Abstract
Purpose
To validate the role of Macklin effect on chest CT imaging in predicting subsequent occurrence of pneumomediastinum/pneumothorax (PMD/PNX) in COVID-19 patients.
Materials and methods
This is an observational, case-control study. Consecutive COVID-19 patients who underwent chest CT scan at hospital admission during the study time period (October 1st, 2020–April 31st, 2021) were identified. Macklin effect accuracy for prediction of spontaneous barotrauma was measured in terms of sensitivity, specificity, positive (PPV) and negative predictive values (NPV).
Results
Overall, 981 COVID-19 patients underwent chest CT scan at hospital arrival during the study time period; 698 patients had radiological signs of interstitial pneumonia and were considered for further evaluation. Among these, Macklin effect was found in 33 (4.7%), including all 32 patients who suffered from barotrauma lately during hospital stay (true positive rate: 96.9%); only 1/33 with Macklin effect did not develop barotrauma (false positive rate: 3.1%). No barotrauma event was recorded in patients without Macklin effect on baseline chest CT scan. Macklin effect yielded a sensitivity of 100% (95% CI: 89.1–100), a specificity of 99.85% (95% CI: 99.2–100), a PPV of 96.7% (95% CI: 80.8–99.5), a NPV of 100% and an accuracy of 99.8% (95% CI: 99.2–100) in predicting PMD/PNX, with a mean advance of 3.2 ± 2.5 days. Moreover, all Macklin-positive patients developed ARDS requiring ICU admission and, in 90.1% of cases, invasive mechanical ventilation.
Conclusions
Macklin effect has high accuracy in predicting PMD/PNX in COVID-19 patients; it is also an excellent predictor of disease severity.
Keywords: Acute respiratory distress syndrome, Mechanical ventilation, Computed tomography, Pneumothorax, Pneumomediastinum, Barotrauma, Intensive care
Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; NPV, negative predictive value; PMD, pneumomediastinum; PNX, pneumothorax; PPV, positive predictive value
1. Introduction
Barotrauma, ranging from asymptomatic air leakage within lung parenchyma to life-threatening conditions such as pneumomediastinum (PMD) and/or tension pneumothorax (PNX) possibly tracking to cervical soft tissue (subcutaneous emphysema) or into the abdomen, occurs frequently in mechanically ventilated patients with coronavirus disease 2019 (COVID-19)-related acute respiratory distress syndrome (ARDS) [[1], [2], [3], [4], [5], [6]]. The reported incidence (on a recent pooled analysis, the rate of barotrauma events was: 16.1% [2,3]), and the difficult, non-standardized management despite employment of lung protective strategies, justify the high mortality rate (higher than 60% [2,3]) associated with this condition [7]. Furthermore, COVID-19 ARDS patients are thought to be more vulnerable to barotrauma as compared with patients with ARDS due to other than COVID-19 causes [1,2]; a higher than expected incidence of barotrauma was also observed in COVID-19 patients not requiring invasive mechanical ventilation [8]. These findings support the existence of a virus-induced frailty of lung parenchyma, possibly triggered by microvascular thrombosis [9], interstitial inflammation, as well as endothelial barrier disruption [10,11], resulting in an extensive, COVID-19-specific diffuse alveolar damage. Moreover, the eventual contribution of any medical treatment is not clearly understood [8].
Taken together, these assumptions provide a firm rationale for searching a tool able to objectively, non-invasively assess this lung frailty and therefore provide early risk stratification in terms of barotrauma susceptibility amongst mechanically ventilated COVID-19 ARDS patients, thus providing an actual aid for their management. In this setting, the so-called Macklin effect [12,13], firstly intended to allow proper differentiation between respiratory and other causes of air leakage in the mediastinum such as tracheobronchial/esophageal injury [12,14,15], has been recently suggested to be a consistent, very accurate radiological predictor of barotrauma development [16,17], in COVID-19 ARDS patients.
Accordingly, we decided to validate the role of Macklin effect in predicting subsequent occurrence of overt PMD/PNX in COVID-19 patients.
2. Materials and Methods
Under Ethics Committee approval, we analyzed all consecutive adult patients admitted for SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) infection and requiring hospitalization between October 1st, 2020 and April 30th, 2021 (corresponding to the period of the second and third Italian pandemic waves). Confirmed infection was defined as positive real-time reverse-transcriptase polymerase chain reaction (RT-PCR) from a nasal and/or throat swab.
