Abstract
Stress is the most common trigger among episodic neurologic disorders. In episodic ataxia type 2 (EA2), physical or emotional stress causes episodes of severe motor dysfunction that manifest as ataxia and dystonia. We used the tottering (tg/tg) mouse, a faithful animal model of EA2, to dissect the mechanisms underlying stress-induced motor attacks. We find that in response to acute stress, activation of α1-adrenergic receptors (α1-Rs) on Purkinje cells by norepinephrine leads to their erratic firing and consequently motor attacks. We show that norepinephrine induces erratic firing of Purkinje cells by disrupting their spontaneous intrinsic pacemaking via a casein kinase 2 (CK2)–dependent signaling pathway, which likely reduces the activity of calcium-dependent potassium channels. Moreover, we report that disruption of this signaling cascade at a number of nodes prevents stress-induced attacks in the tottering mouse. Together, our results suggest that norepinephrine and CK2 are required for the initiation of stress-induced attacks in EA2 and provide previously unidentified targets for therapeutic intervention.
Stress-induced attacks in EA2 are mediated by noradrenergic α1-Rs and CK2-dependent phosphorylation of Purkinje cell SK channels.
INTRODUCTION
Episodic neurologic disorders caused by channelopathies include several common conditions, such as epilepsy, migraine, and movement disorders (1). While seemingly a heterogeneous group of disorders, most result from mutations in ion channels and share a number of features: (i) Despite the chronic expression of the defective gene, the baseline symptoms are often unremarkable or mild, with little or no sign of the disorder; (ii) the primary manifestation of severe symptoms occurs during episodes of attacks, which can occur spontaneously, or in response to triggers; and (iii) the triggers can broadly be classified as chemical stressors, such as caffeine and ethanol, or as physical and psychological stressors (1). The episodic nature of these disorders argues against a role for degeneration as the primary cause of the symptoms and points toward trigger-induced physiologic dysfunction. Moreover, the existence of a common set of triggers among the various episodic neurologic disorders may be indicative of a shared mechanism by which the stressors trigger the attacks in these disorders. Thus, understanding the mechanism by which stressors trigger episodic dysfunction might be valuable for rational design of preventive or therapeutic interventions in episodic neurologic disorders.
Because stress is one of the most common triggers in episodic neurologic disorders, we used an animal model of an episodic movement disorder, episodic ataxia type 2 (EA2), to delineate the mechanism by which stress triggers attacks of motor dysfunction. EA2 is a channelopathy caused by loss-of-function mutations in the CACNA1A gene encoding the α1 pore-forming subunit of P/Q-type voltage-gated calcium channels (2). In EA2, caffeine, ethanol, and physical or emotional stress cause episodes of severe ataxia and dystonia that can last from a few hours to a few days (3). The tottering (tg/tg) mouse is a faithful model of EA2. It suffers from a spontaneous loss-of-function mutation in the pore-forming subunit of P/Q-type calcium channels and, similar to patients with EA2, exhibits a mild baseline ataxia with episodes of severe ataxia and dystonia that are brought about by stress or by injections of caffeine or alcohol (4). Because of the similarity of the mutation in the tottering mice to that seen in humans and the fidelity with which these mice recapitulate the symptoms of EA2, they represent a remarkably good model of EA2 [see Discussion of (5)]. In the tottering mice, the cerebellum is required for the expression of the episodic motor symptoms (6), and the dysfunction of the calcium channels in the cerebellum alone is sufficient to replicate the episodic disorder (7).
Here, we show that the episodes of stress-induced attacks of motor dysfunction in the tottering mouse are caused by avid burst firing of cerebellar Purkinje cells, similar to that seen in caffeine-induced attacks (5). The hypothalamic-pituitary-adrenal (HPA) axis is activated during stress and promotes norepinephrine (NE) release in the brain (8), including in the cerebellum (9). The stress-induced Purkinje cell burst firing is likely brought about by an increase in cerebellar NE and the subsequent activation of α1-adrenergic receptors (α1-Rs). Activation of α1-Rs results in casein kinase 2 (CK2)–dependent phosphorylation of calmodulin (CaM), two molecules that are known to be tightly coupled with small-conductance calcium-activated potassium (SK) channels and to modulate their activity (10). CK2-dependent phosphorylation of the CaM associated with SK channels has been shown to reduce the open probability of the channels (10), and a reduction in the activity of SK channels results in Purkinje cell burst firing (11). We show that pharmacologic or genetic knockdown (KD) of CK2 prevents stress-induced attacks. Thus, on the basis of our finding in the tottering mice, the stress-induced attacks of motor dysfunction in EA2 are likely mediated by an adrenergic, CK2-mediated reduction in the activity of SK channels and thus burst firing of cerebellar Purkinje cells. On the basis of these mechanistic findings, we were able to identify several potential therapeutic intervention points and experimentally validated the efficacy of Food and Drug Administration (FDA)–approved agents targeting these intervention points in preventing stress-induced attacks in the tottering mice. Given the success of this rational therapeutic approach in the tottering mice, we believe that it may be relevant in the clinic for the treatment of patients with EA2.
RESULTS
Stress-induced attacks of motor dysfunction in the tottering are associated with high-frequency burst firing of Purkinje cells
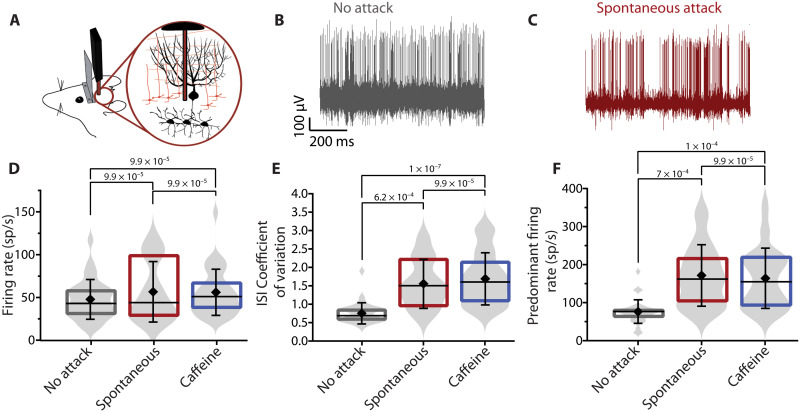
A hallmark of most episodic neurologic disorders, including EA2, is that independent of the nature of the triggers, the features of the symptoms associated with the attacks remain the same. It is thus often contemplated that the different triggers might converge on a common mechanism to precipitate the neurologic attacks. In the tottering mouse, the episodes of motor dysfunction triggered by either stress or pharmacological stressors such as caffeine and ethanol are virtually identical (12). It has been reported that in the tottering mouse, caffeine-induced attacks of motor dysfunction are associated with high-frequency burst firing of Purkinje cells (5). Because Purkinje cells encode the information relevant for motor coordination in their spiking activity, a change in their ability to reliably encode this information, when they fire erratically, impairs cerebellar function and leads to ataxia (13). In the tottering mice, the extent to which the firing of Purkinje cells is irregular, as a caffeine-induced motor attack is initiated and progresses to peak motor dysfunction, is strongly correlated with the severity of the motor symptoms (5). Given that stress produces similar episodes of motor dysfunction as caffeine, one might predict a comparable change in the activity of Purkinje cells with stress-induced attacks. We thus recorded the activity of cerebellar Purkinje cells in the tottering mice under baseline conditions and at the peak of stress-induced attacks triggered spontaneously as a consequence of head restraint or after being restrained in a tube. Similar to that seen with caffeine-induced attacks [data reproduced from (5)], both the interspike interval coefficient of variation (ISI CV; SD of the ISI divided by the mean ISI), which is a measure of regularity of firing, and the predominant firing rate (defined as the reciprocal of the mode of the ISI distribution histogram) significantly increased during attacks (Fig. 1, D to F). Moreover, the extent to which the ISI CV and predominant firing rate of Purkinje cells were increased at the peak of motor dysfunction in stress attacks was similar to that seen with caffeine-induced attacks, consistent with the idea that triggers might be affecting Purkinje cell firing comparably, and perhaps through a shared mechanism.
Fig. 1. Stress causes high-frequency burst firing of tottering Purkinje cells in vivo.
(A) Schematic of extracellular single-unit recordings from Purkinje cells of awake, head-restrained tg/tg mice. (B and C) Representative traces of single-unit recordings of Purkinje cells during baseline when the mouse has no attack (B) and during spontaneous stress attack (C). Note the change in firing regularity from aberrant during baseline to high-frequency bursts during attacks. Firing rate (D), ISI CV (E), and predominant firing rate (F) of Purkinje cells during baseline conditions (gray), spontaneous attacks (red), and caffeine-induced attacks (blue). Baseline: n = 32 cells; spontaneous attack: n = 11 cells in N = 6 mice; caffeine attack: n = 14 in N = 6 mice. Statistical analysis includes nonparametric Kruskal-Wallis test with Dunn’s multiple comparisons test. Note that caffeine data were acquired for a previous study (5), reanalyzed, and presented here for comparison.
Cerebellar adrenergic signaling is necessary and sufficient for stress-induced attacks
We next sought to delineate the signaling pathway through which stress induces motor attacks in the tottering mice. A role for noradrenergic signaling in the initiation of stress-induced attacks in the tottering mice has been ruled out, although systemic block of α1-Rs has been shown to significantly decrease the frequency of stress-induced attacks (14). This conclusion was based on the observation that systemically applied noradrenergic agonists in the tottering mice failed to induce attacks (3). Because of the practical challenges associated with such an approach, we reexamined the role of adrenergic signaling in mediating stress-induced attacks in the tottering mice.
To explore whether noradrenergic signaling is critical for stress-induced attacks, we first manipulated adrenergic signaling by intraperitoneal injection of pharmacological agents that are known to effectively cross the blood-brain barrier and assessed the probability with which restraining the mice for 10 min, a well-established paradigm to trigger stress-induced attacks in the tottering mice, elicited an attack (12, 14). In situ hybridization and autoradiographical studies indicate that α1 and α2 and β1 and β2 receptors are expressed in the cerebellum (15, 16). Noradrenergic α1 and β receptors are primarily expressed postsynaptically, whereas α2 receptors are thought to reside mainly on the presynaptic axons where they act as autoreceptors and, via a negative feedback mechanism, inhibit release of NE, including in the cerebellum (17). We thus used two different strategies to disrupt adrenergic transmission to Purkinje cells. Either we used antagonists to block adrenergic receptors to prevent NE from activating the postsynaptic receptors expressed on Purkinje cells or we selectively activated α2 receptors to reduce the release of NE from parallel fibers to Purkinje cells.
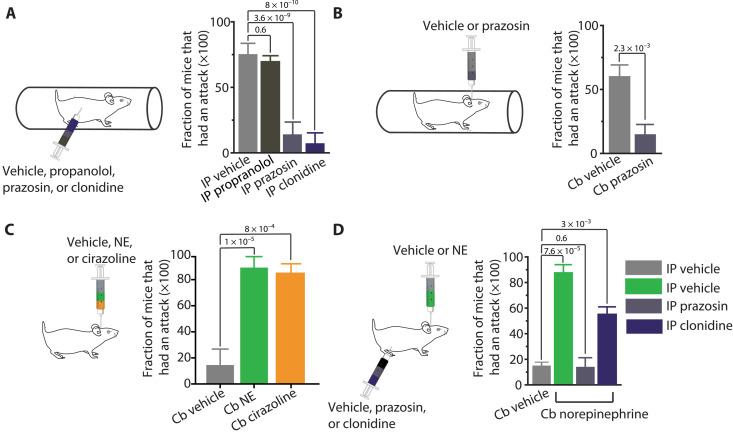
In agreement with previous findings, we found that systemic intraperitoneal injection of the α1-R antagonist prazosin, but not the β-adrenergic receptor antagonist propranolol, decreased the probability with which restraint stress caused attacks (Fig. 2A). Similarly, we found that systemic application of the α2 receptor agonist clonidine to reduce the release of NE onto Purkinje cells also decreased the probability of stress-induced attacks (Fig. 2A).
Fig. 2. Cerebellar adrenergic signaling is required for stress-induced attacks.
(A) Compared to vehicle, systemic injection of the α1 adrenoreceptor antagonist prazosin at 0.5 mg/kg or α2 adrenoreceptor agonist clonidine at 0.05 mg/kg before subjecting tottering mice to restraint stress decreases the frequency with which restraint induces attacks. Injection of the β-adrenoceptor antagonist propranolol at 5 mg/kg does not alter the frequency of stress-induced attacks. Vehicle N = 6 mice, propanolol N = 6 mice, prazosin N = 6 mice, and clonidine N = 6 mice, in each, repeated 5 to 10 times. (B) Pretreatment with intracerebellar (Cb) infusion of 1.5 μg of prazosin decreases frequency of stress-induced attacks compared to pretreatment with vehicle. Vehicle N = 3 mice and prazosin N = 3 mice, in each, repeated four to seven times. (C) The frequency of attacks following intracerebellar (Cb) infusion of 10 μg of NE or 2.5 μg of the α1 agonist cirazoline is significantly higher than that following vehicle infusion. Cb vehicle N = 6 mice, Cb NE N = 5, and Cb cirazoline N = 5 mice, in each, repeated five to nine times. (D) Systemically injecting tottering mice with the α1 antagonist prazosin at 0.5 mg/kg decreases the frequency of attacks following intracerebellar infusions of 10 μg of NE (green) compared to pretreatment with either vehicle (gray) or 0.8 μg of the α2 agonist clonidine (dark blue). Vehicle N = 2 mice, prazosin N = 2 mice, and clonidine N = 3 mice, in each, repeated four to nine times. Data represent means ± SEM. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test.
There is strong evidence that the motor dysfunctions exhibited by tottering mice are cerebellar and, specifically, are mediated by Purkinje cells (7, 18). Moreover, it is clear, on the basis of the comparable changes in the activity of Purkinje cells during stress- or caffeine-induced attacks reported here, that, ultimately, the effects of the triggers may converge on the cerebellum. However, these data do not necessarily dictate that the cerebellum must be the direct target of the triggers: It is plausible that the triggers act on upstream brain regions that ultimately converge onto the cerebellum to cause its dysfunction. If, in the tottering mouse, engagement of the HPA axis during stress activates adrenergic signaling in the cerebellum, then block of α1-Rs only in the cerebellum should be sufficient to block restraint-induced attacks. Using a preimplanted bilateral injection cannula, we injected prazosin directly and selectively into the cerebellum. In agreement with the hypothesis that the cerebellum is the direct target of the HPA axis, we found cerebellar injections of prazosin to be effective in preventing attacks (Fig. 2B). Conversely, we reasoned that direct activation of adrenergic receptors by cerebellar injection of adrenergic agonists should be sufficient to trigger attacks. In agreement with this hypothesis, we found that cerebellar injections of either NE or the selective α1 agonist cirazoline reliably induced attacks (Fig. 2C). Furthermore, as expected, systemic block of α1-Rs by intraperitoneal injection of prazosin significantly reduced the probability with which cerebellar injections of NE caused attacks in the tottering mice (Fig. 2D).
