Abstract
Thyroid disorders are prevalent in pregnant women. Furthermore, thyroid hormone has a critical role in fetal development and thyroid dysfunction can adversely affect obstetric outcomes. Thus, the appropriate management of hyperthyroidism, most commonly caused by Graves disease, and hypothyroidism, which in iodine sufficient regions is most commonly caused by Hashimoto thyroiditis, in pregnancy is important for the health of both pregnant women and their offspring. Gestational transient thyrotoxicosis can also occur during pregnancy and should be differentiated from Graves disease. Effects of thyroid autoimmunity and subclinical hypothyroidism in pregnancy remain controversial. Iodine deficiency is the leading cause of hypothyroidism worldwide. Despite global efforts to eradicate iodine deficiency disorders, pregnant women remain at risk of iodine deficiency due to increased iodine requirements during gestation. The incidence of thyroid cancer is increasing worldwide, including in young adults. As such, the diagnosis of thyroid nodules or thyroid cancer during pregnancy is becoming more frequent. The evaluation and management of thyroid nodules and thyroid cancer in pregnancy pose a particular challenge. Postpartum thyroiditis can occur up to 1 year after delivery and must be differentiated from other forms of thyroid dysfunction, as treatment differs. This Review provides current evidence and recommendations for the evaluation and management of thyroid disorders in pregnancy and in the postpartum period.
Thyroid disorders are fairly common in women of reproductive age. Hashimoto thyroiditis and Graves disease, both autoimmune thyroid disorders, are eight to ten times more frequent in women than in men and have a peak prevalence in early adulthood1. Of note, hypothyroidism is estimated to occur in 4% of pregnancies (0.5% overt and 3.5% subclinical hypothyroidism) and hyperthyroidism in 2.4% of pregnancies (0.6% overt and 1.8% subclinical hyperthyroidism)2. Thyroid hormone is important for both maternal and child health. Although the fetal thyroid gland is present and functional by 10–12 weeks gestation, it does not mature until 18–20 weeks3. Thus, the fetus depends on maternal thyroid hormone delivered via transplacental passage during a critical period of development in early gestation3. Consequently, maternal thyroid dysfunction can lead to adverse pregnancy and child neurodevelopmental outcomes. In addition, in the postpartum period, ~5% of women might experience transient thyroid dysfunction from postpartum thyroiditis within 12 months of delivery4.
Human chorionic gonadotropin (hCG), a hormone produced by the placenta during pregnancy, has a weakly thyrotrophic effect5 and can promote the development and growth of thyroid nodules in pregnancy. The prevalence of thyroid nodules in pregnancy has been reported to be as high as 29%, depending on the iodine status of the population studied6,7. Of note, a worldwide increase has occurred in the incidence of thyroid cancer in young women in the past few decades. Thyroid cancer is now the most common type of cancer diagnosed in women aged 15–29 years and the second most common type in women aged 30–39 years8–10.
In this Review, we present current evidence regarding the diagnosis and management of thyroid disorders during pregnancy and the postpartum period. We note that unless otherwise specified, the term ‘women’ refers to ciswomen, as the research highlighted in this Review included only ciswomen. There is currently a lack of available research regarding thyroid disease in pregnancy in transmen and non-binary pregnant people.
Thyroid dysfunction in pregnancy
Assessment of thyroid hormone levels in pregnancy
During pregnancy, the weakly thyrotrophic effect of hCG on the thyroid gland increases thyroid hormone production (FIG. 1), which in turn decreases thyroid stimulating hormone (TSH) secretion from the pituitary gland via negative feedback11. Thus, in early pregnancy when levels of hCG are high, serum levels of TSH tend to shift downwards compared with those of non-pregnant adults12. Serum levels of TSH typically increase slightly after the first trimester, largely due to a decrease in serum levels of hCG11. The levels of thyroid hormone in pregnancy can differ by race and/or ethnicity, and iodine status of populations. Therefore, the American Thyroid Association (ATA) and the American College of Obstetricians and Gynecologists (ACOG) recommend using population-specific, assay-specific and trimester-specific ranges for thyroid function tests in pregnant women6,13. If such ranges are not available, a TSH reference range of 0.1–4.0 mIU/l can be used in early gestation (for context, the reference range for non-pregnant adults is usually 0.5–4.5 mIU/l)6,13.
Fig. 1 |. Factors increasing maternal dietary iodine requirement in pregnancy.
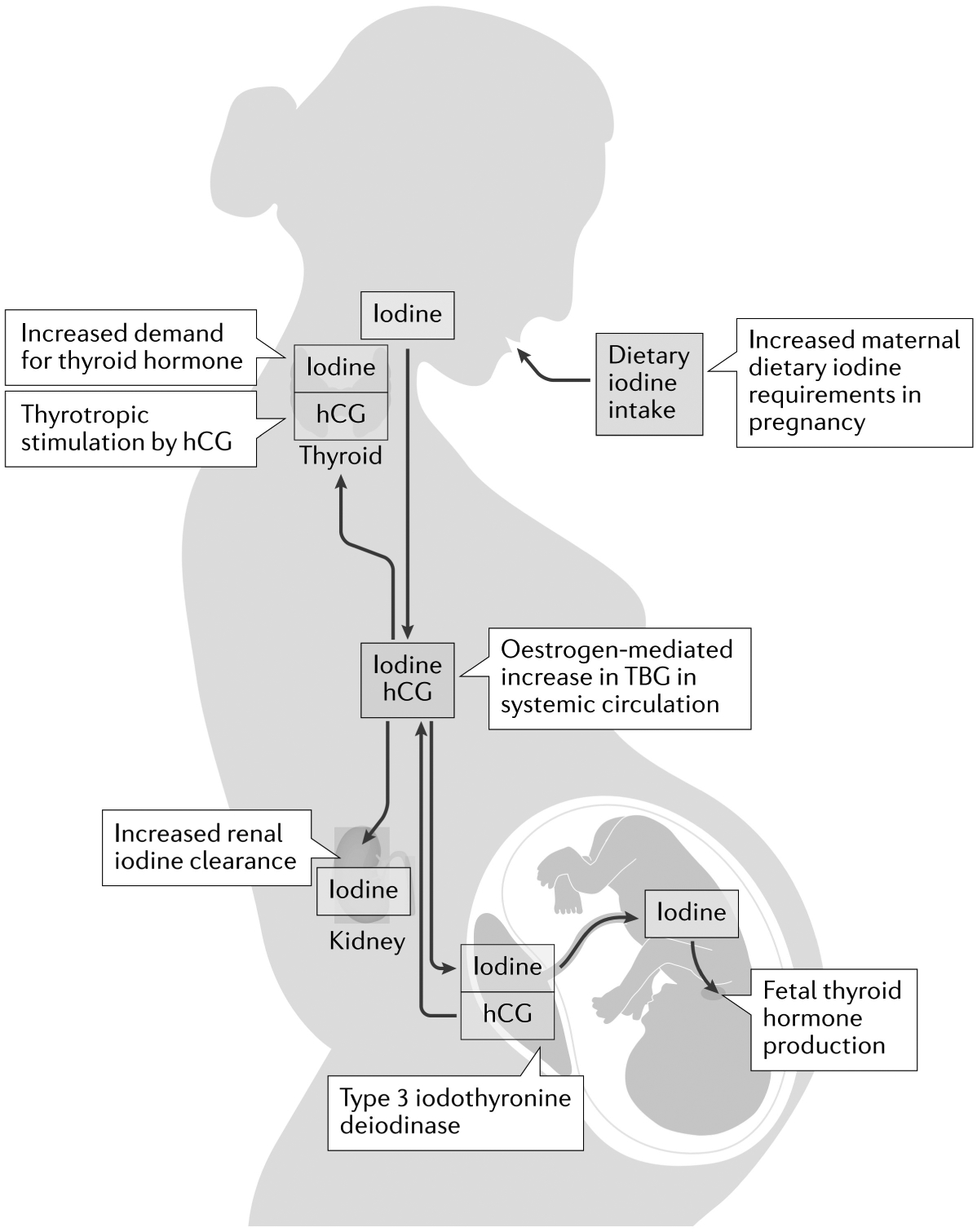
Maternal and fetal factors leading to increased iodine requirements in pregnancy are described. During pregnancy, there is a ~50% increase in demand for thyroid hormone, which requires an additional 50–100 μg daily dietary iodine intake. Maternal thyroid hormone production is in part increased by the thyrotropic action of human chorionic gonadotropin (hCG), a hormone produced by the placenta. Oestrogen mediates a twofold increase in circulating levels of thyroxine-binding globulin (TBG), which binds thyroid hormones in the maternal circulation, leading to a relative decrease in levels of active free T4, which further promotes an increase in thyroid hormone production. Furthermore, placental type 3 iodothyronine deiodinase deactivates T4 in the circulation to reverse T3. Increased maternal renal clearance of iodine (by 30–50%) also occurs due to the increase in maternal blood volume. Finally, iodine is transferred from the maternal systemic circulation (red box) to the fetus for fetal thyroid hormone production.
Serum TSH should be used as the initial screening test for thyroid dysfunction in pregnancy, as TSH is the most sensitive marker for primary thyroid dysfunction. In addition, serum levels of free T4 can be used to distinguish between overt and subclinical thyroid dysfunction. Overt hypothyroidism is defined by high serum levels of TSH with low levels of free T4, whereas in subclinical hypothyroidism serum levels of TSH are elevated but levels of free T4 are normal. Similarly, women with overt hyperthyroidism have low serum levels of TSH with elevated levels of free T4 and those with subclinical hyperthyroidism have low serum levels of TSH with normal levels of free T4.
Accurate measurement of serum levels of free T4 is challenging in pregnant women. High levels of oestrogen in pregnancy cause a twofold increase in serum levels of thyroxine-binding globulin (TBG)14. Indirect free T4 immunoassays can be confounded by high TBG, which can lead to falsely low results, especially in the second and third trimesters of pregnancy. The free T4 index, which utilizes assessment of levels of total T4 and an index of binding to calculate free T4, might be more accurate than indirect immunoassays in high TBG states such as pregnancy15. In 2018, Gronowski argued that the decrease in serum levels of free T4 measured by immunoassay seen in the second and third trimesters of pregnancy might be physiological16. Furthermore, a 2021 study in about 300 euthyroid pregnant Spanish women showed that serum levels of free T4 (measured by two different immunoassays) decreased in the second and third trimesters17. Whether the decrease in free T4 observed in the second and third trimesters is actually physiological remains to be confirmed. Although levels of free T4 measured by immunoassays correlated well with levels of free T4 measured by liquid chromatography with tandem mass spectrometry (LC–MS–MS) in the first trimester, the reference ranges were not interchangeable at any time point and the immunoassay results were not correlated with LC–MS–MS results in the second and third trimesters. Serum levels of free T4 measured by LC–MS–MS remain the gold standard for assessing levels of free thyroid hormone, but this technique is cumbersome and expensive. Although the modern immunoassays for free T4 measurements might be more reliable than older immunoassays in pregnancy, the pregnancy-specific, trimester-specific reference ranges for each assay are needed. If a reliable assay for free T4 or the free T4 index is not available, levels of total T4 or total T3 can be used as surrogates for the measurement of free hormone. Of note, between 7 and 16 weeks gestation, the non-pregnancy assay reference ranges for total T4 or T3 can be increased by 5% per week to account for rises in serum levels of TBG. When serum levels of TBG stabilize beyond 16 weeks gestation, 150% of the upper limit of non-pregnant reference ranges for total T4 and T3 levels can be used18.
Hypothyroidism in pregnancy
Overt hypothyroidism and obstetric outcomes.
