CoV-2 genome is organized similarly to other coronaviruses and the positive-stranded RNA genome has a 5′-cap and a 3′-poly-A tail, allowing its translation from the host translation machinery. A frameshift between two open reading frames (Orf), Orf1a and Orf1b at the 5′-end of the genome allows production of two polypeptides that are then proteolytically processed to produce 16 non-structural proteins (Nsp1–16) that are involved in various processes of the virus infection cycle. The structural S, N, M and E proteins are encoded at the 3′-end. The life cycle of SARS-CoV-2 involves the initial infection of human cells through the host cell receptor angiotensin-converting enzyme 2 (ACE2), together with the cell surface serine protease TMPRSS2 that acts as a coreceptor mediated by the S glycoprotein via its receptor binding domain (RBD). Following entry via endocytosis, the positive-sense RNA genome is translated into a polyprotein with RNA polymerase function; this produces negative-sense sub-genomic mRNA before making positive-sense sub-genomic mRNA ready for translation. Following translation, the components associate in the endoplasmic reticulum Golgi intermediate compartment (ERGIC) before being released by exocytosis. We would like to direct the reader to excellent articles on the structure and life cycle of SARS-CoV-2 and its relationship to other known coronaviruses (V'Kovski et al., 2021; Yang and Rao, 2021) as these aspects will not be discussed in detail in this manuscript. Most of the antiviral development efforts against SARS-CoV-2 have targeted the glycoprotein S which is the major receptor binding protein. This glycoprotein is also the focus of the vaccine development efforts as it is the main target of our immune response. As the coronavirus 3C-like protease 3CLpro (also termed Mpro) is critical for virus polyprotein processing and virus replication this enzyme poses as another viable antiviral target.
Search strategy
The main motivation for this review was to understand how Ayurvedic formulations can support the clinical management of individuals infected with COVID-19. Our search strategy involved five electronic databases: Scopus, PubMed, AYUSH Research Portal, Clinical Trial Registry-India (CTRI), and ClinicalTrials.gov since the journals and websites inception until 22 October 2021. We have also performed updated searches for any additional articles that may be of interest just prior to re-submission of this manuscript (20 March 2022). The keywords used included “SARS-CoV-2” AND/OR “COVID-19” AND “Ayurveda”. The resulting database of research literature and clinical trials included in silico studies, pre-clinical laboratory studies and clinical trials published in English. Clinical trials where no trial data was available were excluded. The various Ayurvedic formulations mentioned in this review and their herbal constituents are listed in Table 1 .
Table 1.
Herbal constituents of Ayurvedic formulations referred to in the text.
| Ayurvedic name | Constituents |
| Adathodai Manapagu | Adhatoda vasica |
| Agnikumara Rasa | Piper nigrum |
| Amalaki Churna | Phyllanthus emblica |
| Amruth | Tinospora cordifolia |
| Amrutha Sanjeevini | Tinospora cordifolia, Phyllanthus emblica, Ocimum tenuiflorum, Andrographis paniculate, Glycyrrhiza glabra, Withania somnifera |
| Amukkara | Withania somnifera, Zingiber officinale, Piper nigrum, Piper longum, Elettaria cardamomum, Cinnamomum verum, Syzygium aromaticum, Saccharum officinaru, Messua ferrea. |
| Anu Taila | Herbal oil with >20 ingredients*, including Ocimum tenuiflorum, Elettaria cardamomum, Cinnamomum zeylancium. |
| Arogya Kashayam-20 | Tinospora cordifolia, Zingiber officinale, Phyllanthus niruri, Glycyrrhiza glabra, Terminalia chebula, Piper longum, Piper nigrum. |
| AyurCoro-3 | Seven ingredients, including Curcuma longa. |
| AYUSH 64 | Alstonia cholaris, Picrorhiza kurroa, Swertia chirata, Caesalpinia crista |
| Bilvadi | Seven herbs and spices, including Zingiber officinale, Coriandrum sativum, Piper longum, Piper nigrum, Aegle marmelos. |
| Brahmananda Bhairavam | Zingiber officinale |
| Bresol | 11 ingredients, including Curcuma longa, Ocimum Sativum, Adathoda vasica, Zingiber Officinale. |
| CIM-MEG19 | Andrographis paniculata |
| CLEVIRA | ten ingredients, including Tinospora cordifolia and Andrographis paniculata |
| CONTAZAP | Andrographis panniculata, Rheum emodi, Embelica officinalis. |
| Dashamula Kwatha | Aegle marmelos, Premna mucronate, Oroxylum indicum, Stereospermem suaveolens, Gmelina afborea, Solanum indicum, Solanum xanthocarpum, Tribulus terrestris, Desmodium gangeticum, Uraria picta |
| Giloy | Tinospora cordifolia |
| Golden Milk, Ojovardhini | Curcuma longa |
| Immunofree | 14 ingredients, including Andrographis paniculata, Phylanthus niruri, Glycirhizza glabra, Ocimum tenuiflorum, Tinospora cordifolia, Curcuma longa. |
| Immuzan | Ocimum tenuiflorum, Curcuma longa, Embelica officinalis, Zingiber officinale |
| Jeevaneeyam | 15 ingredients, including Tinospora cordifolia, Phyllanthus emblica, Ocimum tenuiflorum, Glycyrrhiza glabra |
| Kalmegh | Andrographis paniculata |
| Kantakaryavaleha | 14 ingredients, including Tinospora cordifolia, Piper longum, Piper nigrum, Zingiber officinale. |
| Mahasudarsan Chooranam | Polyherbal with >50 herbal ingredients. |
| Maldevi Chendooram | not a herbal |
| Nagaradi kwath | Zingiber officinale, Terminalia chebula, Tinospora cordifolia, |
| Nochi Kudineer Chooranam | Vitex negundo, Artemisia pallens, Piper nigrum, Allium sativum, Piper betle. |
| NOQ19 | 19 ingredients, including Withania somnifera, Aegle marmelos, Glycyrrhiza glabra Pluchea lanceolata, Adhatoda vasica, Piper longum, Curcuma longa, Cissampelos pareira, Phyllanthus fraternus, Andrographis paniculate, Alstonia scholaris, Ocimum tenuiflorum, Tinospora cordifolia. |
| Oma theeneeer | Trachispermum roxburghianum |
| Pathyadi Kwatha | Terminalia chebula, Terminalia bellirica, Emblica officinalis, Andrographis paniculate, Curcuma longa, Azadireachta indica, Tinospora cordifolia. |
| PINAK | Erythrina indica, Magnefera indica, Eugenia jambolana, Jusminum sambac |
| Pipli | Piper longum |
| Rasona | Allium sativum |
| Reginmune | Echinacea purpurea, Aloe vera, Uncaria tomentosa |
| Sanshamani Vati | Tinospora cordifolia |
| Septilin | ∼35 ingredients, including Commiphora wightii, Tinospora cordifolia, Phyllanthus emblica, Glycyrrhiza glabra |
| Shadangodak | Cyperus rotundus, Fumaria indica, Vetiveria zizanioides, Santalum album, Andropogan vetiveria, Zingiber officinale |
| Shakthi | Emblica officinalis, Withania somnifera, monnieri, Eclipta alba, Tinospora cordifolia, Asparagus racemosus, Convolvulus pluricaulis, Glycyrrhiza glabra. Bacopa |
| Shunthi | Zingiber officinale |
| SSV Formulation | Curcuma longa and other ingredients |
| Surasadi Kadha | Ocimum tenuiflorum, Solanum virginianum, Tinospora cordifolia, Zingiber officinale, Curcuma longa, Adathoda vasica, Elettaria cardamomum, Piper nigrum, Piper longum, Acorus calamus. |
| Swasari Ras | 10 ingredients, including Glycyrrhiza glabra, Syzygium aromaticum, Cinnamomum zeylancium, Pistacia chinensis, Capparis noonii, Zingiber officinale, Piper longum. |
| T-AYU-HM Premium | 11 ingredients, including Terminala chebula, Zingiber officinale, Punica granatum, Asparagus racemosus, Piper longum, Myristica fragrans, Leptadinia reticlata and Tinospora cordifolia. |
| Thaleesadhivadagam | 14 ingredients, including Piper nigrum, Piper longum, Zingiber officinale, Elettaria cardamomum |
| Trikatu Churna | Zingiber officinale, Piper longum, Piper nigrum. |
| Trishun | Proprietary formulation with >50 ingredients. |
| ViroNil | Tinospora cordifolia, Ocimum tenuiflorum, Zingiber officinale, Curcuma longa, Piper nigrum, Phyllanthus emblica including others. |
| Vyaghryadi Kashaya | Solanum xanthocarpum, Zingiber officinalis, Tinospora cordifolia, Piper longum |
| Yastimadhu Ghanavati | Glycyrrhiza glabra |
*other constituents are non-herbal and may include minerals.
In silico investigations
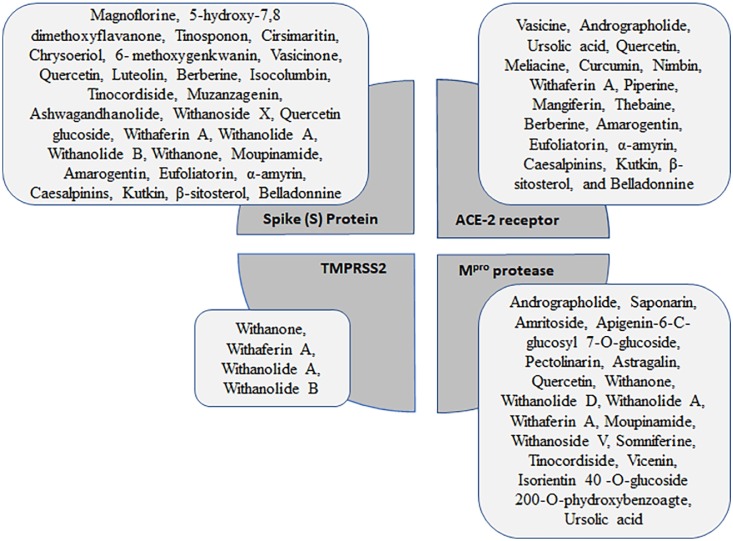
The searches have identified 20 peer reviewed publications of studies utilizing in silico techniques to determine the possible direct antiviral effect of known phytoconstituents of Ayurvedic formulations. The Ayurvedic formulations were composed of natural extracts from multiple plant sources, therefore, it is essential to understand the possible antiviral effect of the phytoconstituents and their metabolites within the extracts. Among various antiviral activities exhibited by plant metabolites, angiotensin converting enzyme 2 (ACE2) inhibitors have been explored in detail and shown to be a possible way to manage SARS-CoV-2 infection (V'Kovski et al., 2021). Ayurvedic formulations have also been studied for their potential inhibition of Mpro activity (Gurung et al., 2020; Havranek and Islam, 2021). Although there are relatively limited studies examining the potential of Ayurvedic formulations to inhibit RNA polymerase activity, these formulations may present excellent candidates for development as RNA polymerase inhibitors as they are usually less toxic than other antiviral strategies (Antonio et al., 2020). The most common targets of the phytoconstituents studied are the S protein, ACE2 receptor, TMPRSS2 (SARS-CoV-2 co-receptor) and Mpro (Fig. 1 ).
