Abstract
BACKGROUND:
Stress produces differential behavioral responses through select molecular modifications to specific neurocircuitry elements. The orexin system targets key components of this neurocircuitry in the basolateral amygdala (BLA).
METHODS:
We assessed the contribution of intra-BLA Orexin 1 receptors (Orx1R) in the expression of stress-induced phenotypes of mice. Using the Stress Alternatives Model (SAM), a social stress paradigm that produces two behavioral phenotypes, we characterized the role of intra-BLA Orx1R using acute pharmacological inhibition (SB-674042) and genetic knockdown (AAV-U6-Orx1R-shRNA) strategies.
RESULTS:
In the BLA, we observed that Orx1R (HCRTR1) mRNA is predominantly expressed in CamKIIα+ glutamatergic neurons and rarely in GABAergic cells. While there is a slight overlap in HCRTR1 and Orexin 2 receptor (Orx2R; HCRTR2) mRNA expression in the BLA, we find that these receptors are most often expressed in separate cells. Antagonism of intra-BLA Orx1R after phenotype formation shifted behavioral expression from stress sensitive (Stay) to resilient (Escape) responses, an effect that was mimicked by genetic knockdown. Acute inhibition of Orx1R in the BLA also reduced contextual and cued fear freezing responses in Stay animals. This phenotype-specific behavioral change was accompanied by biased molecular transcription favoring HCRTR2 over HCRTR1, and MAPK3 over PLCB1 cell signaling cascades and enhanced BDNF mRNA.
CONCLUSIONS:
Functional reorganization of intra-BLA gene expression is produced by antagonism of Orx1R, which promotes elevated HCRTR2, greater MAPK3, and increased BDNF expression. Together, these results provide evidence for a receptor-driven mechanism that balances pro- and anti-stress responses within the BLA.
Keywords: Social stress, Stress Alternatives Model, Hypocretin, Anxious Behavior, resilience, fear conditioning
INTRODUCTION
Stress-induced alterations in neurocircuitry result in divergent behavioral responses. Enhanced stress reactivity (pro-stress) in rodent models is similar to human affective dysfunction in mood disorders like depression, fear-/anxiety-related disorders, or post-traumatic stress disorder (PTSD) (1). Current pharmacotherapies for affective disorders have limited success, and a mechanistic understanding remains elusive.
Balance within key stress circuits may be disrupted during periods of intense or prolonged stress to shift signaling dynamics in pro- or anti-stress pathways (2-4). Stressful stimuli are interpreted, in part, through converging signals in the basolateral amygdala (BLA), where glutamatergic projection neurons are influenced by distinctive GABAergic interneurons, to direct behavioral responses (5). Additionally, activity in the BLA is modified by hypothalamic orexinergic neurons, which are critical for panic (6, 7) and motivation (8, 9).
Orexin A (OrxA) and orexin B (OrxB), neuromodulators derived from a single pre-propeptide, activate two G-coupled protein receptors: orexin 1 receptors (Orx1R) bind OrxA and OrxB (EC50=30nM vs 2,500nM), as do orexin 2 receptors (Orx2R; EC50=38nM vs 36nM)(10, 11). These receptors stimulate Gq proteins which increase intracellular Ca2+ (11) to activate phospholipase C (PLC) pathways (12). The PLCβ1 isozyme variant is transcribed in the amygdala (13), and its dysfunction is linked to psychopathologies like depression (14), bipolar disorder (15), addiction (16), and schizophrenia (17, 18).
Stimulation of Orx1R can also activate extracellular signal-regulated protein kinase (ERK). In the amygdala, recruitment of ERKs is important for consolidation, reconsolidation, and extinction of fear memories (19, 20). While Orx1R in the BLA are important in regulating fear (21, 22), depression (23, 24), and anxiety (25), it is unclear how shifts in molecular signaling cascades mediate such responses and initiate stress-induced phenotype development.
Utilizing the Stress Alternatives Model (SAM), a behavioral paradigm that separates individuals into social stress resilient (Escape; as validated by Social Interaction/Preference [SIP] test) and vulnerable (Stay) populations (26), we explored how Orx1R activity in the BLA is involved in the formation of stress-related phenotypes. As a social interaction and avoidance paradigm in which smaller subjects encounter intense attacks from larger novel aggressors over a four-day period, the SAM produces two separate subsets of animals, exhibiting social avoidance or enhanced fear conditioned responses (27, 28). Unlike a traditional social defeat outcome, the SAM provides mice an opportunity to avoid social aggression by exiting the arena through escape tunnels only large enough for the smaller mouse. By the end of the second day of social interaction, test subjects commit to a phenotype: Escape or Stay. These stable phenotypes may be altered through pharmacological manipulations (Escape reduced by anxiogenic drugs, Stay reduced by anxiolytic drugs) administered on the third day of the SAM (28-30). Thus, the SAM is a useful tool for studying the development of stress-induced phenotypes, while providing an opportunity to explore physiological and clinically relevant molecular mechanisms.
We investigated if inhibition of intra-BLA Orx1R, alters the formation of social stress-induced behavioral phenotypes. We predict that pharmacological inhibition or genetic knockdown will shift behavioral patterns in vulnerable (Stay) populations toward resilience (Escape). Further, we explored if Orx1R inhibition affects conditioned fear responses and alters expression of genes responsible for balancing signaling in pro- and anti-stress neurocircuitries. Together, these results allow us to propose a neurocircuit model that defines the role of intra-BLA Orx1R signaling in the balance of pro- and anti-stress states.
METHODS AND MATERIALS
Social Stress and Choice Paradigm
Aggressive social interactions between larger novel CD1 and smaller male C57BL/6NHsd mice dyads in the SAM apparatus (Fig. 1) involves four trials, lasting up to five minutes each, allows test animals the opportunity to shorten stressful encounters by making use of size-restricted tunnels at the apical end of the oval open field interaction arena. A tone given during isolation in the SAM apparatus prior to social interaction permits comparisons between cued and contextual fear conditioning. The escape routes provide a choice, producing two stable phenotypes: active avoidance (Escape) and accepting confrontation (Stay), which may be modified by drug treatment on Day 3. The treatment regimen allows for statistical comparisons between groups, and within subjects, by comparing responses to SAM interactions before and after treatment. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) and approved by the University of South Dakota Institutional Animal Care and Use Committee.
Figure 1.

The Stress Alternatives Model (SAM) is used to assess the development of stress-induced phenotypes. A) The SAM is a 4-day behavioral paradigm in which (I) a test mouse is placed into an opaque cylinder, (II) presented a tone, (III) exposed to social aggression, and commits to a phenotype: (IV) Escape or (V) Stay. B) The behavioral timelines for (I) pharmacology and (II) genetic knockdown experiments (mice are the same age at testing) include surgeries targeting the BLA, SAM exposure (Days 1-4), and the testing of contextual and cued fear responses (Day 5).
Experimental Overview (see also Supplemental Information)
The primary treatments for these experiments is inhibition of BLA Orx1R, via the antagonist SB-674042 (0.3 nmol/0.3 μL delivered bilaterally intra-BLA, 1h prior to interaction on Day 3), contrasted with Orx1R stimulation (accomplished by OrxA + Orx2R antagonism), or short-hairpin knockdown (bilateral intra-BLA transfection beginning 30 days prior to SAM interaction). Considering the difference in timing of delivery, these treatments were done and analyzed separately, with a priori hypotheses. All behavioral measures were performed during the dark cycle when the animals are active, and included Escape (use of the apical tunnels), Stay (remaining in the SAM arena with the novel aggressor), time spent attentive to the escape hole, latency to escape (for Escape mice), fear conditioned freezing (measured in response to the tone [CS] and context, prior to the social interaction unconditioned stimulus [US], and as a conditioned response [CR on Day 5] in the absence of the US), and food intake. Thus, treatment groups included home cage controls, and intra-BLA SB-674042 (or vehicle, OrxA, OrxA + MK-1064, MK-1064) injection of Escape and Stay mice. In addition, transgenic treatment groups included home cage controls, intra-BLA AAV-Orx1R-shRNA injection, and intra-BLA AAV-scramble-shRNA injection. Brains and blood were collected for visual representations of gene expression (using RNAscope) of HCRTR1, HCRTR2, calbindin (CALB1), Ca++/Calmodulin Kinase type 2 alpha (CAMKIIα), Glutamate Decarboxylase (GAD1), and parvalbumin (PVALB) in BLA, as well as to measure plasma concentrations of the stress hormone corticosterone (by enzyme linked immunosorbent assay). Gene expression (using RT-qPCR) of HCRTR1, HCRTR2, PLCB1, MAPK1, MAPK3, BDNF, and GAPDH (housekeeping gene) were measured in BLA tissue. All experimental designs and statistical analyses were based on a priori hypotheses, using two-way repeated measures ANOVA, two-way ANOVA, one-way ANOVA, Regression analyses, and t-test, followed (where appropriate) by post hoc analyses.
