Abstract
The efficacy of ravuconazole, a new triazole antifungal agent, and the echinocandin LY-303366 were evaluated in an immunosuppressed, temporarily leukopenic rabbit model of invasive aspergillosis. Oral therapy with ravuconazole at a dosage of 30 mg/kg of body weight per day or the echinocandin LY-303366, given intravenously in a dosage of 5 or 10 mg/kg, was begun 24 h after a lethal or sublethal challenge, and results were compared with those for amphotericin B therapy and untreated controls. Prophylaxis was also studied with LY-303366 given at a dosage of 5 or 10 mg/kg/day 48 h before lethal or sublethal challenge. Ravuconazole eliminated mortality, cleared aspergillus antigen from the serum, and eliminated Aspergillus fumigatus organisms from tissues of both lethally and sublethally challenged immunosuppressed animals with invasive aspergillosis. Although LY-303366, at both doses, prolonged survival and reduced aspergillus antigenemia, it did not eliminate aspergillus organisms from organ tissues. The half-lives of ravuconazole and LY-303366 in rabbits were 13 and 12.5 h, respectively, and no accumulation of either drug was seen after 6 days of treatment. Although LY-303366 showed activity in this rabbit model of invasive aspergillosis, ravuconazole was the more active agent, comparable to amphotericin B. Additional studies are needed to determine the potential of ravuconazole for use in the treatment of this infection.
Invasive aspergillosis is a common infection in immunocompromised patients and is associated with significant morbidity and mortality, even when treated with amphotericin B, which is the drug of choice for this infection (1, 3, 6, 7, 16). Thus, novel antifungal therapies with less toxicity and improved efficacy are needed to improve the treatment of invasive aspergillosis.
A number of new antifungal agents have been developed for use in patients with serious fungal infections, particularly newer azoles and echinocandins, which may prove to be effective and less toxic than amphotericin B (2, 10, 11). Some of these newer agents have been or are currently being studied in animal models of fungal disease and in patients with fungal infections (2, 4). One of the newer azoles, ravuconazole (BMS-207147; ER 30346), and a novel echinocandin (LY-303366) have excellent in vitro activity against strains of Aspergillus fumigatus (5, 9, 15, 17, 26).
A number of immunosuppressed and nonimmunosuppressed animal models of invasive aspergillosis have been used to study the pharmacokinetics, toxicology, and therapeutic efficacy of newer antifungal agents (3). The present report describes the efficacies of ravuconazole (BMS-207147) and LY-303366, compared with that of conventional amphotericin B, in our well-established immunosuppressed-rabbit model of invasive aspergillosis (3, 10, 11, 18–24). The infection in our model mimics the clinical dissemination of invasive aspergillosis in humans with extensive infection in the lung, liver, kidney, and brain (3, 10, 11, 18–24). Therapeutic efficacy is determined by the rate of survival, semiquantitative organ cultures to evaluate the reduction or elimination of Aspergillus organisms from specific target organs, and the kinetics of Aspergillus antigenemia in response to antifungal therapy compared to that in untreated control animals (3, 10, 11, 18–24).
(Part of this research was presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September, 1998.)
MATERIALS AND METHODS
Pharmacokinetics.
New Zealand White rabbits (2.0 to 3.0 kg) were each immunosuppressed with a single intravenous dose of 200 mg of cyclophosphamide (Bristol-Myers Pharmaceutical Research and Development, Evansville, Ind.) on the 1st day (day 1) of the experiment. Triamcinolone acetonide was given subcutaneously at 10 mg per rabbit beginning on day 1 and was administered daily for the duration of the experiment. With this immunosuppressive regimen, the rabbits have a reduced leukocyte count through day 7 of the experiment, with a nadir on day 4. Therapy with ravuconazole (Bristol-Myers Squibb Pharmaceutical Research Institute, Wallingford, Conn.) (Fig. 1) or, in separate experiments, LY-303366 (Eli Lilly and Company, Indianapolis, Ind.) (Fig. 2) was initiated 48 h after immunosuppression with cyclophosphamide (day 3). Ravuconazole was prepared by dissolving 240 mg in 4.8 ml of ethanol, then adding 1.2 ml of polysorbate 80, followed by 6.0 ml of polyethylene glycol 400 to a final concentration of 20 mg/ml for oral administration. Three groups of four rabbits each were treated once daily for 6 days with BMS-207147 at either 10, 20, or 30 mg/ kg of body weight/day. Blood was obtained from central ear arteries at 0, 1, 3, 8, and 24 h after the first and after the final dose, and concentrations of BMS-207147 in serum were quantified using high-performance liquid chromatography (HPLC). These samples were used to determine single- and multiple-dose pharmacokinetics as well as mean peak levels of ravuconazole.
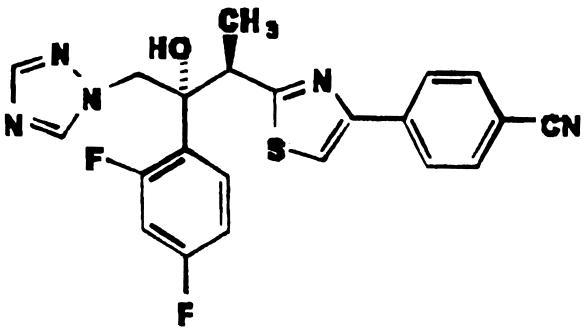
FIG. 1.
Ravuconazole.
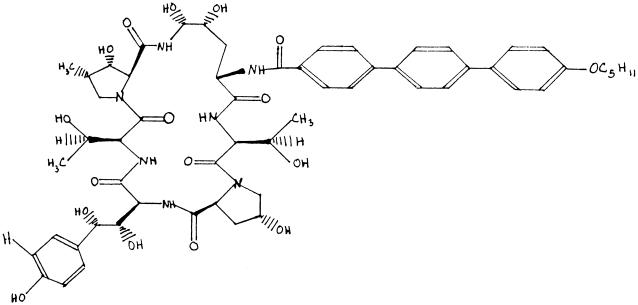
FIG. 2.
LY-303366.
LY-303366 was provided at a concentration of 10 mg/ml in dimethyl sulfoxide (DMSO) by Eli Lilly and Company. The drug was diluted to the appropriate concentration with 5% dextrose in water just prior to treatment. Three groups of six rabbits each were treated with LY-303366 once daily for 6 days at either 1, 5, or 10 mg/kg/day. In these experiments blood was also obtained from central ear arteries at 10 min prior to treatment, and at 10 min and 1, 4, and 8 h after treatment on the first and last days of treatment. Samples were taken at 24-h troughs and 10-min peaks for all other doses. Final blood samples were taken at 24 and 48 h after the final day of therapy. Levels of LY-303366 in serum were measured by HPLC. These results were used to determine single- and multiple-dose pharmacokinetics as well as mean peak levels of LY-303366.
