Abstract
An amoxicillin-resistant (Amoxr) strain of Helicobacter pylori was selected for by culturing an amoxicillin-sensitive (Amoxs) strain in increasingly higher concentrations of amoxicillin, resulting in a 133-fold increase in MIC, from 0.03 to 0.06 μg/ml to 4 to 8 μg/ml. This resistance was stable upon freezing for at least 6 months and conferred cross-resistance to seven other β-lactam antibiotics. β-Lactamase activity was not detected in this Amoxr strain; however, analysis of the penicillin-binding protein (PBP) profiles generated from isolated bacterial membranes of the Amoxs parental strain and the Amoxr strain revealed a significant decrease in labeling of PBP 1 by biotinylated amoxicillin (bio-Amox) in the Amoxr strain. Comparative binding studies of PBP 1 for several β-lactams demonstrated that PBP 1 in the Amoxr strain had decreased affinity for mezlocillin but not significantly decreased affinity for penicillin G. In addition, PBP profiles prepared from whole bacterial cells showed decreased labeling of PBP 1 and PBP 2 in the Amoxr strain at all bio-Amox concentrations tested, suggesting a diffusional barrier to bio-Amox or a possible antibiotic efflux mechanism. Uptake analysis of 14C-labeled penicillin G showed a significant decrease in uptake of the labeled antibiotic by the Amoxr strain compared to the Amoxs strain, which was not affected by pretreatment with carbonyl cyanide m-chlorophenylhydrazone, eliminating the possibility of an efflux mechanism in the resistant strain. These results demonstrate that alterations in PBP 1 and in the uptake of β-lactam antibiotics in H. pylori can be selected for by prolonged exposure to amoxicillin, resulting in increased resistance to this antibiotic.
Helicobacter pylori is the most common cause of gastric and duodenal ulcers and is strongly associated with the development of gastric adenocarcinoma (see references 14 and 32 for reviews). It is estimated that at least a third of the world's population is infected with H. pylori, making it one of the most common infections in humans (14). Successful treatment of H. pylori infections most often employs the use of two or more antibiotics and the addition of either bismuth or a proton pump inhibitor (14, 17, 18). However, H. pylori resistance to many of the commonly used antibiotics in this triple regimen is rising (19), including resistance to metronidazole (1, 27, 36), clarithromycin (1, 6, 9, 27), rifampin or rifabutin (24), and, recently, amoxicillin (11, 12, 13, 22, 38).
Resistance to β-lactam antibiotics by gram-negative bacteria is most commonly due to the production of β-lactamase, either chromosomally encoded or, more often, plasmid mediated (see reference 30 for a review). Other important mechanisms of resistance include alterations in penicillin-binding proteins (PBPs), decreased permeation of the antibiotic into the bacterial cell, or combinations of these resistance strategies (see reference 28 for a review). Active efflux pumps in gram-negative bacteria which excrete drugs, including multidrug efflux pumps, can also confer resistance to β-lactams (see reference 34 for a review).
The PBPs are a set of enzymes involved in the synthesis of the peptidoglycan layer of the bacterial cell wall and include transpeptidases, transglycosylases, endopeptidases, and carboxypeptidases (4, 16). We have previously reported the following molecular masses of four major PBPs in H. pylori ATCC 43579: 66, 63, 60 and 47 kDa (7). Other investigators have also reported three to four PBPs for H. pylori (10, 26, 29). The molecular mass of a small PBP was reported in the range of 30 to 32 kDa by both Dore et al. (10) (named PBPD) and Krishnamurthy et al. (29) (named PBP4). Krishnamurthy et al. (29) also identified three high-molecular-mass PBPs (PBPs 1, 2, and 3) from H. pylori 84–183 in the range of 66 to 55 kDa and indicated that these PBPs corresponded to PBPs A, B, and C, previously described by Ikeda et al. (26). Other PBPs in H. pylori were recently identified by Harris et al. (23) at 72, 62, 54, 50, 44, 33.5, 30.5, and 28 kDa. For consistency with other PBP labeling systems, we shall therefore refer to the three high-molecular-mass proteins identified in our laboratory with apparent molecular masses of 66, 63, and 60 kDa as PBP 1, 2, and 3, respectively. However, we do not believe the PBP of 47 kDa we identified corresponds to either PBP D or PBP 4 (30 to 32 kDa) and will therefore continue to consider this protein from H. pylori ATCC 43579 a putative PBP.
The covalent binding of β-lactam antibiotics to various PBPs results in the inability of the bacterium to build a complete cell wall, ultimately leading to cell lysis and death (15). Alterations in these PBPs which affect the ability of the β-lactams to bind can confer increased resistance of the bacteria to these antibiotics (reviewed in references 21 and 31). Reports of alterations in PBPs which result in resistance to β-lactams include PBPs 3a and 3b of Haemophilus influenzae (35), PBP 1A of Proteus mirabilis (33), PBPs 2b and 2x of Streptococcus pneumoniae (20), and PBPs 1b, 2a, and 2b of S. pneumoniae (25).