Fig. 1.
Common phytoconstituents of Ayurvedic formulations and their targets.
A summary of the results of the in silico studies reviewed is provided. The most common targets of the phytoconstituents studied are shown in the central quardants, while the phytoconstituents are listed in the associated rectangles. The size of the rectangles is relative to the number of compounds.
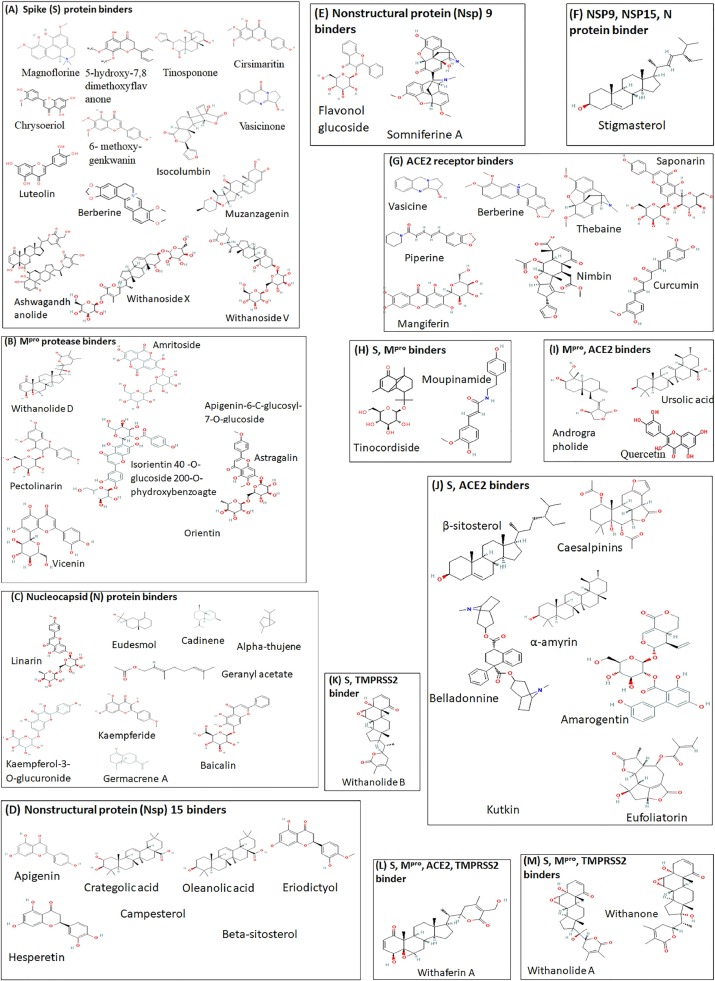
One of the most popular Ayurvedic therapies currently used for treatment of COVID-19 (asymptomatic, mild to moderate) in India, particularly in the state of Tamil Nadu, is the “Kabasura Kudineer” (KSK), a polyherbal formulation containing over 32 plant metabolites that is generally used to treat other respiratory symptoms (Jeyanthi and Kumar, 2020; Kiran et al., 2020; Rao et al., 2020). The KSK constituents include Magnoflorine, scutellarein, chrysoeriol, 5‑hydroxy-7,8 dimethoxyflavanone, tinosponone, cirsimaritin, chrysoeriol, 6- methoxygenkwanin, vasicinone, quercetin and luteolin, obtained from Sida acuta, Andrographis paniculata, Tinospora cordifolia, Plectranthus amboinicus, Justicia adhatoda,and Costus speciosus. All of these were found to have excellent predicted binding affinity with the SARS-CoV-2 S protein (Kiran et al., 2020). The molecular dynamics simulation showed that scutellarein, chrysoeriol, luteolin, cirsimaritin, and magnoflorine have strong affinity towards the spike protein via hydrogen bond contact with amino acids Arg 346, Asn343, Asp364, Cys336, Gly339, Ser373, Thr345, and Val341. The same research group have also found that phytoconstituents vasicine (quinazoline alkaloid), andrographolide (labdane diterpenoid), ursolic acid (pentacyclic triterpenoid acid), quercetin (flavonol) and meliacine (peptide) were predicted to inhibit the ACE2 receptor (Kiran et al., 2020). “Nilavembu Kudineer” (NVK), an Ayurvedic formulation containing nine herbal ingredients, is commonly used to treat respiratory illnesses due to its anti-inflammatory, anti-analgesic, and anti-pyretic effects. One of the most predominant phytoconstituents of NVK is andrographolide, derived from the herb A. paniculata that is used for supporting the immune function and relieve symptoms of the common cold. The findings of in silico studies have indicated that andrographolide can dock successfully in the binding site of SARS-CoV-2 Mpro (free energy −3.094 kJ/mol) and shows further promise in potential therapeutic use due to its relatively high solubility and favourable pharmacodynamics characteristics (Enmozhi et al., 2021). The relevant chemical structures are shown in Fig. 2 .
Fig. 2.
Structure of common phytoconstituents of Ayurvedic formulations.
Chemical structures of compounds, arranged by the target binding proteing, are shown. Compounds that bind to SARS-CoV-2 spike (S) protein (A), large protease Mpro (B), nucleocapsid (N) protein (C), Nonstructural protein Nsp15 (D), Nsp9 (E) are shown. Compounds that bind to ACE2 receptor are shown in (G). Compounds that bind to more than one relevant target are shown in F, H, I, J, K, L, M. TMPRSS2 is a co-receptor for SARS-CoV-2.
T. cordifolia, a common constituent of several different Ayurvedic formulations, including KSK and NVK, has received considerable attention for its potential anti-SARS-CoV-2 properties. The aqueous extract of T. cordifolia, known as “Guduchi Ghan Vati” or “Shilajatu Rasayana”, is used widely in Ayurvedic medicine management of several health conditions (Upadhyay et al., 2010). An in silico molecular docking study found that its phytoconstituents including berberine (alkaloid), isocolumbin (diterpenoid), magnoflorine (quaternary benzylisoquinoline), and tinocordiside (sesquiterpene glycoside) could potentially bind with high efficiency to the S glycoprotein in its prefusion state and to the receptor binding-domain of the mature S glycoprotein (Sagar and Kumar, 2021). These phytoconstituents may also provide preventative measures for COVID-19 by inhibiting the attachment of the virus to its host cells as evidenced by the low IC50 (<1 μM) of tinocordiside and isocolumbin. A study by Mulpuru and Mishra (2021) found that of all T. cordiflora phytoconstituents investigated, saponarin (flavone glucoside) showed the highest affinity for docking with SARS-CoV-2 Mpro, with affinity (8.75 kCal/ml) exceeding that of a nucleotide prodrug of an adenosine analog, Remdesivir (Mulpuru and Mishra, 2021). Remdesivir has been shown to shorten the time of recovery of individuals hospitalized with COVID-19 and evidence of lower respiratory tract infections (Beigel et al., 2020). Additionally, molecular dynamics simulation showed that saponarin-Mpro interaction was stable, without any significant fluctuations. In another in silico molecular docking and dynamics study, two bioactive constituents of T. cordiflora, amritoside (tannin) and apigenin-6-C-glucosyl-7-O-glucoside, were predicted to bind to the Mpro with high affinity, 60.35 and 50.50 kCal/mol respectively (Murugesan et al., 2021). In the same study, pectolinarin (cirsium isolate), a bioactive compound found in extracts of Phyllanthus niruri, as well as astragalin (3-O-glucoside of kaempferol) and quercetin, bioactive constituents of Emblica officinalis extracts, were also found to efficiently occupy the substrate binding cleft of Mpro in silico. P. niruri and E. officinalis are used in Ayurvedic medicine as components of several formulations and on their own as extracts for various conditions.
The “Ayurvedic Rasayana” describes a group of herbs used as dietary supplements that are proposed to exhibit immunomodulatory and antioxidant activities with extracts of T. cordifolia, Withania somnifera, and Asparagus racemosus major constituents of Rasayana therapy. A study by Borse et al. (2021) used an ultra-high-performance liquid chromatographic-photodiode array (uHPLC-PDA) and identified 31 phytoconstituents in the combined extract (Borse et al., 2021). All 31 phytoconstituents were tested for their immunomodulatory potential using an in silico network pharmacology model, followed by in silico molecular docking investigations with the SARS-CoV-2 S, Mpro and polymerase proteins. Network analysis showed that 53 protein targets in 28 of the 31 phytoconstituents were involved in 20 immune pathways, suggesting that the extract could concomitantly modulate multiple immune pathways. Two phytoconstituents, ashwagandhanolide (dimeric withanolide; Fig. 2A) and withacoagin, had high docking scores of 10 and 7.6 kCal/mol respectively, for SARS-CoV-2 S protein, forming strong hydrogen bond interactions with Arg403, Asn501, Ser494, Thr500, and Tyr495. However, the authors raised concerns about their drug-likeness as determined by the Lipinski's rule of five (Borse et al., 2021).
A literature review on the use of withanolides (phytoconstituents of W. somnifera) in COVID-19 (Dhawan et al., 2021) indicated that withanolides inhibit the ACE2 mediated entry of SARS-CoV-2 into host cells and subsequent Mpro activity. There was no direct evidence provided of either action with most of the molecular docking studies included. The authors proposed that withanone, withanolide D, withanolide A, and withaferin A have the potential to alter Mpro activity, while withanoside II, withanoside IV, withanoside V, sitoindoside IX, withanolide R and 2,3‐dihydrowithaferin A interfere with Mpro action (Dhawan et al., 2021; Kumar et al., 2020c; Parida et al., 2020; Tripathi et al., 2021). Interestingly, withanone and withaferin A (Fig. 2K–M) were also predicted to bind and stably interact at the catalytic site of TMPRSS2, the SARS-CoV-2 co-receptor, inducing changes at the allosteric site, which would potentially inhibit virus entry into host cells (Kumar et al., 2020c). Withanoside X and quercetin glucoside (Fig. 2D) may bind to the active site of NSP15 endoribonuclease and S protein RBD (Chikhale et al., 2021). A 100 ns molecular dynamics simulation suggests that withanoside X has high binding affinity with free energy value of 89.42 kCal/mol. Withaferin A, withanolide A, withanolide B, and withanone also have excellent predicted binding affinity to S protein, ACE2 receptor, and Mpro (Srivastava et al., 2020). Additionally, molecular dynamics simulation suggested that withanolides A and B bind stably to the S protein and the papain-like protease (PLpro) of SARS-CoV-2.