HPLC for ravuconazole had a lower limit of quantitation of 10 ng/ml and was validated for a standard curve of 10 to 2,000 ng/ml. The relative standard deviations for inter- and intra-assay precision were <8%, and the accuracy was within ±7% of expected values. HPLC for LY-303366 was validated for a standard curve of 20 to 5,000 ng/ml. The accuracy of the assay ranged from 100.4 to 103.2%, and the precision ranged from 1.2 to 4.7%.
Rabbit model.
Our rabbit model has been described in detail previously (3, 10, 11, 18–24). Rabbits were immunosuppressed on day 1 as described above. On day 2 (24 h after immunosuppression with cyclophosphamide), rabbits were challenged intravenously with 106 (lethal model) or 105 (sublethal model) A. fumigatus conidia. Untreated, lethally challenged rabbits succumb with disseminated aspergillosis within 8 days. Rabbits were given 100 mg of ceftazidime (SmithKline Beecham, Philadelphia, Pa.) per day and 20 mg of gentamicin (Schering-Plough Research, Bloomfield, N.J.) per day intramuscularly to prevent opportunistic bacterial infection. Therapy with ravuconazole, given by gastric gavage, was initiated 24 h after challenge and continued once daily for 6 days. Groups of 10 rabbits, lethally or sublethally challenged, were treated with ravuconazole at 30 mg/kg/day, prepared and administered as described above. Four untreated, infected controls were used with each group of 10 ravuconazole-treated rabbits along with 4 rabbits treated with amphotericin B (Fungizone) at a dose of 1 mg/kg. Amphotericin B was diluted with 5% dextrose in sterile water at a ratio of 1 mg of drug to 10 ml of diluent and was given intravenously over 30 to 60 min once daily for 6 days. Surviving rabbits were killed 72 h after the last dose of ravuconazole (day 11) or amphotericin B by an overdose of ketamine (100 mg; Bristol Laboratories, Syracuse, N.Y.) and xylazine (20 mg; Mobay, Shawnee, Kans.). Tissue samples were cultured at the time of autopsy or sacrifice. Cultures were obtained by placing minced organ samples directly on blood agar and on Sabouraud dextrose agar plates. Samples were considered positive when more than one colony of A. fumigatus was present on ≥1 g of minced organ tissues plated directly onto Sabouraud dextrose or blood agar plates or when semiquantitative cultures of tissue homogenates contained >10 CFU/g of tissue as described previously (3). The tissue burden of A. fumigatus was evaluated with a modification (3, 10, 11) of the semiquantitative culture technique of Graybill and Kaster (13). Samples of liver, kidney, lung, and brain tissues were manually chopped, weighed, diluted 1:10 (wt/vol) with sterile saline, and homogenized for 25 s with an electric tissue homogenizer (TRI-R Instruments, Rockville Center, N.Y.). Then 1.0- and 0.1-ml samples of each organ homogenate were plated in duplicate on Sabouraud dextrose and blood agar plates. The plates were incubated for 48 h at 37°C, and colonies were counted. The combination of these methods detected 2 to 20,000 CFU/g of tissue. Blood was collected at intervals and assayed for circulating levels of A. fumigatus antigen by our competition-inhibition enzyme-linked immunosorbent assay (ELISA) (28), and leukocyte counts were monitored.
Similar experiments were performed with the echinocandin LY-303366. Therapy with LY-303366, given intravenously, was initiated 24 h after challenge and was continued once daily for 6 days. Groups of rabbits, lethally or sublethally challenged, were treated with LY-303366 at either 5 or 10 mg/kg/day, prepared and administered as described above. Also, untreated infected controls were studied with each group of treated rabbits, along with infected rabbits treated with amphotericin B in a dose of 1 mg/kg, also prepared as described above and given once daily for 6 days. Surviving rabbits were killed 72 h after the last treatment dose and were studied exactly as the ravuconazole-treated rabbits described above.
Similar experiments were also performed to evaluate LY-303366 as a prophylactic agent in our model of invasive aspergillosis. Prophylaxis with LY-303366 at either 5 or 10 mg/kg/day was given to groups of rabbits 48 h before lethal or sublethal challenge, and results were compared with those for untreated infected rabbits as well as rabbits given prophylaxis with amphotericin B at 1 mg/kg/day. Prophylaxis was given daily for eight days. Surviving rabbits were killed 72 hours after the last dose of LY-303366 or amphotericin B and were studied as described above.
Criteria for evaluation of efficacy.
Three criteria were used to evaluate therapeutic and prophylactic efficacy in the lethally challenged rabbits compared with the lethally challenged, untreated controls: mortality, tissue burden of A. fumigatus of the target organs, and antigenemia as determined by our ELISA. Only the last two criteria were used to evaluate efficacy in the sublethally challenged rabbits.
Statistical analysis.
The Fisher exact test, the Wilcoxon rank sum test, and the Kruskal-Wallis analysis were used when appropriate. Statistical significance was defined as a P value of <0.05.
RESULTS
Pharmacokinetics. (i) Ravuconazole (single dose).
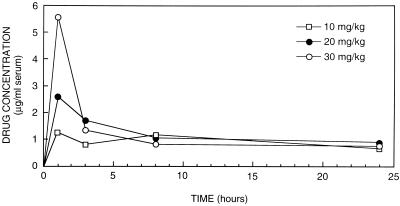
The mean peak level of ravuconazole in serum (± standard deviation) after a single dose of 10, 20, or 30 mg/kg (four rabbits in each group) was 1.25 ± 0.57, 2.58 ± 2.45, or 5.55 ± 1.62 μg/ml, respectively, at 1 h after oral administration (Fig. 3). At 3 h, the mean levels of ravuconazole in serum, for the 10-, 20-, and 30-mg/kg groups, had fallen to 0.8 ± 0.64, 1.83 ± 1.04, and 1.48 ± 0.8 μg/ml, respectively; at 8 h, the levels were 1.17 ± 0.95, 1.03 ± 0.76, and 0.8 ± 0.59 μg/ml, respectively. Ravuconazole was detectable in the sera of all rabbits at 24 h after single-dose therapy, i.e., at 0.65 ± 0.29, 0.88 ± 0.77, and 0.75 ± 0.30 μg/ml for the 10-, 20-, and 30-mg/kg groups, respectively (Fig. 3). These results suggest a half-life for ravuconazole of approximately 13 h.