The adverse obstetric and perinatal effects of maternal overt hyperthyroidism have been well documented. For example, increased rates of gestational hypertension (that is eclampsia, pre-eclampsia and pregnancy-induced hypertension), placental abruption, postpartum haemorrhage, preterm delivery, low birthweight and fetal death were initially reported in women with overt hypothyroidism and their infants in the 1980s to the 1990s19,20. More recently, a 2013 US national cohort study including 223,512 pregnant women similarly showed that untreated maternal overt hypothyroidism increased the risks of pre-eclampsia, gestational diabetes mellitus, preterm birth, caesarean section and infant intensive care unit admission21.
Overt hypothyroidism and child neurodevelopmental outcomes.
Thyroid hormone affects neuronal migration, connection, myelination and synaptogenesis and is thus essential for normal brain development22,23. Thyroid hormone receptors are expressed during cortex development24,25. The adverse effects of maternal thyroid hypofunction in child neurodevelopment were first reported in a series of studies in the 1970s26,27. In a subsequent seminal study published in 1999, 62 children born to hypothyroid women (median TSH 13.2 mIU/l) had an average IQ that was four points lower at 7–9 years of age than that of 124 children born to euthyroid women (median TSH 1.4 mIU/l)28. Furthermore, when 14 women with hypothyroidism who were treated with levothyroxine during pregnancy were excluded from the analysis, the difference in IQ was seven points.
Subclinical hypothyroidism, hypothyroxinaemia and obstetric outcomes.
The effects of maternal subclinical hypothyroidism and isolated hypothyroxinaemia (that is, low serum levels of free T4 with normal levels of TSH) on obstetric outcomes have been debated. For example, several observational studies have found no associations between maternal subclinical hypothyroidism and outcomes including miscarriage, gestational diabetes mellitus, gestational hypertension, pre-eclampsia, preterm labour, premature delivery, low birthweight and high birthweight29–31. Furthermore, a meta-analysis and systematic review of ten cohort studies including 48,684 women (which included some of the observational studies discussed in the previous sentence) similarly showed no significant associations between maternal subclinical hypothyroidism or isolated hypothyroxinaemia and preterm birth32.
On the other hand, multiple other observational cohort studies have found associations between maternal subclinical hypothyroidism and pregnancy loss or miscarriage33,34, prematurity or preterm birth35,36, placental abruption35 and neonatal intensive care unit admission and respiratory distress syndrome in infants35. A meta-analysis of 18 cohort studies including almost 4,000 women found that compared with euthyroidism, maternal subclinical hyperthyroidism is associated with increased risks of pregnancy loss (relative risk (RR) 2.01, 95% CI 1.66–2.44), placental abruption (RR 2.14, 95% CI 1.23–3.70), premature rupture of membranes (RR 1.43, 95% CI 1.04–1.95) and neonatal death (RR 2.58, 95% CI 1.41–4.73)37. In a 2020 retrospective study in 8,413 pregnant women, offspring of women with subclinical hypothyroidism had increased risks of prematurity (RR 2.15, 95% CI 1.14–4.03) and neonatal respiratory distress syndrome (RR 2.8, 95% CI 1.01–7.78) compared with the risks in euthyroid women. Furthermore, the risks of these adverse outcomes were even higher if subclinical hypothyroidism was present in the first trimester38. In addition, an individual-participant level meta-analysis of data published in 2019 from 19 cohort studies (including 47,045 pregnant women) similarly showed increased risks of preterm birth in women with subclinical hypothyroidism (OR 2.9, 95% Cl 1.01–1.64) and isolated hypothyroxinaemia (OR 1.46, 95% Cl 1.12–1.90)39.
These conflicting results regarding the effects of maternal subclinical hypothyroidism across cohort studies might be due to variability in timing of TSH measurements, differences in TSH cut-off values used for the diagnosis of subclinical hypothyroidism, and variability in assessment of thyroid peroxidase (TPO) antibody status. However, evidence is mounting that maternal subclinical hypothyroidism is associated with miscarriage and preterm birth. The effects of isolated maternal hypothyroxinaemia on pregnancy outcomes remain unclear40.
Subclinical hypothyroidism, hypothyroxinaemia and child neurodevelopmental outcomes.
Mild maternal thyroid hypofunction has been shown to have a deleterious effect on child neurodevelopmental outcomes in multiple cohorts. In a study of 3,839 Dutch mother–child pairs, both low and high maternal levels of free T4 in early pregnancy were associated with lower child IQ and higher likelihood of an IQ of <85 at 6–8 years of age compared with normal levels of maternal free T4 (REF.41). This association remained statistically significant even when overt hypothyroidism and overt hyperthyroidism were excluded. Of note, in this analysis, maternal serum TSH values did not predict child IQ. Brain MRI has shown alterations in child neuroanatomy associated with either low or high maternal thyroid function; the strongest effect was observed when thyroid function was measured at 8–14 weeks gestation. These findings suggest that 8–14 weeks gestation is a critical period for the effects of thyroid hormone on fetal brain development42. Of note, a longitudinal study in 4,615 UK mother–child pairs did not show significant associations between maternal levels of TSH or free T4 levels measured in the first trimester and the standardized test scores of children from the age of 54 months through to 15 years43. However, a 2018 meta-analysis of 26 studies found significant associations between maternal subclinical hypothyroidism or hypothyroxinaemia and measures of child intellectual disability such as low IQ, language delay or global developmental delay (OR 2.14, 95% CI 1.2–3.83, for subclinical hypothyroidism and OR 1.63, 95% CI 1.03–2.56, for hypothyroxinaemia, both compared with children of euthyroid women)44. Although questions remain to be answered, observational data strongly suggest an association between maternal levels of free T4 in early pregnancy and child developmental outcomes.
Levothyroxine treatment for subclinical hypothyroidism and hypothyroxinaemia.
Only a few randomized controlled trials (RCTs) have assessed the benefit of levothyroxine treatment for maternal subclinical hypothyroidism and/or hypothyroxinaemia45. A RCT of 366 TPO antibody-negative pregnant Iranian women with TSH 2.5–10 mIU/l assessed the effects of levothyroxine treatment versus no therapy (randomization at 11–12 weeks gestation) on preterm birth46. Overall, no difference was observed in the prevalence of preterm delivery between treated and untreated women. However, in a sensitivity analysis in which the sample was restricted to women with a baseline TSH of 4–10 mIU/l, levothyroxine treatment did decrease preterm delivery risk (7.3% versus 19%, P = 0.04). A 2019 meta-analysis of seven cohort studies and six RCTs (including women undergoing assisted reproductive technology) showed a 17% decrease in risk of pregnancy loss in women with subclinical hypothyroidism who received levothyroxine treatment (95% CI 0.72–0.97), although heterogeneity was noted in the timing of intervention and the TSH cut-off values used47.
A pair of RCTs in the USA randomized 677 pregnant women with subclinical hypothyroidism (defined as TSH ≥4 mIU/l with normal free T4) and 526 women with hypothyroxinaemia (defined as free T4 <0.86 ng/dl) to either levothyroxine or placebo treatment at a median gestational age of 16.7 weeks48. No significant differences were noted in child neurodevelopmental scores at up to 60 months of age between children of the levothyroxine-treated women and the placebo-treated women. In addition, levothyroxine treatment for subclinical hypothyroidism or hypothyroxinaemia did not alter pregnancy outcomes, including preterm birth, gestational hypertension, pre-eclampsia, gestational diabetes mellitus, stillbirth or miscarriage, low birthweight or respiratory distress syndrome. In this study, levels of TSH were monitored throughout pregnancy and the levothyroxine doses in the treatment groups were adjusted for a goal TSH of 0.1–2.5 mIU/l. In another RCT, the Controlled Antenatal Thyroid Screening Study (CATS), 390 pregnant women with subclinical hypothyroidism or hypothyroxinaemia who were treated with 150 μg daily levothyroxine were compared with 404 untreated pregnant women49. Treatment did not result in significant differences in mean child IQ or the proportion of children with an IQ of <85 at 3 years of age. A follow-up study of these children at 9 years of age again showed no difference in IQ between children of treated and children of untreated mothers50. Of note, the levothyroxine dose employed in this study was high, potentially resulting in over-treatment, although a secondary analysis including only those with high free T4 had similar results. A major limitation of both of these trials is that the intervention was initiated well into the second trimester (median gestational age at randomization was 16.7 weeks in the US study48 and 12.3 weeks in CATS)49,50. As the fetus depends entirely on maternal thyroid hormone during the first trimester, both trials may have missed the critical window for assessing the potential benefits of maternal levothyroxine supplementation.
Overall, limited data from RCTs suggest that levothyroxine treatment in pregnant women with subclinical hypothyroidism, especially those with TSH >4 mIU/l, might decrease risks of preterm delivery and pregnancy loss when initiated in early pregnancy. Levothyroxine treatment has not shown any notable benefit for child neurodevelopmental outcomes, but trials have been limited by initiation of therapy fairly late in gestation. Additional RCTs are needed to determine the effect of levothyroxine treatment on adverse obstetric and child neurodevelopment outcomes, when treatment is initiated in the first trimester.
Thyroid autoimmunity.
Thyroid autoimmunity can occur in the presence or absence of thyroid dysfunction. In regions with adequate iodine nutrition, autoimmune thyroiditis (Hashimoto thyroiditis) is the most common cause of hypothyroidism. TPO and thyroglobulin antibodies are frequently elevated in autoimmune thyroiditis. The prevalence of TPO and thyroglobulin antibody positivity (indicators of thyroid autoimmunity) in pregnant women is estimated to be 5–14% and 3–18%, respectively51. Underlying thyroid autoimmunity seems to have an additive or synergistic effect on miscarriage34 and prematurity36 risk, when combined with maternal subclinical hypothyroidism. Thyroid autoimmunity itself might also have adverse effects in pregnancy, even in the absence of thyroid dysfunction. Meta-analyses of pregnancy in euthyroid women have shown a twofold to fourfold increase in the risk of recurrent miscarriage associated with thyroid autoimmunity52,53. Similarly, euthyroid pregnant women with TPO antibody positivity have been shown to have twofold to threefold increased risks of preterm birth34,39,52. The adverse effects of TPO antibody positivity might be due, at least in part, to increased risk of hypothyroidism as pregnancy progresses54. In a Dutch cohort study, TPO antibody-positive women had a higher risk of premature delivery than TPO antibody-negative women; however, this effect was negated if the TPO antibody-positive women showed an appropriate increase in thyroid hormone synthesis in response to hCG55. Of note, TPO antibody positivity could potentially just be a marker for other forms of autoimmunity and the observed increased rates of pregnancy loss in TPO antibody-positive women could be mediated by the presence of non-organ-specific autoantibodies rather than by thyroid status51.
A few RCTs have assessed the benefits of levothyroxine treatment in euthyroid TPO antibody-positive pregnant women. A RCT of 393 euthyroid pregnant Italian women with positive TPO antibody titres found no significant differences in the rates of miscarriage and preterm delivery between levothyroxine-treated and untreated women. However, 49% of women randomized to the no-treatment group were then treated with levothyroxine owing to a serum level of TSH of >3 mIU/l occurring in the second or third trimester56. Both the TPO antibody-positive levothyroxine-treated and untreated groups had higher rates of miscarriage and preterm delivery than the TPO antibody-negative euthyroid women. Another RCT in 131 TPO antibody-positive women showed a decrease in preterm delivery risk in levothyroxine-treated women compared with untreated women (RR 0.3, 95% CI 0.1–0.85), but only in those with a baseline serum TSH of >4 mIU/l57.