A survey of 17 bioactive phytoconstituents of commonly used herbs in Ayurveda for management of fever, inflammation and immune disorders, found that curcumin, nimbin, withaferin A, piperine, mangiferin, thebaine, berberine, and andrographolide have favourable drug-likeness characteristics and may effectively inhibit the interaction of SARS-CoV-2 S protein with its receptor (Maurya et al., 2020b). Nimbin and curcumin had very high docking scores of 148.621 kCal/mol and 141.36 kCal/mol respectively, comparable to the results for the antiviral nafamostat and hydroxychloroquine. Both nimbin and curcumin were predicted to interact via hydrogen bonding with S protein amino acid residues Arg 765, Asn317, Gln314, Lys304, Thr768, and Ser316. In a study by Maurya et al. (2020a), 20 phytoconstituents from herbs commonly used in Ayurveda, Allium cepa, Allium sativum, Alstonia scholaris, Artemisia vulgaris, Atropa belladonna, Caesalpinia crista, Glycyrrhiza glabra, Nigella sativa, Picrorhiza kurroa, Piper nigrum, Swertia chirata, T. cordifolia and Zingiber officinale were investigated for their ability to bind to the SARS-CoV-2 S protein and its ACE2 receptor (Maurya et al., 2020a). Computational structure-based drug design revealed that the compounds amarogentin, eufoliatorin, α-amyrin, caesalpinins, kutkin, β-sitosterol, and belladonnine have potentially high affinity towards both the S protein and the ACE2 receptor (Fig. 2). Amarogentin had the highest docking score of 149.76 kCal/mol against the S protein. Molecular dynamics simulation revealed that it interacts with the S protein via hydrogen bonding with amino acids Arg765, Cys738, Gln314, Ser735, Thr739, Thr768, and Val736, and with ACE2 receptor via hydrogen bonding with Asp350, Asp382, Asn394, and Tyr385.
An important Ayurvedic method for enrichment of active pharmacological agents from herbs involves preparation of Kadha (decoction) for oral consumption. In a molecular docking study by Maurya and Sharma (2020), the potential for phytoconstituents of common Kadha components to bind to SARS-CoV-2 proteins, ACE2 receptor or pro-inflammatory mediators were investigated (Maurya and Sharma, 2020). The authors reported the findings of 108 phytoconstituents from Withania somnifera, Ocimum tenuiflorum, T. cordifolia, Z. officinale, C. longa, P. nigrum, Syzygium aromaticum, Elettaria cardamomum and Citrus limon. Many of the phytochemicals tested were found to have a strong binding affinity with SARS-CoV-2 proteins. Orientin, withanolide, withanolide B, crategolic acid, ursolic acid, withaferin A, apigenin, eriodictyol, hesperetin, oleanolic acid, stigmasterol and withanone had a high binding affinity with NSP15. Whereas, withanone, withanolide B, withaferin A, withanolide, beta-sitosterol, campesterol, stigmasterol, flavonol glucoside and somniferine A had a high binding affinity to the Nsp9 protein (Fig. 2E). Several phytochemicals, including quercetin, withanone, withanolide and stigmasterol (Fig. 2F), also had high binding affinity to the N protein. Importantly, withaferin A, withanolide B, withanolide, withanone, campesterol, cyclocurcumin, somniferine A, stigmasterol, eriodictyol, isopiperine, oleanolic acid, rhamnetin, orientin, quercetin, piperine and vicenin had high binding affinity for inflammatory mediators cyclooxygenase-2 (COX2), phospholipase A2 (PLA2), NF-ĸB-Inducing Kinase (NIK), and interleukin-1 receptor associated kinase 4 (IRAK-4) (Maurya and Sharma, 2020). Molecular dynamics simulations were performed for Mpro and ACE2 receptors with the above-selected phytoconstituents, and root mean square deviations (RMSD) and root mean square fluctuations (RMSF) plot analysis demonstrated that their interactions are stable with very low variability. As COVID-19 is characterized by high inflammatory response (Mishra et al., 2020), the anti-inflammatory action of the phytoconstituents may be very important for potentially reducing disease symptoms. Moupinamide (Fig. 2), one of the phytoconstituents of Kadha used to relieve symptoms of respiratory influenza like illness, was found to have strong potential to bind stably to both the S protein and the Mpro (Shukla et al., 2021).
Based on the previous findings (discussed above), W. somnifera, T. cordifolia, and O. tenuiflorum are commonly used components for various Ayurvedic formulations. Over one hundred different active phytoconstituents of these herbs were identified as potential Mpro binders and further analyzed for drug-likeness. This has resulted in the identification of six potential phytochemical inhibitors with high stability as evidenced by the RMSD values (withanoside V, somniferine, tinocordiside, vicenin, isorientin 4′-O-glucoside 2’’-Op-hydroxybenzoate and ursolic acid) (Shree et al., 2020). Similarly, nine compounds (linarin, eudesmol, cadinene, geranyl acetate, alpha-thujene, germacrene A, kaempferol-3-O-glucuronide, kaempferide, and baicalin) out of 127 phytoconstituents from Mentha arvensis, Coriandrum sativum, and O. tenuiflorum were found to have potentially high binding affinity towards the catalytic pocket of N protein, with high stability and few fluctuations, as well as favourable drug-likeness (Fig. 2C) (Muthumanickam et al., 2021).
A major limitation of all in silico studies that have thus far been included in this review (Banerjee et al., 2021; Borse et al., 2021; Chikhale et al., 2021; Dhawan et al., 2021; Enmozhi et al., 2021; Kiran et al., 2020; Kumar et al., 2020c; Maurya and Sharma, 2020; Maurya et al., 2020a, 2020b; Mulpuru and Mishra, 2021; Murugesan et al., 2021; Muthumanickam et al., 2021; Parida et al., 2020; Sagar and Kumar, 2021; Shree et al., 2020; Shukla et al., 2021; Srivastava et al., 2020; Tripathi et al., 2021; Upadhyay et al., 2020) is that none have investigated the potential synergistic effects. Given that whole herbs are used for Ayurvedic formulations, it is not clear whether the activities attributed to individual phytoconstituents would be functional in practice. Therefore, further laboratory studies using whole herb extracts, and observational clinical studies in cohorts using Ayurvedic formulations are needed to investigate the in silico predictive studies for potential therapeutic properties.
To the best of our knowledge, only one study included in the present review used a disease association model and attempted to predict synergy among component molecules to contribute to the immunomodulatory potential of the whole extract (Banerjee et al., 2021). The 12 phytoconstituents identified in an ethanolic extract of A. paniculata were further analysed in silico by network pharmacology methods. The gene-disease network analysis identified several targets that were associated with viral infections and immunodeficiency. Andrographolide and andrographodin A were predicted to interact with important immune markers (toll like receptors) and to modulate the PI3K-AKT pathway that is implicated in coronavirus infection (Kindrachuk et al., 2015). The authors conclude that there is a synergistic action between phytoconstituents that may affect an anti-inflammatory response.
In summary, in silico studies clearly show that phytoconstituents of commonly used Ayurvedic herbal formulations have potential to inhibit SARS-CoV-2 infection and COVID-19 disease. As most formulations are commonly delivered orally, it is not clear if, and how, the bioactive compounds reach the respiratory system, thus the question whether some of the interactions with host factors take place in circulation and hence reduce systemic disease markers.
Pre-clinical investigations
Based on our searches, only five studies (Balkrishna et al., 2021b, 2021c, 2020; Gheware et al., 2021a; Upadhyay et al., 2020) have tested the ability of selected bioactive phytoconstituents of Ayurvedic herbs to directly bind and/or inhibit the function of SARS-CoV-2 proteins. Terminalia chebula is the major constituent of the Ayurvedic formulation known as Triphala and it has been reported to be beneficial in disease caused by herpes simplex virus-2 (Kesharwani et al., 2017) and Influenza A virus (Li et al., 2020). The aqueous extract of T. chebula was shown to be effective in inhibiting the proteolytic activity of SARS-CoV-2 3CLpro in an in vitro protease assay based on cleavage of casein (Upadhyay et al., 2020).
Withanone, a steroidal lactone, highlighted repeatedly as a potential inhibitor of ACE2-S protein interaction in molecular docking studies, was found to inhibit the interaction of the ACE2 receptor with the RBD of S protein in vitro, in a dose dependent fashion (Balkrishna et al., 2021c). Importantly, a herbal extract of W. somnifera enriched with withanone was able to ameliorate pathological symptoms in a zebrafish xenotransplant model that used the S protein as a marker for SARS-CoV-2 infection. The same group also investigated T. cordifolia (Balkrishna et al., 2021b) and Swasari Vati (Balkrishna et al., 2020) as potential treatment options for COVID-19 using the zebrafish xenotransplant model. Swasari Vati is a calcium rich Ayurvedic mixture of 16 herbs or their extracts, processed into a tablet containing three excipients and is commonly prescribed for chronic cough, common cold and asthma. Treatment with Swasari Vati led to the complete recovery of the zebrafish as measured by renal degeneration, necrosis, and survival. Importantly, Swasari Vati treatment showed progressive recovery of immune cell infiltration with very few pro-inflammatory infiltrates, suggesting its effectiveness in reducing the inflammatory damage caused by SARS-CoV-2 spike protein (Balkrishna et al., 2020). In a similar study, T. cordifolia extract in the form of orally delivered powdered tablet, was also found to be effective in reversing the pathological symptoms in the zebrafish model (Balkrishna et al., 2021b).
Only one group investigated the effect of an Ayurvedic herbal extract in a mouse model of COVID-19 disease (Gheware et al., 2021a). The authors have previously shown that an extract of Justicia adhatoda (one of the component herbs in KSK, see above) has an anti-hypoxic property and can reduce severe airway inflammation induced by an augmented hypoxic response in a mouse model of treatment-resistant asthma (Gheware et al., 2021b). The group extended their investigations to show that J. adhatoda extract can inhibit SARS-CoV-2 replication in cell culture and reverse the pulmonary fibrosis in a bleomycin mice model where treatment with the extract resulted in reduced pro-inflammatory mediators and markers of hypoxia. The authors concluded that J. adhatoda extract may be a potential candidate for development of anti-SARS-CoV-2 treatment.
Clinical trials
A number of clinical trials are currently ongoing or have been completed on the use of Ayurvedic formulations in mild and moderate COVID-19 disease. The clinical trials are mostly observational studies on cohorts of patients who are using Ayurvedic formulations as part of their treatment regime. In this context, the Indian Government has set up guidelines for the clinical management of COVID-19 patients based on Yoga and Ayurveda (Government of India, 2020) that includes preventative measures for general use in the community.
There were 51 completed clinical trials on the use of Ayurvedic formulations to prevent or treat COVID-19 registered at ClinicalTrials.gov (https://clinicaltrials.gov) and Clinical Trial Registry-India (http://ctri.nic.in/Clinicaltrials/login.php) as of 20th March 2022. The protocol details of all completed clinical trials on different Ayurvedic formulations are provided in Table 2 . The majority are small, single-center trials undertaken in different jurisdictions in India in SARS-CoV-2 positive subjects with asymptomatic or mild disease, comparing the Ayurvedic treatments with standard care. The outcomes of these studies varied considerably between the studies; however, trial outcome data was only available for 17 completed trials and these are discussed below.