FIG. 3.
Single-dose pharmacokinetics: mean serum ravuconazole levels in four immunosuppressed rabbits at 1, 3, 8, and 24 h after a single dose of 10, 20, or 30 mg of ravuconazole per kg.
(ii) Ravuconazole (multiple dose).
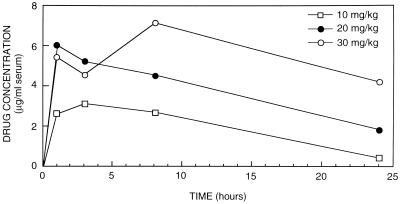
The mean peak level of ravuconazole in serum (± standard deviation) after the 6th day of treatment with 10, 20, or 30 mg/kg (four rabbits per group) was 2.6 ± 0.22, 6.0 ± 3.13, or 5.4 ± 2.91 μg/ml, respectively, at 1 h after oral administration (Fig. 4). At 3 h the mean levels in serum were 3.1 ± 0.86, 5.2 ± 2.63, and 4.55 ± 1.96 μg/ml for the 10-, 20-, and 30-mg/kg groups, respectively; at 8 h the levels were 2.67 ± 0.26, 4.5 ± 2.05, and 7.13 ± 2.67 μg/ml, respectively; and at 24 h they were 0.4 ± 0.08, 1.98 ± 1.84, and 4.35 ± 1.53 μg/ml for the 10-, 20-, and 30-mg/kg groups, respectively (Fig. 4). There was no significant drug accumulation after 6 days of treatment. All animals appeared healthy at the end of treatment, and no pathology was seen in any organs at autopsy. Since ravuconazole did not produce grossly observable toxicity even at the highest dose (30 mg/kg) studied, and since invasive aspergillosis in our immunocompromised-rabbit model is highly lethal, we chose to evaluate the efficacy of a high dose (30 mg/kg) of ravuconazole. A dose of 30 mg/kg/day in rabbits produces mean peak concentrations in serum similar to those produced by a dose of 400 mg/day in human volunteers (unpublished observations).
FIG. 4.
Multiple-dose pharmacokinetics: mean serum ravuconazole levels in four immunosuppressed rabbits at 1, 3, 8, and 24 h after 6 consecutive days of treatment with 10, 20, or 30 mg of ravuconazole per kg.
(iii) LY-303366 (single dose).
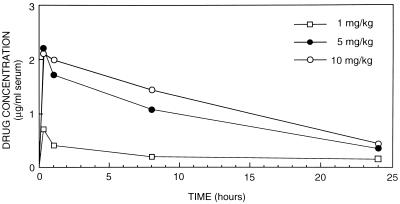
The mean peak level of LY-303366 in serum (± standard deviation) after a singe dose of 1, 5, or 10 mg/kg (three rabbits in each group) was 0.714 ± 0.15, 2.20 ± 0.15, or 2.10 ± 0.30 μg/ml, respectively, for 10 min after intravenous administration (Fig. 5). At 1 h the mean levels, for the 1-, 5-, and 10-mg/kg groups, fell to 0.417 ± 0.09, 1.71 ± 0.16, and 1.99 ± 0.24 μg/ml, respectively; at 8 h the levels were 0.20 ± 0.08, 1.07 ± 0.22, and 1.43 ± 0.29 μg/ml, respectively. LY-303366 was detectable in serum at 24 h after single-dose therapy, i.e., at 0.139 ± 0.04, 0.35 ± 0.06, and 0.44 ± 0.05 μg/ml for the 1-, 5-, and 10-mg/kg groups, respectively (Fig. 5). These results suggest a half-life for LY-303366 of approximately 12.5 h.
FIG. 5.
Single-dose pharmacokinetics: mean serum LY-303366 levels in three immunosuppressed rabbits at 10 min and 1, 8, and 24 h after a single dose of 1, 5, or 10 mg of LY-303366 per kg.
(iv) LY-303366 (multiple dose).
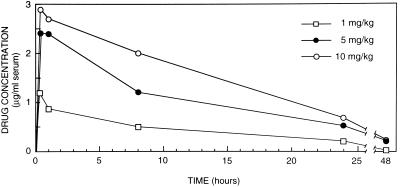
The mean peak level of LY-303366 in serum (± standard deviation) after the 6th day of treatment with 1, 5, or 10 mg/kg (three rabbits in each group) was 1.19 ± 0.11, 2.41 ± 0.20, or 2.90 + 0.57 μg/ml, respectively, at 10 min after intravenous administration (Fig. 6). At 1 h the mean levels were 0.87 ± 0.07, 2.4 + 0.20, and 2.7 ± 0.55 μg/ml for the 1-, 5-, and 10-mg/kg groups, respectively; at 8 h the levels were 0.51 ± 0.08, 1.21 ± 0.09, and 2.01 ± 0.39 μg/ml, respectively; at 24 h they were 0.21 ± 0.06, 0.52 ± 0.06, and 0.74 ± 0.08 μg/ml, respectively; and at 48 h they were 0.03 ± 0.01, 0.21 ± 0.09, and 0.23 ± 0.05 μg/ml, respectively (Fig. 6). There was no significant drug accumulation after 6 days of treatment. Although all treated rabbits appeared to be well at the end of treatment, at autopsy one of three rabbits treated with 5 mg/kg and all three treated with 10 mg/kg had mottled livers and necrotic lungs secondary to infarction. All three rabbits treated with 1 mg/kg appeared normal at autopsy. Since LY-303366 produced gross abnormalities in the livers and lungs of all rabbits treated with 10 mg/kg, we chose not to study LY-303366 at doses higher than 10 mg/kg/day.
FIG. 6.
Multiple-dose pharmacokinetics: mean serum LY-303366 levels in three immunosuppressed rabbits at 10 min and 1, 8, 24 and 48 h after the sixth dose of 1, 5 or 10 mg of LY-303366 per kg.
Mortality. (i) Treatment.
The survival of rabbits treated with ravuconazole beginning 24 h after challenge is shown in Table 1. By day 9, all 4 untreated lethally infected control animals (challenged with 106 A. fumigatus conidia) had died, compared with none of 10 rabbits treated with ravuconazole at 30 mg/kg/day (P < 0.005). Also, all amphotericin B-treated animals survived. Similarly, by day 9, mortality occurred in 2 of 4 sublethally infected control rabbits (challenged with 105 A. fumigatus conidia) compared with none of 10 animals treated with ravuconazole at 30 mg/kg/day and none of 4 rabbits treated with amphotericin B at 1 mg/kg/day.
TABLE 1.
