Abstract
The coronavirus pandemic, COVID-19 has a global impact on the lives and livelihoods of people. It is characterized by a widespread infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), where infected patients may develop serious medical complications or even face death. Development of therapeutic is essential to reduce the morbidity and mortality of infected patients. Chitosan is a versatile biomaterial in nanomedicine and exhibits anti-microbial, anti-cancer and immunomodulatory properties. This review highlights the progress in chitosan design and application pertaining to the anti-viral effects of chitosan and chitosan derivatives (hydroxypropyl trimethylammonium, sulfate, carboxymethyl, bromine, sialylglycopolymer, peptide and phosphonium conjugates) as a function of molecular weight, degree of deacetylation, type of substituents and their degree and site of substitution. The physicochemical attributes of these polymeric therapeutics are identified against the possibility of processing them into nanomedicine which can confer a higher level of anti-viral efficacy. The designs of chitosan for the purpose of targeting SARS-CoV-2, as well as the ever-evolving strains of viruses with a broad spectrum anti-viral activity to meet pandemic preparedness at the early stages of outbreak are discussed.
Keywords: Chitosan, Coronavirus, COVID-19, Nanomedicine, SARS-CoV-2
Graphical abstract
1. Introduction
The COVID-19 pandemic is marked by a widespread Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection. Infected individuals may develop serious medical complications such as acute respiratory distress syndrome or even face death, especially patients with cancer, immunosuppression or comorbidities who may experience increased risks of cardiovascular disorders, thromboembolic stroke, Guillain-Barre syndrome, rhabdomyolysis, brain, hepatic and acute kidney injury (Bourgonje et al., 2020; Murugan et al., 2021; Sanyaolu et al., 2020). With lockdown and safety measures for the prevention of virus spread (Donthu & Gustafsson, 2020), the summative incidences of SARS-CoV-2 infection have a great impact on lives and livelihoods, which warrants the advancement of anti-SARS-CoV-2 therapeutics.
SARS-CoV-2 is a spherical virus classified under the order of Nidovirales, which has a diameter of 100 nm (60–140 nm) and a mass of 1000 MDa (Bar-On, Flamholz, Phillips, & Milo, 2020; Hu, Frieman, & Wolfram, 2020; Murugan et al., 2021). Its transmembrane spike glycoprotein (S protein) presents 10 nm-long trimers. The S protein is divided into two subunits, S1 (N-terminal subunit) and S2 (C-terminal subunit) (Huo et al., 2020; Xu et al., 2020). The SARS-CoV-2 S protein is postulated to interact with Angiotensin Converting Enzyme 2 (ACE2) receptors at the host cell surface, and initiate membrane fusion and viral entry (Walls et al., 2020). S1 initiates viral binding to host ACE2 receptor at C-terminal receptor binding domain that comprises 200 amino acid residues (Huo et al., 2020). S2 then exposes a site cleaved by host proteases to mediate membrane fusion (Yan et al., 2020). SARS-CoV-2 invades host cells via ACE2 receptor-mediated endocytosis, and viral RNA is then released, replicated and translated into viral proteins that mature to infect other host cells (McKee, Sternberg, Stange, Laufer, & Naujokat, 2020). SARS-CoV-2 is highly infectious due to its higher binding affinity for the ACE2 receptor than SARS-CoV-1 (Bourgonje et al., 2020; Zhuang et al., 2020). SARS-CoV-2 first enters ACE2 receptor-expressing epithelial cells in the lower respiratory tract, before infecting other ACE2 receptor-expressing organs such as the heart, kidney and gastrointestinal tract (Bourgonje et al., 2020; McKee et al., 2020).
Severe medical conditions and death of COVID-19 patients are mainly brought about by excessive inflammatory response that SARS-CoV-2 induces in its hosts. It is hypothesised that patients with COVID-19 hyperinflammation have a dysregulated level of macrophage subtypes, reduced level of tissue-resident alveolar macrophages and a spike in the number of inflammatory monocyte-derived macrophages (Merad & Martin, 2020). These inflammatory macrophages may promote fibrosis production and stimulate an interferon signature that induces macrophage hyperactivation (Merad & Martin, 2020).
Vaccines for SARS-CoV-2 are being developed at a rapid pace due to the urgent need to control the morbidity and mortality of COVID-19 (Gallagher, 2020). There are more than 50 COVID-19 vaccine candidates on trials. On 11th December 2020, the United States Food & Drug Administration (FDA) approved the first mRNA vaccine developed by Pfizer Biotech under the purview of emergency use authorization (EUA) to control the COVID-19 pandemic (Mahase, 2020). Following the same month, FDA approved the second COVID-19 vaccine developed by Moderna company (Cohen, 2020). Other examples of vaccines have also been developed by Cansino Biologics and Sinovac Biotech, China, and Gamaleya Research Institute of Epidemiology and Microbiology, Russia (Burki, 2020; Kaur & Gupta, 2020). However, the design and manufacture of vaccine for global supply require a considerable period of time. This is further challenged by SARS-CoV-2 mutating into new strains within a short time, thus affecting the vaccine effectiveness (Gallagher, 2020). On this note, concurrent development of other pharmacological therapeutics is imperative to ensure effective therapeutics are available in the shortest possible time.
1.1. Treatment strategies for COVID-19
COVID-19 patients have been treated with repurposed medications such as favipiravir (Avigan) and hydroxychloroquine that are anti-influenza and anti-malarial drugs respectively (Jean, Lee, & Hsueh, 2020). Four treatment modes have been proposed in search of drug candidates: virus removal or weakening, blocking specific host receptors or enzymes, preventing RNA synthesis and replication, and restoring the host's innate immunity.
Drug candidates upon binding to viruses, are able to remove viruses or inhibit virus-host cell binding. Nanosponges, coated with human cell membranes containing ACE2 & CD147 viral entry receptors, have been designed to bind and remove SARS-CoV-2, preventing it from attaching to human host cells (Zhang et al., 2020). Peptides with structural similarity to the ACE2 receptor and complementary conformation that binds strongly to the receptor binding domain of SARS-CoV-2, have been computationally designed. These peptides have been proposed to serve as multivalent binding sites of nanoparticles for SARS-CoV-2 removal (Han & Král, 2020). Umifenovir blocks the trimerisation of SARS-CoV-2 S-protein needed in host cell viral entry and inhibits membrane fusion between host cell membrane and viral envelope (Vankadari, 2020). Also, Emodin commonly found in Chinese herbs has been shown to bind to SARS-CoV-1 and inhibit the interaction of SARS-CoV-1 S-protein with ACE2 receptor (McKee et al., 2020).
Anti-malarial drugs, chloroquine phosphate and hydroxychloroquine, hinder SARS-CoV-2 replication in vitro (Jean et al., 2020). They are hypothesised to work via hindering the glycosylation of the ACE2 receptor (Devaux, Rolain, Colson, & Raoult, 2020). This impedes SARS-CoV-2 from binding to host cell ACE2 receptors and weakens the ACE2-SARS-CoV-2 interaction. Other proposed mechanisms include alkalinizing the host intracellular pH which inhibits endosome-mediated viral entry, as well as interferes with post-translational modification of viral proteins (Devaux et al., 2020). Chloroquine phosphate also stimulates the host immune system to defend against viral infection by inhibiting pro-inflammatory cytokine activity and synthesis, or by inducing anti-SARS-CoV-2 cytotoxic T lymphocytes (Devaux et al., 2020). Many phytochemicals and natural products, such as luteolin, myricetin, quercetin and kaempferol, have been shown to exhibit a broad-spectrum anti-viral activity and suppress the hyperinflammatory response linked to SARS-CoV-2 infection (McKee et al., 2020; Murugan et al., 2021). However, their mechanism of action is not fully understood (McKee et al., 2020). Phytochemicals such as 3,5,7,3′,4′,5′-hexahydroxy flavanone-3-O-β-D-glucopyranoside, amaranthin, methyl rosmarinate, licoleafol, calceolarioside B, (2S)-eriodictyol 7-O-(6”-O-galloyl)-β-D-glucopyranoside and 5,7,3′,4′-tetrahydoxy-2′-(3,3-dimethylallyl) isoflavone have been reported to be potentially active against the SARS-CoV-2 (Murugan et al., 2021).
Remdesivir triphosphate displays anti-viral activity against SARS-CoV-2 in vitro (Amirian & Levy, 2020). Although there is no conclusive evidence of its effectiveness in clinical trials (Amirian & Levy, 2020), remdesivir is considered the most developed COVID-19 therapeutic and is authorised for use in emergency and severely inflicted patients in the USA (McKee et al., 2020). Lately, remdesivir is permitted to be used in the treatment of suspected or laboratory confirmed COVID-19 in hospitalized paediatric patients (U.S.FDA, 2020). As an adenine nucleotide analogue, remdesivir triphosphate acts via competitively inhibiting natural nucleotide in viral RNA transcription in hosts (Romano, Ruggiero, Squeglia, Maga, & Berisio, 2020). Substitution of nucleotide with remdesivir triphosphate in growing RNA strands results in premature termination of RNA synthesis (Amirian & Levy, 2020), thus hindering viral propagation.
Clinically, the efficacy of many other drugs such as arbidol, baricitinib, camostat mesilate, darunavir, lopinavir, nafamostat mesilate, oseltamivir, recombinant human angiotensin converting enzyme 2, recombinant interferon-α2β, interferon-β1, ribavirin and ritonavir as well as immunomodulatory drugs such as ravulizumab, ruxolitinib, sarilumab and sirolimus have been evaluated against SARS-CoV-2 (Murugan et al., 2021; Tammam et al., 2021). Solid lipid nanoparticles, nanostructured lipid carriers, nanoemulsion, polymeric nanoparticles, metallic nanoparticles, dendrimer, PEGylated cationic liposome, immunoliposome, carbon dots and carbon nanotubes are examples of nanocarrier developed for the delivery of anti-viral drugs (Shah et al., 2021). Nanoformulation of such drugs is envisaged to bring about the following critical advantages: increased drug solubility, prolonged drug residence/circulation time, reduced off-target drug exposure, sustained drug retention or transportation across biological barriers, flexibility in downstream delivery system design, decoratable with targeting ligand for effective binding to host cells and virus, enhanced cellular uptake particularly by the immune cells, and nanoscale geometry sufficient to restrict virus mobility and block its fusion with host (Al-Hatamleh et al., 2021; Tammam et al., 2021; Zhou, Krishnan, Jiang, Fang, & Zhang, 2021).
Lipid nanoparticles have been developed as the carrier of mRNA-based vaccine, which encodes for a full length, prefusion stabilized S protein of SARS-CoV-2 (Al-Hatamleh et al., 2021; Shah et al., 2021). Nanoformulation of remdesivir with PEGylated dendrimer is translated to sustained drug release behaviour and reduced frequent drug dosing (Tammam et al., 2021). Nanoformulation of dexamethasone, administered via injection or inhalation, has been developed to improve anti-COVID-19 treatment efficacy (Lammers et al., 2020). It directs the potent corticosteroid drug to target phagocytic cells that initiate and propagate inflammation in lung, blood, myeloid and lymphoid tissues, which aids to control macrophage activation syndrome and cytokine storm that are responsible for COVID-19 related fatalities. Nanoformulation may potentiate the anti-edema and anti-fibrotic effects of dexamethasone by increasing drug bioavailability and activity in the inflamed lungs. SARS-CoV-2 is believed to gain access through ciliated cells and this was attributed to the high expression of ACE-2 receptors by the cells (Tammam et al., 2021). With reference to nasal application, nanoparticles can be functionalised with cilia binding ligand to promote targeted drug action on the infected ciliated cells. The anionic nanoparticles with diameters greater than 300 nm have been shown to increase cilia beating frequency upon their deposition on the cell target. The surface charge and size of nanoparticles can be tailored to promote viral clearance via the ciliary action. The diffuse alveolar damage in COVID-19 patients is characterized by the formation of fibrin-rich hyaline membranes (Tammam et al., 2021). The fibrin clot in the alveoli, with collagen accumulation, leads to the development of a fibrotic lung. Chitosan nanoparticles have been shown to display an intrinsic collagen binding affinity. Cys-Arg-Glu-Lys-Ala peptide-decorated nanoparticles are endowed with fibrin-fibrinogen binding ability. They are both potentially useful in targeted drug delivery to the infected lung and combat against the SARS-CoV-2.