Table 2.
List of completed clinical studies with Ayurvedic formulations.
| Formulation/ Compound | Study design | clinical phase | Intervention details | Outcome measures | Sample size | clinical study identifier | ||||||
| Dabur™ Chyawanprash (DCP) | Randomised, Parallel-Group Trial | N/A | Adults (13 −70 years): One teaspoonful (approx. 12 gm of Chyawanprash) twice daily, followed by milk Children (5–12 years): Half teaspoonful (approx. 6 gm of Chyawanprash) twice daily, followed by milk |
Comparative assessment of the incidence of COVID-19 in subjects who take DCP and those who do not take it for three months (90 days) | 600 | CTRI/2020/05/024,981 | ||||||
| AYUSH 64 | Randomised, Parallel Group, Active Controlled Trial | Phase 2/ Phase 3 | Capsules (500 mg each) thrice a day after food | Early recovery from COVID-19 infection | 80 | CTRI/2020/05/025,214 | ||||||
| Kabasura Kudineer | Randomised, Parallel-Group Trial | Phase 1/ 2 | Kabasura Kudineer 60 ml twice a day for 14 days | Reduction in Viral load, clinical recovery | 50 | CTRI2020/05/025,215 | ||||||
| Ashwagandha, | Randomised, Parallel-Group, Placebo-Controlled Trial | N/A | 500 mg twice a day after Breakfast/ Dinner | Reduction in viral load | 120 | CTRI/2020/05/025,273 | ||||||
| Giloy, | 1000 mg twice a day after Breakfast/ Dinner | |||||||||||
| Tulsi | 500 mg twice a day after Breakfast/Dinner | |||||||||||
| Anu Taila | 4 drops twice a day | |||||||||||
| Swasari Ras | 2 gm twice a day before Breakfast/ Dinner | |||||||||||
| Chyawanprash | Randomised, Parallel-Group Trial | Phase 3 | Twice a day - on an empty stomach in the morning at least 1 h before breakfast and in the evening two hours after dinner | Percentage of participants with SARS- Cov-2 positivity | 200 | CTRI/2020/05/025,275 | ||||||
| Sanshamani Vati, Nagaradi kwath, |
Single Arm Trial | Phase 3 | 1 gm twice a day with 50 ml kwath decoction | Time and number of patients transitioning from an asymptomatic to a symptomatic state | 50 | CTRI/2020/05/025,276 | ||||||
| Amalaki Churna, | 3 gm with water once a day at 5 pm, for 14 days | |||||||||||
| Golden Milk | Once daily, 9 pm, for 14 days | |||||||||||
| Tab PINAK | Single Arm Trial | Phase 2 | Moderate to severe illness: 4 times a day for five days; Mild: 3 times a day for five days. | Early recovery and reduced mortality | 30 | CTRI/2020/05/025,326 | ||||||
| AYUSH-64 | Single Arm Trial | Phase 3 | 2 Tablets (500 mg) thrice daily | Clinical recovery | 40 | CTRI/2020/05/025,335 | ||||||
| AYUSH −64 | Single Arm Trial | Phase 2/ Phase 3 | 2 Tablets (500 mg each) thrice a day after food | Early recovery and changes in liver enzymes, Renal functions | 40 | CTRI/2020/05/025,338 | ||||||
| Guduchi Ghan Vati | Non-randomised, Multiple Arm Trial | N/A | 500 mg two times a day for 30 days. | Comparative assessment of incidence of infection in clinically stable participants in the community who have already identified at least one confirmed case with the standard prophylaxis control arm | 30,000 | CTRI/2020/05/025,385 | ||||||
| Curcumin with black pepper | Randomised, Parallel-Group Trial | N/A | 525 mg. Two to four tablets a day. | Variations in d-Dimer, CRP, LDH, CBC, Ferritin, Troponin, cardiac myoglobin, PT INR, clinical improvement. | 50 | CTRI/2020/05/025,482 | ||||||
| CLEVIRA | Randomised, Parallel-Group Trial | Phase 3 / Phase 4 |
Tablet twice daily for 14 days | Recovery and reduction of viral load | 100 | CTRI/2020/05/025,483 | ||||||
| Ashwagandha (Shakti) | Single Arm Trial | Phase 3/ Phase 4 | 5 drops three times a day | Early clinical recovery | 50 | CTRI/2020/06/025,592 | ||||||
| Amruth, | 1 tablet twice a day | |||||||||||
| Turmeric, | 1 tablet twice a day | |||||||||||
| Tulsi | 10 drops thrice a day | |||||||||||
| Shunthi | Randomised, Parallel Group, Active Controlled Trial | Phase 2 | 10 day treatment 2 gm twice daily |
To minimize the likelihood of disease progression in terms of severity. Reduction of viral load and clinical recovery |
120 | CTRI/2020/06/025,800 | ||||||
| Rasona | 1 gm once daily | |||||||||||
| Vyaghryadi Kashaya | 50 ml twice daily | |||||||||||
| Samshamani Vati | 500 mg twice daily | |||||||||||
| Agnikumara Rasa | 125 mg twice daily | |||||||||||
| Septilin and Bresol | Randomised, Parallel Group, Active Controlled Trial | Phase 2/ Phase 3 | One tablet twice daily from day one upto14 days. | Early recovery and changes in liver enzymes, Renal functions To minimize the likelihood of disease progression in terms of severity. |
40 | CTRI/2020/06/025,801 | ||||||
| AYUSH-64 | Randomised, Parallel-Group Trial | Phase 2 | 2 Capsules of 500 mg thrice daily for 14 days | Recovery. | 200 | CTRI/2020/06/025,855 | ||||||
| Kabasura Kudineer | Non-randomised, Multiple Arm Trial | Phase 2 | 60 ml twice a day, before food. | Resolution of Symptoms and recovery | 200 | CTRI/2020/06/025,856 | ||||||
| Vasantha kusumakaram | 1 tablet twice a day, after food. | |||||||||||
| Thippili Rasayanam | 2 g twice a day, after food. | |||||||||||
| Adathodai Manapagu | 15 ml twice a day with 30 ml lukewarm water, after food for 14 days | |||||||||||
| Arogya Kashayam-20 | Randomised, Parallel Group, Active Controlled Trial | Phase 2 | 100 ml Twice a day for ten days | Check the progression of the disease | 100 | CTRI/2020/06/026,221 | ||||||
| Dashamula Kwatha, Pathyadi Kwatha, Trikatu Churna |
Randomised, Parallel Group, Active Controlled Trial | N/A | Decoction of 20 ml each of the Kwathas and 2 gm Trikatu Churna twice a day on an empty stomach | Clinical recovery | 25 | CTRI/2020/07/026,433 | ||||||
| Sansamani Vati, | 500 mg two tablets twice a day after meal, | |||||||||||
| AYUSH-64, | Two tablets twice a day after a meal, | |||||||||||
| Yastimadhu Ghanavati | One chewable tablet (500 mg) six times a day every two hours in a daytime | |||||||||||
| Immusante and Guduchi | Randomised, Parallel Group, Active Controlled Trial | Phase 3 | Each tablet: 1 tablet twice daily for 30 days | Improvement in the immune status based on the adapted Immune status | 100 | CTRI/2020/07/026,579 | ||||||
| Surasadi Kadha, | Single Arm Trial | Phase 2/ Phase 3 | 50 ml twice a day for seven days | Clinical recovery | 70 | CTRI/2020/07/026,601 | ||||||
| ViroNil, | Capsule (500 mg) daily for seven days | |||||||||||
| Bilvadi | Capsule twice a day for seven days | |||||||||||
| SSV Formulation | Randomised, Parallel Group, Active Controlled Trial | Phase 3 | Tablet of (500 mg), twice a day immediately after meals for ten days | Reduction of clinical symptoms and disease severity | 200 | CTRI/2020/07/026,839 | ||||||
| Guduchi Ghan vati | Randomised, Parallel Group, Active Controlled Trial | N/A | Two capsules/tablet (250 mg each) twice a day after food | Early recovery. | 30 | CTRI/2020/07/026,840 | ||||||
| Kabasura Kudineer, Nilavembu Kudineer | Double Blinded, Three arm, Single center, Placebo Controlled, Randomized Controlled Trial | N/A | Placebo or NVK or KSK, 60 ml Morning and Night after Food, along with standard Allopathy Treatment for 10 days. |
Reduction in viral load | 125 | CTRI/2020/08/027,286 | ||||||
| Immunofree | Randomised, Parallel-Group Trial | Phase 3/ Phase 4 | Two tablets 500 mg thrice a day for ten days | Early recovery. | 100 | CTRI/2020/08/026,957 | ||||||
| Reginmune | One capsule 750 mg twice a day for ten days | |||||||||||
| Nochi Kudineer Chooranam, | Randomised, Parallel Group, Active Controlled Trial | Phase 2 | Decoction – Two times daily before 30 min of meals for seven days | Reduction of viral load and clinical recovery | 100 | CTRI/2020/08/026,999 | ||||||
| Mahasudarsan Chooranam, |
5 gm – Two times daily after meals for seven days with warm water/ Honey. | |||||||||||
| Maldevi Chendooram, | 100 mg. Two Times daily, after meals for seven days with honey. | |||||||||||
| Adathodai Manapagu, | 10 ml- Morning and Evening for seven days. | |||||||||||
| Omatheeneeer | 10 ml –Twice daily with water after meals for seven days. | |||||||||||
| Shakthi, | N/A | Phase 3/ Phase 4 | 5 drops, three times a day | Changes in anti-oxidant biomarkers (Superoxide dismutase, Catalase, MalondialdehydeGlutathione) and Immune biomarkers (IFN-α, IFN- β) | 100 | CTRI/2020/08/027,009 | ||||||
| Amruth, | 1 tablet, two times a day | |||||||||||
| Turmeric Plus, | 1 tablet, two times a day | |||||||||||
| Tulsi Arka | 10 drops, three times a day | |||||||||||
| Ashwagandha Tablet, | Randomised, Parallel Group, Active Controlled Trial | Phase 2/ Phase 3 | 2 Tablets 250 mg each, twice a day, 2 hrs after food | Early recovery | 60 | CTRI/2020/08/027,224 | ||||||
| Shunti Capsule | 2 capsules 500 mg each, twice a day at least 30 min after food | |||||||||||
| T-AYU-HM Premium | Single Arm Trial | Phase 2 | 600 mg twice a day for 21 days | Changes in clinical symptoms | 30 | CTRI/2020/08/027,477 | ||||||
| Kabasura Kudineer, | Single Arm Trial | Decoction – Two times daily before 30 min of meals for seven days | Reduction in viral load and clinical symptoms | 20 | CTRI/2020/08/027,490 | |||||||
| Amukkara, | 2 tablets - Three times daily after meals for seven days | |||||||||||
| Thaleesadhivadagam, | 2 Chewable tablets – Three times daily after meals for seven days | |||||||||||
| Brammanandhabairavam, | 1 pill - Two times daily after meals for seven days | |||||||||||
| Adathodaimanapagu | 10 ml –Twice daily with warm water after meals for seven days | |||||||||||