Our result confirm previous findings that the block of α1-Rs reduces the frequency of stress-induced attacks in the tottering mice but differ from those previously published where systemic injections of cirazoline was reported to fail to increase the frequency of stress-induced attacks, forcing the authors to conclude that the adrenergic pathway did not play a role in triggering attacks in these mice (14). By injecting the adrenergic modulators directly into the cerebellum of the mice, our experiments bypassed the potential complications and uncertainties associated with absorption of the drugs and their permeation into the brain following their systemic administration, allowing us to directly test the efficacy of NE, in addition to a slew of other agents, in triggering or blocking the episodes of motor dysfunction.
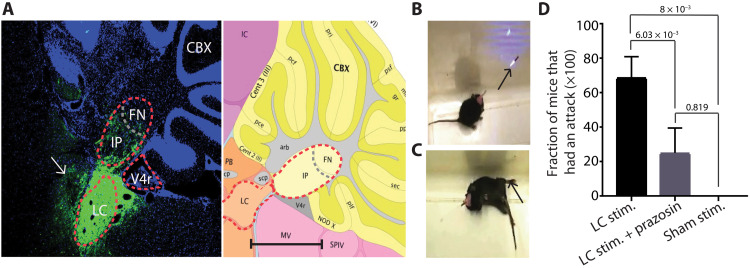
To corroborate our pharmacological finding that activation of α1-Rs in the cerebellum of the tottering mice can trigger attacks, we used an alternative strategy to engage the adrenergic pathway. The noradrenergic terminals in the cerebellum originate exclusively from neurons in the locus coeruleus (LC) (19), and electrical stimulation of the LC has been shown to increase cerebellar NE content (20). It is plausible that activation of the HPA axis during stress results in increased activity of the LC neurons and a subsequent rise in cerebellar NE. The noradrenergic varicosities are mainly restricted to Purkinje cells in mice (19), and thus, an increase in cerebellar NE would invariably result in activation of the adrenergic receptors on Purkinje cells. Because it has been shown that the firing rate of LC neurons increases in response to various stressful stimuli (21), we explored whether promoting release of endogenous NE from the LC using an optogenetic approach would be sufficient to trigger attacks in the tottering mice. We stereotaxically injected an adeno-associated viral (AAV) vector containing channel rhodopsin (ChR2) into the LC and implanted a fiber optic to selectively target the transfected neurons in LC for optogenetic stimulation (Fig. 3A). Optogenetic activation of LC reliably produced attacks in all the mice examined, whereas control experiments with sham stimulations using a laser wavelength that does not activate ChR2 did not cause any attacks (Fig. 3, B to D). The latency of the optogenetically induced attacks was comparable to the latency of stress-induced attacks (fig. S1). Bilateral cerebellar infusion of the α1 antagonist prazosin markedly reduced the probability of inducing an attack following optogenetic stimulation of LC (Fig. 3D), supporting the premise that stimulation of LC triggered attacks primarily by activating cerebellar adrenergic receptors. Thus, the data presented so far suggest that adrenergic signaling is required, and sufficient, to induce attacks in the tottering mice.
Fig. 3. Optogenetic stimulation of the LC induces attacks of dyskinesia in tottering mice.
(A) Left: Channel rhodopsin expression around and including the left LC visualized by green fluorescent protein (GFP) antibody staining and 4′,6-diamidino-2-phenylindole costaining. White arrow points at the fiber optic track used to stimulate the LC. Interpose (IP) nuclei, fastigial nuclei (FN), and fourth ventricle (V4r) are outlined for reference. Fiber tracts (arb), lobule II molecular layer [Cent 2 (II)], precentral fissure (pce), lobule III molecular layer [Cent 3 (III)], preculminate fissure (pcf), Noduluds X (NOD X), posterolateral fissure (plf), secondary fissure (sec), granular layer (gr), posterior superior fissure (psf), and primary fissure (pri). CBX, cerebellar cortex; IC, inferior colliculus; MV, Medial-Ventral; cp, cerebellar peduncle; scp, superior cerebellar peduncle. Right: Picture from the Allen Mouse Brain Atlas showing the same section of the cerebellum from the left with the corresponding structures. Scale bar, 1000 μm. Image credit: Allen Institute, 2017 Allen Institute for Brain Science, Allen Mouse Brain Atlas (2004) (available from http://atlas.brain-map.org/atlas?atlas=2&plate=100884129#atlas=2&plate=100883867&resolution=8.38&x=11952&y=3431&zoom=-3&structure=10710&z=6). (B) Tottering mouse during optogenetic stimulation of the LC. Arrow shows the blue light coming out of the optical fiber. (C) Arrow shows characteristic dystonic posture during a motor attack. Photo credit: Ariel Vitenzon. (D) The frequency of attacks is significantly higher following stimulation compared to sham stimulation at an inactive wavelength (594 nm). The block of α1 receptors in the cerebellum using local application of 1.5 μg of prazosin prevents the effect of LC stimulation. Stimulation: n = 6 trials in total in N = 7 mice. LC stim + prazosin: n = 5 trials in N = 4 mice. Sham: n = 5 trials in total in N = 7 mice. Data represent means ± SD. Multiple t test with a two-stage step-up method of Benjamini, Krieger, and Yekutieli for false discovery rate.
NE decreases regularity of firing of tottering and wild-type Purkinje cells in brain slices
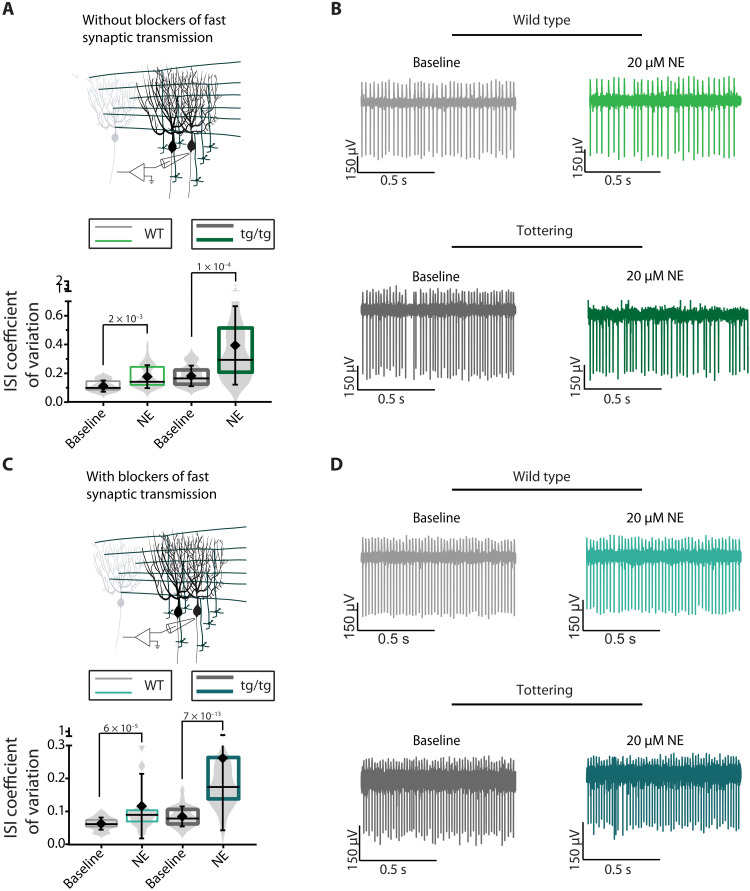
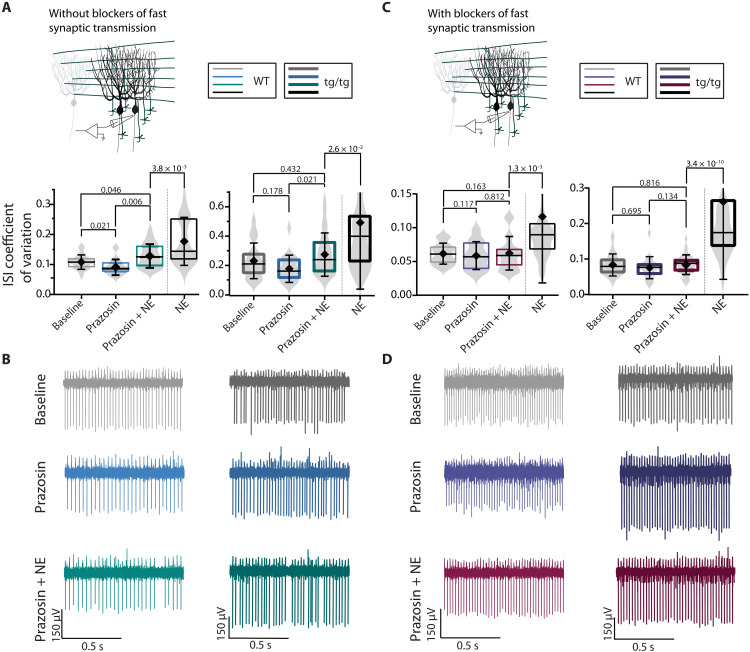
If in the tottering mice stress-induced attacks are mediated by a rise in NE in the cerebellum and the subsequent burst firing of Purkinje cells, then one should be able to observe a change in the activity of Purkinje cells in acutely prepared cerebellar slices when exposed to NE. With synaptic transmission intact, bath application of 20 μM NE, a concentration that was previously shown to activate α1 receptors in Purkinje cells (22), produced highly erratic firing in Purkinje cells of the tottering mice (ISI CV, 0.395) and mild irregularity (ISI CV, 0.178) in Purkinje cells of the wild-type (WT) mice (Fig. 4). Similarly, in the presence of synaptic transmission blockers to allow examination of intrinsic pacemaking of Purkinje cells, bath application of NE markedly increased the irregularity of Purkinje cell firing in tottering mice (ISI CV, 0.262) but only marginally, although significantly (ISI CV, 0.116), affected that of the WT mice. At this concentration, NE did not appreciably affect other firing characteristics of Purkinje cells, including their average firing rate, allowing the use of ISI CV as a good indicator of regularity of firing (fig. S2). The impact of NE on Purkinje cell intrinsic pacemaking in the presence of blockers of fast synaptic transmission supports the assertion that deterioration of the regularity of firing of Purkinje cells in vivo in the tottering mice drives motor dysfunction rather than simply reflect sensory feedback originating from erratic muscle contractions during attacks (5, 23).
Fig. 4. NE decreases regularity of firing of tottering and WT Purkinje cells in vitro.
(A) Schematic of in vitro recordings. ISI CV of WT and tottering Purkinje cells during baseline (WT: light gray; tottering: dark gray) or after bath application of 20 μM NE (WT: light green; tottering: dark green) in the absence of fast synaptic blockers. N = 14 mice; n = 35 cells for WT baseline (mean = 0.117 ± 0.039) and n = 37 for WT NE (mean = 0.177 ± 0.080). N = 12 mice; n = 36 for tottering baseline (mean = 0.182 ± 0.072) and n = 40 for tottering NE (mean = 0.395 ± 0.273). (B) Representative traces of WT and tottering Purkinje cells during baseline (gray) or after bath application of 20 μM NE (green), in the absence of fast synaptic blockers. (C) Schematic of in vitro recordings. ISI CV of WT and tottering Purkinje cells during baseline (WT: light gray; tottering: dark gray) or after bath application of 20 μM NE (WT: light blue; tottering: dark blue) in the presence of fast synaptic blockers. N = 5 WT mice; n = 41 cells for WT baseline (mean = 0.063 ± 0.018) and n = 33 cells for WT NE (mean = 0.1163 ± 0.0.098). N = 9 tottering mice; n = 53 cells for tottering baseline (mean = 0.085 ± 0.030) and n = 42 for tottering NE (mean = 0.262 ± 0.022). (D) Representative traces of WT and tottering Purkinje cells during baseline (gray) or after bath application of 20 μM NE (blue), in the presence of fast synaptic blockers.
We then sought to delineate the nature of the adrenergic receptors that mediated the NE-induced Purkinje cell irregularity. The data presented earlier showed that activation of α1-R is sufficient and necessary to induce attacks in the tottering mice, suggesting that NE may cause Purkinje cells to fire irregularly by activating α1 receptors. To test this hypothesis, we blocked α1 receptors with prazosin and explored the efficacy of NE in altering the activity of Purkinje cells when α1 receptors were blocked (Fig. 5). Given that even in acutely prepared slices there might be some background NE tone, we first examined the impact of the block of α1 receptors on the pacemaking of Purkinje cells. In the absence of blockers of fast synaptic transmission, the block of α1 receptors with prazosin had no effect on the regularity of firing of tottering Purkinje cells (Fig. 5, A and B). However, prazosin made the firing of WT Purkinje cells somewhat more regular, suggesting that the background NE tone had a slight impact on the activity of WT Purkinje cells (Fig. 5, A and B). The α1-mediated effect of the basal NE tone on WT Purkinje cell activity was likely a consequence of its actions on the processes presynaptic to Purkinje cells, because in the presence of blockers of fast synaptic transmission, prazosin did not alter the pacemaking of Purkinje cells. We then explored whether the block of α1 receptors blunted the impact of NE on the firing of Purkinje cells. With synaptic transmission intact, both in WT and tottering mice, prazosin significantly reduced the efficacy of NE in increasing the irregularity of Purkinje cell firing, decreasing the impact of NE by approximately 25 and 50%, respectively (Fig. 5). However, in the presence of blockers of fast synaptic transmission, prazosin completely abolished the impact of NE on the pacemaking of Purkinje cells both in WT and the tottering mice (Fig. 5, C and D).
Fig. 5. The block of α1-Rs prevents NE-induced irregularity in tottering and WT Purkinje cells with fast synaptic transmission blocked but attenuates NE-induced irregularity with fast synaptic transmission intact.