Chitosan was presented as a promising anti-SARS-CoV-2 therapeutic in recent studies (Mohd Rasul et al., 2020). N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride has been shown to strongly interact with the recombinant ectodomain of Human Coronavirus (HCoV) S protein (Ciejka, Wolski, Nowakowska, Pyrc, & Szczubiałka, 2017). Importantly, chitosan was reported to have a high binding affinity for the SARS-CoV-2 S protein trimer cavity (Kalathiya et al., 2020). Chitosan nanoparticles were found to exert anti-inflammatory effects on lipopolysaccharide-inflamed Caco-2 cells where lipopolysaccharide-induced production of tumor necrosis factor-α, interleukin-8 and monocyte chemoattractant protein-1 was inhibited, unlike nanoparticles of cationic polyamidoamine dendrimer where cellular injury of lung was induced via deregulation of renin-angiotensin system owing to their ability to bind to the ACE2 receptors (Tammam et al., 2021).
1.2. Chitosan
Chitin is a natural polysaccharide produced from arthropod shells (Chandra Hembram, Prabha, Chandra, Ahmed, & Nimesh, 2016; Wang et al., 2019). Chitosan is a deacetylated form (> 50%) of chitin, that comprises repeating N-acetyl-D-glucosamine and D-glucosamine units (El Knidri, Belaabed, Addaou, Laajeb, & Lahsini, 2018). Chitosan and chitin have amino and hydroxyl moieties which provide sites for chemical modification to produce the desired physicochemical properties for anti-viral applications (El Knidri et al., 2018). The typical molecular weight of chitin and chitosan is 1030–2500 kDa and 100–500 kDa respectively (Ravi Kumar, 2000). Chitosan possesses a lower acetylation degree and no inter-sheet hydrogen bonds, and is thus more aqueous-soluble than chitin (El Knidri et al., 2018). Chitosan is soluble at pH < 6.5 due to the protonation of amino moieties conferring polycationic characteristics (El Knidri et al., 2018), but is insoluble at pH > 7 (Chandra Hembram et al., 2016). Chemical modification of chitosan increases its aqueous solubility in a broader pH range. This facilitates the use of chitosan as drug carrier and vaccine adjuvant.
The chitosan and chitin have identical molecular structures. They however exhibit different physicochemical attributes. The presence of polycationic moieties allows chitosan to form complexes with heavy metals and polyelectrolytes, undergo specific chemical modifications, and exhibit biological properties (Piegat, Goszczyńska, Idzik, & Niemczyk, 2019). Broadly, chitosan has been reported to exhibit anti-inflammatory, anti-oxidative, anti-fungal, anti-cancer, anti-bacterial and anti-viral activities (Sharma et al., 2019). The anti-viral potential of chitosan and its derivatives is reflected by their affinity to interact with the cell walls of microorganisms as a function of the state of amino protonation, molecular weight and degree of acetylation of chitosan (Dimassi, Tabary, Chai, Blanchemain, & Martel, 2018; Fernandes et al., 2010; Sharma et al., 2019), free radical-scavenging and anti-oxidative activity (Younes et al., 2014), and ability to induce chemotaxis of mononuclear and polymorphonuclear cells (Kumar, Muzzarelli, Muzzarelli, Sashiwa, & Domb, 2004), anti-inflammatory cytokine production (Tripathi & Singh, 2018) and immunomodulation (Adhikari & Yadav, 2018). Chitosan is mucoadhesive (Bonam et al., 2021). It readily adheres to the mucus of the host for a sufficient period of time to exert its anti-viral activity.
While there is a strong research interest on chitosan in drug delivery system development, few studies have focused on chitosan and its derivatives as an anti-viral therapeutic. With reference to COVID-19, inhalable nanovaccine comprising of chitosan and SARS-CoV-2 spike protein has been designed to provide a strong spike protein-specific antibody immune response and augment local mucosal immunity in bronchoalveolar lavage and lungs (Zhuo et al., 2021). Nonetheless, there is very limited knowledge about the relationship of the chitosan derivatives with their application as an anti-SARS-CoV-2 therapeutic. In this critical review, (1) developmental studies using chitosan and its derivatives as anti-viral therapeutics are identified, and the (2) characteristics of these anti-viral chitosan and derivatives are assessed along with their potential to translate into nanomedicine and for SARS-CoV-2 treatment.
2. Anti-viral activities of chitosan and its derivatives
The anti-viral activities of chitosan and its derivatives are affected by three main characteristics—molecular weight, degree of acetylation and degree of substitution—among others. This paper reports and evaluates the effects that the characteristics of chitosan-based therapeutics have on anti-viral activity, in relation to the mechanism of action of chitosan-based anti-viral therapeutics. Chitosan and its derivatives may exhibit anti-viral effects via acting on the virus or by host immunomodulation, where the host refers to the virus-infected organism.
2.1. Chitosan
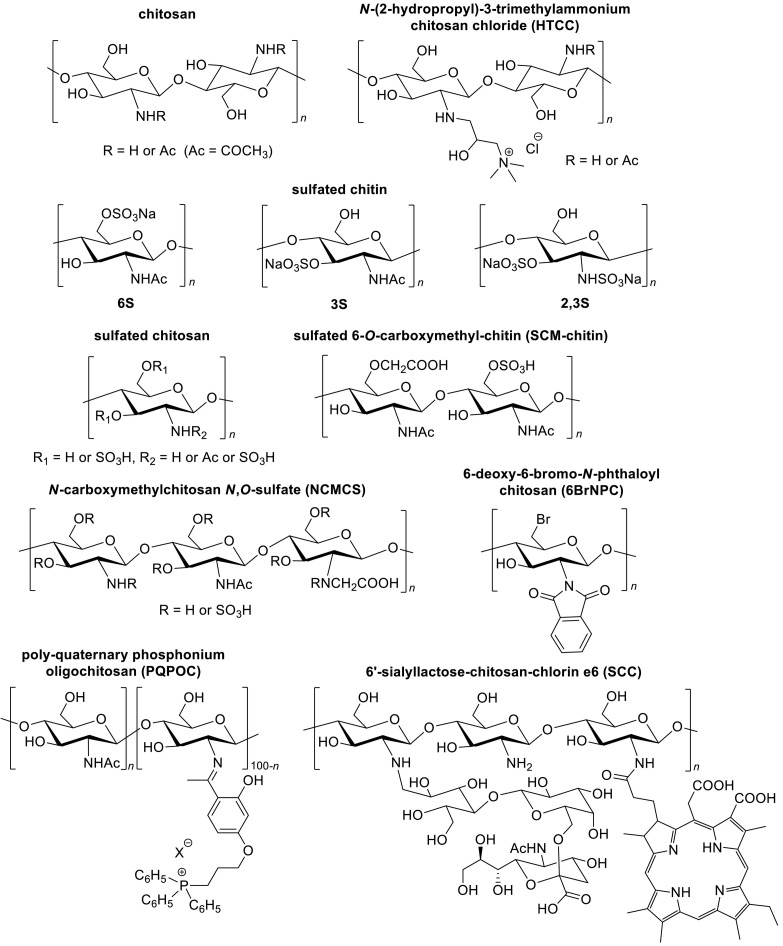
Moderately large to large molecular weight chitosan (53–1150 kDa, 9.1–32.5% degree of acetylation) inhibit the growth of feline calicivirus F-9, bacteriophages MS2 (MS2), phiX174 (Ai, Wang, Xia, Chen, & Lei, 2012; Davis, Zivanovic, D'Souza, & Davidson, 2012), Autographa californica multicapsid nucleopolyhedrovirus, Bombyx mori Nuclear Polyhedrosis (Ai et al., 2012) and Tobacco Mosaic Virus (TMV) (Davydova et al., 2011) (Fig. 1 ). Smaller chitosan with a molecular weight of 3.375–10.491 kDa and < 10% degree of acetylation was found to be active against the Newcastle Disease Virus (NDV) (He et al., 2016).
Fig. 1.
Chemical structures of chitosan and its anti-viral derivatives (adapted from Baranova, Shastina, & Shvets, 2011; Gao, Liu, Wang, Zhang, & Zhao, 2018; He et al., 2019; Ishihara et al., 1993; Jeong, Lee, Lee, & Na, 2020; Milewska et al., 2013; Nishimura et al., 1998; Sofy, Hmed, Abd El Haliem, Zein, & Elshaarawy, 2019; Sosa, Fazely, Koch, Vercellotti, & Ruprecht, 1991).
Increasing the molecular weight of chitosan has different effects on anti-viral activity against different virus types. While the inhibitory effect against MS2 increases with the molecular weight of chitosan (Davis, Zivanovic, Davidson, & D'Souza, 2015; Su, Zivanovic, & D'Souza, 2009), the growth inhibition of feline calicivirus F-9 and phiX174 is less dependent on the molecular weight variation of chitosan (Davis et al., 2012; Davis et al., 2015). Anti-TMV activity is greater with lower molecular weight chitosan at 15–17 kDa below which, there is no further increase in the anti-viral activity (Davydova et al., 2011).
Compared to molecular weight, the degree of acetylation of chitosan weakly affects the anti-viral activity. Generally, the anti-viral influence of chitosan is mediated via an enhancement of direct and/or complement-mediated chemotactic activities of macrophages and polymorphonuclear cells when chitosan of larger molecular weight and/or lower degree of acetylation is concerned (Chirkov, 2002; He et al., 2016). With reference to NDV, β-chitosan (3.375–10.491 kDa, < 10% degree of acetylation) shows a higher anti-viral activity than α-chitosan (He et al., 2016), possibly due to its parallel orientation contributing to a less compact intermolecular hydrogen bond network (Dimassi et al., 2018). This in turn increases its aqueous solubility and enhances its contact with the virus. Chitosan can inhibit neuraminidase, an enzyme involved in the release of influenza virus from the infected host cells (Park, Park, Jo, & Lee, 2010), and is thus potentially useful for negating virus replication. Moderately large to large molecular weight (50–1900 kDa) chitosan inhibits neuraminidase (Park, 2010). Chitosan oligosaccharides, composed of a few monomer units of chitosan derived from chitosan degradation (Guo et al., 2018; Naveed et al., 2019), show greater inhibition toward neuraminidase activity in vitro than hetero-oligosaccharides of lower molecular weight and lower degree of acetylation (Park et al., 2010).
Chitosan can exert its anti-viral effects via stimulating the innate immune system of hosts through the participation of cytokines such as interleukin-2, interferon-β, interferon-α and tumor necrosis factor-α (He et al., 2016). A study reported that the chitosan that induces innate immune responses against H7N9, H1N1 and H9N2 influenza viruses, is accompanied by increased levels of tumor necrosis factor-α, interferon-γ, monocyte chemoattractant protein-1 and interleukin-6 (Zheng et al., 2016). Chitosan regulates the functional activity of macrophages and granulocytes such as chemotaxis, leucocytosis, nitric oxide production, and the release of immune response mediators that stimulate T-helper cells production (Boroumand et al., 2021; Chirkov, 2002). Phagocytosis of chitosan elicits the production of active oxygen species and γ-interferon; these molecules may suppress virus replication via hindering the genomic RNA or early viral mRNA translation. Furthermore, chitosan stimulates the synthesis of leukotriene B4, prostaglandin E2, and inflammatory mediators that are able to activate the alternative pathway of the complement system to promote the clearance of dead pathogen and exert pro-inflammatory effects without inflammatory symptoms (Chirkov, 2002; Denk et al., 2017; Minami, Suzuki, Okamoto, Fujinaga, & Shigemasa, 1998).
Additionally, MS2, phiX174 and enteric virus surrogates (murine norovirus-1, feline calicivirus F-9) have viral capsid proteins with negatively charged functional groups that positively charged chitosan binds to (Zhu et al., 2020). This binding may damage viral structures such as capsid, tail sheath and tail fibres (Davis et al., 2015). Chitosan is reported to chelate trace metals, inhibit enzymatic activity, expose DNA and affect nucleic acid synthesis, thereby inactivating viral infection and replication.