In this study we isolated an amoxicillin-resistant strain of H. pylori and characterized the level of antibiotic resistance in this strain, its stability, its β-lactam cross-resistance, and the mechanism(s) responsible for its amoxicillin resistance.
MATERIALS AND METHODS
Bacterial culture conditions.
Stock cultures of H. pylori ATCC 43579 were streaked for isolation on brucella agar (Becton-Dickinson Microbiology, Cockeysville, Md.) supplemented with 10% defibrinated sheep blood (Colorado Serum Company, Denver, Colo.) and 1% IsoVitaleX (Becton-Dickinson Microbiology) and cultured at 37°C in a humidified 10% CO2 incubator. Liquid cultures were prepared by suspension of H. pylori colonies in brucella broth (Difco Laboratories, Detroit, Mich.) supplemented with 10% fetal bovine serum (Gibco Bethesda Research Laboratories, Grand Island, N.Y.) and 1% IsoVitaleX. Cultures were routinely passed by dilution into fresh media at 48-h intervals; however, in some experiments, bacteria were passed into fresh media with increasing concentrations of antibiotic at 72- to 96-h intervals. Freezer stocks of cultures were prepared by resuspending 24-h cultures in 1% proteose peptone–20% glycerol, flash frozen in liquid nitrogen, and kept at −80°C.
Development of amoxicillin resistance and MIC and MBC determination.
Details for determination of the MIC using the broth microdilution method and determination of the minimal bactericidal concentration (MBC) of the β-lactam antibiotics were published previously (7). Liquid cultures of Amoxs H. pylori ATCC 43579 (MIC, 0.03 to 0.06 μg/ml) were passed by dilution into fresh media with increasing concentrations of antibiotic at 3- to 4-day intervals in order to select for amoxicillin resistance. These resulting amoxicillin-resistant (Amoxr) bacteria (MIC, 4 to 8 μg/ml) were frozen and stored at −80°C. These Amoxr isolates were also subcultured in broth media without antibiotic and after freezing to determine the stability of amoxicillin resistance.
β-Lactamase detection.
The production of β-lactamase by these bacteria was tested by the chromogenic cephalosporin method using nitrocefin BBL DrySlides as directed by the manufacturer (Becton-Dickinson) using β-lactamase-positive Staphylococcus aureus ATCC 27760 as a positive control.
Bio-Amox labeling of PBPs.
H. pylori membranes were prepared as previously described (7) with the following modifications. Membrane fractions were prepared from 4 liters of 48-h cultures of Amoxs and Amoxr H. pylori and frozen at −20°C until analyzed. Biotinylated amoxicillin (bio-Amox) was prepared by the method of Dargis and Malouin (5) and kept frozen in aliquots at −80°C for up to 12 months. Membrane pellets were thawed and resuspended in approximately 750 μl of 0.1 M phosphate buffer, pH 7.2 (PBS), and then adjusted for consistent protein concentrations using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.). Equal membrane protein aliquots (approximately 7 to 14 mg/ml) were reacted with bio-Amox at concentrations of 4, 0.4, and 0.04 μg/ml for 30 min at room temperature. Membrane fractions were then prepared as described previously (7), adjusted for consistent protein concentrations, and separated using sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE). For whole-cell labeling studies, 2 ml of 48-h cultures of Amoxs and Amoxr strains were adjusted for consistent optical densities at 600 nm (∼0.25) and labeled with bio-Amox at concentrations of 4, 0.4, and 0.04 μg/ml for 30 min at room temperature. The bacteria were then washed twice in PBS, resuspended in 12 μl of distilled water, and then disrupted in Laemmli sample buffer, boiled, and separated by SDS–10% PAGE. After electrophoresis, proteins were transferred to nitrocellulose and prepared for detection by chemiluminescence as described previously (7). Membranes were exposed to ECL Hyperfilm (Amersham) for 10 to 60 s until banding patterns appeared. Resulting banding patterns were read for absorbance intensities using densitometric tracings with an Ultroscan XL laser densitometer (LKB Products, Bromma, Sweden).
Affinity of PBP 1 for mezlocillin and penicillin G.
Log-phase H. pylori membrane fractions from the Amoxs and Amoxr strains were prepared as described above and incubated with either mezlocillin (Mezlin; Miles, West Haven, Conn.) or penicillin G (penicillin G potassium; Marsham, Cherry Hill, N.J.) at 1, 10, and 100 times the respective MIC for the Amoxs strain for 30 min at room temperature prior to labeling with bio-Amox. The resulting PBP banding pattern intensity was determined as described above.
Uptake studies using [14C]penicillin G.