(A) Schematic of in vitro recordings. ISI CV of WT (thin lines) and tottering (thick lines) Purkinje cells during baseline (gray), after bath application of 1 nM prazosin alone (blue), and 1 nM prazosin and 20 μM NE (green), compared to application of 20 μM NE alone (black) in the absence of fast synaptic blockers. N = 6 WT mice; n = 31 cells for WT baseline (mean = 0.108 ± 0.024), n = 23 cells for WT prazosin (mean = 0.092 ± 0.026), and n = 38 WT NE + prazosin (mean = 0.129 ± 0.040). N = 10 tottering mice; n = 40 tottering baseline (mean = 0.234 ± 0.121), n = 19 tottering prazosin (mean = 0.180 ± 0.093), and n = 50 tottering NE + prazosin (mean = 0.0277 ± 0.147). (B) Representative traces of WT (first column) and tottering (second column) Purkinje cells during baseline (gray), after bath application of 1 nM prazosin alone (blue), and 1 nM prazosin and 20 μM NE (green). (C) Schematic of in vitro recordings. ISI CV of WT (thin lines) and tottering (thick lines) Purkinje cells during baseline or after bath application of prazosin alone or prazosin and NE, in the presence of fast synaptic blockers. N = 6 WT mice; n = 25 cells for WT baseline (mean = 0.062 ± 0.015), n = 22 cells for WT prazosin (mean = 0.059 ± 0.021), and n = 27 WT NE + prazosin (mean = 0.062 ± 0.025). N = 8 tottering mice; n = 34 tottering baseline (mean = 0.084 ± 0.031), n = 20 tottering prazosin (mean = 0.076 ± 0.031), and n = 32 tottering NE + prazosin (mean = 0.085 ± 0.027). (D) Representative traces of WT (first column) and tottering (second column) Purkinje cells during baseline (gray) or after bath application of prazosin alone (purple) or prazosin and NE (red).
These data suggest that NE alters the precision of intrinsic pacemaking of Purkinje cells via an α1 receptor–mediated signaling pathway. However, other adrenergic receptors also clearly affect the activity of Purkinje cells by targeting processes presynaptic to Purkinje cells. β-Adrenergic receptors, for example, are highly expressed on cerebellar basket cells in addition to Purkinje cells (24).
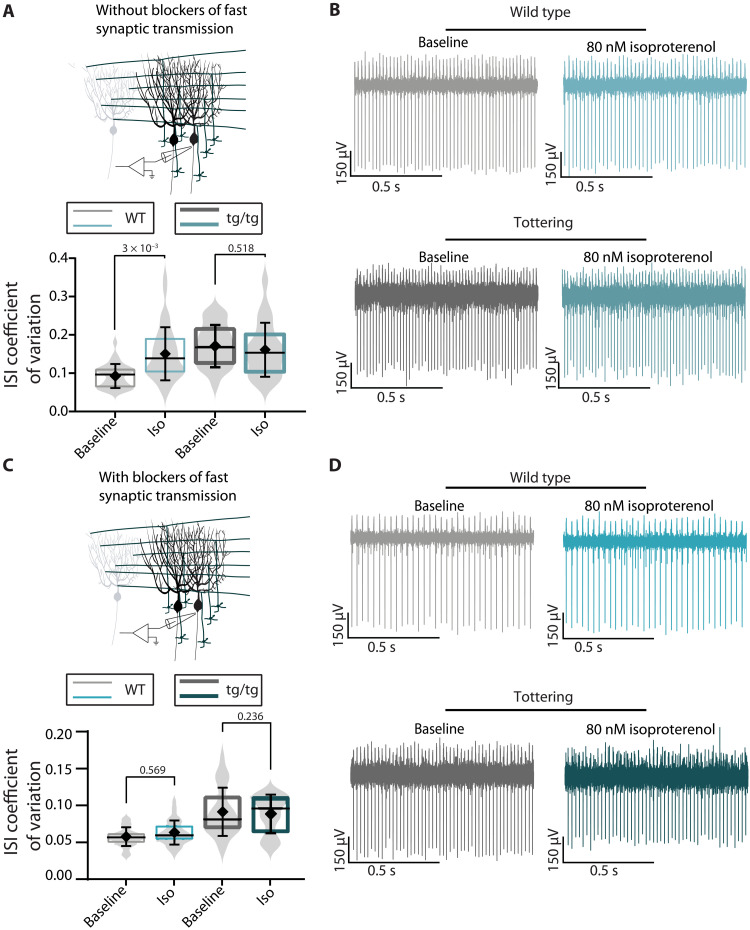
To test a potential role for β-adrenergic signaling in NE-induced irregularity of Purkinje cells, we bath-applied the β-adrenergic receptor agonist isoproterenol onto cerebellar slices in the absence and presence of blockers of fast synaptic transmission. With fast synaptic transmission intact, activating cerebellar β-adrenergic receptors resulted in no significant change in the regularity of firing of Purkinje cells in slices from tottering mice but resulted in a significant decrease in the regularity of firing of Purkinje cells in slices from WT mice (Fig. 6A). In the presence of blockers of fast synaptic transmission, the effect of isoproterenol on Purkinje cells from WT mice was abolished (Fig. 6C). These data suggest that while, in the WT mice, activation of β-adrenergic receptors affects firing of Purkinje cells by targeting presynaptic processes, in the tottering mice, the pathway may be altered and thus does not play a substantial role in modulation of the activity of Purkinje cells.
Fig. 6. Isoproterenol decreases regularity of firing of WT but not tottering Purkinje cells in vitro.
(A) Schematic of in vitro recordings. ISI CV of WT and tottering Purkinje cells during baseline (gray) or after bath application of 80 nM isoproterenol (blue) in the absence of fast synaptic blockers. N = 5 mice; n = 30 cells for WT baseline (mean = 0.094 ± 0.031) and n = 35 for WT isoproterenol (mean = 0.152 ± 0.069); N = 6 mice; n = 31 for tottering baseline (mean = 0.177 ± 0.060) and n = 42 for tottering isoproterenol (mean = 0.162 ± 0.070). (B) Representative traces of WT and tottering Purkinje cells during baseline (gray) or after bath application of 80 nM isoproterenol (blue), in the absence of fast synaptic blockers. (C) Schematic of in vitro recordings. ISI CV of WT and tottering Purkinje cells during baseline (gray) or after bath application of 20 μM NE (blue) in the presence of fast synaptic blockers. N = 5 mice; n = 30 cells for WT baseline (mean = 0.057 ± 0.013) and n = 37 cells for WT isoproterenol (mean = 0.062 ± 0.016). N = 5 tottering mice; n = 26 cells for tottering baseline (mean = 0.090 ± 0.016) and n = 29 for tottering isoproterenol (mean = 0.094 ± 0.032). (D) Representative traces of WT and tottering Purkinje cells during baseline (gray) or after bath application of 80 nM isoproterenol (blue), in the presence of fast synaptic blockers.
The reason for the differences in β-adrenergic receptors in the WT and tottering mice remains to be established. However, the lack of a substantial role for β-adrenergic receptors in inducing irregular activity in the tottering Purkinje cells agrees with our observation that β-adrenergic receptors did not decrease the frequency of stress-induced attacks in tottering mice. Thus, given the efficacy of prazosin in blocking stress- and NE-induced attacks in the tottering mice and the sufficiency of α1 agonists in causing motor attacks, it is clearly the α1-mediated changes in the intrinsic pacemaking of Purkinje cells that constitute the critical pathway in triggering episodes of motor dysfunction in EA2.
Tg/tg mice are more sensitive to progressive block of cerebellar SK channels compared with WT mice
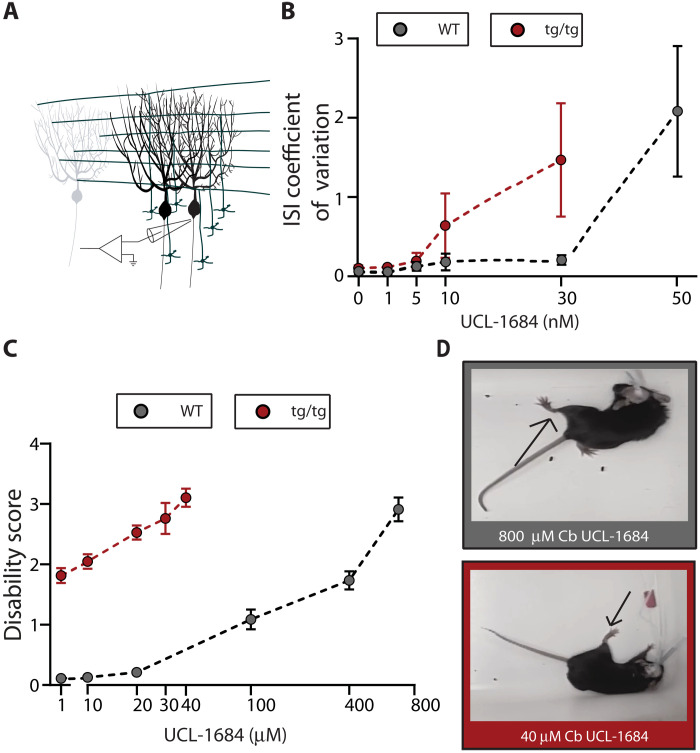
The extent and pattern of erratic firing seen during caffeine- or stress-induced attacks in vivo and that is induced by NE by activation of α1-Rs in vitro resemble the changes in the firing pattern of Purkinje cells when calcium-activated potassium channels are progressively blocked (5, 11). As mentioned, acute activation of cerebellar SK channels during a caffeine-induced episode has been shown to promptly abort a motor attack (5). It is plausible, therefore, that the episodes of motor dysfunction in the tottering mice brought about by either caffeine or stress are caused by diminished activation of SK channels. Activation of α1 receptors by NE has been shown to reduce the activity of SK channels (25). As can be noted, however, the impact of NE on the firing pattern of WT Purkinje cells was less pronounced, suggesting that the tottering Purkinje cells were more sensitive. We have previously shown that, in Purkinje cells, the Cav2.1 and SK channels are tightly coupled (26). Because of the diminished Cav2.1-mediated calcium current caused by the point mutation in the tottering mice, under baseline conditions, each action potential activates fewer SK channels, thereby producing a smaller afterhyperpolarization (AHP), thus leading to irregular firing (13, 27, 28). This irregularity accounts for the mild baseline ataxia seen in the tottering mice (13). The preexisting reduced activity of SK channels in the tottering would make Purkinje cells more susceptible to a further decrease in SK current by NE and most likely accounts for the higher sensitivity of the tottering Purkinje cells to NE: A further reduction in the SK current would result in an even smaller AHP and a more irregular pacemaking, similar to that seen when SK channels are progressively blocked by a channel blocker in the WT cerebellar slices (11, 26). However, if the arguments thus presented are valid, then the firing of tottering Purkinje cells should be more sensitive to partial reductions in the activity of SK channels. We thus explored this possibility. To do so, we bath-perfused increasing concentrations of the SK channel blocker UCL-1684 while recording the activity of Purkinje cells in acutely prepared slices of the cerebellum from WT and tottering mice. We found that the regularity of firing of Purkinje cells of tottering mice was significantly more sensitive to partial block of SK channels compared with that of the WT (Fig. 7A).
Fig. 7. tg/tg cerebella are more sensitive to SK channel block than WT.
(A) Schematic of in vitro recordings. (B) ISI CV of WT (gray dots) and tottering (red dots) Purkinje cells at baseline and in the presence of increasing concentrations of the SK2 channel blocker UCL-1684 in the presence of fast synaptic blockers. X axis depicts the log of the concentration of UCL-1684. For WT UCL-1684, N = 4; n = 81, means: baseline = 0.0681 ± 0.0315, 1 nM = 0.0627 ± 0.0172, 5 nM = 0.1318 ± 0.0547, 10 nM = 0.1858 ± 0.1046, 30 nM = 0.2116 ± 0.0607, and 50 nM = 2.083 ± 0.8202. For tottering UCL-1684, N = 3 mice; n = 21, means: baseline = 0.1049 ± 0.035, 1 nM = 0.1150 ± 0.068, 5 nM = 0.1927 ± 0.104, 10 nM = 0.6404 ± 0.407, and 30 nM = 1.469 ± 0.715. (C) Average disability score with respect to the log of the concentration of UCL-1684 infused in the cerebellum of WT (gray) and tottering (red). For WT, N = 5; for tottering, N = 3. (D) WT (top gray box) and Tottering (bottom red box) mice during motor impairment elicited by cerebellar infusion of 800 and 40 μM UCL-1684, respectively. Arrows show characteristic dystonic posture during a motor impairment. Photo credit: Heather Snell.
We then explored whether progressive pharmacological block of SK channels in the cerebellum of WT mice produced motor dysfunction and, if so, whether the same level of motor dysfunction could be achieved in the tottering mice with lower concentrations of the blocker. To do so, we bilaterally infused different concentrations of UCL-1684 into the cerebella of tottering and WT mice. We found that, indeed, progressive block of SK channels in the cerebellum of the WT mice resulted in motor symptoms that increased in severity, starting with mild ataxia, to severe ataxia, and ultimately to sustained dystonic postures. While the tottering mice showed the same progression in severity of motor dysfunction from their baseline of mild ataxia, the symptoms appeared at far lower concentrations of the blocker (Fig. 7, B to D). These results are consistent with the notion that trigger-induced attacks in tottering might be associated with a greater reduction in SK channel activity. It is noteworthy, in this context, that it has been shown that pharmacologic activation of tottering cerebellar SK channels in the midst of a caffeine-induced attack rescues the precision of Purkinje cell pacemaking and aborts the motor attack (5).
Stress-induced attacks increase the levels of phosphorylated CaM in Purkinje cells of tottering mice
As noted, it is plausible that stress-induced release of NE in the cerebellum of tottering mice activates α1-Rs on Purkinje cells and reduces SK2 channel function, thus causing highly erratic Purkinje cell activity. NE has been shown to reduce SK2 channel activity by acting on α1-Rs and subsequently activating SK2-associated CK2, which, in turn, phosphorylates SK2-associated CaM (10, 25). The phosphorylation of CaM at T80 lowers the affinity of CaM to Ca2+, thereby reducing the open probability of SK2 channels (10, 25). If this is indeed the mechanism by which stress induces attacks, then it would be expected that following stress-induced attacks, the levels of phospho-CaM (p-CaM) in tottering Purkinje cells would be elevated. In the adult mouse cerebellum, CaM is almost exclusively expressed in Purkinje cells and deep cerebellar nuclei neurons (29).