2.2. N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride (HTCC)
HTCC (250 kDa) displays anti-viral activity against human coronaviruses such as HCoV-NL63, HCoV 229E, HCoV OC43, and HCoV HKU1 (Ciejka et al., 2017; Milewska et al., 2013) (Fig. 1). HTCC and HTCC nanospheres/microspheres bind to the recombinant ectodomain of the coronavirus S protein via electrostatic interaction as a function of the ionic strength of medium, resulting in the formation of protein-polymer complexes (Ciejka et al., 2017; Milewska et al., 2016). This in turn prevents viruses such as HCoV-NL63 from interacting with the ACE2 receptor, thus leading to inactivation of the virus. Although the degree of N-(2-hydroxypropyl)-3-trimethylammonium substitution (quaternization) could potentially alter the strength of electrostatic interaction between HTCC and human coronaviruses, there is no observable trend between the degree of N-(2-hydroxypropyl)-3-trimethylammonium substitution and the anti-viral activity level of HTCC. HTCC-63 and HTCC-65 (0.63 and 0.65 degree of N-(2-hydroxypropyl)-3-trimethylammonium substitution respectively) are active against HCoV-NL62, while HTCC-62 and HTCC-77 (0.62 and 0.77 degree of N-(2-hydroxypropyl)-3-trimethylammonium substitution respectively) demonstrate a greater anti-viral activity against HCoV-229E (Milewska et al., 2016). The relationship between cationic character of HTCC and anti-viral action remains elusive. The cationic character however is deemed to contribute to the aqueous solubility of HTCC and enable it to act on the viruses.
2.3. Sulfated chitin
Sulfated chitin is more hydrophilic than chitin. Low molecular weight (16–58 kDa) sulfated chitin exhibits anti-human immunodeficiency virus (HIV) activity (Nishimura et al., 1998) (Fig. 1). The site of sulfation exerts a greater influence on the anti-HIV activity than the total degree of substitution of sulfated chitin (Nishimura et al., 1998). Sulfated chitin 3S with a lower degree of sulfate substitution shows a greater anti-HIV-1 activity than 6S. Sulfation of the N-acetylglucosamine residue at the O-6 position seems to reduce the anti-viral activity of sulfated chitin against HIV-1. It is postulated that the sulfate moieties at O-3 and/or O-2 of chitin sulfate associate with the gp120 of HIV-1 in a specific manner (Nishimura et al., 1998). The binding of negatively charged HIV gp120 to positively charged T lymphocyte receptors via electrostatic forces triggers HIV entry into T lymphocyte host cells (Briz, Poveda, & Soriano, 2006). Through complexing HIV gp120 with 2,3-O-sulfonated chitin, the infection of T lymphocytes by HIV can be negated (Vo & Kim, 2010).
2.4. Sulfated chitosan and sulfated oligochitosan
Sulfated chitosan and sulfated oligochitosan, with molecular weight of 1.277–100 kDa, 0–25% degree of acetylation and 0.82–1.55 degree of sulfate substitution, exhibit anti-viral activity against human papillomavirus, HIV-1, vesicular stomatitis virus and NDV (Baranova et al., 2011; Gao et al., 2018; Karthik, Manigandan, Saravanan, Rajesh, & Chandrika, 2016; Stepanov et al., 2012) (Fig. 1). They inhibit viral entry into host cells via binding to viral surface glycoprotein (gp) receptors or viral capsid proteins; this binding prevents viruses from interacting with target cells, thus preventing virus-cell fusion (Baranova et al., 2011; Boroumand et al., 2021; Gao et al., 2018; Karthik et al., 2016). Alternatively, they down regulate host cell cellular pathway such as the PI3K/Akt/mTOR pathway which in turn, inhibits autophagy needed for viral entry (Gao et al., 2018).
The sulfation of chitosan is important in imparting effective and selective viral inhibition against HIV and other enveloped viruses. The anti-viral activity of sulfated chitosan and sulfated chitin are affected by the sulfation site, while that of sulfated chitosan is also enhanced by a higher sulfation degree (Ishihara et al., 1993; Karthik et al., 2016; Nishimura et al., 1998; Stepanov et al., 2012). Using aqueous-soluble β-chitosan as the polysaccharidic backbone may increase the reactivity of the chitosan derivative (Dimassi et al., 2018). Chitosan derivatives with richer sulfate content and higher molecular weight (100 kDa) display greater anti-viral activity than those of lower molecular weights (25 kDa) (Stepanov et al., 2012), except those that mediate their anti-viral action via immunomodulation. In the latter, the influence of molecular weight on nitric oxide production has mixed reports. Yang et al. (2018) reported that sulfated β-chitosan (5.030 kDa) induces the greatest rise in nitric oxide production in hosts compared to those with higher (14.481 kDa) or lower (3.169 kDa) molecular weights. However, low molecular weight sulfated chitosan oligosaccharides (< 1 kDa parent molecule) decrease nitric oxide production in another study (Kim et al., 2014). The conflicting results may have been confounded by the sulfation degree, position of substituted sulfate group and preparation method of sulfated chitosan, among other factors (Yang et al., 2018). Nitric oxide exhibits anti-viral activity against viruses such as herpes virus, RNA viruses and SARS coronaviruses (Akaike & Maeda, 2000; PeÑarando, Aranda, & RodrÍguez-Ariza, 2019). It inhibits viral replication and respiratory tract infection via altering essential viral proteins and viral RNA synthesis (PeÑarando et al., 2019; Xu, Zheng, Dweik, & Erzurum, 2006). While nitric oxide modulates host immune response, excessive nitric oxide levels may suppress T-cell-mediated immune responses, and contribute to respiratory tract inflammation and injury (PeÑarando et al., 2019). The molecular weight of sulfated chitosan could possibly be adjusted to modulate nitric oxide levels and thus immune response, specific to the inflammatory state of patients.
2.5. Sulfated 6-O-carboxymethyl-chitin (SCM-chitin)
SCM-chitin (430 kDa, 0.0766–1.69 degree of sulfate substitution, 0.56 degree of carboxymethyl substitution) significantly inhibits Friend murine leukemia helper virus (F-MuLV) and herpes simplex virus-1 growth (Ishihara et al., 1993) (Fig. 1). Viruses may infect host cells via specific binding to host cell receptors. Since there is no common host cell adsorption receptor between F-MuLV and herpes simplex virus, SCM-chitin is likely to exhibit its anti-viral effect via non-specific binding to the viruses. A high molecular weight (430 kDa) and/or the presence of sulfate moiety in SCM-chitin could be important contributor(s) to the anti-viral activity, since its non-sulfated counterpart–CM-chitin–of low molecular weight (63 kDa) has no inhibitory effect against F-MuLV and herpes simplex virus (Ishihara et al., 1993).
2.6. N-carboxymethylchitosan N,O-sulfate (NCMCS)
NCMCS is shown to potently inhibit HIV-1 replication in human T helper cells and Rausher murine leukemia virus (Sosa et al., 1991). The anti-viral NCMCS has a molecular weight of 7.4 kDa, 16% degree of acetylation, and 0.18 degree of N-carboxymethyl substitution (Sosa et al., 1991) (Fig. 1). Ampholytic polymer, NCMCS, varies in its charge distribution depending on the degree of sulfate and carboxymethyl substitution, and the pH and ionic strength of the medium (Sosa et al., 1991). The zwitterionic characteristic of NCMCS could enhance its binding to the cell or the virus, and cellular permeability. Moreover, the anti-viral activity of NCMCS is postulated to be affected by the site of sulfation with O-2 and O-3 sulfated derivatives being the most active (Boroumand et al., 2021; Vo & Kim, 2010).
The highly negatively charged and ampholytic NCMCS is electrostatically attracted to and binds to the gp120 of HIV-1 (Sosa et al., 1991). HIV gp120 is a major viral coat glycoprotein receptor of the HIV-1 virus, and is important for HIV-1 entry into host cells (Chirkov, 2002). By binding to the gp120, NCMCS inhibits HIV-1 viruses from adsorbing to specific target CD4+ cell receptors (Sosa et al., 1991). NCMCS also competitively inhibits the virus-specific reverse transcriptase (Chirkov, 2002). Overall, NCMCS is likely to inhibit HIV-1 infection via blocking the fusion of viral and cell membranes and/or inhibition of reverse transcription of the viral genome.
2.7. 6-Deoxy-6-bromo-N-phthaloyl chitosan (6BrNPC)
6BrNPC with a molecular weight of 1160 kDa and 0.5471 degree of bromine substitution shows anti-viral activity against NDV (He et al., 2019) (Fig. 1). It is more active than the non-brominated chitosan derivatives (N-phthaloyl chitosan and 6-aminoethylamino-6-deoxy-N-phthaloyl chitosan). 6BrNPC increases the expression level of tumor necrosis factor-α and interferon-β in hosts; these molecules exert an immune response against NDV, and inhibit virus transcription (He et al., 2019). It was also reported that bromination of chitosan alters its electron density and this greatly improves its anti-viral activity (He et al., 2019). Similar to bromine drugs such as ambroxol, bromocriptine and brivudine, its exact mechanism remains unknown.
2.8. Chitosan-sialyllactose conjugate
Chitosan-sialyllactose conjugate interacts with influenza viruses via high affinity binding with hemagglutinin of the virus through its sialyllactose component, and this binding between the chitosan-sialyllactose conjugate and the virus in turn, inhibits viral binding to the host cells and infection (Li, Wu, Gao, & Cheng, 2011). The chitosan-sialyllactose conjugate is characterized by a molecular weight of 219.6 kDa and 0.156–0.623 degree of sialyloligosaccharide substitution (Fig. 1). Sialic acid analogues in the form of ligands alone do not successfully inhibit influenza virus activity via hindering its binding to viral receptors of the hosts (Umemura et al., 2008). The monomeric 6'sialyllactose-chlorin e6 binds to viral hemagglutinin of influenza in a weaker manner than 6′-sialyllactose-chitosan-chlorin e6 (SCC), which has a higher ligand content (Jeong et al., 2020). The interaction between the sialyllactose ligand and the hemagglutinin of influenza virus depends on their binding affinity and the accessibility of the sialyllactose ligand to the hemagglutinin of influenza virus (Umemura et al., 2008). A higher degree of sialyllactose substitution promotes a greater probability of ligands binding to the hemagglutinin of influenza virus, which results in a greater inhibition of influenza virus infection.
It was reported that SCC displays 23% and 50% higher anti-viral effects on influenza A and B respectively than oseltamivir, the conventional anti-viral drug (Jeong et al., 2020). SCC gives full protection from influenza virus infection in mice subjected to laser irradiation (Jeong et al., 2020). Here, chitosan serves as a backbone for conjugation with multiple 6′-sialyllactose molecules which influenza hemagglutinin recognises and binds to (Jeong et al., 2020). The chlorin e6 moiety produces reactive oxygen species that permanently damage the viral membrane when excited by light irradiation, thus causing irreversible inactivation of the influenza virus (Jeong et al., 2020). Therein, 6′-sialyllactose-chitosan-chlorin e6 possesses both prophylactic and anti-viral potential.
To prevent influenza virus infection, the length of chitosan backbone—governed by its polymerisation degree—should exceed the virus particle diameter (100 nm) to introduce a physical barrier between the virus and the host (Umemura et al., 2008). As the polymerisation degree of sialylglycopolymer-conjugated chitosan increases from 20 to 1430, its strength of inhibition against three strains of human influenza viruses (H1N1, H3N2 and B/Shanghai/ 361/2002) becomes greater. Aqueous solubility is lowered with an increase in the polymerisation degree of chitosan. Chitosan with optimal aqueous solubility and anti-viral activity has been found to associate with a polymerisation degree of 500, 0.15 degree of sialyloligosaccharide substitution and a molecular weight of 219.6 kDa (Umemura et al., 2008). At 500 polymerisation degree, the “tail” of sialyloligosaccharide-conjugated chitosan is sufficiently long to prevent the chitosan-bound virus particles from approaching the host cell surfaces.