Log-phase Amoxs and Amoxr cultures were concentrated to 3 × 109 to 5 × 109 bacteria/ml and then were incubated with 6.7 μg of [14C]penicillin G (1 μCi/ml; Amersham) per ml for 1 h. One-milliliter aliquots were taken at 5, 10, 30, and 60 min, centrifuged, and washed four times in PBS. The resulting pellets and washes were then diluted in scintillation fluid (CytoScint; FisherBiotech) and analyzed for radioactivity in a Beckman LS 6500 scintillation counter (Beckman Instruments, Palo Alto, Calif.). Carbonyl cyanide m-chlorophenylhydrazone (CCCP; Sigma) was prepared and stored per the manufacturer's instructions. Amoxr cultures were prepared as described above and incubated with a final concentration of 40 μM CCCP according to the method of Bina et al. (3) 15 min prior to treatment with [14C]penicillin G and then analyzed as described above.
RESULTS
Development and characterization of amoxicillin resistance.
An Amoxr strain of H. pylori ATCC 43579 was obtained by subculturing an Amoxs parental strain (MIC = 0.03 to 0.06 μg/ml) in increasingly higher concentrations of amoxicillin. As shown in Table 1, after 39 passes (a period of ∼4 months), an isolate for which the MIC was 4 to 8 μg/ml was obtained. There were several noticeable plateaus during this subculturing period, particularly at 0.5 and 2 to 4 μg/ml, where the MICs remained constant for up to a month before increasing to the next MIC. However, after reaching 4 to 8 μg/ml, the MIC remained unchanged even after many repeat subcultures in higher concentrations of amoxicillin, and this isolate, designated Amoxr, was stored frozen at −80°C and used for all subsequent experiments described below.
TABLE 1.
Selection of an amoxicillin-resistant strain of H. pylori
| Passesa | No. of passes | MIC (μg/ml) |
|---|---|---|
| 1–5 | 4 | 0.03–0.06 |
| 6–7 | 2 | 0.125 |
| 8–10 | 3 | 0.25 |
| 11–18 | 8 | 0.5 |
| 19–22 | 4 | 1–2 |
| 23–32 | 10 | 2–4 |
| 33–38 | 6 | 4 |
| 39–44 | 6 | 4–8 |
H. pylori ATCC 43579 was passed by dilution into fresh media with increasing concentrations of amoxicillin at 3- to 4-day intervals over a 4-month period (see Materials and Methods for details).
This level of amoxicillin resistance in Amoxr proved to be stable for at least 6 months at −80°C. Resistance was also found to be stable even after subculturing of the Amoxr strain in media without antibiotic for at least five consecutive passes. The MICs and MBCs of eight β-lactam antibiotics for the Amoxs and Amoxr strains are shown in Table 2. The Amoxr strain demonstrated cross-resistance to each of the β-lactams examined, with MICs increasing between 4- and 133-fold. Most noticeably, the MICs for the Amoxr strain increased significantly for ampicillin and penicillin G (both 133-fold), cefuroxime (67-fold), and mezlocillin (32-fold).
TABLE 2.
Determination of the MIC and MBC of each β-lactam antibiotic for the Amoxs and Amoxr strainsa
| β-Lactam | MIC (μg/ml) for strain
|
MBC (μg/ml) for strain
|
||
|---|---|---|---|---|
| Amoxs | Amoxr | Amoxs | Amoxr | |
| Amoxicillin | 0.03–0.06 | 4–8 | 0.125–0.5 | 4–8 |
| Ampicillin | 0.03–0.06 | 4–8 | 0.03–0.06 | 4–8 |
| Penicillin G | 0.03–0.06 | 4–8 | 0.03–0.06 | 8–16 |
| Oxacillin | 2–4 | 31–62 | 4–8 | 62–125 |
| Mezlocillin | 0.125–0.25 | 4–8 | 0.25–1.0 | 4–16 |
| Cefuroxime | 0.03–0.06 | 2–4 | 0.06–0.5 | 4–8 |
| Ceftriaxone | 0.125–0.25 | 4–8 | 0.125–1.0 | 8–16 |
| Aztreonam | 4–8 | 16–31 | 4–8 | 62–125 |
MICs of each β-lactam were determined after 24 to 48 h of incubation at 37°C using a broth microdilution method (7); MBCs were determined by examination of agar plate subcultures after 72 h of incubation at 37°C (7). Data shown are the averages of three separate experiments.
β-Lactamase production.
β-Lactamase activity was not detected in either the Amoxr or Amoxs strains.
PBP profiles.