Mortality of Aspergillus-infected rabbits treated with ravuconazole, LY-303366, or amphotericin B
| Group and inoculum (no. of organisms) | Druga (mg/kg/day) | No. dead/no. tested (%)b |
|---|---|---|
| Ravuconazole treatment | ||
| Lethal (106) | None | 4/4 (100) |
| Rav (30) | 0/10* (0) | |
| AmB (1.0) | 0/4* (0) | |
| Sublethal (105) | None | 2/4 (50) |
| Rav (30) | 0/10* (0) | |
| AmB (1.0) | 0/4 (0) | |
| LY-303366 | ||
| Treatment | ||
| Lethal (106) | None | 4/4 (100) |
| LY (5) | 0/7* (0) | |
| LY (10) | 0/8* (0) | |
| AmB (1.0) | 0/4* (0) | |
| Sublethal (105) | None | 3/4 (75) |
| LY (5) | 0/8* (0) | |
| LY (10) | 0/7* (0) | |
| AmB (1.0) | 0/4 (0) | |
| Prophylaxis | ||
| Lethal (106) | None | 4/4 (100) |
| LY (5) | 0/6* (0) | |
| LY (10) | 0/6* (0) | |
| AmB (1.0) | 0/4* (0) | |
| Sublethal (105) | None | 2/4 (50) |
| LY (5) | 0/6 (0) | |
| LY (10) | 0/6 (0) | |
| AmB (1.0) | 0/4 (0) |
Rav, ravuconazole; AmB, amphotericin B; LY, LY-303366.
*, P < 0.05 compared to controls using Fisher's exact test.
Similar survival was observed with LY-303366 (Table 1). By day 9, all four untreated lethally infected control animals (challenged with 106 A. fumigatus conidia) had died, compared with none of seven animals treated with LY-303366 at 5 mg/kg/day and none of eight rabbits treated with 10 mg/kg/day. Also, all amphotericin B-treated animals survived. Mortality results in animals receiving a sublethal challenge (105 A. fumigatus conidia) are also shown in Table 1. Three of four untreated animals died, compared with none of eight rabbits treated with LY-303366 at 5 mg/kg/day and none of seven treated with 10 mg/kg/day. All amphotericin B-treated animals also survived.
(ii) Prophylaxis.
Mortality data for rabbits receiving LY-303366 at either 5 or 10 mg/kg/day 48 h before lethal or sublethal challenge are also shown in Table 1, and are compared with those for untreated animals and those for animals given prophylaxis with amphotericin B at 1 mg/kg/day. None of the LY-303366- or amphotericin B-treated rabbits died.
Tissue cultures. (i) Ravuconazole.
The results of semiquantitative cultures of liver, lung, kidney, and brain tissues from lethally challenged animals are shown in Table 2. Extensive infection with A. fumigatus was present in the liver, lung, kidney, and brain tissues of all untreated control rabbits. Treatment with ravuconazole at 30 mg/kg/day reduced the tissue burden by approximately 2 to 3 log units in all organ tissues, as did treatment with amphotericin B at 1 mg/kg/day. All organ cultures from 9 of 10 ravuconazole- and 4 of 4 amphotericin B-treated animals were sterile; only 1 of 10 ravuconazole-treated rabbits had 10 or fewer CFU of A. fumigatus/g of tissue.
TABLE 2.
Results of organ cultures with ravuconazole or amphotericin B begun 24 h after challenge
| Inoculum | Treatmenta and dose (mg/kg) | Colony counts (mean log10 CFU/g of tissue ± SE)
|
No. of rabbits with positive cultures/total no.
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Liver | Lung | Kidney | Brain | Liver | Lung | Kidney | Brain | ||
| Lethal | None (4) | 2.92 ± 0.00 | 3.32 ± 0.22 | 3.08 ± 0.94 | 2.28 ± 0.65 | 4/4 | 4/4 | 4/4 | 4/4 |
| Rav 30 (10) | 0.11 ± 0.33 | 0.07 ± 0.21 | 0.17 ± 0.37 | 0.14 ± 0.31 | 1/10 | 1/10 | 1/10 | 1/10 | |
| AmB 1.0 (4) | 0.00 | 0.00 | 0.00 | 0.00 | 0/4 | 0/4 | 0/4 | 0/4 | |
| Sublethal | None (4) | 2.82 ± 1.35 | 2.44 ± 0.06 | 1.72 ± 0.91 | 0.80 ± 0.71 | 4/4 | 4/4 | 4/4 | 2/4 |
| Rav 30 (10) | 0.09 ± 0.20 | 0.14 ± 0.26 | 0.06 ± 0.13 | 0.09 ± 0.14 | 1/10 | 1/10 | 1/10 | 1/10 | |
| AmB 1.0 (4) | 0.41 ± 0.64 | 0.00 ± 0.00 | 0.60 ± 0.59 | 0.30 ± 0.42 | 1/4 | 1/4 | 1/4 | 1/4 | |
Rav, ravuconazole; AmB, amphotericin B.
The results of organ cultures of liver, lung, kidney, and brain tissues from sublethally challenged animals are also shown in Table 2. Infection with A. fumigatus was present in liver, lung, kidney, and brain tissues of all untreated control rabbits. Treatment with ravuconazole at 30 mg/kg/day or with amphotericin B at 1 mg/kg/day significantly reduced the tissue burden of A. fumigatus in these organs. Similarly, fewer than 10 CFU/g of tissue was recovered in only 1 of 10 ravuconazole-treated and 1 of 4 amphotericin B-treated animals; 9 ravuconazole-treated and 3 amphotericin B-treated rabbits had sterile organ cultures.
(ii) LY-303366.
The results of semiquantitative cultures of liver, lung, kidney, and brain tissues from lethally challenged rabbits are shown in Table 3. Infection with A. fumigatus was found in the liver, lung, kidney, and brain tissues of all untreated rabbits. Treatment with LY-303366 reduced the sizes of A. fumigatus colonies grown on semiquantitative organ cultures from the typical 0.5 to 1.5 cm in diameter to ≤0.1 cm in diameter. These microcolonies were most numerous in the liver and were also present in lung and kidney tissues, but not in brain tissue. Treatment with LY-303366 at 5 or 10 mg /kg did not significantly reduce the tissue burden in the brain. Treatment with amphotericin B (1.0 mg/kg) significantly reduced the tissue burden in the kidney and liver (P < 0.05 compared to controls) and reduced the tissue burden in the brain and lung.
TABLE 3.