2.9. Chitosan-peptide conjugate
Chitosan oligomers (< 1 kDa, 10% degree of acetylation), conjugated with tripeptides containing tryptophan, methionine and glutamine, have been reported to show anti-HIV-1 activity (Karagozlu, Karadeniz, & Kim, 2014). Chitosan-peptide conjugates bind to viral glycoprotein gp41 and thus inhibit gp41 from interacting with the receptors on CD4 host cell membrane, impeding HIV-1 fusion with infected and uninfected cells. The study also reported that chitosan-peptide conjugates protect cells from lysis and inhibit HIV-triggered syncytia formation in a co-culture assay. The type, sequence and number of specific amino acid attached to the oligochitosan backbone could be key factors in the anti-viral effect displayed by chitosan-peptide conjugates. Notably, the peptide sequence was found to be similar to a strong HIV-1 fusion inhibitor (CP621–652), which inhibits the protein binding domain of the HIV-1 gp41 (Karagozlu et al., 2014).
2.10. Poly-quaternary phosphonium oligochitosan (PQPOC)
PQPOC with 0.2855 degree of poly-quaternary phosphonium substitution shows greater anti-viral activity against feline calicivirus, hepatitis A virus, and Coxsackievirus B4 than that of lower substituent content (Sofy et al., 2019) (Fig. 1). PQPOC is constituted of a permanently positive charged moiety in the polymer chain and exhibits a higher ionic character than the zwitterionic chitosan. The positively charged PQPOC is likely to interact with negatively charged viral capsids, which may block viral replication via causing structural injury to the viral capsids. The chitosan backbone in PQPOC could hinder the virus propagation by promoting ribonuclease activity in hosts, which increases viral RNA degradation, and therefore, hinders virus transcription and translation.
3. Insights into anti-viral action of chitosan-based therapeutics
Overview of the literature indicates that the chitosan derivatives that inhibit viral replication via binding to enveloped viruses have a molecular weight of 1–430 kDa, 14–16% degree of acetylation, 0.18–0.56 degree of carboxymethyl substitution, 0.0766–1.69 degree of sulfate substitution and 0.62–0.77 degree of N-(2-hydroxypropyl)-3-trimethylammonium substitution. The chitosan derivatives that inhibit enveloped NDV via stimulation of the host immune response are generally characterized by a molecular weight of 1–1160 kDa, < 10% degree of acetylation, and 0.5471 degree of bromine substitution.
The anti-viral effects of chitosan can be virus-specific and are dependent on the molecular weight, degree of acetylation, and/or conjugate type and its substitution site and extent. Generally, chitosan-based therapeutics express positive charges that are favourable to interact with the protein domain of the virus or negatively charged virus. The positive charges promote aqueous solubility at physiological pHs (Chandra Hembram et al., 2016) and this enhances their contact with the target. Chitosan-based therapeutics are characterized by a polymerisation degree that enables them to protect the viral binding site of the host from the virus.
The relationship of the chemical descriptor with the anti-viral activity, however, may not be linear. A rise or reduction in the molecular weight of a polymeric therapeutic may increase its anti-viral effects. Each chitosan-based therapeutic is likely to have an optimal molecular weight, specific to the virus used in the study. The extent of conjugate substitution may not be critical to combat the virus, when compared to its substitution site or vice versa, probably depending on the target variant of virus and choice of parent polymer. The choice of substituent to be conjugated with chitosan can be critical in the eradication of a specific virus. A study reported that while some sulfonated non-chitosan polymers exhibited anti-H3N2 influenza virus activity, N-sulfonated chitosan did not (Ciejka et al., 2016). A change in chemical characteristics, such as molecular weight, may affect the anti-viral mechanisms of the therapeutics. Chitosan-based therapeutics that directly interact with virus particles tend to be of larger molecular weight. Conversely, the chitosan-based therapeutics that activate and increase the host immune response had mixed reports on their molecular weight effects.
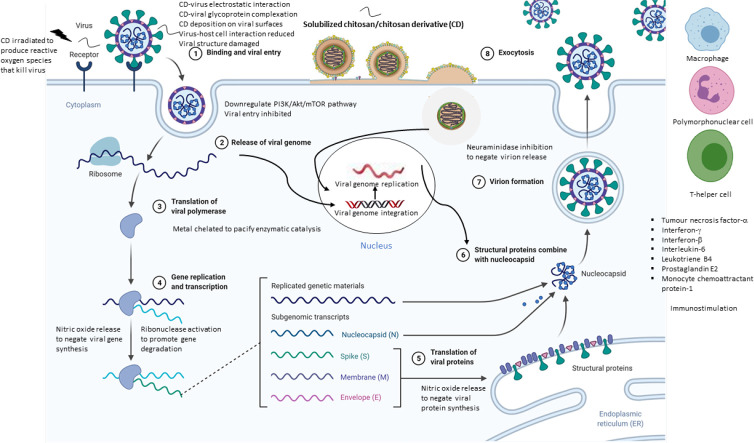
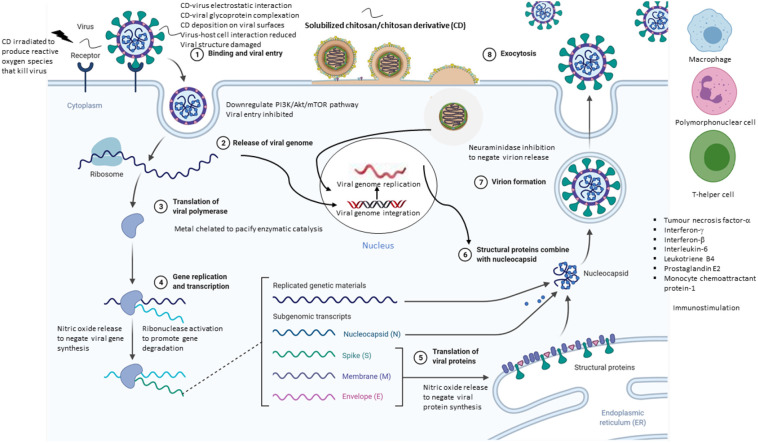
Fig. 2 summarizes the general anti-viral mechanisms of actions of the chitosan and chitosan derivatives. Broadly, chitosan-based therapeutics act on non-enveloped viruses (murine norovirus-1, MS2, feline calicivirus F-9, human papillomavirus, hepatitis A virus, Coxsackievirus B4, phi X174) through viral capsid interaction and (human papillomavirus) through host immunomodulation. One or more mechanisms of action may take place. The positively charged chitosan has been postulated to bind to viral nucleic acid and reduce the viral genome integrity (Zhu et al., 2020). With respect to enveloped viruses, chitosan-based therapeutics may stimulate the immune system (to inhibit NDV), bind to envelope glycoprotein to inhibit viral entry (of HIV-1, influenza) and/or inhibit the virus growth (of herpes simplex virus) in a non-specific manner. The anti-viral actions of such therapeutics and their respective physicochemical constructs can be exploited to eradicate the SARS-CoV-2, another variant of enveloped virus.
Fig. 2.
General anti-viral mechanisms of actions of the chitosan and chitosan derivatives (Created with BioRender.com).
SARS-CoV-2 infection is characterized by pulmonary mucus hypersecretion and mucus plugging in COVID-19 patients (Kumar, Binu, Devan, & Nath, 2021). The mucin gene is overexpressed under the influence of pro-inflammatory cascades involving interferon-γ and interferon-β which activate aryl hydrocarbon receptor signaling thereby leading to mucus hypersecretion, impaired mucociliary clearance, airway obstruction and respiratory distress. Mucus targeting is foreseeably a viable approach in the delivery of drugs to a site where a high viral load is reflected by its pathophysiological conditions. The chitosan is a mucoadhesive (Gulati, Dua, & Dureja, 2021). As an anti-viral therapeutic, the high affinity for mucus enables it to interact with the host and/or virus in a close proximity and exert the biological action. The chitosan derivatives adhere to mucus via hydrogen bonding and/or electrostatic attraction (Wang et al., 2020). Quaternization of chitosan at C2 —N (HTCC) or attaching a phosphonium moiety to the chitosan (PQPOC) provides permanent positive charges that electrostatically bind to the negatively charged sialic acid of the mucus. Carboxymethylation and sulfation could promote chitosan-mucus interaction via the formation of hydrogen bonding. With reference to 6BrNPC and SCC that active against the enveloped viruses, the introduction of phthaloylation and sialyllactose is expected to reduce the intermolecular binding between the chitosan molecules, thus facilitating the chitosan to bind to the mucus. Overall, the binding affinity of chitosan derivatives for mucus is governed by a complex interplay of chemistry of substituents and their degree of substitution, molecular weight and chain flexibility of derivatives, as well as ionic and pH environments of mucus as a function of the disease progression (Collado-Gonzalez, Espinosa, & Goycoolea, 2019).
4. Manufacturability of chitosan-based therapeutics into nanomedicine
Chitosan is widely employed as the excipient of choice in the development of nanomedicine for a wide range of diseases (Alhajj et al., 2020; Calderon et al., 2013; Loutfy et al., 2020; Meng, Sturgis, & Youan, 2011; Xue et al., 2015; Zhao et al., 2014). With reference to anti-viral application, several attempts to formulate chitosan nanoparticles for the treatment of HIV, and hepatitis B and C viruses have been initiated (Loutfy et al., 2020; Meng et al., 2011; Xue et al., 2015). Chitosan derivatives of molecular weight of 3–1000 kDa, 5–27.8% degree of acetylation and 0.07–1.48 degree of conjugate substitution, have been used in nanoparticle development for pharmaceutical applications (Table 1 ). The synthesized nanoparticles exhibit a wide range of size (5.1–1279 nm), polydispersity index (0.034–0.780), and zeta potential (−50.5 to +55.2 mV) that are influenced by choice of pH and/or substituent. They are spherical or cuboidal, and are generally presenting with smooth surfaces. While chitosan is usually partially crystalline, drug-loaded chitosan nanoparticles are typically amorphous with a few exceptions. Drug loading into chitosan nanoparticles has varied or no effect on the size, polydispersity and surface charge of chitosan, depending on its formulation and preparation method.
Table 1.
Physicochemical attributes of chitosan and chitosan-based nanomedicine.