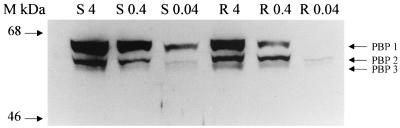
Isolated membrane fractions from both the Amoxs and Amoxr strains were prepared and incubated with bio-Amox as described in the Materials and Methods section, and the PBP profiles for each strain were compared. Results of a representative experiment are shown in Fig. 1, and the data from repeat experiments analyzed quantitatively are presented in Fig. 2. In the range of the MIC for the Amoxs strain (0.04 μg/ml), the banding intensity of PBP 1 in the Amoxr strain was decreased by >95% from that of the Amoxs parental strain and by >50% at 10 times the MIC (0.4 μg/ml). However, at 4 μg/ml (the MIC for the resistant strain), labeling of PBP 1 in both strains was identical. Labeling of PBP 2 and PBP 3 with bio-Amox in the Amoxr strain was comparable to that in the Amoxs strain at each of the bio-Amox concentrations tested.
FIG. 1.
Membranes from Amoxs (S) and Amoxr (R) H. pylori strains were incubated with bio-Amox at 4, 0.4, and 0.04 μg/ml, separated by SDS-PAGE, and visualized on Western blots by chemiluminescence; results of a representative experiment are shown here. Molecular mass markers (M) are on the left.
FIG. 2.
Membranes from Amoxs and Amoxr strains of H. pylori were incubated with bio-Amox at 4, 0.4, and 0.04 μg/ml, separated by SDS-PAGE, and visualized on Western blots by chemiluminescence. These blots were then quantitatively analyzed by laser densitometry. Lightface bars, Amoxs strain; boldface bars, Amoxr strain; open bars, 4 μg of bio-Amox per ml; dotted bars, 0.4 μg of bio-Amox per ml; hatched bars, 0.04 μg of bio-Amox per ml. Abs., absorbance. Data bars represent the means plus standard errors of the means based on three separate experiments.
Additional studies were done using bio-Amox labeling of log-phase whole-cell cultures of the Amoxs and Amoxr strains, and the PBP profiles from these experiments are shown in Fig. 3. In these experiments, decreased labeling of both PBP 1 and PBP 2 was detected at all three bio-Amox concentrations tested. The banding intensity of PBP 1 was decreased by >90% using bio-Amox at the MIC for the Amoxs strain (0.04 μg/ml) and by >50% at 10 and 100 times the MIC. In addition, the banding intensity of PBP 2 was decreased by >75% using bio-Amox at the MIC and by >33 and 50% at 10 and 100 times the MIC, respectively. A decrease in banding intensity for PBP 3 of >75% in the Amoxr strain was detected only with the smallest amount of bio-Amox tested.
FIG. 3.
Intact log-phase cultures of H. pylori cells were incubated with bio-Amox at 4, 0.4, and 0.04 μg/ml, separated by SDS-PAGE, visualized on Western blots by chemiluminescence, and then quantitatively analyzed by laser densitometry. Lightface bars, Amoxs strain; boldface bars, Amoxr strain; open bars, 4 μg of bio-Amox per ml; dotted bars, 0.4 μg of bio-Amox per ml; hatched bars, 0.04 μg of bio-Amox per ml. Abs., absorbance. Data bars represent the means plus standard errors of the means based on three separate experiments.
Affinity of PBP 1 for mezlocillin and penicillin G.
In order to compare the binding affinity of PBP 1 for other β-lactam antibiotics, competitive binding experiments were conducted using 4 μg of bio-Amox per ml, a concentration found previously to just saturate PBP 1 of both strains (data not shown) and above our reported 50% inhibitory concentration (IC50) of amoxicillin for PBP 1 in the Amoxs strain (3 μg/ml) (7). In these studies, isolated membranes from the Amoxs and Amoxr strains were preincubated with various concentrations of mezlocillin or penicillin G prior to addition of bio-Amox. Consequently, the decrease in bio-Amox labeling relative to that in membrane fractions incubated without competing antibiotic represents the ability of each antibiotic to compete with bio-Amox for the binding of PBP 1.
A marked decrease in the affinity of PBP 1 from the Amoxr strain for mezlocillin was demonstrated using 10 times the mezlocillin MIC for the Amoxs strain, with a modest decrease seen at the MIC (Table 3). At 100 times the MIC, labeling of PBP 1 by bio-Amox was nearly identical in both strains. In contrast, there was only a slight drop in affinity of PBP 1 in the Amoxr strain for penicillin G at 10 times the MIC, with no significant difference in bio-Amox labeling of PBP 1 at 1 or 100 times the MIC for the two strains.
TABLE 3.