Results of organ cultures with intravenously administered LY-303366 begun 24 h after challenge
| Inoculum | LY-303366 dose, in mg/kg (no. of rabbits cultured)a | Colony counts (mean log10 CFU/g of tissue ± SE)
|
No. of rabbits with positive cultures/no. cultured
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Liver | Lung | Kidney | Brain | Liver | Lung | Kidney | Brain | ||
| Lethal | None (4) | 3.36 ± 0.5 | 2.11 ± 0.04 | 2.78 ± 0.5 | 1.66 ± 0.5 | 4/4 | 4/4 | 4/4 | 4/4 |
| 10 (8) | 2.72 ± 0.4 | 2.64 ± 0.3 | 2.10 ± 0.5 | 1.10 ± 0.4 | 8/8 | 7/8 | 6/8 | 5/8 | |
| 5 (7) | 2.30 ± 0.6 | 1.86 ± 0.4d | 1.60 ± 0.5 | 0.92 ± 0.4 | 7/7 | 5/7 | 4/7 | 3/7 | |
| AmB 1.0 (3) | 0.0b | 0.75 ± 0.6 | 0.26 ± 0.2b | 0.0 | 0/3c | 1/3 | 0/3c | 1/3 | |
| Sublethal | None (4) | 3.25 ± 0.3 | 1.71 ± 0.5 | 1.88 ± 0.5 | 1.27 ± 0.7 | 4/4 | 3/4 | 2/4 | 3/4 |
| 10 (7) | 1.60 ± 0.5 | 1.42 ± 0.6 | 1.56 ± 0.4 | 0.90 ± 0.5 | 1/7c | 5/7 | 4/7 | 1/7 | |
| 5 (8) | 2.10 ± 0.6 | 2.01 ± 0.5 | 1.69 ± 0.5 | 1.41 ± 0.3 | 5/8 | 6/8 | 5/8 | 2/8 | |
| AmB 1.0 (3) | 0.10 ± 0.08b | 0.20 ± 0.2 | 0.0b | 0.95 ± 0.4 | 0/3c | 1/3 | 1/3 | 0/3 | |
AmB, amphotericin B.
P < 0.05 compared to controls (Wilcoxon rank sum test).
P < 0.05 compared to controls (Fisher's exact test).
P < 0.05 compared to LY-303366 at 10 mg/kg (Wilcoxon rank sum test).
The numbers of positive organ cultures for all rabbits given a lethal challenge of A. fumigatus are also shown in Table 3. There was no significant difference between the recovery of organisms from the organs of animals treated with LY-303366 and that from untreated control animals. Amphotericin B significantly reduced the number of positive liver and kidney cultures compared with that for controls (P < 0.05).
Results of semiquantitative cultures of liver, lung, kidney, and brain tissues of sublethally challenged rabbits are also shown in Table 3. Again, microcolonies were recovered from the liver, lung, and kidney. There was no significant reduction in aspergillus organisms in brains of rabbits treated with LY-303366 at 5 or 10 mg/kg. Treatment with amphotericin B sterilized kidney tissues and significantly reduced the tissue burden in the liver (P < 0.05).
The number of positive organ cultures for sublethally challenged rabbits treated with LY-303366 at 5 mg/kg was slightly less than that seen in the lethally challenged rabbits treated with the same dose of LY-303366 (Table 3). Treatment with LY-303366 at 10 mg/kg significantly reduced the number of positive liver cultures (P < 0.05), as did treatment with amphotericin B (P < 0.05).
The results of semiquantitative cultures of liver, lung, kidney, and brain tissues in lethally challenged rabbits given prophylaxis with LY-303366 at 5 or 10 mg/kg/day are shown in Table 4. Microcolonies of A. fumigatus were again recovered from the liver, lung and kidney, whereas normal-sized colonies of aspergillus were recovered from brain tissue. Lethally challenged rabbits given prophylaxis with amphotericin B had significantly reduced tissue burdens of aspergillus in the liver and lung. Similar results were observed in sublethally challenged rabbits given prophylaxis with LY-303366 at either 5 or 10/kg/day or amphotericin B (Table 4).
TABLE 4.
Results of organ cultures with intravenously administered LY-303366 begun 48 h prior to challenge
| Inoculum | LY-303366 dose, in mg/kg (no. of rabbits cultured)a | Colony counts (mean log10 CFU/g of tissue ± SE)
|
No. of rabbits with positive cultures/no. cultured
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Liver | Lung | Kidney | Brain | Liver | Lung | Kidney | Brain | ||
| Lethal | None (4) | 2.99 ± 0.3 | 3.16 ± 0.2 | 3.10 ± 0.3 | 1.86 ± 0.8 | 4/4 | 4/4 | 4/4 | 3/4 |
| 10 (6) | 2.31 ± 0.5 | 2.84 ± 0.4 | 1.85 ± 0.6 | 0.54 ± 0.2 | 5/6 | 5/6 | 3/6 | 1/6d | |
| 5 (6) | 3.18 ± 0.4 | 3.04 ± 0.4 | 2.38 ± 0.5 | 1.81 ± 0.5 | 6/6 | 6/6 | 6/6 | 5/6 | |
| AmB 1.0 (3) | 0.0b | 0.36 ± 0.2b | 1.31 ± 0.6 | 0.10 ± 0.08 | 0/3c | 0/3c | 1/3 | 0/3 | |
| Sublethal | None (4) | 2.82 ± 0.5 | 1.58 ± 0.2 | 1.08 ± 0.2 | 1.01 ± 0.5 | 3/4 | 3/4 | 4/4 | 1/4 |
| 10 (6) | 1.92 ± 0.4 | 2.12 ± 0.5 | 1.00 ± 0.4 | 0.61 ± 0.3 | 5/6 | 4/6 | 5/6 | 2/6 | |
| 5 (6) | 2.01 ± 0.6 | 1.81 ± 0.5 | 0.95 ± 0.4 | 0.56 ± 0.3 | 6/6 | 3/6 | 1/6ce | 1/6 | |
| AmB 1.0 (4) | 0.08 ± 0.07b | 0.73 ± 0.3 | 0.42 ± 0.2 | 0.23 ± 0.1b | 1/4 | 1/4 | 2/4 | 1/4 | |
AmB, amphotericin B.
P <0.05 compared to controls (Wilcoxon rank sum test).
P <0.05 compared to controls (Fisher's exact test).
P <0.05 compared to LY-303366 at 5 mg/kg (Fisher's exact test).
P <0.05 compared to LY-303366 at 10 mg/kg (Wilcoxon rank sum test).