Results
OrxR Expression in BLA
The glutamatergic marker, CamKIIα identified the vast majority of BLA neurons (~80%; Fig. S2) as well as those expressing HCRTR1 (31, 32) (also in some calbindin-GABAergic neurons; Fig. 2). Few (<20%) BLA HCRTR1-possessing cells express GAD1 (GABAergic marker) and co-express parvalbumin (PV, ~10%; Figs. 2G-K). Our results suggest HCRTR1 is expressed in 10-15% of BLA glutamatergic neurons and ~5% of GABA cells (Fig. 2K). In BLA cells, mRNA for HCRTR1 and HCRTR2 largely do not overlap, ~80% of HCRTR1+ cells do not co-express HCRTR2 (Figs. 2L-O). Specific BLA GABAergic neurons may predominantly localize Orx2R (Fig. 2P) (28).
Figure 2.
In the untreated BLA, Orx1R are expressed predominantly in glutamatergic neurons and are rarely co-expressed with Orx2R. A) Imaged sections containing BLA cells (LA = lateral amygdala) stained with probes targeting mRNA of B) HCRTR1 (red), C) CamKIIα (green), and D) Calb (Magenta) revealed when E) merged (with DAPI) that F) Orx1R+ cells mostly co-express the glutamatergic cell marker, CamKIIα (N = 4, F2,9 = 54.4, p < 0.001; CamKIIα+ vs Calb+: t6 = 10.4, p < 0.001; CamKIIα+ vs Other: t6 = 5.2, p < 0.001; Calb+ vs Other: t6 = 5.2, p < 0.001; bars are statistically different from one another as illustrated with unique letters, e.g. A is significantly different from B and C; p < 0.001). G) Expression of HCRTR1 (red) GAD67 (GAD1) mRNA (yellow) infrequently overlap with H) most HCRTR1+ cells being absent of the GABAergic marker (N = 5, t8 = 29.5, *p < 0.001). I) While a subset of BLA GABAergic neurons produce the calcium-binding protein parvalbumin (Pvalb+), J) HCRTRV (red) cells are mostly absent of Pvalb expression (light blue) with K) less than 10% being both HCRTR1+ and Pvalb+ (N = 4, t6 = 23.1, *p < 0.001). K) Further, more BLA glutamatergic (CamKIIα+) neurons (compared to GABAergic → GAD1+) also express HCRTR1 (N = 9, t7 = 3.2, *p < 0.015). L) Images of BLA cells with fluorescent markers labeling M) HCRTR1 mRNA (red) and HCRTR2 mRNA (green) demonstrate N) most BLA cells express neither HCRTR1 nor HCRTR2 (N = 4, F2,9 = 42.1, p < 0.001; HCRTR1+ vs Other, t6 = 7.5, p < 0.001; HCRTR2+ vs Other, t6 = 8.4, p < 0.001; bars are statistically different from one another as illustrated with unique letters, e.g. A is significantly different from B). O) Most HCRTR1+ cells in the BLA do not express HCRTR2 (N = 4, t6 = 10.1, *p < 0.001), as depicted in P) showing Orx1R on glutamatergic neurons.
Motivation for Active Avoidance (Escape)
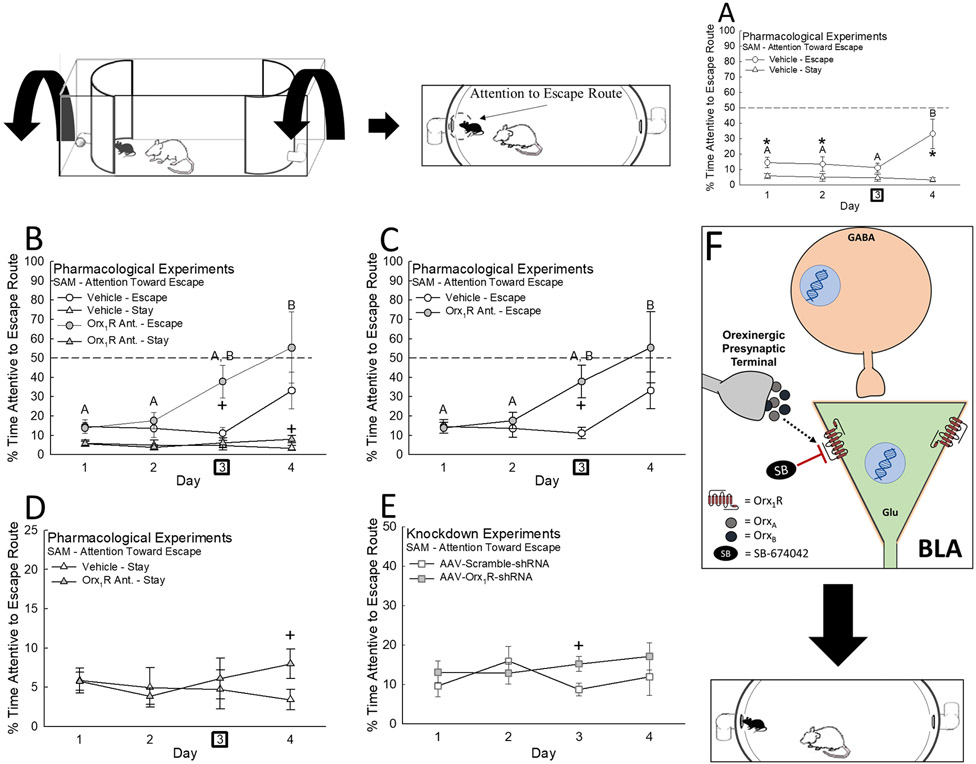
In the SAM, animals evenly choose one of two stable (27-29, 33) behavioral phenotypes, Escape (44.7%) or Stay (55.3%; Figs. 1A, S1B, C)(26, 27, 29, 33, 34). Time spent investigating escape routes predict active avoidance and indicates motivation to escape (28). Time spent attentive to the hole was significantly greater in vehicle-treated Escape mice (Fig. 3A), but intra-BLA infusion of the Orx1R antagonist (Escape: Fig. 3B, C; Stay: Fig. 3B, D) or AAV-U6-Orx1R-shRNA (Fig. 3E) increases attention to the escape route. Further, receptor activation with OrxA reduced time Escape mice spent investigating the escape route (Fig. S3).
Figure 3.
Motivation toward Escape behavior is impacted through inhibition of intra-BLA Orx1R. A) Escape mice, as compared to those expressing the Stay phenotype, spend a greater % of time investigating the SAM escape routes (N = 19, Phenotype Effect: F1,51 = 16.4, p < 0.001; Escape vs Stay: Day 1, t17 = 2.6, *p ≤ 0.018; Day 2, t17 = 2.5, *p ≤ 0.017; Day 4, ti7 = 4.2, *p < 0.001). B) While Escape mice, in general, explore the escape routes more often, C) inhibition of intra-BLA Orx1R promotes even more attention toward the escape tunnels (N = 34, Treatment Effect: F1,30 = 7.7, p ≤ 0.019; Day 3 Vehicle Escape vs Orx1R Ant. Escape, t10 = 2.5, +p ≤ 0.018). D) Antagonism of intra-BLA Orx1R only slightly stimulates escape route exploration in Stay mice (Day 4 Vehicle x Orx1R Ant., t20 = 2.1, +p ≤ 0.05). E) Knockdown of intra-BLA Orx1R temporarily and minimally increases attention toward escape on Day 3 of the SAM (N = 22, Day 3 Scramble vs AAV-Orx1R-shRNA, t20 = 2.4, +p ≤ 0.024). F) Illustration demonstrating inhibition of intra-BLA Orx1R on predominantly on glutamatergic neurons promotes attention toward the escape route in the SAM arena. In Pharmacological Experiments, drug treatment is administered on Day 3 as designated by the bold square. Note, the data plotted in A, C, and D are the same as those graphed in B; we have separated out these individual comparisons for the sake of clarity.
Avoidance (Escape)
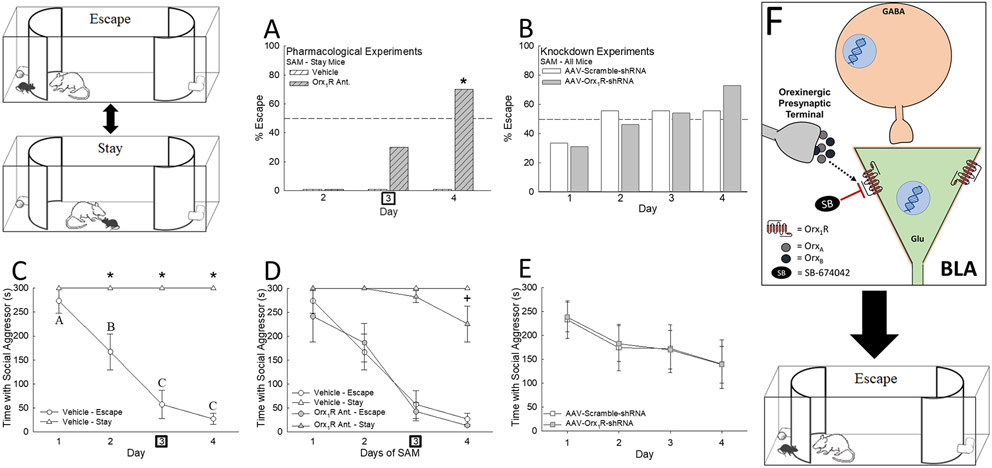
Upon intra-BLA injections of an Orx1R antagonist on SAM Day 3, a substantial number of Stay mice exhibited Escape behavior (Fig. 4A), with a 30% shift that day, and a significant increase the day after (Day 4 = 70% increase). Interestingly, intra-BLA activation of both Orx receptors with OrxA or biased activation of Orx1R (OrxA + Orx2R antagonist) blocked Escape behavior in a small, but not statistically significant, proportion of mice on Days 3 and 4 (Fig. S4), exhibiting deviation from stable phenotype behavioral patterns.