| Kabasura Kudineer | Single Arm Trial | Phase 2 | Decoction – Twice daily 30 min before meals for 7 days | Reduction in viral load and clinical symptoms | 20 | CTRI/2020/08/027,491 | ||||||
| Amukkara Chooranam Mathirai | Two tablets - Thrice daily after meals for 7 days | |||||||||||
| Thaleesadhivadagam | Two tablets – Thrice daily after meals for 7 days | |||||||||||
| Brammanandhabairavam | One pill - Twice daily after meals for 7 days | |||||||||||
| Adathodaimanapagu | 10 ml –Twice daily with warm water after meals for 7 days | |||||||||||
| Elaeocarpus sylvestris var. ellipticus extract | Randomised, Parallel-Group, Placebo-Controlled Trial | Phase 2 | 480 mg per day orally for ten days | Early, changes in mental health, and changes in levels of prostaglandin E2, TNF α, and IL-6 | 60 | CTRI/2020/09/027,775 | ||||||
| Tulsi, | Randomised, Parallel-Group Trial | Phase 2/ Phase 3 | 1 tablet two times a day for 28 days, | Early recovery | 72 | CTRI/2020/09/027,914 | ||||||
| Giloy ki Ghanvati, | 1 tablet two times a day for 28 days | |||||||||||
| Kalmegh, | 1 tablet two times a day for 28 days | |||||||||||
| Dabur™ Chyawanprash | 1 teaspoonful (Approx 10–12 gs) two times a day for 28 days | |||||||||||
| AyurCoro-3 | Single Arm Trial | N/A | 10 ml of liquid, three times a day for one day, repeat every 15 days for three months | Clinical recovery | 120 | CTRI/2020/10/028,324 | ||||||
| AyurCoro-3 | Randomised, Parallel-Group Trial | Phase 3/ Phase 4 | 10 ml three times a day for three days. Medication for a day | Clinical recovery | 500 | CTRI/2020/10/028,333 | ||||||
| Bilwadi Yog, | Randomised, Parallel Group, Active Controlled Trial | Phase 3 | 1 gm twice a day | Early recovery from COVID-19 infection and reduction in viral load | 380 | CTRI/2020/10/028,437 | ||||||
| Kantakaryavaleha | 3 gm twice a day | |||||||||||
| *Shadangodak | 40 ml twice a day | |||||||||||
| NIFAy.C-19 (CONTAZAP) | Randomised, Parallel-Group Trial | Phase 3/ Phase 4 | 640 mg- 2 Capsules, twice daily after meal. | Clinical recovery | 120 | CTRI/2021/01/030,169 | ||||||
| Jeevaneeyam, Ojovardhini, Amrutha Sanjeevini |
Non-randomised, Active Controlled Trial | Phase 3/ Phase 4 | Twice a day after meal for 14 days |
Reduction in clinical symptoms and early recovery | 60 | CTRI/2021/02/031,353 | ||||||
| Kabasura Kudineer | Randomised Parallel | Phase 4 | Two tablets a day thrice before food | Early clincal recovery | 200 | CTRI/2021/04/032,755 | ||||||
| AEV01 (Kutki/ Picrorhizakurroa) | Randomised, Parallel-Group, Placebo-Controlled Trial | Phase 3 | Thrice daily orally after food for 30 days | Alleviation of COVID-19 symptoms. | 70 | CTRI/2021/04/032,804 | ||||||
| Zandu™ Chyawanprash, | Randomised, Parallel-Group Trial | Phase 2/ Phase 3 | 10–12 gm twice daily for 8 weeks | Clinical recovery. Comparative assessment of post-clinical recovery based on signs and symptoms. |
60 | CTRI/2021/01/030,733 | ||||||
| Zandu™ Pure Honey, | 1 teaspoonful twice daily for 8 weeks | |||||||||||
| *Trishun, | 1 tablet twice daily for 2 weeks | |||||||||||
| Immuzan | 2 tablets twice daily for 8 weeks | |||||||||||
| CIM-MEG19 | Non-randomised, Active Controlled Trial | Phase 2 | 2 Tablets twice daily after meal for 21 days | Recovery and improvement in WHO-QOL | 80 | CTRI/2021/05/033,472 | ||||||
| CIM-MEG19 | Randomised, Parallel Group, Active Controlled Trial | Phase 2 | Two tablets two times a day after meal | Changes in time to 2-point improvement (from time of enrolment) on the WHO ordinal scale | 80 | CTRI/2021/05/033,543 | ||||||
| NOQ19 | Randomised, Parallel Group-Placebo Controlled Trial | Phase 4 | Two tablets– Thrice daily after meals for 3 months | Prevention of COVID-19 and symptom severity in infected cases | 5000 | CTRI/2021/07/034,606 | ||||||
| NOQ19 | Randomised, Parallel Group-Placebo Controlled Trial | NA | Two tablets– Thrice daily for 14 days | Duration of hospital stay, resolution of symptoms, and early clinical recovery | 100 | CTRI/2022/01/039,370 | ||||||
| Amukkura chooranam | NA | Phase 2 | 5 gs, twice daily for 15 days | Prevention or reduction of post COVID-19 complications | 1409 | CTRI/2021/07/035,028 | ||||||
| NOQ19 | Randomised, Parallel Group-Placebo Controlled Trial | Phase 2 | Two tablets– Thrice daily for 10 days | Early clinical recovery and symptoms | 155 | CTRI/2021/08/036,025 | ||||||
| NOQ19 | Randomised, Parallel Group-Placebo Controlled Trial | Phase 3/ 4 | Two tablets-Three times a day for 14 days | Reduction in Viral load, clinical recovery | 40 | CTRI2021/10/037,423 | ||||||
| Guduchi Ghan Vati | Retrospective study | N/A | Two tablets (500 mg each) twice daily were given orally after meal for 28 days | Early recovery and reduction in viral load | 91 | NCT04480398 | ||||||
| Guduchi Ghan Vati | Single-arm study | N/A | Two tablets (1000 mg) twice daily for two weeks | Early recovery and reduction in viral load | 46 | NCT04542876 | ||||||
| Shanshamani Vati plus (Guduchi and Pipli) | Single Group Assignment | N/A | Shanshamani Vati Plus was given twice daily | Alleviation of symptoms | 26 | NCT04621903 | ||||||
| Ashwagandha, | Community-Based Participatory Research | N/A | 250 mg – 5 g based on age, weight and severity of symptoms | Early recovery | 28 | NCT04716647 | ||||||
| Giloy | 500 mg – 1 g based on age, weight and severity of symptoms | |||||||||||
| Tulsi | 500 mg–1 g based on age, weight and severity of symptoms | |||||||||||
| Guduchi Ghanvati | Non-Randomized | N/A | 500 mg of Samshamani vati or Giloy Ghanavati twice daily | Clinical recovery | 216 | NCT04920773 | ||||||
Various formulations containing T. cordifolia, A. paniculata, W. somnifera, O. tenuiflorum, Z. officinale, T. chebula, C. longa, and E. officinalis have shown positive results in prophylaxis and treatment of COVID-19. Several clinical studies have been reported on the use of KSK, with reduction in SARS-CoV-2 viral load and faster recovery of COVID-19 patients (CTRI2020/05/025,215, CTRI/2020/08/027,286), (Natarajan et al., 2020, 2021; Srivastava et al., 2021a, 2021b). Patients in the placebo group had a significantly longer stay in the hospital (mean ± SD = 8.4 ± 2.0 days) as compared to patients receiving KSK (mean ± SD = 4.2 ± 1.5 days; Kruskal-Wallis test, P = 0.0001) (Srivastava et al., 2021b). Further study showed that patients given KSK as a decoction had a larger reduction in viral load as compared to the control group of patients who received vitamin C and zinc tablets (no statistical analysis provided) (Natarajan et al., 2021).
In one clinical trial (CTRI/2020/06/025,856), several Ayurvedic formulations were evaluated in combination with a ‘standard treatment’ which consisted of five pharmaceuticals and 2 nutraceuticals. It should be noted that none of the seven drugs have been shown to have any direct effect on SARS-CoV-2 or COVID-19 although some have generalised effects that may be beneficial (Siemieniuk et al., 2020). The pharmaceuticals included were Hydroxychloroquine, Ivermectin, azithromycin, paracetamol, Omez as well as Vitamin C and zinc. The Ayurvedic add on treatment included KSK, Adathodai manapagu, Vasantha kusumakaram mathirai and Thippili rasayanam; all of which are used in the community for relief of respiratory symptoms. The authors reported improved recovery of those given the Ayurvedic formulations as add-on to the ‘standard treatment’ (Chitra et al., 2021), with a reduction in the average number of days taken for symptoms to improve following treatment (3.21 with add-on vs 5.13 with only standard treatment (P < 0.001)). This was not surprising as most of the components in the ‘standard treatment’ have not been shown to have a direct effect on COVID-19 clinical progression (Bartoszko et al., 2021; Siemieniuk et al., 2021).
A trial by Thakar et al. (CTRI/2020/07/026,433) compared the effect of ‘standard treatment’ (Vitamin C, azithromycin, and paracetamol) with an Ayurvedic add-on treatment (Thakar et al., 2021). The Ayurvedic add-on treatment consisted of the State Government recommended combination of Dashamula and Pathyadi kwatha, Trikatu churna, Guduchi ghan vati, AYUSH-64 and Yastimadhu ghanvati, all of which are polyherbals used primarily in respiratory and influenza like illness. Dashamula, alone and in combination with aspirin has previously shown to have anti-inflammatory, analgesic, and anti-platelet effects comparable to aspirin alone (Parekar et al., 2015). Trikatu powder contains black and long pepper and probably augments the metabolic process by improved absorption of nutrients through the action of the phytoconstituent piperine (Zhang et al., 2021). Given its predicted ability to bind to the S protein (Maurya et al., 2020b), piperine may also have a role in inhibiting SARS-CoV-2 infection. Yastimadhu (G. glabra) or licorice is used widely in both Ayurveda and Traditional Chinese Medicine and shown to have antimicrobial activity (Wang et al., 2015). The AYUSH-64 is a polyherbal formulation with its basis in Ayurvedic herbs and has previously been shown to be effective in malaria (Willcox, 2011) and in influenza like illness (Gundeti et al., 2020). In this study participants in the ‘add-on treatment’ group showed similar outcomes to CTRI/2020/06/025,856 clinical trial with shortened duration of symptomatic phase of the infection compared to those on standard treatment alone (mean ± SD = 3.66 ± 1.55 compared to 5.34 ± 3.35 days, p < 0.001); no adverse reactions or drug interactions were observed. There was no difference in progression to symptomatic disease (27.6% compared to 24.6%).