The numbers of positive organ cultures in lethally and sublethally challenged rabbits given prophylaxis with LY-303366 or amphotericin B are also shown in Table 4. No significant differences were observed between animals given LY-303366 prophylaxis and controls, except in the brain tissues of lethally challenged rabbits given prophylaxis with 10 mg/kg/day and in the kidney tissues of sublethally challenged rabbits given prophylaxis with 5 mg/kg/day. Amphotericin B prophylaxis reduced the number of positive organ cultures (Table 4).
Antigenemia was dramatically reduced or eliminated in rabbits treated with ravuconazole at 30 mg/kg/day or amphotericin B at 1 mg/kg/day compared with that in untreated controls, whether they received a lethal or sublethal challenge with A. fumigatus (Table 5). All control rabbits had >50 ng of circulating aspergillus antigen per ml; median antigen values were 800 and 575 ng/ml for lethally and sublethally challenged animals, respectively. In contrast, none of 20 ravuconazole-treated rabbits had circulating antigen values of >50 ng/ml, and the median antigen values for all treated rabbits, both lethally and sublethally challenged, were 0 ng/ml (Table 5). These antigen values were similar to those observed for amphotericin B-treated animals.
TABLE 5.
Serum Aspergillus antigen values for temporarily immunosuppressed rabbits infected with A. fumigatus and treated with ravuconazole
| Inoculum (no. of organisms) | Treatmenta (mg/kg/day) | No. of rabbits with >50 ng of antigen per ml/ no. tested
|
Median ng of antigen/ml (range)
|
||
|---|---|---|---|---|---|
| Day 5b | Day 9c | Day 5b | Day 9c | ||
| Lethal (106) | None | 2/2 | 2/2 | 800 (600–1,000) | 1,000 (1,000) |
| Rav (30) | 0/10 | 0/10 | 0 (0–50) | 0 (0–50) | |
| AmB (1) | 0/4 | 0/4 | 0 (0–33) | 17.5 (0–40) | |
| Sublethal (105) | None | 2/2 | 2/2 | 575 (450–700) | 1,000 (1,000) |
| Rav (30) | 1/10 | 0/9 | 0 (0–100) | 0 (0–50) | |
| AmB (1) | 0/4 | 0/4 | 0 (0) | 23.5 (0–50) | |
Rav, ravuconazole; AmB, amphotericin B.
Day 5 indicates that the median antigen levels were calculated from the 3rd day of treatment.
Day 9 indicates that the median antigen levels were calculated from the final antigen concentration for each rabbit.
Antigenemia was also reduced or eliminated in rabbits either treated or given prophylaxis with LY-303366 at 5 or 10 mg/kg/day or amphotericin B at 1 mg/kg/day compared with that for untreated controls, whether they received a lethal or a sublethal challenge with A. fumigatus (Table 6). Every control animal had >50 ng of circulating aspergillus antigen per ml; median antigen values on day 5 were 1,325 and 350 ng/ml for lethally and sublethally challenged rabbits, respectively. Similar values were observed on day 9. In contrast, a number of animals either treated or given prophylaxis with LY-303366 at 5 or 10 mg/kg/day had circulating antigen values of >50 ng/ml, although the median antigen values in these groups of rabbits were below 50 ng/ml. Only one group of lethally challenged rabbits given prophylaxis with LY-303366 at 5 mg/kg/day had median antigen values of 57.5 ng/ml on day 9 of this study (Table 6). The remaining groups of rabbits had antigen values similar to those observed for animals given amphotericin B treatment or prophylaxis.
TABLE 6.
Serum Aspergillus antigen values for temporarily immunosuppressed rabbits infected with A. fumigatus and treated with LY-303366
| Inoculum and group | Druga (mg/kg/day) | No. of rabbits with >50 ng of antigen per ml/ no. tested
|
Median ng of antigen/ml (range)b
|
||
|---|---|---|---|---|---|
| Day 5c | Day 9d | Day 5c | Day 9d | ||
| Lethal | None | 4/4 | 4/4 | 1,325 (149–6,560) | 1,325 (400–6,560) |
| Treatment | LY (5) | 1/4 | 1/7 | 12* (0–55) | 13* (0–65) |
| LY (10) | 4/8 | 0/8 | 49* (14–105) | 22.5* (0–50) | |
| AmB (1) | 0/3 | 0/4 | 20* (0–33) | 37.5* (0–44) | |
| Prophylaxis | LY (5) | 3/6 | 4/6 | 40* (0–150) | 57.5 (6–140) |
| LY (10) | 0/6 | 0/6 | 2* (0–47) | 10** (0–31) | |
| AmB (1) | 1/4 | 0/4 | 9* (0–80) | 2** (0–22) | |
| Sublethal | None | 4/4 | 4/4 | 350 (110–700) | 385 (350–1,300) |
| Treatment | LY (5) | 2/8 | 2/8 | 11.5** (0–150) | 34* (0–63) |
| LY (10) | 3/7 | 0/7 | 29* (0–80) | 11* (0–40) | |
| AmB (1) | 0/4 | 0/4 | 2* (0–12) | 4.5* (0–11) | |
| Prophylaxis | LY (5) | 1/5 | 0/6 | 0** (0–60) | 14* (0–43) |
| LY (10) | 0/5 | 1/6 | 0* (0–26) | 2* (0–55) | |
| AmB (1) | 0/4 | 0/4 | 7* (0–31) | 0* (0–21) | |
LY, LY-303366; AmB, amphotericin B.
P < 0.01 compared with controls by the Wilcoxon rank sum test; **, P <0.05 compared with controls by the Wilcoxon rank sum test.
Day 5 indicates that the median antigen levels were calculated from the 3rd day of treatment.
Day 9 indicates that the median antigen levels were calculated from the final antigen concentration for each rabbit.
DISCUSSION
Immunocompromised patients with invasive aspergillosis continue to have a poor prognosis despite therapy with antifungal agents (1, 5, 7). Newer antifungal agents, as well as newer preparations of older agents, have been developed in an attempt to increase efficacy, decrease toxicity, and improve our ability to treat invasive aspergillosis. The newer azoles and echinocandins were developed because they appeared to offer potential advantages in the treatment of invasive fungal infections, such as oral and intravenous preparations, minimal acute toxicity, and reduced nephrotoxicity (11, 12).