Figure 4.
Intra-BLA Orx1R mediates stress-related behavioral phenotype development. A) Infusion of an Orx1R antagonist (SB-674042) into the BLA promotes Escape behavior in Stay mice (N = 22, Day 4, χ2: F1 = 9.3, *p < 0.001). B) Knockdown of Orx1R (AAV-Orx1R-shRNA) upsets normal Day 2 phenotype commitment behavior (as observed with AAV-Scramble-shRNA controls), inducing more Escape behavior on Days 3 and 4 (N = 22). C) Escape animals learn to efficiently utilize the escape route to avoid social aggression (Escape latency = Time with Social Aggressor) over the course of 4 days while Stay mice remain with the aggressor (N = 19, Phenotype Effect: F1,45 = 175.3, p < 0.001; Time Effect: F3,45 = 26.1, p < 0.001; Interaction Effect: F3,45 = 26.1, p < 0.001; Escape vs Stay: Day 2, t17 = 5.8, *p < 0.001; Day 3, t17 = 10.6, *p < 0.001; Day 4, t17 = 11.9, *p < 0.001; Within Escape phenotype comparison, F3,18 = 17.8, p < 0.001, Day 1 vs Day 3, t6 = 5.7, p < 0.001; Day 1 vs Day 4, t6 = 6.5, p < 0.001; Day 2 vs Day 3, t6 = 2.9, p ≤ 0.009; Day 2 vs Day 4, t6 = 3.7, p ≤ 0.002; p < 0.05 for Days marked with unique lettering, e.g. A is different from B and C). D) Antagonizing intra-BLA Orx1R promotes aggressor avoidance in Stay mice (N = 34, Time Effect: F3,54 = 2.9, p ≤ 0.043; Interaction Effect: F3,54 = 2.9, p < 0.043; Day 4 Vehicle Stay vs Orx1R Ant. Stay, t20 = 3.4, +p < 0.001), but has no effect on those animals exhibiting the Escape phenotype. E) Knockdown of intra-BLA Orx1R does not impact the overall latency of aggressor avoidance (N = 22). Overall, F) inhibition of Orx1R in the BLA appears to prompt Escape behavior. In Pharmacological Experiments, drug treatment is administered on Day 3 as designated by the bold square.
As knockdown reduced Orx1R expression prior to stressful interactions, we did not expect a dramatic change in behavior over the course of SAM trials, but AAV-U6-Orx1R-shRNA yielded incrementally (though not significantly) more escape on the last two days (Fig. 4B). By Day 4, 72.7% of AAV-U6-Orx1R-shRNA-treated mice displayed Escape compared to 54.5% of the scramble control.
Escape mice spent significantly less time in the SAM arena with the CD1 mouse on Days 2 through 4 (26, 27, 29, 33, 35), thus escape latency was reduced (Fig. 4C). Stay mice remain for the entire 5 min period, unless treated with Orx1R antagonist, significantly reducing time spent with aggressive CD1 mice on Day 4 (Figs. 4D, F). Inhibition of Orx1R did not influence escape latency in Escape animals (Figs. 4D). Importantly neither of the Orx1R manipulations, antagonist or knockdown treatments, influenced arousal/locomotion (Figs. S5), but did result in small but significant decreases in food intake and body weight (Figs. S6).
Cued and Contextual Fear Conditioning
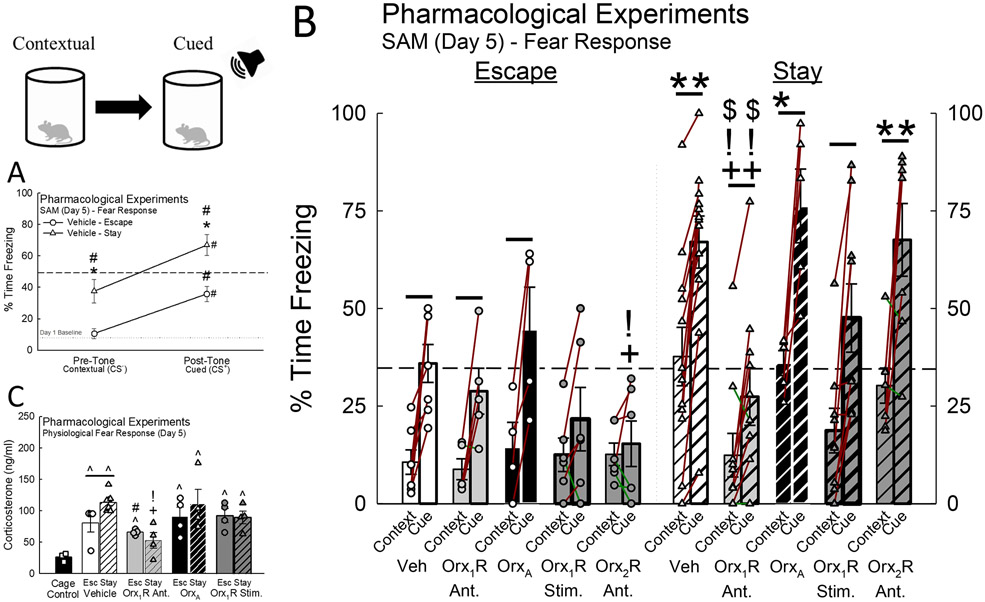
Cued fear responses significantly enhanced freezing in both Escape and Stay phenotypes, and Stay mice displayed heightened freezing behavior to context (opaque cylinder divider) as well (Figs. 5A, B). Although inhibition of intra-BLA Orx1R did not affect the fear freezing profile in Escape mice, antagonist-treated Stay mice exhibited significantly reduced contextual and cued fear responses (Figs. 5B, S7; Table S1). Like mice of the Escape phenotype, knockdown of BLA Orx1R did not affect conditioned freezing behavior (Fig. S8). Importantly, activation of intra-BLA Orx receptors with OrxA did not change the fear freezing profile in Escape or Stay mice compared to vehicle (Figs. 5B, S7; Table S1). However, biased stimulation of Orx1R in the BLA with a combination of OrxA + Orx2R antagonist eliminated the conditioned response in Escape, but not Stay mice (Figs. 5B, S7; Table S1). Further, acute inhibition of Orx2R in the BLA eliminated the cued freezing response in Escape mice and significantly reduced freezing during the post-tone period (Figs. 5B, S7; Table S1). Stay mice treated with an Orx2R antagonist displayed no statistical differences in the levels of contextual and cued freezing (Figs. 5B, S7; Table S1).
Figure 5.
Inhibition of Orx1R in the BLA reduces contextual/cued fear responses and stress hormone concentrations. A) Although both Escape and Stay phenotypes learn to associate a cue (tone, CS+) with social aggression (Phenotype Effect: F1,17 = 7.6, p < 0.013; CS Effect: F1,17 = 47.7, p < 0.001; Escape CS− vs CS+, t6 = 3.9, #p < 0.008; Stay CS− vs CS+, t11 = 5.7, #p < 0.001), Stay mice exhibit heightened freezing behavior to both context (CS−; t17 = 2.8, *p < 0.011) and tone (CS+; t17 = 2.3, *p ≤ 0.033). Baseline measurements of freezing are represented by a dotted line. B) Antagonism of intra-BLA Orx1R reduces conditioned fear responses in Stay animals while Orx2R inhibition diminishes fear freezing in Escape mice (N = 71; * represents significant differences compared to Escape mice in the same treatment group; + signifies significance compared to Vehicle-treated animals in the same phenotype group; ! identifies significant differences compared to OrxA-treated mice; $ denotes significant differences compared to Orx2R antagonist-treated animals). See Supplemental Information Figure S7 for specific a priori hypotheses comparisons. C) Mice exposed to social stress produce elevated levels of stress hormone (N = 39, F2,12 = 24.3, p < 0.001; Cage Control vs Vehicle Escape, t5 = 3.1, ^p ≤ 0.028; Cage Control vs Vehicle Stay, t9 = 9.9, ^p < 0.001); however, Stay animals have the highest concentration (Vehicle Escape vs Stay, t10 = 2.6, p ≤ 0.025). Inhibition of intra-BLA Orx1R reduces corticosterone levels in Stay mice (Vehicle Stay vs Orx1R Ant. Stay, t10 = 5.1, +p < 0.001; Orx1R Ant. Stay vs OrxA Stay, t6 = 3.3, !p ≤ 0.002).