| Chitosan |
Nanomedicine |
Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight (kDa) | Degree of acetylation (%) | Substituent | Degree of conjugate substitution | Site of substitution | Size (nm) |
Polydispersity index | Zeta potential (mV) | Surface morphology | Crystallinity | Processing method | |
| 3 | 9.7 |
|
|
– | 163.8–237.3 | 0.111–0.134 | +25.9 − +34.1 (pH 4.5) + 1.5 − +14.2 (pH 8.5) |
Spherical, uniform diameter | – | Crosslinking; immobilisation of bromelain on nanoparticles surface | Wang et al., 2018 |
| 5 | – | Dithiodipropionic acid mono benzyl ester | 0.059–0.063 | – | 78.8–83.4 | 0.183–0.207 | +12.3 − +13.1 | Spherical | – | – | Xu et al., 2020 |
| 5–20 | – |
|
|
2–NH2 and 6–OH | 134–232 | 0.09–0.27 | −50.5 to −24.2 | Spherical | – | Lyophilisation, centrifugation and dialysis technique | Zhang, Huo, Zhou, Yu, & Wu, 2009 |
| 5; 90 (protasan) |
< 10; 10–25 (protasan) |
– | – | – | 175.2–287.9 (anionic starch-chitosan core polyplexes) 204.6–214.0 (protasan coated anionic core polyplexes) |
0.18–0.27 (anionic starch-chitosan core polyplexes) 0.14–0.17 (protasan coated anionic core polyplexes) |
−29.8 to −14.6 (anionic starch-chitosan core polyplexes) + 25.5 − +28.0 (protasan coated anionic core polyplexes) |
Spherical | – | – | Yasar et al., 2018 |
| 10–50 | 17 | – | – | – | 100–200 | 0.15–0.35 | +30 | – | – | – | Kolonko, Bangel-Ruland, Goycoolea, & Weber, 2020 |
| 23.7 | 20 | Thio group | – | – | 5.10–5.32 | – | +24.8 − +32.8 | Spherical, smooth, uniform size distribution | Crystalline | Ionic gelation/crosslinking | Rajawat, Shinde, & Nair, 2016 |
| 50 | 15.3 | 278 | +14.9 | Spherical, smooth, uniform size distribution | – | Depolymerisation and lyophilisation | Jamali et al., 2018 | ||||
| 50 | 5 | (2-Hydroxy)-propyl-3-trimethylammonium chloride | 0.425 | N-substituted | 87–1025 | 0.15–0.30 | +19.0 − +22.5 (NC); -24.6 – −5.5 (NE) |
Spherical | – | Complexation and lyophilisation | Sun et al., 2019 |
| 50–136 | – | Methylene phosphonic acid | 0.37–0.46 | – | – | – | – | – | Amorphous | – | Ramos et al., 2003 |
| 50–190 | 15–25 | – | – | – | 163–306 | – | +9.8 − +31.4 | – | – | Confined Impinging jets mixing; multi-inlet vortex mixing; solvent displacement method; freeze drying | Zelenkova, Onnainty, Granero, Barresi, & Fissore, 2018 |
| 50–190 | 17.4 | – | – | – | 36 | 0.41 | +30 | Spherical | – | – | Canepa et al., 2017 |
| 50–190 | 8 | – | – | – | 182.4–602.4 | 0.226–0.436 | +46.4 − +55.23 | Spherical | – | – | Meng et al., 2011 |
| 50–190 | 15–25 | – | – | – | 239.2–365.8 | – | +42.1 − +53.3 | Spherical and cuboidal | – | Ionic gelation | Ng, Selvarajah, Hussein, Yeap, & Omar, 2020 |
| 50–190 | 12–13 | – | – | – | 113 | 0.104 | −34 | Spherical, smooth, uniform size distribution, non-aggregated, well distributed | Amorphous | Ionic gelation | Singh et al., 2018 |
| 60–170 | 15 | – | – | – | 213.7–276.1 | 0.301–0.416 | +9.84 − +13.12 | Spherical | – | Physical adsorption | Li et al., 2019 |
| 190 | 5 | a) Pelargonic acid b) Lauric acid |
a) 0.07 b) 0.085 |
– | 147–192 | 0.13–0.18 | +18.9 − +35.1 | – | – | Stepwise extrusion through inorganic membranes | Kozhikhova et al., 2018 |
| 190–310 | 15 | – | – | – | 208–497 | 0.26–0.78 | +14.3 − +24.5 | – | – | Ionotropic gelation/crosslinking | Russo et al., 2014 |
| 190–310 | 15–25 | Carboxymethyl group | – | O- and N,O-substituted | 80–100 | high | −2 to −13 | Core-shell formation | – | One-step nanoprecipitation | Shanavas et al., 2019 |
| 190–310 | 15–25 |
|
– | – | 15–25 | – | – | Core-shell formation | Crystalline | – | Hasantabar, Tashakkorian, & Golpour, 2019 |
| 250 (glycol chitosan) | 10 | Farnesyl group | 0.109–0.158 | – | 200–500 | 0.15–0.28 (pH 5) | +25 − +47 | Spherical | – | Facile synthesis | Ho et al., 2018 |
| 282 | 27.8 | – | – | – | 40–400 | 0.31–0.67 | +18.9 − +34.6 | Spherical | – | – | Chiesa et al., 2019 |
| 310–375 | ≤ 25 | – | – | – | 136–379 | 0.24–0.47 | +0.1 − +28.5 | Core-shell formation | – | Ionic gelation | Ngo, Ezoulin, Murowchick, Gounev, & Youan, 2016 |
| 1000 | 5 | – | – | – | ~10 (carboxylic multiwalled carbon nanotube) | – | – | Ill-defined interface between inorganic and organic phases | – | in situ precipitation | Chen, Hu, Shen, & Tong, 2013 |
| – | 17.6 | – | – | – | 324.6–1279.0 | – | – | Spherical, smooth, uniform size distribution | – | Ionic gelation and freeze drying | Soh et al., 2019 |
| – | 15 | Hydroxypropyl-trimethyl-ammonium chloride | 0.3404 | O-substituted | 199.42–317.05 | – | +46.36 | Spherical | Amorphous | Ionic crosslinking | Dai et al., 2015 |
| – | <10 | – | – | – | 50 | – | −26.4 to −28.1 | Spherical | – | Double crosslinking | Zhou et al., 2020 |
| – | 15–25 | – | – | – | 39.4 | 0.034 | +32.4 | – | – | – | Moodley & Singh, 2020 |
| – | – | – | – | – | 299.1–387.1 | 0.221–0.242 | +22.3 − +38.2 | Spherical | – | Emulsifier-free emulsion polymerisation | Hunsawong et al., 2015 |
The molecular characteristics of chitosan that are used in the nanomedicine development resemble those of chitosan derivatives that show positive anti-viral effects and can potentially be developed as anti-SARS-CoV-2 polymeric therapeutics. It is hypothesised that the chitosan-based therapeutics, with proven anti-SARS-CoV-2 if any, can be easily processed into nanomedicine that can possibly bring about a higher interaction propensity with the viral transmembrane spike glycoprotein. As the chitosan derivatives are largely ionic in nature, their required aqueous solubility for nanoparticle preparation can be met via simple solution pH modulation or solubilization process. The nanofabrication of chitosan-based anti-SARS-CoV-2 therapeutics may be introduced with an additional anti-viral agent to synergistically promote the therapeutic effect. An example is PQPOC, which synergistically stabilises silver nanoparticles that have the ability to bind to the viral envelope glycoprotein, through the formation of a PQPOC‑silver nanoparticle biocomposite (Karagozlu et al., 2014; Sofy et al., 2019). This prevents the virus from binding and entering into the host cell to a greater extent than the chitosan therapeutics alone.
Polymersomes are artificial vesicles that are constituted of amphiphilic block or grafted copolymers (Al-Hatamleh et al., 2021). They exhibit a high colloidal stability and ease of being decorated with targeting ligand. Lately, the polymersomes have been advocated as an anti-viral drug carrier. In comparison to liposomes (3–5 nm), the introduction of polymers provides a resultant bilayer thickness of 5–50 nm. The polymersomes are physicochemically more stable than liposomes. With reference to anti-viral chitosan therapeutics that are polymeric in nature, their use in the development of polymersomes is expected to lead to the formation of a strong and mucoadhesive nanostructure with prolonged residence time and biological action in the nanoscale. Combination of chitosan therapeutic with anti-viral drug in polymersomes can have their anti-viral actions synergized with chitosan controlling drug release and activity.
5. Conclusion and future perspectives
The present review highlights that chitosan is a viable biopolymer for the development of broad-spectrum anti-viral therapeutics. Structural modification of chitosan through conjugating with hydroxypropyl trimethylammonium, sulfate, carboxymethyl, bromine, sialyllactose, peptide and phosphonium moieties grants or greatly improves the anti-viral activity of the parent polymer. Chitosan conjugates—such as HTCC, sulfated chitosan, SCM-chitin, NCMCS, 6BrNPC and SCC —exhibit broad-spectrum anti-viral activity against human coronavirus, HIV, herpes simplex virus, influenza virus, NDV, human papillomavirus and F-MuLV. Their anti-viral effectiveness depends on the interplay of molecular weight, degree of acetylation, degree and site of conjugate substitution. One or more substituents may be introduced to a chitosan monomeric residue. The resultant conjugate may be blended with alternative anti-viral agents such as silver nanoparticles in the advancement of a more efficacious nanomedicine (Sofy et al., 2019). Further, it is envisaged that the anti-viral chitosan therapeutics can be processed into nanomedicine as their physicochemical characteristics are similar to those of chitosan and derivatives used in nanomedicine design. The nano-enabling process is foreseen to promote the anti-viral activity of the chitosan therapeutics to a greater extent.
Generally, the chitosan and its derivatives are associated with low cytotoxicity levels at the effective in vitro therapeutic doses (Artan, Karadeniz, Karagozlu, Kim, & Kim, 2010; Ciejka et al., 2016; Gao et al., 2018; Karagozlu et al., 2014; Milewska et al., 2013, Milewska et al., 2016; Nishimura et al., 1998; Sofy et al., 2019; Sosa et al., 1991; Stepanov et al., 2012). Several in vivo studies conducted in mice and chicken eggs similarly suggest that the chitosan-based anti-viral therapeutics are relatively safe (He et al., 2019; Ishihara et al., 1993; Jeong et al., 2020). The median inhibitory concentration (IC50) values of repurposed drugs such as chloroquine and remdesivir are 9.12 μM (4.71 μg/mL) and 11.41 μM (6.88 μg/mL) respectively against SARS-CoV-2 (Jeon et al., 2020). The chitosan-based therapeutics are characterized by IC50 values of 0.28–100 μg/mL against various viruses (Gao et al., 2018; Karagozlu et al., 2014; Milewska et al., 2013; Sosa et al., 1991; Stepanov et al., 2012; Umemura et al., 2008). Current studies of chitosan and its derivatives as anti-viral therapeutics are mainly conducted at the in vitro level (Ai et al., 2012; Artan et al., 2010; Ciejka et al., 2016, Ciejka et al., 2017; Davis et al., 2012; Davis et al., 2015; Gao et al., 2018; Karagozlu et al., 2014; Karthik et al., 2016; Li et al., 2011; Milewska et al., 2013, Milewska et al., 2016; Nishimura et al., 1998; Park, 2010; Park et al., 2010; Sofy et al., 2019; Sosa et al., 1991; Stepanov et al., 2012; Su et al., 2009; Umemura et al., 2008; Zhu et al., 2020). Strong chitosan-based anti-viral therapeutics with relatively low IC50 values may be effective against SARS-CoV-2. However, in vitro studies by means of cell cultures may not fully account for their anti-viral effects in organs and complex systems of organisms. In vivo evaluation is required to fully elucidate the actual potential and side effects of chitosan-based therapeutics.
Chitosan can potentially inhibit SARS-CoV-2 via binding with the SARS-CoV-2 spike glycoprotein trimer cavity (Kalathiya et al., 2020). SARS-CoV-2, as an enveloped virus, may be treated with chitosan-based therapeutics developed with due consideration to the structure-activity relationship of chitosan derivatives substituted with carboxymethyl, sulfate, bromine, N-(2-hydroxypropyl)-3-trimethylammonium, phthaloyl and sialyllactose moieties that are active against other enveloped virus variants. Future studies may also research on the optimal characteristics— molecular weight, degree of acetylation, degree and site of conjugate substitution—of chitosan which will provide the greatest anti-viral effect against SARS-CoV-2. Further, conjugating chitosan with optimal anti-viral characteristics by other anti-SARS-CoV-2 ligands, may confer additive or synergistic effects, and greater effectiveness in inhibiting SARS-CoV-2 replication.
The current pandemic preparedness planning is focused on expediting vaccine research and repurposing the use of approved anti-virals and other drugs (Cho & Glenn, 2020). Such an approach may be insufficient in promptly controlling the spread of contagious viral infections such as COVID-19. It has been highlighted that investing time and resources to develop an anti-viral therapeutic for a single virus may not be worthwhile (Cho & Glenn, 2020). Therefore, an effective broad-spectrum anti-viral strategy will be the next generation solution to work against various viruses, including COVID-19 and other novel ones. Such an approach is foreseeable to enable potential viable therapeutics to greatly improve pandemic preparedness at early stages of outbreaks. The anti-viral chitosan derivatives described herein are characterized by a broad spectrum anti-viral activity. They can be the potential lead in the development of anti-SARS-CoV-2 therapeutics and novel ones in future.
CRediT authorship contribution statement
Rebecca Shu Ling Tan: Validation, Formal analysis, Investigation, Data curation, Writing – original draft. Pouya Hassandarvish: Validation, Data curation, Writing – review & editing. Chin Fei Chee: Validation, Data curation, Writing – review & editing. Lai Wah Chan: Methodology, Resources, Writing – review & editing, Supervision. Tin Wui Wong: Conceptualization, Methodology, Validation, Formal analysis, Resources, Writing – review & editing, Supervision.