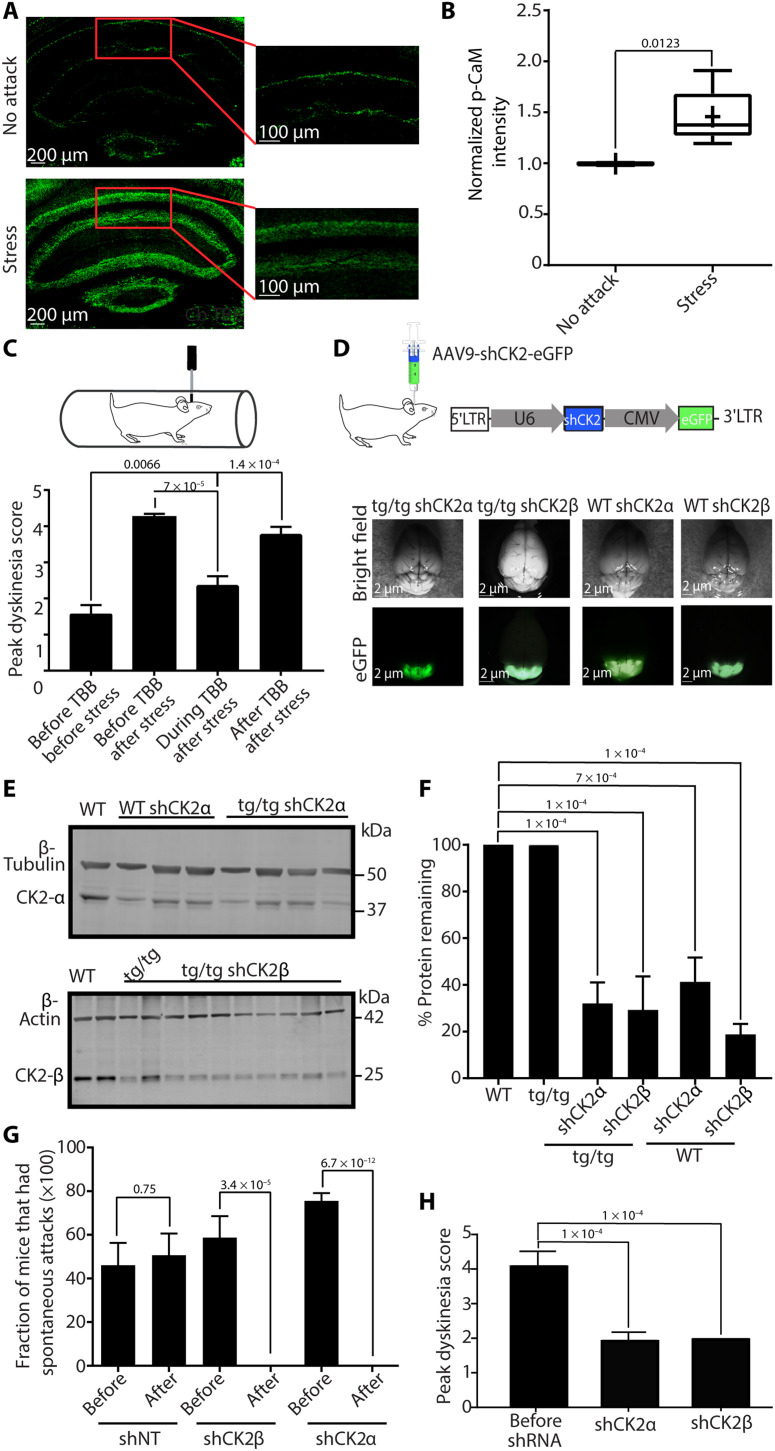
To determine whether the increase in p-CaM occurs in Purkinje cells, we used immunofluorescence and compared p-CaM levels in Purkinje cells of mice immediately after stress-induced attacks with mice that were not stressed. Consistent with the literature (29), we found that Purkinje cell soma throughout the cerebellum expresses high levels of p-CaM, whereas almost no expression was observed in the soma of granule cells (Fig. 8A). Compared to the mice that were not stressed, we found that the p-CaM levels in somas of tottering mice were significantly higher soon after stress-induced attacks (Fig. 8B).
Fig. 8. KD of CK2 in the cerebellum prevents stress attacks.
(A) Representative images of cerebella from a tottering mouse that was undergoing stress attack and a tottering mouse that was not undergoing attack at the time of sacrifice. (B) Normalized p-CaM levels from Purkinje cell somas of mice undergoing stress attacks compared to mice not undergoing attacks. Stress, N = 6; no attack, N = 5. One-sample t test. (C) The frequency of stress-induced attacks is decreased during the chronic perfusion of 20 mM CK2 inhibitor (TBB) into the cerebellum of tottering mice. One-way ANOVA with Dunnett’s multiple comparisons test. (D) Top: Schematic of the AAV9 construct used to drive shCK2 in vivo. Bottom: Representative whole brains of tottering and WT mice injected with shCK2β or shCK2α. Photo credit: Ariel Vitenzon. (E) Top: Cropped immunoblot from the cerebellum of a WT mouse and three WT and four tottering mice injected with shCK2α, showing bands at 45 kDa for CK2α and 50 kDa for β-tubulin. Bottom: Cropped immunoblot from the cerebellum of WT noninjected tottering and 10 tottering mice injected with shCK2β, showing bands at 25 kDa for CK2β and at ~42 kDa for β-actin. (F) Quantification of the above blots showing average KD of CK2α and CK2β in tottering cerebellum (N = 8 for CK2α and N = 10 for CK2β) and WT cerebellum (N = 3 for CK2α and N = 4 for CK2β). Error bars represent SD. One-way ANOVA with Tukey’s multiple comparisons test. (G) Frequency of spontaneous stress attacks in tottering mice injected with shNT, shCK2β, and shCK2α before and after shRNA expression. Error bars represent SEM. N = 7 shNT, N = 8 shCK2α, and N = 8 shCK2β. Student’s t test. (H) Average peak dyskinesia scores of tottering mice before and after injection of shCK2α and shCK2β, 40 min after restraint stress. Error bars represent SEM. Baseline: N = 10; shCK2α: N = 10; shCK2β: N = 4. One-way ANOVA with Dunnett’s multiple comparisons test.
To specifically measure SK2-associated changes in p-CaM levels during stress-induced attacks, we attempted to coimmunoprecipitate (Co-IP) SK2 channels and CaM (fig. S6). However, we were unsuccessful despite numerous attempts and the use of several different commercial or published SK2 antibodies (10). We found that this is due to the poor quality of the available antibodies and because of the low abundance of p-CaM in cerebellar lysates. We further attempted to generate novel monoclonal antibodies specifically targeting 12 highly antigenic sequences on the SK2 receptor (fig. S6A). Unfortunately, a Western blot screen of 28 hybridoma clones (Abmart) using cerebellar lysates from WT and SK2 knockout mice (10, 30) as controls revealed that, similar to the six existing antibodies that we tested (NeuroMab-K78/29, Aviva, Alomone-APC-028, Nittobo Medical Co LTD), there were no high-quality SK2-targeted antibodies that could be used for Co-IPs (figs. S6B and S7 and table S1).
Pharmacologic block of cerebellar CK2 prevents stress-induced attacks
The data reported above are in agreement with the hypothesis that a rise in cerebellar NE levels brought about by stress results in phosphorylation of CaM, perhaps via a CK2-dependent pathway, decreases the open probability of SK channels in Purkinje cells, and thereby causes them to fire erratically. If, as implied, CK2-dependent phosphorylation in the cerebellum is required for stress-induced attacks, then blocking CK2 should prevent attacks. To test this hypothesis, we pharmacologically blocked CK2 by chronic perfusion of a highly selective CK2 inhibitor, 4,5,6,7-tetrabromobenzotriazole (TBB) (31), directly in the cerebellum of tottering mice using osmotic pumps. Within 48 hours of starting cerebellar perfusion of TBB, the tottering mice stopped having stress-induced attacks. The stress-induced attacks, however, returned when the osmotic pumps delivering TBB depleted 14 days after implantation (Fig. 8C).
Cerebellar, small hairpin RNA–mediated KD of CK2 prevents stress-induced attacks
To corroborate our pharmacological results, we used small hairpin RNAs (shRNAs) to KD CK2 in the cerebellum of tottering mice. To control for any potential off-target effects of the shRNA, we used two different sequences with nonoverlapping domains. To do so, we took advantage of the fact that the SK2-associated CK2 is (i) a holoenzyme that is composed of two catalytic α subunits and two regulatory/structural β subunits and (ii) both the α and β subunits are required for its function (25, 32). The α subunit is the catalytic part of the enzyme (32); thus, KD of this subunit will block CK2 activity. The β subunit is required for the assembly of the holoenzyme in complexes (32); thus, KD of this subunit would prevent CK2 from forming a complex with SK2 but will leave its catalytic function intact. Because, with this approach, each subunit is targeted with an shRNA sequence that is completely different form the other, the chances of overlapping off-target effects are reduced.
We stereotaxically injected AAV9-shCK2α (shCK2α), AAV9-shCK2β (shCK2β), or AAV9-shNT (nontargeting, shNT) into the cerebellum of tottering and WT mice. Purkinje cells throughout the cerebellum of tottering mice injected with shCK2β were positive for green fluorescent protein (GFP) (fig. S8A), confirming wide spread of the virus. Moreover, consistent with previous reports, little to no GFP expression was seen in the granular cell layer, whereas the Purkinje cells highly expressed GFP, suggesting that AAV9 preferentially targets Purkinje cells in the cerebellum (fig. S8A) (33, 34). Western blot quantification showed 60 to 80% KD of the targeted CK2 subunits in vivo (Fig. 8, D to F). shNT-injected tottering mice also showed good spread of the virus in the cerebellum with no significant KD of CK2 as expected (fig. S8, C to G), confirming that the shCK2 sequences used were indeed responsible for the observed KD of the protein detailed earlier.
To explore the impact of KD of CK2 on stress-induced attacks in the tottering mice, we started behavioral assessment 7 days before shRNA injection. We counted the frequency of these spontaneous attacks before and 2 to 4 weeks after shRNA injections and found that after expression of shCK2α or shCK2β, the mice stopped having spontaneous stress-induced attacks (Fig. 8G), suggesting that CK2 activity is required for spontaneous stress-induced attacks in tottering mice. In contrast, the occurrence of spontaneous attacks in tottering mice targeted with shNT was not different before or after shRNA injection.
Expression of shRNAs delivered by AAVs and KD of the corresponding proteins usually take about 2 to 3 weeks after injection as the shRNA is fully expressed, and the existing proteins have turned over (35, 36). Because, during the first few weeks, one does not expect to see any alterations in stress-induced motor attacks, it can be used as a control for complications arising from surgery, unintended brain damage, or any other unforeseen complications. This is particularly pertinent in this case because damage to the cerebellum of tottering mice is known to prevent motor attacks (37). We thus explored the timeline during which the tottering mice stopped having spontaneous attacks. Reassuringly, the mice continued having spontaneous attacks for the first 2 weeks after injection in the case of shCK2β and for the first 4 weeks after injection in the case of shCK2α, after which spontaneous attacks completely ceased (fig. S10, A and B).
As discussed, examination of the mice during the 2-hour acclimation period allowed us to explore whether the mice had spontaneous stress-induced attacks. After this 2-hour acclimation period and only when the mice returned to their baseline disability score for at least half an hour if they had an attack, each mouse was subjected to the standard procedure for restraint stress–induced attacks. At the peak of their motor dysfunction, about 30 to 40 min after restraining, the average dyskinesia score of the tottering mice is above 4. With both CK2 shRNAs, 2 to 4 weeks after injection, the mice did not exhibit any motor attacks, and their average dyskinesia score 40 min after stress was less than 2 (Fig. 8H and movie S1). In control experiments on WT mice, although one of the CK2 shRNAs slightly affected motor function, the other sequence did not have a notable effect on motor function despite both sequences effectively reducing the CK2 protein levels. This suggests that the subtle motor dysfunction seen with the first shRNA was unrelated to KD of CK2 in the cerebellum (fig. S10, C and D). Together, the data presented here strongly suggest that CK2 activity in the cerebellum is required for motor attacks in tottering mice.
KD of CK2 blocks NE-induced irregularity of tottering Purkinje cells in vitro
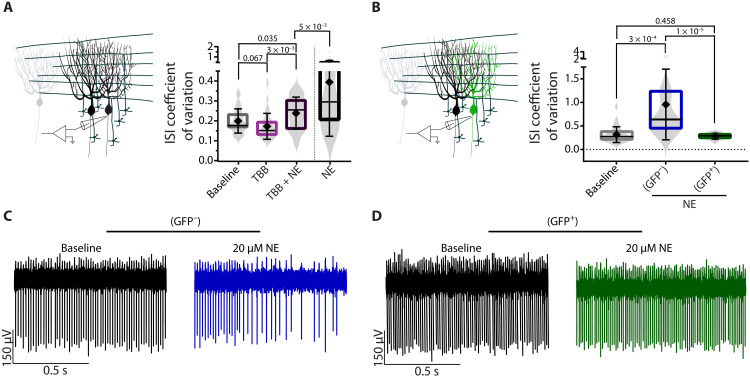
Our working hypothesis, motivated and supported by the data presented so far, posits that stress-induced increase in cerebellar NE further reduces the regularity of pacemaking in Purkinje cells by decreasing the open probability of SK channels via CK2-dependent phosphorylation of their associated CaM. A prediction of this hypothesis is that blocking CK2 activity should prevent the decrease in regularity of Purkinje cells when they are exposed to NE in acutely prepared brain slices. To test this hypothesis, we first assessed whether pharmacologic block of CK2 with TBB reduced the impact of NE on the activity of tottering Purkinje cells and found this to be the case (Fig. 9A). We next examined whether shRNA-mediated KD of CK2 was effective in preventing NE-induced firing irregularity of tottering Purkinje cells in vitro. After injecting shCK2α into the cerebellum of the tottering mice and verifying that doing so had abolished stress-induced motor attacks, we used GFP as a proxy for expression of the shRNA and recorded from GFP-positive and GFP-negative Purkinje cells in acutely prepared cerebellar slices. Consistent with our working hypothesis, NE did not affect the firing pattern of the CK2 shRNA–expressing GFP-positive cells, although the firing of adjacent Purkinje cells that did not express GFP still became more irregular (Fig. 9, B to D). These data suggest that CK2 activity is required for the effects of NE on tottering Purkinje cell regularity.