A retrospective, open label clinical study by Balkrishna et al. (2021a) found that Ayurvedic treatment alone led to shorter time to recovery of COVID-19 patients, when compared to patients receiving the Ayurvedic treatment as an add-on with standard drug treatment (azithromycin with Vitamin-C and anti-histamines (not specified) and acetaminophen as prescribed) (Balkrishna et al., 2021a). The Ayurvedic treatment consisted of the polyherbal formulations Swasari Ras, Tulsi Ghanvati and Anu Taila, and herbal extracts of T. cordifolia and W. somnifera. A trial by Devpura et al. (2021) in a geographically distant region of India (CTRI/2020/05/025,273) compared the anti-COVID-19 efficacy of the same Ayurvedic treatment with placebo (Devpura et al., 2021) and found that treatment was better than placebo in reducing time to a negative diagnostic test. Several phytoconstituents of both T. cordifolia and W. somnifera are predicted to bind to SARS-CoV-2 proteins S and Mpro and the ACE2 receptor in molecular docking studies (refer to section above) and oral T. cordifolia treatment reversed pathology in a zebrafish xenotransplant model of COVID-19 (Balkrishna et al., 2021b). Interestingly, totality of evidence from in silico studies, animal models and clinical trials strongly suggest a multipronged mechanism underlying the observed effects of T. cordifolia on COVID-19.
A trial by Rais et al. (CTRI/2020/06/025,800) evaluated the efficacy of Vyaghryadi kashaya and Samshamani vati or Shunthi churna and Rasona kalka relative to ‘standard treatment’ of paracetamol with Vitamin C (Rais et al., 2021) in SARS-CoV-2 positive subjects with very mild or no clinical symptoms. Participants on either Ayurvedic treatment had a shorter period to recovery. 98–100% of participants on the Ayurvedic ‘add-on’ treatments tested negative for COVID-19 on day 7, compared to 68% in the ‘standard treatment’ group (p < 0.001). There was some variation in the efficacy of the two Ayurvedic treatments in relation to specific clinical symptoms of fever, sore throat, cough, and loss of taste.
A treatment regime of T. cordifolia, O. tenuiflorum, A. paniculata tablets and Chyawanprash, an Ayurvedic supplement, as an add-on to (unspecified) ‘standard of care’ was evaluated for efficacy in mild to moderate COVID-19 hospitalized patients (CTRI/2020/09/027,914). The authors found that participants given the add-on treatment with the ‘standard of care’ had a shorter period to recovery (mean ± SD = 10.77 ± 3.24 days), compared to those on ‘standard of care’ alone (16.30 ± 5.93; p < 0.001)(Gupta et al., 2021).
Two separate retrospective clinical trials studied the efficacy of T. cordifolia extract in confirmed asymptomatic COVID-19 patients (NCT04480398) compared to ‘standard of care’ (isolation and clinical monitoring). Although no results have been posted on the Clinical Trials site, preliminary findings are included in a preprint article (Kumar et al., 2020b). The treatment group remained asymptomatic and cleared the virus in a shorter period relative to the group on ‘standard of care’. A companion single arm study of the efficacy of T. cordifolia extract with no control group was also conducted by the same group (NCT04542876) (Kumar et al., 2020a) on a cohort of asymptomatic SARS-CoV-2 positive subjects. Of the subjects who completed the study, none developed any symptoms and 95% returned a negative diagnostic test on day 7.
A pilot community-based participatory research study (Back to Roots, NCT04716647) investigated the safety and efficacy of T. cordifolia and P. longum extracts in patients in Leicester, UK, with mild to moderate COVID-19. The primary measures were clinical improvement and deterioration in clinical status to severe/critical. The study outcomes are available as a MedRxiv preprint and showed no participants were hospitalised for medical or oxygen requirements and none developed breathing difficulties with average time to recovery being 4.85 (±.1.8) days compared to 13.5.±.6.4 days in the control group (p < 0.0001) (Kulkarni et al., 2021).
Curcumin, and its bioactive formulations have gained significant attention in recent years for the treatment of inflammatory diseases. One randomized clinical trial (CTRI/2020/05/025,482) investigated the usefulness of Bioperine®, containing curcumin with piperine, as adjuvant therapy for COVID-19 patients (Pawar et al., 2021). In line with the other trials discussed in this review, the authors found that Ayurvedic treatment resulted in early symptomatic recovery compared to the control group who received probiotics. This is the only trial we have found that included patients with mild, moderate and severe disease.
A randomized, controlled, multi-center clinical trial compared W. somnifera extract with hydroxychloroquine for efficacy in pre-exposure prophylaxis (CTRI/2020/08/027,163). The interim results (Chopra et al., 2021) showed that W. somnifera extract was no worse than hydroxychloroquine; the latter had been recommended for such use by the Indian Council for Medical Research. One participant in the W. somnifera group and three in the hydroxychloroquine group contracted COVID-19 during the initial eight weeks.
A recent randomized, placebo controlled, single center clinical trial (CTRI/2021/10/037,423) investigated the efficacy of the polyherbal NOQ19 on the rate of recovery and clinical improvement among moderate to severe COVID-19 patients who were not on a ventilator or in intensive care unit. The outcomes of the trial are available as a preprint on ResearchSquare (Arun et al., 2021). Participants enrolled in the study received either NOQ19 or placebo, along with the ‘standard of care’ which included Doxycycline, Azithromycin, Ivermectin, Vitamin C, Zinc and paracetamol. Hospitalised participants also received supportive care, e.g. intravenous fluids and oxygen therapy, as determined by the overseeing physician. There were more participants with ‘severe’ disease in the NOQ19 arm (59%) compared to the placebo arm (22%) and all enrolled participants were monitored for 14 days. There was no statistically significant difference in recovery times between the two arms as a whole. However, a significant increase in the number of participants returning a negative test for COVID-19 on day 7 was observed in the NOQ19 arm when ‘moderate’ and ‘severe’ participants were considered separately.
The discussion of the limited trials data available is strongly suggestive of some advantage in the use of specific Ayurvedic formulations in the clinical management of COVID-19, with no adverse events associated with such treatment. All treatments used in clinical trials were those used commonly for respiratory indications, and hence did not present a significant risk. As Ayurvedic treatments are well accepted in the global Indian community, and the Government of India has recommended specific Ayurvedic treatments in its COVID-19 management guidelines, the trials have been conducted primarily within that population group.
Conclusions
The emergence of SARS-CoV-2 and the resulting COVID-19 global pandemic has seen a resurgence of research into the antiviral efficacy of Ayurvedic herbal formulations. This is primarily due to the current lack of effective, safe, widely available antiviral treatments in addition to the herbal treatments being relatively cheap and easy to transport without requirement for specific storage conditions. Furthermore, the widespread use of Ayurvedic formulations in India presented an opportunity for these treatments to be examined using well designed and controlled clinical trials.
In the current review article, the evaluation of in silico, in vitro, in vivo and clinical evidence for use of Ayurvedic formulations and their herbal constituents is presented:
-
a
In silico techniques were reported in 20 peer review publications. It appears that a variety of different Ayurvedic formulations exhibit antiviral activities as ACE2 and/or 3CLpro inhibitors. Despite relatively limited literature describing the RNA polymerase inhibitions, these formulations may be considered as a platform for development of future antiviral treatments.
-
b
The findings of pre-clinical animal models were reported in only five studies where Ayurvedic herbs were found to inhibit the proteolytic activity of 3CLpro of SARS-CoV-2 proteins.
-
c
Over 50 clinical trials were completed where Ayurvedic formulations were used to prevent or treat COVID-19 symptoms. A majority of the trials were relatively small in size (less than 30 participants), single-centered, and in participants who were asymptomatic or had mild self-reported symptoms.
Many of the studies in this review have focussed on in silico investigations and the potential of individual phytoconstituents to bind to SARS-CoV-2 proteins. As these formulations are almost always taken as herbal extracts, these studies, although useful in themselves, provide relatively limited understanding of the clinical mechanisms that result in reduced period to recovery observed in clinical trials. Clearly, use of Ayurvedic formulations is warranted in all levels of COVID-19 disease as it is, at least, as effective as some of the other currently available treatments and may be rather beneficial in shortening period to recovery. Furthermore, use of Ayurvedic formulations as an “add-on” treatment to the already established medical interventions can also produce beneficial outcomes in individuals at different levels of SARS-CoV-2 infection (asymptomatic and mildly symptomatic).
Limitations of this literature review include its narrative design and that the studies included are reporting on observational findings with relatively limited descriptions of mechanisms of action. Nevertheless, the findings of this literature review also highlight the possibility of applying knowledge gained from traditional medicine with modern medicine approaches across patient populations in addition to those with COVID-19.
Funding
None
CRediT authorship contribution statement
Anees Ahmed Mahaboob Ali: Investigation, Writing – original draft, Writing – review & editing, Formal analysis. Andrea Bugarcic: Writing – review & editing. Nenad Naumovski: Writing – review & editing, Visualization. Reena Ghildyal: Conceptualization, Writing – original draft, Writing – review & editing, Validation, Visualization.
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
The authors acknowledge the help of Mrs Amanda Bulman in proofreading the final manuscript for English language.