Declaration of competing interest
There are no conflicts of interest to declare.
Acknowledgment
The authors wish to express their heart-felt thanks to the National University of Singapore for the facility provided throughout the study.
References
- Adhikari H., Yadav P. Anticancer activity of chitosan, chitosan derivatives, and their mechanism of action. International Journal of Biomaterials. 2018;2018:1–29. doi: 10.1155/2018/2952085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai H., Wang F., Xia Y., Chen X., Lei C. Antioxidant, antifungal and antiviral activities of chitosan from the larvae of housefly. Musca domestica L. Food Chemistry. 2012;132(1):493–498. doi: 10.1016/j.foodchem.2011.11.033. [DOI] [PubMed] [Google Scholar]
- Akaike T., Maeda H. Nitric oxide and virus infection. Immunology. 2000;101(3):300–308. doi: 10.1046/j.1365-2567.2000.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhajj N., Zakaria Z., Naharudin I., Ahsan F., Li W., Wong T.W. Critical physicochemical attributes of chitosan nanoparticles admixed lactose-PEG3000 microparticles in pulmonary inhalation. Asian Journal of Pharmaceutical Sciences. 2020;12:374–384. doi: 10.1016/j.ajps.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hatamleh M.A.I., Hatmal M.M., Alshaer W., Rahman E.N.S.E.A., Mohd Zahid M.H., Alhaj-Qasem D.M., Chan Y.Y., Alias I.Z., Jaafar J., Ferji K., Six J.-L., Uskokovic V., Yabu H., Mohamud R. COVID-19 infection and nanomedicne applications for development of vaccines and therapeutics: An overview and future perspectives based on polymersomes. European Journal of Pharmacology. 2021;896 doi: 10.1016/j.ejphar.2021.173930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirian E.S., Levy J.K. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020;9 doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artan M., Karadeniz F., Karagozlu M.Z., Kim M.M., Kim S.K. Anti-HIV-1 activity of low molecular weight sulfated chitooligosaccharides. Carbohydrate Research. 2010;345(5):656–662. doi: 10.1016/j.carres.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Baranova E.O., Shastina N.S., Shvets V.I. Polyanionic inhibitors of HIV adsorption. Russian Journal of Bioorganic Chemistry. 2011;37(5):527–542. doi: 10.1134/s1068162011050037. [DOI] [PubMed] [Google Scholar]
- Bar-On Y.M., Flamholz A., Phillips R., Milo R. SARS-CoV-2 (COVID-19) by the numbers. eLife. 2020;9 doi: 10.7554/eLife.57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonam S.R., Kotla N.G., Bohara R.A., Rochev Y., Webster T.J., Bayry J. Potential immuno-nanomedicine strategies to fight COVID-19 like pulmonary infections. Nano Today. 2021;36 doi: 10.1016/j.nantod.2020.101051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroumand H., Badie F., Mazaheri S., Seyedi Z.S., Nahand J.S., Nejati M., Baghi H.B., Abbasi-Kolli M., Badehnoosh B., Ghandali M., Hamblin M.R., Mirzaei H. Chitosan-based nanoparticles against viral infections. Frontiers in Cellular and Infection Microbiology. 2021;11 doi: 10.3389/fcimb.2021.643953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., Bolling M.C., Dijkstra G., Voors A.A., Osterhaus A.D.M.E., van der Voort P.H.J., Mulder D.J., van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) Journal of Pathology. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briz V., Poveda E., Soriano V. HIV entry inhibitors: Mechanisms of action and resistance pathways. Journal of Antimicrobial Chemotherapy. 2006;57(4):619–627. doi: 10.1093/jac/dkl027. [DOI] [PubMed] [Google Scholar]
- Burki T.K. The russian vaccine for COVID-19. The Lancet Respiratory Medicine. 2020;8(11):e85–e86. doi: 10.1016/S2213-2600(20)30402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon L., Harris R., Cordoba-Diaz M., Elorza M., Elorza B., Lenoir J., Adriaens E., Remon J.P., Heras A., Cordoba-Diaz D. Nano and rnicroparticulate chitosan-based systems for antiviral topical delivery. European Journal of Pharmaceutical Sciences. 2013;48(1–2):216–222. doi: 10.1016/j.ejps.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Canepa C., Imperiale J.C., Berini C.A., Lewicki M., Sosnik A., Biglione M.M. Development of a drug delivery system based on chitosan nanoparticles for oral administration of interferon-alpha. Biomacromolecules. 2017;18(10):3302–3309. doi: 10.1021/acs.biomac.7b00959. [DOI] [PubMed] [Google Scholar]
- Chandra Hembram K., Prabha S., Chandra R., Ahmed B., Nimesh S. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artificial Cells, Nanomedicine, and Biotechnology. 2016;44(1):305–314. doi: 10.3109/21691401.2014.948548. [DOI] [PubMed] [Google Scholar]
- Chen L., Hu J.X., Shen X.Y., Tong H. Synthesis and characterization of chitosan-multiwalled carbon nanotubes/hydroxyapatite nanocomposites for bone tissue engineering. Journal of Materials Science-Materials in Medicine. 2013;24(8):1843–1851. doi: 10.1007/s10856-013-4954-x. [DOI] [PubMed] [Google Scholar]
- Chiesa E., Greco A., Riva F., Tosca E.M., Dorati R., Pisani S., Modena T., Conti B., Genta I. Staggered herringbone microfluid device for the manufacturing of chitosan/TPP nanoparticles: Systematic optimization and preliminary biological evaluation. International Journal of Molecular Sciences. 2019;20(24) doi: 10.3390/ijms20246212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirkov S.N. The antiviral activity of chitosan (review) Applied Biochemistry and Microbiology. 2002;38(1):1–8. [PubMed] [Google Scholar]
- Cho N.J., Glenn J.S. Materials science approaches in the development of broad-spectrum antiviral therapies. Nature Materials. 2020;19(8):813–816. doi: 10.1038/s41563-020-0698-4. [DOI] [PubMed] [Google Scholar]
- Ciejka J., Milewska A., Wytrwal M., Wojarski J., Golda A., Ochman M., Nowakowska M., Szczubialka K., Pyrc K. Novel polyanions inhibiting replication of influenza viruses. Antimicrobial Agents and Chemotherapy. 2016;60(4):1955–1966. doi: 10.1128/AAC.02183-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciejka J., Wolski K., Nowakowska M., Pyrc K., Szczubiałka K. Biopolymeric nano/microspheres for selective and reversible adsorption of coronaviruses. Materials Science and Engineering C: Materials for Biological Applications. 2017;76:735–742. doi: 10.1016/j.msec.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Vaccine designers take first shots at COVID-19. Science. 2020;368(6486):14–16. doi: 10.1126/science.368.6486.14. [DOI] [PubMed] [Google Scholar]
- Collado-Gonzalez M., Espinosa Y.G., Goycoolea F.M. Interaction between chitosan and mucin: Fundamentals and applications. Biomimetics. 2019;4(2):32. doi: 10.3390/biomimetics4020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C., Kang H., Yang W., Sun J., Liu C., Cheng G., Rong G., Wang X., Wang X., Jin Z., Zhao K. O-2 '-hydroxypropyltrimethyl ammonium chloride chitosan nanoparticles for the delivery of live Newcastle disease vaccine. Carbohydrate Polymers. 2015;130:280–289. doi: 10.1016/j.carbpol.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Davis R., Zivanovic S., Davidson P.M., D'Souza D.H. Enteric viral surrogate reduction by chitosan. Food and Environmental Virology. 2015;7(4):359–365. doi: 10.1007/s12560-015-9208-2. [DOI] [PubMed] [Google Scholar]
- Davis R., Zivanovic S., D'Souza D.H., Davidson P.M. Effectiveness of chitosan on the inactivation of enteric viral surrogates. Food Microbiology. 2012;32(1):57–62. doi: 10.1016/j.fm.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Davydova V.N., Nagorskaya V.P., Gorbach V.I., Kalitnik A.A., Reunov A.V., Solov'eva T.F., Ermak I.M. Chitosan antiviral activity: Dependence on structure and depolymerization method. Applied Biochemistry and Microbiology. 2011;47(1):103–108. [PubMed] [Google Scholar]
- Denk S., Taylor R.P., Wiegner R., Cook E.M., Lindorfer M.A., Pfeiffer K., Paschke S., Eiseler T., Weiss M., Barth E., Lambris J.D., Kalbitz M., Martin T., Barth H., Messerer D.A.C., Gebhard F., Huber-Lang M.S. Complement C5a-induced changes in neutrophil morphology during inflammation. Scandinavian Journal of Immunology. 2017;86(3):143–155. doi: 10.1111/sji.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? International Journal of Antimicrobial Agents. 2020;55(5):105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimassi S., Tabary N., Chai F., Blanchemain N., Martel B. Sulfonated and sulfated chitosan derivatives for biomedical applications: A review. Carbohydrate Polymers. 2018;202:382–396. doi: 10.1016/j.carbpol.2018.09.011. [DOI] [PubMed] [Google Scholar]
- Donthu N., Gustafsson A. Effects of COVID-19 on business and research. Journal of Business Research. 2020;117:284–289. doi: 10.1016/j.jbusres.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Knidri H., Belaabed R., Addaou A., Laajeb A., Lahsini A. Extraction, chemical modification and characterization of chitin and chitosan. International Journal of Biological Macromolecules. 2018;120:1181–1189. doi: 10.1016/j.ijbiomac.2018.08.139. [DOI] [PubMed] [Google Scholar]
- Fernandes J.C., Tavaria F.K., Fonseca S.C., Ramos Ó.S., Pintado M.E., Malcata F.X. In vitro screening for antimicrobial activity of chitosans and chitooligosaccharides, aiming at potential uses in functional textiles. Journal of Microbiology and Biotechnology. 2010;20(2):311–318. doi: 10.4014/jmb.0904.04038. [DOI] [PubMed] [Google Scholar]
- Gallagher J. Vol. 2020. British Broadcasting Corporation (BBC); 2020. Coronavirus vaccine: When will we have one? [Google Scholar]
- Gao Y., Liu W., Wang W., Zhang X., Zhao X. The inhibitory effects and mechanisms of 3,6-O-sulfated chitosan against human papillomavirus infection. Carbohydrate Polymers. 2018;198:329–338. doi: 10.1016/j.carbpol.2018.06.096. [DOI] [PubMed] [Google Scholar]
- Gulati N., Dua K., Dureja H. Role of chitosan based nanomedicines in the treatment of chronic respiratory diseases. International Journal of Biological Macromolecules. 2021;185:20–30. doi: 10.1016/j.ijbiomac.2021.06.035. [DOI] [PubMed] [Google Scholar]
- Guo X., Sun T., Zhong R., Ma L., You C., Tian M., Li H., Wang C. Effects of chitosan oligosaccharides on human blood components. Frontiers in Pharmacology. 2018;9:1412. doi: 10.3389/fphar.2018.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Král P. Computational design of ACE2-based peptide inhibitors of SARS-CoV-2. ACS Nano. 2020;14(4):5143–5147. doi: 10.1021/acsnano.0c02857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasantabar V., Tashakkorian H., Golpour M. Fabrication of chitosan based magnetic nanocomposite by click reaction strategy; evaluation of nanometric and cytotoxic characteristics. Carbohydrate Polymers. 2019;224 doi: 10.1016/j.carbpol.2019.115163. [DOI] [PubMed] [Google Scholar]
- He X., Xing R., Li K., Qin Y., Zou P., Liu S., Yu H., Li P. Beta-chitosan extracted from loligo japonica for a potential use to inhibit Newcastle disease. International Journal of Biological Macromolecules. 2016;82:614–620. doi: 10.1016/j.ijbiomac.2015.10.059. [DOI] [PubMed] [Google Scholar]
- He X., Xing R., Liu S., Qin Y., Li K., Yu H., Li P. The improved antiviral activities of amino-modified chitosan derivatives on Newcastle virus. Drug and Chemical Toxicology. 2019;44(4):335–340. doi: 10.1080/01480545.2019.1620264. [DOI] [PubMed] [Google Scholar]