Corticosterone Concentrations
Social stress in SAM interactions increases corticosterone concentrations in both Escape and Stay animals (27, 28, 33), although Stay mice have higher levels of corticosterone compared to Escape. Inhibition of BLA Orx1R decreased Stay corticosterone concentrations compared to vehicle-treated Stay animals; and did not differ significantly from non-stressed mice (Fig. 5C). Treatments with OrxA or the combination of OrxA and an Orx2R antagonist did not change corticosterone levels relative to vehicle-treated controls, however, the differences between Escape and Stay were eliminated and levels were elevated compared to Orx1R antagonist-treated mice (Fig. 5C). Inhibition of BLA Orx1R not only reduces social fear responses, but also reverses social stress responsiveness.
Antagonism of intra-BLA OrxR Recruits Alternative Signaling
Although HCRTR1 expression was unaltered following vehicle treatment, Orx1R antagonism reduced intra-BLA HCRTR1 in Escape mice compared to non-stressed cage controls (Fig. 6A), and simultaneously elevated HCRTR2 expression in Stay mice compared to Escape and vehicle-treated Stay mice (Fig. 6B; Table S2). In vehicle controls HCRTR2 expression was higher in Escape mice compared to both Stay and Orx1R antagonist-treated Escape mice (Fig. 6B; Table S2). A reduction in HCRTR1 gene expression after Orx2R antagonism was observed, but only in Stay animals relative to vehicle (Fig. 6A; Table S2). Expression of HCRTR2 in the BLA was reduced in both Escape and Stay phenotypes after blocking Orx2R, contrasting with Orx1R antagonism, which enhanced HCRTR2 mRNA levels in Stay mice (Fig. 6B; Table S2).
Figure 6.
Transcriptional changes (relative to home-cage naïve controls) in the BLA after Orx1R or Orx2R antagonism shifts signaling profile. A) Antagonism of Orx1R in the BLA reduces HCRTR1 expression (N = 45, Treatment Effect: F2,27 = 3.5, p ≤ 0.043), but only significantly so in animals expressing the Escape phenotype (Cage Control vs Orx1R Ant. Escape, t11 = 2.2, ^p ≤ 0.050); whereas infusion of an Orx2R antagonist in the BLA reduces HCRTR1 expression in Stay mice compared to vehicle animals of the same phenotype (t10 = 2.2, +p < 0.044). B) While Escape mice (Treatment Effect: F2,27 = 9.8, p < 0.001; Interaction Effect: F2,27 = 8.6, p < 0.001) treated with vehicle express higher HCRTR2 levels compared to Stay mice (t9 = 3.0; *p < 0.016) and Orx1R- or Orx2R-antagonist-treated Escape animals (Vehicle vs Orx1R Ant., t7 = 2.6, +p < 0.035; Vehicle vs Orx2R Ant.: t7 = 4.5, +p < 0.001; Orx1R Ant. vs Orx2R Ant.: t8 = 3.5, !p < 0.001), Orx1R antagonism results in elevated levels (Escape vs Stay, t10 = 2.2, *p ≤ 0.05; Vehicle vs Orx1R Ant., t12 = 2.4, +p < 0.034) while Orx2R inhibition leads to a reduction (Vehicle vs Orx2R Ant.: t10 = 3.5, +p < 0.002; Orx1R Ant. vs Orx2R Ant.: t10 = 4.7, !p < 0.001) of HCRTR2 in Stay mice. C) A reduction of PLCB1 (Phenotype Effect: F1,27 = 19.1, p < 0.001; Interaction Effect: F2,27 = 4.3, p ≤ 0.023) that is found in Escape mice under control conditions (Cage control vs Vehicle Escape, t10 = 5.1, ^p < 0.001; Escape vs Stay, t9 = 5.0, *p < 0.001) and Orx1R antagonism (Escape vs Stay, t10 = 3.1, *p < 0.012; Cage Control vs Orx1R Ant., t11 = 3.3, ^p ≤ 0.007) is eliminated with intra-BLA Orx2R antagonism (Vehicle vs Orx2R Ant.: t7 = 2.8, +p < 0.017). D) While Stay mice treated with an Orx1R antagonist express higher levels of MAPK3 (Phenotype Effect: F1,27 = 11.3, p ≤ 0.002; Treatment Effect: F2,27 = 4.3, p ≤ 0.023; Interaction Effect: F2,27 = 5.1, p ≤ 0.013) in the BLA compared to Vehicle controls (t12 = 3.1, +p < 0.001), administration of an Orx2R antagonist does not induce the same transcriptional response (Orx1R Ant. vs Orx2R Ant.: t10 = 2.7, !p ≤ 0.022). E) Expression of BDNF in the BLA after treatment (Interaction Effect: F2,27 = 10.6, p < 0.001) with an Orx2R antagonist is enhanced in Escape mice (Orx2R Ant. Escape vs Stay: t8 = 2.9, *p ≤ 0.019; Vehicle vs Orx2R Ant.: t7 = 2.7, +p ≤ 0.013; Orx1R Ant. vs Orx2R Ant.: t8 = 2.5, !p ≤ 0.017) and reduced in Stay animals (Vehicle vs Orx2R Ant.: t10 = 2.2, +p < 0.05; Orx1R Ant. vs Orx2R Ant.: t10 = 3.9, !p < 0.001); a phenotypically opposite effect is observed after Orx1R antagonism (Escape vs Stay, t10 = 2.8, *p ≤ 0.018; Orx1R Ant. Stay vs Vehicle Stay, t12 = 2.2, +p ≤ 0.049). Transcriptional changes after F) intra-BLA Orx1R antagonism and G) Orx2R inhibition are differentially regulated in a phenotype-dependent fashion.
Transcription of BLA PLCβ1 (PLCB1) mRNA (13) is important for Orx1R signaling (36). We predicted Orx1R antagonist might limit PLCB1 expression levels (Fig. 6C). Escape mice in both vehicle and Orx1R antagonist groups expressed lower amounts of PLCB1 compared to Stay and cage control animals (Fig. 6C). Further, greater PLCB1 followed intra-BLA Orx2R inhibition compared to vehicle-treated Escape mice (Fig. 6C; Table S2).
Alternative molecular pathways recruited during Gq activation are driven by ERK genes (MAPK1 & MAPK3). In Stay mice Orx1R antagonism resulted in a significant increase in MAPK3 expression (MAPK1 mRNA was unaffected, Fig. S9) compared to similarly treated Escape, vehicle-treated Stay, and non-stressed cage control mice (Fig. 6D; Table S2). Inhibition of intra-BLA Orx2R did not alter MAPK3 gene expression (Fig. 6D; Table S2).
The transcription of brain-derived neurotrophic factor (BDNF) is tied to neuroplasticity (37, 38) and behavioral changes like extinction of fear memories (39), so we predicted an increase in BDNF might be associated with intra-BLA Orx1R inhibition (Fig. 6E). As hypothesized, intra-BLA Orx1R antagonism resulted in elevated BDNF in Stay compared to Escape mice and vehicle-treated Stay mice (Fig. 6E; Table S2). Finally, Orx2R antagonist treatment enhanced BDNF expression in Escape mice, while diminishing transcription in Stay animals, an effect that is phenotypically opposite to that observed after Orx1R inhibition (Fig. 6E; Table S2). As Stay mice treated with an Orx1R antagonist experienced shifts from stress-vulnerable to resilient behavioral responses, the alterations in gene expression reported here (Figs. 6F, G) may be implicit in this behavioral plasticity.
Molecular Restructuring is Related to Fear Responsiveness
Expression levels of HCRTR2, but not HCRTR1, in both vehicle- and Orx1R antagonist-treated mice are negatively correlated with cued freezing (Figs. 7A, B). Relative expression levels of PLCB1 were positively correlated with cued freezing behavior in vehicle-treated mice (Fig. 7C); however, this relationship is not observed after intra-BLA Orx1R inhibition (Fig. 7D). Contextual freezing behavior was associated with MAPK3 expression in only vehicle-treated mice (Figs. S10I). By contrast, intra-BLA antagonism of Orx1R cued freezing behavior was negatively correlated to MAPK3 expression (Fig. 7F), but not in vehicle-treated mice (Fig. 7E). The lack of gene expression correlations with cued fear freezing when phenotypes were assessed independently (Figs. S11), indicates that behavioral and transcriptional relationships exist within collective operational adaptations that link behavioral change to molecular modification. Importantly, no relationships between gene expression and conditioned fear freezing were observed for any of the tested cell signaling markers after Orx2R antagonism except for BDNF (not Orx1R antagonism), in which a significant negative correlation was revealed (Fig. S12E). Together, these results suggest a functional connection between Orx1R antagonist-induced shifts in gene expression and fear-related behaviors.
Figure 7.