Binding affinities of PBP 1 for mezlocillin and penicillin G in the Amoxs and Amoxr strainsa
| Abx | Phenotype | Control abs. | 1× MIC
|
10× MIC
|
100× MIC
|
|||
|---|---|---|---|---|---|---|---|---|
| Abs. | % Decreaseb | Abs. | % Decrease | Abs. | % Decrease | |||
| Mezl | S | 2.45 ± 0.03 | 2.04 ± 0.39 | 17 | 0.39 ± 0.19 | 84 | 0.07 ± 0.01 | 97 |
| Mezl | R | 2.51 ± 0.14 | 2.39 ± 0.17 | ND | 1.37 ± 0.38 | 45 | 0.04 ± 0.01 | 98 |
| Pen G | S | 2.87 ± 0.02 | 2.89 ± 0.05 | ND | 2.41 ± 0.14 | 16 | 0.59 ± 0.23 | 79 |
| Pen G | R | 2.57 ± 0.04 | 2.68 ± 0.12 | ND | 2.57 ± 0.11 | ND | 0.48 ± 0.17 | 81 |
H. pylori membrane fractions were preincubated with either mezlocillin or penicillin G at 1, 10, or 100 times the MIC prior to labeling with 4 μg of bio-Amox per ml, separated by SDS-PAGE, and visualized on Western blots. These blots were then quantitatively examined by laser densitometry. Control values represent bio-Amox labeling without preincubation with a competing antibiotic. Abx, antibiotic treatment; Abs., absorbance; Mezl, mezlocillin; Pen G, penicillin G; S, Amoxs H. pylori; R, Amoxr H. pylori; ND, decrease of ≤5%. Data represent the means ± standard errors of the means determined from three separate experiments.
% Decrease, percent decrease in absorbance compared to control.
Uptake of [14C]penicillin G by the Amoxs and Amoxr strains.
Comparison of the quantitative uptake of 14C-labeled penicillin G by the Amoxs and Amoxr strains demonstrated that the Amoxr strain accumulated >40% less [14C]penicillin G than equal numbers of Amoxs bacteria at each time point examined (Fig. 4). When these studies were repeated using the proton translocator CCCP (which would affect the proton motive force), the same level of uptake of [14C]penicillin G by the Amoxr strain was observed (Fig. 5).
FIG. 4.
Log-phase H. pylori 43579 Amoxs and Amoxr cultures were incubated with [14C]penicillin G and 1-ml aliquots were taken at 5, 10, 30, and 60 min, centrifuged, and washed; the resulting pellets and washes were then analyzed for radioactivity in a scintillation counter. Squares, Amoxs strain; triangles, Amoxr strain. Data bars represent the means ± standard errors of the means based on five separate experiments.
FIG. 5.
Log-phase H. pylori 43579 Amoxr cultures were preincubated with either PBS or CCCP for 15 min prior to incubation with [14C]penicillin G for 1 h. One-milliliter aliquots were taken at 5, 10, 30, and 60 min, centrifuged, and washed; the resulting pellets and washes were then analyzed for radioactivity in a scintillation counter. Triangles, Amoxr strain without CCCP treatment; circles, Amoxr strain with CCCP treatment. Data bars represent the means ± standard errors of the means based on three separate experiments.
DISCUSSION
Previous studies (7) have shown that H. pylori strain ATCC 43579 is very susceptible to amoxicillin, one of the major antibiotics used in treatment of H. pylori infections. In this study we isolated an Amoxr strain of H. pylori by subculturing the Amoxs parental strain in increasingly higher concentrations of amoxicillin over 4 months, resulting in a final MIC of 4 to 8 μg/ml, 133-fold higher than the MIC for the original Amoxs strain. Cross-resistance to other β-lactams was also noted, with MICs increasing between 4- and 133-fold. Amoxicillin resistance in β-lactamase-negative H. pylori clinical isolates has also been associated with cross-resistance to other β-lactams (11).
This amoxicillin resistance proved to be quite stable, both to freezer storage and to subculture in media without amoxicillin. Stable amoxicillin resistance has also been described by van Zwet et al. (38) with an H. pylori strain resistant to 8 μg of amoxicillin per ml. Han et al. (22) described both stable and unstable amoxicillin resistance (256 μg/ml) in H. pylori clinical isolates, with the unstable isolates losing resistance after freezer storage. Selection for amoxicillin resistance did not appear to adversely affect the metabolic fitness of the Amoxr strain even after freezer storage, as differences in growth rates between the Amoxs and Amoxr freezer stocks were not detected (data not shown).
Amoxicillin resistance in this Amoxr isolate was not found to be due to the acquisition or expression of a β-lactamase, since β-lactamase activity was not detected in either strain. This was not too surprising, since there are no β-lactamase homologue genes in either of the two strains of H. pylori which have been sequenced (2, 37).
PBP profiles generated by labeling isolated H. pylori membrane fractions showed significantly decreased bio-Amox labeling of PBP 1 in the Amoxr strain compared to that of the Amoxs strain. Previous studies in our laboratory have shown PBP 1 to be a major target for the binding of β-lactam antibiotics (7). In contrast, no significant decrease in bio-Amox labeling of PBP 2 or PBP 3 was observed in the Amoxr strain in these experiments. From these studies it would appear that amoxicillin resistance in H. pylori ATCC 43579 is due, at least in part, to an alteration in PBP 1.