Fig. 9. Block or KD of CK2 prevents NE’s effect on tottering Purkinje cells in vitro.
(A) Schematic of in vitro recordings. ISI CV of tottering Purkinje cells recorded in the absence of fast synaptic blockers during baseline (gray), bath application of 200 nM TBB (pink), or coapplication of 200 nM TBB and 20 μM NE (dark purple) compared to application of 20 μM NE alone (black). N = 6 tottering mice; n = 29 for baseline (mean = 0.199 ± 0.061), n = 30 for TBB (mean = 0.172 ± 0.065), and n = 29 for TBB + NE (mean = 0.247 ± 0.088). (B) Schematic of in vitro recordings. Green cell denotes GFP-positive cell with CK2 KD. ISI CV of Purkinje cells from tottering cerebellar slices recorded in the absence of fast synaptic blockers during baseline or bath application of 20 μM NE in GFP-negative (blue) and GFP-positive (green) cells. N = 6 tottering mice; n = 31 for tottering baseline (mean = 0.313 ± 0.167), n = 14 for tottering GFP negative (blue) (mean = 0.953 ± 0.750), and n = 17 for GFP positive (green) (mean = 0.281 ± 0.062). (C) Sample traces of GFP-negative (blue) Purkinje cells from tottering cerebellar slices recorded in the absence of fast synaptic blockers during baseline (left) or bath application of 20 μM NE (right, blue). (D) GFP-positive (green) Purkinje cells from tottering cerebellar slices recorded in the absence of fast synaptic blockers during baseline (left) or bath application of 20 μM NE (green).
The block of CK2 by CX-4945 prevents stress-induced attacks and NE’s effects on tottering Purkinje cells
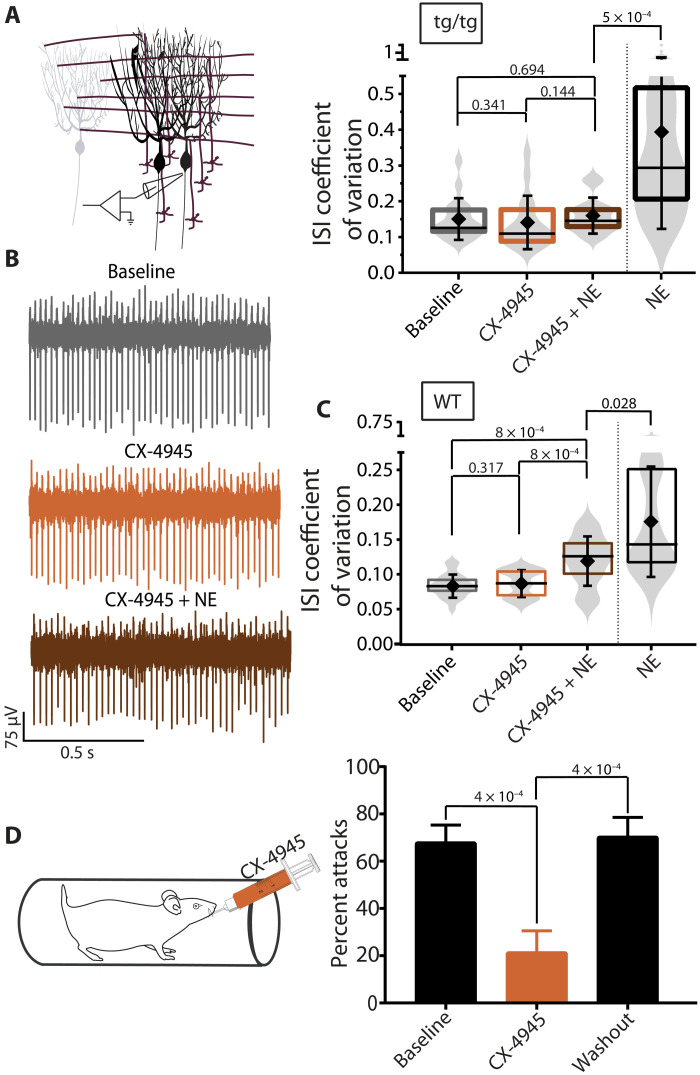
The data so far strongly suggest that CK2-dependent phosphorylation in Purkinje cells is required for stress-induced attacks in tottering mice and that CK2 might be a potential pharmacologic intervention point for prevention of stress-induced attacks in patients suffering from EA2. We thus sought to explore the utility of systemic application of an FDA-approved, potent and selective CK2 blocker, CX-4945 (silmitasertib), that has been shown to cross the blood-brain barrier in mice when administered orally (38), as a potential therapeutic agent for prevention of stress-induced attacks in EA2. We first assessed whether, similar to TBB, CX-4945 could prevent NE-induced irregularity of tottering Purkinje cell activity in acutely prepared cerebellar slices. At a concentration of 100 nM, CX-4945 effectively prevented the NE-induced aberrant Purkinje cell activity in tottering mice (Fig. 10, A and B) and attenuated, although did not completely alleviate, NE-induced aberrant Purkinje cell activity in WT mice (Fig. 10C). Given this success, we examined the efficacy of CX-4945 in blocking restraint-induced stress attacks in the tottering mice and found it to be highly efficacious (Fig. 10D). Thus, systemic administration of CX-4945 might be a potentially suitable prophylactic treatment for patients with EA2.
Fig. 10. The block of CK2 by CX-4945 prevents stress-induced attacks and NE’s effects on tottering Purkinje cells.
(A) Schematic of in vitro recordings. ISI CV of tottering Purkinje cells recorded during baseline (gray), bath application of 10 nM CX-4945 (orange), and coapplication of 10 nM CX-4945 and 20 μM NE (brown) compared to application of 20 μM NE alone (black) in the absence of fast synaptic blockers. N = 3 tottering mice, n = 17 for baseline (mean = 0.150 ± 0.059), n = 16 for CX-4945 (mean = 0.140 ± 0.075), and n = 14 for CX-4945 + NE (mean = 0.159 ± 0.051). (B) Sample traces of tottering Purkinje cells recorded during baseline, bath application of 10 nM CX-4945, and coapplication of 10 nM CX-4945 and 20 μM NE in the absence of fast synaptic blockers. (C) ISI CV of WT Purkinje cells recorded during baseline (gray), bath application of CX-4945 (orange), and coapplication of CX-4945 and NE (brown) compared to application of NE alone (black) in the absence of fast synaptic blockers. N = 4 WT mice, n = 24 for baseline (mean = 0.084 ± 0.017), n = 17 for CX-4945 (mean = 0.088 ± 0.020), and n = 20 for CX-4945 + NE (mean = 0.120 ± 0.036). (D) Schematic of the experimental paradigm. Frequency of attacks of tottering mice exposed to restraint stress during baseline, after 3 days of receiving CX-4945 (75 mg/kg) via gavage, and during washout periods. N = 5 and n = 3 to 5 trials. One-way ANOVA with Tukey’s multiple comparisons test.
DISCUSSION
An intriguing feature of many neurological disorders is their episodic nature. Typically, the patients often show relatively few and mild symptoms for a stretch of time, with the disorder manifesting, often abruptly, after a trigger. One of the most common triggers, prevalent among several diverse neurological disorders, is stress. We currently have, at best, a superficial understanding of how stress affects the brain to trigger the episodes of dysfunction in these disorders.
Here, we used the tottering mice, a faithful model of EA2, to tease out the mechanism by which stress triggers episodes of motor dysfunction. The data presented here are consistent with the working hypothesis that stress triggers the episodes of motor attacks by elevating cerebellar NE levels. The source of NE in the cerebellum is the LC, and it is known that in the tottering mice, this pathway is altered and LC hyperinnervates the cerebellum (2, 39–41). The higher NE levels in the cerebellum activate α1-Rs expressed on Purkinje cells, which, in turn, via a CK2-dependent mechanism, phosphorylate the CaM associated with SK channels. The phosphorylated CaM has a lower affinity for calcium and thus results in the reduced open probability of the SK channels. Because SK channels are crucial for setting the regularity of pacemaking in Purkinje cells (13, 26), their diminished activity further decreases the precision of the Purkinje cell intrinsic activity. This decreased fidelity with which Purkinje cells encode movement-related synaptic information in their firing leads to the more severe motor dysfunction seen during the attacks, consistent with prior observations in the tottering mice where the severity of motor symptoms was found to be correlated with the irregularity of Purkinje cell activity (5). It is noteworthy that the return of irregularity of Purkinje firing, and thus motor dysfunction, to baseline levels requires dephosphorylation of CaM, a step that is likely to be much slower than the time course of the rise and fall of NE in the cerebellum. The time course of this dephosphorylation step, therefore, likely dictates the duration of the motor attacks in EA2, which outlast the time course of NE rise in the brain.
Our work has clear therapeutic implications for patients with EA2. Given the prominent role of the adrenergic α1 receptors in mediating stress-evoked attacks, their selective FDA-approved blockers, such as prazosin or silodosin, might be of therapeutic value. Moreover, given our finding that CK2 activity was also required for stress-evoked attacks, this enzyme, too, could be a impactful therapeutic target. In our experiments, we found that oral administration of the CK2 inhibitor CX-4945 was quite effective. CX-4945 (silmitasertib) is FDA approved for use in cancer and seems to have less side effects than other CK2 inhibitors (42). The potential utility of drugs targeting these two pathways in the prevention of stress-induced attacks in patients with EA2, therefore, merits closer scrutiny.
While, in the tottering mice, only the adrenergic α1 receptors were required and sufficient to trigger the attacks, in the WT mice, by targeting presynaptic processes, β-adrenergic receptors also contributed to the NE-induced irregularity of Purkinje cells. Thus, it is plausible that in other episodic movement disorders, adrenergic β receptors may also be a contributing factor in triggering the onset of cerebellar symptoms. For example, many patients with essential tremor have reported that their tremor worsens with stress (43), and stress- and anxiety-induced hand, limb, or voice shaking and tremor are relatively common occurrences in the general population and can be debilitating for many (44). Intriguingly, β blockers such as propranolol have been used clinically to treat these disorders (45, 46) for 40 years without a clear understanding of their mechanism of action (47). EA1 is another example of an episodic movement disorder in which stress is a prominent trigger. EA1 is caused by mutations in the Kv1.1 channel that is expressed both in cerebellar basket cells and on the dendrites of Purkinje cells (48). While a small fraction of patients show persistent cerebellar symptoms, most of them display interictal myokymia with bouts of ataxia that can be triggered by physical and emotional stress, startle response, or abrupt movement (49). In a mouse model of EA1, injection of an adrenergic agonist before exercise on the treadmill, as a proxy for stress fear, caused a significant increase in foot slips (50), possibly by activation of cerebellar adrenergic receptors and increasing irregularity of Purkinje cell firing as described here. Thus, the pathways delineated here might also be involved in EA1 and could constitute meaningful therapeutic targets.
Ataxias and tremors are not the only movement disorders in which stress worsens the symptoms, and the disease might be associated, at least in part, with the cerebellum. Cerebellar dysfunction is thought to contribute to motor dysfunction in a number of hereditary movement disorders where the symptoms include athetosis and dystonia, such as DYT1 (51, 52), DYT11 (53, 54), DYT12 (55), ataxia telangiectasia (56), and paroxysmal movement disorders such as paroxysmal kinesigenic dyskinesia (57). In many of these disorders, stress is known to exaggerate and worsen the motor symptoms (58, 59), and again, it is quite likely that cerebellar α1- and β-adrenergic receptors may play a prominent role in mediating the adverse effects of stress as delineated here.
Because stress is a common trigger among a diverse group of episodic neurologic disorders and not just in movement disorders, it is plausible that activation of cerebellar adrenergic receptors and changes in cerebellar output might also be the mechanism by which stress triggers episodes of dysfunction in other disorders as well. For this to be the case, a prerequisite, however, would be that in these disorders, either the cerebellum should be directly implicated in the disease or its dysfunction should somehow affect the brain region(s) that cause(s) the neurologic disorder. Allelic gain-of-function missense mutations in the CACNA1A, the gene implicated in EA2, cause familial hemiplegic migraine type 1 (FHM1). In FHM1, bouts of hemiplegia, spontaneous seizure, and migraine are triggered by stress (60, 61) and, on occasions, are preceded by motor aura (60). Given the allelic comorbidity between EA2 and FHM1 and the fact that about half of patients with EA2 also suffer from migraines (49), it is plausible that dysfunction of the cerebellum may also contribute to the nonmotor symptoms. Aberrant firing of cerebellar Purkinje cells and motor incoordination have been noted in a mouse model of FHM1 (62). It is plausible, therefore, that a further stress-induced increase in the irregularity of Purkinje cell activity as detailed here could trigger the episodic symptoms exhibited by patients with FHM1.
It is also worth noting that migraine and epilepsy are often found to be comorbid (63), and changes in cerebellar output have been shown to be capable of initiating or aborting seizures in several animal models (64, 65). Given the episodic nature of many forms of epilepsy and the prominent role of stress in triggering them (66), the role of the adrenergic pathway described here in triggering seizures merits further scrutiny.
Without doubt, the impact of stress on the brain is not limited to changes in the firing pattern of Purkinje cells. Even within the cerebellum, NE has a myriad of additional effects, including modulation of synaptic plasticity (24, 67, 68). Stress also causes the release of several neuropeptides, including adrenocorticotropic hormones and corticotropin-releasing hormone (69), which, in turn, have profound effects on the brain’s excitability and function (70, 71). Nonetheless, our data in the tottering mice clearly demonstrate the requirement for, and the sufficiency of, cerebellar noradrenergic signaling in triggering episodes of motor dysfunction after stress. While future studies need to delineate the exact contribution of other stress-induced neuropeptides to episodic neurologic disorders, as discussed, the data presented here offer a number of therapeutic targets for improving the quality of life for patients with EA2 and possibly a number of other episodic neurologic disorders.
Experimental model and subject details
Experiments were performed on 3- to 9-month-old male or female tottering (tottering) and WT mice on a C57/Bl6 background, in accord with the guidelines set and approved by the Institutional Animal Care and Use Committee (IACUC) of the Albert Einstein College of Medicine. Tottering (tottering) and heterozygous (tg/+) mice were bred in-house, and tottering mice were born in a Mendelian fashion.
Method details
Restraint-induced attacks
Stress attacks were induced by restraining mice in a 60-ml syringe for 10 min (stress-induced attacks), a commonly used paradigm to trigger attacks in tottering mice (12).