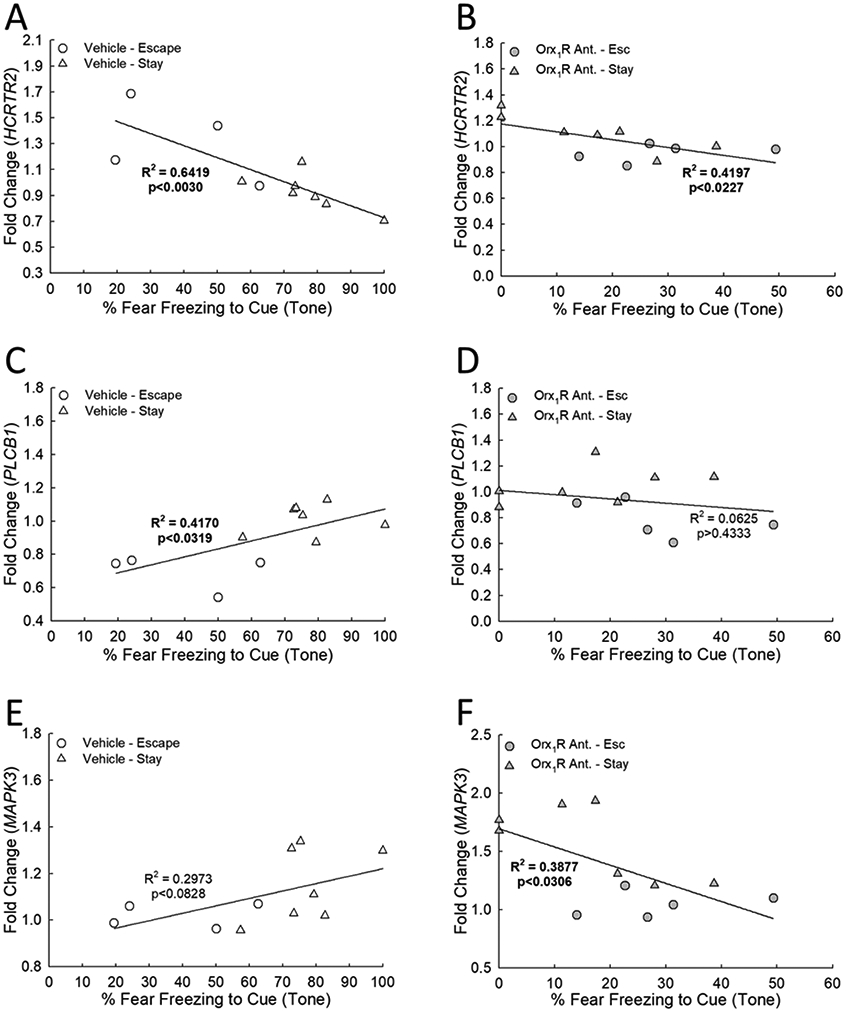
Conditioned fear freezing response is related to gene expression changes (fold change relative to home-cage naïve controls) resulting from intra-BLA Orx1R antagonism. In both A) vehicle- (N = 11, F1,9 = 16.1, R2 = 0.6419, p ≤ 0.003) and B) Orx1R antagonist-treated animals (N = 12, F1,10 = 7.2, R2 = 0.4197, p ≤ 0.023) a negative correlation exists between HCRTR2 expression and cued fear freezing. C) With vehicle treatment, relative PLCB1 expression is positively associated with cued fear freezing (F1,9 = 6.4, R2 = 0.417, p ≤ 0.0319). D) This relationship is not observed in mice that were administered an Orx1R antagonist (F1,10 = 0.7, R2 = 0.0625, p ≥ 0.4333). E) While there is not a significant association between MAPK3 expression and cued fear freezing after vehicle treatment (F1,9 = 3.8, R2 = 0.2973, p ≥ 0.0828), F) a significant negative correlation is observed after Orx1R antagonism (F1,10 = 6.3, R2 = 0.3877, p < 0.0306).
Potential molecular mechanism behind intra-BLA Orx1R antagonism
To help generate a theoretical mechanism to explain the physiological basis surrounding the observed behavioral (and phenotypic) shifts resulting from intra-BLA inhibition of Orx1R, we explored transcriptional relationships in systems that exhibited similar regression patterns (Fig. 8). With antagonism of Orx1R, there is a strongly positive relationship between HCRTR2 and MAPK3 expression (Fig. 8A). Importantly, this association does not exist after vehicle or Orx2R antagonist treatment (Fig. S13). While there are no observed relationships between BDNF and HCRTR2 expression levels (Figs. 8B, S13), BDNF expression is positively correlated to MAPK3 expression in animals treated with an Orx1R antagonist (Fig. 8C). Notably, no relationships exist between FICRTR1 expression and the other genes of interest (Figs. S13). These data allowed us to predict a working model to explain how BLA Orx1R may function to establish behavioral patterns consistent with stress-induced phenotype development (Fig. 9).
Figure 8.
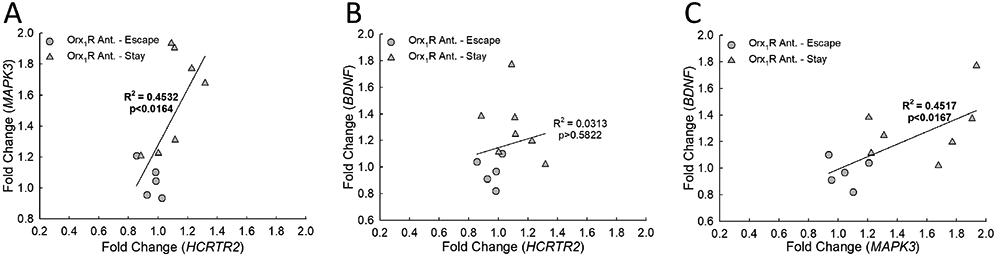
The BLA transcriptional changes (relative to home-cage naïve controls) that result from Orx1R antagonism form relationships that hint at molecular timelines and signaling dynamics. A) While relative gene expression of MAPK3 is positively correlated to the transcriptional changes of HCRTR2 (N = 12, F1,10 = 8.3, R2 = 0.4532 p ≤ 0.0164), B) there is no association between BDNFand HCRTR2 (F1,10 = 0.3, R2 = 0.0313, p ≥ 0.5822). However, C) a positive relationship emerges when comparing BDNF expression to that of MAPK3 (F1,10 = 8.2, R2 = 0.4517, p < 0.0167).
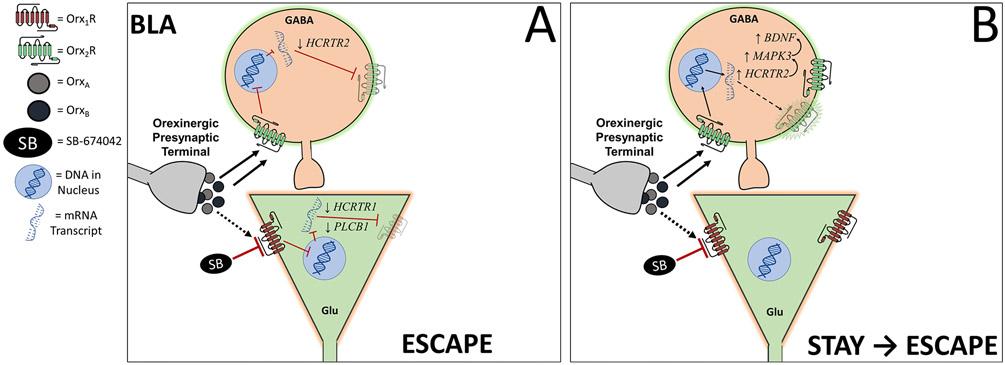
Figure 9.
Predicted circuit demonstrates the influence of intra-BLA Orx1R antagonism, during endogenous stimulation through OrxA and OrxB release, on microcircuit dynamics in a phenotype-dependent fashion. A) Escape mice treated with an Orx1R Antagonist (SB-674042) undergo molecular shifts, including a feedforward reduction of FICRTR1 and reduced PLCB1 transcription, leading to diminished orexin activity on glutamatergic neurons in the BLA. Escape mice also have a feedforward decrease in FICRTR2 expression, potentially via (un-diagrammed) negative circuit feedback, even while Orx2R are stimulated. B) While OrxB and OrxA maintain stimulation of some GABAergic neurons through Orx2R, antagonism of some pyramidal neurons via intra-BLA Orx1R inhibition differentially modifies molecular mechanisms in Stay mice through enhancement of Orx2R (HCRTR2), ERK1 (MAPK3), and BDNF transcription and increased orexin activity in Orx2R-containing neurons (likely GABAergic cells).
Discussion
Antagonism of Orx1R in the BLA can reverse or diminish expression of stress-related behavior. Our results suggest BLA Orx1R play a central role in stress responsiveness (40, 41) and related behavioral, physiological, and molecular outcomes that are important components of affective disorders (42, 43), such as anxiety (7), depression, and PTSD. Acute inhibition of intra-BLA Orx1R promotes Escape over Stay responses and limits freezing during fear conditioning in a phenotype-dependent way. Further, inhibition of Orx1R alters gene expression associated with critical signaling cascades. Following intra-BLA Orx1R antagonism, transcription for receptors and intracellular signaling becomes biased toward Orx2R (FICRTR2) over Orx1R (FICRTR1), and ERKi (MAPK3) over PLCβ1 (PLCB1) pathways. Importantly, even when BLA Orx1R are inhibited, native OrxA and OrxB will bind Orx2R. The relationship of these behavioral and molecular changes to enhanced expression of FICRTR2 mRNA, potentially in BLA neurons that do not contain Orx1R (Figs. 2L-O), suggests receptor-mediated mechanisms that balance pro- and anti-stress responses in BLA microcircuits.