Other studies have explored the impact of levothyroxine treatment initiated before conception. The Pregnancy Outcomes Study in Euthyroid Women with Thyroid Autoimmunity after Levothyroxine trial assessed the benefit of levothyroxine treatment in 565 Chinese TPO-positive euthyroid women who were undergoing in vitro fertilization58. Levothyroxine treatment, initiated before conception and continued through delivery, did not have a significant effect on rates of miscarriage or live birth. Similarly, the Thyroid Antibodies and Levothyroxine trial in 952 euthyroid UK women with positive TPO antibody titres and a history of miscarriage or infertility showed no differences in the rates of live births, pregnancy loss, preterm birth or neonatal outcomes when levothyroxine was initiated before conception59. A 2021 systematic review and meta-analysis of six RCTs assessing the effect of levothyroxine treatment in euthyroid women with thyroid autoimmunity did not find any statistically significant differences in the relative risks of pregnancy achieved, miscarriage, preterm delivery or live birth60.
Current recommendations for screening for hypothyroidism in pregnancy.
There is general agreement that any pregnant woman with symptoms and signs suggestive of overt hypothyroidism should have her thyroid function evaluated, although many symptoms might overlap the normal effects of pregnancy. The presence of underlying risk factors for thyroid dysfunction (BOX 1) could be helpful in determining who should be tested. However, universal screening for thyroid dysfunction in asymptomatic pregnant women is controversial. Such universal screening strategies would detect overt hypothyroidism, for which there is a dear treatment benefit, in about 0.5% of pregnant women. However, universal screening would also uncover far more cases of subclinical hypothyroidism, in about 3.5% of pregnant women, for which the benefits of treatment remain uncertain2. Therefore, there is currently no consensus regarding routine screening for thyroid dysfunction in pregnant women.
Box 1 |. Risk factors for developing thyroid hypofunction in pregnancy.
The risk factors for developing thyroid hypofunction are reviewed in the ATA guidelines6 and can be summarized as follows:
History of thyroid dysfunction
Symptoms or signs of thyroid dysfunction
Presence of a goitre
Known thyroid antibody positivity
Age >30 years
History of type 1 diabetes mellitus or other autoimmune disease
History of head or neck radiation or prior thyroid surgery
Family history of autoimmune thyroid disease or thyroid dysfunction
Presence of morbid obesity (defined as BMI ≥40 kg/m2)
Use of amiodarone, lithium or administration of intravenous contrast agent within the past 3 months
Two or more prior pregnancies
History of pregnancy loss, preterm delivery or infertility
Residing in area of moderate to severe iodine deficiency
The ATA and European Thyroid Association (ETA) both state that insufficient evidence exists to recommend for or against universal TSH screening at the first trimester antenatal visit6,61. By contrast, ACOG and the American Society of Reproductive Medicine recommend against universal screening for thyroid disease in pregnant women13,62. Of note, the ATA does recommend aggressive case-finding strategies to identify pregnant women who are at increased risk for thyroid hypofunction (BOX 1). A retrospective single-centre study has suggested that these case-finding criteria identify fewer pregnant women with subclinical hypothyroidism but a comparable proportion with overt hypothyroidism, compared with a universal screening strategy63. On the other hand, several observational studies and a meta-analysis have suggested that such a case-finding approach, even based on the recommended criteria by the ATA, might miss up to 40–50% of pregnant women with thyroid dysfunction in whom treatment would be recommended64–66.
Recommendations for treatment of hypothyroidism in pregnancy.
Overt hypothyroidism should be treated to prevent adverse effects on pregnancy and child developmental outcomes6,13. Regarding maternal subclinical hypothyroidism, the 2017 ATA guidelines recommend utilizing TPO antibody status along with serum levels of TSH to guide treatment decisions (TABLE 1). Neither ATA nor ACOG currently recommends levothyroxine treatment for isolated hypothyroxinaemia6,13. However, ATA and ACOG do have differences in their recommendations due to differing interpretations of currently available evidence. For example, ACOG does not recommend treatment of subclinical hypothyroidism in pregnancy due to the lack of clear benefit of levothyroxine treatment for child neurodevelopmental outcomes. By contrast, the ATA recommends treatment of subclinical hypothyroidism with levothyroxine, especially in the presence of thyroid autoimmunity. This recommendation is based on the mounting observational evidence regarding increased risk of adverse obstetric outcomes in pregnant women with subclinical hypothyroidism, and data from small trials suggesting a treatment benefit. Given the currently available evidence, and the safety and ease of levothyroxine treatment, the authors agree with the treatment of pregnant women with serum concentrations of TSH that are greater than pregnancy-specific reference ranges or >4 mIU/l, regardless of TPO antibody status.
Table 1 |.
Current US recommendations for treatment of thyroid hypofunction in pregnancy
| Laboratory data | ATA guidelines6 | ACOC guidelines13 |
|---|---|---|
| TPO antibody-negative and TSH >10 mlU/l | Treat with levothyroxine | Treat with levothyroxine only if free T4 is low |
| TPO antibody-positive and TSH greater than the pregnancy-specific range (or >4 mlU/l) | Treat with levothyroxine | Treat with levothyroxine only if free T4 is low |
| TPO antibody-positive and TSH >2.5 mlU/l but less than the pregnancy-specific reference range (or <4 mlU/l) | Consider treatment with levothyroxine | No treatment |
| TPO antibody-negative and TSH greater than the pregnancy-specific range (or >4 mlU/l) but <10 mlU/l | Consider treatment with levothyroxine | Treat with levothyroxine only if free T4 is low |
| Isolated hypothyroxinaemia | No treatment | Not discussed |
ACOG, American College of Obstetricians and Gynecologists; ATA, American Thyroid Association; TPO, thyroid peroxidase; TSH, thyroid stimulating hormone.
During pregnancy, levothyroxine monotherapy should be used for thyroid hormone replacement6,13. The use of liothyronine, levothyroxine–liothyronine combination therapy, or desiccated thyroid is not recommended in pregnancy, as liothyronine does not cross the blood–brain barrier to the fetal brain67. Consequently, liothyronine-containing treatment options might normalize maternal serum levels of TSH but result in inadequate thyroid hormone availability for the fetus. Once levothyroxine therapy is started in a pregnant woman, serum levels of TSH should be measured every 4–6 weeks until mid-gestation, then once in the second and third trimesters for a goal serum level of TSH of <2.5 mIU/l6,13. Most women who are on levothyroxine before conception will require a 25–30% increase in their levothyroxine dose once pregnant, in order to maintain euthyroidism in the setting of the oestrogen-induced increase in serum levels of TBG68,69. Levothyroxine can be decreased back to the pre-pregnancy dose after delivery.
Hyperthyroidism in pregnancy
Aetiology of hyperthyroidism in pregnancy.
Graves disease and gestational transient thyrotoxicosis (GTT) are the two most common causes of hyperthyroidism in pregnancy. The prevalence of new-onset Graves disease in pregnant women is estimated to be 0.05%70, whereas that of GTT ranges from 2% to 11%71,72. As the body’s autoimmune response generally decreases as pregnancy progresses, the incidence of Graves hyperthyroidism decreases in the second and third trimesters after a slight increase in the first trimester73,74. Distinguishing between the two forms of hyperthyroidism seen in pregnancy is important, as the disease course and treatment options differ. GTT is mediated by high serum levels of hCG occurring in early pregnancy. As high levels of hCG are also associated with hyperemesis75,76, GTT is usually associated with symptoms of nausea and/or vomiting. In both GTT and Graves disease, laboratory testing will demonstrate suppressed serum levels of TSH and elevated levels of thyroid hormone. Patients with Graves disease often have an increased ratio of T3 to T4 (≥20:1), as preferential production of T3 occurs in Graves disease. By contrast, serum levels of T3 are often lower in women with GTT in the setting of hyperemesis.
Of note, antibodies specific for the thyrotropin receptor (TRAb) are generally detectable in Graves disease but are absent in GTT70. TRAb can be assessed as TSH receptor-binding immunoglobulin, which measures both stimulating and blocking antibodies without distinction. Alternatively, thyroid-stimulating immunoglobulin can be measured, which specifically measures stimulating TRAb77. Women with Graves disease typically report thyrotoxic symptoms, such as unintentional weight loss, palpitations, hand tremors and heat intolerance, which might have been present prior to pregnancy. By contrast, patients with GTT rarely have severe thyrotoxic symptoms and they lack typical signs of Graves disease such as diffuse goitre and Graves ophthalmopathy. GTT spontaneously resolves as hCG levels decrease after about 10–12 weeks gestation and the condition is not associated with adverse pregnancy outcomes78,79. Therefore, patients with GTT can be treated with supportive measures for nausea, vomiting and any electrolyte imbalance or volume depletion if they have concurrent hyperemesis gravidarum, but these patients do not require antithyroid drug therapy.
Effects of hyperthyroidism on pregnancy outcomes.
Other than GTT, untreated overt hyperthyroidism in pregnancy is associated with maternal and fetal complications including pre-eclampsia, pregnancy loss, maternal and fetal congestive heart failure, maternal thyroid storm, preterm labour, intrauterine growth retardation, low birthweight, and fetal and/or neonatal hyperthyroidism19,21,80,81. However, subclinical hyperthyroidism is not associated with such adverse obstetric outcomes82.
Treatment options for Graves hyperthyroidism in pregnancy.
If treatment of overt hyperthyroidism is indicated, antithyroid drugs or thyroidectomy can be considered in pregnancy. Radioactive iodine ablation is contraindicated during gestation given its teratogenic effects6. Methimazole and propylthiouracil are antithyroid drugs available in the USA and Europe. In Europe, carbimazole (a drug that is rapidly metabolized to methimazole after ingestion) is also available. All antithyroid drugs are equally effective in treating Graves disease, but their adverse effects differ. Methimazole, carbimazole and propylthiouracil can increase serum levels of liver enzymes, which generally normalize after improvement of thyrotoxicosis83 or discontinuation of medication84. A meta-analysis of 15 studies including both non-pregnant adults and pregnant women showed increased odds of elevated levels of liver enzymes associated with propylthiouracil compared with methimazole (OR 2.4, 95% CI 1.16–4.96)85. Propylthiouracil has also been associated with rare but potentially fatal fulminant hepatic failure, including in pregnant women86,87. Thus, methimazole or carbimazole is generally preferred over propylthiouracil in non-pregnant patients.
The risk of teratogenicity must be considered when choosing antithyroid drugs in pregnancy and the preconception period. Previously, only methimazole or carbimazole, but not propylthiouracil, was thought to cause birth defects. However, studies from the past decade have shown that methimazole, carbimazole and propylthiouracil are all teratogenic88–92. A Danish registry study found that 8–9% of children exposed to these antithyroid drugs in utero had birth defects compared with about 5–6% of non-exposed children92. Similarly, a large Korean study found a 7.2% risk of birth defects with antithyroid drug exposure in the first trimester compared with 5.9% without antithyroid drug exposure91. Compared with children of non-exposed women (whose rate of birth defects was 5.94 cases per 1,000 live births), the absolute risk of birth defects was higher in children of women exposed to antithyroid drugs during pregnancy: 8.81, 17.05 and 16.53 cases per 1,000 live births following exposure during the first trimester to propylthiouracil alone, methimazole alone and both propylthiouracil and methimazole, respectively91. A 2020 meta-analysis of seven studies of first-trimester pregnant women also showed a higher risk of birth defects in women exposed to methimazole or carbimazole compared with propylthiouracil (OR 1.29, 95% CI 1.09–1.53)85. Methimazole or carbimazole exposure is associated with more severe birth defects, including aplasia cutis, oesophageal or choanal atresia, and ventricular septal defects, whereas propylthiouracil exposure is associated with less severe defects including cystic malformations of the face or neck, hydronephrosis, and cysts in the urinary tract88. Given these findings, propylthiouracil is the preferred antithyroid drug in the first trimester. If a pregnant woman continues to require antithyroid drugs beyond 16 weeks gestation, it is unclear whether the antithyroid drug should be changed to methimazole, given concern for suboptimal control of hyperthyroidism during dose titration of the new antithyroid drug. If needed, a propylthiouracil to methimazole dose ratio of 20 to 1 can be used to switch6.