We enrolled all patients that underwent at least one chest computed tomography (CT) scan at hospital admission. CT scan, as well as contrast medium administration, was performed at the discretion of the attending physicians, according to clinical needs. We excluded patients with no pneumonia at CT scan; those who already had PMD/PNX at the time of the first available chest CT imaging were also excluded.
Four experienced radiologists, unaware of patients’ symptoms, carefully reviewed all selected baseline CT images (i.e. the first in-hospital CT scan demonstrating interstitial pneumonia), searching for Macklin effect (Fig. 1 ), PMD and PNX (Fig. 2 ); in case of differences in the evaluation, consensus was reached amongst them via discussion.
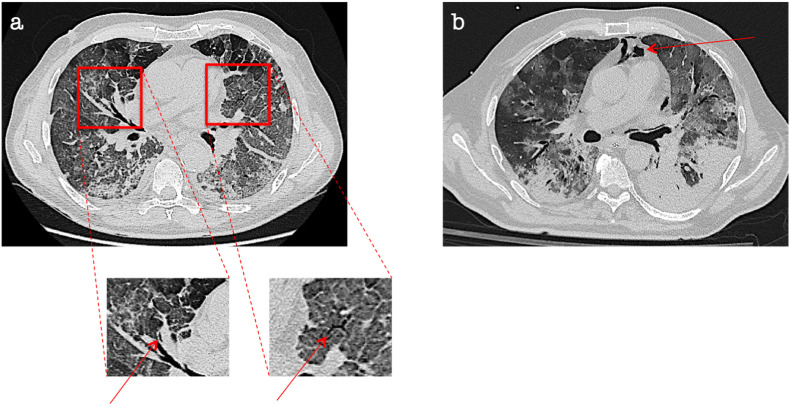
Fig. 1.
Macklin effect in a COVID-19 patient. Lung parenchyma windowed CT images demonstrate [a] a crescent collection of air contiguous to the middle lobar bronchovascular sheath as well as interstitial emphysema in the left upper lobe, both representing Macklin effect (red arrows). Seven days later [b], pneumomediastinum occurred. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
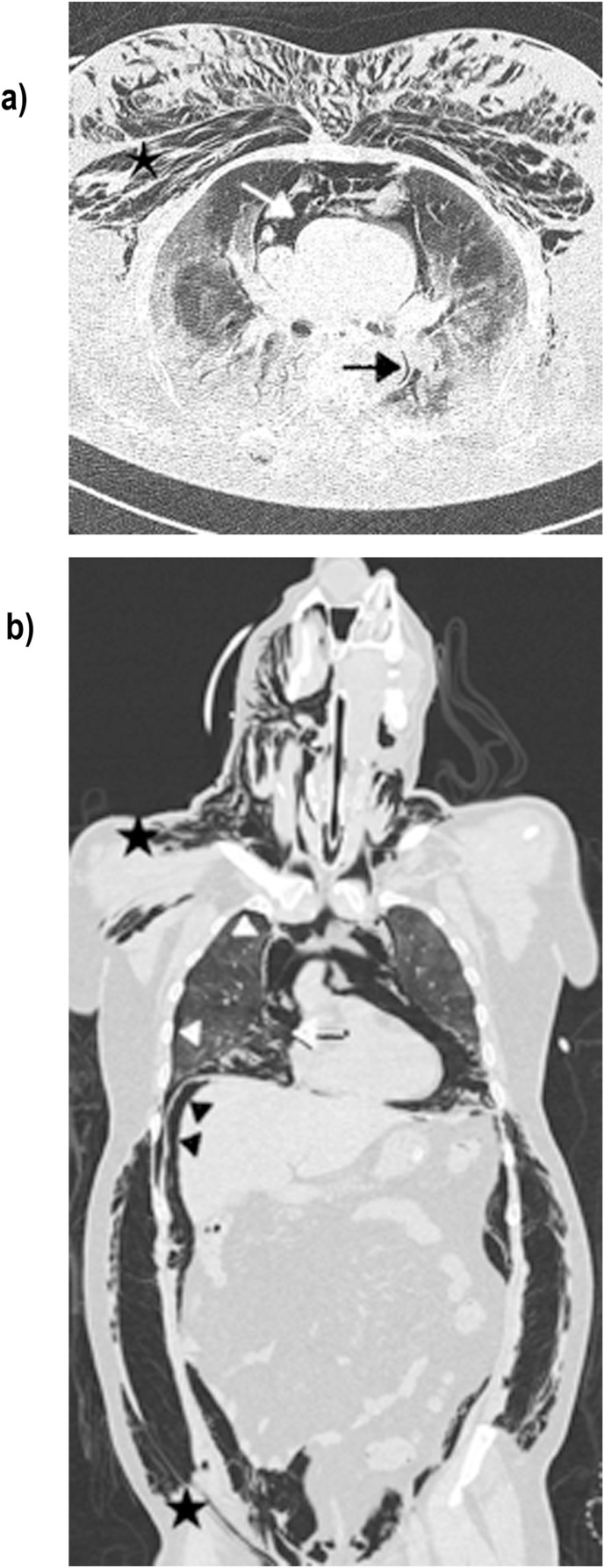
Fig. 2.