The decreased labeling of PBP 1 by bio-Amox in the Amoxr strain could have been due to either a decreased number of PBP 1 molecules produced or a decreased affinity of PBP 1 for amoxicillin. To address this, the relative affinity of PBP 1 in the Amoxr and Amoxs strains for two other β-lactam antibiotics was investigated. For these studies we selected mezlocillin, which has a low IC50 for PBP 1 in the Amoxs strain (0.125 μg/ml, the same as the MIC for the Amoxs strain), representing a strong affinity of PBP 1 for this β-lactam, and penicillin G, which has a relatively high IC50 for PBP 1 in the Amoxs strain (>3 μg/ml, which is >100 times the MIC for the Amoxs strain), representing a much lower affinity for this β-lactam (7).
From these experiments, it was found that PBP 1 from the Amoxr strain had significantly decreased binding affinity for mezlocillin but had an affinity for binding to penicillin G similar to that of PBP 1 from the Amoxs strain. The decrease in mezlocillin's ability to compete with the bio-Amox label for PBP 1 in the Amoxr strain is consistent with a change in affinity of this PBP for the antibiotic. However, the fact that penicillin G competed comparably in the Amoxs and Amoxr strains indicates that the same number of PBP 1 proteins are produced in these two strains but that their affinity for amoxicillin and mezlocillin has been reduced in the Amoxr strain, rendering this strain more resistant to the effects of amoxicillin.
When the PBP profiles of bio-Amox labeling in the Amoxs and Amoxr strains were examined using whole-cell labeling studies, decreased labeling of all three major PBPs was observed in the Amoxr strain, with significant decreases in PBP 1 and PBP 2 detected at all bio-Amox concentrations tested. Since we had not noted a significant decrease in PBP 2 or PBP 3 labeling using isolated membranes, this suggested the possibility that the bacterial cell membrane of the Amoxr strain was less permeable to amoxicillin than the Amoxs strain. This was confirmed by demonstrating that the Amoxr strain accumulated >40% less [14C]penicillin G than the Amoxs strain. When the Amoxr strain was pretreated with the proton translocator CCCP, which would effectively knock out active transport mechanisms, the level of uptake of [14C]penicillin G by the Amoxr strain was unaltered. These studies demonstrated that the decreased accumulation of [14C]penicillin G by the Amoxr strain is not due to an active efflux mechanism, a result consistent with the observation reported by Bina et al. (3) that active efflux does not play a role in antibiotic resistance in H. pylori. These results suggest that amoxicillin resistance in the Amoxr strain is due in part to an increased diffusional barrier to β-lactam antibiotics in the Amoxr strain compared to the Amoxs strain. The uptake change could be due to an alteration in an outer membrane protein serving as a porin. One candidate for this would be HopE, a nonspecific porin protein in H. pylori, with a large channel through which antibiotics are likely to be able to cross the outer membrane (8).
We were surprised to identify two different antibiotic resistance mechanisms in the Amoxr isolate. However, it seems unlikely that the full increase in the MIC for the Amoxr strain (4 to 8 μg/ml) could be completely explained by the decrease in affinity of PBP 1, because when the highest level of bio-Amox was used (4 μg/ml), there was equivalent binding of bio-Amox to PBP 1 in the Amoxs and Amoxr strain using isolated membranes. When whole cells were labeled by the same method, not only did PBP 1 show a decrease in bio-Amox labeling at 4 μg/ml, but there was also a decrease in PBP 2 labeling in the Amoxr strain. These results pointed to a second mechanism of β-lactam resistance, namely, an increased permeability barrier in the Amoxr strain. This is also supported by the increased resistance of the Amoxr strain to penicillin G without a significant change in affinity of PBP 1 for this antibiotic. Interestingly, there were several plateaus noted during the subculturing experiments which led to the final selection of this Amoxr strain. Thus, one mechanism of resistance may have been selected for prior to a second mechanism of resistance appearing. At what MIC each mechanism was selected for is unknown; future studies are planned to address this question.
The only amoxicillin resistance mechanism of H. pylori previously described was one reported in a study by Dore et al. (10), in which amoxicillin resistance was associated with a loss of PBP D (molecular mass of 30 to 32 kDa) in amoxicillin-resistant clinical isolates. The amoxicillin resistance was associated with an amoxicillin tolerance MBC/MIC ratio of ≥32. In contrast, the amoxicillin resistance characterized in this study appears not only to be stable but also to have MBC/MIC ratios in the range of 1 to 4, in contrast to that observed in strains displaying amoxicillin tolerance as described by Dore et al. (12).
In conclusion, we have identified several mechanisms of amoxicillin resistance in H. pylori ATCC 43579 related to a decreased affinity of PBP 1 for amoxicillin as well as a decrease in uptake of β-lactam antibiotics. Future studies are planned to characterize the change in PBP 1 as well as the mechanism conferring a decreased uptake of β-lactam antibiotics in this Amoxr strain of H. pylori. It will be interesting to determine whether these mechanisms of amoxicillin resistance are present in some of the emerging clinical isolates of H. pylori. A better understanding of antibiotic resistance mechanisms in H. pylori is important in guiding therapeutic choices for treatment and in suggesting alternative strategies at combating these infections.