- Ho D.K., Frisch S., Biehl A., Terriac E., de Rossi C., Schwarzkopf K., Lautenschlager F., Loretz B., Murgia X., Lehr C.M. Farnesylated glycol chitosan as a platform for drug delivery: Synthesis, characterization, and investigation of mucus-particle interactions. Biomacromolecules. 2018;19(8):3489–3501. doi: 10.1021/acs.biomac.8b00795. [DOI] [PubMed] [Google Scholar]
- Hu T.Y., Frieman M., Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nature Nanotechnology. 2020;15(4):247–249. doi: 10.1038/s41565-020-0674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsawong T., Sunintaboon P., Warit S., Thaisomboonsuk B., Jarman R.G., Yoon I.K., Ubol S., Fernandez S. Immunogenic properties of a BCG adjuvanted chitosan nanoparticle-based dengue vaccine in human dendritic cells. PLoS Neglected Tropical Diseases. 2015;9(9) doi: 10.1371/journal.pntd.0003958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo J., Le Bas A., Ruza R.R., Duyvesteyn H.M.E., Mikolajek H., Malinauskas T., Tan T.K., Rijal P., Dumoux M., Ward P.N., Ren J., Zhou D., Harrison P.J., Weckener M., Clare D.K., Vogirala V.K., Radecke J., Moynié L., Zhao Y.…Naismith J.H. Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nature Structural and Molecular Biology. 2020;2020 doi: 10.1038/s41594-020-0469-6. [DOI] [PubMed] [Google Scholar]
- Ishihara C., Yoshimatsu K., Tsuji M., Arikawa J., Saiki I., Tokura S., Azuma I. Antiviral activity of sulfated chitin derivatives against friend murine leukemia and herpes-simplex type-1 viruses. Vaccine. 1993;11(6):670–674. doi: 10.1016/0264-410x(93)90315-o. [DOI] [PubMed] [Google Scholar]
- Jamali A., Mottaghitalab F., Abdoli A., Dinarvand M., Esmailie A., Kheiri M.T., Atyabi F. Inhibiting influenza virus replication and inducing protection against lethal influenza virus challenge through chitosan nanoparticles loaded by siRNA. Drug Delivery and Translational Research. 2018;8(1):12–20. doi: 10.1007/s13346-017-0426-z. [DOI] [PubMed] [Google Scholar]
- Jean S.S., Lee P.I., Hsueh P.R. Treatment options for COVID-19: The reality and challenges. Journal of Microbiology, Immunology and Infection. 2020;53(3):436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S., Ko M., Lee J., Choi I., Byun S.Y., Park S., Shum D., Kim S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrobial Agents and Chemotherapy. 2020;64(7):e00819–e00820. doi: 10.1128/AAC.00819-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H., Lee J.J., Lee J., Na K. A multiligand architectural photosensitizer that targets hemagglutinin on envelope of influenza virus for photodynamic inactivation. Small. 2020;16(20) doi: 10.1002/smll.202000556. [DOI] [PubMed] [Google Scholar]
- Kalathiya U., Padariya M., Mayordomo M., Lisowska M., Nicholson J., Singh A., Baginski M., Fahraeus R., Carragher N., Ball K., Haas J., Daniels A., Hupp T.R., Alfaro J.A. Highly conserved homotrimer cavity formed by the SARS-CoV-2 spike glycoprotein: A novel binding site. Journal of Clinical Medicine. 2020;9(5):1473. doi: 10.3390/jcm9051473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagozlu M.Z., Karadeniz F., Kim S.K. Anti-HIV activities of novel synthetic peptide conjugated chitosan oligomers. International Journal of Biological Macromolecules. 2014;66:260–266. doi: 10.1016/j.ijbiomac.2014.02.020. [DOI] [PubMed] [Google Scholar]
- Karthik R., Manigandan V., Saravanan R., Rajesh R.P., Chandrika B. Structural characterization and in vitro biomedical activities of sulfated chitosan from Sepia pharaonis. International Journal of Biological Macromolecules. 2016;84:319–328. doi: 10.1016/j.ijbiomac.2015.12.030. [DOI] [PubMed] [Google Scholar]
- Kaur S.P., Gupta V. COVID-19 vaccine: A comprehensive status report. Virus Research. 2020;288 doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Kim Y.S., Hwang J.W., Han Y.K., Lee J.S., Kim S.K., Jeon Y.J., Moon S.H., Jeon B.T., Bahk Y.Y., Park P.J. Sulfated chitosan oligosaccharides suppress LPS-induced NO production via JNK and NF-κB inactivation. Molecules. 2014;19(11):18232–18247. doi: 10.3390/molecules191118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolonko A.K., Bangel-Ruland N., Goycoolea F.M., Weber W.M. Chitosan nanocomplexes for the delivery of ENaC antisense oligonucleotides to airway epithelial cells. Biomolecules. 2020;10(4):553. doi: 10.3390/biom10040553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhikhova K.V., Ivantsova M.N., Tokareva M.I., Shulepov I.D., Tretiyakov A.V., Shaidarov L.V., Rusinov V.L., Mironov M.A. Preparation of chitosan-coated liposomes as a novel carrier system for the antiviral drug triazavirin. Pharmaceutical Development and Technology. 2018;23(4):334–342. doi: 10.1080/10837450.2016.1242624. [DOI] [PubMed] [Google Scholar]
- Kumar M.N.V.R., Muzzarelli R.A.A., Muzzarelli C., Sashiwa H., Domb A.J. Chitosan chemistry and pharmaceutical perspectives. Chemical Reviews. 2004;104(12):6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- Kumar S.S., Binu A., Devan A.R., Nath L.R. Mucus targeting as a plausible approach to improve lung function in COVID-19 patients. Medical Hypotheses. 2021;156 doi: 10.1016/j.mehy.2021.110680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers T., Sofias A.M., van der Meel R., Schiffelers R., Storm G., Tacke F., Koschmieder S., Brümmendorf T.H., Kiessling F., Metselaar J.M. Dexamethasone nanomedicines for COVID-19. Nature Nanotechnology. 2020;15(8):622–624. doi: 10.1038/s41565-020-0752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wu P., Gao G.F., Cheng S. Carbohydrate-functionalized chitosan fiber for influenza virus capture. Biomacromolecules. 2011;12(11):3962–3969. doi: 10.1021/bm200970x. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang C., Sun Z., Xiao J., Yan X., Chen Y., Yu J., Wu Y. Simultaneous intramuscular and intranasal administration of chitosan nanoparticles-adjuvanted chlamydia vaccine elicits elevated protective responses in the lung. International Journal of Nanomedicine. 2019;14:8179–8193. doi: 10.2147/IJN.S218456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutfy S.A., Elberry M.H., Farroh K.Y., Mohamed H.T., Mohamed A.A., Mohamed E.B., Faraag A.H.I., Mousa S.A. Antiviral activity of chitosan nanoparticles encapsulating curcumin against hepatitis C virus genotype 4a in human hepatoma cell lines. International Journal of Nanomedicine. 2020;15:2699–2715. doi: 10.2147/IJN.S241702. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mahase E. Covid-19: Pfizer and BioNTech submit vaccine for US authorisation. BMJ. 2020;371 doi: 10.1136/bmj.m4552. [DOI] [PubMed] [Google Scholar]
- McKee D.L., Sternberg A., Stange U., Laufer S., Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacological Research. 2020;157:104859. doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Sturgis T.F., Youan B.B.C. Engineering tenofovir loaded chitosan nanoparticles to maximize microbicide mucoadhesion. European Journal of Pharmaceutical Sciences. 2011;44(1–2):57–67. doi: 10.1016/j.ejps.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nature Review Immunology. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewska A., Ciejka J., Kaminski K., Karewicz A., Bielska D., Zeglen S., Karolak W., Nowakowska M., Potempa J., Bosch B.J., Pyrc K., Szczubialka K. Novel polymeric inhibitors of HCoV-NL63. Antiviral Research. 2013;97(2):112–121. doi: 10.1016/j.antiviral.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewska A., Kaminski K., Ciejka J., Kosowicz K., Zeglen S., Wojarski J., Nowakowska M., Szczubiałka K., Pyrc K. HTCC: Broad range inhibitor of coronavirus entry. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0156552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami S., Suzuki H., Okamoto Y., Fujinaga T., Shigemasa Y. Chitin and chitosan activate complement via the alternative pathway. Carbohydrate Polymers. 1998;36(2):151–155. [Google Scholar]
- Mohd Rasul R., Muniandy M.T., Zakaria Z., Shah K., Chee C.F., Dabbagh A., Abd Rahman N., Wong T.W., AP A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers. Carbohydrate Polymers. 2020;250:116800. doi: 10.1016/j.carbpol.2020.116800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley T., Singh M. Sterically stabilised polymeric mesoporous silica nanoparticles improve doxorubicin efficiency: Tailored cancer therapy. Molecules. 2020;25(3) doi: 10.3390/molecules25030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan C., Ramamoorthy S., Kuppuswamy G., Murugan R.K., Sivalingam Y., Sundaramurthy A. COVID-19: A review of newly formed viral clades, pathophysiology, therapeutic strategies and current vaccination tasks. International Journal of Biological Macromolecules. 2021;193:1165–1200. doi: 10.1016/j.ijbiomac.2021.10.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveed M., Phil L., Sohail M., Hasnat M., Baig M.M.F.A., Ihsan A.U., Shumzaid M., Kakar M.U., Mehmood Khan T., Akabar M.D., Hussain M.I., Zhou Q.G. Chitosan oligosaccharide (COS): An overview. International Journal of Biological Macromolecules. 2019;129:827–843. doi: 10.1016/j.ijbiomac.2019.01.192. [DOI] [PubMed] [Google Scholar]
- Ng S.W., Selvarajah G.T., Hussein M.Z., Yeap S.K., Omar A.R. In vitro evaluation of curcumin-encapsulated chitosan nanoparticles against feline infectious peritonitis virus and pharmacokinetics study in cats. Biomedical Research International. 2020;2020 doi: 10.1155/2020/3012198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo A.N., Ezoulin M.J.M., Murowchick J.B., Gounev A.D., Youan B.B.C. Sodium acetate coated tenofovir-loaded chitosan nanoparticles for improved physico-chemical properties. Pharmaceutical Research. 2016;33(2):367–383. doi: 10.1007/s11095-015-1795-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S., Kai H., Shinada K., Yoshida T., Tokura S., Kurita K., Nakashima H., Yamamoto N., Uryu T. Regioselective syntheses of sulfated polysaccharides: Specific anti-HIV-1 activity of novel chitin sulfates. Carbohydrate Research. 1998;306(3):427–433. doi: 10.1016/s0008-6215(97)10081-7. [DOI] [PubMed] [Google Scholar]
- Park J.K. Inhibitory effect of chitosan oligosaccharides on sialidase activity in vitro. Journal of Chitin and Chitosan. 2010;15(2):88–91. [Google Scholar]
- Park Y.I., Park J.K., Jo H.S., Lee C.G. Significance of the molecular weight and degree of N-deacetylation of chitosan-oligosaccharides on the biological applications. Journal of Chitin and Chitosan. 2010;15(4):195–202. [Google Scholar]
- PeÑarando J., Aranda E., RodrÍguez-Ariza A. Immunomodulatory roles of nitric oxide in cancer: Tumor microenvironment says “NO” to antitumor immune response. Translational Research. 2019;210:99–108. doi: 10.1016/j.trsl.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Piegat A., Goszczyńska A., Idzik T., Niemczyk A. The importance of reaction conditions on the chemical structure of N. O-acylated chitosan derivatives. Molecules. 2019;24(17):3047. doi: 10.3390/molecules24173047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajawat G.S., Shinde U.A., Nair H.A. Chitosan-N-acetyl cysteine microspheres for ocular delivery of acyclovir: Synthesis and in vitro/in vivo evaluation. Journal of Drug Delivery Science and Technology. 2016;35:333–342. [Google Scholar]