In pregnant women with severe hyperthyroidism who cannot tolerate antithyroid drugs, or when hyperthyroidism is not adequately controlled with medical treatment, thyroidectomy can be considered. The second trimester is the safest for thyroidectomy, given the potential risk of teratogenicity from anaesthesia in the first trimester and increased risk of preterm delivery in the third trimester6.
Adequate thyroid hormone is critical for normal fetal development, especially in early pregnancy. Antithyroid drugs cross the placenta and can affect fetal thyroid hormone production once the fetal thyroid becomes functional at mid-gestation. Therefore, it is important to avoid over-treatment of hyperthyroidism in pregnancy. A Japanese study demonstrated that antithyroid drugs might cause fetal hypothyroidism even when maternal thyroid function is normal93. Thus, the ATA recommends targeting a maternal level of free T4 that is high-normal to mildly elevated when treating hyperthyroidism in pregnancy. Furthermore, thyroid function should be monitored every 2–4 weeks until a pregnant woman is on a stable dose of antithyroid drugs, then every 4 weeks thereafter6. Of note, thyroid autoimmunity might improve in the second trimester due to pregnancy-induced immune tolerance, therefore pregnant women might require decreasing doses of antithyroid drugs. The ‘block-and-replace’ strategy, using both antithyroid drugs and levothyroxine, is not recommended in pregnancy, as antithyroid drugs cross the placenta to a much greater extent than levothyroxine and this strategy can lead to fetal hypothyroidism.
TRAb can cross the placenta and cause fetal hyperthyroidism and goitre when present in high levels. Furthermore, TRAb titres can remain elevated for years after thyroidectomy or radioactive iodine ablation of the thyroid gland. Therefore, all pregnant women with active Graves hyperthyroidism or a history of ablative treatment for Graves disease should have serum TRAb titres measured in the first trimester. If the TRAb titre is more than 3 times the upper limit of the reference range, the measurement should be made again at 18–22 weeks gestation when the fetal thyroid gland fully matures. When the TRAb titre remains elevated above 3 times the upper limit of the reference range by mid-gestation, consultation with maternal-fetal medicine and neonatology is warranted to monitor for fetal and neonatal hyperthyroidism and goitre.
Preconception counselling and treatment options for women with Graves disease.
Women of reproductive age who are diagnosed with Graves disease should be counselled regarding plans for pregnancy, given the potential adverse effects of both antithyroid drugs and uncontrolled overt hyperthyroidism in pregnancy. Pregnancy should be postponed until women are euthyroid. Propylthiouracil might be preferred over methimazole in the preconception setting to avoid methimazole exposure during the period of organogenesis in early pregnancy6. In some women, antithyroid drugs can be discontinued as soon as pregnancy is confirmed, with close thyroid function monitoring undertaken, in the hope that hyperthyroidism will not recur until the second trimester when the potential teratogenic effects of antithyroid drugs are no longer a concern. This strategy is appropriate only in carefully selected women who require a low dose of antithyroid drugs to maintain euthyroidism (up to 5–10 mg of methimazole a day or 100–200 mg of propylthiouracil a day), have been treated with antithyroid drugs for at least 6 months, do not have Graves ophthalmopathy and have a low or negative TRAb titre6.
If women prefer to avoid antithyroid drug use during pregnancy, definitive therapy with thyroidectomy or radioactive iodine ablation can be considered before conception. Serum levels of TRAb generally decrease within a year after thyroidectomy, but can transiently increase and remain elevated for years after radioactive iodine treatment94. After definitive treatment, it is prudent to avoid conception until women are euthyroid on a stable dose of levothyroxine. Pregnancy should be avoided for 6 months after radioactive iodine treatment95.
Iodine nutrition in pregnancy
Iodine requirements in pregnancy
Iodine is an essential micronutrient that is required for thyroid hormone production. Iodine requirements increase in pregnancy due to several factors. First, increased demand occurs for maternal thyroid hormone production owing to the thyrotrophic effects of hCG and oestrogen-mediated increases in TBG. Second, iodine is transferred to the fetus, especially after the fetal thyroid gland matures at 18–20 weeks gestation. Third, increases in renal clearance of iodine occur, owing to increased blood volume and glomerular filtration in pregnancy96 (FIG. 1). Thus, increased iodine intake from 150 μg per day in non-pregnant adults to 220–250 μg per day during pregnancy is recommended97,98 (TABLE 2).
Table 2 |.
Recommended daily iodine intakes by life stage
| Life stage | Iodine intake (μg per day) |
|---|---|
| US Institute of Medicine 97 | |
| Age 0–6 months | 110 |
| Age 7–12 months | 130 |
| Age 1–8 years | 90 |
| Age 9–13 years | 120 |
| Age >13 years | 150 |
| During pregnancy | 220 |
| During lactation | 290 |
| WHO 98 | |
| Age 0–5 years | 90 |
| Age 6–12 years | 120 |
| Age >12 years | 150 |
| During pregnancy | 250 |
| During lactation | 250 |
Spot urinary iodine concentrations (UIC) can be used to assess iodine status at the population level99,100. A median UIC of 150–249 μg/l is consistent with adequate iodine status99 (TABLE 3). Currently, no biomarker exists for chronic iodine nutritional status at the individual level, as UIC only reflects recent (past 6–8 h) iodine intake101.
Table 3 |.
Median urinary iodine concentration ranges for assessment of population-level iodine status
| Croup for assessment | Median UIC (μg/l) | Iodine status |
|---|---|---|
| School-age children or non-pregnant, non-lactating adults | <20 | Severely insufficient |
| 20–49 | Moderately insufficient | |
| 50–99 | Mildly insufficient | |
| 100–299 | Adequate | |
| ≥300 | Excessive | |
| Pregnant women | <150 | Insufficient |
| 150–249 | Adequate | |
| 250–499 | More than adequate | |
| ≥500 | Excessive |
Epidemiology of iodine deficiency
Given the increased iodine requirements in gestation, pregnant women might be at risk of iodine deficiency even when the general population is iodine-sufficient102. Only one-third of the 31 European countries with available data report adequate iodine intake in pregnancy103. In addition, pregnant women in the USA. are currently considered mildly iodine-deficient, with a median UIC of 144 μg/l in assessments performed during 2007–2014 (REFS104,105). A fourfold increase was noted in the proportion of US women of childbearing age with UIC <50 μg/l in assessments performed during 2005–2010 compared with prior years104,106,107.
Severe iodine deficiency in pregnancy
Severe iodine deficiency in pregnancy can cause maternal hypothyroidism. Severe iodine deficiency has been associated with increased risks of miscarriage, premature birth and stillbirth108,109. Chronically low iodine intakes of <50 μg per day might also lead to maternal and fetal goitre110,111. Iodine intakes of <25 μg per day in pregnancy could lead to children being born with cretinism, characterized by severe intellectual disability, deaf-mutism and stunted growth112,113. Meta-analyses have shown that children born to women with severe iodine deficiency have an IQ lower by 7.4 to 12 points compared with children born to iodine-sufficient women114,115.
Mild-moderate iodine deficiency in pregnancy
Many observational studies have found associations between mild-to-moderate iodine deficiency in pregnancy and risk of miscarriage116, preterm birth117–119, pre-eclampsia118, low birthweight117,118, and neonatal intensive care unit admission119. However, these findings have not been universal120. The effects of maternal mild-to-moderate iodine deficiency on child neurodevelopment are unclear. Observational studies have shown lower verbal IQ and reading skills at 8 years of age121 and lower language skills assessment score at 9 years of age122 in children born to mothers with UIC <150 μg/g creatinine or 150 μg/l compared with children born to mothers with higher UIC levels. Other studies have shown that children born to mothers with maternal mild-to-moderate iodine deficiency show poorer working memory at 4 years of age123, lower scores in psychomotor development assessment at 12 months of age124, lower verbal IQ at 6–12 years of age125, and lower cognitive, language and motor scores in children at 18 months of age126, compared with those born to iodine-sufficient mothers. A 2019 meta-analysis of individual data from three population-based cohorts including 6,180 mother-child pairs also showed associations between maternal UIC <150 μg/g creatinine or ≥500 μg/g creatinine with decreased verbal IQ scores assessed at 1.5–8 years of age127. However, other observational studies have failed to show notable associations between maternal iodine deficiency and child IQ127,128. The few available RCTs assessing iodine supplementation for women with mild-to-moderate iodine deficiency in pregnancy have not shown a clear benefit for child neurodevelopmental outcomes. Of note, these studies have been limited by non-randomized or inadequately powered designs, initiation of supplements too late in pregnancy or by being conducted in areas of only borderline iodine deficiency129,130.
Current recommendations for iodine intake
Universal salt iodization is considered the most effective way to improve iodine status at the population level131. However, salt iodization has not been mandated in many countries including the USA and much of Europe. Therefore, many societies, including the ATA6, the Endocrine Society132, the US Teratology Society133, the American Academy of Pediatrics134 and the ETA61, currently recommend that women who are planning to be pregnant, are pregnant or are breastfeeding should take a daily oral supplement containing 150 μg of iodine.
Thyroid nodules and cancer in pregnancy
Thyroid nodules in pregnancy
The estimated prevalence of thyroid nodules in pregnant women ranges from 3% to 29%, depending on iodine intake6·7. Older age, increased number of previous pregnancies, and low or excessive iodine nutritional status are associated with an increased risk of thyroid nodules in pregnancy135,136. A prospective cohort study of 221 pregnant Chinese women found a 15% prevalence of thyroid nodules ≥2 mm in the first trimester, with a median increase of 5 mm in nodule size occurring during pregnancy135. Nodule size might increase during gestation due to the thyrotrophic effects of hCG and due to TSH stimulation resulting from depletion of iodine stores137,138. In addition to the structural similarity between hCG and TSH, structural similarities also exist between hCG and TSH receptors5. Consequently, high serum levels of hCG can potentially stimulate TSH receptors on the thyroid gland; this effect can lead to the development of thyroid nodules or gland hyperplasia, in addition to increased thyroid hormone production.
Thyroid ultrasonography is the best imaging modality for the evaluation of thyroid nodules found in pregnant women. This modality can also be used for patient risk stratification using the same characteristics as in non-pregnant adults. Using pregnancy-specific reference ranges, serum levels of TSH should be checked in pregnant women with thyroid nodules to screen for hyperfunctioning nodules, which are unlikely to be malignant. Of note, nuclear thyroid scans using radioactive iodine tracer are contraindicated in pregnancy. In pregnant women with thyroid nodules, a serum level of TSH that remains suppressed beyond 16 weeks gestation suggests the presence of a toxic adenoma (that is, a single hyperfunctioning thyroid nodule) and fine needle aspiration (FNA) biopsy can be delayed until nuclear scanning is feasible after delivery. If toxic adenoma is unlikely and the nodule meets criteria for cytological evaluation, FNA biopsy of thyroid nodule(s) can be safely performed in pregnancy138. Nodules that are cytologically benign can be managed in the same way as in non-pregnant adults. If cytology shows indeterminate results, including atypia of unknown significance or follicular lesion of unknown significance, FNA biopsy can be repeated for further evaluation. Molecular testing is not currently recommended in pregnancy, as it is not yet clear how pregnancy affects the results of molecular markers138. For indeterminate nodules without characteristics suggestive of an aggressive cancer, repeat FNA biopsy can be deferred until after pregnancy.