ACKNOWLEDGMENTS
We thank Sacred Heart Medical Center of Spokane, Wash., for supplying the antibiotics at cost and R. Hatch for photographic assistance.
REFERENCES
- 1.Alarcon T, Domingo D, Lopez-Brea M. Antibiotic resistance problems with Helicobacter pylori. Int J Antimicrob Agents. 1999;12:19–26. doi: 10.1016/s0924-8579(99)00051-5. [DOI] [PubMed] [Google Scholar]
- 2.Alm R A, Ling L S, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, Carmel G, Tummino P J, Caruso A, Uria-Nickelsen M, Mills D M, Ives C, Gibson R, Merberg D, Mills S D, Jiang Q, Taylor D E, Vovis G F, Trust T J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;14:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 3.Bina J E, Alm R A, Uria-Nickelsen M, Thomas S R, Trust T J, Hancock R E W. Helicobacter pylori uptake and efflux: basis for intrinsic susceptibility to antibiotics in vitro. Antimicrob Agents Chemother. 2000;44:248–254. doi: 10.1128/aac.44.2.248-254.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blumberg P M, Strominger J L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974;38:291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dargis M, Malouin F. Use of biotinylated β-lactams and chemiluminescence for study and purification of penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1994;38:973–980. doi: 10.1128/aac.38.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debets-Ossenkopp Y J, Sparrius M, Kusters J G, Kolkman J J, Vandenbroucke-Grauls C M J E. Mechanism of clarithromycin resistance in clinical isolates of Helicobacter pylori. FEMS Microbiol Lett. 1996;142:37–42. doi: 10.1111/j.1574-6968.1996.tb08404.x. [DOI] [PubMed] [Google Scholar]
- 7.DeLoney C R, Schiller N L. Competition of various β-lactam antibiotics for the major penicillin-binding proteins of Helicobacter pylori: antibacterial activity and effects on bacterial morphology. Antimicrob Agents Chemother. 1999;43:2702–2709. doi: 10.1128/aac.43.11.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doig P, Exner M M, Hancock R E W, Trust T J. Isolation and characterization of a conserved porin protein from Helicobacter pylori. J Bacteriol. 1995;177:5447–5452. doi: 10.1128/jb.177.19.5447-5452.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domingo D, Alarcon T, Sanz J C, Sanchez I, Lopez-Brea M. High frequency of mutations at position 2144 of the 23S rRNA gene in clarithromycin-resistant Helicobacter pylori strains isolated in Spain. J Antimicrob Chemother. 1998;41:573–574. doi: 10.1093/jac/41.5.573. [DOI] [PubMed] [Google Scholar]
- 10.Dore M P, Graham D Y, Sepulveda A R. Different penicillin-binding protein profiles in amoxicillin-resistant Helicobacter pylori. Helicobacter. 1999;4:154–161. doi: 10.1046/j.1523-5378.1999.99310.x. [DOI] [PubMed] [Google Scholar]
- 11.Dore M P, Graham D Y, Sepulveda A R, Realdi G, Osato M S. Sensitivity of amoxicillin-resistant Helicobacter pylori to other penicillins. Antimicrob Agents Chemother. 1999;43:1803–1804. doi: 10.1128/aac.43.7.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dore M P, Osato M S, Realdi G, Mura I, Graham D Y, Sepulveda AR. Amoxycillin tolerance in Helicobacter pylori. J Antimicrob Chemother. 1999;43:47–54. doi: 10.1093/jac/43.1.47. [DOI] [PubMed] [Google Scholar]
- 13.Dore M P, Piana A, Carta M, Atzei A, Are B M, Mura I, Massarelli G, Maida A, Sepulveda A R, Graham D Y, Realdi G. Amoxycillin resistance is one reason for failure of amoxycillin-omeprazole treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 1998;12:635–639. doi: 10.1046/j.1365-2036.1998.00350.x. [DOI] [PubMed] [Google Scholar]
- 14.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgopapadakou N H. Penicillin-binding proteins and bacterial resistance to β-lactams. Antimicrob Agents Chemother. 1993;37:2045–2053. doi: 10.1128/aac.37.10.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghuysen J M. Penicillin-binding proteins. Wall peptidoglycan assembly and resistance to penicillin: facts, doubts and hopes. Int J Antimicrob Agents. 1997;8:45–60. doi: 10.1016/s0924-8579(96)00358-5. [DOI] [PubMed] [Google Scholar]
- 17.Gibaldi M. Helicobacter pylori and gastrointestinal disease. J Clin Pharmacol. 1995;35:647–654. doi: 10.1002/j.1552-4604.1995.tb04103.x. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin C S. Antimicrobial treatment of Helicobacter pylori infection. Clin Infect Dis. 1997;25:1023–1026. doi: 10.1086/516078. [DOI] [PubMed] [Google Scholar]