Lung parenchyma window axial (a) and coronal (b) CT scans of patient suffering from SARS-CoV-2 infection, subjected to invasive mechanics ventilation, show: central Macklin effect (black arrow) in association with pneumomediastinum (white arrow), pneumothorax (white arrow heads), subcutaneous emphysema (black star) and pneumoperitoneum (black arrow head).
Macklin effect (also known as Macklin-like radiological sign or interstitial emphysema) was defined, on lung parenchyma windowed CT images, as a linear collection of air contiguous to the bronchovascular sheaths [12]. It was analyzed in terms of presence/absence and topographical distribution within the lungs (adjacent to peripheral [segmental/subsegmental] vs. central [lobar] bronchial branches). All contrast-enhanced CT images were reviewed also to detect the eventual occurrence of pulmonary vascular thrombosis, defined as filling defects in the branches of the pulmonary arteries.
In all patients with Macklin effect on baseline CT scan, demographic and anamnestic data, together with therapeutic treatment, were systematically collected. Time from symptoms onset to hospital admission and eventual intubation, ICU (Intensive Care Unit) length of stay, and overall hospital length of stay were also recorded. For patients admitted in the ICU we also collected daily clinical respiratory parameters throughout the first 7 days of mechanical ventilation.
2.1. Statistical analysis
A convenience sample was considered for this analysis, with consecutive patients included until the latest follow-up. A formal sample size calculation was, therefore, not performed; yet the study power retrospectively calculated was 97%, considering a null hypothesis proportion of barotrauma 0.02,[[18], [19], [20], [21]] and an actual proportion of 0.046, with an alpha of 0.05.
Continuous variables are presented as mean and standard deviation in case of normal distribution or medians, and interquartile range in case of non-normal distribution, and categorical variables as number and percentages. Macklin effect accuracy in predicting spontaneous PMD/PNX was measured in terms of sensitivity, specificity, positive and negative predictive values (PPV and NPV, respectively).
3. Results
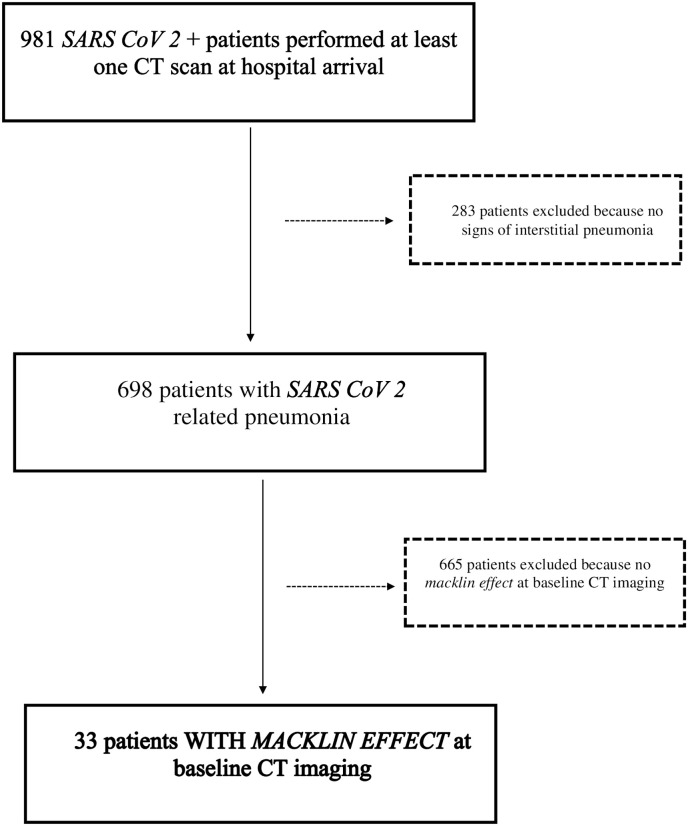
Overall, 981 COVID-19 patients underwent chest CT scan at hospital arrival during the study time period; 698 patients had radiological signs of interstitial pneumonia and were considered for further evaluation. Among these, Macklin effect was found in 33 (4.7%) (10 female [30.3%]; mean age: 65.8 years [± 8.6] – patients’ characteristics are summarized in Table 1 ) (Fig. 3 ), including all 32 patients (4.6%) who suffered from barotrauma lately during their hospital stay. Specifically, 14 patients had PMD, 8 PNX, whereas 10 suffered from both; 11 patients also had extensive chest wall soft tissues emphysema, and in one case the air leakage extended into the abdomen. Of note, even if none of these 33 patients was receiving invasive mechanical ventilation at the time of baseline CT scan, they all required subsequent ICU admission because of ARDS development (respiratory parameters for the first 7 days after ICU admission are reported in Table 2 ).