- Ramos V.M., Rodriguez N.M., Diaz M.F., Rodriguez M.S., Heras A., Agullo E. N-methylene phosphonic chitosan. Effect of preparation methods on its properties. Carbohydrate Polymers. 2003;52(1):39–46. [Google Scholar]
- Ravi Kumar M.N.V. A review of chitin and chitosan applications. Reactive and Functional Polymers. 2000;46(1):1–27. [Google Scholar]
- Romano M., Ruggiero A., Squeglia F., Maga G., Berisio R. A structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells. 2020;9(5):1267. doi: 10.3390/cells9051267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E., Gaglianone N., Baldassari S., Parodi B., Cafaggi S., Zibana C., Donalisio M., Cagno V., Lembo D., Caviglioli G. Preparation, characterization and in vitro antiviral activity evaluation of foscarnet-chitosan nanoparticles. Colloids and Surfaces B-Biointerfaces. 2014;118:117–125. doi: 10.1016/j.colsurfb.2014.03.037. [DOI] [PubMed] [Google Scholar]
- Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., Hosein Z., Padda I., Mangat J., Altaf M. Comorbidity and its impact on patients with COVID-19. SN Comprehensive Clinical Medicine. 2020:1–8. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S., Chougule M.B., Kotha A.K., Kashikar R., Godugu C., Raghuvanshi R.S., Singh S.B., Srivastava S. Nanomedicine based approaches for combating viral infections. Journal of Controlled Release. 2021;338:80–104. doi: 10.1016/j.jconrel.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanavas A., Jain N.K., Kaur N., Thummuri D., Prasanna M., Prasad R., Naidu V.G.M., Bahadur D., Srivastava R. Polymeric core-shell combinatorial nanomedicine for synergistic anticancer therapy. ACS Omega. 2019;4(22):19614–19622. doi: 10.1021/acsomega.9b02167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., Singh D., Rani R., Sharma D., Pandey H., Agarwal V. Nanomaterials in plants, algae and microorganisms. Vol. 2. Elsevier; 2019. Chitosan and its nanocarriers: Applications and opportunities; pp. 267–286. Chapter 13. [Google Scholar]
- Singh P.K., Srivastava A.K., Dev A., Kaundal B., Choudhury S.R., Karmakar S. 1, 3 Beta-glucan anchored, paclitaxel loaded chitosan nanocarrier endows enhanced hemocompatibility with efficient anti-glioblastoma stem cells therapy. Carbohydrate Polymers. 2018;180:365–375. doi: 10.1016/j.carbpol.2017.10.030. [DOI] [PubMed] [Google Scholar]
- Sofy A.R., Hmed A.A., Abd El Haliem N.F., Zein M.A.E., Elshaarawy R.F.M. Polyphosphonium-oligochitosans decorated with nanosilver as new prospective inhibitors for common human enteric viruses. Carbohydrate Polymers. 2019;226 doi: 10.1016/j.carbpol.2019.115261. [DOI] [PubMed] [Google Scholar]
- Soh S.H., Shim S., Im Y.B., Park H.T., Cho C.S., Yoo H.S. Induction of Th2-related immune responses and production of systemic IgA in mice intranasally immunized with brucella abortus malate dehydrogenase loaded chitosan nanoparticles. Vaccine. 2019;37(12):1554–1564. doi: 10.1016/j.vaccine.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Sosa M.A.G., Fazely F., Koch J.A., Vercellotti S.V., Ruprecht R.M. N-carboxymethylchitosan-N, O-sulfate as an anti-HIV-1 agent. Biochemical and Biophysical Research Communications. 1991;174(2):489–496. doi: 10.1016/0006-291x(91)91443-g. [DOI] [PubMed] [Google Scholar]
- Stepanov O.A., Prokof'eva M.M., Stocking K., Varlamov V.P., Levov A.N., Vikhoreva G.A., Spirin P.V., Mikhailov S.N., Prassolov V.S. Replication-competent gamma-retrovirus mo-MuLV expressing green fluorescent protein as efficient tool for screening of inhibitors of retroviruses that use heparan sulfate as primary cell receptor. Molecular Biology. 2012;46(3):457–466. [PubMed] [Google Scholar]
- Su X., Zivanovic S., D'Souza D.H. Effect of chitosan on the infectivity of murine norovirus, feline calicivirus, and bacteriophage MS2. Journal of Food Protection. 2009;72(12):2623–2628. doi: 10.4315/0362-028x-72.12.2623. [DOI] [PubMed] [Google Scholar]
- Sun L.L., Liu Z.J., Tian H.K., Le Z.C., Liu L.X., Leong K.W., Mao H.Q., Chen Y.M. Scalable manufacturing of enteric encapsulation systems for site specific oral insulin delivery. Biomacromolecules. 2019;20(1):528–538. doi: 10.1021/acs.biomac.8b01530. [DOI] [PubMed] [Google Scholar]
- Tammam S.N., Safy S.E., Ramadan S., Arjune S., Krakor E., Mathur S. Repurpose but also (nano)-reformulate! The potential role of nanomedicine in the battle against SARS-CoV2. Journal of Controlled Release. 2021;337:258–284. doi: 10.1016/j.jconrel.2021.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi K., Singh A. Chitin, chitosan and their pharmacological activities: A review. International Journal of Pharmaceutical Sciences and Research. 2018;9(7):2626–2635. [Google Scholar]
- U.S.FDA FDA's approval of Veklury (remdesivir) for the treatment of COVID-19—The science of safety and effectiveness. 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fdas-approval-veklury-remdesivir-treatment-covid-19-science-safety-and-effectiveness (Accessed 5 Nov 2020)
- Umemura M., Itoh M., Makimura Y., Yamazaki K., Umekawa M., Masui A., Matahira Y., Shibata M., Ashida H., Yamamoto K. Design of a sialylglycopolymer with a chitosan backbone having efficient inhibitory activity against influenza virus infection. Journal of Medicinal Chemistry. 2008;51(15):4496–4503. doi: 10.1021/jm8000967. [DOI] [PubMed] [Google Scholar]
- Vankadari N. Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein. International Journal of Antimicrobial Agents. 2020;56(2) doi: 10.1016/j.ijantimicag.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo T.S., Kim S.K. Potential anti-HIV agents from marine resources: An overview. MarineDrugs. 2010;8(12):2871–2892. doi: 10.3390/md8122871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Meng Q., Li Q., Liu J., Zhou M., Jin Z., Zhao K. Chitosan derivatives and their application in biomedicine. International Journal of Molecular Sciences. 2020;21:487. doi: 10.3390/ijms21020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., He L., Wei B., Yan G.Q., Wang J., Tang R.P. Bromelain-immobilized and lactobionic acid-modified chitosan nanoparticles for enhanced drug penetration in tumor tissues. International Journal of Biological Macromolecules. 2018;115:129–142. doi: 10.1016/j.ijbiomac.2018.04.076. [DOI] [PubMed] [Google Scholar]
- Wang Y., Su Y., Wang W., Fang Y., Riffat S.B., Jiang F. The advances of polysaccharide-based aerogels: Preparation and potential application. Carbohydrate Polymers. 2019;226 doi: 10.1016/j.carbpol.2019.115242. [DOI] [PubMed] [Google Scholar]
- Xu W., Zheng S., Dweik R.A., Erzurum S.C. Role of epithelial nitric oxide in airway viral infection. Free Radical Biology and Medicine. 2006;41(1):19–28. doi: 10.1016/j.freeradbiomed.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Science China Life Sciences. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Liu K.P., Jiao B.B., Luo K.J., Ren J., Zhang G., Yu Q.S., Gan Z.H. Mucoadhesive nanoparticles based on ROS activated gambogic acid prodrug for safe and efficient intravesical instillation chemotherapy of bladder cancer. Journal of Controlled Release. 2020;324:493–504. doi: 10.1016/j.jconrel.2020.03.028. [DOI] [PubMed] [Google Scholar]
- Xue M., Hu S., Lu Y., Zhang Y., Jiang X., An S., Guo Y., Zhou X., Hou H., Jiang C. Development of chitosan nanoparticles as drug delivery system for a prototype capsid inhibitor. International Journal of Pharmaceutics. 2015;495(2):771–782. doi: 10.1016/j.ijpharm.2015.08.056. [DOI] [PubMed] [Google Scholar]
- Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Xing R., Liu S., Qin Y., Li K., Yu H., Li P. Immunostimulatory effects of sulfated chitosans on RAW 264.7 mouse macrophages via the activation of PI3K/Akt signaling pathway. International Journal of Biological Macromolecules. 2018;108:1310–1321. doi: 10.1016/j.ijbiomac.2017.11.042. [DOI] [PubMed] [Google Scholar]
- Yasar H., Ho D.K., De Rossi C., Herrmann J., Gordon S., Loretz B., Lehr C.M. Starch-chitosan polyplexes: A versatile carrier system for anti-infectives and gene delivery. Polymers. 2018;10(3) doi: 10.3390/polym10030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes I., Hajji S., Frachet V., Rinaudo M., Jellouli K., Nasri M. Chitin extraction from shrimp shell using enzymatic treatment. Antitumor, antioxidant and antimicrobial activities of chitosan. International Journal of Biological Macromolecules. 2014;69:489–498. doi: 10.1016/j.ijbiomac.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Zelenkova T., Onnainty R., Granero G.E., Barresi A.A., Fissore D. Use of microreactors and freeze-drying in the manufacturing process of chitosan coated PCL nanoparticles. European Journal of Pharmaceutical Sciences. 2018;119:135–146. doi: 10.1016/j.ejps.2018.04.006. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Honko A., Zhou J., Gong H., Downs S.N., Vasquez J.H., Fang R.H., Gao W., Griffiths A., Zhang L. Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Letters. 2020;20(7):5570–5574. doi: 10.1021/acs.nanolett.0c02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Huo M., Zhou J., Yu D., Wu Y. Potential of amphiphilically modified low molecular weight chitosan as a novel carrier for hydrophobic anticancer drug: Synthesis, characterization, micellization and cytotoxicity evaluation. Carbohydrate Polymers. 2009;77(2):231–238. [Google Scholar]
- Zhao K., Zhang Y., Zhang X., Shi C., Wang X., Wang X., Jin Z., Cui S. Chitosan-coated poly(lactic-co-glycolic) acid nanoparticles as an efficient delivery system for Newcastle disease virus DNA vaccine. International Journal of Nanomedicine. 2014;9:4609–4619. doi: 10.2147/IJN.S70633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Qu D., Wang H., Sun Z., Liu X., Chen J., Li C., Li X., Chen Z. Intranasal administration of chitosan against influenza a (H7N9) virus infection in a mouse model. Scientific Reports. 2016;6 doi: 10.1038/srep28729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Liu S.N., Hu Y.J., Yang S.W., Zhao B., Zheng K.K., Zhang Y.H., He P.X., Mo G.Y., Li Y.L. Tumor-mediated shape-transformable nanogels with pH/redox/enzymatic-sensitivity for anticancer therapy. Journal of Materials Chemistry B. 2020;8(17):3801–3813. doi: 10.1039/d0tb00143k. [DOI] [PubMed] [Google Scholar]
- Zhou J., Krishnan N., Jiang Y., Fang R.H., Zhang L. Nanotechnology for virus treatment. Nano Today. 2021;36 doi: 10.1016/j.nantod.2020.101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Barnes C., Bhar S., Hoyeck P., Galbraith A.N., Devabhaktuni D., Karst S.M., Montazeri N., Jones M.K. Survival of human norovirus surrogates in water upon exposure to thermal and non-thermal antiviral treatments. Viruses. 2020;12(4) doi: 10.3390/v12040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang M.W., Cheng Y., Zhang J., Jiang X.M., Wang L., Deng J., Wang P.H. Increasing host cellular receptor-angiotensin-converting enzyme 2 expression by coronavirus may facilitate 2019-nCoV (or SARS-CoV-2) infection. Journal of Medical Virology. 2020;92(11):2693–2701. doi: 10.1002/jmv.26139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo S.-H., Wu J.-J., Zhao L., Li W.-H., Zhao Y.-F., Li Y.-M. A chitosan-mediated inhalable nanovaccine against SARS-CoV-2. Nano Research. 2021 doi: 10.1007/s12274-021-4012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]