Aggressive social interactions in the SAM produced two behavioral phenotypes that represent risk assessment and choice: Escape and Stay. These phenotypes, like those exposed to social defeat paradigms (44, 45), exhibit resilience (tightly linked to Escape) and susceptibility (highly correlated with Stay) in the SIP test (28). However, unlike traditional social defeat, SAM-separated phenotypes are expressed early in the behavioral paradigm, providing insight into the development and progression of stress-induced behavior and pathophysiology. Anxiolytic drugs (such as CRF1 receptor antagonist antalarmin and the Orx2R agonist [Ala11, d-Leu15]–OrxB) promote escape, while anxiogenic drugs (such as the α2 antagonist yohimbine and the Orx2R antagonist MK-1064) delay and/or block escape behavior (28, 29). Surprisingly, neither the Orx1R antagonist (Fig. 4D) nor knockdown (Fig. 4E) influenced escape latency, although it is reduced by anxiolytic factors such as exercise, Neuropeptide S, antalarmin, and increased by anxiogenic factors like yohimbine (29). We posit that enhanced escape on Day 4, following BLA Orx1R inhibition (on Day 3, drug treatment), is a reflection of the shift toward anti-stress signaling indicated by downregulation in pro-stress signaling (HCRTR1), and upregulation of anti-stress systems (HCRTR2, MAPK3, BDNF). Thus, BLA dual Orx1R/Orx2R inhibition (DORA) may not promote behavioral change. These stress-induced effects are paired with important learning and motivational components during SAM interactions (27, 29, 33, 35), and in human affective disorders (46).
In addition to species-specific anxious behavior and learning, social stress promotes behavioral inhibition, depressed behavioral drive and motivation in some individuals (47), plus a lower rate of adaptive behavior (48). Behavioral depression reveals two distinctive phenotypes related to stress responsiveness in humans and other animals (45, 49, 50). In SAM social interactions, Stay animals do less exploration of the escape route (Fig. 3A) and show indecisiveness relative to escape (35). Measuring motivation in the SAM is derived from a simple choice process, Escape or Stay (26, 27). Antagonism and knockdown of Orx1R increases interest in the escape route for both Stay and Escape mice (Figs. 3C, D). Thus, BLA Orx1R regulate stress-induced motivational behaviors; greatest in Escape mice, but marking a dramatic behavioral reversal in Stay mice that typically avoid the escape route (Figs. 3B, C). Attention to the escape route happens prior to escape, and is thus the first evidence of phenotypic differentiation in the SAM (28, 35). Latency to escape, and escape behavior also are influenced by motivation, although as previously demonstrated, these behaviors are strongly affected by stress and fearfulness associated with familiarity of the SAM or social interaction (27-29, 33, 35). Our results, like those of others, suggest Orx activity plays a fundamental role in motivation (8, 51), and in this case, specifically in the BLA for behaviors associated with stress-related motivation and choice.
Understanding the development of choice and motivation in the SAM is enhanced by pairing aversive aggression (US) with a non-threatening stimulus (tone CS) prior to interaction, promoting potent cued and contextual conditioned responses (CR) similar to standard fear conditioning approaches that utilize foot shock as a US (52). While the CRs elicited are similar, e.g. freezing (53), the ethological and ecological relevance of the US to the subject are not. By associating naturally aversive US with a benign stimulus (54), the SAM allows views into development of fear learning as it relates to the etiology of stress-provoked neurocircuitry changes, and demonstrates a connection between stress-induced fear expression and phenotype (Fig. 5). While early work suggested only Stay mice exhibited cued fear learning (27, 33), it is now clear both Stay and Escape respond to auditory cues with enhanced freezing compared to pre-tone freezing, and Stay mice also show contextual (prior to the cue) fear conditioning (Fig. 5A).
Fear responses are mediated through Orx1R activity in the amygdala, and in locus ceruleus (LC), which connects to the amygdala (22, 55-57). Our results similarly demonstrate Orx1R, but not Orx2R, inhibition diminishes both contextual and cued conditioned fear freezing in Stay animals (Figs. 5B; Table S1). While antagonizing Orx1R reduces fear/panic-induced freezing (7, 56, 58), Orx2R antagonism appears to eliminate fear learning in Escape mice, suggesting a phenotype-dependent effect (Figs. 5B; Table S1). Importantly, although Orx2R antagonism in the BLA reduced cued freezing only in Escape mice, we have previously demonstrated a potential anxiogenic effect of blocking receptor function (25, 28). This response may be dependent on brain region, as Orx2R activity in the nucleus accumbens shell and prelimbic prefrontal cortex may enhance anxious behavior (59, 60). Further, Orx2R antagonism has demonstrated antidepressive capabilities in a clinical setting (61).
Stimulation of intra-BLA Orx1R and Orx2R receptors using OrxA in Stay mice produces no reduction in contextual or cued fear conditioning (Fig. 5B; Table S1), suggesting the inhibition of both types of learned fear responses result specifically from Orx1R inhibition in Stay mice. To clarify the roles of Orx1R and Orx2R, we administered OrxA while concurrently inhibiting Orx2R (MK1064), leaving Orx1R stimulated, and again there was no statistically significant reduction in either type of fear conditioning response (Fig. 5B; Table S1). Interestingly, knockdown of Orx1R did not affect the fear freezing profile (Fig. S8). As knockdown occurred before the introduction of social stress, activity levels of Orx1R after SAM exposure allowed for fear learning (higher freezing after CS), but did not diminish freezing as observed with acute antagonism after stress and phenotype development (Fig. 5B).
Molecular gene expression during SAM fear conditioning and phenotype development indicated potential shifts in receptor-linked intracellular signaling cascades (Fig. 6). Acute inhibition of intra-BLA Orx1R, by means of a feedforward rather than feedback mechanisms, lowered HCRTR1 expression in Escape mice while enhancing HCRTR2 mRNA in Stay animals (Figs. 6A, B). Antagonism of Orx2R in BLA did the opposite, reducing HCRTR1 mRNA only in Stay mice, and in a similar feedforward way, decreasing HCRTR2 expression in both phenotypes (Figs. 6A, B). Mice exhibiting escape and reduced fear freezing, expressed lower PLCB1 compared to the Stay phenotype; an effect unaltered by SB-674042 treatment, but reversed by Orx2R antagonism (Fig. 6C). However, intra-BLA Orx1R antagonism increased MAPK3 and BDNF expression in Stay animals only, with Orx2R inhibition having no effect on expression of MAPK3, and enhancing BDNF, but only in Escape mice, while reducing BDNF in Stay mice (Figs. 6D-G; Table S2). These results suggest social stress disrupts gene expression, and potentially alters BLA signaling pathways depending on an individual’s stress state. Therefore, pharmacological interventions (like acute Orx1R antagonism) may functionally amend behavior through signaling adaptations that are phenotype dependent.
Fear conditioning responses appear to be related to specific transcriptional reorganization taking place during/after intra-BLA Orx1R inhibition (Fig. 7). In treated animals, negative regressions exist between cued fear freezing behavior and FICRTR2 as well as MAPK3 (62) transcriptional changes (Figs. 7B, F). Without treatment (vehicle), cued freezing was positively linked to PLCB1 gene expression (Fig. 7C), an effect not observed with Orx1R antagonism (Fig. 7D). These associations provide evidence for potential mechanistic remodeling (Fig. 9) in the BLA during periods of stress that is tied to phenotype formation and involves Orx receptor activity. This balancing act between Orx1R and Orx2R creates an influence over BLA microcircuits, which further defines downstream signaling dynamics, in a way that can modify stress-induced behavior (2). Since changes in HCRTR2 expression after intra-BLA Orx1R inhibition are positively associated with MAPK3 but not BDNF transcription levels (Figs. 8A, B), it appears the adjusted bias of Orx2R over Orx1R activity favors ERKi signaling (Fig. 9). Amplification of ERK1, in turn, may lead to enhanced BDNF expression (Fig. 8C) and plastic changes within BLA microcircuits (Fig. 9) (62, 63). Importantly, these findings highlight a role of intra-BLA Orx1R in establishing pro-stress behavioral states; but exposes a receptor-driven balance that takes part in the fluid, not static, appearance of phenotype-specific behavior.