Spontaneous attacks
The stress associated with the daily moving of the tottering mice from the animal facility to the laboratory, which was situated several floors away, often resulted in a spontaneous attack that, on average, started approximately 10 min after transfer. Therefore, after transferring the mice from the animal facility to the laboratory, we monitored them for at least 2 hours. If a mouse had an attack, this was noted, and the animal was allowed to recover and return fully to baseline for at least 2 hours before the start of an experiment.
Systemic drug administration
Mice were transferred from the animal facility to the laboratory and allowed to habituate for at least 2 hours before injections. On any given day, each mouse received either the vehicle or drug, with a total of six trials of the same drug on alternate days. All the drugs used had a half-life between 2 and 4 hours in rodents (72, 73). Thus, at the concentrations used and given our experimental design that had a 24-hour gap between the injections, sufficient time was ensured for drug clearance between each trial. A volume of 10 ml/kg of each drug was injected intraperitoneally. Clonidine, propranolol, and prazosin were obtained from Tocris, and fresh solutions were prepared every day. Clonidine and propranolol were dissolved in 0.9% saline. Prazosin was first dissolved in dimethyl sulfoxide (DMSO) and then prepared in 0.9% saline with a final DMSO concentration of 0.5%. The concentrations of clonidine (0.05 mg/kg), prazosin (0.5 mg/kg), and propranolol (5 mg/kg) were based on the prior published efficacy of the drugs in inhibiting attacks in the tottering mice (14). These concentrations were below that which alters locomotor behavior (74, 75). In all cases, the control solutions were identical in composition to the vehicle used for delivery of the drugs, except that the drug itself was omitted. In all experiments, the behavior of the mice was monitored to ensure that the drugs did not produce adverse behavioral effects, such as sedation, anxiety, or other unusual behaviors.
Intracerebellar injections
For intracerebellar injections, in initial experiments, a single injection cannula (Plastics One Inc.) was stereotaxically implanted at midline (Anterior-Posterior (AP): −6.90 mm from bregma; Dorsal-Ventral DV: 2 mm) under isoflurane anesthesia. To ensure maximal coverage of the cerebellum, in most subsequent experiments, a bilateral cannula was implanted at AP: −6.90 from bregma, ML: ±0.75 from midline, and DV: 2 mm, although for the most part, little difference was found between the data obtained from single midline cannula injections and the bilateral ones. Following recovery, a total volume of 3 or 5 μl of the desired solution was injected over a period of 15 min using an automated pump (World Precision Instruments). UCL-1684 (Tocris), cirazoline, prazosin, and NE (Sigma-Aldrich) were prepared in a DMSO stock solution and, for experiments, were diluted in 0.9% saline, fresh daily with a final DMSO concentration of 0.5%. Intracerebellar injections of UCL-1684 ranged from 1 to 40 μM for tottering and 1 to 800 μM for WT mice, while cirazoline, prazosin, and NE were injected at 2.5, 1.5, and 10 μg, respectively.
Chronic perfusion of the cerebellum
Chronic perfusion of the cerebellum was done as previously described (76). Briefly, a single cannula (Plastics One) was stereotaxically implanted at midline (AP: −6.90 mm from bregma; DV: 2 mm) and connected to an osmotic pump (0.25 μl/hour; model 1007D, Alzet). The pump was then placed under the skin on the back of the mice. Pumps were filled with solutions of 20 mM TBB (Tocris) made in 5% DMSO in 0.1% cyclodextrin and 0.01% methylene blue, which allowed for postmortem examination of the perfusion site. Using this technique, we have shown that the slow rate of perfusion allows the drug to remain contained within the cerebellum, with its concentration sharply dropping within 1 mm of the perfusion site (76). Following surgery and every 12 hours thereafter, the long-lasting nonsteroidal anti-inflammatory drug pain reliever flunixin was administered subcutaneously.
Optogenetic stimulation of the LC
Virus (1.2 μl or 400 nl) [AAV2/1. CamKIIa.hChR2 (H134R)-EYFP; University of Pennsylvania Vector Core] was injected targeting the LC bilaterally (AP: −5.8 mm; ML: ±1 mm; DV: −2.8 mm). While the 400-nl volume allowed for less spread of the virus, no differences were found in motor response between the experiments in which 1.2 μl or 400 nl of virus was injected. Two optical fibers (200 μm in diameter, 0.48 numerical aperture; Thorlabs) were implanted to target the LC. Light from a 473- or 450-nm laser (OEM Laser Systems) was delivered using fiber optic ferrules (Kientec Systems) to the implanted fibers. Attenuation over the connector was measured before implantation to calculate output at the fiber tip during experiments. Stimuli were delivered as 10-ms light pulses at 10 Hz for 30 s at intensities ranging from <1 to 15 mW. As described, in some experiments, 30 min before stimulation of LC, 5 μl of 1.5-μg prazosin was injected into the cerebellum using bilateral cannulas over a period of 15 min using an automated pump (World Precision Instruments).
shRNA constructs
shRNA sequences against Mus musculus CSNK2A1 and CSNK2B were obtained from the Albert Einstein College of Medicine Viral Core (CSNK2B clone 364: GCTCCGTGGTAATGAATTCTT; clone 365: GCATCGCACAAATGTTGGAAA; clone 366: CTCTATGGTTTCAAGATCCAT; CSNK2A1 clone 3203: CCGAGTTGCTTCTCGATATTT; clone 3205: CTGGACAAGCTGCTTCGATAT). Lentivirus was produced from each construct at a titer of 2.5 × 106 TU (transduction units) by the Albert Einstein College of Medicine Viral Core. Cortical cultures were infected at 4 to 5 days after plating with lentivirus expressing each shRNA. Lysates were prepared from the infected cultures with ice-cold lysis buffer at 9 days after infection, and KD of CK2α or CK2β was confirmed by Western blots [lysis buffer: 50 mM tris (pH 7.5), 2% SDS, and protease and phosphatase inhibitors from Roche]. After KD of CK2 was confirmed in cortical cultures, these shRNA sequences were packaged into AAV (AAV9) by Virovek. AAVs used include the following: AAV9-U6-sh364-CMV-GFP at a titer of 1.3 × 1013 vg/ml; AAV9-U6-sh3203-CMV-GFP at a titer of 1.6 × 1013 vg/ml; AAV9-U6-shNon-targeted-CMV-GFP at a titer of 2.3 × 1013 vg/ml. We chose AAVs rather than lentiviruses because of their larger spread and their higher titer and overall safety (77). To test the efficacy of the AAV9 particles carrying our shRNA sequences, we again infected mouse cortical cultures with varying volumes of AAV9 particles carrying shRNA sequences against the target proteins or nontargeted (NT) sequences.
Preparation of cortical cultures
Cortical cultures were prepared from WT C57BL6/J mice at embryonic days 15 and 16. Embryos were removed and placed in a 10-cm dish containing cold, sterile Hepes-glucose [2.38 g of Hepes and 6 g of glucose dissolved in 1 liter of 1× phosphate-buffered saline (PBS) and filtered (pH 7.3)]. The cortices were removed from the embryos and incubated in a 15-ml tube containing 4.5 ml of Hepes-glucose buffer. After all the cortices are removed and placed in the Hepes-glucose buffer, 0.5 ml of 0.5% trypsin is added to the tube and incubated in a 37°C water bath for 15 min. The buffer containing trypsin is now removed and replaced with 10 ml of Hepes-glucose. The tube is tilted gently from side to side three times to wash the tissue. The Hepes-glucose is removed carefully to not remove the tissue. This washing process is repeated three more times. After the last wash, 2 ml of plating media (Dulbecco’s modified Eagle’s medium, 10% fetal bovine serum, and penicillin/streptomycin (Penn/Strep)] is added to the tube. The tissue is then triturated with a Pasteur pipette that has been heated to reduce the bore size. After dissociation, an additional 8 ml of plating media is added, and the cells are counted. Cells are plated in a 12-well or a 24-well plate coated overnight in poly-l-lysine at 150,000 cells per 12 wells in 1 ml of plating media. After 2 hours when the cells have adhered to the plate, the plating media is carefully removed and replaced with Neurobasal media (Neurobasal, B27 supplement, Penn/Strep, and Glutamax). Cultures were incubated in a humidified incubator at 37°C and 5% CO2.
Stereotaxic viral injections
For all injections, mice were anesthetized with isoflurane, placed on a stereotaxic surgery apparatus, and stabilized with ear bars. Isoflurane anesthesia was maintained throughout the surgery with the use of a vaporizer. Animals were also maintained at 35° to 37°C during surgery using a heating pad. Virus was injected stereotaxically into anesthetized 3- to 6-month-old tottering or 2- to 4-month-old WT C57BL6 mice. Virus (2 μl) was infused into the cerebellum at four sites at a rate of 0.12 μl/min with a stereotaxic injector (Stoelting) and using a 30-gauge Hamilton syringe (needle length, 1.905 cm; point style, 3). Coordinates of viral infusion from bregma are as follows: AP: −6 mm, ML: 0 mm, and DV: −1.5 mm; AP: −6.96 mm, ML: 0 mm, and DV: 1.5 mm; AP: −6 mm, ML: 1.8 mm, and DV: 2.3 mm; AP: −6 mm, ML: −1.8 mm, and DV: 2.3 mm. After the injection, the syringe was left in place for a minimum of 10 min before it was slowly removed. All mice underwent behavioral testing, and at the end of all experiments, their cerebella were removed, immediately imaged to record the spread of the virus, and then subsequently subjected to either detailed histological examination or Western blot analysis.
Evaluating motor behavior
On the experimental day, mice were collected from the animal facility and allowed to acclimate to the laboratory in their home cages for at least 2 hours. During this acclimation period, the mice were assessed for the presence of spontaneous stress-induced attacks that were often triggered by their transportation from the animal facility, which is housed on a separate floor of the building, to the laboratory where the experiments were conducted. The use of 16 surveillance cameras (Lorex Full 960H Security) enabled us to monitor and record the activity of up to 16 individually caged mice at once. Representative 30-s clips were made from each video. The severity of motor dysfunction under various experimental conditions was determined by four independent observers who were blinded to the nature of the experiment using the scale described below. The scorers were trained with a video set containing representative examples with key characteristics highlighted for each score. Mice were scored on the basis of a previously published disability scale (78) as follows: 0 = normal motor behavior; 1 = slightly slowed or abnormal movements; 2 = mild impairments and limited ambulation unless disturbed; 3 = moderate impairment, limited ambulation even when disturbed, and frequent abnormal postures; 4 = severe impairment, almost no ambulation, and sustained abnormal postures; 5 = prolonged immobility in abnormal postures. In tottering mice, independent of the nature of the trigger (caffeine-, ethanol-, and restraint-induced stress, spontaneous attacks), the episodes of motor dysfunction are very similar and stereotypic. Initially, the baseline ataxia transforms to severe ataxia, and then dysfunction progresses to dyskinesia and dystonia. In cases where we calculated the fraction of the animals that had an attack, an animal that had a score of ≥2 on the disability scale was defined as having an attack.
Balance beam
The balance beam, a plexiglass rectangular rod of 11 mm in width, was mounted on a stand. At one end, there was a dark chamber, while the other end had a bright light attached. Mice were acclimated to the balance beam for 1 week before testing began. Each week after injection, all mice were individually tested on the balance beam. The mouse was placed on the end where the light source was positioned and was allowed to walk to the opposite side of the beam toward the dark chamber, a length of 80 cm. After one trial, where the mouse crossed once, it was placed in its home cage. All other mice were also subjected to one trial. After 10-min resting periods, each mouse was subjected to the second and third trials. Videos were analyzed after the experiment, the number of hindlimb slips made by each mouse was counted, and the average number of slips per mouse per week was noted.
Western blots
Mice were euthanized, and their brains were quickly removed and imaged using a fluorescence microscope to evaluate the spread of the virus. The cerebellum was dissected, lysed, and Dounce-homogenized in 1 ml of lysis buffer [lysis buffer: 50 mM tris (pH 7.5), 2% SDS, and protease and phosphatase inhibitors from Roche]. The lysates were sonicated for 15 s (10% amplitude and 5 s on and 5 s off × 3) and incubated at room temperature on a rocker for 1 hour. The lysates were then centrifuged at 10,000g for 20 min at room temperature. The supernatant was then aliquoted and stored at −80°C.
Lysates were subsequently thawed on ice, and protein concentration was determined using a Bicinchoninic acid (BCA) protein assay. Samples were prepared for loading with 10 to 20 μg of protein, 75 mM dithiothreitol (DTT), Lithium dodecyl sulfate (LDS)-based Laemmli sample buffer, and water and then incubated at 95°C for 5 min. Each sample was loaded onto a 4 to 12% or 10% Nu-PAGE Bis-Tris gel (Life Technologies). Running buffer used was a standard 1× Mops buffer supplemented with 5 mM sodium metabisulfite. The gel was run for 1.5 hours at a constant voltage of 130 V and transferred onto a polyvinylidene difluoride membrane for 1 to 1.5 hours at a constant current of 330 mA. Transfer buffer contained 1× Nu-PAGE transfer buffer with 10% methanol. After transfer, the membrane was rinsed with deionized water and blocked with LI-COR blocking buffer [diluted with tris-buffered saline (TBS) 1:1 (v/v); part#: 927-50000] for 1 hour at room temperature.
The membrane was then incubated with primary antibody overnight at 4°C according to the following dilutions in TBS-based LI-COR blocking buffer diluted 1:1 with TBS-T (0.1% Tween 20): mouse anti-CK2β at 1:1000 (6D5, Calbiochem) and rabbit anti-CK2α at 1:1000 (Millipore). Mouse anti–β-actin and mouse anti–β-tubulin (Sigma-Aldrich) at 1:40,000 were incubated for 1 hour on the rocker at room temperature. After primary antibody incubation, the membrane was washed 3× 10 min each with TBS-T and then incubated with secondary antibody (Licor IRDye 680 or IRDye 800) at 1:20,000 for 1 hour on a rocker at room temperature. The secondary antibody was prepared in LI-COR blocking buffer diluted 1:1 with TBS-T + 0.02% SDS. The secondary antibody was removed, and the membrane was washed 3× 10 min each with TBS-T and a final rinse with TBS. The membrane was then visualized using LI-COR Odyssey CLx imaging System.