Table 1.
Baseline characteristics and clinical course of the 33 patients with Macklin effect on baseline chest CT scan.
| Variable | Value (N = 33) |
|---|---|
| Patients Characteristics | |
|
23 (69.9) |
|
65.8 ± 8.6 |
|
29.4 ± 5.3 |
| Comorbidities | |
|
11 (39.3) |
|
9 (27.2) |
|
10 (30.3) |
|
10 (30.3) |
|
2 (6.1) |
|
5 (15.1) |
| O2 supplementation at the time of the first CT scan | |
|
0 (0) |
|
33 (100) |
|
0 (0) |
|
0 (0) |
| Barotrauma characteristics | |
|
32 (96.9) |
|
14 (42.4) |
|
8 (24.2) |
|
10 (30.3) |
|
11 (33.3) |
BMI: body mass index; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CPAP: continuous positive airway pressure; ICU: intensive care unit; SpO2: peripheral oxygen saturation; °C: Celsius degrees.
Fig. 3.
Inclusion/exclusion flowchart.
Table 2.
Respiratory parameters for the first 7 days after Intensive Care Unit admission.
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|---|---|---|
| PaO2/FiO2 ratio | 89.8 ± 38.5 | 85.3 ± 19.3 | 91.1 ± 19.7 | 91.2 ± 21.9 | 90.3 ± 22.7 | 86.1 ± 16.4 | 82.2 ± 19.7 |
| PaCO2 (mmHg) | 47.7 ± 8.9 | 47.5 ± 9.3 | 47.3 ± 9.7 | 46.8 ± 9.2 | 45.3 ± 10.0 | 45.0 ± 11.4 | 46.9 ± 0.1 |
| PEEP (cmH2O) | 9.2 ± 1.8 | 9.1 ± 2.3 | 9.5 ± 2.3 | 10.2 ± 3.0 | 9.6 ± 1.5 | 9.3 ± 1.7 | 9.7 ± 2.4 |
Continuous variables are presented as mean ± standard deviation.
PaO2/FiO2 ratio: ratio between arterial partial oxygen pressure (PaO2) and oxygen inspired fraction (FiO2); PaCO2: Carbon dioxide partial arterial pressure; PEEP: Positive End Expiratory Pressure.
Only one patient with baseline CT signs of interstitial pneumonia and Macklin effect did not develop barotrauma during the study period (false positive rate: 3.1%). On the contrary, there is no patient with barotrauma without baseline Macklin effect (true positive rate: 96.9%); mean time from Macklin effect detection on baseline chest CT scan and development of clinically overt barotrauma was 3.2 days (±2.5). Furthermore, no false negative result was recorded when considering the whole population with radiological evidence of pneumonia (0/665, true negative rate of 100%). As a consequence, in our population Macklin effect yielded a sensitivity of 100% (95% CI: 89.1–100), a specificity of 99.85% (95% CI: 99.2–100), a PPV of 96.7% (95% CI: 80.8–99.5), a NPV of 100% and an overall accuracy of 99.8% (95% CI: 99.2–100) in predicting subsequent development of barotrauma.