Thyroid cancer in pregnancy and postpartum
Although the prevalence of thyroid cancer in pregnant women is difficult to estimate, the incidence of thyroid cancer in young adults has been increasing in the last two to three decades, especially in young women of reproductive age8–10. A large retrospective study using the 1991–1999 California Cancer Registry showed that thyroid cancer was the second most common cancer in the perinatal period among all cancers recorded in the registry, with a prevalence of 14.4%. This study showed 3.3 occurrences of thyroid cancer per 100,000 cancer cases found within 9 months before pregnancy, 3 occurrences of thyroid cancer per 100,000 cancer cases found at delivery, and 10.8 occurrences of thyroid cancer per 100,000 cancer cases found within 1 year of delivery139. An assessment of 595 women diagnosed with thyroid cancer between 9 months before to 12 months after delivery from the same cohort in 1991–1995 did not show any statistically significant differences in maternal or neonatal outcomes compared with pregnant women without thyroid cancer140. In addition, no difference was observed in survival between women with thyroid cancer diagnosed in the perinatal period and non-pregnant women with thyroid cancer.
Given the overall good prognosis of differentiated thyroid cancer (DTC) in young women and the potential anxiety associated with a new cancer diagnosis141, FNA biopsy of many thyroid nodules can be deferred until after pregnancy, if the patient prefers. Small studies have shown that delaying diagnosis and treatment of thyroid cancer until after pregnancy does not result in worse outcomes. For example, no differences were observed in long-term survival142, changes in tumour volume143,144 or development of lymph node metastases143,144 between those who were treated during pregnancy and those who deferred treatment. Therefore, the ATA recommends that most pregnant women with newly diagnosed DTC can be monitored conservatively with serial thyroid ultrasonography during pregnancy. If evidence is found of notable tumour growth by mid-gestation or development of metastatic disease, thyroid surgery can be considered. When required, thyroidectomy is safest in the second trimester138. No evidence is available to support the use of suppressive therapy with levothyroxine when thyroid cancer surgery is deferred.
In a small study, stimulation of TSH and oestrogen receptors by hCG in pregnancy has been implicated in increased tumour aggressiveness in DTC145. Two Italian studies found an increased risk of persistent or recurrent disease in patients when DTC is diagnosed during pregnancy145,146. However, both of these studies defined recurrent or persistent disease based on serum levels of thyroglobulin only, without evaluation for structural disease. Furthermore, neither study showed an increased risk of recurrent or persistent disease when surgery was delayed until after pregnancy. In light of current evidence regarding long-term outcomes in patients with DTC diagnosed in pregnancy, we agree with the current recommendation by the ATA for diagnosis and treatment of DTC diagnosed in pregnancy as described above.
Studies assessing the potential effects of pregnancy on anaplastic or medullary thyroid cancer are lacking. However, thyroid surgery in pregnancy should be strongly considered given the aggressive nature of these cancers.
If radioactive iodine ablation is indicated as an adjunctive treatment for thyroid cancer, it should be delayed until after delivery. Iodine is taken up into the lactating mammary gland via the sodium iodide symporter147. As such, women should be counselled to stop breastfeeding at least 6 weeks to 3 months prior to I131 radioactive iodine treatment in order to prevent potential adverse effects of radiation on breast tissues and breastfed infants95.
Those with a history of thyroid cancer
Having a history of thyroid cancer should not deter patients from pursuing pregnancy. A large Korean study utilizing national insurance data from 2006–2014 showed no statistically significant increase in adverse pregnancy outcomes including caesarean section, preterm birth, pre-eclampsia, placental abruption, placental previa, stillbirth, low birthweight and large for gestational age, in women with a history of thyroid cancer148. In a study of 235 pregnant women in the USA with a history of DTC, disease recurrence and progression were monitored using imaging (ultrasonography, CT scan or radioactive iodine scans), serum levels of thyroglobulin and thyroglobulin antibody, and clinical evaluation within 12 months before and after pregnancy149. Women who had an excellent, indeterminate or biochemical incomplete response to treatment per the 2015 ATA guidelines138 prior to pregnancy had no evidence of structural recurrence or progression of thyroid cancer on evaluation at 3–12 months after delivery, although a small number of women had an increase in serum levels of thyroglobulin and thyroglobulin antibody. By contrast, 29% of women who had a structural incomplete response to therapy prior to pregnancy had tumour progression, defined as a ≥3 mm increase in the size of pre-existing disease or identification of a new metastasis, at postpartum evaluation. Therefore, women with a response to therapy classified as excellent or indeterminate according to ATA guidelines prior to pregnancy do not require increased surveillance, but those with an incomplete response before pregnancy should be closely monitored during gestation. As subclinical hyperthyroidism is not associated with adverse obstetric outcomes82, serum TSH goals for cancer survivors are the same in pregnant women as in non-pregnant adults138. Most women will require an increase in their levothyroxine dose by 25–30% during pregnancy to maintain TSH targets, due to increased TBG levels.
Postpartum thyroiditis
Epidemiology
Postpartum thyroiditis is a destructive thyroiditis that occurs in 5–8% of women within the first 12 months after delivery150. The aetiology is thought to be a rebound of underlying thyroid autoimmunity occurring after the immune tolerance induced by pregnancy has ended. This mechanism is proposed because postpartum thyroiditis is more commonly seen in women who are positive for TPO antibodies151 or those with a personal or family history of autoimmune diseases152. Postpartum thyroiditis can also occur after miscarriage or pregnancy loss, as well as in women on levothyroxine replacement for underlying Hashimoto thyroiditis153.
Disease course
Postpartum thyroiditis classically presents as thyrotoxicosis within 4–8 weeks of delivery, due to release of preformed thyroid hormones from an inflamed thyroid gland. This thyrotoxic phase typically lasts for 1–2 months and can be followed by transient hypothyroidism lasting 4–6 months until the thyroid gland recovers (FIG. 2). However, the disease course can vary; ~25% of women only have a thyrotoxic phase and 50% of women only have a hypothyroid phase150. The majority of women recover completely and return to a euthyroid state within a year, but up to 50% will develop persistent hypothyroidism even after initial recovery154,155. Women who are positive for serum TPO antibodies are at increased risk of developing permanent hypothyroidism after postpartum thyroiditis155. Postpartum thyroiditis recurs after up to 70% of subsequent pregnancies150.
Fig. 2 |. Time course of postpartum thyroiditis.
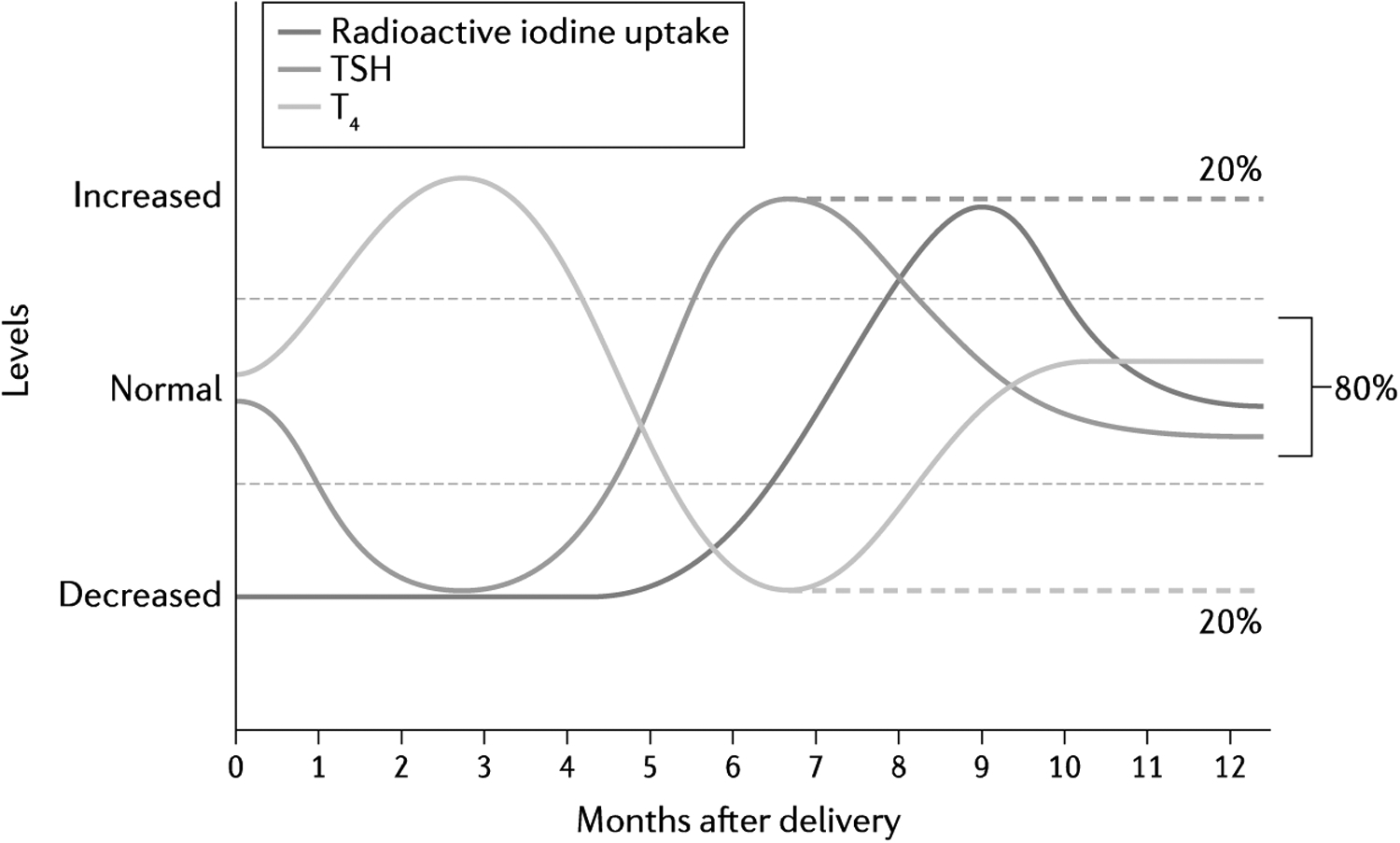
Typical time course in changes in serum levels of thyroid stimulating hormone (TSH), T4 and radioactive iodine uptake in postpartum thyroiditis up to 12 months after delivery is depicted. Transient thyrotoxicosis is generally followed by transient hypothyroidism, before full recovery to euthyroidism. However, about 20% of women with postpartum thyroiditis might develop permanent hypothyroidism (as indicated by the dashed lines).
Evaluation and treatment
Distinguishing the thyrotoxic phase of postpartum thyroiditis from new or recurrent Graves disease is important, as treatment options differ. Antibody testing for TRAb is helpful, as it is a highly specific and sensitive biomarker for Graves disease. By contrast, serum levels of TPO antibody can be elevated in both postpartum thyroiditis and Graves hyperthyroidism156. The ratio of T3 to T4 is often higher (>20:1) in Graves disease due to preferential T3 production157. Graves disease most frequently presents at 6–12 months after delivery, compared with a presentation at 1–4 months after delivery for postpartum thyroiditis152. Of note, I123 radioactive iodine uptake and scan can be employed to differentiate postpartum thyroiditis from Graves disease if needed. Iodine uptake is low in the thyrotoxic phase of postpartum thyroiditis, whereas it is elevated in Graves disease. However, TRAb measurement is usually adequate to differentiate between the two entities.
As the thyrotoxic phase of postpartum thyroiditis is transient and results from the release of preformed hormones, rather than increased thyroid hormone synthesis, antithyroid drugs are not useful. Notable thyrotoxic symptoms, such as palpitations or anxiety, can be managed with β-adrenergic agonists that are safe for use during lactation (for example, metoprolol or propranolol). Levothyroxine can be employed for the hypothyroid phase if women are symptomatic or are actively trying to conceive. Around 6–12 months after diagnosis, levothyroxine treatment can be tapered as hypothyroidism is usually transient. However, given the increased risk of recurrent hypothyroidism in women who recover from postpartum thyroiditis, these women should have yearly monitoring of TSH and should be monitored for recurrent postpartum thyroiditis after subsequent pregnancies. Selenium supplementation has been explored for its possible anti-inflammatory effect in autoimmune thyroiditis51. However, trials of selenium supplementation in pregnancy have shown mixed results158. Thus, selenium supplementation for prevention or treatment of postpartum thyroiditis is currently not recommended6.