Coimmunoprecipitation
WT mice were euthanized, and the cerebellum was dissected and lysed in 1 ml of ice-cold lysis buffer [190 mM NaCl, 10 mM KCl, 10 mM Hepes, 1 mM EGTA, and 1% β-d-dodecyl maltoside (DDM)], supplemented with protease (Roche cOmplete, 04693159001) and phosphatase (Roche PhosSTOP, PHOSS-RO) inhibitors (pH 7.4). The lysates were sonicated for 15 s (10% amplitude and 5 s on and 5 s off × 3) and incubated on ice for 1 hour. The lysates were then centrifuged at 10,000g for 20 min at 4°C. Protein-containing supernatant (1 mg) was diluted 1:10 with the same lysis buffer containing no DDM to dilute the concentration of DDM to 0.1%. The diluted supernatant was then incubated with the appropriate anti-SK2 primary antibody for 16 hours on a rocker at 4°C. The lysates were then incubated with magnetic Dynabeads Protein G for immunoprecipitation (Invitrogen, catalog no. 10003D) for 1 hour on a rocker at 4°C and then washed three times with 0.1% DDM–containing lysis buffer. The bound proteins were eluted using 2× LDS-based Laemmli sample buffer without DDT. The eluted samples were then diluted to 1× Laemmli buffer with water, and 75 mM DTT was added before incubation at 95°C for 5 min. Samples were then subjected to Western blotting.
Immunostaining
Mice were euthanized with isoflurane in accordance with the IACUC guidelines of Albert Einstein College of Medicine. Perfusions were performed with 4% paraformaldehyde (PFA) to fix the brain. The brain was removed from the skull and fixed overnight in 4% PFA. The brain was then transferred to a solution of 15% sucrose followed by 30% sucrose prepared in 1× PBS. After the brain had sunk in sucrose, it was then cryopreserved in a mold by carefully positioning within a medium of Optimal cutting temperature (OCT) compound on dry ice. Sagittal or coronal sections were prepared on a cryostat (Leica) at 30 to 50 μm in thickness. Sections were collected in a 24-well plate containing a cryoprotectant (glycerol and ethylene glycol). Sections were stored at −20°C until they were ready for use. For the p-CaM experiment, mice were stressed as previously reported, and 20 to 30 min after stress, the mice were euthanized with isoflurane, and the cerebellum was removed and processed for immunohistochemistry. About five to nine coronal sections per mouse were randomly chosen between −5 and −7 mm from bregma.
Sections were transferred to wells containing 1× PBS and washed three times at 10 min per wash. The sections were blocked with a blocking buffer [10% goat serum, 1% bovine serum albumin (BSA), and 0.02% saponin in 1× PBS] for 1 hour at room temperature. The blocking buffer was then removed, and the sections were incubated overnight at 4°C in primary antibody prepared in blocking buffer: chicken anti-GFP at 1:1000 (Abcam) and rabbit anti–p-CaM (Thr79 and Ser81) at 1:50 (Thermo Fisher Scientific). The sections were washed three times for 10 min per wash with 1× PBS containing 0.02% saponin. The sections were then incubated in secondary antibody prepared in a secondary blocking buffer (5% goat serum, 1% BSA, and 0.02% saponin in 1× PBS) for 1 hour at room temperature as follows: goat anti-chicken Alexa Fluor 488 (1:400), goat anti-rabbit Alexa Fluor 488 (1:400), and Hoechst 33342 (1:2000). The sections were washed with 1× PBS with 0.02% saponin two times followed by two washes with 1× PBS. The sections were then placed on Superfrost slides and mounted with Fluoromount-G (SouthernBiotech) or ProLong gold (Cell Signaling Technology) and a coverslip.
Imaging and image analysis
For the shCK2 study, slides were imaged using a Zeiss Axio Observer Z1 fluorescence motorized microscope. For the p-CaM study, sections were imaged using a Zeiss Axio Scan.Z1 slide scanner with the accompanying Zen Blue 2 software. A 20× objective was used for imaging, and three Z stacks (8 μm per stack) were acquired for each section with identical settings for all conditions. Images were then stitched with 10% overlap using Zen Blue 2 software and exported as 16-bit TIF images. Fluorescence intensity of Purkinje cell somas was quantified using Metamorph v.7.8.13.0 image analysis software by two of the authors in a blinded fashion. From each section, 500 to 1500 somas were used as regions of interest. The experiment was carried out four times; at each experiment, the average intensity from each section was normalized to the average intensity of all sections of the no-attack condition and multiplied by 100. For each experiment, three to five sections were stained with only the secondary antibodies (goat anti-rabbit 488 Alexa Fluor and Hoechst 33342), excluding the primary antibody, to be used as background removal controls. The background was obtained by choosing 15 regions of interest in the background control sections, and the average intensity +4x(SD) of these regions was used as background removal.
In vivo electrophysiology
All surgical procedures were performed under isoflurane anesthesia (5% induction and 2% maintenance). A custom-made “L”-shaped bracket was fixed onto the skull with three bone screws (Plastics One Inc., Wallingford, CT) and dental cement (M&S Dental Supply, Jamaica, NY). A recording chamber 3 mm in diameter was drilled in the skull above the cerebellum, surrounded with dental cement, and covered with Surgifoam (Ethicon, Somerville, NJ) and bone wax (Ethicon, Somerville, NJ). Following surgery, mice were allowed to recover for 1 week before the recording sessions.
To record neural activity, the mouse’s head was immobilized by fixing the previously implanted head bracket to the stereotaxic apparatus with a screw, with the mouse standing on a flat surface, allowing the experimenter to observe its movements. The Surgifoam and bone wax were removed, and the recording chamber was filled with agar. Single-unit activity of Purkinje cells was recorded using a platinum-quartz electrode (2 to 3 megohm; Thomas Recording GmBH, Giessen, Germany), which was advanced into the cerebellum until the Purkinje cell layer was reached. For electrical reference, we either put a screw above the cortex or placed a wire in the saline bathing the craniotomy. Purkinje cells were identified by the brief pause in their activity following each complex spike, their location, and their characteristic firing rate in vivo (79). Each cell was recorded for at least 5 min and attributed to the attack condition when motor signs could visually be identified and quantified. To aid in accurate quantification of the severity of motor abnormalities, periodically, mice were removed from the stereotaxic apparatus and placed in an open cage, videotaped, and the motor signs quantified as described below. Cells were only classified as baseline when mice exhibited no motor abnormalities for at least 10 min before and after recording. The tottering mice show attacks of motor dysfunction after moderate stress; even their handling or transferring to a new environment frequently triggers an attack. To make unambiguous single-unit recordings that corresponded to their baseline unstressed condition, we habituated the mice to the in vivo recording setup during the course of a few weeks. Under these conditions, we found that mice no longer had attacks while head-restrained, unless if we purposefully stressed them further by inserting them in a 60-ml tube.
Neural signals were band-pass–filtered (80 Hz to 20 kHz), amplified (2000×), and digitized (20 kHz) using a data acquisition card (PCI-MIO-16XE) and an in-house written software based on LabView (National Instruments, Austin, TX). Waveforms were sorted using Offline Sorter software (Plexon Inc., Dallas, TX, USA) using principal components analysis.
In vitro electrophysiology
Adult mice were anesthetized with isoflurane and, once unresponsive to touch, decapitated. The cerebellum was removed, and 300-μm sagittal slices were cut at 35°C. Slices were incubated at 35°C for a minimum of 1 hour and then were transferred to room temperature for the remainder of the experiment. Extracellular electrophysiological recordings were conducted from Purkinje cells using a custom-built differential amplifier and glass pipette electrode back-filled with extracellular solution [135 mM NaCl, 2.5 mM KCl, 26 mM NaHCO3, 1.25 mM sodium phosphate buffer, 1 mM MgCl2, 2 mM CaCl2, and 10 mM glucose (pH 7.4), gassed with 5% CO2:95% O2]. An upright microscope was used for recordings (Olympus). Slices were kept at 35°C and constantly perfused at a rate of 1.5 ml/min during recording sessions. In each experimental setup, the tested drug was added to the extracellular solution and perfused over the slice. Time of drug perfusion began once the drug/artificial cerebrospinal fluid (ACSF) solution reached the recording chamber. All experiments were conducted blind to the drug and the genotype of the mouse (tottering or C57/Bl6 WT), which were revealed after analysis of data was complete.
NE (Sigma-Aldrich), prazosin (Tocris), isoproterenol (Tocris), TBB (Tocris), and CX-4945 (MedChemExpress) were diluted in DMSO to make stock solutions and then added to ACSF on the day of experiments to make their final concentrations of 20 μM, 1 nM, 80 nM, 200 nM, and 10 nM, respectively. In all cases, the baseline control solution was identical to the drug solution except that it lacked the test drug. In all experiments, the concentration of DMSO never exceeded 0.1%, a concentration that we have experimentally determined does not affect the activity of Purkinje cells. An analog-to-digital converter was used to acquire all extracellular data (National Instruments, PCI-MIO-16XE), and analysis was conducted using LabView software. To block fast synaptic transmission, picrotoxin (Tocris) at 10 μM to block gamma-Aminobutyric acid type A (GABA-A) receptors, 6-cyano-7-nitroquinoxaline-2,3-dione or 6,7-dinitroquinoxaline-2,3-dione (DNQX) at 10 μM (Tocris) to block α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors, and CGP55845 (Tocris) at 1 μM to block gamma-aminobutyric acid type b (GABA-B) receptors were added to ACSF before experiments.
Gavage of CX-4945
CX-4945 was dissolved at a concentration of 75 mg/kg in a solution composed of 5% DMSO:40% polyethylene glycol 400:55% water. To ensure that CX-4945 had fully dissolved, it was vortexed for 1 min and then sonicated for 3 to 5 min, with this procedure repeated at least three times. For three consecutive days, CX-4945 was given to tottering mice via gavage using a plastic feeding tube (INSTECH Laboratory Inc.). On the third day, mice underwent restraint stress 30 min after the gavage of CX-4945, and the fraction of the mice that had an attack was determined using the 16–surveillance camera setup described earlier.
Statistics
Statistical analysis was performed using GraphPad Prism software v.7 for Windows. Depending on the data distribution, mean comparisons were performed with either one-way analysis of variance (ANOVA)/two-tailed unpaired Student’s t test, Kolmogorov-Smirnov test, or the Kruskal-Wallis/Mann-Whitney U test. The comparison between the means of dependent samples was accomplished with the two-tailed paired Student’s t test and proportions with the chi-square (x2) test. In the optogenetic experiments, a multiple t test was used with a two-stage step-up method of Benjamini, Krieger, and Yekutieli for false discovery rate approach. When one-way ANOVA was used, it was followed by Tukey’s multiple comparisons test to determine significance. Violin plots display the distribution of the data. Overlayed box plot displays the median (horizontal black line) and the first and third quartiles (colored lines of the box plot), in addition to the mean (black diamond or +) and the SD (vertical black lines) of the data. Exact P values are reported for each statistical comparison.
Acknowledgments
Funding: This study was funded, in part, by the National Institute of Neurological Disorders and Stroke (NINDS) RNS050808.