The exact topographical distribution within the lung of Macklin effect was found to be central (adjacent to lobar bronchial branches) in the vast majority of patients (29/33 [87.8%]). All patients except one had bilateral distribution of Macklin effect.
All Macklin-positive patients underwent at least one contrast enhanced CT scan during ICU stay: of these, six (18.1%) had concurrent pulmonary vascular thrombosis.
4. Discussion
In this observational study, we found that, among patients with COVID-19 pneumonia, detection of Macklin effect on baseline chest CT scan accurately predicts subsequent development of barotrauma. In this respect, our findings confirmed the observation that COVID-19 patients with Macklin effect on baseline CT imaging, are to be considered at very high-risk for clinically overt PMD/PNX during hospital stay [2,16,17]. Macklin effect demonstrated indeed almost perfect accuracy in predicting barotrauma occurrence (sensitivity: 100% [95% CI: 89.1–100]; specificity: 99.85% [95% CI: 99.2–100]). Moreover, to the best of our knowledge, this is the first report of Macklin effect in non-intubated COVID-19 patients, corroborating the existence of a virus-induced frailty of lung parenchyma [1,10,16], regardless of the type of ventilation. Finally, all patients with Macklin effect at the time of the first in-hospital CT scan later developed ARDS requiring ICU admission and, in 90.1% of cases, invasive mechanical ventilation. On that note, Macklin effect can be considered a reliable marker of severity of disease.
Contrary to what other authors reported, we observed a significantly shorter mean time interval (3.2 days) between Macklin effect on baseline CT scan and the first radiological evidence of barotrauma. Specifically, Belletti et al. [15] reported a median gap of about 12 days, whereas Palumbo et al. [16] of 8.5 days. A possible explanation for this discrepancy could lie in the different topographical distribution within the lungs of Macklin effect, which was found to be, in our cohort, almost always central, differently from the distribution reported by previous authors (predominantly peripheral). In this regard, our results are consistent with previous literature. According to Palumbo and colleagues [16], there is a relationship between topographical distribution of Macklin effect and temporal advance before the first radiological evidence of PMD/PNX, justifying the shorter delay observed in our population; a central distribution of Macklin effect is indeed associated with significantly shorter temporal delay before barotrauma occurrence when compared with a peripheral distribution. Taken together, these findings validate the hypothesis of Macklin effect as a sort of “radiological countdown” for PMD/PNX development, capturing air leakage centripetal movement along the pulmonary interstitium. Furthermore, the central distribution of the Macklin effect could also justify the higher sensitivity in predicting barotrauma in our cohort, when compared with previous literature (100% vs. 89.2% in a previous report [17]): the presence of air along pulmonary hila structures implies indeed the near certainty of barotrauma development.
Of note, in our cohort we found that a significant proportion (18.1%) of Macklin-positive patients had concurrent pulmonary vascular thrombosis. Whether this finding could provide one of the earliest evidence of the mechanism underlying COVID-19 specific lung frailty [10,11], with microvascular diffuse thrombosis being a main contributor, needs further confirmation.
Our study may have several clinical implications. First of all, identification of Macklin effect may help to stratify baseline risk of clinical deterioration of COVID-19 pneumonia, and therefore allowing early referral to intensive care unit. This might be particularly important in the course of pandemic waves, in settings of limited resources. In addition, our findings could also provide baseline data for the potential development of a therapeutic algorithm for patients thought to be at high-risk for barotrauma development and lung damage progression, identifying those most likely to benefit from various strategies (early application of ultraprotective mechanical ventilation or extracorporeal support) [[22], [23], [24], [25]]. However, future studies should investigate whether different management strategies could actually prevent barotrauma development, and whether these will translate in outcome improvement. Finally, our findings might also apply to non-COVID-19 ARDS, and future investigations also different (non-COVID-19) settings are therefore warranted.
The present study has some limitations, the main ones being its retrospective nature and its relative small sample size. However, it includes almost 700 patients, being the largest performed so far on this topic. Moreover, the study lacks standardization both in terms of radiological examinations timing and patients’ management.