- 19.Graham D Y. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology. 1998;115:1272–1277. doi: 10.1016/s0016-5085(98)70100-3. [DOI] [PubMed] [Google Scholar]
- 20.Grebe T, Hakenbeck R. Penicillin-binding proteins 2b and 2x of Streptococcus pneumoniae are primary resistance determinants for different classes of β-lactam antibiotics. Antimicrob Agents Chemother. 1996;40:829–834. doi: 10.1128/aac.40.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakenbeck R. Target-mediated resistance to β-lactam antibiotics. Biochem Pharmacol. 1995;50:1121–1127. doi: 10.1016/0006-2952(95)00158-v. [DOI] [PubMed] [Google Scholar]
- 22.Han S-R, Bhakdi S, Maeurer M J, Schneider T, Gehring S. Stable and unstable amoxicillin resistance in Helicobacter pylori: should antibiotic resistance testing be perfomed prior to eradication therapy? J Clin Microbiol. 1999;37:2740–2741. doi: 10.1128/jcm.37.8.2740-2741.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris A G, Hazell S L, Netting A G. Use of digoxigenin-labelled ampicillin in the identification of penicillin-binding proteins in Helicobacter pylori. J Antimicrob Chemother. 2000;45:591–598. doi: 10.1093/jac/45.5.591. [DOI] [PubMed] [Google Scholar]
- 24.Heep M, Beck D, Bayerdörffer E, Lehn N. Rifampin and rifabutin resistance mechanism in Helicobacter pylori. Antimicrob Agents Chemother. 1999;43:1497–1499. doi: 10.1128/aac.43.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda F, Yokota Y, Ikemoto A, Teratani N, Shimomura K, Kanno H. Interaction of beta-lactam antibiotics with the penicillin-binding proteins of penicillin-resistant Streptococcus pneumoniae. Chemotherapy. 1995;41:159–164. doi: 10.1159/000239338. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda F, Yokota Y, Mine Y, Tatsuta M. Activity of cefixime against Helicobacter pylori and affinities for the penicillin-binding proteins. Antimicrob Agents Chemother. 1990;34:2426–2428. doi: 10.1128/aac.34.12.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iovene M R, Romano M, Pilloni A P, Giordano B, Montella F, Caliendo S, Tufano M A. Prevalence of antimicrobial resistance in eighty clinical isolates of Helicobacter pylori. Chemotherapy. 1999;45:8–14. doi: 10.1159/000007159. [DOI] [PubMed] [Google Scholar]
- 28.Jacoby G A, Archer G L. New mechanisms of bacterial resistance to antimicrobial agents. N Engl J Med. 1991;324:601–612. doi: 10.1056/NEJM199102283240906. [DOI] [PubMed] [Google Scholar]
- 29.Krishnamurthy P, Parlow M H, Schneider J, Burroughs S, Wickland C, Vakil N B, Dunn B E, Phadnis S H. Identification of a novel penicillin-binding protein from Helicobacter pylori. J Bacteriol. 1999;181:5107–5110. doi: 10.1128/jb.181.16.5107-5110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livermore D M. β-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malouin F, Bryan L E. Modification of penicillin-binding proteins as mechanisms of β-lactam resistance. Antimicrob Agents Chemother. 1986;30:1–5. doi: 10.1128/aac.30.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McColl K E L. Helicobacter pylori 1988–1998. Eur J Gastroenterol Hepatol. 1999;11:13–16. doi: 10.1097/00042737-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Neuwirth C, Siebor E, Duez J-M, Pechinot A, Kazmierczak A. Imipenem resistance in clinical isolates of Proteus mirabilis associated with alterations in penicillin-binding proteins. J Antimicrob Chemother. 1995;36:335–342. doi: 10.1093/jac/36.2.335. [DOI] [PubMed] [Google Scholar]
- 34.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parr T R, Jr, Bryan L E. Mechanism of resistance of an ampicillin-resistant, β-lactamase-negative clinical isolate of Haemophilus influenzae type b to β-lactam antibiotics. Antimicrob Agents Chemother. 1984;25:747–753. doi: 10.1128/aac.25.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sörberg M, Hanberger H, Nilsson M, Björkman A, Nilsson LE. Risk of development of in vitro resistance to amoxicillin, clarithromycin, and metronidazole in Helicobacter pylori. Antimicrob Agents Chemother. 1998;42:1222–1228. doi: 10.1128/aac.42.5.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzgerald L M, Lee N, Adams M D, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 38.van Zwet A A, Vandenbrouke-Grauls C M J E, Thijs J C, van der Wouden E J, Gerrits M M, Kusters J G. Stable amoxicillin resistance in Helicobacter pylori. Lancet. 1998;352:1595. doi: 10.1016/s0140-6736(98)00064-6. [DOI] [PubMed] [Google Scholar]