Conclusions
Thyroid diseases are fairly common in pregnancy and their management presents specific challenges. Due to physiological changes in pregnancy, pregnancy-specific reference ranges should be used to assess thyroid function in pregnant women. Given the importance of normal thyroid function for maternal and child health, both maternal and fetal outcomes should be considered in making management decisions for treatment of thyroid dysfunction in pregnancy. Similarly, the evaluation and treatment of thyroid nodules or thyroid cancer might require special considerations in pregnancy. Thyroid dysfunction is also frequent in the immediate postpartum period.
Many questions related to the care of pregnant women with thyroid disorders remain unanswered. Levothyroxine treatment of maternal subclinical hypothyroidism and hypothyroxinaemia initiated in the second trimester has not shown any benefit; however, it is not known whether levothyroxine treatment initiated earlier in gestation would improve adverse obstetric and child neurodevelopmental outcomes. The mechanism for the observed increased rates of pregnancy loss in TPO-positive euthyroid pregnant women remains unknown; future studies will be needed to determine whether there is an effective therapy for these women. The safest approach to antithyroid drug use for overt hyperthyroidism during gestation is also not entirely clear. Although iodine supplementation for women in mildly-to-moderately iodine-deficient regions is now widely recommended, clinical trials will be needed to determine whether this supplementation improves child developmental outcomes. Furthermore, the appropriate dose and timing of supplementation remains to be elucidated. Regarding thyroid cancer, we do not fully understand the effects of pregnancy on the more aggressive types, such as anaplastic or medullary thyroid cancer. Finally, it is not known whether any intervention could prevent progression of postpartum thyroiditis to permanent hypothyroidism. Additional studies are needed to answer these questions and to further improve the care of women with thyroid disease during gestation and the postpartum period.
Key points.
Serum levels of thyroid stimulating hormone might be lower in pregnant women during early gestation than levels outside the pregnancy setting, due to increased production of thyroid hormone and stimulation from high levels of human chorionic gonadotropin.
Levothyroxine treatment for maternal subclinical hypothyroidism in pregnancy remains controversial because currently available studies have not shown a clear benefit of treatment on obstetric or child neurodevelopmental outcomes.
Treatment of Graves disease in pregnancy requires careful consideration of the adverse effects of uncontrolled hyperthyroidism, antithyroid drugs and overtreatment of hyperthyroidism on pregnancy outcomes.
Women who are planning to be pregnant, are pregnant or are breastfeeding should take a daily oral supplement containing 150 μg of iodine to prevent adverse effects of iodine deficiency.
Treatment of differentiated thyroid cancer diagnosed in pregnancy can be safely deferred until after pregnancy with close monitoring for any tumour size increase or spread during pregnancy.
Postpartum thyroiditis is a destructive thyroiditis that typically presents with thyrotoxicosis followed by transient hypothyroidism up to the first 12 months after childbirth.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Taylor PN et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol 14, 301–316 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Dong AC & Stagnaro-Green A Differences in diagnostic criteria mask the true prevalence of thyroid disease in pregnancy: a systematic review and meta-analysis. Thyroid 29, 278–289 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Burrow GN, Fisher DA & Larsen PR Maternal and fetal thyroid function. N. Engl. J. Med 331, 1072–1078 (1994). [DOI] [PubMed] [Google Scholar]
- 4.Stagnaro-Green A Approach to the patient with postpartum thyroiditis. J. Clin. Endocrinol. Metab 97, 334–342 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura M & Hershman JM Thyrotropic action of human chorionic gonadotropin. Thyroid 5, 425–434 (1995). [DOI] [PubMed] [Google Scholar]
- 6.Alexander EK et al. 2017 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 27, 315–389 (2017). [DOI] [PubMed] [Google Scholar]; This paper describes the most current ATA guideline on diagnosis and management of thyroid disease in pregnancy and the postpartum period.
- 7.Ollero MD et al. Thyroid function reference values in healthy iodine-sufficient pregnant women and influence of thyroid nodules on thyrotropin and free thyroxine values. Thyroid 29, 421–429 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Lim H, Devesa SS, Sosa JA, Check D & Kitahara CM Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 317, 1338–1348 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Gosnell JE & Roman SA Geographic influences in the global rise of thyroid cancer. Nat. Rev. Endocrinol 16, 17–29 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Miller KD et al. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin 70, 443–459 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Glinoer D et al. Regulation of maternal thyroid during pregnancy. J. Clin. Endocrinol. Metab 71, 276–287 (1990). [DOI] [PubMed] [Google Scholar]
- 12.Li C et al. Assessment of thyroid function during first-trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? J. Clin. Endocrinol. Metab 99, 73–79 (2014). [DOI] [PubMed] [Google Scholar]
- 13.[No authors listed] Thyroid disease in pregnancy: ACOG Practice Bulletin, Number 223. Obstet. Gynecol 135, e261–e274 (2020). [DOI] [PubMed] [Google Scholar]; This paper describes the most recent ACOG recommendations on the diagnosis and management of thyroid disease in pregnancy.
- 14.Krassas GE, Poppe K & Glinoer D Thyroid function and human reproductive health. Endocr. Rev 31, 702–755 (2010). [DOI] [PubMed] [Google Scholar]; This is a comprehensive review on the interactions between thyroid function and human reproduction.
- 15.Lee RH et al. Free T4 immunoassays are flawed during pregnancy. Am. J. Obstet. Gynecol 200, 260.e1–250.e6 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Gronowski AM Evaluation of thyroid function during pregnancy: have we taken a wrong turn? Clin. Chem 64, 439–441 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Hernández JM et al. Reference intervals of thyroid function tests assessed by immunoassay and mass spectrometry in healthy pregnant women living in Catalonia. J. Clin. Med 10, 2444 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weeke J et al. A longitudinal study of serum TSH, and total and free iodothyronines during normal pregnancy. Acta Endocrinol. 101, 531–537 (1982). [DOI] [PubMed] [Google Scholar]
- 19.Davis LE, Leveno KJ & Cunningham FG Hypothyroidism complicating pregnancy. Obstet. Gynecol 72, 108–112(1988). [PubMed] [Google Scholar]
- 20.Leung AS, Millar LK, Koonings PP, Montoro M & Mestman JH Perinatal outcome in hypothyroid pregnancies. Obstet. Gynecol 81, 349–353 (1993). [PubMed] [Google Scholar]
- 21.Männistö T et al. Thyroid diseases and adverse pregnancy outcomes in a contemporary US cohort. J. Clin. Endocrinol. Metab 98, 2725–2733 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howdeshell KL A model of the development of the brain as a construct of the thyroid system. Environ. Health Perspect 110, 337–348 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernal J, Guadaño-Ferraz A & Morte B Perspectives in the study of thyroid hormone action on brain development and function. Thyroid 13, 1005–1012 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Moog NK et al. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 342, 68–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Escobar GM, Obregón MJ & del Rey FE Maternal thyroid hormones early in pregnancy and fetal brain development. Best. Pract. Res. Clin. Endocrinol. Metab 18, 225–248 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Man EB, Jones WS, Holden RH & Mellits ED Thyroid function in human pregnancy. 8. Retardation of progeny aged 7 years; relationships to maternal age and maternal thyroid function. Am. J. Obstet. Gynecol 111, 905–916 (1971). [PubMed] [Google Scholar]
- 27.Man EB & Serunian SA Thyroid function in human pregnancy. IX. Development or retardation of 7-year-old progeny of hypothyroxinemic women. Am. J. Obstet. Gynecol 125, 949 (1976). [PubMed] [Google Scholar]
- 28.Haddow JE et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med 341, 549–555 (1999). [DOI] [PubMed] [Google Scholar]
- 29.Cleary-Goldman J et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet. Gynecol 112, 85–92 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Männistö T et al. Perinatal outcome of children born to mothers with thyroid dysfunction or antibodies: a prospective population-based cohort study. J. Clin. Endocrinol. Metab 94, 772–779 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Männistö T et al. Thyroid dysfunction and autoantibodies during pregnancy as predictive factors of pregnancy complications and maternal morbidity in later life. J. Clin. Endocrinol. Metab 95, 1084–1094 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Sheehan PM, Nankervis A, Araujo Júnior E & Da Silva Costa F Maternal thyroid disease and preterm birth: systematic review and meta-analysis. J. Clin. Endocrinol. Metab 100, 4325–4331 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Negro R et al. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J. Clin. Endocrinol. Metab 95, E44–E48 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Liu H et al. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study. Thyroid 24, 1642–1649 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casey BM et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet. Gynecol 105, 239–245 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Korevaar TIM et al. Hypothyroxinemia and TPO-antibody positivity are risk factors for premature delivery: the generation R study. J. Clin. Endocrinol. Metab 98, 4382–4390 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Maraka S et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 26, 580–590 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This systematic review and meta-analysis summarizes results of randomized trials and cohort studies on the effects of subclinical hypothyroidism in pregnancy published up to 2015.
- 38.Lee SY, Cabral HJ, Aschengrau A & Pearce EN Associations between maternal thyroid function in pregnancy and obstetric and perinatal outcomes. J. Clin. Endocrinol. Metab 105, e2015–e2023 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Consortium on Thyroid and Pregnancy — Study Group on Preterm Birth, et al. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA 322, 632–641 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study assesses risk of preterm birth associated with maternal thyroid hypofunction and thyroid autoimmunity using individual data from 47,045 pregnant women from 19 cohort studies.
- 40.Ramezani Tehrani F, Nazarpour S & Behboudi-Gandevani S Isolated maternal hypothyroxinemia and adverse pregnancy outcomes: a systematic review. J. Gynecol. Obstet Hum. Reprod 50, 102057 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Korevaar TIM et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol. 4, 35–43 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Jansen TA et al. Maternal thyroid function during pregnancy and child brain morphology: a time window-specific analysis of a prospective cohort. Lancet Diabetes Endocrinol. 7, 629–637 (2019). [DOI] [PubMed] [Google Scholar]; This study assessed offspring brain morphology by MRI at a median age of 10 years in 1,981 mother-child pairs with maternal thyroid function measurements during pregnancy.
- 43.Nelson SM et al. Maternal thyroid function and child educational attainment: prospective cohort study. BMJ 360, k452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson W et al. Maternal thyroid hormone insufficiency during pregnancy and risk of neurodevelopmental disorders in offspring: a systematic review and meta-analysis. Clin. Endocrinol 88, 575–584 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto JM, Benham JL, Nerenberg KA & Donovan LE Impact of levothyroxine therapy on obstetric, neonatal and childhood outcomes in women with subclinical hypothyroidism diagnosed in pregnancy: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 8, e022837 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nazarpour S et al. Effects of levothyroxine on pregnant women with subclinical hypothyroidism, negative for thyroid peroxidase antibodies. J. Clin. Endocrinol. Metab 103, 926–935 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Nazarpour S, Ramezani Tehrani F, Amiri M, Bidhendi Yarandi R &Azizi F Levothyroxine treatment and pregnancy outcomes in women with subclinical hypothyroidism: a systematic review and meta-analysis. Arch. Gynecol. Obstet 300,805–819 (2019). [DOI] [PubMed] [Google Scholar]; This systematic review and meta-analysis summarizes results of 13 randomized controlled trials and cohort studies on the effects of levothyroxine treatment for maternal subclinical hypothyroidism on pregnancy outcomes.
- 48.Casey BM et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N. Engl. J. Med 376, 815–825 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This US randomized controlled trial assessed the effects of levothyroxine treatment of maternal thyroid hypofunction on pregnancy outcomes and child neurodevelopmental outcomes at a median age of 5 years.