5. Conclusions
Our observational study confirmed, in an independent population, that identification of Macklin effect on baseline chest CT scan can accurately predict development of clinically overt barotrauma in patients with COVID-19 pneumonia. Furthermore, this radiological sign could be considered a reliable marker of disease severity, anticipating the need for ICU admission and mechanical ventilation. Further studies are needed to confirm these results in non-COVID-19 settings.
Conflict of interest disclosures
None.
Funding/support
None.
CRediT authorship contribution statement
Gianluca Paternoster: study conception and design, data interpretation, manuscript drafting. Gianfranco Belmonte: data acquisition, analysis and interpretation, critical review of the manuscript. Enrico Scarano: data acquisition, analysis and interpretation, critical review of the manuscript. Pietro Rotondo: data acquisition, analysis and interpretation, critical review of the manuscript. Diego Palumbo: study conception and design, data interpretation, manuscript drafting. Alessandro Belletti: study conception and design, data interpretation, critical review of the manuscript. Francesco Corradi: data acquisition, analysis and interpretation, critical review of the manuscript. Pietro Bertini: data acquisition, analysis and interpretation, critical review of the manuscript. Giovanni Landoni: study conception and design, data interpretation, manuscript drafting. Fabio Guarracino: study conception and design, data interpretation, critical review of the manuscript, All Authors read and approved the final version of the manuscript.
Declaration of competing interest
We declare that we do not have conflicts of interest.
Acknowledgements
None.
Contributor Information
for the COVID-Macklin Study Group:
Alessandro Isirdi, Diego Costanzo, Matteo Romani, Luigi De Simone, Roberto Mozzo, Alessia Palmaccio, Giorgia Guazzarotti, Renato Pennella, and Francesca Calabrese
References
- 1.Lemmers D.H.L., Abu Hilal M., Bnà C., et al. Pneumomediastinum and subcutaneous emphysema in COVID-19: barotrauma or lung frailty? ERJ Open Res. 2020;6(4) doi: 10.1183/23120541.00385-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belletti A., Todaro G., Valsecchi G., et al. Barotrauma in coronavirus disease 2019 patients undergoing invasive mechanical ventilation: a systematic literature review. Crit. Care Med. 2022;50(3):491–500. doi: 10.1097/CCM.0000000000005283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belletti A., Landoni G., Zangrillo A. Pneumothorax and barotrauma in invasively ventilated patients with COVID-19. Respir. Med. 2021;187 doi: 10.1016/j.rmed.2021.106552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn M.R., Watson R.L., Thetford J.T., Wong J.I., Kamangar N. High incidence of barotrauma in patients with severe coronavirus disease 2019. J. Intensive Care Med. 2021;36(6):646–654. doi: 10.1177/0885066621989959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chopra A., Al-Tarbsheh A.H., Shah N.J., et al. Pneumothorax in critically ill patients with COVID-19 infection: incidence, clinical characteristics and outcomes in a case control multicenter study. Respir. Med. 2021;184 doi: 10.1016/j.rmed.2021.106464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capaccione K.M., D’souza B., Leb J., et al. Pneumothorax rate in intubated patients with COVID-19. Acute Crit. Care. 2021;36(1):81–84. doi: 10.4266/acc.2020.00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Azzawi M., Douedi S., Alshami A., Al-Saoudi G., Mikhail J. Spontaneous subcutaneous emphysema and pneumomediastinum in COVID-19 patients: an indicator of poor prognosis? Am. J. Case Rep. 2020;21 doi: 10.12659/AJCR.925557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palumbo D., Campochiaro C., Belletti A., et al. COVID-BioB Study Group Pneumothorax/pneumomediastinum in non-intubated COVID-19 patients: differences between first and second Italian pandemic wave. Eur. J. Intern. Med. 2021;88:144–146. doi: 10.1016/j.ejim.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Cobelli F., Palumbo D., Ciceri F., et al. Pulmonary vascular thrombosis in COVID-19 pneumonia. J. Cardiothorac. Vasc. Anesth. 2021;35(12):3631–3641. doi: 10.1053/j.jvca.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciceri F., Beretta L., Scandroglio A.M., et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit. Care Resusc. 