References
- Antonio A.d.S., Wiedemann L.S.M., Veiga-Junior V.F. Natural products’ role against COVID-19. RSC Adv. 2020;10:23379–23393. doi: 10.1039/d0ra03774e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun A., Gupta A., Subramanian S., Kanchibhotla D. Evaluation of an Ayurvedic formulation in clinical recovery of COVID-19 patients: a placebo controlled pilot study among moderate-severe patients. Res. Square. 2021 [Google Scholar]
- Ayatollahi S.A., Sharifi-Rad J., Tsouh Fokou P.V., Mahady G.B., Ansar Rasul Suleria H., Krishna Kapuganti S., Gadhave K., Giri R., Garg N., Sharma R., Ribeiro D., Rodrigues C.F., Reiner Z., Taheri Y., Cruz-Martins N. Naturally occurring bioactives as antivirals: emphasis on coronavirus infection. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.575877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkrishna A., Bhatt A.B., Singh P., Haldar S., Varshney A. Comparative retrospective open-label study of ayurvedic medicines and their combination with allopathic drugs on asymptomatic and mildly-symptomatic COVID-19 patients. J. Herb. Med. 2021;29 doi: 10.1016/j.hermed.2021.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkrishna A., Khandrika L., Varshney A. Giloy ghanvati (Tinospora cordifolia (willd.) Hook. F. and Thomson) reversed SARS-CoV-2 viral spike-protein induced disease phenotype in the xenotransplant model of humanized zebrafish. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.635510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkrishna A., Pokhrel S., Singh H., Joshi M., Mulay V.P., Haldar S., Varshney A. Withanone from Withania somnifera attenuates SARS-CoV-2 RBD and host ACE2 interactions to rescue spike protein induced pathologies in humanized zebrafish model. Drug Des. Devel. Ther. 2021;15:1111–1133. doi: 10.2147/DDDT.S292805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkrishna A., Verma S., Solleti S.K., Khandrika L., Varshney A. Calcio-herbal medicine Divya-Swasari-Vati ameliorates SARS-CoV-2 spike protein-induced pathological features and inflammation in humanized zebrafish model by moderating IL-6 and TNF-alpha cytokines. J. Inflamm. Res. 2020;13:1219–1243. doi: 10.2147/JIR.S286199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., Kar A., Mukherjee P.K., Haldar P.K., Sharma N., Katiyar C.K. Immunoprotective potential of Ayurvedic herb Kalmegh (Andrographis paniculata) against respiratory viral infections - LC-MS/MS and network pharmacology analysis. Phytochem. Anal. 2021;32:629–639. doi: 10.1002/pca.3011. [DOI] [PubMed] [Google Scholar]
- Bartoszko J.J., Siemieniuk R.A.C., Kum E., Qasim A., Zeraatkar D., Ge L., Han M.A., Sadeghirad B., Agarwal A., Agoritsas T., Chu D.K., Couban R., Darzi A.J., Devji T., Ghadimi M., Honarmand K., Izcovich A., Khamis A., Lamontagne F., Loeb M., Marcucci M., McLeod S.L., Motaghi S., Murthy S., Mustafa R.A., Neary J.D., Pardo-Hernandez H., Rada G., Rochwerg B., Switzer C., Tendal B., Thabane L., Vandvik P.O., Vernooij R.W.M., Viteri-Garcia A., Wang Y., Yao L., Ye Z., Guyatt G.H., Brignardello-Petersen R. Prophylaxis against covid-19: living systematic review and network meta-analysis. BMJ. 2021;373:n949. doi: 10.1136/bmj.n949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of Covid-19 - final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borse S., Joshi M., Saggam A., Bhat V., Walia S., Marathe A., Sagar S., Chavan-Gautam P., Girme A., Hingorani L., Tillu G. Ayurveda botanicals in COVID-19 management: an in silico multi-target approach. PLoS ONE. 2021;16 doi: 10.1371/journal.pone.0248479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y., Raju Allam V.S.R., Paudel K.R., Singh S.K., Gulati M., Dhanasekaran M., Gupta P.K., Jha N.K., Devkota H.P., Gupta G., Hansbro P.M., Oliver B.G.G., Chellappan D.K., Dua K. Nutraceuticals: unlocking newer paradigms in the mitigation of inflammatory lung diseases. Crit. Rev. Food Sci. Nutr. 2021:1–31. doi: 10.1080/10408398.2021.1986467. [DOI] [PubMed] [Google Scholar]
- Chen H.Y., Ma C.H., Cao K.J., Chung-Man Ho J., Ziea E., Wong V.T., Zhang Z.J. A systematic review and meta-analysis of herbal medicine on chronic obstructive pulmonary diseases. Evid. Based Complement. Alternat. Med. 2014 doi: 10.1155/2014/925069. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikhale R.V., Gurav S.S., Patil R.B., Sinha S.K., Prasad S.K., Shakya A., Shrivastava S.K., Gurav N.S., Prasad R.S. Sars-cov-2 host entry and replication inhibitors from Indian ginseng: an in-silico approach. J. Biomol. Struct. Dyn. 2021;39:4510–4521. doi: 10.1080/07391102.2020.1778539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitra S.M., Mallika P., Anbu N., NarayanaBabu R., SugunaBai A., David Paul Raj R.S., Premnath D. An open clinical evaluation of selected Siddha regimen in expediting the management of COVID-19 -a randomized controlled study. J. Ayurveda Integr. Med. 2021 doi: 10.1016/j.jaim.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra A., Srikanth N., Patwardhan B., Group A.C.R. Withania somnifera as a safer option to hydroxychloroquine in the chemoprophylaxis of COVID-19: results of interim analysis. Complement. Ther. Med. 2021;62 doi: 10.1016/j.ctim.2021.102768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devpura G., Tomar B.S., Nathiya D., Sharma A., Bhandari D., Haldar S., Balkrishna A., Varshney A. Randomized placebo-controlled pilot clinical trial on the efficacy of ayurvedic treatment regime on COVID-19 positive patients. Phytomedicine. 2021;84 doi: 10.1016/j.phymed.2021.153494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan M., Parmar M., Sharun K., Tiwari R., Bilal M., Dhama K. Medicinal and therapeutic potential of withanolides from Withania somnifera against COVID-19. J. Appl. Pharm. Sci. 2021 [Google Scholar]
- Drozdzal S., Rosik J., Lechowicz K., Machaj F., Szostak B., Przybycinski J., Lorzadeh S., Kotfis K., Ghavami S., Los M.J. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist. Updat. 2021 doi: 10.1016/j.drup.2021.100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enmozhi S.K., Raja K., Sebastine I., Joseph J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach. J. Biomol. Struct. Dyn. 2021;39:3092–3098. doi: 10.1080/07391102.2020.1760136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.S., Liu D.X. Human coronavirus: host-pathogen interaction. Annu. Rev. Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- Gheware A., Dholakia D., Kannan S., Panda L., Rani R., Pattnaik B.R., Jain V., Parekh Y., Enayathullah M.G., Bokara K.K., Subramanian V., Mukerji M., Agrawal A., Prasher B. Adhatoda Vasica attenuates inflammatory and hypoxic responses in preclinical mouse models: potential for repurposing in COVID-19-like conditions. Respir. Res. 2021;22:99. doi: 10.1186/s12931-021-01698-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheware A., Panda L., Khanna K., Bhatraju N.K., Jain V., Sagar S., Kumar M., Singh V.P., Kannan S., Subramanian V., Mukerji M., Agrawal A., Prasher B. Adhatoda vasica rescues the hypoxia-dependent severe asthma symptoms and mitochondrial dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021;320:L757–L769. doi: 10.1152/ajplung.00511.2020. [DOI] [PubMed] [Google Scholar]
- Gundeti M.S., Bhurke L.W., Mundada P.S., Murudkar S., Surve A., Sharma R., Mata S., Rana R., Singhal R., Vyas N., Khanduri S., Sharma B.S., Srikanth N., Dhiman K.S. AYUSH 64, a polyherbal Ayurvedic formulation in Influenza-like illness - Results of a pilot study. J. Ayurveda Integr. Med. 2020 doi: 10.1016/j.jaim.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Vedula S., Srivastava R., Tamoli S., Mundhe N., Wagh D.N., Batra S., Patil M., Pawar H.B., Rai R.K. Prospective, randomized, open-label, blinded end point, two-arm, comparative clinical study to evaluate the efficacy and safety of a fixed Ayurvedic regimen (FAR) as add-on to conventional treatment in the management of mild and moderate COVID-19 patients. J. Pharm. Bioallied. Sci. 2021;13:256–267. doi: 10.4103/jpbs.jpbs_242_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung A.B., Ali M.A., Lee J., Abul Farah M., Al-Anazi K.M. In silico screening of FDA approved drugs reveals ergotamine and dihydroergotamine as potential coronavirus main protease enzyme inhibitors. Saudi J. Biol. Sci. 2020;27:2674–2682. doi: 10.1016/j.sjbs.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havranek B., Islam S.M. An in silico approach for identification of novel inhibitors as potential therapeutics targeting COVID-19 main protease. J. Biomol. Struct. Dyn. 2021;39:4304–4315. doi: 10.1080/07391102.2020.1776158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of India . Ministry of AYUSH. Government of India; 2020. National clinical management protocol based on Ayurveda and Yoga for management of Covid-19. [Google Scholar]
- Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyanthi V., Kumar G.V. COVID-19 outbreak: an overview and India’s perspectives on the managementof infection. Indian J. Sci. Technol. 2020;13:3716–3724. [Google Scholar]
- Kesharwani A., Polachira S.K., Nair R., Agarwal A., Mishra N.N., Gupta S.K. Anti-HSV-2 activity of Terminalia chebula Retz extract and its constituents, chebulagic and chebulinic acids. BMC Complement. Altern. Med. 2017;17:110. doi: 10.1186/s12906-017-1620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindrachuk J., Ork B., Hart B.J., Mazur S., Holbrook M.R., Frieman M.B., Traynor D., Johnson R.F., Dyall J., Kuhn J.H., Olinger G.G., Hensley L.E., Jahrling P.B. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agents Chemother. 2015;59:1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran G., Karthik L., Shree Devi M.S., Sathiyarajeswaran P., Kanakavalli K., Kumar K.M., Ramesh Kumar D. In silico computational screening of Kabasura Kudineer - Official Siddha formulation and JACOM against SARS-CoV-2 spike protein. J. Ayurveda Integr. Med. 2020 doi: 10.1016/j.jaim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni, V., Sharma, N., Modi, D., Kumar, A., Joshi, J., Krishnamurthy, N., 2021. A Community-based participatory research to assess the feasibility of Ayurveda intervention in patients with mild-to-moderate COVID-19. medRxiv, 2021.2001.2020.21250198.
- Kumar, A., Prasad, G., Srivastav, S., Gautam, V.K., Sharma, N., 2020a. Efficacy and safety of guduchi ghan vati in the management of asymptomatic COVID-19 infection: an open label feasibility study. medRxiv, 2020.2009.2020.20198515.
- Kumar, A., Prasad, G., Srivastav, S., Gautam, V.K., Sharma, N., 2020b. A retrospective study on efficacy and safety of guduchi ghan vati for COVID-19 asymptomatic patients. medRxiv, 2020.2007.2023.20160424.