- 49.Lazarus JH et al. Antenatal thyroid screening and childhood cognitive function. N. Engl. J. Med 366, 493–501 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; This UK randomized controlled trial assessed the effects of levothyroxine treatment of maternal thyroid hypofunction on child neurodevelopmental outcomes at 3 years of age.
- 50.Hales C et al. Controlled antenatal thyroid screening II: effect of treating maternal suboptimal thyroid function on child cognition. J. Clin. Endocrinol. Metab 103, 1583–1591 (2018). [DOI] [PubMed] [Google Scholar]
- 51.De Leo S & Pearce EN Autoimmune thyroid disease during pregnancy. Lancet Diabetes Endocrinol. 6, 575–586 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Thangaratinam S et al. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ 342, d2616 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie J et al. Effect of antithyroid antibodies on women with recurrent miscarriage: a meta-analysis. Am. J. Reprod. Immunol 83, e13238 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glinoer D, Riahi M, Grüm JP & Kinthaert J Risk of subclinical hypothyroidism in pregnant women with asymptomatic autoimmune thyroid disorders. J. Clin. Endocrinol. Metab 79, 197–204 (1994). [DOI] [PubMed] [Google Scholar]
- 55.Korevaar TIM et al. Thyroid autoimmunity impairs the thyroidal response to human chorionic gonadotropin: two population-based prospective cohort studies. J. Clin. Endocrinol. Metab 102, 69–77 (2017). [DOI] [PubMed] [Google Scholar]; This cohort study showed a suboptimal thyroidal response to hCG stimulation in pregnant women with positive TPO antibody status.
- 56.Negro R, Schwartz A & Stagnaro-Green A Impact of levothyroxine in miscarriage and preterm delivery rates in first trimester thyroid antibody-positive women with TSH less than 2.5 mIU/L. J. Clin. Endocrinol. Metab 101, 3685–3690 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Nazarpour S et al. Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur. J. Endocrinol 176, 253–265 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Wang H et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA 318, 2190–2198 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Dhillon-Smith RK et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N. Engl. J. Med 380, 1316–1325 (2019). [DOI] [PubMed] [Google Scholar]; This UK randomized controlled trial assessed the effects of levothyroxine treatment starting preconception for euthyroid women with positive TPO antibodies and a history of miscarriage or infertility on live birth rates.
- 60.Lau L, Benham JL, Lemieux P, Yamamoto J & Donovan LE Impact of levothyroxine in women with positive thyroid antibodies on pregnancy outcomes: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 11, e043751 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lazarus J et al. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur. Thyroid. J 3, 76–94 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil. Steril 104, 545–553 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Sitoris G et al. Screening for thyroid dysfunction in pregnancy with targeted high-risk case finding: can it be improved? J. Clin. Endocrinol. Metab 104, 2346–2354 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Pop VJ, Broeren MA, Wiersinga WM & Stagnaro-Green A Thyroid disease symptoms during early pregnancy do not identify women with thyroid hypofunction that should be treated. Clin. Endocrinol 87, 838–843 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Rosario PW Selective screening for thyroid dysfunction in pregnant women: how often do low-risk women cease to be treated following the new guidelines of the American Thyroid Association? Arch. Endocrinol. Metab 62, 641–643 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jouyandeh Z, Hasani-Ranjbar S, Qorbani M & Larijani B Universal screening versus selective case-based screening for thyroid disorders in pregnancy. Endocrine 48, 116–123 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Calvo R, Obregón MJ, Ruiz de Oña C, Escobar del Rey F & Morreale de Escobar G Congenital hypothyroidism, as studied in rats. Crucial role of maternal thyroxine but not of 3,5,3′-triiodothyronine in the protection of the fetal brain. J. Clin. Invest 86, 889–899 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arafah BM Increased need for thyroxine in women with hypothyroidism during estrogen therapy. N. Engl. J. Med 344, 1743–1749 (2001). [DOI] [PubMed] [Google Scholar]
- 69.Yassa L, Marqusee E, Fawcett R & Alexander EK Thyroid hormone early adjustment in pregnancy (the THERAPY) trial. J. Clin. Endocrinol. Metab 95, 3234–3241 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Cooper DS & Laurberg P Hyperthyroidism in pregnancy. Lancet Diabetes Endocrinol. 1, 238–249 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Glinoer D The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr. Rev 18, 404–433 (1997). [DOI] [PubMed] [Google Scholar]
- 72.Yeo CP et al. Prevalence of gestational thyrotoxicosis in Asian women evaluated in the 8th to 14th weeks of pregnancy: correlations with total and free beta human chorionic gonadotrophin. Clin. Endocrinol 55, 391–398 (2001). [DOI] [PubMed] [Google Scholar]
- 73.Andersen SL, Olsen J, Carlé A & Laurberg P Hyperthyroidism incidence fluctuates widely in and around pregnancy and is at variance with some other autoimmune diseases: a Danish population-based study. J. Clin. Endocrinol. Metab 100, 1164–1171 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Laurberg P & Andersen SL Endocrinology in pregnancy: pregnancy and the incidence, diagnosing and therapy of Graves’ disease. Eur. J. Endocrinol 175, R219–R230 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Goodwin TM, Montoro M, Mestman JH, Pekary AE & Hershman JM The role of chorionic gonadotropin in transient hyperthyroidism of hyperemesis gravidarum. J. Clin. Endocrinol. Metab 75, 1333–1337 (1992). [DOI] [PubMed] [Google Scholar]
- 76.Goodwin TM, Montoro M & Mestman JH Transient hyperthyroidism and hyperemesis gravidarum: clinical aspects. Am. J. Obstet. Gynecol 167, 648–652 (1992). [DOI] [PubMed] [Google Scholar]
- 77.Kahaly GJ, Diana T & Olivo PD TSH receptor antibodies: relevance & utility. Endocr. Pract 26, 97–106 (2020). [DOI] [PubMed] [Google Scholar]
- 78.Bouillon R et al. Thyroid function in patients with hyperemesis gravidarum. Am. J. Obstet. Gynecol 143, 922–926(1982). [DOI] [PubMed] [Google Scholar]
- 79.Kinomoto-Kondo S et al. The effects of gestational transient thyrotoxicosis on the perinatal outcomes: a case-control study. Arch. Gynecol. Obstet 295, 87–93 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Sheffield JS & Cunningham FG Thyrotoxicosis and heart failure that complicate pregnancy. Am. J. Obstet. Gynecol 190, 211–217 (2004). [DOI] [PubMed] [Google Scholar]
- 81.Millar LK et al. Low birth weight and preeclampsia in pregnancies complicated by hyperthyroidism. Obstet. Gynecol 84, 946–949 (1994). [PubMed] [Google Scholar]
- 82.Casey BM et al. Subclinical hyperthyroidism and pregnancy outcomes. Obstet. Gynecol 107, 337–341 (2006). [DOI] [PubMed] [Google Scholar]
- 83.Scappaticcio L et al. Abnormal liver blood tests in patients with hyperthyroidism: systematic review and meta-analysis. Thyroid 31, 884–894 (2021). [DOI] [PubMed] [Google Scholar]
- 84.Suzuki N et al. Analysis of antithyroid drug-induced severe liver injury in 18,558 newly diagnosed patients with Graves’ disease in Japan. Thyroid 29, 1390–1398 (2019). [DOI] [PubMed] [Google Scholar]
- 85.Yu W et al. Side effects of PTU and MMI in the treatment of hyperthyroidism: a systematic review and meta-analysis. Endocr. Pract 26, 207–217 (2020). [DOI] [PubMed] [Google Scholar]
- 86.Rivkees SA & Szarfman A Dissimilar hepatotoxicity profiles of propylthiouracil and methimazole in children. J. Clin. Endocrinol. Metab 95, 3260–3267 (2010). [DOI] [PubMed] [Google Scholar]
- 87.Cooper DS & Rivkees SA Putting propylthiouracil in perspective. J. Clin. Endocrinol. Metab 94, 1881–1882 (2009). [DOI] [PubMed] [Google Scholar]
- 88.Andersen SL, Olsen J, Wu CS & Laurberg P Birth defects after early pregnancy use of antithyroid drugs: a Danish nationwide study. J. Clin. Endocrinol. Metab 98, 4373–4381 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Andersen SL, Olsen J, Wu CS & Laurberg P Severity of birth defects after propylthiouracil exposure in early pregnancy. Thyroid 24, 1533–1540 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Andersen SL, Lönn S, Vestergaard P & Törring O Birth defects after use of antithyroid drugs in early pregnancy: a Swedish nationwide study. Eur. J. Endocrinol 177, 369–378 (2017). [DOI] [PubMed] [Google Scholar]
- 91.Seo GH, Kim TH & Chung JH Antithyroid drugs and congenital malformations: a nationwide Korean cohort study. Ann. Intern. Med 168, 405–413 (2018). [DOI] [PubMed] [Google Scholar]
- 92.Andersen SL, Knøsgaard L, Olsen J, Vestergaard P & Andersen S Maternal thyroid function, use of antithyroid drugs in early pregnancy, and birth defects. J. Clin. Endocrinol. Metab 104, 6040–6048 (2019). [DOI] [PubMed] [Google Scholar]; This large study assessed the effects of antithyroid drug use in early pregnancy on risk of birth defects utilizing the Danish national registry.
- 93.Momotani N, Noh JY, Ishikawa N & Ito K Effects of propylthiouracil and methimazole on fetal thyroid status in mothers with Graves’ hyperthyroidism. J. Clin, Endocrinol. Metab 82, 3633–3636 (1997). [DOI] [PubMed] [Google Scholar]
- 94.Laurberg P et al. TSH-receptor autoimmunity in Graves’ disease after therapy with anti-thyroid drugs, surgery, or radioiodine: a 5-year prospective randomized study. Eur. J. Endocrinol 158, 69–75 (2008). [DOI] [PubMed] [Google Scholar]
- 95.American Thyroid Association Taskforce on Radioiodine Safety, et al. Radiation safety in the treatment of patients with thyroid diseases by radioiodine 131I: practice recommendations of the American Thyroid Association. Thyroid 21, 335–346 (2011). [DOI] [PubMed] [Google Scholar]
- 96.Leung AM, Pearce EN & Braverman LE Iodine nutrition in pregnancy and lactation. Endocrinol. Metab. Clin. North Am 40, 765–777 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (National Academies Press; (US: ), 2001). [PubMed] [Google Scholar]
- 98.WHO Secretariat, Andersson M, de Benoist B, Delange F & Zupan J Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation. Public Health Nutr 10, 1606–1611 (2007). [DOI] [PubMed] [Google Scholar]; This documents reviews recommendations regarding iodine intake in pregnant and lactating women and children to prevent iodine deficiency.