Ravuconazole is a new oral triazole antifungal agent with excellent broad-spectrum in vitro activity against most yeasts and molds including Candida, Cryptococcus, and Aspergillus spp. (9, 15, 27). Ravuconazole, like other azoles, exerts its antifungal effect by inhibition of cytochrome P450-dependent C-14 α-demethylase, which is responsible for the conversion of lanosterol to ergosterol (2). An intravenous preparation of ravuconazole is under development. LY-303366 is an investigational, semisynthetic antifungal agent derived from echinocandin B which has in vitro and in vivo activity against Candida spp., including fluconazole-resistant isolates, A. fumigatus, Histoplasma capsulatum, and Blastomyces dermatitidis but lacks activity against cryptococci (2, 8). The echinocandins prevent cell wall synthesis by noncompetitive inhibition of 1,3-β-glucan synthase (2). Pharmacokinetic studies indicated a long half-life, i.e., 13 h for ravuconazole and 12.5 h for LY-303366, in our immunosuppressed rabbit model which permitted once daily dosing. Levels in serum were higher with increasing doses of both antifungal agents, and no significant drug accumulation was observed after 6 days of therapy with either drug.
In earlier studies with our immunosuppressed rabbit model of invasive aspergillosis, amphotericin B deoxycholate alone as well as a liposomal preparation of amphotericin B, high-dose fluconazole alone, saperconazole alone, an experimental azole, SCH 39304, alone, and the new azole voriconazole (UK 109496) alone significantly reduced mortality as well as the level of aspergillus antigen in the serum, which correlated with a reduced tissue burden of A. fumigatus compared with that for untreated control animals (3, 10, 11, 18–24, 28). However, in these earlier studies, only amphotericin deoxycholate sterilized tissues (3, 24, 25). In the present studies, oral treatment with ravuconazole at 30 mg/kg/day not only reduced mortality and serum aspergillus antigen levels to zero but also significantly reduced the tissue burden of Aspergillus organisms and sterilized these tissues in 90% of lethally and sublethally challenged rabbits. The treatment dose of 30 mg/kg/day was selected based on the pharmacokinetic experiments, which were performed prior to the efficacy studies, i.e., peak serum drug levels in the rabbit with this dose are comparable to those observed in humans given a daily dose of 400 mg orally. These observations were comparable to the results we observed with amphotericin B deoxycholate therapy. These results also support the previous observations of the efficacy of ravuconazole in the treatment of murine models of A. fumigatus, Candida albicans, and Cryptococcus neoformans infections (14).
Intravenous treatment with the echinocandin LY-303366 reduced both mortality and serum aspergillus antigen levels in lethally and sublethally challenged rabbits as well as in animals given prophylaxis 2 days before challenge, compared with untreated controls. However, the tissue burden of Aspergillus organisms was not reduced. In fact, significant numbers of microcolonies of A. fumigatus were recovered from all organs cultured at the end of the experimental period. Furthermore, these microcolonies, after further in vitro incubation, reverted to macrocolonies of A. fumigatus. Although mortality in LY-303366-treated animals was reduced and serum aspergillus antigen levels were lowered, antigen was not completely cleared from the serum to the same extent as was observed in ravuconazole-treated rabbits. Our results with LY-303366 support the previous observations of the efficacy of similar doses of LY-303366 in a rabbit model of pulmonary aspergillosis reported by others (25). In fact, our observation that neither the 5- nor the 10-mg/kg dose of LY-303366 given as treatment or prophylaxis reduced the tissue burden of Aspergillus organisms is consistent with the observations of Petraitis and colleagues reported earlier (25). Furthermore, their studies identified a dose-dependent damage of hyphal structures in lung tissues of LY-303366-treated rabbits characterized by “a progressive reduction in length and increasing swelling of hyphal elements” (25). These investigators also concluded that the individual damaged hyphal units appeared to remain viable (25). Although we did not perform similar histopathological studies, our gross observations of microcolonies on culture, as well as microscopic examination of these colonies, strongly support their observations. Also, subcultures of these microcolonies resulted in macrocolonies similar to the original organism used to challenge the animals, indicating that the organisms recovered as microcolonies were clearly viable.
Our experimental model, like all models of lethal infection, does not permit simultaneous culturing of organ tissues from untreated controls and from treated animals, since the untreated controls die before the final day of the experimental protocol. However, in previous studies, using a sublethal challenge, we have shown that untreated control animals surviving until sacrifice have a tissue burden virtually identical to that of untreated controls for which cultures were made at autopsy (20).
During the past 15 years we have used this immunosuppressed-rabbit model of invasive aspergillosis to study the efficacy of amphotericin B deoxycholate, liposomal amphotericin B, and the azoles fluconazole, saperconazole, SCH 39304, itraconazole and UK 109496 (voriconazole) (3, 10, 11, 18–24, 28). Only amphotericin B preparations consistently eliminated A. fumigatus from organ tissues of both lethally and sublethally challenged rabbits. Based upon the observations reported here, the new triazole ravuconazole is the only azole which we have studied to date that has antifungal activity comparable to that of amphotericin B in our immunosuppressed-rabbit model of invasive aspergillosis.
In conclusion, the echinocandin LY-303366 prolonged survival and reduced aspergillus antigenemia but did not eliminate aspergillus organisms from organ tissues. In contrast, the new triazole ravuconazole eliminated mortality, cleared aspergillus antigen from the serum, and eliminated A. fumigatus organisms from tissues in almost all immunosuppressed animals with invasive aspergillosis. Further studies are needed to determine the therapeutic potential of ravuconazole in the treatment of invasive aspergillosis.
ACKNOWLEDGMENTS
This work was supported by grants from Eli Lilly and Company and by the Bristol-Myers Squibb Pharmaceutical Research Institute.