Conclusions
Modulation of BLA stress-regulatory pathways via Orx1 receptors found predominantly on glutamatergic pyramidal neurons modifies gene expression and behavior. Modulation of pro-stress BLA microcircuits via Orx1R inhibition reduces stress-induced behavior. In the process, Orx1R BLA inhibition modifies gene expression of HCRTR2 which impedes pro-stress responses. Concurrently, transcription levels for downstream molecular signaling systems associated with Orx receptor signaling are also tilted toward increased ERK1 (MAPK3), rather than PLCβ1 (PLCB1) signaling pathways, potentially altering behavior.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Chemical Compound or Drug | SB-674042 Selective Orx1R Antagonist | MedChemExpress, Monmouth Junction, NJ | Catalog Number HY-10898 | |
| Chemical Compound or Drug | MK-1064 Selective Orx2R Antagonist | MedChemExpress, Monmouth Junction, NJ | Catalog Number HY-19914 | |
| Commercial Assay Or Kit | RNAscope Fluorescent Multplex Detection Reagents | Advanced Cell Diagnostics, Newark, CA | Catalog Number 320851 | |
| Commercial Assay Or Kit | RNAscope HCRTR1 Probe | Advanced Cell Diagnostics, Newark, CA | Catalog Number 466631 | |
| Commercial Assay Or Kit | RNAscope HCRTR2 Probe | Advanced Cell Diagnostics, Newark, CA | Catalog Number 460881 | |
| Commercial Assay Or Kit | RNAscope CAMKIIα Probe | Advanced Cell Diagnostics, Newark, CA | Catalog Number 445231 | |
| Commercial Assay Or Kit | RNAscope CALB1 Probe | Advanced Cell Diagnostics, Newark, CA | Catalog Number 428431 | |
| Commercial Assay Or Kit | RNAscope GAD1 Probe | Advanced Cell Diagnostics, Newark, CA | Catalog Number 400951 | |
| Commercial Assay Or Kit | RNAscope PVALB Probe | Advanced Cell Diagnostics, Newark, CA | Catalog Number 421939 | |
| Commercial Assay Or Kit | Corticosterone ELISA Kit | Enzo Life Sciences, Farmingdale, NY | Catalog Number ADI-900-097 | |
| Commercial Assay Or Kit | One-step RT-qPCR Kit | Thermo Fisher Scientific, Waltham, MA | Catalog Number 4392653 | |
| Commercial Assay Or Kit | RT-qPCR HCRTR1 Assay | Thermo Fisher Scientific, Waltham, MA | Catalog Number 4351370, Mm01185776_m1 | |
| Commercial Assay Or Kit | RT-qPCR HCRTR2 Assay | Thermo Fisher Scientific, Waltham, MA | Catalog Number 4351370, Mm01179312_m1 | |
| Commercial Assay Or Kit | RT-qPCR PLCB1 Assay | Thermo Fisher Scientific, Waltham, MA | Catalog Number 4351370, Mm01329382_m1 | |
| Commercial Assay Or Kit | RT-qPCR MAPK1 Assay | Thermo Fisher Scientific, Waltham, MA | Catalog Number 4448892, Mm00442479_m1 | |
| Commercial Assay Or Kit | RT-qPCR MAPK3 Assay | Thermo Fisher Scientific, Waltham, MA | Catalog Number 4351370, Mm01278702_gH | |
| Commercial Assay Or Kit | RT-qPCR BDNF Assay | Thermo Fisher Scientific, Waltham, MA | Catalog Number 4351370, Mm04230607_s1 | |
| Commercial Assay Or Kit | RT-qPCR GAPDH Assay | Thermo Fisher Scientific, Waltham, MA | Catalog Number 44533200, Mm99999915_g1 | |
| Organism/Strain | Male C57BL/6NHsd Mice | Envigo (an inotiv company), Indianapolis, IN | Item Number 4405M | |
| Organism/Strain | Male Hsd:ICR (CD-1) Retired Breeder Mice | Envigo (an inotiv company), Indianapolis, IN | Item Number 3017M | |
| Peptide, Recombinant Protein | Orexin A (human, rat, mouse) | Tocris, Minneapolis, MN | Catalog Number 1455 | |
| Software; Algorithm | ImageJ | doi:10.1038/nmeth.2089 | RRID: SCR_003070 | |
| Software; Algorithm | ANY-maze Video Tacking Software (Version 6.0) | Stoelting Co., Wood Dale, IL | RRID: SCR_014289 | |
| Transfected Construct | AAV-U6-Orx1R-shRNA, AAV-scramble-shRNA | DiLeone Lab, Yale University, New Haven, CT, https://doi.org/10.1016/j.psyneuen.2013.10.010 | ||
| Other | Stress Alternatives Model (SAM) | Summers Lab, University of South Dakota, Vermillion, SD, https://doi.org/10.3389/fnbeh.2014.00121 |
ACKNOWLEDGMENTS AND DISCLOSURES
We would like to thank Jacob Nordman for comments on this manuscript, J. J. Gale for helping with RNA extraction, Ashley M. Potter for assisting with behavioral analyses, Kelly R. Graber for guiding us on microscope imaging and acquisition, and Raegan Skelton for PCR consultations. We further recognize and commend efforts in the scientific community that stand up against discrimination and social injustices. Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health, USA, under Award Numbers R15 MH104485 and R15 MH125306, through support (for JDWY & KTK) by the National Science Foundation (NSF) Research Training Program, USD-N3 Grant DgE-1633213, by a USD Center for Brain and Behavior Research (CBBRe) pilot grant, and by the Nolop Endowment via the USD Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, NSF, the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.van Praag HM (2004): Can stress cause depression? Progress in Neuro-Psychopharmacology and Biological Psychiatry. 28:891–907. [DOI] [PubMed] [Google Scholar]
- 2.Yaeger JD, Krupp KT, Gale JJ, Summers CH (2020): Counterbalanced microcircuits for Orx1 and Orx2 regulation of stress reactivity. Medicine in Drug Discovery. 100059. [Google Scholar]
- 3.Kim J, Pignatelli M, Xu S, Itohara S, Tonegawa S (2016): Antagonistic negative and positive neurons of the basolateral amygdala. Nature neuroscience. 19:1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giardino WJ, Eban-Rothschild A, Christoffel DJ, Li S-B, Malenka RC, de Lecea L (2018):Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nature Neuroscience. 21:1084–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tovote P, Fadok JP, Lüthi A (2015): Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience. 16:317. [DOI] [PubMed] [Google Scholar]
- 6.Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A (2012): Orexin, stress, and anxiety/panic states. Progress in brain research: Elsevier, pp 133–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, et al. (2010): A key role for orexin in panic anxiety. Nature medicine. 16:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G (2014): Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nature Neuroscience. 17:1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyree SM, Borniger JC, de Lecea L (2018): Hypocretin as a Hub for Arousal and Motivation. Frontiers in Neurology. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ammoun S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, et al. (2003): Distinct Recognition of OX1 and OX2 Receptors by Orexin Peptides. Journal of Pharmacology and Experimental Therapeutics. 305:507. [DOI] [PubMed] [Google Scholar]
- 11.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. (1998): Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 92:573–585. [DOI] [PubMed] [Google Scholar]
- 12.Holmqvist T, Åkerman KE, Kukkonen JP (2002): Orexin signaling in recombinant neuron-like cells. FEBS letters. 526:11–14. [DOI] [PubMed] [Google Scholar]
- 13.Ross CA, MacCumber MW, Glatt CE, Snyder SH (1989): Brain phospholipase C isozymes: differential mRNA localizations by in situ hybridization. Proc Natl Acad Sci U S A. 86:2923–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao CF, Jia P, Zhao Z, Kuo PH (2012): Enriched pathways for major depressive disorder identified from a genome-wide association study. The international journal of neuropsychopharmacology. 15:1401–1411. [DOI] [PubMed] [Google Scholar]
- 15.Lo Vasco VR, Longo L, Polonia P (2013): Phosphoinositide-specific Phospholipase C β1 gene deletion in bipolar disorder affected patient. J Cell Commun Signal. 7:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabana-Domínguez J, Roncero C, Pineda-Cirera L, Palma-Álvarez RF, Ros-Cucurull E, Grau-López L, et al. (2017): Association of the PLCB1 gene with drug dependence. Scientific Reports. 7:10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S-W, Cho T, Lee S (2015): Phospholipase C-β1 hypofunction in the pathogenesis of schizophrenia. Frontiers in Psychiatry. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Udawela M, Scarr E, Boer S, Um JY, Hannan AJ, McOmish C, et al. (2017): Isoform specific differences in phospholipase C beta 1 expression in the prefrontal cortex in schizophrenia and suicide. npj Schizophrenia. 3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cestari V, Rossi-Arnaud C, Saraulli D, Costanzi M (2014): The MAP(K) of fear: From memory consolidation to memory extinction. Brain Research Bulletin. 105:8–16. [DOI] [PubMed] [Google Scholar]
- 20.Merlo E, Milton AL, Everitt BJ (2018): A novel retrieval-dependent memory process revealed by the arrest of ERK1/2 activation in the basolateral amygdala. Journal of Neuroscience. 38:3199–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flores Á, Herry C, Maldonado R, Berrendero F (2017): Facilitation of contextual fear extinction by orexin-1 receptor antagonism is associated with the activation of specific amygdala cell subpopulations. International Journal of Neuropsychopharmacology. 20:654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flores Á, Saravia R, Maldonado R, Berrendero F (2015): Orexins and fear: implications for the treatment of anxiety disorders. Trends in Neurosciences. 38:550–559. [DOI] [PubMed] [Google Scholar]