2020;22(2):95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palumbo D., Guazzarotti G., De Cobelli F. Spontaneous major hemorrhage in COVID-19 patients: another brick in the wall of SARS-CoV-2-associated coagulation disorders? J. Vasc. Intervent. Radiol. 2020;31(9):1494–1496. doi: 10.1016/J.JVIR.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murayama S., Gibo S. Spontaneous pneumomediastinum and Macklin effect: overview and appearance on computed tomography. World J. Radiol. 2014;6(11):850–854. doi: 10.4329/wjr.v6.i11.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macklin C.C. Transport of air along sheaths of pulmonic blood vessels from alveoli to mediastinum: clinical implications. Arch. Intern. Med. 1939;64(5):913–926. doi: 10.1001/archinte.1939.00190050019003. [DOI] [Google Scholar]
- 14.Sakai M., Murayama S., Gibo M., Akamine T., Nagata O. Frequent cause of the Macklin effect in spontaneous pneumomediastinum: demonstration by multidetector-row computed tomography. J. Comput. Assist. Tomogr. 2006;30(1):92–94. doi: 10.1097/01.RCT.0000187416.07698.8D. [DOI] [PubMed] [Google Scholar]
- 15.Russell D.W., Watts J.R., Powers T.A. Searching for the source of the leak: PIE and the Macklin effect. Ann. Am. Thorac. Soc. 2018;15(11):1354–1356. doi: 10.1513/ANNALSATS.201803-200CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belletti A., Palumbo D., Zangrillo A., et al. COVID-BioB Study Group Predictors of pneumothorax/pneumomediastinum in mechanically ventilated COVID-19 patients. J. Cardiothorac. Vasc. Anesth. 2021;35(12):3642–3651. doi: 10.1053/j.jvca.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palumbo D., Zangrillo A., Belletti A., et al. COVID-BioB Study Group A radiological predictor for pneumomediastinum/pneumothorax in COVID-19 ARDS patients. J. Crit. Care. 2021;66:14–19. doi: 10.1016/j.jcrc.2021.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ò Miró, Llorens P., Jiménez S., et al. Frequency, risk factors, clinical characteristics, and outcomes of spontaneous pneumothorax in patients with coronavirus disease 2019: a case-control, emergency medicine-based multicenter study. Chest. 2021;159(3):1241–1255. doi: 10.1016/J.CHEST.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong W.H., Saha B.K., Hu K., Chopra A. The incidence, clinical characteristics, and outcomes of pneumothorax in hospitalized COVID-19 patients: a systematic review. Heart Lung. 2021;50(5):599–608. doi: 10.1016/J.HRTLNG.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escalon J.G., Toy D., Groner L., et al. Incidence, clinical associations and outcomes of intrathoracic complications with and without ARDS in COVID-19 pneumonia. Clin. Imag. 2022;85:106–114. doi: 10.1016/J.CLINIMAG.2022.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrestha D.B., Sedhai Y.R., Budhathoki P., et al. Pulmonary barotrauma in COVID-19: a systematic review and meta-analysis. Ann. Med. Surg. 2022;73:103221. doi: 10.1016/J.AMSU.2021.103221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt M., Pineton de Chambrun M., Lebreton G., et al. Extracorporeal membrane oxygenation instead of invasive mechanical ventilation in a patient with severe COVID-19-associated acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2021;203(12):1571–1573. doi: 10.1164/rccm.202102-0259le. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loyalka P., Cheema F.H., Rao H., Rame J.E., Rajagopal K. Early usage of extracorporeal membrane oxygenation in the absence of invasive mechanical ventilation to treat COVID-19-related hypoxemic respiratory failure. ASAIO J. 2021;67(4):392–394. doi: 10.1097/MAT.0000000000001393. [DOI] [PubMed] [Google Scholar]
- 24.Azzam M.H., Mufti H.N., Bahaudden H., Ragab A.Z., Othman M.M., Tashkandi W.A. Awake extracorporeal membrane oxygenation in coronavirus disease 2019 patients without invasive mechanical ventilation. Crit. Care Explor. 2021;3(6) doi: 10.1097/CCE.0000000000000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paternoster G., Bertini P., Belletti A., et al. Veno-venous extracorporeal membrane oxygenation in awake non-intubated patients with COVID-19 ARDS at high risk for barotrauma. J. Cardiothorac Vasc. Anesth. 2022 doi: 10.1053/j.jvca.2022.03.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]