Author contributions: Conceptualization: K.K., E.T., A.V., and H.D.S. Methodology: K.K., E.T., A.V., H.D.S., B.A.J., and C.C. Investigation: A.V., E.T., H.D.S., C.C., and J.T. Visualization and supervision: K.K. Writing—original draft: K.K., A.V., H.D.S., and E.T. Writing—review and editing: K.K., H.D.S., and A.V.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S11
Table S1
REFERENCES AND NOTES
- 1.Kullmann D. M., Hanna M. G., Neurological disorders caused by inherited ion-channel mutations. Lancet Neurol. 1, 157–166 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Levitt P., Noebels J. L., Mutant mouse tottering: Selective increase of locus ceruleus axons in a defined single-locus mutation. Proc. Natl. Acad. Sci. U.S.A. 78, 4630–4634 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirley T. L., Rao L. M., Hess E. J., Jinnah H. A., Paroxysmal dyskinesias in mice. Mov. Disord. 23, 259–264 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raike R. S., Weisz C., Hoebeek F. E., Terzi M. C., de Zeeuw C. I., van den Maagdenberg A. M., Jinnah H. A., Hess E. J., Stress, caffeine and ethanol trigger transient neurological dysfunction through shared mechanisms in a mouse calcium channelopathy. Neurobiol. Dis. 50, 151–159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tara E., Vitenzon A., Hess E., Khodakhah K., Aberrant cerebellar Purkinje cell activity as the cause of motor attacks in a mouse model of episodic ataxia type 2. Dis. Model. Mech. 11, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell D. B., North J. B., Hess E. J., Tottering mouse motor dysfunction is abolished on the Purkinje cell degeneration (pcd) mutant background. Exp. Neurol. 160, 268–278 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Todorov B., Kros L., Shyti R., Plak P., Haasdijk E. D., Raike R. S., Frants R. R., Hess E. J., Hoebeek F. E., De Zeeuw C. I., van den Maagdenberg A. M. J. M., Purkinje cell-specific ablation of Ca(V)2.1 channels is sufficient to cause cerebellar ataxia in mice. Cerebellum 11, 246–258 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borodovitsyna O., Joshi N., Chandler D., Persistent stress-induced neuroplastic changes in the locus coeruleus/norepinephrine system. Neural Plast. 2018, 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saavedra J. M., Kvetnansky R., Kopin I. J., Adrenaline, noradrenaline and dopamine levels in specific brain stem areas of acutely immobilized rats. Brain Res. 160, 271–280 (1979). [DOI] [PubMed] [Google Scholar]
- 10.Bildl W., Strassmaier T., Thurm H., Andersen J., Eble S., Oliver D., Knipper M., Mann M., Schulte U., Adelman J. P., Fakler B., Protein kinase CK2 is coassembled with small conductance Ca(2+)-activated K+ channels and regulates channel gating. Neuron 43, 847–858 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Womack M. D., Khodakhah K., Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar purkinje neurons. J.Neurosci. 23, 2600–2607 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fureman B. E., Jinnah H. A., Hess E. J., Triggers of paroxysmal dyskinesia in the calcium channel mouse mutant tottering. Pharmacol. Biochem. Behav. 73, 631–637 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Walter J. T., Alvina K., Womack M. D., Chevez C., Khodakhah K., Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat. Neurosci. 9, 389–397 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Fureman B. E., Hess E. J., Noradrenergic blockade prevents attacks in a model of episodic dysfunction caused by a channelopathy. Neurobiol. Dis. 20, 227–232 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Russo-Neustadt A., Cotman C. W., Adrenergic receptors in Alzheimer’s disease brain: Selective increases in the cerebella of aggressive patients. J. Neurosci. 17, 5573–5580 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorton D., Davis J. N., The distribution of beta-1- and beta-2-adrenergic receptors of normal and reeler mouse brain: An in vitro autoradiographic study. Neuroscience 23, 199–210 (1987). [DOI] [PubMed] [Google Scholar]
- 17.Namima M., Okamoto K., Modulatory action of prostaglandin D2 on the release of 3H-norepinephrine from rat cerebellar slices. Jpn. J. Pharmacol. 43, 197–204 (1987). [DOI] [PubMed] [Google Scholar]
- 18.Mark M. D., Maejima T., Kuckelsberg D., Yoo J. W., Hyde R. A., Shah V., Gutierrez D., Moreno R. L., Kruse W., Noebels J. L., Herlitze S., Delayed postnatal loss of P/Q-type calcium channels recapitulates the absence epilepsy, dyskinesia, and ataxia phenotypes of genomic Cacna1a mutations. J. Neurosci. 31, 4311–4326 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloom F. E., Hoffer B. J., Siggins G. R., Studies on norepinephrine-containing afferents to Purkinje cells of art cerebellum. I. Localization of the fibers and their synapses. Brain Res. 25, 501–521 (1971). [DOI] [PubMed] [Google Scholar]
- 20.Bickford-Wimer P., Pang K., Rose G. M., Gerhardt G. A., Electrically-evoked release of norepinephrine in the rat cerebellum: An in vivo electrochemical and electrophysiological study. Brain Res. 558, 305–311 (1991). [DOI] [PubMed] [Google Scholar]
- 21.Abercrombie E. D., Jacobs B. L., Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and nonstressful stimuli. J. Neurosci. 7, 2837–2843 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basile A. S., Dunwiddie T. V., Norepinephrine elicits both excitatory and inhibitory responses from Purkinje cells in the in vitro rat cerebellar slice. Brain Res. 296, 15–25 (1984). [DOI] [PubMed] [Google Scholar]
- 23.Hoebeek F. E., Stahl J. S., van Alphen A. M., Schonewille M., Luo C., Rutteman M., van den Maagdenberg A. M. J. M., Molenaar P. C., Goossens H. H. L. M., Frens M. A., de Zeeuw C. I., Increased noise level of purkinje cell activities minimizes impact of their modulation during sensorimotor control. Neuron 45, 953–965 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Saitow F., Satake S., Yamada J., Konishi S., Beta-adrenergic receptor-mediated presynaptic facilitation of inhibitory GABAergic transmission at cerebellar interneuron-Purkinje cell synapses. J. Neurophysiol. 84, 2016–2025 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Maingret F., Coste B., Hao J., Giamarchi A., Allen D., Crest M., Litchfield D. W., Adelman J. P., Delmas P., Neurotransmitter modulation of small-conductance Ca2+−activated K+ channels by regulation of Ca2+ gating. Neuron 59, 439–449 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Womack M. D., Chevez C., Khodakhah K., Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J.Neurosci. 24, 8818–8822 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvina K., Khodakhah K., The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia. J.Neurosci. 30, 7258–7268 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvina K., Khodakhah K., KCa channels as therapeutic targets in episodic ataxia type-2. J. Neurosci. 30, 7249–7257 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caceres A., Bender P., Snavely L., Rebhun L. I., Steward O., Distribution and subcellular localization of calmodulin in adult and developing brain tissue. Neuroscience 10, 449–461 (1983). [DOI] [PubMed] [Google Scholar]
- 30.Grasselli G., Boele H. J., Titley H. K., Bradford N., van Beers L., Jay L., Beekhof G. C., Busch S. E., de Zeeuw C. I., Schonewille M., Hansel C., SK2 channels in cerebellar Purkinje cells contribute to excitability modulation in motor-learning-specific memory traces. PLoS Biol. 18, e3000596 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarno S., Reddy H., Meggio F., Ruzzene M., Davies S. P., Donella-Deana A., Shugar D., Pinna L. A., Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (‘casein kinase-2’). FEBS Lett. 496, 44–48 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Litchfield D. W., Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem. J. 369, 1–15 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samaranch L., Salegio E. A., San Sebastian W., Kells A. P., Foust K. D., Bringas J. R., Lamarre C., Forsayeth J., Kaspar B. K., Bankiewicz K. S., Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum. Gene Ther. 23, 382–389 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Broekman M. L., Comer L. A., Hyman B. T., Sena-Esteves M., Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or −2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience 138, 501–510 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Lo W. D., Qu G., Sferra T. J., Clark R., Chen R., Johnson P. R., Adeno-associated virus-mediated gene transfer to the brain: Duration and modulation of expression. Hum. Gene Ther. 10, 201–213 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Hommel J. D., Sears R. M., Georgescu D., Simmons D. L., DiLeone R. J., Local gene knockdown in the brain using viral-mediated RNA interference. Nat. Med. 9, 1539–1544 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Abbott L. C., Bump M., Brandl A., De Laune S., Investigation of the role of the cerebellum in the myoclonic-like movement disorder exhibited by tottering mice. Mov. Disord. 15 ( suppl. 1), 53–59 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Son Y. H., Song J. S., Kim S. H., Kim J., Pharmacokinetic characterization of CK2 inhibitor CX-4945. Arch. Pharm. Res. 36, 840–845 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Noebels J. L., A single gene error of noradrenergic axon growth synchronizes central neurones. Nature 310, 409–411 (1984). [DOI] [PubMed] [Google Scholar]
- 40.Noebels J. L., Sidman R. L., Inherited epilepsy: Spike-wave and focal motor seizures in the mutant mouse tottering. Science 204, 1334–1336 (1979). [DOI] [PubMed] [Google Scholar]
- 41.Fletcher C. F., Lutz C. M., O’Sullivan T. N., Shaughnessy J. D. Jr., Hawkes R., Frankel W. N., Copeland N. G., Jenkins N. A., Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell 87, 607–617 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Castello J., Ragnauth A., Friedman E., Rebholz H., CK2-an emerging target for neurological and psychiatric disorders. Pharmaceuticals (Basel) 10, 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winkler G. F., Young R. R., Efficacy of chronic propranolol therapy in action tremors of the familial, senile or essential varieties. N. Engl. J. Med. 290, 984–988 (1974). [DOI] [PubMed] [Google Scholar]
- 44.Thenganatt M. A., Jankovic J., Psychogenic tremor: A video guide to its distinguishing features. Tremor Other Hyperkinet. Mov. 4, 253 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abila B., Wilson J. F., Marshall R. W., Richens A., The tremorolytic action of beta-adrenoceptor blockers in essential, physiological and isoprenaline-induced tremor is mediated by beta-adrenoceptors located in a deep peripheral compartment. Br. J. Clin. Pharmacol. 20, 369–376 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hedera P., Cibulcik F., Davis T. L., Pharmacotherapy of essential tremor. J. Cent. Nerv. Syst. Dis. 5, 43–55 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgan M. H., Hewer R. L., Cooper R., Effect of the beta adrenergic blocking agent propranolol on essential tremor. J. Neurol. Neurosurg. Psychiatry 36, 618–624 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khavandgar S., Walter J. T., Sageser K., Khodakhah K., Kv1 channels selectively prevent dendritic hyperexcitability in rat Purkinje cells. J. Physiol. 569, 545–557 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jen J. C., Graves T. D., Hess E. J., Hanna M. G., Griggs R. C., Baloh R. W.; CINCH investigators , Primary episodic ataxias: Diagnosis, pathogenesis and treatment. Brain 130, 2484–2493 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Herson P. S., Virk M., Rustay N. R., Bond C. T., Crabbe J. C., Adelman J. P., Maylie J., A mouse model of episodic ataxia type-1. Nat. Neurosci. 6, 378–383 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Fremont R., Tewari A., Angueyra C., Khodakhah K., A role for cerebellum in the hereditary dystonia DYT1. eLife 6, e22775 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uluğ A. M., Vo A., Argyelan M., Tanabe L., Schiffer W. K., Dewey S., Dauer W. T., Eidelberg D., Cerebellothalamocortical pathway abnormalities in torsinA DYT1 knock-in mice. Proc. Natl. Acad. Sci. U.S.A. 108, 6638–6643 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weissbach A., Werner E., Bally J. F., Tunc S., Löns S., Timmann D., Zeuner K. E., Tadic V., Brüggemann N., Lang A., Klein C., Münchau A., Bäumer T., Alcohol improves cerebellar learning deficit in myoclonus-dystonia: A clinical and electrophysiological investigation. Ann. Neurol. 82, 543–553 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Washburn S., Fremont R., Moreno-Escobar M. C., Angueyra C., Khodakhah K., Acute cerebellar knockdown of Sgce reproduces salient features of myoclonus-dystonia (DYT11) in mice. eLife 8, e52101 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fremont R., Calderon D. P., Maleki S., Khodakhah K., Abnormal high-frequency burst firing of cerebellar neurons in rapid-onset dystonia-parkinsonism. J. Neurosci. 34, 11723–11732 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shiloh Y., The cerebellar degeneration in ataxia-telangiectasia: A case for genome instability. DNA Repair (Amst) 95, 102950 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Tan G. H., Liu Y. Y., Wang L., Li K., Zhang Z. Q., Li H. F., Yang Z. F., Li Y., Li D., Wu M. Y., Yu C. L., Long J. J., Chen R. C., Li L. X., Yin L. P., Liu J. W., Cheng X. W., Shen Q., Shu Y. S., Sakimura K., Liao L. J., Wu Z. Y., Xiong Z. Q., PRRT2 deficiency induces paroxysmal kinesigenic dyskinesia by regulating synaptic transmission in cerebellum. Cell Res. 28, 90–110 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhatia K. P., Paroxysmal dyskinesias. Mov. Disord. 26, 1157–1165 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Kyllerman M., Forsgren L., Sanner G., Holmgren G., Wahlström J., Drugge U., Alcohol-responsive myoclonic dystonia in a large family: Dominant inheritance and phenotypic variation. Mov. Disord. 5, 270–279 (1990). [DOI] [PubMed] [Google Scholar]
- 60.Barrett C. F., van den Maagdenberg A., Frants R. R., Ferrari M. D., Familial hemiplegic migraine. Adv. Genet. 63, 57–83 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Ryan D. P., Ptacek L. J., Episodic neurological channelopathies. Neuron 68, 282–292 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Gao Z., Todorov B., Barrett C. F., van Dorp S., Ferrari M. D., van den Maagdenberg A. M. J. M., De Zeeuw C. I., Hoebeek F. E., Cerebellar ataxia by enhanced Ca(V)2.1 currents is alleviated by Ca2+−dependent K+-channel activators in Cacna1a(S218L) mutant mice. J. Neurosci. 32, 15533–15546 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toldo I., Perissinotto E., Menegazzo F., Boniver C., Sartori S., Salviati L., Clementi M., Montagna P., Battistella P. A., Comorbidity between headache and epilepsy in a pediatric headache center. J. Headache Pain 11, 235–240 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kros L., Eelkman Rooda O. H. J., Spanke J. K., Alva P., Dongen M. N., Karapatis A., Tolner E. A., Strydis C., Davey N., Winkelman B. H. J., Negrello M., Serdijn W. A., Steuber V., van den Maagdenberg A. M. J. M., de Zeeuw C. I., Hoebeek F. E., Cerebellar output controls generalized spike-and-wave discharge occurrence. Ann. Neurol. 77, 1027–1049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krook-Magnuson E., Szabo G. G., Armstrong C., Oijala M., Soltesz I., Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. eNeuro 1, ENEURO.0005–ENEU14.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galtrey C. M., Mula M., Cock H. R., Stress and epilepsy: Fact or fiction, and what can we do about it? Pract. Neurol. 16, 270–278 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Carey M. R., Regehr W. G., Noradrenergic control of associative synaptic plasticity by selective modulation of instructive signals. Neuron 62, 112–122 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inoshita T., Hirano T., Norepinephrine facilitates induction of long-term depression through β-adrenergic receptor at parallel fiber-to-purkinje cell synapses in the flocculus. Neuroscience 462, 141–150 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Binder E. B., Nemeroff C. B., The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol. Psychiatry 15, 574–588 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zemkova H., Tomic M., Kucka M., Aguilera G., Stojilkovic S. S., Spontaneous and CRH-induced excitability and calcium signaling in mice corticotrophs involves sodium, calcium, and cation-conducting channels. Endocrinology 157, 1576–1589 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paretkar T., Dimitrov E., The central amygdala corticotropin-releasing hormone (CRH) neurons modulation of anxiety-like behavior and hippocampus-dependent memory in mice. Neuroscience 390, 187–197 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taylor J. A., Twomey T. M., von Wittenau M. S., The metabolic fate of prazosin. Xenobiotica 7, 357–364 (1977). [DOI] [PubMed] [Google Scholar]
- 73.Conway E. L., Jarrott B., Tissue pharmacokinetics of clonidine in rats. J. Pharmacokinet. Biopharm. 10, 187–200 (1982). [DOI] [PubMed] [Google Scholar]
- 74.Archer T., Fredriksson A., Effects of clonidine and alpha-adrenoceptor antagonists on motor activity in DSP4-treated mice I: Dose-, time- and parameter-dependency. Neurotox. Res. 1, 235–247 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Concas A., Santoro G., Mascia M. P., Maciocco E., Dazzi L., Ongini E., Biggio G., Anticonvulsant doses of 2-chloro-N6-cyclopentyladenosine, an adenosine A1 receptor agonist, reduce GABAergic transmission in different areas of the mouse brain. J. Pharmacol. Exp. Ther. 267, 844–851 (1993). [PubMed] [Google Scholar]
- 76.Calderon D. P., Fremont R., Kraenzlin F., Khodakhah K., The neural substrates of rapid-onset dystonia-Parkinsonism. Nat. Neurosci. 14, 357–365 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gray S. J., Woodard K. T., Samulski R. J., Viral vectors and delivery strategies for CNS gene therapy. Ther. Deliv. 1, 517–534 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weisz C. J., Raike R. S., Soria-Jasso L. E., Hess E. J., Potassium channel blockers inhibit the triggers of attacks in the calcium channel mouse mutant tottering. J.Neurosci. 25, 4141–4145 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoebeek F. E., Khosrovani S., Witter L., De Zeeuw C. I., Purkinje cell input to cerebellar nuclei in tottering: Ultrastructure and physiology. Cerebellum 7, 547–558 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S11
Table S1