- 23.Kim T-K, Kim J-E, Park J-Y, Lee J-E, Choi J, Kim H, et al. (2015): Antidepressant effects of exercise are produced via suppression of hypocretin/orexin and melanin-concentrating hormone in the basolateral amygdala. Neurobiology of disease. 79:59–69. [DOI] [PubMed] [Google Scholar]
- 24.Arendt DH, Ronan PJ, Oliver KD, Callahan LB, Summers TR, Summers CH (2013): Depressive behavior and activation of the orexin/hypocretin system. Behavioral neuroscience. 127:86. [DOI] [PubMed] [Google Scholar]
- 25.Arendt DH, Hassell J, Li H, Achua JK, Guarnieri DJ, DiLeone RJ, et al. (2014): Anxiolytic function of the orexin 2/hypocretin A receptor in the basolateral amygdala. Psychoneuroendocrinology. 40:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson JM, Prince MA, Achua JK, Carpenter RE, Arendt DH, Smith JP, et al. (2015): Nuance and behavioral cogency: How the Visible Burrow System inspired the Stress-Alternatives Model and conceptualization of the continuum of anxiety. Physiol Behav. 146:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith JP, Achua JK, Summers TR, Ronan PJ, Summers CH (2014): Neuropeptide S and BDNF gene expression in the amygdala are influenced by social decision-making under stress. Frontiers in Behavioral Neuroscience. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staton CD, Yaeger JD, Khalid D, Haroun F, Fernandez BS, Fernandez JS, et al. (2018): Orexin 2 receptor stimulation enhances resilience, while orexin 2 inhibition promotes susceptibility, to social stress, anxiety and depression. Neuropharmacology. 143:79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JP, Prince MA, Achua JK, Robertson JM, Anderson RT, Ronan PJ, et al. (2016): Intensity of anxiety is modified via complex integrative stress circuitries. Psychoneuroendocrinology. 63:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Summers CH, Yaeger JDW, Staton CD, Arendt DH, Summers TR (2020): Orexin/hypocretin receptor modulation of anxiolytic and antidepressive responses during social stress and decision-making: Potential for therapy. Brain Research. 1731:146085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald AJ (1984): Neuronal organization of the lateral and basolateral amygdaloid nuclei in the rat. Journal of Comparative Neurology. 222:589–606. [DOI] [PubMed] [Google Scholar]
- 32.McDonald AJ, Mascagni F (2001): Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience. 105:681–693. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter RE, Summers CH (2009): Learning strategies during fear conditioning. Neurobiology of learning and memory. 91:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson JM, Achua JK, Smith JP, Prince MA, Staton CD, Ronan PJ, et al. (2017): Anxious behavior induces elevated hippocampal Cb2 receptor gene expression. Neuroscience. 352:273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Summers TR, Summers TL, Carpenter RE, Smith JP, Young SL, Meyerink B, et al. (2017): Learning and CRF-Induced Indecision during Escape and Submission in Rainbow Trout during Socially Aggressive Interactions in the Stress-Alternatives Model. Frontiers in Neuroscience. 11:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y, Miwa Y, Yamanaka A, Yada T, Shibahara M, Abe Y, et al. (2003): Orexin Receptor Type-1 Couples Exclusively to Pertussis Toxin-Insensitive G-Proteins, While Orexin Receptor Type-2 Couples to Both Pertussis Toxin-Sensitive and -Insensitive G-Proteins. Journal of Pharmacological Sciences. 92:259–266. [DOI] [PubMed] [Google Scholar]
- 37.Brunoni AR, Lopes M, Fregni F (2008): A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. International Journal of Neuropsychopharmacology. 11:1169–1180. [DOI] [PubMed] [Google Scholar]
- 38.Knaepen K, Goekint M, Heyman EM, Meeusen R (2010): Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med. 40:765–801. [DOI] [PubMed] [Google Scholar]
- 39.Notaras M, van den Buuse M (2020): Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Molecular psychiatry. 25:2251–2274. [DOI] [PubMed] [Google Scholar]
- 40.Giardino WJ, de Lecea L (2014): Hypocretin (orexin) neuromodulation of stress and reward pathways. Current opinion in neurobiology. 29:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sargin D (2019): The role of the orexin system in stress response. Neuropharmacology. 154:68–78. [DOI] [PubMed] [Google Scholar]
- 42.James MH, Campbell EJ, Dayas CV (2017): Role of the Orexin/Hypocretin System in Stress-Related Psychiatric Disorders. In: Lawrence AJ, de Lecea L, editors. Behavioral Neuroscience of Orexin/Hypocretin. Cham: Springer International Publishing, pp 197–219. [Google Scholar]
- 43.Han D, Han F, Shi Y, Zheng S, Wen L (2020): Mechanisms of memory impairment induced by orexin-A via orexin 1 and orexin 2 receptors in post-traumatic stress disorder rats. Neuroscience. 432:126–136. [DOI] [PubMed] [Google Scholar]
- 44.Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, et al. (2006): Essential Role of BDNF in the Mesolimbic Dopamine Pathway in Social Defeat Stress. Science. 311:864–868. [DOI] [PubMed] [Google Scholar]
- 45.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. (2007): Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 131:391–404. [DOI] [PubMed] [Google Scholar]
- 46.Nissen C, Holz J, Blechert J, Feige B, Riemann D, Voderholzer U, et al. (2010): Learning as a Model for Neural Plasticity in Major Depression. Biological Psychiatry. 68:544–552. [DOI] [PubMed] [Google Scholar]
- 47.Zimmerman M, Martin J, McGonigal P, Harris L, Kerr S, Balling C, et al. (2019): Validity of the DSM-5 anxious distress specifier for major depressive disorder. Depression and anxiety. 36:31–38. [DOI] [PubMed] [Google Scholar]
- 48.Vindas MA, Helland-Riise SH, Nilsson GE, Øverli Ø (2019): Depression-like state behavioural outputs may confer beneficial outcomes in risky environments. Scientific Reports. 9:3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Björkqvist K (2001): Social defeat as a stressor in humans. Physiology & behavior. 73:435–442. [DOI] [PubMed] [Google Scholar]
- 50.Kessler RC (1997): The effects of stressful life events on depression. Annual review of psychology. 48:191–214. [DOI] [PubMed] [Google Scholar]
- 51.Tsujino N, Sakurai T (2013): Role of orexin in modulating arousal, feeding, and motivation. Frontiers in behavioral neuroscience. 7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rescorla RA (1968): Probability of shock in the presence and absence of CS in fear conditioning. Journal of comparative and physiological psychology. 66:1. [DOI] [PubMed] [Google Scholar]
- 53.Blanchard RJ, Blanchard DC (1969): Passive and active reactions to fear-eliciting stimuli. Journal of comparative and physiological psychology. 68:129. [DOI] [PubMed] [Google Scholar]
- 54.Zoicas I, Menon R, Neumann ID (2016): Neuropeptide S reduces fear and avoidance of con-specifics induced by social fear conditioning and social defeat, respectively. Neuropharmacology. 108:284–291. [DOI] [PubMed] [Google Scholar]
- 55.Soya S, Shoji H, Hasegawa E, Hondo M, Miyakawa T, Yanagisawa M, et al. (2013): Orexin receptor-1 in the locus coeruleus plays an important role in cue-dependent fear memory consolidation. Journal of Neuroscience. 33:14549–14557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flores Á, Valls-Comamala V, Costa G, Saravia R, Maldonado R, Berrendero F (2014): The hypocretin/orexin system mediates the extinction of fear memories. Neuropsychopharmacology. 39:2732–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sears RM, Fink AE, Wigestrand MB, Farb CR, De Lecea L, LeDoux JE (2013): Orexin/hypocretin system modulates amygdala-dependent threat learning through the locus coeruleus. Proceedings of the National Academy of Sciences. 110:20260–20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salehabadi S, Abrari K, Salmani ME, Nasiri M, Lashkarbolouki T (2020): Investigating the role of the amygdala orexin receptor 1 in memory acquisition and extinction in a rat model of PTSD. Behavioural Brain Research. 384:112455. [DOI] [PubMed] [Google Scholar]
- 59.Li B, Chang L, Peng X (2021): Orexin 2 receptor in the nucleus accumbens is critical for the modulation of acute stress-induced anxiety. Psychoneuroendocrinology. 131:105317. [DOI] [PubMed] [Google Scholar]
- 60.Soares VP, de Andrade TG, Canteras NS, Coimbra NC, Wotjak CT, Almada RC (2021): Orexin 1 and 2 receptors in the prelimbic cortex modulate threat valuation. Neuroscience. [DOI] [PubMed] [Google Scholar]
- 61.Recourt K, de Boer P, Zuiker R, Luthringer R, Kent J, van der Ark P, et al. (2019): The selective orexin-2 antagonist seltorexant (JNJ-42847922/MIN-202) shows antidepressant and sleep-promoting effects in patients with major depressive disorder. Translational psychiatry. 9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu KT, Walker DL, Davis M (2001): Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. The Journal of neuroscience : the official journal of the Society for Neuroscience. 21:Rc162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ (2006): Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nature Neuroscience. 9:870–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.