- 99.WHO, UNICEF, ICCIDD. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3rd ed. http://whqlibdoc.who.int/publications/2007/9789241595827_eng.pdf (2007). [Google Scholar]
- 100.Andersen S, Karmisholt J, Pedersen KM & Laurberg P Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br. J. Nutr 99, 813–818 (2008). [DOI] [PubMed] [Google Scholar]
- 101.Pearce EN & Caldwell KL Urinary iodine, thyroid function, and thyroglobulin as biomarkers of iodine status. Am. J. Clin. Nutr 104, 898S–901S (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wong EM, Sullivan KM, Perrine CG, Rogers LM & Peña-Rosas JP Comparison of median urinary iodine concentration as an indicator of iodine status among pregnant women, school-age children, and nonpregnant women. Food Nutr. Bull 32, 206–212 (2011). [DOI] [PubMed] [Google Scholar]
- 103.Zimmermann MB, Gizak M, Abbott K, Andersson M & Lazarus JH Iodine deficiency in pregnant women in Europe. Lancet Diabetes Endocrinol. 3, 672–674 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Caldwell KL et al. Iodine status in pregnant women in the National Children’s Study and in U.S. women (15–44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 23, 927–937 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perrine CG, Herrick KA, Gupta PM & Caldwell KL Iodine status of pregnant women and women of reproductive age in the United States. Thyroid 29, 153–154 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Caldwell KL, Makhmudov A, Ely E, Jones RL & Wang RY Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid 21, 419–427 (2011). [DOI] [PubMed] [Google Scholar]
- 107.Caldwell KL, Miller GA, Wang RY, Jain RB & Jones RL Iodine status of the U.S. population, National Health and Nutrition Examination Survey 2003–2004. Thyroid 18, 1207–1214 (2008). [DOI] [PubMed] [Google Scholar]
- 108.Yarrington C & Pearce EN Iodine and pregnancy. J. Thyroid Res 2011, 934104 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abuye C & Berhane Y The goitre rate, its association with reproductive failure, and the knowledge of iodine deficiency disorders (IDD) among women in Ethiopia: cross-section community based study. BMC Public Health 7, 316 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pearce EN Effects of iodine deficiency in pregnancy. J. Trace Elem. Med. Biol 26, 131–133 (2012). [DOI] [PubMed] [Google Scholar]
- 111.Glinoer D The importance of iodine nutrition during pregnancy. Public Health Nutr. 10, 1542–1546 (2007). [DOI] [PubMed] [Google Scholar]
- 112.Pharoah PO, Ellis SM, Ekins RP & Williams ES Maternal thyroid function, iodine deficiency and fetal development. Clin. Endocrinol 5, 159–166 (1976). [DOI] [PubMed] [Google Scholar]
- 113.Pharoah PO, Buttfield IH & Hetzel BS Neurological damage to the fetus resulting from severe iodine deficiency during pregnancy. Lancet 1, 308–310 (1971). [DOI] [PubMed] [Google Scholar]
- 114.Bougma K, Aboud FE, Harding KB & Marquis GS Iodine and mental development of children 5 years old and under: a systematic review and meta-analysis. Nutrients 5, 1384–1416 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qian M et al. The effects of iodine on intelligence in children: a meta-analysis of studies conducted in China. Asia Pac. J. Clin. Nutr 14, 32–42 (2005). [PubMed] [Google Scholar]
- 116.Jiskra J et al. Mild iodine deficiency in women after spontaneous abortions living in an iodine-sufficient area of Czech Republic: prevalence and impact on reproductive health. Clin. Endocrinol 80, 452–458 (2014). [DOI] [PubMed] [Google Scholar]
- 117.Charoenratana C, Leelapat P, Traisrisilp K & Tongsong T Maternal iodine insufficiency and adverse pregnancy outcomes. Matern. Child Nutr 12, 680–687 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abel MH et al. Insufficient maternal iodine intake is associated with subfecundity, reduced foetal growth, and adverse pregnancy outcomes in the Norwegian Mother, Father and Child Cohort Study. BMC Med. 18, 211 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gargari SS et al. Maternal and neonatal outcomes and determinants of iodine deficiency in third trimester of pregnancy in an iodine sufficient area. BMC Pregnancy Childbirth 20, 174 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Torlinska B, Bath SC, Janjua A, Boelaert K & Chan S-Y Iodine status during pregnancy in a region of mild-to-moderate iodine deficiency is not associated with adverse obstetric outcomes; results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Nutrients 10, 291 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bath SC, Steer CD, Golding J, Emmett P & Rayman MP Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 382, 331–337 (2013). [DOI] [PubMed] [Google Scholar]
- 122.Hynes KL, Otahal P, Hay I & Burgess JR Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J. Clin. Endocrinol. Metab 98, 1954–1962 (2013). [DOI] [PubMed] [Google Scholar]
- 123.Mil NHV et al. Low urinary iodine excretion during early pregnancy is associated with alterations in executive functioning in children. J. Nutr 142, 2167–2174 (2012). [DOI] [PubMed] [Google Scholar]
- 124.Costeira MJ et al. Psychomotor development of children from an iodine-deficient region. J. Pediatr 159, 447–453 (2011). [DOI] [PubMed] [Google Scholar]
- 125.Moleti M et al. Effects of maternal iodine nutrition and thyroid status on cognitive development in offspring: a pilot study. Thyroid 26, 296–305 (2016). [DOI] [PubMed] [Google Scholar]
- 126.Zhou SJ et al. Association between maternal iodine intake in pregnancy and childhood neurodevelopment at age 18 months. Am. J. Epidemiol 188, 332–338 (2019). [DOI] [PubMed] [Google Scholar]
- 127.Levie D et al. Association of maternal iodine status with child IQ: a meta-analysis of individual participant data. J. Clin. Endocrinol. Metab 104, 5957–5967 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ghassabian A et al. Maternal urinary iodine concentration in pregnancy and children’s cognition: results from a population-based birth cohort in an iodine-sufficient area. BMJ Open 4, e005520 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nazeri P, Shariat M &Azizi F Effects of iodine supplementation during pregnancy on pregnant women and their offspring: a systematic review and meta-analysis of trials over the past 3 decades. Eur. J. Endocrinol 184, 91–106 (2021). [DOI] [PubMed] [Google Scholar]
- 130.Dineva M, Fishpool H, Rayman MP, Mendis J & Bath SC Systematic review and meta-analysis of the effects of iodine supplementation on thyroid function and child neurodevelopment in mildly-to-moderately iodine-deficient pregnant women. Am. J. Clin. Nutr 112, 389–412 (2020). [DOI] [PubMed] [Google Scholar]; This systematic review and meta-analysis summarizes findings of 37 randomized controlled trials, intervention studies, and observational studies on the effects of iodine supplementation in mildly-to-moderately iodine deficiency pregnant women on maternal and infant thyroid function and child neurodevelopmental outcomes.
- 131.World Health Organization. Iodine supplementation in pregnant and lactating women. WHO http://www.who.int/elena/titles/iodine_pregnancy/en/ (2021). [Google Scholar]
- 132.De Groot L et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab 97, 2543–2565 (2012). [DOI] [PubMed] [Google Scholar]
- 133.Obican SG, Jahnke GD, Soldin OP & Scialli AR Teratology public affairs committee position paper: iodine deficiency in pregnancy. Birth Defects Res. A Clin. Mol. Teratol 94, 677–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leung AM, Pearce EN, Braverman LE & Stagnaro-Green A AAP recommendations on iodine nutrition during pregnancy and lactation. Pediatrics 134, e1 282 (2014). [DOI] [PubMed] [Google Scholar]
- 135.Kung AWC, Chau MT, Lao TT, Tam SCF & Low LCK The effect of pregnancy on thyroid nodule formation. J. Clin. Endocrinol. Metab 87, 1010–1014(2002). [DOI] [PubMed] [Google Scholar]
- 136.Gao M et al. Excessive iodine intake is associated with formation of thyroid nodules in pregnant Chinese women. Nutr. Res 66, 61–67 (2019). [DOI] [PubMed] [Google Scholar]
- 137.Sahin SB et al. Alterations of thyroid volume and nodular size during and after pregnancy in a severe iodine-deficient area. Clin. Endocrinol 81, 762–768 (2014). [DOI] [PubMed] [Google Scholar]
- 138.Haugen BR et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26, 1–133 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smith LH, Danielsen B, Allen ME & Cress R Cancer associated with obstetric delivery: results of linkage with the California cancer registry. Am. J. Obstet. Gynecol 189, 1128–1135 (2003). [DOI] [PubMed] [Google Scholar]
- 140.Yasmeen S et al. Thyroid cancer in pregnancy. Int. J. Gynaecol. Obstet 91, 15–20 (2005). [DOI] [PubMed] [Google Scholar]
- 141.Pitt SC et al. Patients’ reaction to diagnosis with thyroid cancer or an indeterminate thyroid nodule. Thyroid 31, 580–588 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moosa M & Mazzaferri EL Outcome of differentiated thyroid cancer diagnosed in pregnant women. J. Clin. Endocrinol. Metab 82, 2862–2866 (1997). [DOI] [PubMed] [Google Scholar]
- 143.Oh H-S et al. Serial neck ultrasonographic evaluation of changes in papillary thyroid carcinoma during pregnancy Thyroid 27, 773–777 (2017). [DOI] [PubMed] [Google Scholar]
- 144.Ito Y et al. Effects of pregnancy on papillary microcarcinomas of the thyroid re-evaluated in the entire patient series at Kuma hospital. Thyroid 26, 156–160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vannucchi G et al. Clinical and molecular features of differentiated thyroid cancer diagnosed during pregnancy. Eur. J. Endocrinol 162, 145–151 (2010). [DOI] [PubMed] [Google Scholar]
- 146.Messuti I et al. Impact of pregnancy on prognosis of differentiated thyroid cancer: clinical and molecular features. Eur. J. Endocrinol 170, 659–666 (2014). [DOI] [PubMed] [Google Scholar]
- 147.Tazebay UH et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat. Med 6, 871–878 (2000). [DOI] [PubMed] [Google Scholar]
- 148.Cho GJ et al. Risk of adverse obstetric outcomes and the abnormal growth of offspring in women with a history of thyroid cancer. Thyroid 29, 879–885 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rakhlin L, Fish S & Tuttle RM Response to therapy status is an excellent predictor of pregnancy-associated structural disease progression in patients previously treated for differentiated thyroid, cancer. Thyroid 27, 396–401 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study assessed structural disease recurrence and progression during pregnancy in women with a history of papillary thyroid cancer.
- 150.Pearce EN Management of thyrotoxicosis: preconception, pregnancy, and the postpartum period. Endocr. Pract 25, 62–68 (2019). [DOI] [PubMed] [Google Scholar]
- 151.Premawardhana LDKE, Parkes AB, John R, Harris B & Lazarus JH Thyroid peroxidase antibodies in early pregnancy: utility for prediction of postpartum thyroid dysfunction and implications for screening. Thyroid 14, 610–615 (2004). [DOI] [PubMed] [Google Scholar]
- 152.Amino N & Arata N Thyroid dysfunction following pregnancy and implications for breastfeeding. Best. Pract. Res. Clin. Endocrinol. Metab 34, 101438 (2020). [DOI] [PubMed] [Google Scholar]
- 153.Moleti M et al. Postpartum thyroiditis in women with euthyroid and hypothyroid hashimoto’s thyroiditis antedating pregnancy. J. Clin. Endocrinol. Metab 105, e2421–e2428 (2020). [DOI] [PubMed] [Google Scholar]
- 154.Lucas A et al. Postpartum thyroiditis: long-term follow-up. Thyroid 15, 1177–1181 (2005). [DOI] [PubMed] [Google Scholar]
- 155.Premawardhana LD et al. Postpartum thyroiditis and long-term thyroid status: prognostic influence of thyroid peroxidase antibodies and ultrasound echogenicity. J. Clin. Endocrinol. Metab 85, 71–75 (2000). [DOI] [PubMed] [Google Scholar]
- 156.Ide A et al. Differentiation of postpartum Graves’ thyrotoxicosis from postpartum destructive thyrotoxicosis using antithyrotropin receptor antibodies and thyroid blood flow. Thyroid 24, 1027–1031 (2014). [DOI] [PubMed] [Google Scholar]
- 157.Amino N et al. Serum ratio of triiodothyronine to thyroxine, and thyroxine-binding globulin and calcitonin concentrations in Graves’ disease and destruction-induced thyrotoxicosis. J. Clin. Endocrinol. Metab 53, 113–116 (1981). [DOI] [PubMed] [Google Scholar]
- 158.Hubalewska-Dydejczyk A, Duntas L & Gilis-Januszewska A Pregnancy, thyroid, and the potential use of selenium. Hormones 19, 47–53 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.UNICEF. Guidance on the monitoring of salt iodization programmes and determination of population iodine status. UNICEF https://www.ign.org/p142003099.html?from=0142002801 (2018). [Google Scholar]