REFERENCES
- 1.Andriole V T. Aspergillus infections: problems in diagnosis and treatment. Infect Agents Dis. 1996;54:47–54. [PubMed] [Google Scholar]
- 2.Andriole V T. The 1998 Garrod Lecture. Current and future antifungal therapy: new targets for antifungal agents. J Antimicrob Chemother. 1999;44:151–162. doi: 10.1093/jac/44.2.151. [DOI] [PubMed] [Google Scholar]
- 3.Andriole V T, Miniter P, George D, Kordick D, Patterson T F. Animal models: usefulness for studies of fungal pathogenesis and drug efficacy in aspergillosis. Clin Infect Dis. 1992;14(Suppl.):S134–S138. doi: 10.1093/clinids/14.supplement_1.s134. [DOI] [PubMed] [Google Scholar]
- 4.Bartroli J, Turmo E, Alguero M, Boncompte E, Vericat M L, Conte L, Ramis J, Merlos M, Garcia-Rafanell J, Forn J. New azole antifungals. 3. Synthesis and antifungal activity of 3-substituted-4(3H)-quinazolinones. J Med Chem. 1998;4:1869–1882. doi: 10.1021/jm9707277. [DOI] [PubMed] [Google Scholar]
- 5.Bodey G P, Vartivarian S. Aspergillosis. Eur J Clin Microbiol Infect Dis. 1989;5:413–437. doi: 10.1007/BF01964057. [DOI] [PubMed] [Google Scholar]
- 6.DeGregorio M W, Lee W M F, Linker C A, Jacobs R A, Ries C A. Fungal infections in patients with acute leukemia. Am J Med. 1982;73:543–548. doi: 10.1016/0002-9343(82)90334-5. [DOI] [PubMed] [Google Scholar]
- 7.Denning D W, Stevens D A. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev Infect Dis. 1990;12:1147–1201. doi: 10.1093/clinids/12.6.1147. [DOI] [PubMed] [Google Scholar]
- 8.Ernst M E, Klepser M E, Wolfe E J, Pfaller M A. Antifungal dynamics of LY-303366, an investigational echinocandin B analog, against Candida spp. Diagn Microbiol Infect Dis. 1996;26:125–131. doi: 10.1016/s0732-8893(96)00202-7. [DOI] [PubMed] [Google Scholar]
- 9.Fung-Tomc J C, Huczko E, Minassian B, Bonner D P. In vitro activity of new oral triazole, BMS-207147 (ER-30346) Antimicrob Agents Chemother. 1998;42:313–318. doi: 10.1128/aac.42.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George D, Kordick D, Miniter P, Patterson T F, Andriole V T. Combination therapy in experimental invasive aspergillosis. J Infect Dis. 1993;168:692–698. doi: 10.1093/infdis/168.3.692. [DOI] [PubMed] [Google Scholar]
- 11.George D, Miniter P, Andriole V T. Efficacy of UK-109496, a new azole antifungal agent, in an experimental model of invasive aspergillosis. Antimicrob Agents Chemother. 1996;40:86–91. doi: 10.1128/aac.40.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graybill J R. New antifungal agents. Eur J Clin Microbiol Infect Dis. 1989;5:402–412. doi: 10.1007/BF01964056. [DOI] [PubMed] [Google Scholar]
- 13.Graybill J R, Kaster S R. Experimental murine aspergillosis. Comparison of amphotericin B and a new polyene antifungal drug, SCH 28191. Am Rev Respir Dis. 1984;129:292–295. [PubMed] [Google Scholar]
- 14.Hata K, Kimutra J, Miki H, Toyosawa T, Moriyama M, Katsu K. Efficacy of ER-30346, a novel oral triazole antifungal agent, in experimental models of aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1996;40:2243–2247. doi: 10.1128/aac.40.10.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hata K, Kimutra J, Miki H, Toyosawa T, Nakamura T, Katsu K. In vitro and in vivo antifungal activities of ER-30346, a novel oral triazole with a broad antifungal spectrum. Antimicrob Agents Chemother. 1996;40:2237–2242. doi: 10.1128/aac.40.10.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer R D, Young L S, Armstrong D, Yu B. Aspergillosis complicating neoplastic disease. Am J Med. 1973;54:6–15. doi: 10.1016/0002-9343(73)90077-6. [DOI] [PubMed] [Google Scholar]
- 17.Oakley K L, Moore C B, Denning D W. In vitro activity of the echinocandin antifungal agent LY-303366 in comparison with itraconazole and amphotericin B against Aspergillus spp. Antimicrob Agents Chemother. 1998;42:2726–2730. doi: 10.1128/aac.42.10.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson T F, Fothergill A W, Rinaldi M G. Efficacy of itraconazole solution in a rabbit model of invasive aspergillosis. Antimicrob Agents Chemother. 1993;37:2307–2310. doi: 10.1128/aac.37.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson T F, George D, Ingersoll R, Miniter P, Andriole V T. Efficacy of SCH 39304 in treatment of experimental invasive aspergillosis. Antimicrob Agents Chemother. 1991;35:1985–1988. doi: 10.1128/aac.35.10.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson T F, George D, Miniter P, Andriole V T. The role of fluconazole in the early treatment and prophylaxis of experimental invasive aspergillosis. J Infect Dis. 1991;164:575–580. doi: 10.1093/infdis/164.3.575. [DOI] [PubMed] [Google Scholar]
- 21.Patterson T F, George D, Miniter P, Andriole V T. Saperconazole therapy in a rabbit model of invasive aspergillosis. Antimicrob Agents Chemother. 1992;36:2681–2685. doi: 10.1128/aac.36.12.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson T F, Miniter P, Andriole V T. Efficacy of fluconazole in experimental invasive aspergillosis. Rev Infect Dis. 1990;12(Suppl. 3):S281–S285. doi: 10.1093/clinids/12.supplement_3.s281. [DOI] [PubMed] [Google Scholar]
- 23.Patterson T F, Miniter P, Dijkstra J, Szoka F C, Ryan J L, Andriole V T. Treatment of experimental invasive aspergillosis with novel amphotericin B/cholesterol-sulfate complexes. J Infect Dis. 1989;159:717–724. doi: 10.1093/infdis/159.4.717. [DOI] [PubMed] [Google Scholar]
- 24.Patterson T F, Miniter P, Ryan J L, Andriole V T. Effect of immunosuppression and amphotericin B on aspergillus antigen in an experimental model. J Infect Dis. 1988;158:415–422. doi: 10.1093/infdis/158.2.415. [DOI] [PubMed] [Google Scholar]
- 25.Petraitis V, Petraitiene R, Groll A H, Bell A, Callender D P, Sein T, Schaufele R L, McMillian C L, Bacher J, Walsh T J. Antifungal efficacy, safety, and single-dose pharmacokinetics of LY-303366, a novel echinocandin B, in experimental pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob Agents Chemother. 1998;42:2898–2905. doi: 10.1128/aac.42.11.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaller M A, Marco F, Messer S A, Jones R N. In vitro activity of two echinocandin derivatives, LY-303366 and MK-0991 (L-763,792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn Microbiol Infect Dis. 1998;30:251–255. doi: 10.1016/s0732-8893(97)00246-0. [DOI] [PubMed] [Google Scholar]
- 27.Pfaller M A, Messer S A, Hollis R J, Jones R N, Doern G V, Brandt M E, Hajjeh R A. In vitro susceptibilities of Candida bloodstream isolates to the new triazole antifungal agents BMS-207147, SCH 56592, and voriconazole. Antimicrob Agents Chemother. 1998;42:3242–3244. doi: 10.1128/aac.42.12.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabetta J R, Miniter P, Andriole V T. The diagnosis of invasive aspergillosis by enzyme-linked immunosorbent assay for circulating antigen. J Infect Dis. 1985;152:946–953. doi: 10.1093/infdis/152.5.946. [DOI] [PubMed] [Google Scholar]