- Kumar V., Dhanjal J.K., Bhargava P., Kaul A., Wang J., Zhang H., Kaul S.C., Wadhwa R., Sundar D. Withanone and Withaferin-A are predicted to interact with transmembrane protease serine 2 (TMPRSS2) and block entry of SARS-CoV-2 into cells. J. Biomol. Struct. Dyn. 2020:1–13. doi: 10.1080/07391102.2020.1775704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Du R., Wang Y., Hou X., Wang L., Zhao X., Zhan P., Liu X., Rong L., Cui Q. Identification of chebulinic acid and chebulagic acid as novel influenza viral neuraminidase inhibitors. Front. Microbiol. 2020;11:182. doi: 10.3389/fmicb.2020.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya D.K., Sharma D. Evaluation of traditional ayurvedic Kadha for prevention and management of the novel Coronavirus (SARS-CoV-2) using in silico approach. J. Biomol. Struct. Dyn. 2020:1–16. doi: 10.1080/07391102.2020.1852119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya V.K., Kumar S., Bhatt M.L.B., Saxena S.K. Antiviral activity of traditional medicinal plants from Ayurveda against SARS-CoV-2 infection. J. Biomol. Struct. Dyn. 2020:1–17. doi: 10.1080/07391102.2020.1832577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya V.K., Kumar S., Prasad A.K., Bhatt M.L.B., Saxena S.K. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. Virusdisease. 2020;31:179–193. doi: 10.1007/s13337-020-00598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra K.P., Singh A.K., Singh S.B. Hyperinflammation and immune response generation in COVID-19. NeuroImmunoModulation. 2020;27:80–86. doi: 10.1159/000513198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulpuru, V., Mishra, N., 2021. Computational identification of SARS-CoV-2 inhibitor in Tinospora cordifolia, Cinnamomum zeylanicum and Myristica fragrans. Virusdisease, 1–7. [DOI] [PMC free article] [PubMed]
- Murugesan S., Kottekad S., Crasta I., Sreevathsan S., Usharani D., Perumal M.K., Mudliar S.N. Targeting COVID-19 (SARS-CoV-2) main protease through active phytocompounds of ayurvedic medicinal plants - Emblica officinalis (Amla), Phyllanthus niruri Linn. (Bhumi Amla) and Tinospora cordifolia (Giloy) - a molecular docking and simulation study. Comput. Biol. Med. 2021;136 doi: 10.1016/j.compbiomed.2021.104683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumanickam S., Kamaladevi A., Boomi P., Gowrishankar S., Pandian S.K. Indian ethnomedicinal phytochemicals as promising inhibitors of RNA-binding domain of SARS-CoV-2 nucleocapsid phosphoprotein: an in silico study. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.637329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan S., Anbarasi C., Sathiyarajeswaran P., Manickam P., Geetha S., Kathiravan R., Prathiba P., Pitchiahkumar M., Parthiban P., Kanakavalli K., Balaji P. The efficacy of Siddha Medicine, Kabasura Kudineer (KSK) compared to vitamin C & Zinc (CZ) supplementation in the management of asymptomatic COVID-19 cases: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:892. doi: 10.1186/s13063-020-04823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan S., Anbarasi C., Sathiyarajeswaran P., Manickam P., Geetha S., Kathiravan R., Prathiba P., Pitchiahkumar M., Parthiban P., Kanakavalli K., Balaji P. Kabasura Kudineer (KSK), a poly-herbal Siddha medicine, reduced SARS-CoV-2 viral load in asymptomatic COVID-19 individuals as compared to vitamin C and zinc supplementation: findings from a prospective, exploratory, open-labeled, comparative, randomized controlled trial, Tamil Nadu, India. Trials. 2021;22:623. doi: 10.1186/s13063-021-05583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Complementary and Integrative Health, N., 2022. Ayurvedic medicine: in depth, in: Health, N.I.o. (Ed.).
- Parekar R.R., Bolegave S.S., Marathe P.A., Rege N.N. Experimental evaluation of analgesic, anti-inflammatory and anti-platelet potential of Dashamoola. J. Ayurveda Integr. Med. 2015;6:11–18. doi: 10.4103/0975-9476.146565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida P.K., Paul D., Chakravorty D. The natural way forward: molecular dynamics simulation analysis of phytochemicals from Indian medicinal plants as potential inhibitors of SARS-CoV-2 targets. Phytother. Res. 2020;34:3420–3433. doi: 10.1002/ptr.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi G.F., Carota G., Castruccio Castracani C., Spampinato M., Manti S., Papale M., Di Rosa M., Barbagallo I., Leonardi S. Nutraceuticals in the prevention of viral infections, including COVID-19, among the pediatric population: a review of the literature. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22052465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar K.S., Mastud R.N., Pawar S.K., Pawar S.S., Bhoite R.R., Bhoite R.R., Kulkarni M.V., Deshpande A.R. Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: a randomized clinical trial. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.669362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rais A., Negi D.S., Yadav A., Arya H., Verma R., Galib R., Ahmad A., Kumar Yadav M., Ahirwar P.N. A Randomized open label parallel group pilot study to evaluate efficacy of Ayurveda interventions in the management of asymptomatic and mild COVID-19 patients-experiences of a Lucknow based level 2 hospital of Uttar Pradesh, India. J. Ayurveda Integr. Med. 2021 doi: 10.1016/j.jaim.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, A., Ranganatha, R., Vikneswaran, G., Sagar, C., Mathu, R., Sherin, M., Sigamani, A., Reddy, M.R.K., 2020. AYUSH medicine as add-on therapy for mild category COVID-19; an open label randomised, controlled clinical trial. medRxiv 12, 20245019.
- Reis G., Dos Santos Moreira-Silva E.A., Silva D.C.M., Thabane L. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob. Health. 2022;10:e42–e51. doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar V., Kumar A.H.S. Efficacy of natural compounds from Tinospora cordifolia against SARS-CoV-2 protease, surface glycoprotein and RNA polymerase. Res. Square. 2021 [Google Scholar]
- Shree P., Mishra P., Selvaraj C., Singh S.K., Chaube R., Garg N., Tripathi Y.B. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants - Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi) - a molecular docking study. J. Biomol. Struct. Dyn. 2020:1–14. doi: 10.1080/07391102.2020.1810778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla R., Singh S., Singh A., Misra K. Two pronged approach for prevention and therapy of COVID-19 (Sars-CoV-2) by a multi-targeted herbal drug, a component of ayurvedic decoction. Eur. J. Integr. Med. 2021;43 doi: 10.1016/j.eujim.2020.101268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemieniuk R.A., Bartoszko J.J., Diaz Martinez J.P., Kum E., Qasim A., Zeraatkar D., Izcovich A., Mangala S., Ge L., Han M.A., Agoritsas T., Arnold D., Avila C., Chu D.K., Couban R., Cusano E., Darzi A.J., Devji T., Foroutan F., Ghadimi M., Khamis A., Lamontagne F., Loeb M., Miroshnychenko A., Motaghi S., Murthy S., Mustafa R.A., Rada G., Rochwerg B., Switzer C., Vandvik P.O., Vernooij R.W., Wang Y., Yao L., Guyatt G.H., Brignardello-Petersen R. Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis. BMJ. 2021;374:n2231. doi: 10.1136/bmj.n2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemieniuk R.A., Bartoszko J.J., Ge L., Zeraatkar D., Izcovich A., Kum E., Pardo-Hernandez H., Qasim A., Martinez J.P.D., Rochwerg B., Lamontagne F., Han M.A., Liu Q., Agarwal A., Agoritsas T., Chu D.K., Couban R., Cusano E., Darzi A., Devji T., Fang B., Fang C., Flottorp S.A., Foroutan F., Ghadimi M., Heels-Ansdell D., Honarmand K., Hou L., Hou X., Ibrahim Q., Khamis A., Lam B., Loeb M., Marcucci M., McLeod S.L., Motaghi S., Murthy S., Mustafa R.A., Neary J.D., Rada G., Riaz I.B., Sadeghirad B., Sekercioglu N., Sheng L., Sreekanta A., Switzer C., Tendal B., Thabane L., Tomlinson G., Turner T., Vandvik P.O., Vernooij R.W., Viteri-Garcia A., Wang Y., Yao L., Ye Z., Guyatt G.H., Brignardello-Petersen R. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Bhargava S., Ganeshan S., Kaur R., Sethi T., Sharma M., Chauhan M., Chauhan N., Chauhan R., Chauhan P., Brahmachari S.K. Big data analysis of traditional knowledge-based Ayurveda medicine. Prog. Prevent. Med. 2018;3:e0020. [Google Scholar]
- Singh S., Kola P., Kaur D., Singla G., Mishra V., Panesar P.S., Mallikarjunan K., Krishania M. Therapeutic potential of nutraceuticals and dietary supplements in the prevention of viral diseases: a review. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.679312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Rengaraju M., Srivastava S., Narayan V., Gupta V., Upadhayay R. A double blinded placebo controlled comparative clinical trial to evaluate the effectiveness of Siddha medicines, Kaba Sura Kudineer (KSK) & Nilavembu Kudineer (NVK) along with standard Allopathy treatment in the management of symptomatic COVID 19 patients - a structured summary of a study protocol for a randomized controlled trial. Trials. 2021;22:130. doi: 10.1186/s13063-021-05041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Rengaraju M., Srivastava S., Narayanan V., Gupta V., Upadhayay R., Kumar J., Parameswaran S., KanakavalliKadarkarai AarthiVelmurugan. Efficacy of two siddha polyherbal decoctions, Nilavembu Kudineer and Kaba Sura Kudineer, along with standard allopathy treatment in the management of mild to moderate symptomatic COVID-19 patients-a double-blind, placebo-controlled, clinical trial. Trials. 2021;22:570. doi: 10.1186/s13063-021-05478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Siddiqui S., Ahmad R., Mehrotra S., Ahmad B., Srivastava A.N. Exploring nature's bounty: identification of Withania somnifera as a promising source of therapeutic agents against COVID-19 by virtual screening and in silico evaluation. J. Biomol. Struct. Dyn. 2020:1–51. doi: 10.1080/07391102.2020.1835725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel A., Wardle J., Lloyd I. The potential contribution of traditional, complementary and integrative treatments in acute viral respiratory tract infections: rapid Reviews in response to the COVID-19 pandemic. Adv. Integr. Med. 2020;7:181–182. doi: 10.1016/j.aimed.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakar A., Panara K., Patel F., Bhagiya S., Goyal M., Bhinde S., Chaudhari S., Chaturvedi S. Add-on Ayurveda treatment for early stage COVID-19: a single center retrospective cohort study from Gujarat, India. J. Evid. Based Integr. Med. 2021;26 doi: 10.1177/2515690X211020685. 2515690X211020685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi M.K., Singh P., Sharma S., Singh T.P., Ethayathulla A.S., Kaur P. Identification of bioactive molecule from Withania somnifera (Ashwagandha) as SARS-CoV-2 main protease inhibitor. J. Biomol. Struct. Dyn. 2021;39:5668–5681. doi: 10.1080/07391102.2020.1790425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay A.K., Kumar K., Kumar A., Mishra H.S. Tinospora cordifolia (Willd.) Hook. f. and Thoms. (Guduchi) - validation of the Ayurvedic pharmacology through experimental and clinical studies. Int. J. Ayurveda Res. 2010;1:112–121. doi: 10.4103/0974-7788.64405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay S., Tripathi P.K., Singh M., Raghavendhar S., Bhardwaj M., Patel A.K. Evaluation of medicinal herbs as a potential therapeutic option against SARS-CoV-2 targeting its main protease. Phytother. Res. 2020;34:3411–3419. doi: 10.1002/ptr.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- V'Kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Yang R., Yuan B., Liu Y., Liu C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B. 2015;5:310–315. doi: 10.1016/j.apsb.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2021a. Coronavirus disease (COVID-19).
- WHO, 2021b . Weekly operational update on COVID-19 - 3 November 2021.
- Willcox M. Improved traditional phytomedicines in current use for the clinical treatment of malaria. Planta Med. 2011;77:662–671. doi: 10.1055/s-0030-1250548. [DOI] [PubMed] [Google Scholar]
- Yang H., Rao Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat. Rev. Microbiol. 2021;19:685–700. doi: 10.1038/s41579-021-00630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zheng Q., Song M., Xiao J., Cao Y., Huang Q., Ho C.T., Lu M. A review on the bioavailability, bio-efficacies and novel delivery systems for piperine. Food Funct. 2021;12:8867–8881. doi: 10.1039/d1fo01971f. [DOI] [PubMed] [Google Scholar]